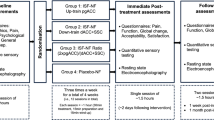
Abstract
Purpose
Chronic low back pain (cLBP) affects a quarter of a population during its lifetime. The most severe cases include patients not responding to interventions such as 5-week-long in-hospital multi-disciplinary protocols. This document reports on a pilot study offering an alpha-phase synchronization (APS) brain rehabilitation intervention to a population of n = 16 multi-resistant cLBP patients.
Methods
The intervention consists of 20 sessions of highly controlled electroencephalography (EEG) APS operant conditioning (neurofeedback) paradigm delivered in the form of visual feedback. Visual analogue scale for pain, Dallas, Hamilton, and HAD were measured before, after, at 6-month and 12-month follow-up. Full-scalp EEG data were analyzed to study significant changes in the brain’s electrical activity.
Results
The intervention showed a great and lasting response of most measured clinical scales. The clinical improvement was lasting beyond the 6-month follow-up endpoints. The EEG data confirm that patients did control (intra-session trends) and learned to better control (intersession trends) their APS neuromarker resulting in (nonsignificant) baseline changes in their resting state activity. Last and most significantly, the alpha-phase concentration (APC) neuromarker, specific to phase rather than amplitude, was found to correlate significantly with the reduction in clinical symptoms in a typical dose–response effect.
Conclusion
This first experiment highlights the role of the APC neuromarker in relation to the nucleus accumbens activity and its role on nociception and the chronicity of pain. This study suggests APC rehabilitation could be used clinically for the most severe cases of cLBP. Its excellent safety profile and availability as a home-use intervention makes it a potentially disruptive tool in the context of nonsteroidal anti-inflammatory drugs and opioid abuses.
Graphic abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lifetime prevalence of low back pain (LBP) is reported to be as high as 84%, and the prevalence of chronic LBP (cLBP) is about 23%, with 11–12% of the population being disabled by LBP [2]. Nonspecific LBP is the leading cause of years of unhealthy life in the world [48]. The prognosis of LBP patients is generally good since 90% of people recover within 3 months [45] thanks to a large therapeutic offering [29] including both pharmaceutical (nonsteroidal anti-inflammatory drugs (NSAIDS), opioids, paracetamol) and nonpharmaceutical interventions (massage, physical therapy, acupuncture) [36] including neuromodulation techniques such as repeated Magnetic Stimulation (rTMS) and transcranial Direct Current Stimulations (tDCS) [4].
The transition to chronicity has a major impact on the individual in his professional and personal life. For instance, it is the third leading cause of disability among those over 45 years of age [22]. A minority of patients, about 10%, remain at work after 6 months [42]. These chronic cases account for up to 80% of global costs of LBP; in France, summing up to €2.7b annually [17].
While most chronic patients eventually recover, there exists a severe subset of the cLBP patients that do not respond to offered therapeutic interventions, even including multi-disciplinary interventions [47]. These patients desperately need innovative and effective management of their condition.
The recent findings in chronic LBP suggest a functional reorganization of both the somatosensory and the motor system in relation to the level of chronicity [18]. This highlights the role of cortical plasticity in the development of chronic pain and suggests it could be appropriately leveraged using brain modulation methods such as neurofeedback (NFB). NFB is a self-paced brain neuromodulation technique that represents one’s brain activity in real-time using auditory or visual modulations, on which learning paradigms—such as operant conditioning [41] or voluntary control—can be applied. It has long been demonstrated that human is able to voluntarily modulate their brain activity [16]. There is also a long-standing evidence that several neuromodulation sessions mobilize brain plasticity and generates anatomical and functional changes in the brain [21]. Those changes have long been associated with clinical improvement in various dysfunction of the central nervous system (CNS) such as attention deficit disorder (ADD) [11] and post-traumatic stress disorder (PTSD) [38]. However, the clinical evidence for most of these fields still lingers due to technical and methodological shortcomings [11].
Existing literature on neurofeedback and chronic pain disorders is encouraging. In one study [35], chronic pain patients (n = 132, 90% respondents) were treated with alpha-synchronicity protocol in frontal areas leading to 90% of patients reporting alleviation of symptoms. Interestingly, the trained neuromarkers (alpha-amplitude) had long been associated with meditative states [24] suggesting a mode of action related to relaxation. In another study, patients with fibromyalgia were given forty NFB sessions; the participants reported significant decreases in physician-assessed tenderness, self-reported pain, attention and fatigue [12]. A study reported by Jensen et al. [27] suggests that the cortical modulation of chronic pain is reflected in EEG bandwidth activity including alpha. In a following study [28], the group reports a retrospective analysis of 18 patients with complex regional pain syndrome type I (CRPS-1) who had been given NFB training showing a statistically significant pre- to post-session decrease in pain intensity at the primary pain site.
The purpose of this project is the clinical evaluation of a novel therapeutic intervention intended to alleviate the symptoms of chronic LBP patients nonresponding to any other available therapeutic options. The secondary objective was to evaluate the clinical and neurofunctional changes induced by a brain rehabilitation program in a chronic LBP population.
Materials and methods
Participants
Patients were enrolled during 2016 at “Centre de l’Arche”, a tertiary day-care clinic for physical medicine and rehabilitation after giving their informed consent about the research. Inclusion criteria included the presence of cLBP resistant to any form of standard therapeutic approach including a 5-week-long intensive multi-disciplinary approach composed of pharmacological and nonpharmacological treatments such as physiotherapy and psychotherapy [8].
The functional rehabilitation of this program is centered around specific exercises, training in functional tasks, education, and work simulation/hardening. This treatment is guided by repeated testing, with an emphasis on feeding progress data back to the patient to enhance spinal mobility and strength. Psychological intervention involved a multi-modal disability management program consisting of four major areas [34]: (1) behavioral stress management training; (2) cognitive-behavioral skills training; (3) individual and group counseling emphasizing a crisis-intervention model; and (4) family counseling.
In order to limit the heterogeneity of the EEG recorded, only female participants were included in the study so as to discard known gender-related sources of EEG variability [5] on this small sample. Patients were told they could leave the research protocol at any time.
Ethical statements
All procedures involving human participants in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The protocol received clearance from a local ethical committee (Comité d’Ethique du CHU d’Angers, France, referenced Etude-2018/49).
Protocol and experimental setup
The treatment consists of 20 sessions of electroencephalographic (EEG) alpha-synchrony neurofeedback training. Each session consisted in six blocks of 5 min, preceded by a 2-min EEG recording under standard eyes-open (EO) and eyes-closed (EC) conditions. Sessions lasted approximately 1 h including EEG cap setup and removal.
The EEG signals were acquired on a 19-channels SmartBCI EEG system (NovaTech EEG Inc., Mesa, Az) with 19 silver chloride electrodes fitted to a neoprene cap at standard 10–20 locations: Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2, while referenced to linked ear lobes A1 and A2. Each electrode was connected to the patient’s scalp by the mean of electrolyte gel (bio-medical, USA) using a syringe with a blunt needle.
The EEG signal was digitized at 250 Hz and transmitted wirelessly via Bluetooth to a laptop running Mensia NeuroRT Training v2.3.1.0 (Mensia Technologies SA, Paris, France). NeuroRT Training is a general neurofeedback software for research offering a patient database management, online eye blink removal by blind source separation techniques and artifact detection by Riemannian geometry techniques. The software allows spectral surface (sensors) and tomographic (source) neurofeedback as well as the ability to build custom neurofeedback pipelines. For this study, a custom neurofeedback pipeline was implemented in order to reinforce alpha-synchrony (upward training) as an attempt to replicate and characterize learning response and specificity of training from previous studies [35]. The effectiveness of the device lies in the patient’s ability to modulate its brain activity, either by operant conditioning or voluntary control. To that effect, the digitized brain signals are analyzed to extract a representation of the targeted cortical area real-time activity, which is translated into a visual or auditory cue. The sensory feedback constitutes the rewards mechanism that promotes learning.
All data were stored in full resolution on a local computer for further analysis.
Clinical endpoints
Two types of clinical endpoints were considered for this study: electromyographic (EMG) and clinical scales, both recorded before and after the NFB training, for all subjects. In addition to this, the clinical scales were taken at 6- and 12-month follow-up.
EMG scores
The EMG scores represent the lateralized (left/right) median frequencies of the spinal erector muscle [15] during a hip motion with tight leg measured for three phases of the trunk flexion (forward bending starting from standing position and return): flexion, interphase flexion–extension and extension. A higher median frequency indicates a gain in the recruitment of motor units with bigger diameters and more dynamics, which is anti-correlated with muscular tiredness.
Clinical scales
In order to assess the clinical progression of these patients, several health questionnaires were taken:
-
A self-reported Body Pain visual analogue scale (VAS), from zero (no pain) to ten (maximal pain one can imagine);
-
The Hospital Anxiety and Depression scale (HAD) [52], an international self-reported scale used to assess depression or anxiety state from zero (normal) to twenty-one points for each marker; each subscale is considered clinically significant over eleven and the maximal accumulated score is forty-two; the HAD is used for its sensitivity to variations of mental state;
-
The Dallas scale [30] is a self-reported disability scale for patients with low back pain assessing the impact on everyday quality of life: work and leisure, anxiety, depression, and sociability; each subpart varies from zero to one hundred percent.
-
The Hamilton anxiety rating scale [23] includes fourteen items (quality of sleep, muscular tension, fears, somatic symptoms in cardiovascular and respiratory systems, neurovegetative…) each scoring from zero to four, summing up to a maximum value of fifty-six, which is considered clinically significant above twenty; this scale is particularly useful for our patients who are suffering from multiple pain and specifically muscle pain; it also proves relevant to capture variation.
All four clinical scales were taken before and after the neurofeedback intervention as well as at 6 and 12 months after the end of the intervention.
Real-time EEG data processing for training sessions
The processing of EEG time series in real-time is an essential component of the therapeutic intervention described here. Because, its details are lengthy and highly technical, yet necessary to provide full replicability of this work, there are provided in the Supporting Material. It describes the few steps that are essential to achieve a specific brain training and deliver therapeutic efficacy: preprocessing of the EEG, real-time eye blink artifact removal using blind source separation, the real-time estimation of signal quality using Riemannian geometry, the extraction of the alpha-phase synchrony neuromarker (APS), and the automated thresholding of the neuromarker to obtain rewards.
Post-hoc analysis of neuromarkers
Presence of learning and specificity
In terms of learning, one expects a subject to gradually learn to control a neuromarkers during a session (intra-session learning) and/or the changes to occur from session to sessions (intersession), reflecting lasting effects. We present in this section the results of these two learning modes at the group level. In order to gain a better understanding of the specificity of learning and its relation to clinical efficacy, different levels of learning were investigated:
-
Control To ensure that subjects did indeed acquire control of the targeted brain activity, its averaged activity within a session that is from block to block (each NFB session is composed of six blocks of 5 min of active training) was study to reveal the presence of a trend. Showing control of the neuromarker enables subsequent learning and lasting changes;
-
Learning To demonstrate that subjects did learn to control the neuromarker, we looked at the improvement in its averaged value across sessions. A positive trend, would arguably relate to an increased control over the targeted brain activity and demonstrate that learning has taken place;
-
Baseline changes Finally, we suggest changes in baseline activity as EO and EC resting state recordings taken at the beginning of each session would reflect lasting changes and reorganization the baseline brain activity.
To study these different levels of changes, the entire dataset was post-processed to extract the evolution of two prespecified neuromarkers: the APS defined above, sensitive to both amplitude and phase which was trained and the alpha-phase concentration (APC) [14] solely sensitive to phase and arguably relating more specifically to the modulation of the nucleus accumbens [26], which we believe relates more specifically to the symptoms of chronic pain and their evolution. Please refer to the Supporting Material for more details.
Mode of action
Finally, in order to explore the possible modes of actions, we are relying on a framework for the pathophysiology of cLBP that we detail in Fig. 1. This model builds on existing models of pain and chronic pain, in which ascending nociceptive inputs are modulated by attentional and emotional states [1, 10]. In these models, ascending pain pathways (in red in Fig. 1) first project into the limbic circuitry triggering increased arousal and setting up ground for learning mechanisms (involving the nucleus accumbens (NAc) [3, 6, 44] and the thalamus). From there, the painful experience develops across three networks expressing its localization and duration, its amplitude, and its emotional valence. First, the posterior (post.) insula activates the somatosensorial areas (S1 and S2) where the localization and duration of the stimulus are integrated. The amplitude is estimated by areas located in the insula, the intraparietal sulcus, and the ventral prefrontal cortex. Information is then projected to the anterior cingulate cortex (ACC) and the medial prefrontal cortex (med. PFC) where it acquires is emotional dimension [19, 39]. Finally, in the presence of a lasting painful experience, “anticipation” develops around the ACC, the anterior insula (ant.), and the NAc, which interacts with ascending pathways. Ultimately, the long-term potentiation of the painful episode will be sealed by the amygdala (AMY) responsible for memory consolidation and emotional learning.
Proposed framework for the pathophysiology of chronic low back. The literature describes pain as a model involving where “ascending nociceptive pathways” (red) are modulated by “Anticipation,” “Attentional regulation” (purple) and “Emotional states” (pink). In presence of low back pain, we identify three physiological responses: (1) “physical” response (green), (2) “cognitive” response (blue), and (3) the “emotional” response (yellow). The self-regulation of APC (box: bottom-right) aims at the regulation of the NAc, effectively tuning down the emotional up-regulation of nociceptive inputs; in turn, the resulting change in pain valence is expected to reduce attentional up-regulation and reduce pain sustainably. The sequence of these changes can be monitored thanks to cortical EEG-biomarkers (rounded gray-blue boxes). ɑ-amp alpha-amplitude, APC alpha-phase concentration, APS alpha-phase synchrony, ACC anterior cingulate cortex, AMY amygdala, fmθ frontal midline theta, Hippo hippocampus, PAG periaqueductal gray, PFC prefrontal cortex, S1 primary somatosensory cortex, S2 secondary somatosensory cortex, SLP superior parietal lobe, SMR sensorimotor rhythms, TPC theta-phase concentration
In the presence of low back pain, we identify three natural remediation strategies. First, the “physical” response (numbered 1 in green in Fig. 1) leads to an immediate change in posture. Then, the “cognitive” response (numbered 2 in blue in Fig. 1) strategizes the avoidance of pain stimuli by increasing anticipation (ACC), memory consolidation (Hippo.), and increased control on movement preparation (MC). Finally, the “emotional” response (numbered 3 in yellow in Fig. 1) mobilizes the medial PFC and the striatal functions of the basal ganglia aiming at more radical and lasting behavioral changes intended to disrupt the origin of the pain. Interestingly, each strategy operates at increasingly long time-frames ranging from seconds to months.
Unfortunately, as illustrated by Fig. 1 (gray box on the bottom-left), each of these coping mechanisms also has the potential to make matters worse by increasing painful inputs, attentional, and affective up-regulation, respectively. We suggest that the self-regulation of the NAc eventually enables the patients to break out this negative feedback loop by disrupting the behavioral feedback loop (reward + emotional circuitry). Consequently, we expect to witness a specific causality between changes observed in proxy neuromarkers of attentional and emotional regulations. More precisely, proxy EEG-biomarkers described in Table 1 should reflect the following sequence: trained neuromarker (APS) triggers changes in NAc activity (TPC), which modulate emotional (APS, ɑ-amplitude) and attentional (fmθ) response; ultimately one would expect changes in the motor cortex (SMR).
The causality between these neuromarkers available longitudinally over the course of the treatment was therefore investigated with a Granger-causality method, which essentially explains a variable with another that is temporally shifted. To further investigate test our model, the Pearson product-moment correlation r between the evolution of medical results (i.e., EMG scores and clinical scales) and the slope of each proxy neuromarker was also investigated.
Statistical analysis
All statistical analyses were carried out in python programming language (v3.6) using the pandas (v0.21.1), scipy (v1.0.0), and matplotlib (v2.1.1) packages.
Populations
It is anticipated that values in such clinical dataset with repeated measures may not be missing entirely at random. For instance, one might anticipate that a patient feeling no improvement lacks incentive to participate and thereby drops out. To account for this, missing values were imputed with the last available score (also called “zero-order interpolation” or “hold and sample”). This interpolation method hypothesizes that there is no clinical improvement from one visit to another. With such interpolation, all patients are kept for the analysis and results are therefore presented on the intend-to-treat (ITT) population.
Evidence of learning
In order to demonstrate control, learning, and baseline changes, statistically significant trends had to be reliably demonstrated in the progression of EEG neuromarkers. Doing so for each individual and for many neuromarkers exposes the analysis to the risk of getting false positives (multiple-testing problem). Using a Bonferroni correction in the context of correlated variables would lead to an undesirable lack of statistical power. To circumvent this limitation, permutation statistics can be implemented where the distribution of the chosen statistics to be tested is estimated from the data permuted under the null hypothesis [51]. Specifically, the longitudinal time series (EO, EC, and training) for each neuromarkers (APS and APC, see Table 1) were permuted under the null hypothesis that the data showed no temporal monotonic trend. At each permutation, the time vector is shuffle and the absolute value |r| of the Pearson product-moment correlation between each neuromarker and the time vector was computed. The maximum value across all neuromarkers was taken (k = 6) resulting, after all permutations, in a distribution of |rmax| statistics under the null hypothesis. Finally, the r value resulting from the real data is compared to the 95th percentile of that distribution to assess statistical significance. Not only this test procedure keeps the probability of false positive controlled at the nominal level, but it also adjusts automatically to the degree of correlations among neuromarkers.
Specificity of learning
Finally, to assess the specificity of the statistical temporal trends identified with respect to the clinical endpoints, a Pearson’s correlation coefficient was computed between the slope of each neuromarker (estimated with a linear regression) and the progression in each clinical scale computed as the raw difference before and after the intervention (post–pre). The statistical significance accounted for multiple correlations tests using two levels of corrections: first, the rmax method was applied to correct for the multiple clinical scales tested (k = 5) and a Holm–Bonferroni corrections to correct for the different neuromarkers investigated (k = 7).
Clinical data
For the evolution of clinical criteria, i.e., EMG scores and clinical scales, between pre- and post-NFB intervention, a Wilcoxon signed-rank T test is applied for each score on paired values and corrected with a Tmin permutation test. The evolution of clinical scores before, after, and at follow-ups was assessed with a nonlinear correlation coefficient (Spearman’s rank correlation), whose significance was assessed with the same ρmax permutation technique used to assess the progression of neuromarkers. To assess the significance of the regression slope (called a) fitted to the plots (see Figs. 3, 4, 5, 6), a first-order linear regression (y = ax + b) was fitted to individual points and coefficients significance was assessed with a-max permutation test. Clinical data and EMG were analyzed (and corrected) together. All tests were one-sided. All permutations were exhaustively computed whenever possible (nperm = 4! = 24 leading to a minimum possible p value of 0.04 for Spearman) or 15,000 random permutations were otherwise sampled.
Validation of pathophysiological model
In order to validate the hypothesis presented in Fig. 1, additional neuromarkers listed in Table 1 were extracted and averaged at each EEG session for each patient: APS, APC, alpha-amplitude, SMR and frontal midline theta. The multi-variate time series with 20 temporal observations (for each patient) were analyzed by means of Granger-causality [37] so as to identify which neuromarkers is leading which. The chosen autoregressive model order was one, meaning that each variable was tentatively explained with the sample temporally preceding at all variables.
Results
Patients
In total, n = 16 patient were included in the study. They were all females aged 15–52 years old (mean 37). Two patients missed the 6-month follow-up visit but were present at the 12-month follow-up visit. Two patients missed the 12-month follow-up visit but were present at the 6-month follow-up visit. Three patients dropped after the post-intervention assessment and did not complete either the 6-month follow-up nor 12-month follow-up. Only three and five patients completed the EMG assessment at 6 and 12 months, respectively. These dropouts are believed to be associated with the organizational load required by the protocol (visit planning and transportation). However, because it cannot be discarded that patients lack interest because of lack of perceived or actual efficacy, these patients were kept in the analysis (ITT). In total, 345 neurofeedback sessions were recorded totaling more than 50 Go of raw EEG data to be analyzed.
Presence of learning in neuromarkers at the group level
Control over neuromarker: intra-session learning
The first analysis is meant to verify that patients can regulate specifically the trained neuromarker and looks at intra-session changes over blocks averaged across all patients. Each block is averaged across 20 sessions for 16 patients. We look at the evolution of alpha-phase synchrony (APS) and phase concentration (APC) at each 5-min-long training blocks showing a significant increase (permutation test: p < 0.001) in the median alpha-synchrony (APS) across patients from block 1 to 6. This increase reflects changes in brain activity induced by the participant doing the NFB training task.
Intersession learning
To ensure learning, the averaged activity across subject from session to session is investigated showing a statistically significant positive trend (permutation test: p = 0.0006) for the NFB block alpha-phase synchrony (APS) neuromarker from session to session. It is also worth noting that no statistically significant trends could be found for the EO and EC condition, for any of the neuromarker (APC and APS).
In Fig. 2, we plot the progression of APS over blocks and sessions, averaged across patients. The increase over these two axes is fitted by a plane. The progression along both axes shows a block-by-session interaction indicating that patients get better and better at controlling the APS. The plot also shows an upward and a downward trend toward EO and EC APS, respectively.
Alpha-phase synchrony evolution over blocks and sessions for eyes open (EO in blue), eyes closed (EC in gray), and during NFB training (in green). Block number is seen on the right horizontal axis, session number on the left horizontal axis, and the normalized alpha-phase synchrony is displayed on the vertical axis. The green plane indicates the significant positive trend over the blocks and sessions. The gray and black lines, respectively, indicate the (nonsignificant) trends over the sessions for EO and EC
Clinical endpoints
Changes in neurophysiological activity are only meaningful if associated with changes in clinical symptoms. Figure 3 shows the progression for VAS, Dallas, Hamilton, and HAD clinical scales, and Fig. 4 shows the progression for EMG scores. Median and 95% confidence intervals are plotted before (pre) and after (post) the neurofeedback sessions, as well as at 6- (FU6m) and 12-month (FU12m) follow-up sessions. Figures 5 and 6 detail progression for Dallas and HAD subscales. On these plots, clinical scales mainly show negative slopes (associated with a reduction in symptoms), whereas EMG scores mainly show positive slopes (associated with an improvement in muscular function). Only the “total Dallas” regression line was found to decreases significantly over time with slope a = − 2.77 (permuted p < 0.001 after correction for multiple tests).
Progression of EMG scores for 16 patients recorded for different phases of the trunk flexion: flexion of the right muscle (right flex), flexion left (left flex), interphase flexion–extension right (right interphase), interphase flexion–extension left (left interphase), extension right (right ext), extension left (left ext). Scores were recorded before (PRE) and after (POST) the NFB treatment as well as at 6 (FU6m) and 12-month (FU12m) follow-up sessions
In terms, of pre-/post-intervention changes, only the Dallas social interest subscale was found significantly improved (T = 6.0, corrected p = 0.05). The analysis of nonlinear temporal changes (Spearman correlation) from admission to 12-month follow-up indicates that the “HAD anxiety” and the “Dallas work and leisure” both show significant improvement over time (ρ = 1.0, permuted p = 0.04). Similarly, the progression of the left-interface EMG median frequency was also found to improve significantly over time (ρ = 1.0, permuted p = 0.04).
Correlation between the evolution in clinical endpoints and neuromarker value
A negative correlation between the changes in Dallas 2 and the changes in APC over the training sessions was found significant (r = − 0.69, permutation test: p = 0.0031) and we provide a visual representation of the two variables and their correlation in Fig. 7.
Correlation between the slope of the APC neuromarker progression over the training sessions (x-axis) and the change in the Dallas 2 clinical score (y-axis). Each of the 16 points represents a patient. The correlation value is R = − 0.69, the original p value = 0.0031, and the corrected value for multiple testing is p = 0.02
Validation of pathophysiological hypothesis
No variable was found to Granger-cause any other variable.
Discussion
This pilot experiment included 16 chronic lower back pain (cLBP) patients who previously resisted to all suggested interventions—including a 5-week-long intensive rehabilitation program [34]. They were offered 20 sessions of NFB training during which full-scalp EEG data was recorded. Considering the severity of the population involved in this trial, the reduction in severity that was observed on all clinical scales immediately after the intervention (significant only for the Dallas Social Interest, T = 6.0, p = 0.05) seems of promising clinical significance. However, these clinical results cannot constitute a breakthrough of its own. Specifically, this study has important methodological shortcomings that should be accounted for. The consistency of the results provided would at best offer a solid scientific base to build clinical evidence upon.
This study has several important limitations including the lack of placebo control, blinding, and randomization procedures. Controlling for placebo effect is of particular importance in pain studies [31, 46]. It is true that some pills placebo arms have reported a VAS improvement that is of similar magnitude to the one reported in this study [43]. However, these results are hard to compare given the multi-resistant nature of the population included in this study, for which an equivalent change is arguably clinically more significant. In addition to this, the progression recorded on clinical scales is also corroborated by statistically significant improvement in the EMG measures, an objective and quantitative endpoint, that was previously reported to not be sensitive to large placebo effect in chronic pain patients [9]. Last but not least, the stability (and even improvement) of the clinical efficacy at the 6-month follow-up tends to reduce the possibility of a strong placebo effect in this population. These limitations will be adequately addressed in one ongoing and one upcoming study. The NEMOLOC trial (Nîmes CHU, France, RCB 2018-AOA02935-50) will investigate NAc activity using fMRI before and after the EEG-based NFB sessions. Another upcoming multi-centric triple blind RCT study will compare three randomized interventional arms: an active APC NFB group, an active alpha-NFB group, and an EMG control group. While the absence of a control procedure undeniably constitutes the primary limitation to this study, we believe the consistency provided across all measures (including the neuromarker analysis discussed below) remains tremendously promising.
The data available for neuromarker analysis consisted of nearly 430-min-long EEG sessions totaling more than 50 Go raw EEG data. The unique granularity of this dataset enabled the exhaustive investigation of the evolution of the neuromarkers using permutation-based statistical framework that are known to offer high sensitivity with low family-wise error rates [32]. To the best of our knowledge, this study is the first of its kind to ever report on a full-scalp and full temporal resolution EEG dataset collected before and during each training brain rehabilitation session. Comparing APS distribution recorded during resting state EO and EC reveals an increased alpha-activity on the EC values, which is consistent we what we know of EC resting state activity. This constitutes a good consistency check for the data analyzed and gives confidence in our findings. Likewise, the absence of this elevation for the APC marker confirms that it is not sensitive to amplitude but only to phase concentration (that is synchronicity irrespective to alpha-amplitude).
This analysis first suggests that patients have control on the targeted neuromarker (as instructed) because a positive trend is reported over the six 5-min-long training blocks for the APS neuromarker (r = 0.95, p < 0.01). There is a possibility that the changes in alpha-synchrony observed within sessions can be imputed to a natural change in physiology such as dizziness, which can be unrelated to neurofeedback training itself. However, the fact that the APC neuromarker, which is not sensitive to changes in amplitude, also shows a positive trend (r = 0.95, p < 0.01) tends to discard an effect mediated by large alpha-amplitude that are associated with dizziness. Nonetheless, it is recommended that a future design monitors dizziness and attention during a session so as to further study this possible confounding factor. The progression of APS neuromarkers from session to session (r = 0.63, p < 0.01) suggests that patients learn to better control, meaning that they improve their ability to activate/deactivate/synchronize the associated neural population. This result should be placed in perspective with the (nonsignificant) reduction in the baseline EC APS and the increase in the baseline EO APS activity. They tend to indicate that the significant improvement seen in the training (NFB) activity transfers in smaller (and nonsignificant) baseline changes in the resting state EEG. It is not clear at this stage why would the EC APS decrease while the EO APS increases. Our finding extends results from previous studies reporting on similar examples of long-term electrophysiological changes induced by self-paced neuromodulation either captured in resting states condition [49] or evoked potentials studies [33].
This paper presents a simple model of cLBP involving anxiety, depression, motor cortex, and pain pathways. Each state can be estimated with a different proxy neuromarkers, presented in Table 1, for which EEG data are available longitudinally. It was attempted to investigate the causality between the different proxy neuromarkers so as to gain understanding of the possible modes of action. Unfortunately, the Granger-causality could not be established between any proxy neuromarkers and the pathophysiological model could not be validated. There are several possible explanations in alternative to rejecting the model’s hypothesis. First, the obvious lack of statistical power since only 20 data points (one per sessions) were used to validate the hypothesis. Second, the notoriously low signal-to-noise ratio (SNR) affecting EEG measures that were used as proxy for the brain functions investigated. Third, the validity of the temporal hypothesis (first-order model) requiring the values at a given time to be directly influenced by those recorded at the session directly preceding. Given those, it is only reasonable to report on this negative finding and invite researchers continuing this work to keep investigating the temporal relationship between the different proxy neuromarkers available.
What constitutes undeniably the most significant finding of this work amount to the equivalent of a dose–response effect. Overall possible combinations of neuromarkers (k = 2) and available clinical endpoints (k = 6), the correlation between the Dallas total score (disability state) and the APC neuromarker slope (r = − 0.69) was found statistically significant after two rounds of correction for multiple testing (p < 0.01). This result indicates very meaningfully that patients who gained control on their APC improved proportionally on their disability state, which is somewhat similar to what one would expect from a dose–response effect in a typical pharmacological study. There are a few studies previously reporting on a correlation between a neuromarker progression and a clinical improvement but those hardly ever corrected for the multiplicity of statistical tests. For instance, Gevensleben et al. [20] showed a statistically significant relation between changes in parietal theta-midline induced by EEG NFB training and changes in the German score for inattention and hyperactivity. Similarly, Wangler et al. [50] could relate the changes contingent negative variation to a similar clinical scale. More recently, Heinrich et al. [25] reported a correlation between the theta–beta-ratio and the reaction time to a continuous performance task in different population of ADHD children.
This correlation stresses the importance of the APC neuromarker, which was not primarily targeted, in relation to our clinical population and specifically the mechanisms of nociception and the chronicity of pain. During an experiment involving six healthy participants with simultaneous recording of the NAc with implanted electrodes and scalp EEG, it was demonstrated that EEG alpha-synchrony was involved in the corticostriatal communication and, more specifically, in the modulation of the NAc activity [26]. In this model, the APC exerts a specific control over the NAc and offers a mechanism by which the self-modulation of a cortical area using operant condition (as in the protocol implemented in this work) offers leverage on deeper brain structure of the basal forebrain (here the NAc) that can otherwise not be measured with EEG. The clinical significance of this result holds to the important role the ACC plays in nociception and the chronicity of pain. Specifically, the nucleus accumbens participates in the central representation of pain but also gates activity in ascending nociceptive pathways [40]. There is also a growing body of evidence [7] suggesting that the chronification of pain rewires the NAc in way that also affects motivations and learning (reward) circuitry involving dopaminergic pathways. In the very context of chronic pain, it has also been reported experimentally that patients’ NAc activation during noxious stimulation was very different to that of a nonchronic population [3]. More recent work on animal model even suggests that the changes affecting the NAc in presence chronic pain are both macroscopic and molecular [13]. The impact of chronic pain on the NAc also has indirect consequence of more remote areas of the brain. For instance, the drop in motivation elicited in mice by two different models of chronic pain was proved to require a galanin receptor 1–triggered depression of excitatory synaptic transmission in indirect pathway NAc medium spiny neurons [44]. These studies not only show the importance of the NAc in the perception and affective processing of pain but also stress the importance of lasting changes induced by chronic pain in this area as well as networks of the prefrontal cortex affecting mental health status.
Conclusion
This document reports on a pilot study offering an alpha-phase synchronization brain rehabilitation intervention to a population of multi-resistant cLBP patients. The intervention showed a significant and lasting response of most measured clinical scales, which is even more relevant considering the severity of the population. First, clinical scales show a post–pre-treatment reduction, but significative only for the Dallas work and leisure. Second, patients did control (intra-session trends) and learned to better control (intersession trends) their APS neuromarker. Third, the APC neuromarker was found to correlate significantly with the reduction in clinical symptoms in a typical dose–response effect. Taken together these results remain of interest despite the small sample size and the absence of blinding. Finally, the intervention favorable safety profile and its availability as a home-use intervention makes it a potentially disruptive tool for less severe population in the context of nonsteroidal anti-inflammatory drugs (NSAID) and opioid abuses.
Change history
01 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s00586-020-06666-3
References
Apkarian AV, Hashmi JA, Baliki MN (2011) Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152(3 Suppl):S49–S64
Balague F, Troussier B, Salminen JJ (1999) Non-specific low back pain in children and adolescents: risk factors. Eur Spine J 8:429–438
Baliki MN, Geha PY, Fields HL, Apkarian AV (2010) Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 66:149–160
Baptista AF, Fernandes AMBL, Sá KN, Okano AH, Brunoni AR, Lara-Solares A, Iskandar AJ, Guerrero C, Amescua-García C, Kraychete DC et al (2019) Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC2-NIN-CP). Pain Rep 4:e692
Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Rushby JA (2004) Age and gender effects in EEG coherence: I. Developmental trends in normal children. Clin Neurophysiol 115:2252–2258
Becerra L, Borsook D (2008) Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain 12:866–869
Benarroch EE (2016) Involvement of the nucleus accumbens and dopamine system in chronic pain. Neurology 87(16):1720–1726
Bontoux L, Roquelaure Y, Billabert C, Dubus V, Sancho PO, Colin D, Brami L, Moisan S, Fanello S, Penneau-Fontbonne D, Richard I (2004) Étude du devenir à un an de lombalgiques chroniques inclus dans un programme associant reconditionnement à l’effort et action ergonomique. recherche de facteurs prédictifs de retour et de maintien au travail. Annales de réadaptation et de médecine physique 47:563–572
Brena SF, Wolf SL, Chapman SL, Hammonds WD (1980) Chronic back pain: electromyographic, motion and behavioral assessments following sympathetic nerve blocks and placebos. Pain 8:1–10
Bushnell MC, Ceko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14(7):502–511
Bussalb A, Congedo M, Barthélemy Q, Ojeda D, Acquaviva E, Delorme R, Mayaud L (2019) Clinical and experimental factors influencing the efficacy of neurofeedback in ADHD: a meta-analysis. Front Psychiatry 10:35
Caro XJ, Winter EF (2011) EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl Psychophysiol Biofeedback 36:193–200
Chang P-C, Pollema-Mays SL, Centeno MV, Procissi D, Contini M, Baria AT, Martina M, Apkarian AV (2014) Role of nucleus accumbens in neuropathic pain: linked multi-scale evidence in the rat transitioning to neuropathic pain. Pain 155:1128–1139
Cohen MX (2014) Analyzing neural time series data: theory and practice. MIT Press, Cambridge
Dolan P, Mannion AF, Adams MA (1995) Fatigue of the erector spinae muscles. A quantitative assessment using “frequency banding” of the surface electromyography signal. Spine 20:149–159
Durup G, Fessard A (1935) I. l’électrencéphalogramme de l’homme. Observations psycho-physiologiques relatives à l’action des stimuli visuels et auditifs. L’année psychologique 36:1–32
Fassier J-B (2011) Prévalence, coûts et enjeux sociétaux de la lombalgie. Revue du rhumatisme 78:S38–S41
Flor H (2014) Role of cortical reorganization in the rehabilitation of chronic pain. In: Replace, repair, restore, relieve—bridging clinical and engineering solutions in neurorehabilitation. Springer, pp 1–2. https://doi.org/10.1007/978-3-319-08072-7_1
Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML (2014) The anterior cingulate cortex and pain processing. Front Integr Neurosci 8:35
Gevensleben H, Holl B, Albrecht B, Vogel C, Schlamp D, Kratz O, Studer P, Rothenberger A, Moll GH, Heinrich H (2009) Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J Child Psychol Psychiatry 50(7):780–789
Ghaziri J, Tucholka A, Larue V, Blanchette-Sylvestre M, Reyburn G, Gilbert G, Lévesque J, Beauregard M (2013) Neurofeedback training induces changes in white and gray matter. Clin EEG Neurosci 44(4):265–272
Gourmelen J, Chastang J-F, Ozguler A, Lanoë J-L, Ravaud J-F, Leclerc A (2007) Frequency of low back pain among men and women aged 30 to 64 years in France. Results of two national surveys. Annales de réadaptation et de médecine physique 50:640–644
Hamilton MAX (1959) The assessment of anxiety states by rating. Br J Med Psychol 32(1):50–55
Hebert R, Lehmann D, Tan G, Travis F, Arenander A (2005) Enhanced EEG alpha time-domain phase synchrony during transcendental meditation: implications for cortical integration theory. Signal Process 85(11):2213–2232
Heinrich H, Busch K, Studer P, Erbe K, Moll GH, Kratz O (2014) EEG spectral analysis of attention in ADHD: implications for neurofeedback training? Front Hum Neurosci 8:611
Horschig JM, Smolders R, Bonnefond M, Schoffelen J-M, Van den Munckhof P, Schuurman PR, Cools R, Denys D, Jensen O (2015) Directed communication between nucleus accumbens and neocortex in humans is differentially supported by synchronization in the theta and alpha band. PLoS ONE 10(9):e0138685
Jensen MP, Grierson C, Tracy-Smith V, Bacigalupi SC, Othmer S (2007) Neurofeedback treatment for pain associated with complex regional pain syndrome type I. J Neurother 11(1):45–53
Jensen MP, Hakimian S, Sherlin LH, Fregni F (2008) New insights into neuromodulatory approaches for the treatment of pain. J Pain 9(3):193–199
Koes BW, van Tulder M, Lin CWC, Macedo LG, McAuley J, Maher C (2010) An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J 19:2075–2094
Lawlis GF, Cuencas R, Selby D, McCoy CE (1989) The development of the Dallas pain questionnaire. An assessment of the impact of spinal pain on behavior. Spine 14(5):511–516
Machado LAC, Kamper SJ, Herbert RD, Maher CG, McAuley JH (2008) Imperfect placebos are common in low back pain trials: a systematic review of the literature. Eur Spine J 17(7):889
Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG-and MEG-data. J Neurosci Methods 164:177–190
Mayer K, Wyckoff SN, Schulz U, Strehl U (2012) Neurofeedback for adult attention-deficit/hyperactivity disorder: investigation of slow cortical potential neurofeedback—preliminary results. J Neurother 16:37–45
Mayer TG, Gatchel RJ, Mayer H, Kishino ND, Keeley J, Mooney V (1987) A prospective two-year study of functional restoration in industrial low back injury: an objective assessment procedure. JAMA 258:1763–1767
McKnight JT, Fehmi LG (2001) Attention and neurofeedback synchrony training: clinical results and their significance. J Neurother 5:45–61
Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin C-WC, Chenot J-F, van Tulder M, Koes BW (2018) Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J 27:2791–2803
Pearl J (2009) Causal inference in statistics: an overview. Stat Surv 3:96–146
Peniston EG, Kulkosky PJ (1991) Alpha-theta brainwave neurofeedback for Vietnam veterans with combat-related post-traumatic stress disorder. Med Psychother 4:47–60
Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971
Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ (2016) The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 19(2):220–222
Reynolds GS (1975) A primer of operant conditioning, rev. APA PsycNET
Rossignol M, Rozenberg S, Leclerc A (2009) Epidemiology of low back pain: what’s new? Joint Bone Spine 76:608–613
Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M (2003) Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin Ther 25:1123–1141
Schwartz N, Temkin P, Jurado S, Lim BK, Heifets BD, Polepalli JS, Malenka RC (2014) Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345:535–542
Spengler DM, Bigos SJ, Martin NA, Zeh J, Fisher L, Nachemson A (1986) Back injuries in industry: a retrospective study. I. Overview and cost analysis. Spine 11:241–245
van Laarhoven AIM, Vogelaar ML, Wilder-Smith OH, van Riel PLCM, van de Kerkhof PCM, Kraaimaat FW, Evers AWM (2011) Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain 152(7):1486–1494
van Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, Koes BW, van Tulder MW (2011) A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J 20:19–39
Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF et al (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet 390:1211–1259
Wang J-R, Hsieh S (2013) Neurofeedback training improves attention and working memory performance. Clin Neurophysiol 124:2406–2420
Wangler S, Gevensleben H, Albrecht B, Studer P, Rothenberger A, Moll GH, Heinrich H (2011) Neurofeedback in children with ADHD: specific event-related potential findings of a randomized controlled trial. Clin Neurophysiol 122:942–950
Westfall PH, Young SS (1993) Resampling-based multiple testing: examples and methods for p-value adjustment. Wiley, Hoboken
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370
Funding
This work was supported by Covea and Mensia Technologies.
Author information
Authors and Affiliations
Contributions
LM contributed to this work’s hypotheses, the clinical protocol, the technical environment setup, the data analysis, and manuscript redaction; HW completed all the sessions with patients; QB implemented the methods for real-time processing, contributed to the data analysis, and manuscript redaction; PF contributed to the clinical protocol and the interpretation of results; YD supported the analysis (EMG), MC consulted on the analysis of EEG data and the real-time methods; AD supported the interpretation of neurophysiological results; MR included all patients and contributed to the clinical protocol and interpretation of data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Mensia Technologies is a medical device company offering therapeutic solutions for psychiatric and neurological disorders. LM and QB were employed and shared owner at Mensia Technologies at the time this study was carried out; MC was scientific advisor at Mensia Technologies at the time this study was carried out.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mayaud, L., Wu, H., Barthélemy, Q. et al. Alpha-phase synchrony EEG training for multi-resistant chronic low back pain patients: an open-label pilot study. Eur Spine J 28, 2487–2501 (2019). https://doi.org/10.1007/s00586-019-06051-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06051-9