Abstract
The area of nanotechnology has been enjoying enormous research interest recently because of the unique characteristics of nanoscale substances. Silver nanoparticles (AgNPs) are attractive for their broad spectrum of utilization in various sectors. Among the several ways of AgNPs preparation, we focused on green synthesis using phytochemicals present in plants without the use of chemicals and surfactants that are harmful for humans and environment. In this chapter, we provide a comprehensive review of the various options of green preparation of silver nanoparticles including bio-mechanochemical synthesis. Just few reports on the bio-mechanochemical synthesis combining tools of mechanochemistry (ball milling) and of green synthesis (plant material) were reported until now, and these studies are briefly reviewed. They show green and sustainable character of this method and underline the growing interest of scientists towards mechanochemistry. Silver and its compounds are known as strong antibacterial agents, so we have focused on description of the methods frequently applied to determine the antimicrobial properties of AgNPs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
7.1 Introduction
Due to small particle size, various shapes and high surface areas, silver nanoparticles show different chemical, physical and biological properties and lead to the broad spectrum of their applications in pharmaceutical field, catalysis, sensors, spectroscopy or electronics (Abbasi et al. 2016; Prasad et al. 2016). Silver provides activity against many bacterial species, so it is commonly used in medical industry, including creams with silver content to avoid local infections after open wounds or burns, soaps, catheters, pastes and textiles (Becker 1999; Catauro et al. 2004; Dallas et al. 2011; García-Barrasa et al. 2011).
Preparation of AgNPs can be realized by various ways. In general, we can classify these techniques as chemical, biological and physical. Some of these techniques are simple with good nanoparticle size control and can be achieved by affecting the process of reaction, but on the other hand, there are some difficulties and complications with stability of the products (Kowshik et al. 2002). Despite that, many of the reactants and surfactants engaged in these processes are dangerous to human health and the environment, and so, a “green” approach for metal nanoparticles is necessary.
Methods of classical chemical or physical approach to nanoparticles synthesis are environmentally toxic and economically demanding (Kalaiarasi et al. 2013). These objectives have forced the scientific community to search for new, easy and environmentally friendly choice that would not be harmful for humans, animals and environment. These facts shifted the attempts to synthesize Ag nanoparticles using green synthesis methods that have been indicated to be easy, economically advantageous and environmentally friendly (Prasad 2014). The green preparation of silver nanoparticles means preparing nanoparticles using biological routes for Ag(I) ion reduction by natural sources of reducing agents. These sources include microorganisms (algae, yeast, fungi, bacteria), plants and plant extracts or their products, such as small biomolecules including vitamins, amino acids or polysaccharides (Sharma et al. 2009; Ahmed et al. 2016; Mohammadlou et al. 2016; Prasad et al. 2016, 2018). Nanoparticles prepared by green approach are much superior to nanoparticles prepared by chemical and physical processes, as green methods exclude the use of toxic and consumptive chemicals and save the energy needed for synthesis (Clark et al. 2002; Kharissova et al. 2013; Prasad et al. 2016).
Nowadays, investigation on nanometals’ biosynthesis by plant extracts has become immensely popular. This alternative green approach to the synthesis of AgNPs, which utilizes plant sources in the synthesis of metal nanoparticles, has developed in the new area of “phytonanotechnology” (Rajan et al. 2015; Prasad 2019). This method reflects the newest epoch in rapid and harmless methods for nanoparticles synthesis. For that reason, the present work has focused on silver nanoparticles synthesis using plant extracts. As already mentioned before, there are several ways of silver nanoparticles preparation. Among them, an environmentally friendly solvent-free approach called mechanochemistry has been used. However, the reports on bio-mechanochemical synthesis (co-milling of plant material and silver nitrate) are rather scarce (Rak et al. 2016; Arancon et al. 2017; Baláž et al. 2017a, b).
This chapter provides a brief introduction into the topic of green synthesis of silver NPs, outlining the most important group of compounds responsible for reduction, commonly used characterization techniques and methods used for antibacterial activity determination. The second part is devoted to the bio-mechanochemical preparation of silver NPs.
7.2 Green Synthesis of Silver Nanoparticles
The term “green chemistry” is generally understood as a principle to reduce chemistry-related impacts on human health and to eliminate the environmental pollution. Green chemistry represents a different way of thinking how chemistry can be done, for example, by reducing waste, saving energy, using non-toxic chemicals and solvents (de Marco et al. 2018; Joshi et al. 2018).
Nature is an endless source of compounds which can be used for silver nanoparticles synthesis by green chemistry approach. Synthetic methods using biomaterials are in line with the principles of “green” chemistry, as safe and non-toxic reagents are used to prepare nanoparticles. This part of the chapter is focused on green approach for the Ag nanoparticles synthesis using plant extracts. These procedures are performed outside of a living organism using an extract without cells producing reducing molecules with reducing capacity.
Green approach to nanoparticles synthesis is based on bottom-up approach, where the reducing agents contained in plants are liable for the Ag(I) ion bioreduction to prepare AgNPs. This process starts after incubation of the plant extracts with silver salts (silver nitrate is mostly used). In general, the biosynthesis of Ag nanoparticles involves three steps. The first is an induction step involving the reduction of Ag(I) ions followed by the second phase of growth, where larger aggregates are produced, and finally, the third, stabilizing step, in which the stabilization of prepared nanoparticles is carried out (Marchiol et al. 2014). The green approaches include the choice of suitable solvent used for the Ag nanoparticles synthesis and the choice of environmentally friendly reducing agent and non-toxic material as a capping agent for nanoparticle stabilization. The green reduction of silver ion is completed by natural material, mainly extracts obtained from plants, which contain reducing compounds, for example phenolics, flavonoids, terpenoids or saccharides (Prabhu and Poulose 2012), which also are responsible for their stabilization. In the plant-mediated nanotechnology field, a variety of plant parts (flowers, fruit, leaves, root, stem, seed, peel) are used (Kharissova et al. 2013; Mittal et al. 2013; Ahmed et al. 2016; Carmona et al. 2017).
In the concept of green approach of AgNPs synthesis, elimination of the use of toxic chemical is expected. Extractable phytochemicals therefore play a dual role as both reducing and stabilizing representatives for the production of metal nanoparticles containing AgNPs simultaneously without any involvement of chemicals. This approach is dependent on the amount of reductants contained in the extract, and therefore, the key step is to extract these reductants and capping agents.
7.2.1 Extraction
The ways of efficient extraction of the bioactive compounds were intensively studied in literature (Azwanida 2015). Several techniques have been studied to optimalize the extraction process of these compounds, such as drying methods, temperature of extraction and extraction solvent type. Extraction of different plant parts can be done by several extraction procedures. The initial stage in the plant extract production is the preparation of plant samples. Different parts of plants such as leaves (Ajitha et al. 2015; Khatoon et al. 2018), barks (Velayutham et al. 2013), roots (Behravan et al. 2019), fruits and flowers can be extracted from fresh or dried plant material prior to AgNPs synthesis. Generally, after the collection of plant/plant parts, the cleaning of plant material by washing it twice or more times with distilled water follows. Both, fresh and dried plant samples are used in plant extract preparation. After drying procedure, the plants are powdered or ground. Lowering the particle size allows better contact between sample and extraction solvent.
The solvent types used in the extraction importantly influence the quantity of the extracting reducing agents. The most frequently used solvent for extraction of bioreducing compound is water. There are few reports regarding the use of organic solvents for extraction, like Murraya koenigii methanol leaf extract (Suganya et al. 2013), Ziziphora tenuior methanol extract (Sadeghi and Gholamhoseinpoor 2015), Malva parviflora ethanol/water extract (Zayed et al. 2012). In most cases, the solvent was evaporated and the residue diluted in water for silver nanoparticles synthesis.
Traditional solvent extractions of biologically active compounds from plant raw material are carried out in aqueous solution or organic solvents and by application of heat and mixing. The most frequently used traditional methods include soxhlet extraction (Sadeghi and Gholamhoseinpoor 2015), maceration (Zayed et al. 2012), percolation (Padalia et al. 2015) and sonication.
Instead of conventional extraction techniques, several modern extraction techniques were developed. Mechanochemical-assisted extraction (MCAE) belongs to the innovative pre-extraction techniques (Wu et al. 2017), the forming of which is nearly connected with the development of mechanochemistry (Baláž et al. 2013). This method has been established and put in an application for the bioactive compounds’ extraction. In addition to the extraction method of heat reflux, the method of MCAE has higher yield of extraction, shortened time of extraction and lower temperature of extraction, so it can decrease or evade the reduction of thermolabile compounds’ amount. MCAE’s main goal is mechanical treatment of powder mixtures of plant materials and suitable reagents in special mill activators to solubilize the biologically active compounds (Lomovsky et al. 2017). In general, the MCAE procedures involve three steps, including sample preparation, mechanochemical treatment of plant material with solid reagent in ball mill and subsequent extraction procedure. There are several ways how MCAE provides improvement of extractable biomolecules: (a) the particle size reduction leading to the total contact surface area increase, so the probability of the reaction of biologically active compounds with the solid agents increases; (b) cell wall damage; and (c) chemical form transformation. The MCAE also allows the chemical conversion of compounds to enhance the solubility in water, so water can be used as an solvent of extraction as an alternative to organic solvents (Wu et al. 2017). This method was used for improved extraction, for example flavonoids from bamboo (Xie et al. 2013), chondroitin sulphate from shark cartilage (Wang and Tang 2009), polysaccharides extraction from Ganoderma lucidum spores (Zhu et al. 2012) or alkaloids from Stephania tetrandra, Stephania moore (Wang et al. 2019).
Plant extracts can be also obtained via microwave-assisted extraction (MAE). The MAE is a versatile technique which offers lower extraction period, higher extraction yield and less energy consumption. In addition, the microwave oven produces high temperature, which facilitates the breakage of the cell wall when the plant material is extracted, and as a result, active substances extraction is facilitated in the solvent. Water can be used in MAE under controlled temperatures. Different plants were subjected to MAE prior to AgNPs synthesis like red cabbage (Demirbas et al. 2016), green tea (Sökmen et al. 2017) and Anthriscus cerefolium (L.) Hoffm. (Apiaceae) (Ortan et al. 2015).
Safarpoor used ultrasound-assisted extraction (UAE) of 40 kHz frequency for preparation of Thymus daenensis and Silybum marianum ethanolic extract prior to AgNPs synthesis. (Safarpoor et al. 2018). Ultrasound-assisted extraction by effect of acoustic cavitation from the ultrasound facilitates inorganic and organic substances leaching from plant matrix. The extraction mechanism can be described by the diffusion through the cell wall and washing the contents of cell after destruction of the cell walls. The procedure is simple to use and relatively cheap that facilitates release of compounds. The UAE or sonification can be advantageously used for shortening the extraction time and extraction of termolabile biological compounds (Azmir et al. 2013; Azwanida 2015).
7.2.2 Biomolecules with Reducing Ability
The green synthesis of nanoparticles is based on the bioactive compounds extracted from plants that are able to reduce the metal ions to their elemental form. These metabolites are from group of terpenoids, flavonoids, tannins, phenolic acids, saponins, steroids, alkaloids, saccharides, proteins, amino acids, enzymes or vitamins (Kulkarni and Muddapur 2014). The mentioned substances containing reducing groups (OH, CH=O, NH2, SH) are able to reduce silver ions to the elementary form Ag(0) and produce the Ag nanoparticles, but also play an important role in their stability. Metal nanoparticles prepared by plant extracts were found to be functionalized with biomolecules that have primary amino, carbonyl, hydroxyl or other stabilizing functional groups (Kulkarni and Muddapur 2014; Sadeghi and Gholamhoseinpoor 2015). Different concentrations and ratio of active compound in plant have effect on the morphological diversity of nanoparticles (triangles, cubes, spherical, ellipsoids and others) and their properties (Makarov et al. 2014).
7.2.2.1 Flavonoids
Flavonoids represent a wide group of plant secondary phenolic compounds based on the flavan nucleus and can be divided into isoflavonoids, flavonols, flavones, flavanones, chalcones and anthocyanins (Kelly et al. 2002). The number, positions and types of substitutions influence the preparation and stabilization of nanoparticles. Tautomeric transformation of enol-form of flavonoid molecule to its oxo-form is responsible for the synthesis of nanoparticles, because it may release hydrogen which can reduce metal ions to NPs. For example; it is predicted that luteolin and rosmarinic acid present in Ocimum basilicum are responsible for AgNPs formation from silver ions by changing their tautomeric forms (Ahmad et al. 2010). Dihydromyricetin-mediated silver nanoparticles were synthesized by green way from AgNO3 and flavonoid dihydromyricetin (also known as ampelopsin) isolated from Ampelopsis grossedentata. Hydroxyl and carbonyl groups of dihydromyricetin were involved in formation of spherical Ag nanoparticles (Ameen et al. 2018).
7.2.2.2 Terpenoids
Terpenoids are secondary plant metabolites constructed from two or more isoprene units. Silver nanoparticles were prepared, for example, by rapid biosynthesis using leaf extract of Thuja occidentalis (Kanawaria et al. 2018). The nanoparticles were also encapsulated and stabilized by organic layer which contains mainly terpenes and terpenoids. These secondary metabolites take a part in preparation of nanoparticles and in their stabilization; it means prevention of agglomeration (Kanawaria et al. 2018). The main secondary metabolites from leaf extracts of Azadirachta indica (flavonoids and terpenoids) act as a reducing agent for the preparation of silver nanoparticles from AgNO3 and also as a capping agent (Roy et al. 2017). Ag nanoparticles were prepared in a very short time using clove extract. The main constituent of clove extract is terpenoid eugenol which is responsible for bioreduction of metallic ions to nanoparticles. The mechanism of bioreduction via eugenol terpenoid found in the clove has been described. The induction effect is mediated by methoxy and allyl groups, which are located in the eugenol structure in the para and ortho positions to the -OH group capable of releasing the proton, thereby forming a resonance-stabilized anionic form. It is then able to release two electrons from one eugenol molecule (Singh et al. 2010).
7.2.2.3 Phenolic Acids
High nucleophilicity of aromatic ring may support the chelating ability. Phenolic acids containing hydroxyl and carbonyl groups are considered as the major phytochemicals. For example, in rice husk, phenolic acids were investigated for their reducing ability to Ag(0) ion. An alkaline medium fits the transfer of the electron from the phenolic acids to reduce Ag(I) to create AgNPs, and in Fig. 7.1, the proposed mechanism for generating AgNPs prepared by caffeic acid (CA) in the rice husk extracts is shown. The reduction process of Ag(I) includes releasing of electrons to reduce Ag(I) to Ag(0), form caffeic acid-derived free radical, and consequently, another Ag(I) is reduced and CA is oxidized to o-quinone. Additionally, a caffeic acid dimer is produced which provides four electrons for Ag(I) reduction and is oxidized itself to quinone form (Fig. 7.1). The quinone derivatives can be combined to create a steric hindrance around the AgNPs, which avoids aggregation and enhances the stability of NPs (Liu et al. 2018).
Supposed mechanism of the AgNPs formation using caffeic acid in rice husk extract (Liu et al. 2018)
7.2.2.4 Saccharides
One of the biomolecules used as bioreducing agents are saccharides. Saccharides represent a wide group of polyhydroxy derivatives. The reducing ability of saccharides depends on two factors. The first is the content of individual monosaccharides and the second is binding between them. Saccharides that are used for synthesis of nanoparticles have many advantages; (a) availability, (b) low price (c) environmental reasons (d) capping ability (Panigrahi et al. 2004).
Glucose is supposed to be a strong reducing agent than fructose. However, it was found that fructose is better for synthesis of smaller nanoparticles and hence utilized for the preparation of Au–AgNPs composite (Au-core, Ag-shell) of ∼10 nm (Panigrahi et al. 2005).
It was shown that isolated polysaccharides and extract of biological material containing polysaccharides and other substances are suitable for biosynthesis of nanoparticles. Isolated polysaccharides such as starch or heparin serve as both a capping agent and a reducing agent (Raveendran et al. 2003; Huang and Yang 2004). However, also some additional substances, such as β-d-glucose, can serve as a reducing reagent and starch can serve as a capping agent (Raveendran et al. 2003; Vigneshwaran et al. 2006).
7.2.2.5 Proteins
Synthesis of nanoparticles is also connected with presence of amino acids and proteins in plants extracts. It was observed that proteins act as both reducing and stabilizing agents. The big advantage of such prepared nanoparticles is their biocompatibility (Tan et al. 2010). On the other hand, the disadvantage is the speed of reaction. The synthesis is relatively slow (hours, days) in comparison to the other plant metabolites (terpenoids, phenolic acids, flavonoids, saccharides), which took only few minutes (Ashraf et al. 2016; Baláž et al. 2017a). AgNPs synthesized by AgNO3 using extract of Malva parviflora were stabilized in solution containing proteins produced by the biomass (Zayed et al. 2012). Nitrogen of the amide group and silver ion coordination confirm the fact that the NH2 group of proteins has the strong capacity to capture the metal. It indicates that the proteins from plant extracts could cover the nanoparticles by layer, which has ability to avoid agglomeration and thereby to stabilize the solutions containing nanoparticles (Zayed et al. 2012). Although amino acids and proteins have the ability to reduce precursors of nanoparticles, they are very rarely used. Usually, different agents are used as reducers and proteins or amino acids serve only as capping agents , for example in bio-mechanochemical synthesis (Baláž et al. 2017a), simulated sunlight radiation (Huang et al. 2016) and sonochemical route (Ghiyasiyan-Arani et al. 2018).
7.2.3 Synthesis Procedure
After the extraction the solid residue is filtered off, the filtrate is used for nanoparticles synthesis. From literature survey, it follows that myriad of different plant extracts has already been used for the green synthesis of AgNPs (Ahmed et al. 2016; Ismail et al. 2016). We have selected just few recent interesting examples in our chapter. However, the principle of all the studies is always the same (Fig. 7.2).
The mostly used source of Ag(I) ions is silver nitrate aqueous solution in various concentrations. For example, aqueous extracts of Adiantum capillus-veneris L. were used for AgNPs biosynthesis by 1, 2 and 3 mM silver nitrate solution at room temperature via mechanical stirring (Omidi et al. 2018). The change of colour from yellow to dark brown was indication of silver nanoparticles formation. According to transmission electron microscopy (TEM) analysis, they synthesized nanoparticles of 18.4 nm size. Strong antibacterial activity was evidenced against both Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacterial strains.
Green synthesis of AgNPs using a root extract of Lepidium draba weed was successfully implemented by Benakashani et al. (Benakashani et al. 2017). As source of Ag(I) ions, they used 0.01 M AgNO3 with various amounts of root extract (2, 2.5, 3, 3.5 mL). The evidence of nanoparticles generating was visually controlled by colour change from green to dark brown as is generally known. The tested antibacterial activity was determined as higher as Ag(I) ions.
Seeds used for silver NPs synthesis were used by Ping and his colleagues (Ping et al. 2018). As reducing and stabilizing agent, they used grape seed extract and 0.01 M AgNO3 was reduced. The average size of prepared AgNPs was 54.8 nm when synthesized at room temperature. Antibacterial activities have not been detected yet.
The very interesting and improved green synthesis method by photoreduction was used by Viet and his working group using the Buddleja globosa leaf extracts (Van Viet et al. 2018). For Ag nanoparticles synthesis, they used low-power UV light in the presence of poly (vinyl pyrrolidone) (PVP) as the stabilizing agent. The procedure was optimized (ratio of ethanol/water/silver nitrate; pH value and time of reduction) to obtain spherical Ag nanoparticles with average size of 16 ± 2 nm. The prepared Ag nanoparticles were tested against E. coli strains with approximately 100% elimination.
7.3 Characterization Techniques
Characterization of Ag nanoparticles by various physical methods is very important in order to evaluate their physicochemical properties that can affect their behaviour, bio-distribution or safety. Synthesized silver nanoparticles are characterized by wide spectrum of methods including UV-Vis spectroscopy, infrared spectroscopy with Fourier transform (FTIR), X-ray photoelectron spectroscopy (XPS), dynamic light scattering (DLS), zeta potential, X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). These techniques are very briefly reviewed below.
Very often, discrepancies in the papers regarding particle size determined by various methods occur. It is mainly because some methods focus on the individual nanoparticles, whereas the others on the large grains. This is explained by a very simplified figure (Fig. 7.3).
7.3.1 Ultraviolet and Visible Spectroscopy
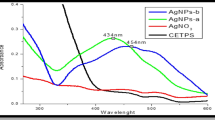
Ultraviolet and visible spectroscopy (UV-Vis) is a broadly used method due to its availability, simplicity and sensitivity of measurement and is a relevant technique for the initial characterization of synthesized AgNPs by monitoring the synthesis during the process (Patil and Sastry 1997). In silver nanoparticles, the valence and conduction bands lie closely to each other, and electrons are able to move freely. This action leads to the formation of a surface plasmon resonance (SPR) absorption band occurring as a result of oscillation of AgNPs electrons with the wave of light (Noginov et al. 2007; Van Der Merwe 2010). The absorption of Ag nanoparticle depends on the size of particles, dielectric medium and chemical environment (Noginov et al. 2007). The SPR band can serve as a simple detection of biosynthesized nanoparticles’ stability with respect to unchanged wavelength of this absorption band (Zhang et al. 2016). As an example, the UV-Vis spectra showing surface plasmon resonance bands of AgNPs prepared by using the Origanum vulgare L. water extract (whole plant) and silver nitrate water solution (Baláž et al. 2017a) are shown in Fig. 7.4.
Time-dependent absorption spectra during AgNPs synthesis and observation of SPR at 445 nm (Baláž et al. 2017a), copyright by Springer
The time-dependent investigation of Vis spectra (Fig. 7.4) showed that soon after mixing O. vulgare L. water extract (showing two peaks in UV region) and silver nitrate water solution, the absorbance at 445 nm occurred, corresponding to the spherical silver nanoparticles. With reaction time, its intensity increased and after 7 min, the absorbance reached the maximum and after that, there were only slight changes (Baláž et al. 2017a).
7.3.2 Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR) represents accurate and reproducible possibility to detect small changes in functional groups by measuring absorbance. FTIR spectroscopy is relatively commonly technique used to identify which molecules are responsible for bioreduction of Ag(I) ions in synthesis of AgNPs. In infrared spectra, the interactions between nanomaterials and biomolecules or the conformational states of bounded biomolecules (e.g. proteins) can be observed (Jiang et al. 2005; Shang et al. 2007; Lin et al. 2014). In addition to classical potassium bromide-based method, attenuated total reflection (ATR)-FTIR spectroscopy can also serve as a method for observation of the chemical changes on the surface (Lin et al. 2014). Sample preparation is much easier for conventional FTIR as there is no need to prepare pellet. ATR-FTIR method seems to be a good candidate for investigation of the surface characteristics of nanomaterial, but it is not a very sensitive technique at nanoscale due to its penetration depth and also because it has the same order of magnitude as the wavelength of IR (Liu and Webster 2007). As an example, ATR-FTIR spectra from the same study from where the UV-Vis spectrum, as shown, are provided (Fig. 7.5).
ATR-FTIR spectra of silver nitrate, pure Origanum vulgare L. extract (ORE), polyvinyl pyrrolidone and both PVP-stabilized and non-stabilized AgNPs (Baláž et al. 2017a), copyright by Springer
This complex, as shown in Fig. 7.5, provides a comparison of the FTIR spectra of all species included in the synthesis of AgNPs in the mentioned study. The spectra are described in detail in Fig. 7.5; however, the presence of –OH and the main peaks of the polymer in the spectrum of the final stabilized product can be emphasized. Also, no peaks of silver nitrate were observed in X-ray diffraction.
7.3.3 X-Ray Diffraction
X-ray diffraction (XRD) is very extended analytical method which can be used to analyse the structure of molecules as well as the crystal structures, qualitative identification of studied structures and, last but not least, the particle sizes (Singh et al. 2013). The X-ray light reflects on crystal, and this reflection leads to the formation of diffraction peaks that reflect structural characteristics of studied crystal. By XRD technique, a broad spectrum of materials from biomolecules, inorganic catalysts to polymers and nanoparticles and nanostructures can be analysed (Vaia and Liu 2002; Macaluso 2009; Khan et al. 2013). A rich plethora of compounds can be easily detected, as almost every phase has a unique diffraction pattern. There are, of course, exceptions, and then other techniques are necessary. It is also possible to identify impurities; however, the detection limit of commercial X-ray diffractometers is around 5%. This method has also some disadvantages including problems with crystal growth and the ability to obtain data related to single conformation (Sapsford et al. 2011). With regard to silver NPs preparation, it is very good method, as silver nitrate and silver are well-distinguishable. However, it is necessary to dry obtained powder for analysis. XRD patterns showing the progress of the bio-mechanochemical synthesis of AgNPs when using Origanum vulgare L. plant and eggshell membrane are shown in Fig. 7.6.
XRD patterns of AgNPs synthesized using AgNO3 and (a) ESM (eggshell membrane) mixture; (b) ORE (Origanum vulgare) mixture. Time of milling is included in figure, where green lines respond to the reflections of Ag (JCPDS 01–087-0717) and violet lines to WC (JCPDS 00–051-0939). All the non-marked peaks in (b) correspond to silver nitrate, AgNO3 (JCPDS 01–074-2076) (Baláž et al. 2017b), copyright by Elsevier
It can be seen that after 15 min, the peaks corresponding to elemental silver were already present in both studied samples. The synthesis was completed a little bit faster for the eggshell membrane and took only 30 min. In case of Origanum vulgare, L. after 30 min of milling, some peaks of AgNO3 were still visible, so the milling was conducted for 45 min in order to consume the remaining AgNO3. In Fig. 7.6, also the reflections corresponding to qusongite (WC, material of milling chamber and balls) can be seen after 45 min, which is a proof of the wear from milling media. Therefore, it was concluded that for Ag/ESM system, 30 min of milling is enough.
7.3.4 Dynamic Light Scattering
The dynamic light scattering (DLS) method is also commonly used for physicochemical characterization of synthesized nanoparticles and plays an important role in size distribution of small particles in suspension or solution (Sapsford et al. 2011; Lin et al. 2014; Zhang et al. 2016). The mentioned method measures the scattered laser light passing through a studied sample and is based on interaction of light with the studied particles. DLS is a technique used for determining the scattered light intensity as a function of time, and the average hydrodynamic size of prepared nanoparticles dispersed in liquids can be determined (Sapsford et al. 2011; Zhang et al. 2016). An example of particle size distribution determined by DLS and results of zeta potential measurement (described below) for AgNPs prepared by green synthesis using Origanum vulgare L. is provided in Fig. 7.7. The particle size is bimodal , with the average value of 136 nm (Sankar et al. 2013).
Particle size distribution determined by dynamic light scattering (a) and zeta potential measurement (b) of the silver NPs prepared by green synthesis (Baláž et al. 2017a), copyright by Springer
7.3.5 Zeta Potential
Zeta (ξ) potential of nanomaterials in solution provides information about the charge of a nanoparticle-bioconjugate and its stability. Zeta potential is usually determined by application of an electric field across the prepared sample and measures the velocity at which charged species move to electrode (Pons et al. 2006). Zeta potential can also be applied to infer nanoparticles stability. Values with potential of 30 mV or more indicate stability, while values with potential lower than 30 mV predict particles with instability or agglomeration trend (Sapsford et al. 2011). However, this depends on the utilization of various stabilizers, as some of them act as steric ones, which do not affect the final zeta potential value then. The zeta potential measurement showing the detected value of −26 mV is shown in Fig. 7.7b.
7.3.6 Transmission Electron Microscopy
Transmission electron microscopy (TEM) is a very important, valuable and frequently applied method for nanoparticles characterization. The electrons penetrate through the sample in this case. TEM technique is used to quantitatively measure particle size, morphology and size distribution. The TEM analysis provides good spatial resolution and possibility for other analytical measurements, but on the other hand, it requires high vacuum and thin sample section, so the preparation of the sample is very important in order to get high-quality and representative pictures (Lin et al. 2014; Zhang et al. 2016). For high magnification, high-resolution TEM (HRTEM) is used. Moreover, selected area diffraction (SAD) indicating the exact phase composition of the particular area in the samples can be recorded and subsequently compared with the XRD measurements. This analysis results in the rings or points in the reciprocal space, from which the interplanar distance can be calculated and individual crystallographic planes can be indexed. TEM is often connected with the energy-dispersive X-ray spectroscopy (EDX or EDS) providing information about individual elements present in the system. The example of TEM analysis of silver nanoparticles prepared by Baláž and his co-workers from water extract of Origanum vulgare L. after the stabilization with the polymer including SAD pattern and EDS spectra is provided in Fig. 7.8 (Baláž et al. 2017a).
(a) TEM images of the Ag/ORE/PVP indicating differentiation of the smaller and larger fractions of AgNPs. (b) SAD pattern of larger AgNPs fraction. (c) EDS spectrum of the larger and smaller AgNPs areas (Baláž et al. 2017a)
Regarding particle size, two different populations can be detected from the samples, larger ones of about 38 nm and smaller ones of about 7 nm. The shape of the larger particles seems to be pseudooctahedral, whereas the smaller ones exhibit spherical shape. These groups of nanoparticles are separated. The SAD patterns presented in Fig. 7.8b show the presence of silver with cubic crystal structure. The EDS spectra of the sample after stabilization (Fig. 7.8c) did not show only silver, but also other elements presented in the plant matrix.
7.3.7 Scanning Electron Microscopy
Scanning electron microscopy (SEM) method represents a valuable technique in surface imaging used in nanomaterials studies. In contrast with TEM, the electron beam ends on the sample. This method is appropriate for investigating particle size, size distribution and shape of nanoparticles and surface of prepared particles. By SEM technique, we can study the morphology of prepared nanoparticles and obtain histogram from pictures by measuring and counting the particles manually or by software (Fissan et al. 2014; Lin et al. 2014; Zhang et al. 2016). The SEM method is also very often combined with EDX method; however, it is less accurate than in combination with TEM, as also bulk material (not seen on the photograph) is analysed (the beam goes deep into the material), Also, elemental mapping showing the distribution of individual elements is often used. The disadvantage of SEM is inability to resolve the internal structure of particles , as it is impossible to zoom so much to see individual NPs, but we can obtain valuable information about purity and aggregation of nanoparticles (Zhang et al. 2016).
7.3.8 X-Ray Photoelectron Spectroscopy
X-ray photoelectron microscopy (XPS) is an electron spectroscopy for chemical analysis (ESCA) and represents a valuable method to obtain qualitative and quantitative spectroscopic information of surface chemical analysis to obtain an estimated empirical formulae (Demathieu et al. 1999; Manna et al. 2001; Zhang et al. 2016). XPS technique is carried out under vacuum; X-ray irradiation of the nanoparticles leads to the emission of electrons, and the amount of escaped electrons from the surface of nanoparticles gives XPS spectra. The main advantage is that it can distinguish among different oxidation states of elements. An example of XPS spectra of bio-mechanochemically synthesized AgNPs showing only the peaks corresponding to elemental Ag(0) is shown in Fig. 7.9.
XPS spectra of AgNPs containing composite (Rak et al. 2016), copyright by Royal Society of Chemistry
7.4 Antibacterial Activity
Due to development of bacterial resistance of classical antibiotics, scientists are forced to find out more effective antibacterial compounds. There are many studies dealing with the synthesis and antibacterial and toxicological properties’ testing of NPs. The silver NPs make no exception. The interest of the scientific community on silver nanoparticles as antibacterial agents has been increased in the last few decades. Unusual properties of AgNPs have led to their utilization in medicinal field, and the main attention is focused on their antimicrobial properties.
Many authors confirmed that Ag nanoparticles show antimicrobial effects and eliminate the bacteria even at low concentrations (mg L−1) (Panáček et al. 2006) without toxic influence on human cells (Carlson et al. 2008; AshaRani et al. 2009). As silver nanoparticles have shown potential as antibacterial substances, several methods are being used to evaluate their effect on different Gram-positive and Gram-negative bacterial strains. They will be briefly reviewed below.
7.4.1 Evaluation of Antibacterial Activity
Antibacterial activity studies are performed by different techniques, which lead to obtaining the results in different ways so the comparison of acquired data can be complicated. The mostly used methods of antibacterial activity evaluation are the determination of: (a) minimal inhibitory concentration (MIC), (b) half effective concentration (EC50), (c) minimal bactericidal concentration (MBC) and then (d) time-kill test, (e) disc-diffusion method and (f) well-diffusion method.
The commonly used method of evaluation of antibacterial activity is the disc-diffusion method. This well-known method is based on preparation of agar plates inoculated by tested microorganism. After incubation, the discs are impregnated with silver nanoparticles at desired concentration. Prepared agar plates are incubated under appropriate conditions for examined bacterial strains, and sensitivity analysis to AgNPs is carried out by measurement of the inhibition zone diameter around the disc or well. These methods are advantageous for simplicity and economic reasons and are commonly used for antibacterial activity of silver nanoparticles determination (Balouiri et al. 2016).
Antibacterial characteristics of AgNPs are most often studied by dilution methods and quantitative assays for the determination of MIC. MIC values represent the lowest concentrations of silver nanoparticles that completely inhibit the growth of tested microorganisms. The less common method for quantification of antibacterial activity in comparison with MIC method is MBC (minimum bactericidal concentration) and is defined as the lowest concentration of tested antibacterial compound that kills 99.9% of microorganisms after 24 h of incubation. The most suitable technique for the antibacterial effect is its determination by the time-kill test (or time-kill curve) and can also be applied for establishing the synergistic effect for combination of two or more antimicrobial substances. These experiments offer the data about the dynamic interactions between various bacterial strains and tested antibacterial compounds. The time-kill test provides a time-dependent (usually 0, 4, 6, 8, 10, 12 and 24 h) or concentration-dependent antibacterial activity, and obtained data are typically presented in the form of graph (Balouiri et al. 2016). EC50 (effective concentration) and IC50 (inhibitory concentration) are used in discovery processes to determine suitability of the drugs. The EC50 is half maximal effective concentration reffering of drug, which induces half-maximal response. The IC50 is the half maximal inhibitory concentration corresponding to the 50% control (Sebaugh 2011; Mohammadlou et al. 2016).
7.4.2 Antibacterial Potential of AgNPs
Silver in Ag(0) oxidation state is well-known as antibacterial agent and has been applied as therapeutics of some diseases since ancient time (Russell and Hugo 1994). Silver nanoparticles prepared by different ways were broadly examined on a large number of pathogens including Gram-positive and Gram-negative bacteria. Some works have shown that the silver nanoparticles are more effective against Gram-negative bacteria (Chopade et al. 2013; Kokila et al. 2016), but on the other hand, the opposite results have also been reported (El Kassas and Attia 2014). These differences in sensitivity can be explained by the differentiation in the thickness and composition of biomolecules presented in the biomembranes as the cell wall of Gram-positive bacteria is composed by peptidoglycans and is thicker than Gram-negative bacteria cell wall (Franci et al. 2015; Dakal et al. 2016; de Jesús Ruíz-Baltazar et al. 2018). The importance of antibacterial activity determination in various bacterial species becomes the essence of the need to understand the mechanism, resistance and future applications of Ag nanoparticles. Ultimate reports on antimicrobial activity are summarized in Table 7.1.
Though the antibacterial activity of AgNPs has been widely considered and reported, some factors affecting the AgNPs antimicrobial properties are arising, e.g. shape, size and concentration of NPs and capping agents (Ahmed et al. 2016; Aziz et al. 2015, 2016). Nakkala et al. (2017) studied Ag nanoparticles with average size of 21 nm and the size distribution was observed to be 1–69 nm when synthesized using medicinal plant Ficus religiosa. These synthesized silver NPs resulted in strong antibacterial effect against Pseudomonas fluorescens, Salmonella typhi, Escherichia coli and Bacillus subtilis. Lower concentrations of silver nanoparticles indicated delayed effect on bacterial cell growth which may be caused by the bacteriostatic effect, while at the concentrations of 60 and 100 μg, the Ag nanoparticles showed bactericidal effect and no cell growth was detected. The green synthesized silver nanoparticles using the Cynara cardunculus leaf extract exhibited an antibacterial effect on E. coli and S. aureus at concentration of 20 mM; at lower concentration, the effect is minimal (de Jesús Ruíz-Baltazar et al. 2018). Antibacterial effect of AgNPs prepared by aqueous extracts from Berberis vulgaris using disc-diffusion method at (1, 3, 5 mM) concentrations were evaluated on S. aureus and E. coli. As a negative control, water was used and as a positive control were chosen gentamicin and streptomycin. The MIC tests showed much higher antibacterial effect against Gram-negative bacteria, E. coli, than against Gram-positive, S. aureus (Behravan et al. 2019). Authors predict that silver nanoparticles have high affinity to sulphur and phosphorus-containing biomolecules, which is the main reason of AgNPs antibacterial properties. It means that silver nanoparticles influence the proteins containing sulphur which are concentrated inside and outside of the cell biomembrane and impact the possibilities of bacteria cell apoptosis (Yamanaka et al. 2005; Tamboli and Lee 2013). Antibacterial activity of silver nanoparticles is also affected by size and shape. It has been found that the smaller nanoparticles with larger surface-to-volume ratio exhibit higher antimicrobial effect than larger particles (Gurunathan 2015; Ethiraj et al. 2016; Deng et al. 2016). In general, higher concentration of antimicrobial agent results in more effective antimicrobial effect, but particles with small size can lead to apoptosis of bacterial cells at a lower concentration. As we mentioned before, the shape of nanoparticles also influences the interaction with Gram-negative bacteria, for example E. coli (Ge et al. 2014; Deng et al. 2016). The interaction between AgNPs with different shapes and E. coli has been studied (Pal et al. 2007). The interactions were similar, but inhibition results were different. Authors discussed that AgNPs with the same surface area, but different shapes, may have unequal effective surface area in considerations of active facets. At that time, authors were unable to give an estimation of how the surface area of different NPs influences their ability to kill bacteria (Pal et al. 2007).
Many authors and reviewers have reported the mechanism of action of AgNPs in detail, but the strict mechanism of the antibacterial effect of silver and silver nanoparticles is not fully elucidated. Most of studies consider multiple mechanisms of action, but the main three different mechanisms are expected: (a) irreversible damage of bacterial cell membrane through direct contact; (b) generation of reactive oxygen species (ROS); and (c) interaction with DNA and proteins (Sintubin et al. 2012; Durán et al. 2016; Sheng and Liu 2017; Rajeshkumar and Bharath 2017; Aziz et al. 2015, 2016, 2019) (Fig. 7.10).
Fields of applications of mechanochemistry (Baláž et al. 2019)
7.5 Bio-mechanochemical Synthesis
Mechanochemistry has been in existence for a very long time, but its name was established much later. For example, creating fire by just banging two stones together in ancient times can be also considered mechanochemistry. Valuable works regarding history of this branch of chemistry were written by Takacs (Takacs 2004, 2013, 2018). Among many others, at least two important mechanochemists from the past can be mentioned, namely Carey Lea and Ostwald. The first one found out that the application of energy in the form of pressure and temperature yields different results (Lea 1893). Particularly, if temperature is applied on mercury or silver chloride, just sublimation occurs, whereas pressure effect results in the decomposition of these compounds into elements. Ostwald put the term “mechanochemistry” on the same level as thermochemistry, photochemistry and electrochemistry (Ostwald 1919). Since these preliminary works, mechanochemistry underwent a great development. Recently, it has penetrated into almost every branch of chemistry and materials science, for example pharmacy, organic synthesis, waste management, extraction of biologically active compounds from natural resources, nanotechnology, catalysis, etc. (Boldyreva 2013).
The term “mechanochemistry” is composed of two words: mechanics and chemistry. In simple words, by supplying the mechanical energy, it is possible to perform chemical reactions which would normally need higher temperatures or organic solvents under ambient conditions directly in solid state, which brings about very important environmental aspect. From the physical point of view, the energy is supplied to the powders by milling. The materials can be treated for two reasons, either to activate material or to perform reactions. The milling process can be conducted in dry or wet environment. There are many types of mills applying many different regimes (like pressing, shearing, impact, etc.). The principles of work of some basic high-energy mills are described in Fig. 7.11 (Baláž et al. 2019).
Working principles of selected mechanochemical devices: (a) mortar and pestle, (b) shaker/vibratory mill (horizontal and vertical oscillations are possible), (c) planetary ball mill, (d) continuous twin-screw extruder. Adapted from ref. (Baláž et al. 2019)
Most mechanochemical experiments are performed on a laboratory scale; however, the scale-up of this method is also gaining importance, namely, its wide usage in pharmaceutical industry (Al Shaal et al. 2010).
The mechanochemical approach is also very important for nanomaterials synthesis (Baláž et al. 2013). Particularly, it represents a top-down approach by creating nanoparticles by comminution of coarse materials. The other strategy is to use the micro-sized elements for the preparation of the compounds with crystallite sizes in nano-range. This can be achieved by high-energy milling. Another possibility is the utilization of ultrafine milling (e.g. wet stirred media milling) (Romeis et al. 2016), where the size of the product is diminished into very small particles, which can be applied, for example, in biomedicine as nanosuspensions (Peltonen and Hirvonen 2010).
At the very beginning of the new millennium, nanoparticles synthesis by this method was just starting and a short review paper by McCormick and Tsuzuki was elaborated (Tsuzuki and McCormick 2004). Different metallic nanoparticles were successfully prepared by reduction of corresponding chloride with sodium metal. The authors also introduced a concept of dilution agent (NaCl in this case), because the reactions were exothermic, and it was necessary to avoid combustion. Also, the use of smaller grinding balls was beneficial as it also delayed the combustion. Many binary oxides and sulphides with particle size in the nanorange were successfully synthesized by the same research group from the corresponding chlorides. Since that time, the field broadened significantly, as pointed out earlier (Baláž et al. 2013).
There is a large number of papers on silver nanoparticles synthesis using mechanochemistry. One of the approaches is the utilization of AgCl as silver precursor, and the reduction is achieved via displacement reaction with another metal (Keskinen et al. 2001; Le et al. 2008; Khakan et al. 2017). In other works, composites of AgNPs with different compounds for various applications were prepared (Wang et al. 2010; Dai et al. 2016; Chambers et al. 2017). A cryomilling of micro-sized elemental Ag was also used (Kumar et al. 2016). The mechanochemical reduction of Ag2O (Khayati and Janghorban 2012) or AgNO3 was also used (Tiimob et al. 2017).
However, the reports utilizing natural materials for mechanochemical synthesis of AgNPs are very scarce. Namely, the utilization of materials of plant origin is an unexplored field. Such an approach can be called bio-mechanochemical synthesis and in further text, few works in which the combination of mechanochemistry and plant material for the production of AgNPs has been applied are discussed.
The first paper elaborated by Rak et al. in 2016 reports on the preparation of silver nanoparticles by ball milling method, in which lignin serves as a reducing agent and polyacrylamide (PAM) as a supporting polymer (Rak et al. 2016). During the synthesis, AgNO3 was used as a source of silver. Regarding reducing agents, two types of lignin were used, namely commercially available one (Kraft lignin—KLig) and synthetic one (Thermomechanical pulp lignin—TMPLig). In the second case, lignin was prepared from TMP by oxidation prior to experiments. The difference between the two lignins is in the fibre size, as TMP lignin is larger and its structure is very similar to original lignin, so it can be also can called natural lignin. At first only solid AgNO3 and lignin were milled for 90 min and AgNPs were obtained. However, as the authors wanted to use the product in the antibacterial filters, an introduction of the third component, namely polyacrylamide (PAM), was necessary. At first, lignin was left to form a composite with PAM, and then it was co-milled with AgNO3 under similar conditions as in the case of PAM-free experiments. In Fig. 7.12, the scheme of the whole synthetic process is demonstrated.
Scheme of mechanochemical synthesis of AgNPs/lignin/polyacrylamide composites (Rak et al. 2016)
Using TEM, the authors have shown that the prepared AgNPs were embedded in the organic lignin matrix. In the samples with PAM, AgNPs are encased in the polymer. The AgNPs were spherical and polydisperse with same range of sizes in all prepared samples so the PAM or the used type of lignin did not affect studied AgNPs in matter of size and shape.
The results of PXRD confirmed the presence of elemental silver. The authors also used XPS method (the spectrum was already shown in Fig. 7.9) and observed a peak located at 368.5 eV, which supports the presence of Ag in the zero-valence form. FTIR spectra have shown the peaks corresponding to lignin and PAM also in the AgNP-containing samples, thus showing that the structure of the materials was maintained.
The antibacterial activity was tested on two Gram-positive (S. aureus, Enterococcus faecium) and three Gram-negative (Pseudomonas aeruginosa, Klebsiella pneumoniae, E. coli) bacteria. For antimicrobial tests, the Kraft lignin was selected and two types of porous plugs were prepared. One consisted of AgNPs, Kraft lignin and PAM while another only Kraft lignin and PAM without any silver in it. Afterwards, the bacterial dispersion was passed through the plugs, and filtrates were diluted and inoculated onto agar plates. On the agar plates incubated with solutions that were passed through the silver-containing composites, no bacterial growth was noticed. On the contrary, no inhibition of bacterial growth was observed on the agar plates that were treated with the AgNPs-free solution. The scheme of this process and the photographs of the agar plates are presented in Fig. 7.13a and b, respectively.
(a) Schematic illustration showing the antibacterial activity of AgNP@KLig/PAM; (b) photos of agar plates incubated with a solution of K. pneumonia that were passed through the filter plug with a silver-containing composite AgNP@KLig/PAM (top), and the silver-free KLig/PAM control (bottom). Reprinted from (Rak et al. 2016) [17], copyright by Royal Society of Chemistry
The authors showed that mechanochemically prepared AgNPs@KLig/PAM possesses antibacterial activity and generated adequate amounts of Ag(I) to be able to eliminate all studied bacterial strains. The mechanism of antibacterial activity of silver nanoparticles most probably lies in the local oxidative generation of Ag(I) ions at aerobic conditions, which releases antimicrobially active silver ions in contact with water (Xiu et al. 2012).
The utilization of biomass-derived polysaccharides as a reducing agent for AgNPs synthesis was realized by Arancon et al. in 2017 (Arancon et al. 2017), namely, the polysaccharides were isolated from the fungi Abortiporus biennis (PS1), Lentinus tigrinus (PS2), Rigidoporus microporus (PS5) and Ginkgo polypore (PS10). The polysaccharides were then ball-milled with AgNO3 in the ratio 1:2 for 30 min. After milling, the obtained powders were calcined at 600 °C to remove the unbound organic matrix. Finally, the toxicity of the products was investigated by MTT assay.
The first part of the paper is devoted to the characterization of the prepared AgNPs. The authors also focus on the interaction between polysaccharides and AgNPs by using FTIR, and they registered some bands corresponding to organics species, as some functional groups could still be detected in the spectra, despite the calcination step. This points to successful interaction. The calcined Ag-PS10 powders were further investigated by X-ray diffraction (Fig. 7.14).
XRD pattern of Ag-PS10 sample (Arancon et al. 2017), copyright by Elsevier
A non-traditional result pointing to the preparation of silver in hexagonal crystal structures was observed. The authors claim that they have also cubic silver, but the intensity of the main peak, which should be located at 2 theta value at 38.1°, is really low, and the same situation is also with the third most intensive peak of cubic phase which should be located at 64.5°. The authors claim that they have the combination of both crystal structures based on the TEM analysis; however, the actual crystal structure does not have to be the same as the actual shape of the particles. Namely, in most papers, spherical AgNPs are observed (mostly claimed based on UV-Vis measurements), but when XRD is done, the authors observe cubic reflections (Sankar et al. 2013). The authors have also used XPS method with the conclusion that they have Ag in elemental form, as the position of Ag peak was at 368.1 eV and it fits in the range between 368.0 and 368.3, which, according to the authors, corresponds to Ag in this oxidation state. Also, the thermal decomposition during calcination showing the evaporation of residual moisture and decomposition of unbound organics was observed.
The second part of paper is devoted to the MTT assay to show the toxicity on two human cell lines, namely A549 lung adenocarcinoma and SH-SY5Y neuroblastoma cell line. The products have shown almost no toxicity towards the studied cell lines; a statistically significant decrease in viability was observed only in one case when the highest concentration was used. The example is presented for Ag-PS10 sample (Fig. 7.15).
The percentage cell viability estimated by MTT test on (a) A549 and (b) SH-SY5Y cell lines using increasing concentration of Ag-PS10 NPs (0, 25, 50, 100, 200 and 400 μg mL−1) after exposition time of 24, 48 and 72 h. An OD value of control cells (unexposed cells) was taken as 100% viability (0% cytotoxicity). Results were displayed as mean ± SD of three independent experiments (∗significantly different from control; p < 0.05). Reprinted from (Arancon et al. 2017), copyright by Elsevier
In the end, the authors also analysed the Ag content in the soluble silver supernatant of Ag–PS nanohybrid dispersions in supplemented cell culture media, and they detected higher amount of Ag in the media of A549 cell line.
The mechanochemical approach was also applied as a stabilization procedure after the classical green synthesis (Baláž et al. 2017a). Firstly, Ag nanoparticles have been prepared by a classical green synthesis in liquid medium by mixing silver nitrate water solution and aqueous extract of Origanum vulgare, L. (whole plant was used). Generation of AgNPs was monitored by measuring the UV-Vis spectra (Fig. 7.4) and also by visual colour change of the solution from light brownish yellow at the beginning to dark brownish red at the end of the reaction. The reaction was completed after 15 min and subsequently, the stabilizing agent, polyvinylpyrrolidone (PVP) was added to the prepared nanosuspension. The effect of milling on the particle size distribution was studied. The authors found out that before the milling with PVP, two fractions of particles were present with sizes ranging between 110 and 330 nm for the larger one and between 15 and 80 nm for the smaller one. After the milling for 60 min, bimodal particle size distribution was also observed, but the milling decreased the average size of nanoparticles.
The increase in the intensity of the SPR peak with reaction time was already described earlier (Fig. 7.4). After the stabilization with PVP, this peak was red-shifted to 472 nm.
The final sample after green synthesis and the PVP-capped NPs were investigated in terms of photoluminescent properties. In the case of PVP-free AgNPs, just two weak emission peaks were recorded. On the other hand, sample of AgNPs with PVP exhibited one strong emission peak, which indicated that the addition of PVP enhances the photoluminescence emission intensity. Also, infrared spectroscopy (FTIR) was employed for the characterization (Fig. 7.5).
The transmission electron microscopy (TEM) was used to analyse the size, morphology and distribution of AgNPs both in the absence and presence of PVP. The results for the PVP-stabilized sample are shown in Fig. 7.8. The TEM analysis of the sample prior to stabilization is shown in Fig. 7.16.
Transmission electron microscopy (TEM) images of Ag/ORE sample. (a) Particle size distribution at low magnification (b) SAD pattern (c) larger AgNPs is the presence of parallel twins; a small particle of AgCl displaying weaker contrast due to the lower average density is encircled (d) EDS spectrum of larger AgNP and (e) EDS spectrum of small AgNPs in ORE matrix (Baláž et al. 2017a), copyright by Springer
As already mentioned, two different populations were found in both samples. In the sample without PVP, the populations were intermixed and dispersed in O. vulgare matrix, whereas after the stabilization, the separation of these groups occurred. The SAD pattern presented in Fig. 7.16b shows mainly the presence of silver with cubic crystal structure, but also the presence of AgCl was detected. Such particle is shown in Fig. 7.16c. Also, twinning phenomenon, common for silver NPs, was observed as it is shown in the same figure. From the EDS spectra in Fig. 7.16d and e, almost pure silver in the case of larger particle and considerable amount of chlorine in the case of smaller AgNPs with Origanum remains were detected.
The last part was devoted to the stability studies. Zeta potential and photon cross-correlation spectroscopy (PCCS) (quite similar to DLS method) were the techniques used for demonstrating the stability of prepared nanoparticles. After the addition of PVP, the zeta potential decreased, which is the proof of stabilization of the system. PCCS has shown the formation of agglomerates and a quick increase in average hydrodynamic particle diameter during storage of the PVP-free sample (this can be well seen for Ag/ORE sample after 8 weeks). As can be seen in Fig. 7.17, the introduction of PVP into the system led to a noticeable enhancement in the stability of the nanosuspensions. The average hydrodynamic particle diameter did not increase significantly during 26 weeks storage at 4 °C.
Stability of the Ag/ORE/PVP and Ag/ORE nanosuspensions. (a) The hydrodynamic particle diameter, (x50) change with the time of storage. (b) Particle size distribution (Baláž et al. 2017a), copyright by Springer
The main added value of this paper is the introduction of wet stirred media milling for the stabilization of silver NPs. The antibacterial activity was not investigated in this study.
The last paper to be discussed in this sub-chapter is the completely solid-state approach (Baláž et al. 2017b). An eggshell membrane (ESM) and O. vulgare, L. (ORE) plant were used as natural reducing agents. As a silver precursor, silver nitrate was used. The whole synthesis was accomplished in planetary ball mill with the ratio of AgNO3 to eggshell membrane or O. vulgare, L. plant 1:1. The course of reaction was followed by X-ray diffraction, as already described in Fig. 7.6.
From the SEM images, the AgNPs embedded within the organic matrices of both materials could be identified. In the case of Ag/ORE sample, the NPs seemed to be denser. Besides silver, the EDS analysis has shown the presence of phosphorous and chlorine coming from the matrix of Ag/ORE sample. Also, zeta potential in water was measured, yielding more negative value for Ag/ORE sample, which suggests its better stability.
TEM analysis of both samples confirmed the presence of AgNPs dispersed in the organic matrix (Fig. 7.18). The AgNPs differ in size, namely Ag/eggshell membrane sample is comprised of smaller particles than Ag/O. vulgare sample. In the latter sample, the separate nanoparticles have tendency to merge and form larger clusters. Therefore, two different populations of nanoparticles are present in this sample. This phenomenon was not observed for Ag/eggshell membrane composite.
(a) TEM images of Ag/ESM and (b) Ag/ORE at low magnification with inserted SAD patterns indicating the crystallinity of AgNPs in both samples. (c) HRTEM image of well-dispersed AgNPs in ESM matrix and (d) agglomeration of AgNPs in the ORE matrix. (Baláž et al. 2017b), copyright by Elsevier
Both samples were also tested for antibacterial activity on six strains of bacteria (three Gram-positive and three Gram-negative) at four different concentrations (1, 2.5, 5 and 10 mM). The antibacterial activity is expressed as the percentage of relative inhibition zone diameter (RIZD) with respect to gentamicin standard antibiotic. In the case of pure biomaterials, no antibacterial activity was detected. In Ag-containing samples, the antibacterial activity was observed. The antibacterial activity was higher for the nanoparticles prepared with Origanum vulgare, L. plant. Also, the lowest concentration (1 mM) was not effective in inhibiting bacterial growth, whereas in the case of L. monocytogenes and S. aureus, small antibacterial activity of sample Ag/O. vulgare was observed. The Ag/O. vulgare nanoparticles at concentrations 2.5, 5 and 10 mM reduced the growth of all tested bacteria. The concentration 2.5 mM was quite interesting because in four of six studied bacteria, the antibacterial activity was higher than those with 5 mM concentration. It is possible that the silver ions had better distribution in Origanum vulgare, L. matrix and thus better effect. The antibacterial activity towards all studied bacteria for Ag/eggshell membrane sample was observed at 5 and 10 mMAg AgNO3 concentration.
The last discussed study could ignite a new research field, which could be called bio-mechanochemical synthesis as it combines the tools of mechanochemistry and green synthesis (bio-approach) using plants. Together with the studies mentioned earlier, it definitely opens new possibilities and brings mechanochemical research closer to nature.
7.6 Conclusion
From the economical (low cost) and environmental point of view, the plant- and plant extract-based synthesis represents a green chemistry-based approach for silver nanoparticles synthesis and has opened new ways in treating and/or controlling various diseases of humans. This approach avoids the problem of using hazardous chemicals and subsequent addition of stabilization agents. Nature is the source of enormous number of extractable biomolecules (e.g. terpenoids, sugars, alkaloids, polyphenols, flavonoids, proteins) suitable for reduction of silver ions. Commonly used characterization techniques (UV-Vis, FTIR, XRD, SEM, TEM, etc.) can be also used to describe green-synthesized nanoparticles to evaluate and control the particle size, size distribution, shape of nanoparticles or to detect the degree of agglomeration. The last part was devoted to bio-mechanochemical synthesis of AgNPs, which represents a combination of mechanochemistry being a relatively old branch of chemistry, with biosynthesis using plants. This approach is still very rarely described in literature. The combination of mechanochemical-assisted extraction (MCAE) of plant materials and bio-reduction of silver ions in one-step procedure and could open a new research pathway.
Generally, nanoparticles with antimicrobial properties are qualified as a promising variant to antibiotics. They represent an appreciable future prospective in consideration in the development of pathogen-resistant solution. Ultimately, silver nanoparticles used in medical and pharmaceutical field are a rapidly developing field of science where the green synthesis provides easy and environmentally acceptable access to them.
References
Abbasi E, Milani M, Fekri Aval S et al (2016) Silver nanoparticles: synthesis methods, bio-applications and properties. Crit Rev Microbiol 42:173–180. https://doi.org/10.3109/1040841X.2014.912200
Ahmad N, Sharma S, Alam MK et al (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloid Surf B 81:81–86. https://doi.org/10.1016/j.colsurfb.2010.06.029
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res 7:17–28. https://doi.org/10.1016/j.jare.2015.02.007
Ajitha B, Ashok Kumar Reddy Y, Sreedhara Reddy P (2015) Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater Sci Eng C 49:373–381. https://doi.org/10.1016/j.msec.2015.01.035
Al Shaal L, Müller RH, Shegokar R (2010) SmartCrystal combination technology - scale up from lab to pilot scale and long term stability. Pharmazie 65:877–884. https://doi.org/10.1691/ph.2010.0181
Ameen F, AlYahya SA, Bakhrebah MA et al (2018) Flavonoid dihydromyricetin-mediated silver nanoparticles as potential nanomedicine for biomedical treatment of infections caused by opportunistic fungal pathogens. Res Chem Intermed 44:5063–5073. https://doi.org/10.1007/s11164-018-3409-x
Arancon RAD, Balu AM, Romero AA et al (2017) Mechanochemically synthesized Ag-based nanohybrids with unprecedented low toxicity in biomedical applications. Environ Res 154:204–211. https://doi.org/10.1016/j.envres.2017.01.010
AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290. https://doi.org/10.1021/nn800596w
Ashraf JM, Ansari MA, Khan HM et al (2016) Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci Rep 6:20414. https://doi.org/10.1038/srep20414
Ashraf A, Zafar S, Zahid K et al (2018) Synthesis, characterization, and antibacterial potential of silver nanoparticles synthesized from Coriandrum sativum L. J Infect Public Health 12:275. https://doi.org/10.1016/j.jiph.2018.11.002
Aziz N, Faraz M, Pandey R, Sakir M, Fatma T, Varma A, Barman I, Prasad R (2015) Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial and photocatalytic properties. Langmuir 31:11605–11612. https://doi.org/10.1021/acs.langmuir.5b03081
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Aziz N, Faraz M, Sherwani MA, Fatma T, Prasad R (2019) Illuminating the anticancerous efficacy of a new fungal chassis for silver nanoparticle synthesis. Front Chem 7:65. https://doi.org/10.3389/fchem.2019.00065
Azmir J, Zaidul ISM, Rahman MM et al (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117:426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Azwanida NN (2015) A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 4:3–8. https://doi.org/10.4172/2167-0412.1000196
Baláž P, Achimovicová M, Baláž M et al (2013) Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev 42:7571–7637. https://doi.org/10.1039/c3cs35468g
Baláž M, Balážová Ľ, Daneu N et al (2017a) Plant-mediated synthesis of silver Nanoparticles and their stabilization by wet stirred media milling. Nanoscale Res Lett 12:83–91. https://doi.org/10.1186/s11671-017-1860-z
Baláž M, Daneu N, Balážová Ľ et al (2017b) Bio-mechanochemical synthesis of silver nanoparticles with antibacterial activity. Adv Powder Technol 28:3307–3312. https://doi.org/10.1016/j.apt.2017.09.028
Baláž M, Vella-Zarb L, Hernández JG et al (2019) Mechanochemistry: a disruptive innovation for the industry of the future. Chimica Oggi - Chemistry Today 37:32–34
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Becker RO (1999) Silver ions in the treatment of local infections. Met Based Drugs 6:311–314. https://doi.org/10.1155/MBD.1999.311
Behravan M, Hossein Panahi A, Naghizadeh A et al (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154. https://doi.org/10.1016/j.ijbiomac.2018.11.101
Benakashani F, Allafchian A, Jalali SAH (2017) Green synthesis, characterization and antibacterial activity of silver nanoparticles from root extract of Lepidium draba weed. Green Chem Lett Rev 10:324–330. https://doi.org/10.1080/17518253.2017.1363297
Boldyreva E (2013) Mechanochemistry of inorganic and organic systems: what is similar, what is different? Chem Soc Rev 42:7719–7738. https://doi.org/10.1039/c3cs60052a
Carlson C, Hussein SM, Schrand AM et al (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112:13608–13619. https://doi.org/10.1021/jp712087m
Carmona ER, Benito N, Plaza T, Recio-Sánchez G (2017) Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa hope. Green Chem Lett Rev 10:250–256. https://doi.org/10.1080/17518253.2017.1360400
Catauro M, Raucci MG, Gaetano FDE, Marotta A (2004) Antibacterial and bioactive silver-containing Na2O.CaO.2SiO2 glass prepared by sol-gel method. J Mater Sci Mater Med 15:831–837. https://doi.org/10.1023/B:JMSM.0000032825.51052.00
Chambers C, Stewart SB, Su B et al (2017) Silver doped titanium dioxide nanoparticles as antimicrobial additives to dental polymers. Dent Mater 33:e115–e123. https://doi.org/10.1016/j.dental.2016.11.008
Chopade BA, Singh R, Wagh P et al (2013) Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int J Nanomedicine 8:4277. https://doi.org/10.2147/IJN.S48913
Clark JH, Lancaster M, Holder JV et al (2002) Handbook of green chemistry and technology. Blackwell Science Ltd, Oxford, UK
Dai Y, Yan XH, Wu X et al (2016) Facile self-assembly of AgNPs/WS2nanocomposites with enhanced electrochemical properties. Mater Lett 173:203–206. https://doi.org/10.1016/j.matlet.2016.03.050
Dakal TC, Kumar A, Majumdar RS, Yadav V (2016) Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol 7:1–17. https://doi.org/10.3389/fmicb.2016.01831
Dallas P, Sharma VK, Zboril R (2011) Silver polymeric nanocomposites as advanced antimicrobial agents: classification, synthetic paths, applications, and perspectives. Adv Colloid Interf Sci 166:119–135. https://doi.org/10.1016/j.cis.2011.05.008
de Jesús Ruíz-Baltazar Á, Reyes-López SY, de Lourdes Mondragón-Sánchez M et al (2018) Biosynthesis of Ag nanoparticles using Cynara cardunculus leaf extract: evaluation of their antibacterial and electrochemical activity. Results Phys 11:1142–1149. https://doi.org/10.1016/j.rinp.2018.11.032
de Marco BA, Rechelo BS, Tótoli EG et al (2018) Evolution of green chemistry and its multidimensional impacts: a review. Saudi Pharm J 27:1–8. https://doi.org/10.1016/j.jsps.2018.07.011
Demathieu C, Chehimi MM, Lipskier JF et al (1999) Characterization of dendrimers by X-ray photoelectron spectroscopy. Appl Spectrosc 53:1277–1281. https://doi.org/10.1366/0003702991945524
Demirbas A, Welt BA, Ocsoy I (2016) Biosynthesis of red cabbage extract directed Ag NPs and their effect on the loss of antioxidant activity. Mater Lett 179:20–23. https://doi.org/10.1016/j.matlet.2016.05.056
Deng H, McShan D, Zhang Y et al (2016) Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol 50:8840–8848. https://doi.org/10.1021/acs.est.6b00998
Durán N, Durán M, de Jesus MB et al (2016) Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine 12:789–799
El Kassas HY, Attia AA (2014) Bactericidal application and cytotoxic activity of biosynthesized silver Nanoparticles with an extract of the red seaweed Pterocladiella capillacea on the HepG 2 cell line. Asian Pacific J Cancer Prev 15:1299–1306. https://doi.org/10.7314/APJCP.2014.15.3.1299
Ethiraj AS, Jayanthi S, Ramalingam C, Banerjee C (2016) Control of size and antimicrobial activity of green synthesized silver nanoparticles. Mater Lett 185:526–529. https://doi.org/10.1016/j.matlet.2016.07.114
Fissan H, Ristig S, Kaminski H et al (2014) Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal Methods 6:7324–7334. https://doi.org/10.1039/c4ay01203h
Franci G, Falanga A, Galdiero S et al (2015) Silver Nanoparticles as potential antibacterial agents. Molecules 20:8856–8874. https://doi.org/10.3390/molecules20058856
García-Barrasa J, López-de-Luzuriaga JM, Monge M (2011) Silver nanoparticles: synthesis through chemical methods in solution and biomedical applications. Cent Eur J Chem 9:7–19. https://doi.org/10.2478/s11532-010-0124-x
Ge L, Li Q, Wang M et al (2014) Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int J Nanomedicine 9:2399–2407. https://doi.org/10.2147/IJN.S55015
Ghiyasiyan-Arani M, Salavati-Niasari M, Masjedi-Arani M, Mazloom F (2018) An easy sonochemical route for synthesis, characterization and photocatalytic performance of nanosized FeVO4 in the presence of aminoacids as green capping agents. J Mater Sci Mater Electron 29:474–485. https://doi.org/10.1007/s10854-017-7936-9
Gurunathan S (2015) Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J Ind Eng Chem 29:217–226. https://doi.org/10.1016/j.jiec.2015.04.005
Hemmati S, Rashtiani A, Zangeneh MM et al (2019) Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 158:8–14. https://doi.org/10.1016/j.poly.2018.10.049
Huang H, Yang X (2004) Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydr Res 339:2627–2631. https://doi.org/10.1016/j.carres.2004.08.005
Huang M, Du L, Feng J-X (2016) Photochemical synthesis of silver nanoparticles/eggshell membrane composite, its characterization and antibacterial activity. Sci Adv Mater 8:1641–1647. https://doi.org/10.1166/sam.2016.2777
Ismail M, Gul S, Khan MA, Khan MI (2016) Plant mediated green synthesis of anti- microbial silver nanoparticles—a review on recent trends. Rev Nanosci Nanotechnol 5:119–135. https://doi.org/10.1166/rnn.2016.1073
Jayaprakash N, Vijaya JJ, Kaviyarasu K et al (2017) Green synthesis of Ag nanoparticles using tamarind fruit extract for the antibacterial studies. J Photochem Photobiol B Biol 169:178–185. https://doi.org/10.1016/j.jphotobiol.2017.03.013
Jiang X, Jiang J, Jin Y et al (2005) Effect of colloidal gold size on the conformational changes of adsorbed cytochrome c: probing by circular dichroism, UV-visible, and infrared spectroscopy. Biomacromolecules 6:46–53. https://doi.org/10.1021/bm049744l
Joshi N, Jain N, Pathak A, Singh J, Prasad R, Upadhyaya CP (2018) Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J Sol-Gel Sci Techn 86(3):682–689. https://doi.org/10.1007/s10971-018-4666-2
Kalaiarasi R, Prasannaraj G, Venkatachalam P (2013) A rapid biological synthesis of silver nanoparticles using leaf broth of Rauvolfia Tetraphylla and their promising antibacterial activity. Indo Am J Pharm Res 3:8052–8062
Kanawaria SK, Sankhla A, Jatav PK et al (2018) Rapid biosynthesis and characterization of silver nanoparticles: an assessment of antibacterial and antimycotic activity. Appl Phys A Mater Sci Process 124:1–10. https://doi.org/10.1007/s00339-018-1701-7
Kelly EH, Dennis JB, Anthony RT (2002) Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem 13:572–584. https://doi.org/10.1016/S0955-2863(02)00208-5
Keskinen J, Ruuskanen P, Karttunen M, Hannula SP (2001) Synthesis of silver powder using a mechanochemical process. Appl Organomet Chem 15:393–395. https://doi.org/10.1002/aoc.159
Khakan B, Miandashti AR, Ashjari M (2017) Mechanochemical synthesis of silver nanoparticles. J Powder Metall Min 6:2–5. https://doi.org/10.4172/2168-9806.1000174
Khan A, Asiri AM, Rub MA et al (2013) Synthesis, characterization of silver nanoparticle embedded polyaniline tungstophosphate-nanocomposite cation exchanger and its application for heavy metal selective membrane. Compos Part B Eng 45:1486–1492. https://doi.org/10.1016/j.compositesb.2012.09.023
Kharissova OV, Dias HVR, Kharisov BI et al (2013) The greener synthesis of nanoparticles. Trends Biotechnol 31:240–248. https://doi.org/10.1016/j.tibtech.2013.01.003
Khatoon A, Khan F, Ahmad N et al (2018) Silver nanoparticles from leaf extract of Mentha piperita: eco-friendly synthesis and effect on acetylcholinesterase activity. Life Sci 209:430–434. https://doi.org/10.1016/j.lfs.2018.08.046
Khayati GR, Janghorban K (2012) The nanostructure evolution of Ag powder synthesized by high energy ball milling. Adv Powder Technol 23:393–397. https://doi.org/10.1016/j.apt.2011.05.005
Kokila T, Ramesh PS, Geetha D (2016) Biosynthesis of AgNPs using Carica Papaya peel extract and evaluation of its antioxidant and antimicrobial activities. Ecotoxicol Environ Saf 134:467–473. https://doi.org/10.1016/j.ecoenv.2016.03.021
Kowshik M, Deshmukh N, Vogel W et al (2002) Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol Bioeng 78:583–588. https://doi.org/10.1002/bit.10233
Kulkarni N, Muddapur U (2014) Biosynthesis of metal nanoparticles: a review. J Nanotechnol 2014:510246. https://doi.org/10.1155/2014/510246
Kumar N, Biswas K, Gupta RK (2016) Green synthesis of Ag nanoparticles in large quantity by cryomilling. RSC Adv 6:111380–111388. https://doi.org/10.1039/c6ra23120a
Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K (2018) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int J Mod Sci 4:61–68. https://doi.org/10.1016/j.kijoms.2017.10.007
Le MT, Kim DJ, Kim CG et al (2008) Nanoparticles of silver powder obtained by mechano-chemical process. J Exp Nanosci 3:223–228. https://doi.org/10.1080/17458080802301997
Lea MC (1893) On endothermic decompositions obtained by pressure (second part.) transformations of energy by shearing stress. Am J Sci 46:413–420
Lin PC, Lin S, Wang PC, Sridhar R (2014) Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv 32:711–726. https://doi.org/10.1016/j.biotechadv.2013.11.006
Liu H, Webster TJ (2007) Nanomedicine for implants: a review of studies and necessary experimental tools. Biomaterials 28:354–369. https://doi.org/10.1016/j.biomaterials.2006.08.049
Liu YS, Chang YC, Chen HH (2018) Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J Food Drug Anal 26:649–656. https://doi.org/10.1016/j.jfda.2017.07.005
Logeswari P, Silambarasan S, Abraham J (2015) Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc 19:311–317. https://doi.org/10.1016/j.jscs.2012.04.007
Lomovsky OI, Lomovskiy IO, Orlov DV (2017) Mechanochemical solid acid/base reactions for obtaining biologically active preparations and extracting plant materials. Green Chem Lett Rev 10:171–185. https://doi.org/10.1080/17518253.2017.1339832
Macaluso RT (2009) Introduction to powder diffraction and its application to nanoscale and heterogeneous materials. ACS Symp Ser 1010:75–86. https://doi.org/10.1021/bk-2009-1010.ch006
Makarov VV, Love AJ, Sinitsyna OV et al (2014) “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat 6:35–44. https://doi.org/10.1039/c1gc15386b
Manna A, Imae T, Aoi K et al (2001) Synthesis of dendrimer-passivated noble metal nanoparticles in a polar medium: comparison of size between silver and gold particles. Chem Mater 13:1674–1681. https://doi.org/10.1021/cm000416b
Marchiol L, Mattiello A, Pošćić F et al (2014) In vivo synthesis of nanomaterials in plants: location of silver nanoparticles and plant metabolism. Nanoscale Res Lett 9:101. https://doi.org/10.1186/1556-276X-9-101
Mittal AK, Chisti Y, Banerjee UC (2013) Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv 31:346–356. https://doi.org/10.1016/j.biotechadv.2013.01.003
Mohammadlou M, Maghsoudi H, Jafarizadeh-Malmiri H (2016) A review on green silver nanoparticles based on plants: synthesis, potential applications and eco-friendly approach. Int Food Res J 23:446–463
Nakkala JR, Mata R, Sadras SR (2017) Green synthesized nano silver: synthesis, physicochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J Colloid Interface Sci 499:33–45. https://doi.org/10.1016/j.jcis.2017.03.090
Noginov MA, Zhu G, Bahoura M et al (2007) The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl Phys B Lasers Opt 86:455–460. https://doi.org/10.1007/s00340-006-2401-0
Omidi S, Sedaghat S, Tahvildari K et al (2018) Biosynthesis of silver nanoparticles with adiantum capillus-veneris L leaf extract in the batch process and assessment of antibacterial activity. Green Chem Lett Rev 11:544–551. https://doi.org/10.1080/17518253.2018.1546410
Ortan A, Fierascu I, Ungureanu C et al (2015) Innovative phytosynthesized silver nanoarchitectures with enhanced antifungal and antioxidant properties. Appl Surf Sci 358:540–548. https://doi.org/10.1016/j.apsusc.2015.07.160
Padalia H, Moteriya P, Chanda S (2015) Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab J Chem 8:732–741. https://doi.org/10.1016/j.arabjc.2014.11.015
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720. https://doi.org/10.1128/AEM.02218-06
Panáček A, Kvítek L, Prucek R et al (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253. https://doi.org/10.1021/jp063826h
Panigrahi S, Kundu S, Ghosh SK et al (2004) General method of synthesis for metal nanoparticles. J Nanopart Res 6:411–414. https://doi.org/10.1007/s11051-004-6575-2
Panigrahi S, Kundu S, Ghosh SK et al (2005) Sugar assisted evolution of mono- and bimetallic nanoparticles. Colloid Surf A 264:133–138. https://doi.org/10.1016/j.colsurfa.2005.04.017
Parthiban E, Manivannan N, Ramanibai R, Mathivanan N (2018) Green synthesis of silver-nanoparticles from Annona reticulata leaves aqueous extract and its mosquito larvicidal and anti-microbial activity on human pathogens. Biotechnol Reports 20:e00297. https://doi.org/10.1016/j.btre.2018.e00297
Patil V, Sastry M (1997) Electrostatically controlled diffusion of carboxylic acid derivatized Q-state CdS nanoparticles in thermally evaporated fatty amine films. J Chem Soc 93:4347–4353. https://doi.org/10.1039/a704355d
Peltonen L, Hirvonen J (2010) Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol 62:1569–1579. https://doi.org/10.1111/j.2042-7158.2010.01022.x
Pethakamsetty L, Kothapenta K, Nammi HR et al (2017) Green synthesis, characterization and antimicrobial activity of silver nanoparticles using methanolic root extracts of Diospyros sylvatica. J Environ Sci 55:157–163. https://doi.org/10.1016/j.jes.2016.04.027
Ping Y, Zhang J, Xing T et al (2018) Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of direct Orange 26. J Ind Eng Chem 58:74–79. https://doi.org/10.1016/j.jiec.2017.09.009
Pons T, Uyeda HT, Medintz IL, Mattoussi H (2006) Hydrodynamic dimensions, electrophoretic mobility, and stability of hydrophilic quantum dots. J Phys Chem B 110:20308–20316. https://doi.org/10.1021/jp065041h
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:32. https://doi.org/10.1186/2228-5326-2-32
Prasad R (2014) Synthesis of silver nanoparticles in photosynthetic plants. J Nanopart 2014:963961. https://doi.org/10.1155/2014/963961
Prasad R, Pandey R, Barman I (2016) Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed Nanobiotechnol 8:316–330. https://doi.org/10.1002/wnan.1363
Prasad R, Jha A, Prasad K (2018) Exploring the realms of nature for nanosynthesis. Springer International Publishing, New York. ISBN 978-3-319-99570-0. https://www.springer.com/978-3-319-99570-0
Prasad R (2019) Plant Nanobionics: Approaches in Nanoparticles Biosynthesis and Toxicity. Springer International Publishing (ISBN 978-3-030-16379-2). https://www.springer.com/gp/book/9783030163785
Rajan R, Chandran K, Harper SL et al (2015) Plant extract synthesized silver nanoparticles: an ongoing source of novel biocompatible materials. Ind Crop Prod 70:356–373. https://doi.org/10.1016/j.indcrop.2015.03.015
Rajeshkumar S, Bharath LV (2017) Mechanism of plant-mediated synthesis of silver nanoparticles—a review on biomolecules involved, characterisation and antibacterial activity. Chem Biol Interact 273:219–227. https://doi.org/10.1016/j.cbi.2017.06.019
Rak MJ, Friščić T, Moores A (2016) One-step, solvent-free mechanosynthesis of silver nanoparticle-infused lignin composites for use as highly active multidrug resistant antibacterial filters. RSC Adv 6:58365–58370. https://doi.org/10.1039/c6ra03711a
Raveendran P, Fu J, Wallen SL (2003) Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc 125:13940–13941. https://doi.org/10.1021/ja029267j
Romeis S, Schmidt J, Peukert W (2016) Mechanochemical aspects in wet stirred media milling. Int J Miner Process 156:24–31. https://doi.org/10.1016/j.minpro.2016.05.018
Roy P, Das B, Mohanty A, Mohapatra S (2017) Green synthesis of silver nanoparticles using Azadirachta indica leaf extract and its antimicrobial study. Appl Nanosci 7:843–850. https://doi.org/10.1007/s13204-017-0621-8
Russell AD, Hugo WB (1994) 7 antimicrobial activity and action of silver. Prog Med Chem 31:351–370. https://doi.org/10.1016/S0079-6468(08)70024-9
Sadeghi B, Gholamhoseinpoor F (2015) A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta 134:310–315. https://doi.org/10.1016/j.saa.2014.06.046
Safarpoor M, Ghaedi M, Asfaram A et al (2018) Ultrasound-assisted extraction of antimicrobial compounds from Thymus daenensis and Silybum marianum: antimicrobial activity with and without the presence of natural silver nanoparticles. Ultrason Sonochem 42:76–83. https://doi.org/10.1016/j.ultsonch.2017.11.001
Sankar R, Karthik A, Prabu A et al (2013) Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloid Surf B 108:80–84. https://doi.org/10.1016/j.colsurfb.2013.02.033
Sapsford KE, Tyner KM, Dair BJ et al (2011) Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem 83:4453–4488. https://doi.org/10.1021/ac200853a
Sebaugh JL (2011) Guidelines for accurate EC50/IC50 estimation. Pharm Stat 10:128–134. https://doi.org/10.1002/pst.426
Şeker Karatoprak G, Aydin G, Altinsoy B et al (2017) The effect of Pelargonium endlicherianum Fenzl. Root extracts on formation of nanoparticles and their antimicrobial activities. Enzym Microb Technol 97:21–26. https://doi.org/10.1016/j.enzmictec.2016.10.019
Shang L, Wang Y, Jiang J, Dong S (2007) PH-dependent protein conformational changes in albumin:gold nanoparticle bioconjugates: a spectroscopic study. Langmuir 23:2714–2721. https://doi.org/10.1021/la062064e
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interf Sci 145:83–96. https://doi.org/10.1016/j.cis.2008.09.002
Sheng Z, Liu Y (2017) Potential impacts of silver nanoparticles on bacteria in the aquatic environment. J Environ Manag 191:290–296. https://doi.org/10.1016/j.jenvman.2017.01.028
Shriniwas PP, Subhash KT (2017) Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem Biophys Reports 10:76–81. https://doi.org/10.1016/j.bbrep.2017.03.002
Singh AK, Talat M, Singh DP, Srivastava ON (2010) Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J Nanopart Res 12:1667–1675. https://doi.org/10.1007/s11051-009-9835-3
Singh DK, Pandey DK, Yadav RR, Singh D (2013) A study of ZnO nanoparticles and ZnO-EG nanofluid. J Exp Nanosci 8:567–577. https://doi.org/10.1080/17458080.2011.602369
Sintubin L, Verstraete W, Boon N (2012) Biologically produced nanosilver: current state and future perspectives. Biotechnol Bioeng 109:2422–2436. https://doi.org/10.1002/bit.24570
Sökmen M, Alomar SY, Albay C, Serdar G (2017) Microwave assisted production of silver nanoparticles using green tea extracts. J Alloys Compd 725:190–198. https://doi.org/10.1016/j.jallcom.2017.07.094
Suganya A, Murugan K, Kovendan K et al (2013) Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol Res 112:1385–1397. https://doi.org/10.1007/s00436-012-3269-z
Takacs L (2004) M. Carey Lea, the first mechanochemist. J Mater Sci 39:4987–4993. https://doi.org/10.1023/B:JMSC.0000039175.73904.93
Takacs L (2013) The historical development of mechanochemistry. Chem Soc Rev 42:7649–7659. https://doi.org/10.1039/c2cs35442j
Takacs L (2018) Two important periods in the history of mechanochemistry. J Mater Sci 53:13324–13330. https://doi.org/10.1007/s10853-018-2198-3
Tamboli DP, Lee DS (2013) Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against Gram positive and Gram negative bacteria. J Hazard Mater 260:878–884. https://doi.org/10.1016/j.jhazmat.2013.06.003
Tan YN, Lee JY, Wang DIC (2010) Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc 132:5677–5686. https://doi.org/10.1021/ja907454f
Tiimob BJ, Mwinyelle G, Abdela W et al (2017) Nanoengineered eggshell-silver tailored Copolyester polymer blend film with antimicrobial properties. J Agric Food Chem 65:1967–1976. https://doi.org/10.1021/acs.jafc.7b00133
Tsuzuki T, McCormick PG (2004) Mechanochemical synthesis of nanoparticles. J Mater Sci 39:5143–5146. https://doi.org/10.1023/B:JMSC.0000039199.56155.f9
Vaia RA, Liu W (2002) X-ray powder diffraction of polymer/layered silicate nanocomposites: model and practice. J Polym Sci Part B Polym Phys 40:1590–1600. https://doi.org/10.1002/polb.10214
Van Der Merwe PA (2010) Surface plasmon resonance. Physics (College Park Md) 627:1–50. https://doi.org/10.1007/978-1-60761-670-2
Van Viet P, Sang TT, Bich NHN, Thi CM (2018) An improved green synthesis method and Escherichia coli antibacterial activity of silver nanoparticles. J Photochem Photobiol B Biol 182:108–114. https://doi.org/10.1016/j.jphotobiol.2018.04.002
Velayutham K, Rahuman AA, Rajakumar G et al (2013) Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac J Trop Med 6:95–101. https://doi.org/10.1016/S1995-7645(13)60002-4
Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan PV (2006) A novel one-pot “green” synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res 341:2012–2018. https://doi.org/10.1016/j.carres.2006.04.042
Wang P, Tang J (2009) Solvent-free mechanochemical extraction of chondroitin sulfate from shark cartilage. Chem Eng Process Process Intensif 48:1187–1191. https://doi.org/10.1016/j.cep.2009.04.003
Wang J, White WB, Adair JH (2010) Optical properties of core-shell structured Ag/SiO2nanocomposites. Mater Sci Eng B 166:235–238. https://doi.org/10.1016/j.mseb.2009.11.026
Wang S, Zhang R, Song X et al (2019) Mechanochemical-assisted extraction of active alkaloids from plant with solid acids. ACS Sustain Chem Eng 7:197–207. https://doi.org/10.1021/acssuschemeng.8b02902
Wu K, Ju T, Deng Y, Xi J (2017) Mechanochemical assisted extraction: a novel, efficient, eco-friendly technology. Trends Food Sci Technol 66:166–175. https://doi.org/10.1016/j.tifs.2017.06.011
Xie J, Lin YS, Shi XJ et al (2013) Mechanochemical-assisted extraction of flavonoids from bamboo (Phyllostachys edulis) leaves. Ind Crop Prod 43:276–282. https://doi.org/10.1016/j.indcrop.2012.07.041
Xiu Z, Zhang Q, Puppala HL et al (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275. https://doi.org/10.1021/nl301934w
Yamanaka M, Hara K, Kudo J (2005) Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl Environ Microbiol 71:7589–7593. https://doi.org/10.1128/AEM.71.11.7589-7593.2005
Zayed MF, Eisa WH, Shabaka AA (2012) Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc 98:423–428. https://doi.org/10.1016/j.saa.2012.08.072
Zhang X-F, Liu Z-G, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1534. https://doi.org/10.3390/ijms17091534
Zhu X, Chen X, Xie J et al (2012) Mechanochemical-assisted extraction and antioxidant activity of polysaccharides from Ganoderma lucidum spores. Int J Food Sci Technol 47:927–932. https://doi.org/10.1111/j.1365-2621.2011.02923.x
Acknowledgments
The authors wish to acknowledge the Slovak Research and Development Agency (VEGA 2/0044/18) and Slovak Research and Development Agency (APVV-18-0357) for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Baláž, M., Bedlovičová, Z., Kováčová, M., Salayová, A., Balážová, Ľ. (2020). Green and Bio-Mechanochemical Approach to Silver Nanoparticles Synthesis, Characterization and Antibacterial Potential. In: Prasad, R., Siddhardha, B., Dyavaiah, M. (eds) Nanostructures for Antimicrobial and Antibiofilm Applications. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-40337-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-40337-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-40336-2
Online ISBN: 978-3-030-40337-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)