Abstract
Objectives
Mindfulness meditation (MM) is an attention and acceptance–based intervention effective for managing chronic pain. Current literature predominately focuses on the behavioral effects of short-term mindfulness-based programs for pain reduction. However, the long-term potential of MM and its effect on pain processing are less well understood. Furthermore, it is possible that short- and long-term effects of MM are underpinned by different neural processes. This systematic review was undertaken to better understand the short- and long-term effects of MM on brain processes related to pain by comparing pain-related neural process in novice and expert MM.
Methods
A literature search was performed to identify relevant studies using MRI/fMRI and EEG/MEG.
Results
A total of 14 studies were selected: 1 MEG and fMRI, 5 EEG, and 8 MRI/fMRI. Overall, findings across studies are consistent in reporting reduced pain ratings in both novice and expert meditators. However, different brain processes appeared to underlie this effect with experts showing greater activity in the somatosensory regions and novices showing reduced activity. The available evidence also indicates a greater dissociation between pain salience and pain unpleasantness in expert meditators along with greater changes in the respective brain regions, suggesting a dissociation between sensory and the cognitive-affective dimensions of pain. For novice meditators, however, the evidence is less conclusive.
Conclusions
Given the ongoing nature of chronic pain, the long-term effects of mindfulness meditation should be explored to assess whether the effects of short-term programs remain post treatment.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
When pain persists beyond the typical tissue healing period of 3 months, it is classified as chronic pain (Treede et al. 2015). In the majority of cases, the experience of ongoing pain has a significant impact on quality of life and general well-being (Cimmino et al. 2011; Holmes et al. 2013; Skinner et al. 2004). Pain perception, like all sensory-related experiences, is a multidimensional process, influenced by a combination of biological, cognitive, affective, and social factors (Linton and Shaw 2011; Raja et al. 2020). Management options, however, have traditionally focused on treating the sensory experience of pain through invasive options such as surgery and prescriptive medication. Although some relief can be achieved, surgical procedures are limited to a select sample of patients (Farrell et al. 2018), while pharmaceutical interventions carry the risk of addiction and have relatively limited evidence for their long-term use (Crofford 2010; Palmer et al. 2015). Furthermore, complete elimination of pain is seldom achieved through these options alone (Noble et al. 2010). Pain management programs therefore require multiple disciplines to address not only the sensory but also the psychological and emotional factors that shape pain perception. Cognitive behavioral therapy (CBT), for example, targets maladaptive belief systems that contribute to the ongoing maintenance of pain. However, evidence for the efficacy of CBT in the treatment of chronic pain shows moderate effect sizes (Burger et al. 2016; Williams et al. 2012), with some patients reporting little to no benefit (Vlaeyen and Morley 2005). Consequently, mindfulness meditation (MM) training/or practice has gained increasing popularity as a therapeutic option with promising evidence to support its pain-relieving properties (Hilton et al. 2017; Kabat-Zinn 1982; Veehof et al. 2011; Zeidan et al. 2011).
Mindfulness meditation is operationalized as (1) self-regulation of attention to the features of internal and external events, (2) developing an understanding that these experiences are transitory and momentary, and (3) reduced appraisal or reactions to these experiences (Zeidan et al. 2012). In pain management, mindfulness-based pain interventions are utilized to reduce the psychological and emotional impact of prolonged pain (e.g., pain-related worrying and distress) by building pain acceptance (Marchand 2012). Pain acceptance has been described as “acknowledging that one has pain, giving up unproductive attempts to control pain, acting as if pain does not necessarily imply disability, and being able to commit one’s efforts toward living a satisfying life despite pain” (McCracken 1998, p. 22). This attitudinal change has been shown to influence functioning with greater acceptance predicting reduced pain reports, lower mood scores, and greater engagement in activities (Esteve et al. 2007; McCracken 1998; Morone et al. 2008; Vowles et al. 2007). Many living with chronic pain develop maladaptive emotional and behavioral responses as a result; for example, pain-related anxiety is often greater in patients with chronic pain and is typically correlated with a greater level of avoidance for physical activities (Linton and Shaw 2011). Cultivating acceptance to unpleasant experiences is thought to uncouple cognitive-affective components from sensory pain, thereby reducing the emotional and psychological impact of pain, improving any associated functional impairments (Grant and Rainville 2009; Kabat-Zinn et al. 1986). Thus, mindfulness-based pain interventions are better characterized within a functional framework, where the emphasis is not placed on changing the pain felt, but modifying one’s behavior and emotional response to their pain, improving functioning and quality of life (McCracken et al. 2007).
Early evidence for the effectiveness of mindfulness-based pain interventions indicate that short-term structured programs can improve pain symptoms and enhance quality of life (Hilton et al. 2017; Salomons and Kucyi 2011; Veehof et al. 2011). Marikar Bawa et al. (2015) reported that mindfulness-based interventions had a combined effect size of 0.16 (95% CI = − 0.03 to 0.36) on pain intensity across eight studies. A more recent analysis (Hilton et al. 2017) of 30 randomized controlled trials (RCTs) found a significant treatment effect of mindfulness meditation on chronic pain (standard mean difference (SMD) = 0.32; 95% CI = 0.09 to 0.54) compared with treatment as usual and passive control conditions. Another review and meta-analysis in the same year focused on mindfulness-based treatments for low back pain (Anheyer et al. 2017). Congruent with other reviews, the authors found a significant effect (SMD = − 0.96; 95% CI = − 1.64 to − 0.34) of mindfulness-based interventions on pain intensity ratings. Overall, the evidence is in favor of the growing optimism for short-term mindfulness-based pain reduction. Less certain, however, are conclusions about the long-term potential of MM for pain. In the reviews cited, follow-up periods varied across studies with Hilton et al. (2017) reporting a range of 4 to 60 weeks across 30 studies. In the analysis of Anheyer et al. (2017), they reported that the short-term outcomes found in 4 studies were not sustained at long-term follow-ups. Additionally, studies across the reviews predominately adopted pain intensity as the primary outcome to assess the effectiveness of mindfulness-based interventions. Pain intensity ratings do not provide insight to the uncoupling of cognitive-affective and sensory pain dimensions which is the proposed mechanism by which mindfulness improves associated functional impairments (Grant and Rainville 2009; Zorn et al. 2020). Other measures such as quality of life, disability ratings, and the emotional and psychological responses to pain are equally if not more characteristic of MM-related effects on pain. However, few studies included these measures in their outcomes limiting the conclusions drawn.
Furthermore, while pain intensity ratings have been shown to decrease, whether pain acceptance and the uncoupling of cognitive-affective and sensory pain dimensions are achievable through short-term practices is uncertain. In the early phase of MM practice, the initial intention for chronic pain patients is likely motivated by a desire to improve pain symptoms, with intensity reduction as the main focus (Marchand 2014). Across time, however, the goal evolves from pain reduction to pain acceptance (Marchand 2014). Thus, the uncoupling of cognitive-affective from sensory dimensions of pain is less likely in the early phase of MM practice when the focus is placed on reducing sensory pain. This has been demonstrated in studies examining differences between novice and expert meditators. For example, Zorn et al. (2020) examined differences in pain perception (sensory and affective) between novice (approximately 20 h) and expert (more than 10,000 h) meditators. The authors used heat to induce pain and amplified the cognitive-affective aspects of pain by manipulating anticipation (pain anxiety). Assessing both pain intensity and pain unpleasantness ratings, a larger sensory-affective uncoupling effect was found in expert meditators compared to novices, as demonstrated by lower pain unpleasantness ratings in experts than novices, while pain intensity between groups remained comparable. The authors suggested the result was likely reflective of a key difference in pain perception between novice and expert meditators, indicating that expert meditators were more accepting of their experiences responding with less fear and worry. Furthermore, differences in emotional reactivity between novice and experienced meditators have been shown in separate studies examining brain function. For example, activation of the right amygdala to affective pictures (a putative measure of emotional reactivity) was found to be lower in both novice and experienced meditators compared to controls. However, experienced meditators scored lower on a questionnaire measuring emotional reactivity than novices (Five-Facet Mindfulness Questionnaire), while novice meditators did not differ from controls (Kral et al. 2018). In the same study, experienced meditators rated a greater number of affective pictures as neutral compared with novices, mirroring the functional magnetic resonance imaging (fMRI) data of amygdala activation. These results suggest that MM may reduce brain responses associated with emotional reactivity compared to controls; however, only experienced meditators have corresponding behavioral reductions. It is then conceivable that brief meditation training for pain is limited in its efficacy, and chronic pain management requires ongoing commitment to continued practice.
The heavy emphasis of using pain reduction as the primary outcome in examining the efficacy of mindfulness-based pain interventions does not accurately characterize its intended effects. Furthermore, whether short-term MM practice can foster pain acceptance, uncoupling sensory and cognitive-affective pain dimensions is unclear. As mentioned in the preceding paragraphs, one method to delineate whether novice and expert meditators process pain differently and assess the potential uncoupling of sensory and cognitive-affective dimensions is by comparing differences in pain-related neural processes. Brain imaging studies of MM-related changes in brain regions associated with pain processing have largely used two research methods, namely fMRI and electroencephalography (EEG). fMRI is a technique used to measure brain activity by detecting changes in blood flow (Chapin et al. 2012), while EEG records electrical brain activity with high temporal resolution, allowing for accurate examinations of event-related neural responses in the order of milliseconds (Kakigi et al. 2005a, b). In order to explore potential differences as a result of different levels of experience in MM, the current review seeks to add to the existing literature by systematically examining both functional neuroimaging and EEG studies assessing the influence of mindfulness on pain processing in novice and expert meditators, and to characterize the differences in pain processing between healthy mindfulness practitioners, participants with chronic pain, and healthy controls. The primary outcome of interest was the region-specific effects from mindfulness-related pain modulation. This includes fMRI event–related blood-oxygen-level-dependent (BOLD) response, EEG event–related response, EEG oscillation power, and EEG connectivity. The study sought to examine the significance of these activation and connectivity patterns and discuss whether they indicate practical importance as well as possible long-term effects.
Methods
Protocol and Registration
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; Moher et al. 2009). The protocol for this systematic review was registered on PROSPERO (CRD42019143227).
Search Strategy
A broad electronic search strategy was developed in consultation with the review team. The literature search was performed using the Ovid software in MEDLINE; Ovid MEDLINE® and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions®; Ovid PsycINFO; Ovid Embase; Ovid Cochrane Central Register of Controlled Trials; as well as the Cochrane Database of Systematic Reviews.
A combination of keywords was used across the selected databases (e.g., mindfulness, pain, neuroimaging). Search terms were adjusted across databases, and no date or language limitations were placed on the search. References of the selected studies were then searched manually to identify additional material. The searches were conducted again before final analyses, and new studies were screened for.
Two independent reviewers (MYW and JEP) were trained didactically prior to the literature search. Training included detailed summaries of the study’s aims, variables and outcomes being investigated, inclusion and exclusion criteria, and search methods for identification of studies and was concluded with a pilot screening using a random selection of sample abstracts. Official screening of studies underwent two phases. In phase 1, reviewers selected studies based on titles and abstracts deemed relevant for the present review. This was followed by full-text screenings of selected articles (phase 2 screening). Disagreements between reviewers regarding eligibility of selected studies were resolved through consultation with two independent reviewers (NWB and BMF) until consensus was reached.
Selection Criteria
The present review sought to examine the published effects of meditation on neural pain processes; thus, studies were included if they met the following a priori criteria: studies were included if (1) pain was induced in experimental settings (e.g., via thermal stimulus) or was related to an ongoing chronic pain disorder, (2) measures included a form of neuroimaging (fMRI, EEG/magnetoencephalography (MEG)), (3) the study included programs or training with a core focus on meditation, and (4) the article was published in peer-reviewed journals and written in English. Studies were excluded if they consisted solely of theoretical approaches or commentaries without statistical analysis.
Outcome Measures
The primary outcome of interest was the region-specific effects of MM practice on pain modulation. This included fMRI event–related BOLD response, gray matter density/volume, as well as EEG or MEG event–related responses, power, or connectivity. Behavioral pain measures commonly found in mindfulness and pain literature were also included, such as pain intensity, pain unpleasantness, and pain threshold.
Risk of Bias
A two-step process was developed to assess the risk of bias in selected studies. First, an adapted version of the Lievense criteria (Lievense et al. 2002) was used to assess the methodological quality. The Lievense criteria are a standardized set of 15 items assessing the internal validity of studies and can be modified to assess cohort, cross-sectional, and case-control studies. Each item is scored either positive (1) or negative (0), and results determine the overall study quality. Results from the Lievense criteria were then assessed using a tool adapted from the Cochrane Collaboration for cohort studies to determine the overall risk of bias for selected studies (Higgins and Green 2008). The adapted Cochrane tool consisted of 4 items for cross-sectional studies and 5 items for cohort studies. Items are rated (“low,” “moderate,” or “high”) in relation to scores on the Lievense criteria. Scores from the adapted Cochrane tool determined the overall risk of bias for each study; low (all items rated low), low-moderate (1 item rated moderate), moderate (2 items rated moderate), or high (> 2 items rated moderate or any of the items rated high). The stringent scoring criteria require all studies to be scored “low” on all items to be considered low risk (see Supplementary Tables 1 and 2 for a copy of Lievense criteria and Cochrane tool). Two reviewers (MYW and JEP) assessed the risk of bias for each selected study, independently. When there was a disagreement, results were compared and discussed until a consensus was reached.
Planned Meta-analysis
An activation likelihood estimation (ALE) meta-analysis was initially planned for this review (see PROSPERO registration). However, this was not feasible due to the small number of studies and lack of data appropriate for the execution of an ALE analysis. To aid the design of future research on this topic, we have provided our intended methodology in the supplementary materials.
Results
Study Characteristics
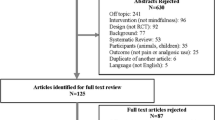
A total of 2118 records were identified in the online search (Fig. 1). After careful examination of abstracts, 2102 records were excluded. The remaining 14 studies were selected for the systematic review. Of these studies, one used MEG and fMRI, six used EEG/MEG, and nine studies used MRI/fMRI in their design. Of the nine MRI/fMRI studies, only one explored structural volumes with the remaining studies exploring task-related brain activation. Five of the EEG/MEG studies explored between-group differences while two were within-group studies. All MRI/fMRI studies used between-group experimental designs. Characteristics of selected studies are presented in Table 1.
Sample sizes of selected studies ranged from 1 to 61. Ten studies consisted of both genders, and four studies either recruited participants of one gender (N = 32) or did not mention the gender of subjects (N = 4). Nine of the eleven between-group studies recruited demographically matched participants in gender and age. Eleven studies induced experimental pain through a variety of methods (i.e., thermal, electrical, and laser stimuli), two studies investigated chronic pain, and another study explored EEG activity in novice meditators during a biopsy procedure without sedation.
In the 14 studies, there were a total of 16 experimental contrasts, eight of which explored pain-related neural effects in expert meditators, while the remaining investigated the effects of short meditation training. Meditation style and tradition varied across the eight experimental contrasts that included expert meditators. Expertise was uniquely defined in each experiment and ranged from hours to months and years. Four experiments compared differences between experts and controls, while two investigated differences between experts and novice meditators. Among studies that recruited participants for short-term meditation practice (novices), the length and type of training provided were again varied, with novice participants in one study having 5 months of meditation practice (Orme-Johnson et al. 2006), while another having 20 min of brief training prior to testing (Jensen et al. 2014).
Our assessment of risk of bias in the selected studies rated two studies without a comparison control group along with low participant numbers as high risk of bias, while twelve studies were rated low to moderate risk of bias (Tables 2 and 3). No studies met the full criteria for low risk of bias.
Pain Intensity and Unpleasantness Self-Reports
Eight studies differentiated between pain intensity and unpleasantness (Brown and Jones 2010, 2013; Gard et al. 2012; Grant et al. 2011; Lutz et al. 2008; Su et al. 2016; Zeidan et al. 2011, 2015), while seven studies only assessed pain intensity or sensitivity without mention of affective measures (Anand et al. 1960; Grant et al. 2010; Jensen et al. 2014; Kakigi et al. 2005a, b; Orme-Johnson et al. 2006; Ratcliff et al. 2018).
Of the studies that differentiated between the pain intensity and pain unpleasantness, the MM experts’ ratings of pain from three studies did not differ from controls in intensity or unpleasantness (Brown and Jones 2010; Gard et al. 2012; Grant et al. 2011). However, one study (Brown and Jones 2010) found significant results with experts reporting lower unpleasantness, when analysis only included expert meditators with six or more years of experience, compared with age-matched controls. In the same study, greater meditation experience within the expert group also predicted lower pain unpleasantness.
In novice meditators, three studies found reductions in both intensity and unpleasantness ratings post meditation training (Su et al. 2016; Zeidan et al. 2011, 2015), while another study (Brown and Jones 2013) did not find pain rating differences in participants with chronic pain after an 8-week mindfulness program. Only one study compared expert and novice meditators directly (Lutz et al. 2008), reporting similar intensity ratings between groups and lower pain unpleasantness ratings in the expert group relative to novices.
Oscillatory Activity (EEG and MEG)
Two studies (Anand et al. 1960; Kakigi et al. 2005a, b) reported prominent global alpha activity (power) in expert meditators at rest and a marked increase during meditation. When exposed to noxious stimuli (NS), experienced meditators in both studies showed stable and consistent activity, again with increased alpha power during meditation compared to rest. In one study (Anand et al. 1960), noxious stimulation inhibited alpha activity at rest in expert meditators. However, during meditation, consistent parietal alpha amplitudes were recorded even when sensory afferents were expected to be projecting in this region, indicating that noxious stimulation did not disturb the relaxed wakefulness state of expert meditators during meditation. However, it is important to note that both studies had low expert sample sizes (n = 4 and n = 1, respectively).
Two studies examined oscillatory activity in novice meditators. In one study, participants with chronic pain (Jensen et al. 2014) were asked to report pain ratings while they performed a cognitive task before and after meditation training. Participants with lower baseline alpha responded more to the meditative intervention, resulting in higher pain reduction following training (Jensen et al. 2014). This suggests the potential for individualized treatment recommendations or response prediction in future research. Another study (Ratcliff et al. 2018) used current source density (CSD) analysis to estimate the current sources generating measured potentials (Wójcik 2014). Novice meditators were asked to meditate while undergoing a biopsy procedure. The results showed higher beta CSD in the insular compared to controls and increases in theta CSD at the medial prefrontal cortex (mPFC), insular, and anterior cingulate cortex (ACC)—regions associated with self-awareness (Modinos et al. 2009; Nejad et al. 2019; Philippi et al. 2012). Novice meditators also reported less anxiety during the pain anticipation phase than controls, but their pain intensity ratings did not differ, demonstrating a dissociation between the emotional and sensory aspects of pain.
Meditation Effects on Pain-Related Brain Activity
Somatosensory Regions
Within the somatosensory regions, neuroimaging research has shown that while both novices and expert meditators show different patterns of neural activity in response to pain compared to controls, the direction of change sometimes differed between the two groups, perhaps suggesting a different mechanism of pain reduction for short- vs. long-term meditation. In two fMRI studies comparing pain-evoked activity during meditation and at rest, expert meditators showed greater pain-evoked activity in the SII and posterior insular compared to pain-evoked activity at rest (Gard et al. 2012), while novices showed significantly lower pain-evoked activation in the SI during meditation than rest (Zeidan et al. 2011). Cortical thickness in the SII and insular was also found to predict pain threshold in expert meditators (Grant et al. 2010). Similar results were also found in two EEG studies of participants at rest, with MM experts in one study showing lower pain-evoked responses in the SII and posterior insular compared to controls (Brown and Jones 2013), and another study reporting higher activity in the SII and insular post-meditation training for novices (Brown and Jones 2010). In a fMRI study directly comparing experts and novices during meditation, pain-evoked activity in the posterior insular and SII was not significantly different between groups (Lutz et al. 2008). The results indicate a difference in pain processing between novice and expert meditators, with greater activation in the primary somatosensory regions in experienced meditators and reduced activation in novice meditators.
Pain-evoked activity in the thalamus was consistent across fMRI studies for both expert and novice meditators. Lower thalamic response to pain stimuli was found in more experienced meditators (Grant et al. 2011; Kakigi et al. 2005a, b; Orme-Johnson et al. 2006). Similar to experts, three studies with novice meditators (Orme-Johnson et al. 2006; Zeidan et al. 2011, 2015) found reduced pain-evoked response in the thalamus post meditation training.
Cognitive-Affective Regions
For cognitive-affective region, consistent evidence across studies showed reduced activation in experienced meditators during noxious stimulation compared to controls. One fMRI study of expert meditators at rest (Orme-Johnson et al. 2006) showed lower pain-evoked responses in the ACC and prefrontal cortex (PFC) compared to controls. Greater meditation experience was also shown to predict lower pain-related ACC and prefrontal activation at rest (Grant et al. 2011), and correlated with thicker gray matter in the ACC (Grant et al. 2010). Two studies (Gard et al. 2012; Kakigi et al. 2005a, b) reported greater pain-related reduction in regions of the PFC during meditation compared to rest. Pain sensitivity (threshold needed to induce pain) was also predicted by a lower connectivity between dorsolateral prefrontal cortex (DLPFC) and dACC in experts (Grant et al. 2011). Furthermore, a negative relationship between pain-evoked activations of mPFC and pregenual ACC and pain unpleasantness ratings was found for expert meditators, while a positive relationship between these variables was found in controls, suggesting again a dissociation between affective and sensory regions in experts. For fMRI studies of novice meditators, one study reported lower pain unpleasantness ratings associated with greater activation of the orbitofrontal cortex (OFC) during meditation. The consistent reports of reduced activity in the ACC and prefrontal regions for expert meditators perhaps suggest that mindfulness-related analgesia functions by modulating affective mechanisms. However, it is unclear whether this effect is present in novice meditators.
Network Effects
Two studies on expert meditators reported congruent findings of lower baseline activity (without pain) in the salience network. The salience network is made up of the posterior insular, anterior insular, and midcingulate cortex (MCC) and is believed to be involved in the anticipation and assessment of pain-related cues (Ahmad and Abdul Aziz 2014; Craig et al. 2000; Iannetti and Mouraux 2010). One EEG study using source localization (Brown and Jones 2010) reported lower anticipatory MCC and insular activity in expert meditators compared to healthy controls at rest. Anticipatory MCC activity also correlated with pain unpleasantness in meditators, with lower activity predicting less unpleasantness. This effect was not found in controls. This finding, however, should be interpreted with caution, given the limited spatial resolution of EEG and difficulty localizing brain activity with EEG when not using MRI templates to assist source localization algorithms (Ploner and May 2018). Similar results were reported in a fMRI study comparing experts and novice meditators (Lutz et al. 2013), and baseline activity in the salience network (MCC and anterior insular) was greater for novice meditators than experts. Meditation experience also predicted lower baseline MCC (Brown and Jones 2010) and insular activity (Grant et al. 2011; Lutz et al. 2013), with the most experienced meditators showing the least activity.
In terms of pain-evoked responses, Brown and Jones (2010) found lower pain-evoked MCC and insular activity in experts compared to controls at rest. Comparing experts and novice meditators directly, greater activity in the MCC and anterior insular was found in experts during meditation than rest compared to controls (Lutz et al. 2013). In the same study, pain unpleasantness ratings correlated positively with activation in the left AI and aMCC (Lutz et al. 2013), but this relationship was not present for experts, even though expert meditators demonstrated greater activation in these clusters in response to pain during meditation. This indicates that during meditation, experts were more attuned to the sensory sensations of the painful stimuli; however, they did not perceive this as unpleasant, suggesting the dissociation between salience and unpleasantness, while this was not the case for novice meditators.
Discussion
This review aimed to assess pain-related neural processing differences between expert and novice meditators. Fourteen studies were selected, including both MRI/fMRI and EEG/MEG analyses. Our review suggested that the literature demonstrates common findings with regard to greater alpha power modulation in expert meditators during noxious stimulation. Differences in published results between novice and expert meditators were reported in brain regions related to pain processing, with greater activation in the primary somatosensory regions in experienced meditators and reduced activation in novice meditators. For cognitive-affective regions, consistent evidence of reduced activity in the ACC and prefrontal regions was found for expert meditators; however, the evidence is less consistent for whether this effect is present in novice meditators.
The evidence across studies indicates that meditation practice likely modulates alpha activity in both expert and novice meditators. This has been previously suggested by Kerr et al. (2013) whom argued that meditation practice increases alpha modulation, which functions to suppress the processing of sensory input. Greater alpha power has also been shown to predict lower pain scores (Nir et al. 2012), and enhancing alpha phase synchrony using neurofeedback can improve pain symptoms (Mayaud et al. 2019). The results also indicated that participants with lower baseline alpha responded more to the meditative intervention, resulting in higher pain reduction following training. This result suggests the potential for individualized treatment recommendations or response prediction in future research. However, it is important to note that although some evidence point to meditation-related changes in alpha power during noxious stimulation, two of the studies with expert meditators were rated as high in risk of bias with a small sample and no comparison group.
The contrasting activation patterns between novice and expert meditators in the somatosensory regions may be explained by the specific meditation instructions given to participants during the study. For example, in the study by Gard et al. (2012), expert meditators were asked to “bring their attention to the skin surface underneath the electrode on their forearm and to observe the sensations related to the stimuli, making sure to be mindful, accepting, and being aware of the transient nature of the stimuli” (p. 2694). While novice meditators in the study by Zeidan et al. (2011) were asked to “meditate by focusing on the changing sensations of the breath” (p. 5541). Somatosensory activity can be modulated by cognitive factors such as attention; for example, when attention is directed away from the pain stimulus, activity in the somatosensory region is reduced compared to when attention is focused on the stimulus (Bushnell et al. 1999). Thus, the differences found between groups may lie in the unique meditation instructions participants received. The evidence therefore does not indicate whether unique mechanisms were adopted by groups to modulate pain. Despite this, there is evidence to suggest that meditation training improves modulation of the somatosensory regions, resulting in pain reduction. Furthermore, the thalamus is considered a relay hub of sensory information (Iannetti and Mouraux 2010; Tracey and Mantyh 2007). The reduction of thalamic activity in response to pain in both experts and novices across the studies indicates that sensory signals were modulated before they reached the thalamus or that thalamus did not activate as strong signals, further suggesting a possible meditation-related effect on somatosensory processing.
The consistent reports of reduced activity in the ACC and prefrontal regions for expert meditators perhaps suggest that mindfulness-related analgesia functions by modulating affective mechanisms in more experienced meditators. The ACC is linked to the affective-motivational dimension of pain—emotional evaluation and behavioral response to pain (Lithwick et al. 2013; Moseley 2003), while three regions of the PFC, including the DLPFC, mPFC, and OFC, form to represent the cognitive-evaluative dimension of pain, involved in sensory discrimination, memory, and emotional response (Lithwick et al. 2013; Ong et al. 2019; Seminowicz and Moayedi 2017). Reduced pain-evoked activity in these regions indicates that expert meditators were less emotionally and psychologically reactive to the experience of pain, suggesting dissociation between salience and unpleasantness.
Finally, a notable finding in the present review relates to the homogenous reporting of lower or reduced pain ratings in both expert and novice meditators compared to controls. Notwithstanding the varying and at times conflicting neural activation patterns between groups, findings across studies are consistent in reporting reduced pain ratings in both novices and experts. The combination of consistent reports of pain reduction but varied reports of neural activation may suggest a distinct effect of meditation practice on pain attenuation across experience levels, with meditators likely engage in unique mechanisms to attenuate pain at different stages of practice, while still resulting in a similar behavioral report when measuring pain with simple self-report methods. However, as only eight studies differentiated between intensity and unpleasantness, it is less certain whether the reduction in pain reports relates to a dissociation between salience (the sensory aspect of pain) and unpleasantness (the cognitive and emotional aspect of pain which might be considered to be more reduced by a theoretical perspective on MM).
In summary, while preliminary evidence indicates modulation in both experts and novices of early sensory regions during noxious stimulation, the degree of change in higher pain processing regions (cognitive-affective) in novice meditators remains unclear. The results of existing studies suggest that expertise in meditation likely results in a greater dissociation between pain salience and unpleasantness, highlighting the cumulative nature of the effects of meditation practice, specifically relevant to conditions such chronic pain.
Limitations and Future Research
Overall, most studies were low in sample size and varied in study designs using a selection of meditation practices. Meditation training techniques can vary across traditions with unique effects on the brain. For example, in a morphometric neuroimaging review on meditation-related brain structure changes, structural changes in the insular cortex was found to depend on the particular type of meditative practice (Fox et al. 2014). In another review, discernible differences in neural activation and deactivation were observed in participants practicing different meditative techniques such as focused attention and mantra recitation (Fox et al. 2016). Thus, the type of meditation practiced by expert meditators may have unique effects on pain processing and thus may not be generalizable to all meditative practices. The conclusions we can draw from the field as it currently stands are also limited as study results tended to vary, with inconsistent evidence across different protocols. Several studies compared groups during meditation, while other compared groups at rest. It is uncertain whether meditation-induced state effects generalize to periods outside of meditation, and thus, the applicability of the evidence for chronic conditions remains unclear. Furthermore, studies predominantly evoked cortical responses using tonic pain which engage unique neural mechanisms to clinical pain (Apkarian et al. 2005). As such, any extrapolation of the results to chronic pain management needs to be interpreted with caution.
Existing studies have largely focused on assessing MM-related effects on pain intensity. Perceived pain reduction is a critical component of pain management, but this measure does not provide insight on whether MM enhances the dissociation of cognitive-affective and sensory pain dimensions. Associating brain changes with pain ratings does not necessarily provide insight to the functional impact of MM practice for individuals. Future studies could therefore investigate cognitive or behavioral functioning (e.g., attention, memory, reflexive response) in chronic pain patients before and after meditative practice and the associated neural changes, along with disability, quality of life, and well-being measures. Additionally, using phasic pain to assess neural habituation differences between novice and expert meditators at rest will provide insight to the level of adaptability and trait differences between short- and long-term meditation practices.
As both MM and chronic pain have been linked to multiple brain networks, research in other regions may be relevant. For example, connectivity of the default mode network (DMN) hubs is implicated in pain processing, self-orientated attention, and mind wandering (Baliki et al. 2014). There is evidence of DMN regions deactivating and activating during pain anticipation and perception, and greater DMN connectivity was associated with higher ratings of clinical pain (Loggia et al. 2013; Ter Minassian et al. 2013). Mindfulness has been shown to modulate the connectivity and network responses of the DMN, with considerable evidence demonstrating lower activation of DMN network clusters during pain in expert meditators compared to controls (Brewer et al. 2011; Ter Minassian et al. 2013; Wells et al. 2013). Thus, research into DMN function differences between novice and expert meditators may elucidate whether short-term meditation programs reduce attention to pain. Lastly, longitudinal studies of continued practice with concurrent brain imaging data are needed to determine the dose-response relationship of MM practice, thus informing clinical application of MM for pain treatment.
Overall, the available evidence indicates a greater dissociation between pain salience and pain unpleasantness in expert meditators, along with meditation-related changes in the respective brain regions. For novice meditators, however, the evidence remains less conclusive. However, findings across studies are consistent in reporting reduced pain ratings in both expert and novice meditators. Thus, irrespective of the degree of meditation-related changes in brain regions associated with emotional and cognitive aspects of pain perception, beginner meditators may still benefit from meditation practice. Future research may consider expanding self-report pain outcomes to interrogate the reported pain-related neural effects in experienced meditators.
References
References marked with an asterisk (*) are studies included in the systematic review.
Ahmad, A. H., & Abdul Aziz, C. B. (2014). The brain in pain. Malaysian Journal of Medical Sciences, 21, 45–53.
*Anand, B. K., Chhina, G. S., & Singh, B. (1960). Some aspects of electroencephalographic studies in Yogis. Electroencephalography and Clinical Neurophysiology, 13(3), 452–456. https://doi.org/10.1016/0013-4694(61)90015-3.
Anheyer, D., Haller, H., Barth, J., Lauche, R., Dobos, G., & Cramer, H. (2017). Mindfulness-based stress reduction for treating low back pain: A systematic review and meta-analysis. Annals of Internal Medicine, 166(11), 799–807. https://doi.org/10.7326/M16-1997.
Apkarian, A. V., Bushnell, M. C., Treede, R. D., & Zubieta, J. K. (2005). Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain, 9(4), 463. https://doi.org/10.1016/j.ejpain.2004.11.001.
Baliki, M. N., Mansour, A. R., Baria, A. T., & Apkarian, A. V. (2014). Functional reorganization of the default mode network across chronic pain conditions. PLoS One, 9(9). https://doi.org/10.1371/journal.pone.0106133.
Brewer, J. A., Worhunsky, P. D., Gray, J. R., Tang, Y. Y., Weber, J., & Kober, H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America, 108(50), 20254–20259. https://doi.org/10.1073/pnas.1112029108.
*Brown, C. A., & Jones, A. K. P. (2010). Meditation experience predicts less negative appraisal of pain: Electrophysiological evidence for the involvement of anticipatory neural responses. Pain, 150(3), 428–438. https://doi.org/10.1016/j.pain.2010.04.017
*Brown, C. A., & Jones, A. K. P. (2013). Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness-based pain management program. Clinical Journal of Pain, 29(3), 233–244. https://doi.org/10.1097/AJP.0b013e31824c5d9f
Burger, A. J., Lumley, M. A., Carty, J. N., Latsch, D. V., Thakur, E. R., Hyde-Nolan, M. E., Hijazi, A. M., & Schubiner, H. (2016). The effects of a novel psychological attribution and emotional awareness and expression therapy for chronic musculoskeletal pain: A preliminary, uncontrolled trial. Journal of Psychosomatic Research, 81, 1–8. https://doi.org/10.1016/j.jpsychores.2015.12.003.
Bushnell, M. C., Duncan, G. H., Hofbauer, R. K., Ha, B., Chen, J. I., & Carrier, B. (1999). Pain perception: Is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences of the United States of America, 96(14), 7705–7709. https://doi.org/10.1073/pnas.96.14.7705.
Chapin, H., Bagarinao, E., & Mackey, S. (2012). Real-time fMRI applied to pain management. Neuroscience Letters, 520(2), 174–181. https://doi.org/10.1016/j.neulet.2012.02.076.
Cimmino, M. A., Ferrone, C., & Cutolo, M. (2011). Epidemiology of chronic musculoskeletal pain. Best Practice and Research: Clinical Rheumatology, 25(2), 173–183. https://doi.org/10.1016/j.berh.2010.01.012.
Craig, A. D., Chen, K., Bandy, D., & Reiman, E. M. (2000). Thermosensory activation of insular cortex. Nature Neuroscience, 3(2), 184–190. https://doi.org/10.1038/72131.
Crofford, L. J. (2010). Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nature Reviews Rheumatology, 6(4), 191–197. https://doi.org/10.1038/nrrheum.2010.24.
Esteve, R., Ramírez-Maestre, C., & López-Martínez, A. E. (2007). Adjustment to chronic pain: The role of pain acceptance, coping strategies, and pain-related cognitions. Annals of Behavioral Medicine, 33(2), 179–188. https://doi.org/10.1007/BF02879899.
Farrell, S. M., Green, A., & Aziz, T. (2018). The current state of deep brain stimulation for chronic pain and its context in other forms of neuromodulation. Brain Sciences, 8(8). https://doi.org/10.3390/brainsci8080158.
Fox, K. C. R., Dixon, M. L., Nijeboer, S., Girn, M., Floman, J. L., Lifshitz, M., Ellamil, M., Sedlmeier, P., & Christoff, K. (2016). Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neuroscience and Biobehavioral Reviews, 65, 208–228. https://doi.org/10.1016/j.neubiorev.2016.03.021.
Fox, K. C. R., Nijeboer, S., Dixon, M. L., Floman, J. L., Ellamil, M., Rumak, S. P., Sedlmeier, P., & Christoff, K. (2014). Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neuroscience and Biobehavioral Reviews, 43, 48–73. https://doi.org/10.1016/j.neubiorev.2014.03.016.
*Gard, T., Hölzel, B. K., Sack, A. T., Hempel, H., Lazar, S. W., Vaitl, D., & Ott, U. (2012). Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cerebral Cortex, 22(11), 2692–2702. https://doi.org/10.1093/cercor/bhr352
*Grant, J. A., Courtemanche, J., Duerden, E. G., Duncan, G. H., & Rainville, P. (2010). Cortical thickness and pain sensitivity in Zen meditators. Emotion, 10(1), 43–53. https://doi.org/10.1037/a0018334
*Grant, J. A., Courtemanche, J., & Rainville, P. (2011). A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain, 152(1), 150–156. https://doi.org/10.1016/j.pain.2010.10.006
Grant, J. A., & Rainville, P. (2009). Pain sensitivity and analgesic effects of mindful states in Zen meditators: A cross-sectional study. Psychosomatic Medicine, 71(1), 106–114. https://doi.org/10.1097/PSY.0b013e31818f52ee.
Higgins, J. P., & Green, S. (2008). Cochrane handbook for systematic reviews of interventions: Cochrane book series. In Cochrane handbook for systematic reviews of interventions: Cochrane book series. https://doi.org/10.1002/9780470712184.
Hilton, L., Hempel, S., Ewing, B. A., Apaydin, E., Xenakis, L., Newberry, S., Colaiaco, B., Maher, A. R., Shanman, R. M., Sorbero, M. E., & Maglione, M. A. (2017). Mindfulness meditation for chronic pain: Systematic review and meta-analysis. Annals of Behavioral Medicine, 51(2), 199–213. https://doi.org/10.1007/s12160-016-9844-2.
Holmes, A., Christelis, N., & Arnold, C. (2013). Depression and chronic pain. The Medical Journal of Australia, 199(6), S17–S20. https://doi.org/10.5694/mja12.10589.
Iannetti, G. D., & Mouraux, A. (2010). From the neuromatrix to the pain matrix (and back). Experimental Brain Research, 205(1), 1–12. https://doi.org/10.1007/s00221-010-2340-1.
*Jensen, M. P., Sherlin, L. H., Fregni, F., Gianas, A., Howe, J. D., & Hakimian, S. (2014). Baseline brain activity predicts response to neuromodulatory pain treatment. Pain Medicine (United States), 15(12), 2055–2063. https://doi.org/10.1111/pme.12546.
Kabat-Zinn, J. (1982). An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry, 4 (1), 33–47. https://doi.org/10.1016/0163-8343(82)90026-3.
Kabat-Zinn, J., Lipworth, L., Burney, R., & Sellers, W. (1986). Four-year follow-up of a meditation-based program for the self-regulation of chronic pain: Treatment outcomes and compliance. Clinical Journal of Pain, 2(3), 159–173. https://doi.org/10.1097/00002508-198703010-00010.
*Kakigi, R., Inui, K., & Tamura, Y. (2005a). Electrophysiological studies on human pain perception. Clinical Neurophysiology, 116(4), 743–763. https://doi.org/10.1016/j.clinph.2004.11.016
Kakigi, R., Nakata, H., Inui, K., Hiroe, N., Nagata, O., Honda, M., Tanaka, S., Sadato, N., & Kawakami, M. (2005b). Intracerebral pain processing in a yoga master who claims not to feel pain during meditation. European Journal of Pain, 9(5), 581. https://doi.org/10.1016/j.ejpain.2004.12.006.
Kerr, C. E., Sacchet, M. D., Lazar, S. W., Moore, C. I., & Jones, S. R. (2013). Title: Mindfulness starts with the body: Somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Frontiers in Human Neuroscience, JAN. https://doi.org/10.3389/fnhum.2013.00012.
Kral, T. R. A., Schuyler, B. S., Mumford, J. A., Rosenkranz, M. A., Lutz, A., & Davidson, R. J. (2018). Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. NeuroImage, 181, 301–313. https://doi.org/10.1016/j.neuroimage.2018.07.013.
Lievense, A. M., Bierma-Zeinstra, S. M. A., Verhagen, A. P., van Baar, M. E., Verhaar, J. A. N., & Koes, B. W. (2002). Influence of obesity on the development of osteoarthritis of the hip: A systematic review. Rheumatology (Oxford, England), 41(10), 1155–1162 http://www.ncbi.nlm.nih.gov/pubmed/12364636.
Linton, S. J., & Shaw, W. S. (2011). Impact of psychological factors in the experience of pain. Physical Therapy, 91(5), 700–711. https://doi.org/10.2522/ptj.20100330.
Lithwick, A., Lev, S., & Binshtok, A. M. (2013). Chronic pain-related remodeling of cerebral cortex – ‘pain memory’: A possible target for treatment of chronic pain. Pain Management, 3(1), 35–45. https://doi.org/10.2217/pmt.12.74.
Loggia, M. L., Kim, J., Gollub, R. L., Vangel, M. G., Kirsch, I., Kong, J., Wasan, A. D., & Napadow, V. (2013). Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain, 154(1), 24–33. https://doi.org/10.1016/j.pain.2012.07.029.
Lutz, A., Brefczynski-Lewis, J., Johnstone, T., & Davidson, R. J. (2008). Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS One, 3(3). https://doi.org/10.1371/journal.pone.0001897.
*Lutz, A., McFarlin, D. R., Perlman, D. M., Salomons, T. V., & Davidson, R. J. (2013). Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. NeuroImage, 64(1), 538–546. https://doi.org/10.1016/j.neuroimage.2012.09.030
Marchand, W. R. (2012). Mindfulness-based stress reduction, mindfulness-based cognitive therapy, and Zen meditation for depression, anxiety, pain, and psychological distress. Journal of Psychiatric Practice, 18(4), 233–252. https://doi.org/10.1097/01.pra.0000416014.53215.86.
Marchand, W. R. (2014). Neural mechanisms of mindfulness and meditation: Evidence from neuroimaging studies. World Journal of Radiology, 6(7), 471. https://doi.org/10.4329/wjr.v6.i7.471.
Marikar Bawa, F. L., Mercer, S. W., Atherton, R. J., Clague, F., Keen, A., Scott, N. W., & Bond, C. M. (2015). Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. British Journal of General Practice, 65(635), e387–e400. https://doi.org/10.3399/bjgp15X685297.
Mayaud, L., Wu, H., Barthélemy, Q., Favennec, P., Delpierre, Y., Congedo, M., Dupeyron, A., & Ritz, M. (2019). Alpha-phase synchrony EEG training for multi-resistant chronic low back pain patients: An open-label pilot study. European Spine Journal, 28(11), 2487–2501. https://doi.org/10.1007/s00586-019-06051-9.
McCracken, L. M. (1998). Learning to live with the pain: Acceptance of pain predicts adjustment in persons with chronic pain. Pain, 74(1), 21–27. https://doi.org/10.1016/S0304-3959(97)00146-2.
McCracken, L. M., Gauntlett-Gilbert, J., & Vowles, K. E. (2007). The role of mindfulness in a contextual cognitive-behavioral analysis of chronic pain-related suffering and disability. Pain, 131(1–2), 63–69. https://doi.org/10.1016/j.pain.2006.12.013.
Modinos, G., Ormel, J., & Aleman, A. (2009). Activation of anterior insula during self-reflection. PLoS One, 4(2). https://doi.org/10.1371/journal.pone.0004618.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62(10), 1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005.
Morone, N. E., Greco, C. M., & Weiner, D. K. (2008). Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study. Pain, 134(3), 310–319. https://doi.org/10.1016/j.pain.2007.04.038.
Moseley, G. L. (2003). A pain neuromatrix approach to patients with chronic pain. Manual Therapy, 8(3), 130–140. https://doi.org/10.1016/S1356-689X(03)00051-1.
Nejad, A. B., Rotgé, J. Y., Valabregue, R., Guérin-Langlois, C., Hoertel, N., Gorwood, P., Dubertret, C., Limosin, F., Fossati, P., & Lemogne, C. (2019). Medial prefrontal disengagement during self-focus in formerly depressed patients prone to rumination. Journal of Affective Disorders, 247, 36–44. https://doi.org/10.1016/j.jad.2019.01.004.
Nir, R. R., Sinai, A., Moont, R., Harari, E., & Yarnitsky, D. (2012). Tonic pain and continuous EEG: Prediction of subjective pain perception by alpha-1 power during stimulation and at rest. Clinical Neurophysiology, 123(3), 605–612. https://doi.org/10.1016/j.clinph.2011.08.006.
Noble, M., Treadwell, J. R., Tregear, S. J., Coates, V. H., Wiffen, P. J., Akafomo, C., Schoelles, K. M., & Chou, R. (2010). Long-term opioid management for chronic noncancer pain. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.cd006605.pub2.
Ong, W. Y., Stohler, C. S., & Herr, D. R. (2019). Role of the prefrontal cortex in pain processing. Molecular Neurobiology, 56(2), 1137–1166. https://doi.org/10.1007/s12035-018-1130-9.
*Orme-Johnson, D. W., Schneider, R. H., Son, Y. D., Nidich, S., & Cho, Z. H. (2006). Neuroimaging of meditation’s effect on brain reactivity to pain. NeuroReport, 17(12), 1359–1363. https://doi.org/10.1097/01.wnr.0000233094.67289.a8
Palmer, R. E., Carrell, D. S., Cronkite, D., Saunders, K., Gross, D. E., Masters, E., Donevan, S., Hylan, T. R., & Von Kroff, M. (2015). The prevalence of problem opioid use in patients receiving chronic opioid therapy: Computer-assisted review of electronic health record clinical notes. Pain, 156(7), 1208–1214. https://doi.org/10.1097/j.pain.0000000000000145.
Philippi, C. L., Feinstein, J. S., Khalsa, S. S., Damasio, A., Tranel, D., Landini, G., Williford, K., & Rudrauf, D. (2012). Preserved self-awareness following extensive bilateral brain damage to the insula, anterior cingulate, and medial prefrontal cortices. PLoS One, 7(8). https://doi.org/10.1371/journal.pone.0038413.
Ploner, M., & May, E. S. (2018). Electroencephalography and magnetoencephalography in pain research - Current state and future perspectives. Pain, 159(2), 206–211. https://doi.org/10.1097/j.pain.0000000000001087.
Raja, S. N., Carr, D. B., Cohen, M., Finnerup, N. B., Flor, H., Gibson, S., Keefe, F. J., Mogil, J. S., Ringkamp, M., Sluka, K. A., Song, X.-J., Stevens, B., Sullivan, M. D., Tutelman, P. R., Ushida, T., & Vader, K. (2020). The revised International Association for the Study of Pain definition of pain. Pain. https://doi.org/10.1097/j.pain.0000000000001939.
*Ratcliff, C. G., Prinsloo, S., Chaoul, A., Zepeda, S. G., Cannon, R., Spelman, A., Yang, W. T., & Cohen, L. (2018). A randomized controlled trial of brief mindfulness meditation for women undergoing stereotactic breast biopsy. Journal of the American College of Radiology, 16(5), 691–699. https://doi.org/10.1016/j.jacr.2018.09.009
Salomons, T. V., & Kucyi, A. (2011). Does meditation reduce pain through a unique neural mechanism? Journal of Neuroscience, 31(36), 12705–12707. https://doi.org/10.1523/JNEUROSCI.2843-11.2011.
Seminowicz, D. A., & Moayedi, M. (2017). The dorsolateral prefrontal cortex in acute and chronic pain. Journal of Pain, 18(9), 1027–1035. https://doi.org/10.1016/j.jpain.2017.03.008.
Skinner, M. A., Zautra, A. J., & Reich, J. W. (2004). Financial stress predictors and the emotional and physical health of chronic pain patients. Cognitive Therapy and Research, 28(5), 695–713. https://doi.org/10.1023/B:COTR.0000045572.33750.6c.
*Su, I. W., Wu, F. W., Liang, K. C., Cheng, K. Y., Hsieh, S. T., Sun, W. Z., & Chou, T. L. (2016). Pain perception can be modulated by mindfulness training: A resting-state fMRI study. Frontiers in Human Neuroscience, 10(NOV2016). https://doi.org/10.3389/fnhum.2016.00570
Ter Minassian, A., Ricalens, E., Humbert, S., Duc, F., Aubé, C., & Beydon, L. (2013). Dissociating anticipation from perception: Acute pain activates default mode network. Human Brain Mapping, 34(9), 2228–2243. https://doi.org/10.1002/hbm.22062.
Tracey, I., & Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron, 55(3), 377–391. https://doi.org/10.1016/j.neuron.2007.07.012.
Treede, R. D., Rief, W., Barke, A., Aziz, Q., Bennett, M. I., Benoliel, R., Cohen, M., Evers, S., Finnerup, N. B., First, M. B., Giamberardino, M. A., Kaasa, S., Kosek, E., Lavand’homme, P., Nicholas, M., Perrot, S., Scholz, J., Schug, S., Smith, B. H., et al. (2015). A classification of chronic pain for ICD-11. Pain, 156(6), 1003–1007. https://doi.org/10.1097/j.pain.0000000000000160.
Veehof, M. M., Oskam, M. J., Schreurs, K. M. G., & Bohlmeijer, E. T. (2011). Acceptance-based interventions for the treatment of chronic pain: A systematic review and meta-analysis. Pain, 152(3), 533–542. https://doi.org/10.1016/j.pain.2010.11.002.
Vlaeyen, J. W. S., & Morley, S. (2005). Cognitive-behavioral treatments for chronic pain: What works for whom? Clinical Journal of Pain, 21(1), 1–8. https://doi.org/10.1097/00002508-200501000-00001.
Vowles, K. E., McCracken, L. M., & Eccleston, C. (2007). Processes of change in treatment for chronic pain: The contributions of pain, acceptance, and catastrophizing. European Journal of Pain, 11(7), 779–787. https://doi.org/10.1016/j.ejpain.2006.12.007.
Wells, R. E., Yeh, G. Y., Kerr, C. E., Wolkin, J., Davis, R. B., Tan, Y., Spaeth, R., Wall, R. B., Walsh, J., Kaptchuk, T. J., Press, D., Phillips, R. S., & Kong, J. (2013). Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: Pilot study. Neuroscience Letters, 556, 15–19. https://doi.org/10.1016/j.neulet.2013.10.001.
Williams, A. C. D. C., Eccleston, C., & Morley, S. (2012). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD007407.pub3.
Wójcik, D. K. (2014). Current source density (CSD) analysis. Encyclopedia of Computational Neuroscience. https://doi.org/10.1007/978-1-4614-7320-6_544-1.
Zeidan, F., Grant, J. A., Brown, C. A., McHaffie, J. G., & Coghill, R. C. (2012). Mindfulness meditation-related pain relief: Evidence for unique brain mechanisms in the regulation of pain. Neuroscience Letters, 520(2), 165–173. https://doi.org/10.1016/j.neulet.2012.03.082.
*Zeidan, Fadel, Emerson, N. M., Farris, S. R., Ray, J. N., Jung, Y., McHaffie, J. G., & Coghill, R. C. (2015). Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. Journal of Neuroscience, 35(46), 15307–15325. https://doi.org/10.1523/JNEUROSCI.2542-15.2015
*Zeidan, Fadel, Martucci, K. T., Kraft, R. A., Gordon, N. S., Mchaffie, J. G., & Coghill, R. C. (2011). Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience, 31(14), 5540–5548. https://doi.org/10.1523/JNEUROSCI.5791-10.2011
Zorn, J., Abdoun, O., Bouet, R., & Lutz, A. (2020). Mindfulness meditation is related to sensory-affective uncoupling of pain in trained novice and expert practitioners. European Journal of Pain (United Kingdom), 24(7), 1301–1313. https://doi.org/10.1002/ejp.1576.
Funding
PBF is supported by a National Health and Medical Research Council of Australia Practitioner Fellowship 659 (6069070).
Author information
Authors and Affiliations
Contributions
MYW designed and executed the review and also wrote the paper. NWB provided mindfulness research expertise and collaborated in the writing and editing of the final manuscript. JEP acted as an independent reviewer of the literature search and collaborated in the writing and editing of the final manuscript. PBF collaborated in the writing and editing of the final manuscript. BMF provided pain research expertise and collaborated in the writing and editing of the final manuscript. All author approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of Interest
All authors have no conflicts to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Wang, M.Y., Bailey, N.W., Payne, J.E. et al. A Systematic Review of Pain-Related Neural Processes in Expert and Novice Meditator. Mindfulness 12, 799–814 (2021). https://doi.org/10.1007/s12671-020-01558-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12671-020-01558-5