Abstract
Sulfate-bearing soils is widely distributed around the world, and this type of soil is prone to rock and soil disasters such as dissolution, corrosion of foundations, and swell when exposed to water. Cement is a frequently used stabilizer to treat sulfate-bearing soils. However, sulfate-bearing soils usually include various types of sulfates, such as, calcium sulfate (CaSO4), sodium sulfate (Na2SO4), potassium sulfate (K2SO4), and magnesium sulfate (MgSO4). So far, the effect of sulfate type on the strength and swelling properties of sulfate-bearing soil stabilized with cement has not been clarified. Therefore, in this study, the strength and swelling properties of four sulfate-bearing soils treated with cement were studied using unconfined compressive strength tests, and swelling tests. X-ray diffraction (XRD), scanning electron microscopy, and inductively coupled plasma spectroscopy were employed to study mineralogical, micro-structural properties, and concentrations of calcium ion of stabilized soils, to explore stabilization mechanisms. The results showed that the formation of magnesium silicate hydrate and highest concentration of free Ca2+ in the stabilized Mg-sulfate-soil caused its lowest strength. The reduction in free Ca2+ concentration was greater in the stabilized Na-sulfate-soil and K-sulfate-soil compared to stabilized Mg-sulfate-soil and Ca-sulfate-soil, contributing to the formation of more calcium silicate hydrate and ettringite. Therefore, the stabilized Na-sulfate-soil and K-sulfate-soil had greater swelling and strength compared to other soils. As the cement content increases, there are abundant in the sulfated cement stabilized soil observed in XRD and SEM photos. Overall, sulfates with monovalent cations increased the strength of cement-stabilized soils more than those with divalent cations, while sulfates with divalent cations improved the resistance to swelling of cement-stabilized soils. Before treating sulfate-bearing soils with cement, it is necessary to first determine the cations type in the soil. If the soil contains Mg2+, seek cement alternatives. If the other three cations are present, choose an appropriate cement content for stabilization. This study provides some references for the stabilization of sulfate-bearing soils with cement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Problematic sulfate-bearing soils are widespread globally (Shivanshi et al., 2022; Solis and Zhang 2008). Soil with insufficient geotechnical performance is classified as unacceptable and cannot be used in its natural state to support civil engineering structures (Wang et al. 2021). Previous studies demonstrated that cement was an effective stabilizer that was frequently used for the treatment of sulfate-bearing soils (Behnood 2018; Knopp and Moormann 2016; Puppala et al. 2004). Furthermore, other calcium-based stabilizers such as fly ash and lime were utilized to enhance the soil capacity to withstand the loads imposed by the constructed structures (Gadouri et al. 2019b; Hozatlıoğlu and Yılmaz 2021). Interactions between sulfate types and soil stabilization have been extensively explored in contemporary research. Zhu et al. (2022) examined soil water dynamics using stable isotopes, which provided essential insights into soil-water interactions critical for sulfate-bearing soils. He et al. (2024) introduced nanomaterials to enhance cement hydration, significantly improving strength and durability. Wei et al. (2023) focused on the seismic performance of composite columns, offering valuable data on ensuring resilience against dynamic loads. These studies collectively lay a foundation for understanding the multifaceted challenges posed by sulfate-bearing soils and the need for innovative solutions. Sun et al. (2023) studied sea sand coral concrete with FRP bars, addressing challenges in marine environments, which were particularly relevant to sulfate-bearing soils. Cui et al. (2024) and Huang et al. (2021) investigated fiber-reinforced concrete under impact loading, highlighting the mechanical properties necessary for stabilized soils to endure sudden stresses. These findings complemented the previous research by providing practical strategies for enhancing the durability of soil stabilization methods in harsh environmental conditions. Li et al. (2023b) explored the synergistic use of red mud, ultra-fine fly ash, and GGBS in cementless composites, presenting a sustainable alternative to traditional cement. Lu et al. 2017, 2019a, b) developed fractional elastoplastic constitutive models for soils and concrete, providing advanced tools for predicting geomaterial behavior. These advancements contributed to developing comprehensive approaches for stabilizing sulfate-bearing soils, considering both material sustainability and predictive modeling. Diao et al. (2024) enhanced the dynamic compressive performance of mortars modified with silicon carbide whiskers, showcasing the potential of advanced materials in improving the mechanical properties of stabilized soils. Su et al. (2023) developed a deep learning model for underground utilities localization using GPR, which was essential for assessing subsurface conditions in sulfate-bearing soils before and after stabilization. These innovative methodologies facilitate the improvement of monitoring and optimizing soil stabilization efforts in sulfate-rich environments. However, sulfate-bearing soils stabilized with cement or other calcium-based binders will swell when in contact with water due to the formation of an expansive mineral, ettringite (Cheshomi et al. 2017; Hunter 1988; Li et al. 2022; Little et al. 2009; Mitchell 1986; Puppala et al. 2004, 2005). Ettringite infiltrated between soil particles, thereby disrupting and deteriorating the soil structure, increasing volume changes (Puppala et al. 2012; Wang et al. 2021). The ettringite-induced swelling may threaten the stability of pavements and foundations (Higgins 2005; Puppala et al. 2018, 2019). Most of these studies focused on the stabilization of soils that consist of only one type of sulfate, but infield sulfate-bearing soils probably contain more than one as multiple sulfates were identified in these soils, such as sodium sulfate (Na2SO4), magnesium sulfate (MgSO4), gypsum (CaSO4), and potassium sulfate (K2SO4) (Wild et al. 1999). Therefore, it is necessary to explore the effect of sulfate types on the properties of cement-stabilized sulfate-bearing soils.
Table 1 summarizes previous studies on lime- and cement-stabilized soils suffering from sulphate attack. It can be found that the effect of sulfate type on the compaction, strength, and swelling characteristics of lime-stabilized soils has been investigated by Kinuthia et al. (1999) and Kinuthia and Wild (2001). In such studies, it was reported that sulfates with divalent cations (i.e. Ca2+ and Mg2+) were more beneficial for compaction characteristics of stabilized soils than those with monovalent cations (i.e. Na+ and K+). Additionally, the sulfate type had a significant effect on the performance of lime-stabilized soils, and the effects of the sulfates containing sodium, magnesium and potassium were more deleterious than those containing calcium (Kinuthia and Wild 2001). Aldaood et al. (2014) also pointed out that the presence of CaSO4 was beneficial for the resistance to swelling development of lime-stabilized soils. Gadouri et al. (2017a, b, 2018, 2019a, b) compared the effects of Na2SO4 and CaSO4 on the physical properties of lime-stabilized soil, and found that Na2SO4 increased the pH value of stabilized soil more than CaSO4. The addition of Na2SO4 to lime-stabilized kaolinite-soil caused significant swelling due to the abundant formation of ettringite, which resulted from the pH increased of soil (Tsatsos and Dermatas 1998). Shivanshi et al. (2022) reported that Na2SO4 concentrations influenced the strength of the lime-stabilized soils and the highest strength of stabilized soils occurred at 16,000 ppm of Na2SO4, after which the strength decreased with the increase of Na2SO4 concentration. Gadouri (2023) compared two types of clay with the same lime content and sulfate content, and found that Na-sulfate-soil had a higher swelling potential than Ca-sulfate-soil. Lime-stabilized soils were soaked in solutions containing sulfate concentrations of 3000 ppm and 30,000 ppm for 28 days, after which UCS tests were conducted. The results showed that, at the same sulfate concentrations, lime-stabilized soils soaked in Na2SO4 solutions had a greater adverse effect on the strength of the soil compared to those soaked in CaSO4 solutions (Shivanshi et al., 2023).
The difference in performance of lime-stabilized soils containing different sulfate types is likely related to the solubilities of sulfates (e.g. CaSO4, Na2SO4, MgSO4, and K2SO4) (Hunter 1988; Kota et al. 1996; Mitchell 1986). Due to differences in solubility and cation configuration each sulfate behaved in soil, the interaction between soils and sulfates could affect cation exchange and pozzolanic reaction (Shivanshi et al., 2022). It has been proved that SO42− was the main factor for ettringite formation. High-solubility sulfates (such as Na2SO4, MgSO4, K2SO4) were more likely to hydrolyze and produce SO42−, which reacted with Ca2+ in lime-stabilized soils to form ettringite, causing greater swelling damage of stabilized sulfate-bearing soil.
Consequently, the sulfate type probably has a significant impact on the swelling and strength properties of cement stabilized sulfate-bearing soils. Nevertheless, from the literature review, sulfate-bearing soils subjected to cement stabilization usually contained a single sulfate, preferably Na2SO4 or CaSO4, (as shown in Table 1). As well as this, these studies mainly focused on the ettringite-induced adverse effects of the cement-stabilized sulfate-bearing soils rather than the comparison in performance of such soils containing different sulfates. Hence, limited information about the effect of the sulfate type on the performance is available in the case of cement stabilized soils.
To understand the effect of the sulfate type on properties of cement stabilized soils, this study prepared four sulfate-bearing soils, containing CaSO4, Na2SO4, MgSO4, and K2SO4, respectively. The strength and swelling properties of four sulfate-bearing soils treated with cement were studied using unconfined compressive strength (UCS) tests and swelling tests. X-ray diffraction (XRD), scanning electron microscopy and inductively coupled plasma spectroscopy (ICP) were employed to study mineralogical, micro-structural properties, and concentrations of calcium ion of stabilized soils, to explore stabilization mechanisms.
Materials and methods
Materials
In this study, four sulfate-bearing soils were prepared using kaolin and sodium sulfate decahydrate (Na2SO4·10H2O), magnesium sulfate heptahydrate (MgSO4·7H2O), gypsum (CaSO4·2H2O), and potassium sulfate (K2SO4). These artificially prepared soils were named as Na-sulfate-soil, Mg-sulfate-soil, Ca-sulfate-soil and K-sulfate-soil, respectively. The liquid limit of kaolin was 53.8% and the plastic limit was 34.6%, both tested according to BSI (1990). Kaolin was purchased from Chuangke New Material Technology Co., Ltd, Guangzhou. Regarding all sulfate-bearing soils, the sulfate concentration was set as 20,000 ppm, which was a comparatively high sulfate level, as seen in Table 1.
Na2SO4·10H2O was obtained from Shanghai Yien Chemical Technology Co., Ltd. MgSO4·7H2O was supplied by Shanxi Nanfeng Group Chemical Co., Ltd. CaSO4·2H2O was produced by Wuhan Xianglong Building Materials Co., Ltd. K2SO4·2H2O was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. Moreover, ordinary Portland cement, CEMI42.5 R, came from Dengfeng Zhonglian Dengdian Cement Co., Ltd. The chemical composition of the soil, sulfates and cement used in this study were analyzed by X-ray fluorescence spectroscopy, and the results are shown in Table 2.
Mixing and sample compaction
As shown in Table 1, the content of binders commonly used to treat roadbeds ranged from 5 to 10%, so the cement content was determined to be 5% and 10% by the weight of the dry soils (Li et al. 2019b; Puppala et al. 2005). The four prepared sulfate-bearing soils were mixed with cement separately, and then the appropriate amount of water was added. Afterwards, the mixed materials were compacted into a mold with a size of Φ50 mm×100 mm. Each specimen was compacted in three layers, and each layer required 30 compactions. In this study, a compaction hammer with a weight of 0.51 kg was used for compaction, and the hammer was allowed to freely fall along a steel bar with a height of 270 mm. The unit compaction work was 606.73 kJ/m3, which was close to the standard Proctor test of 605.60 kJ/m3 (ASTM 2012). After compaction, the specimen was demolded, sealed with plastic film, and then cured at room temperature (20 °C) until the specified curing period. The specimens used for strength and swelling tests were all prepared at their respective optimum moisture content (OMC).
Experimental studies
Vertical Swelling tests
Similar to previous studies (Kinuthia and Wild 2001; Li et al. 2019b, 2020, 2023a), the swelling test used in this study was a three-dimensional swelling test, with only vertical swelling recorded. After the specimen was prepared, it was sealed and cured for 7 days. The swelling of the specimen during curing was recorded. After cured for 7 days, the specimen was subjected to the swelling test through being immersed in water. The specimen was placed in a flat-bottomed beaker. The specimen with a filter stone placed on top. A dial gauge was fixed, and its probe stood on the top surface of the filter stone to record the swelling deformation. Tap water was used for the swelling test and added to the beaker until the water level reached half the height of the filter stone. Readings were recorded at designed time. The recording intervals on the first day were 15, 30, 60, 75, 180, 360, 720 min, and then 1440 min (i.e. 24 h), until swelling stabilized for three consecutive days.
UCS
The specimens underwent UCS test after completing the swelling test. To emphasize the UCS variation due to swelling, the specimens were also tested for UCS without soaking. An electronic universal testing machine produced by Jinan Nair Testing Machine Co., Ltd was used to obtain the strength of soils. The maximum load of the device was 20 kN and the loading rate was 1 mm/min determined according to ASTM (2017). In the UCS test, two identical samples were used, peak stress values were obtained from the stress-strain curves, and the average value was calculated for the two test samples.
XRD
Soils samples were collected after the UCS test. They were vacuum dried using a YTLG-12 C freeze dryer produced by Shanghai Yetuo Technology Co., Ltd. The soils samples were weighed before drying, and during the drying process, the soil sample was weighed again until no further change in mass occurred, indicating that drying was completed. After dried, a portion of sample was ground, and then screened using a 0.075 mm sieve. The mineralogical property of the sieved samples was analyzed by XRD, which was an Empyrean X-ray diffractometer manufactured by PANalytical Instruments. The scanning angle in this test was 10–80° (2θ) and scanning rate was 5°/min.
SEM
A SU8010 high-resolution field emission scanning electron microscope manufactured by Hitachi, Japan was used for SEM testing. A small piece of dried soil sample was taken and crushed with a metal hammer. The flat soil sample was then adhered to the sample stage using conductive adhesive. After completion of the adhesion process, the soil sample surface was blown clean with an air dusting bulb to ensure tight and firm adhesion. Following the treatment, the samples on the sample stage were sprayed with gold for pre-treatment to reduce surface charging interference during scanning. The SEM images of samples were magnified 5000 and 10,000 times.
Concentration of free calcium ions
The concentration of free calcium ions could reflect the change of calcium ions occurring in the process of the soil stabilization, which would contribute to analyzing the formation of calcium-bearing minerals (i.e. ettringite and calcium silicate hydrate (CSH). This could facilitate the understanding of the stabilization mechanism for different sulfate-bearing soils. The leaching test was used to extract free calcium ions contained in stabilized soil. Part of vacuum-dried samples were prepared for the leaching test, which was conducted according to BSI (2002). 90 g of dried sample was placed in a 2-liter bottle. 900 ml of deionized water was then slowly added to the bottle. The bottle was secured on a flip oscillator and shaken at a speed of 10 rpm/min for 1440 min (i.e. 24 h). After the shaking was completed, the bottle was removed and the pH of the soil-water mixture was assessed by a pH meter. Then the supernatant was carefully filtered through a 0.45 micron filter to obtain a leachate. A 10 mL portion of the filtrate was transferred into a 100 mL volumetric flask using a pipette, and the volume was made up to capacity with concentrated nitric acid solution. Subsequently, dilutions were made to prepare filtrate standard solutions of different concentrations. Further analysis of the leachate was conducted using an ICP spectrometer produced by Leeman Labs Inc., USA, and the concentration of free calcium ions was determined based on the spectral characteristics of calcium elements.
Test results and discussion
Compaction characteristics
Figure 1 shows the compaction curves of four sulfate-bearing soils treated with different contents of cement (i.e. 5% and 10%). The OMC and maximum dry density (MDD) were summarized in Fig. 2. In Fig. 2a, the OMCs of sulfate-bearing soils treated with cement was significantly higher than those of untreated soils. This was because the cement addition triggered a hydration reaction that used up more water, increasing the OMCs of the four types of cement-treated sulfate-bearing soil. As the cement content increased from 0 to 5%, the OMC for each soil increased, and then tended to stabilize for cement content of 5–10%. Figure 2b shows MDDs of four sulfate-bearing soils treated with different cement contents. It could be observed that the MDDs of cement-treated soils were lower than that of untreated soil. They all had a trend of first decreasing, and then increasing with the increase of cement content, which was consistent with the results reported by Hossain et al. (2007) and Rahman (1986).
K-sulfate-soil stabilized with 5% cement had the highest OMC among other stabilized soils. The crystalline water in Na2SO4·10H2O, MgSO4·7H2O and CaSO4·2H2O were decomposed and released water during compaction. K2SO4 did not contain crystalline water, so more water had to be artificially provided, resulting in a higher OMC for K-sulfate-soil than other soils. The OMCs of stabilized Mg-sulfate-soil and Na-sulfate-soil were lower than that of stabilized Ca-sulfate-soil. The trends in MDDs and OMCs for 5% cement-stabilized soils were in agreement with the MDD and OMC results found in Kinuthia et al. (1999). The following order was observed for soils treated with 5% cement:
OMC: K-sulfate-soil (37%) > Ca-sulfate-soil (36%) > Mg-sulfate-soil (32%) > Na-sulfate-soil (28%).
MDD: Na-sulfate-soil > Mg-sulfate-soil > Ca-sulfate-soil > K-sulfate-soil.
When 10% cement was used, Na2SO4, CaSO4 and K2SO4 had the same effect on the OMCs and MDDs of stabilized soils as 5% cement-stabilized soils. However, the MDD of cement-stabilized Mg-sulfate-soil was lower than that of other stabilized soils. This may be due to that more magnesium silicate hydrate (MSH) and gypsum were formed in cement-stabilized Mg-sulfate-soil with increasing cement content (Hekal et al. 2002). The limited cementing properties of MSH caused a loose soils structure and a lower dry density of stabilized soils (Xing et al. 2009; Zhang et al. 2014). For soils treated with 10% cement, the following order could be obtained:
OMC: K-sulfate-soil (37%) > Ca-sulfate-soil (36%) > Mg-sulfate-soil (34%) > Na-sulfate-soil (29%).
MDD: Na-sulfate-soil > Ca-sulfate-soil > K-sulfate-soil > Mg-sulfate-soil.
Vertical swelling
Figure 3a shows the vertical swelling percentage of four sulfate-bearing soils treated with 5% cement. During curing, four sulfate-bearing soils showed shrinkage (shrinkage percentage of 0.003–0.136%). Ettringite formation causes a slight volume decrease at the initial stages (Mehta 1973, 1983). After these stabilized specimens were soaked in water, Ca-sulfate-soil swelled slowly and became stable within 7 days with a maximum swelling percentage of 0.44%. However, Na-sulfate-soil, Mg-sulfate-soil, and K-sulfate-soil swelled more quickly, and reached the stable swelling level in a longer period (> 10 days). For Na-sulfate-soil, the swelling percentage increased significantly to 4.90%, and stabilized after 12 days. K-sulfate-soil had a similar swelling-stabilized time of 12 days, but its maximum swelling percentage reached 6.71%. The largest maximum swelling percentage of 13.36% occurred for a Mg-sulfate-soil, which needed 45 days to achieve the status of stable swelling. The maximum swelling percentages for soils treated with 5% cement could be ranked as follows:
Mg-sulfate-soil (13.36%) > K-sulfate-soil (6.71%) > Na-sulfate-soil (4.90%) > Ca-sulfate-soil (0.44%).
The time required for achieving a stable swelling was as follows:
Mg-sulfate-soil (45 days) > K-sulfate-soil (12 days) > Na-sulfate-soil (12 days) > Ca-sulfate-soil (7 days).
Vertical swelling of four sulfate-bearing soils treated with 10% cement is provided in Fig. 3b. During curing, similar to those with 5% cement, each specimen of stabilized sulfate-bearing soils also shrank, with a percentage of 0.17–0.73%, and then began to swell after immersed in water. During soaking, soils with 10% cement had higher maximum swelling percentage except for Mg-sulfate-soil, relative to soils with 5% cement. Additionally, with the increase of cement content (5–10%), longer time was required to achieve the stable swelling for stabilized soils. The lowest maximum swelling percentage for four sulfate-bearing soils with 10% cement was 1.25%, occurring at Ca-sulfate-soil, and took about 14 days for reaching a stable state. Na-sulfate-soil and K-sulfate-soil had similar swelling-stabilized time and maximum swelling percentage. Additionally, about 45 days were needed for Mg-sulfate-soil to reach a stable swelling percentage, and the maximum swelling percentage was 5.39%. It can be obtained that the maximum swelling percentages for soils treated with 10% cement were in the order:
K-sulfate-soil (8.86%) > Na-sulfate-soil (8.57%) > Mg-sulfate-soil (5.39%) > Ca-sulfate-soil (1.25%).
The swelling-stabilized time of such soils was as follows:
Mg-sulfate-soil (60 days) > Na-sulfate-soil (40 days) > K-sulfate-soil (35 days) > Ca-sulfate-soil (15 days).
The above results illustrate that the sulfate type had a significant influence on the swelling. After cement stabilization, Ca-sulfate-soil was superior to other types of sulfate-bearing soils in resisting the soil swelling. The increase of the cement content improved the capability of stabilized Mg-sulfate-soil to resist the swelling. This could have been related to the competition between Mg2+ and Ca2+ in binding to hydrated silicates, with MSH being more likely to form than CSH (Dehwah 2007; Xing et al. 2009). Compared to the other three sulfate-bearing soils, when the soils treated with 5% cement, Mg-sulfate-soil produced relatively less CSH and more ettringite, resulting in higher swelling. As the cement content increased, the Mg2+ and SO42- in Mg-sulfate-soil were rapidly consumed. Therefore, a relatively larger amount of free Ca2+ participated in the reaction to generate CSH, enhancing the stabilized Mg-sulfate-soil to resist swelling. The increase in cement content reduced the capability of the other three sulfate-bearing soils to resist swelling. This could be attributed to that with the increase in cement content, free Ca2+ promoted the occurrence of CSH and consequently led to more ettringite formation. For instance, the Texas Department of Transportation in the USA requires that the swelling percentage of treated gypseous soil should be less than 1.2% (Harris et al. 2006). At both cement contents, stabilized Na-sulfate-soil and K-sulfate-soil always had very high swelling percentages.
UCS results
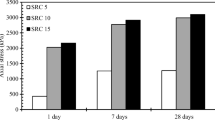
Figure 4 shows the UCS of four sulfate-bearing soils treated with 5% and 10% cement at different curing period and after soaking. The UCS of untreated four sulfate-bearing soils specimens was provided for comparison purpose. “Cured for 7 or 28 days” and “before soaking” represent that the stabilized specimens were cured for 7 or 28 days without soaking. “After soaking” means that the swelling test for the stabilized specimens was completed. Some specimens during the swelling test collapsed and could not be tested for UCS, so their UCS after soaking was recorded as 0. The UCS of untreated K-sulfate-soil, Na-sulfate-soil, Ca-sulfate-soil, and Mg-sulfate-soil were 0.27 MPa, 0.23 MPa, 0.18 MPa and 0.13 MPa, respectively, represented by red lines in Fig. 4. It could be found that the 7-day and 28-day UCS of cement stabilized soils was significantly higher than that of untreated soils, indicating that cement addition would improve the soil strength.
Figure 4a shows that the UCS results of soils stabilized with 5% cement. From 7 to 28 days curing, the UCS of Mg-sulfate-soil increased from 0.68 MPa to 1.16 MPa with the highest UCS increment of 70.59%. This was followed by Na-sulfate-soil (increased from 1.12 MPa to 1.73 MPa) and Ca-sulfate-soil (increased from 1.35 MPa to 1.85 MPa), with UCS increment of 54.46% and 37.04%, respectively. The lowest UCS increment was observed on K-sulfate-soil, only 25.98%, although its 7-day UCS (1.27 MPa) and 28-day UCS (1.6 MPa) were not the lowest compared to other types of soils. The UCS of the soils treated with 5% cement had the following ranking:
Cued for 7 days: Ca-sulfate-soil (1.35 MPa) > K-sulfate-soil (1.27 MPa) > Na-sulfate-soil (1.12 MPa) > Mg-sulfate-soil (0.68 MPa).
Cued for 28 days: Ca-sulfate-soil (1.85 MPa) > Na-sulfate-soil (1.73 MPa) > K-sulfate-soil (1.6 MPa) > Mg-sulfate-soil (1.16 MPa).
Figure 4(b) shows that when the cement content was increased to 10%, at curing periods of 7 days and 28 days, Na-sulfate-soil has the largest UCS (2.41 MPa and 3.81 MPa), respectively. And it had the largest increment in UCS according to curing period (59.34%). Although the UCS of Mg-sulfate-soil results were lower than those of other stabilized soils, it had a second highest increment in UCS according to curing period. Ca-sulfate-soil and K-sulfate-soil still maintained their lower increment in UCS when treated with 10% cement (increment were 41.99% and 34.42%, respectively). The following ranking was observed for the UCS of the soils treated with 10% cement:
Cued for 7 days: Na-sulfate-soil (2.41 MPa) > K-sulfate-soil (2.15 MPa) > Ca-sulfate-soil (1.81 MPa) > Mg-sulfate-soil (0.85 MPa).
Cued for 28 days: Na-sulfate-soil (3.84 MPa) > K-sulfate-soil (2.89 MPa) > Ca-sulfate-soil (2.57 MPa) > Mg-sulfate-soil (1.31 MPa).
The UCS results indicate that the sulfate type significantly affected the UCS of cement-stabilized soils. The UCS of cement-stabilized Mg-sulfate-soil was the lowest among all soils. The development of strength is severely hindered by MgSO4 (Kinuthia and Wild 2001). Except for Mg-sulfate-soil, the UCS of stabilized soils with 5% cement did not vary significantly. With 10% cement, Na-sulfate-soil had the highest UCS and surpassed other stabilized soils. At low concentrations (less than 2%), Na2SO4 served as a catalyst for the volcanic ash reaction, thereby enhancing the soils of strength without altering the stabilization process (Gadouri et al. 2019b). When cement content was 5%, Ca-sulfate-soil had a largest UCS of 1.22 MPa after soaking, but with cement content increased to 10%, the UCS of Ca-sulfate-soil after soaking was only 0.8 MPa, decreased by 34.43%. When Na-sulfate-soil was treated with 5% cement, the UCS after soaking was 0.05 MPa, and then slightly increased to 0.07 MPa with the cement content increasing to 10%. For K-sulfate-soil, its UCS after soaking appeared to be unaffected by the cement content. Mg-sulfate-soil lost bearing capacity after the swelling test when treated with 5% cement, so its UCS after soaking recorded as 0, but this UCS increased to 0.22 MPa after being treated with 10% cement. The UCS after soaking of cement-stabilized soils was ranked as follows:
5% cement: Ca-sulfate-soil (1.22 MPa) > Na-sulfate-soil (0.05 MPa) > K-sulfate-soil (0.04 MPa) > Mg-sulfate-soil (0 MPa).
10% cement: Ca-sulfate-soil (0.8 MPa) > Mg-sulfate-soil (0.22 MPa) > Na-sulfate-soil (0.07 MPa) > K-sulfate-soil (0.05 MPa).
XRD and SEM
Figure 5a and b shows the XRD result of soils stabilized with 5% cement before and after soaking, respectively. Kaolinite, quartz, and muscovite found in the soils came from kaolin. CSH, detected at around 30°, was the main source of strength improvement in stabilized soils. The main product of cement hydration, Ca(OH)2, was not detected in any stabilized soils and should be consumed in the formation of ettringite in cement-stabilized soils (Li et al. 2019b). For Na-sulfate-soil, gypsum was detected at around 32° without soaking but not detected after soaking. This was due to the formation of gypsum in the early reaction of Na2SO4 with Ca(OH)2, which could further lead to the formation of ettringite in the presence of aluminates (Massazza 1993). Regarding Mg-sulfate-soil, gypsum was detected at around 12° before and after soaking. This was consistent with Massazza (1993), who reported that MgSO4 combined with cement hydrates to form gypsum, which at a later stage contributed to the formation of ettringite. It was found that MgSO4 in Mg-sulfate-soil caused CSH to decalcify and form MSH (Hekal et al. 2002), and MSH had a peak around 22.5°. Additionally, no gypsum peak was observed in Ca-sulfate-soil and K-sulfate-soil.
Figure 5c and d show the XRD patterns of sulfate-bearing soils stabilized with 10% cement before and after soaking. Most stabilized soils showed insignificant change in CSH with increasing cement content from 5 to 10%. Gypsum was detected in the XRD pattern of Ca-sulfate-soil after soaking, the CSH peak was significantly reduced. This might explain why the UCS of 10% cement-stabilized Ca-sulfate-soil after soaking was lower than that of 5% cement. A new peak of ettringite around 8° was detected in the XRD pattern of Na-sulfate-soil, compared with 5% cement, so the increased ettringite formation caused more swelling in Na-sulfate-soil with 10% cement than the soils with 5% cement. For K-sulfate-soil after soaking, a peak at 8° signified the presence of ettringite. After soaking, the peaks of gypsum and MSH disappeared in 10% cement-stabilized Mg-sulfate-soil, which might account for its greater UCS and less swelling than those of 5% cement-stabilized Mg-sulfate-soil.
The samples used for XRD tests were used for SEM tests. The detection of ettringite was the focus of this SEM testing. Figure 6 shows the SEM photos of 5% cement-stabilized soils. Unfortunately, only a small amount of ettringite was found in the SEM photos of stabilized Na-sulfate-soil and Ca-sulfate-soil after soaking, as shown in Fig. 6b and f. However, in Fig. 7, each sample of 10% cement-stabilized soils contained a large amount of needle-like ettringite. The formation of ettringite in stabilized soils increased significantly with cement content, consistent with the XRD results.
SEM photos of soils stabilized with 5% cement: a Na-sulfate-soil, before soaking, b Na-sulfate-soil, after soaking, c Mg-sulfate-soil, before soaking, d Mg-sulfate-soil, after soaking, e Ca-sulfate-soil, before soaking, f Ca-sulfate-soil, after soaking, g K-sulfate-soil, before soaking, h K-sulfate-soil, after soaking
SEM photos of soils stabilized with 5% cement: a Na-sulfate-soil, before soaking, b Na-sulfate-soil, after soaking, c Mg-sulfate-soil, before soaking, d Mg-sulfate-soil, after soaking, e Ca-sulfate-soil, before soaking, f Ca-sulfate-soil, after soaking, g K-sulfate-soil, before soaking, h K-sulfate-soil, after soaking
Leachate pH and concentration of free calcium ions
Figure 8 shows pH of soils treated with 5% and 10% cement. The filtrate sample at 0-day curing was obtained by immediately mixing sulfate-bearing soils, cement, and water. For soils stabilized with 5% cement, the pH ranged from 10.73 to 11.51 at both 0 and 7 days of curing. After soaking, the pH of the stabilized soils (except stabilized Mg-sulfate-soil) decreased to some extent, but remained above 10.76, promoting the formation of ettringite and CSH. This was because ettringite was a stable mineral above pH value of 10.7 while CSH was more easily produced above pH value of 9.5 (Myneni et al. 1998b; Song et al. 2000). The pH of 5% cement stabilized Mg-sulfate-soil decreased significantly to 9.94 after soaking. For 10% cement-stabilized soils, the pH was between 11.58 and 12.08 after cured for 0 or 7 days. Soaking lowered the pH of the soils to a range of 9.69 to 11.64, in contrast to its value after 7 days of curing. The pH of the soils stabilized with 10% cement was higher than that with 5% cement, for both 0-day and 7-day curing without soaking, indicating that the increase of cement content increased the pH of soils. This was consistent with the findings of Al-Mukhtar et al. (2010). However, there was a risk of dissolution of ettringite only at neutral or lower pH values (Myneni et al. 1998a). As the pH increased of soils, the migration water rich in released sulfate and Ca2+ facilitated the recrystallization of ettringite (Kowalska et al. 2023). This explains the reason for the increased swelling of the other three types of stabilized sulfate-bearing soils (except stabilized Mg-sulfate-soil) as the cement content increases.
Figure 9 shows the concentration of free Ca2+ in the filtrate of stabilized soils with 5% and 10% cement at different curing (or soaking) period. In Fig. 9a, it can be seen that as the curing period increased from 0 to 7 days, the concentration of free Ca2+ in all stabilized soils with 5% cement decreased significantly, indicating that free Ca2+ was consumed in cement hydration reaction. Free Ca2+ in the soils formed water-insoluble CSH and ettringite during cement hydration, lowering its concentration. After immersed in water, Mg-sulfate-soil and Ca-sulfate-soil had a decreasing concentration of free Ca2+ due to continuous cement hydration reaction. However, for Na-sulfate-soil and K-sulfate-soil, the concentration of free Ca2+ after soaking slightly increased compared to that for 7-day curing. It was deduced that the pH of the stabilized Na-sulfate-soil and K-sulfate-soil had dropped to about 10.7 after soaking, leading to a slight instability of ettringite, which released free Ca2+ (Hobbs and Taylor 2000). Overall, the concentration of free Ca2+ in each 5% cement-stabilized soils decreased from 0-day curing to after soaking, but the degree of decrease varied. Specifically, the degrees by which the concentrations of free Ca2+ decreased were in the following order:
Na-sulfate-soil (71.18%) > K-sulfate-soil (61.83%) > Ca-sulfate-soil (53.51%) > Mg-sulfate-soil (32.17%).
The concentration of free Ca2+ decreased to a higher degree in Na-sulfate-soil and K-sulfate-soil with 5% cement compared to the other two soils. The monovalent cations reacted with cement hydration products to produce NaOH and KOH, which increased the soils alkalinity more easily and enhanced the cement hydration reaction (Gadouri et al. 2019b; Shivanshi et al., 2022; Sridharan et al. 1995). Regarding the soils containing divalent cations, due to the introduction of Mg2+, CSH would be decalcified to form gypsum and MSH was formed in Mg-sulfate-soil stabilized with cement (Li et al. 2023a; Massazza 1993), resulting in the release of free Ca2+. However, most of free Ca2+ in Ca-sulfate-soil participated in the formation of insoluble CSH and calcium aluminate hydrate (CAH) (Jha and Sivapullaiah 2018). Therefore, the concentration of free Ca2+ decreased much more in Ca-sulfate-soil than in Mg-sulfate-soil.
Higher content of cement (i.e. 10%) increased the concentrations of free Ca2+ in the soils at the beginning of curing (i.e. 0 day curing), as shown in Fig. 9b. Like 5% cement-stabilized soil, the concentration of free Ca2+ in 10% cement-stabilized Ca-sulfate-soil and Mg-sulfate-soil decreased continuously from 0-day curing to after soaking. For stabilized K-sulfate-soil and Na-sulfate-soil, the concentration of free Ca2+ showed a decrease from 0 to 7 days curing, and then a slight increase. From 0-day curing to after soaking, 10% cement-stabilized K-sulfate-soil had the highest decrease of free Ca2+ concentration. The stabilized Na-sulfate-soil had a lower decrease in free Ca2+ concentration than the K-sulfate-soil. This might be due to that the pH value of stabilized Na-sulfate-soil after soaking was 9.69, which was not high enough to make ettringite stable. Additionally, the reduction in free Ca2+ concentration was higher in stabilized Mg-sulfate-soil compared to Ca-sulfate-soil. The degrees by which the concentrations of free Ca2+ decreased from 0-day curing to after soaking was ranked as follows:
K-sulfate-soil (89.60%) > Na-sulfate-soil (85.77%) > Mg-sulfate-soil (72.29%) > Ca-sulfate-soil (68.38%).
For both 5% and 10% cement addition, sulfate-bearing soils with divalent cations consistently had higher free Ca2+ concentration than those with monovalent cations from 0-day to after soaking. As per soils with divalent cations, the free Ca2+ concentration of stabilized Mg-sulfate-soil was always higher than that of stabilized Ca-sulfate-soil. This is because Mg2+ in stabilized Mg-sulfate-soil reacts preferentially with cement hydration products to form MSH (Hekal et al. 2002; Li et al. 2023a). After Mg2+ depletion, Ca2+ would bind silicate hydrate to forms CSH and ettringite. Regardless of cement content, for soils containing monovalent cations, the concentration of free Ca2+ was quite closed between K-sulfate-soil and Na-sulfate-soil at each curing (and soaking) stage.
Discussion of mechanism
Calcium-based stabilizers such as cement or those having pozzolanic properties involve five processes to improve the properties of clay soils(Al-Mukhtar et al. 2012; Lin et al. 2007; Prusinski and Bhattacharja 1999; Sol-Sánchez et al. 2016): (a) hydration, (b) cation exchange, (c) flocculation and agglomeration, (d) pozzolonaic reaction, and (e) potential carbonation. Cation exchange process due to the mixing of stabilizers with soil causes flocculation and agglomeration of soil particles, which in turn produces a soil with coarser particle size distribution, higher permeability and lower plasticity. Cement, due to its rapid hydration reaction, provides better strength characteristics and requires a shorter curing time compared to lime and other soil stabilizers.
The testing results show that the sulfate type affected the properties of cement-stabilized soils. When cement reacts with water, the reaction yields CAH, CSH and other hydration products (Eq. (1)) (Xing et al. 2009). The development of soil strength was often attributed to the hydration of cement, which formed hydrates such as CSH and CAH. These hydrates crystallized over time and interlocked with the soil system (Behnood 2018).
For Ca-sulfate-soil, when CaSO4 was introduced into soil-cement system, it would dissolve into Ca2+ and SO42- in water (Eq. (2)).
Ca2+ ions sourced from CaSO4 would participate in the reaction (indicated by Eq. (1) to form CSH and CAH, while SO42- promoted the formation of ettringite (as shown in Eq. (3). Previous studies had shown that the formation of ettringite could occur under two different conditions. Naturally occurring ettringite was typically formed by the reaction of sulfate and alumina in the presence of Ca2+ at pH > 10 (Chen et al. 2020; Sun et al. 2020; Zhang et al. 2021). The formation of ettringite and CSH can be confirmed by the XRD and SEM results of the Ca-sulfate-soil (Figs. 5, 6 and 7). CSH, CAH, and ettringite, are all insoluble in water (Li et al. 2019a; Rajasekaran 2005), leading to a considerable reduction on the concentration of free Ca2+ (Fig. 9). Although ettringite formation could cause swelling of soils, the occurrence of cementitious compounds, such as CSH and CAH, might limit the ettringite-induced swelling and improve soil strength to some extent (Aldaood et al. 2014; Jha and Sivapullaiah 2018; Yilmaz and Civelekoglu 2009b). Hence, the cement-treated Ca-sulfate-soil swelled less than any other soil.
When MgSO4 was added into the soil-cement system, MgSO4 caused the decalcification of CSH, producing gypsum and MSH, which resulted in the formation of more ettringite at a later stage (Hekal et al. 2002; Massazza 1993). SO42- contained four oxygen atoms with strong electronegativity. The distance between Ca2+ and the silicate chain structure was relatively large. When SO42- and Mg2+ simultaneously corroded CSH, Ca2+ was decalcified due to the attraction of SO42−(Cao et al. 2023). The reaction could be simplified as Eq. (4). MSH and gypsum formation in Mg-sulfate-soil was confirmed by XRD patterns (Fig. 5). This is why cement-stabilized Mg-sulfate-soil had the lower decrease of free Ca2+ concentration. Compared to CSH, MSH had a significantly lower binding capacity, which caused greater swelling and less strength compared to other soils (Figs. 3 and 4).
As Hekal et al. (2002) and Yi et al. (2014) stated, MSH formation was the primary cause of strength reduction.
With the introduction of Na2SO4 and K2SO4 into the soil-cement system, they would react with the cement hydration products as follows:
As shown in Eqs. (5) and (6), monovalent ions were more likely to increase soil alkalinity in the process of cement stabilization (Gadouri et al. 2018, 2019b; Shivanshi et al., 2022; Wu et al. 1990). XRD patterns also verified the formation of gypsum in Eqs. (5) and (6) (Fig. 5). As shown in Fig. 9, the concentration of free Ca2+ was much lower in sulfate-bearing soils containing monovalent cations than in soils containing divalent cations. With the increase of soil alkalinity, more Al2O3 and SiO2 dissolved in stabilized soil (Mitchell 1986; Sridharan et al. 1995). This process promoted the reaction of free Ca2+ with Al2O3 and SiO2 to form CSH and CAH in stabilized Na-sulfate-soil and K-sulfate-soil (Eq. (1) (Wild et al. 1993), resulting in higher strength of Na-sulfate-soil and K-sulfate-soil (Fig. 4).
Summary and concluding remarks
-
The OMC order of the four sulfate-bearing soils stabilized with 5% cement was K-sulfate-soil > Ca-sulfate-soil > Mg-sulfate-soil > Na-sulfate-soil. The MDD order of these soils was opposed to the OMCs order. For soils treated with 10% cement, the order of their OMCs remained unchanged as mentioned above. The MDD of 10% cement-stabilized Mg-sulfate-soil was lower than that of other soils.
-
The maximum swelling of 5% cement stabilized Mg-sulfate-soil was the highest, while the maximum swelling of Ca-sulfate-soil was the lowest. Their UCS trends were opposite at 7 and 28 days. The swelling of 10% cement stabilized monovalent cationic sulfate-bearing soil was higher than that of divalent cationic sulfate-bearing soil, and the unconfined compressive strength of monovalent cationic sulfate-bearing soil at 7 and 28 days was higher than that of divalent cationic sulfate-bearing soil.
-
Nevertheless, the UCS after soaking of Ca-sulfate-soil was the highest among all stabilized soils. The XRD and SEM results revealed the presence of ettringite and CSH in all cement-stabilized soils. The formation of MSH and highest concentration of free Ca2+ in stabilized Mg-sulfate-soil was the main cause of its lowest strength and greater swelling.
-
From 0-day curing to after soaking, both types of divalent cation sulfate soils in cement-stabilized sulfate-bearing soils with two different dosages maintained a relatively high level of consumption of Ca2+. Sulfates with monovalent cations demonstrated a higher ability to facilitate the consumption of free Ca2+, leading to an increased production of ettringite and CSH compared to sulfates with divalent cations. Therefore, stabilized Na-sulfate-soil and K-sulfate-soil stabilization resulted in higher strength and swelling.
In summary, sulfates with monovalent cations had more positive effects on the strength of cement-stabilized soils, while sulfates with divalent cations had better capacity to resist the swelling of cement-stabilized soils.
Data availability
Data generated or analyzed during this study are provided in full within the published article.
References
Abdi M, Wild S (1993) Sulphate expansion of lime-stabilized kaolinite: I. Physical characteristics. Clay Miner 28:555–567. https://doi.org/10.1180/claymin.1993.028.4.06
Adeleke B, Kinuthia J, Oti J (2020) Strength and swell performance of high-sulphate kaolinite clay soil. Sustainability. https://doi.org/10.3390/su122310164
Al-Mukhtar M, Lasledj A, Alcover J-F (2010) Behaviour and mineralogy changes in lime-treated expansive soil at 20 C. Appl Clay Sci 50:191–198. https://doi.org/10.1016/j.clay.2010.07.023
Al-Mukhtar M, Khattab S, Alcover J-F (2012) Microstructure and geotechnical properties of lime-treated expansive clayey soil. Eng Geol 139:17–27. https://doi.org/10.1016/j.enggeo.2012.04.004
Aldaood A, Bouasker M, Al-Mukhtar M (2014) Free swell potential of lime-treated gypseous soil. Appl Clay Sci 102:93–103. https://doi.org/10.1016/j.clay.2014.10.015
ASTM (2012). Standard test methods for laboratory compaction characteristics of soil using standard effort (12 400 ft-lbf/ft3 (600 kN-m/m3)), D698-12e2. ASTM, West Conshohocken
ASTM (2017) Standard method for compressive strength of molded soil-cement cylinders. D1633. ASTM, West Conshohocken
Behnood A (2018) Soil and clay stabilization with calcium-and non-calcium-based additives: a state-of-the-art review of challenges, approaches and techniques. Transp Geotechnics 17:14–32. https://doi.org/10.1016/j.trgeo.2018.08.002
BSI (1990) Methods of test for soils for civil engineering purposes. Part 2: classification tests. BSI 1377-2. BSI, London
BSI (2002) Characterisation of waste-Leaching-Compliance test for leaching of granular waste materials and sludges Part 1: One stage batch test at a liquid to solid ratio of 2L/kg for materials with high solid content and with particle size below 4 mm (without or with size reduction). BSI EN 12457-2. BSI, London
Cao K, Wu D, Chen K, Mao N (2023) Erosion experiments and molecular dynamics simulations of hydrated calcium silicate under the action of sulfate. J Non-cryst Solids 613:122362. https://doi.org/10.1016/j.jnoncrysol.2023.122362
Caselles LD, Hot J, Roosz C, Cyr M (2020) Stabilization of soils containing sulfates by using alternative hydraulic binders. Appl Geochem 113:104494. https://doi.org/10.1016/j.apgeochem.2019.104494
Celik E, Nalbantoglu Z (2013) Effects of ground granulated blastfurnace slag (GGBS) on the swelling properties of lime-stabilized sulfate-bearing soils. Eng Geol 163:20–25. https://doi.org/10.1016/j.enggeo.2013.05.016
Chen W, Huang B, Yuan Y, Deng M (2020) Deterioration process of concrete exposed to internal sulfate attack. Materials 13:1336. https://doi.org/10.3390/ma13061336
Cheshomi A, Eshaghi A, Hassanpour J (2017) Effect of lime and fly ash on swelling percentage and Atterberg limits of sulfate-bearing clay. Appl Clay Sci 135:190–198. https://doi.org/10.1016/j.clay.2016.09.019
Cui D, Wang L, Zhang C, Xue H, Gao D, Chen F (2024) Dynamic splitting performance and energy dissipation of fiber-reinforced concrete under impact loading. Materials 17:421. https://doi.org/10.3390/ma17020421
Dehwah H (2007) Effect of sulfate concentration and associated cation type on concrete deterioration and morphological changes in cement hydrates. Constr Build Mater 21:29–39. https://doi.org/10.1016/j.conbuildmat.2005.07.010
Diao X, Song Y, Pan S, Cai X, Shi T, Wang L (2024) Enhanced dynamic compressive performance of silicon carbide whiskers-modified mortars. Constr Build Mater 432:136626. https://doi.org/10.1016/j.conbuildmat.2024.136626
Gadouri H (2023) Behavior of natural Pozzolana-lime-stabilized clayey soils artificially contaminated by sulfates. Jordan J Civil Eng. https://doi.org/10.14525/JJCE.v17i4.07
Gadouri H, Harichane K, Ghrici M (2017a) Effects of Na2SO4 on the geotechnical properties of clayey soils stabilised with mineral additives. Int J Geotech Eng 11:500–512. https://doi.org/10.1080/19386362.2016.1238562
Gadouri H, Harichane K, Ghrici M (2017b) Effect of calcium sulphate on the geotechnical properties of stabilized clayey soils. Periodica Polytech Civil Eng 61:256–271. https://doi.org/10.3311/PPci.9359
Gadouri H, Harichane K, Ghrici M (2018) Assessment of sulphates effect on the classification of soil–lime–natural pozzolana mixtures based on the unified soil classification system (USCS). Int J Geotech Eng 12:293–301. https://doi.org/10.1080/19386362.2016.1275429
Gadouri H, Harichane K, Ghrici M (2019a) Effect of sulphates and curing period on stress–strain curves and failure modes of soil–lime–natural pozzolana mixtures. Mar Georesources Geotechnol 37:1130–1148. https://doi.org/10.1080/1064119X.2018.1537321
Gadouri H, Harichane K, Ghrici M (2019b) A comparison study between CaSO4· 2H2O and Na2SO4 effects on geotechnical properties of clayey soils stabilised with mineral additives to recommend adequate mixtures as materials for road pavements. Int J Geotech Eng 13:61–82. https://doi.org/10.1080/19386362.2017.1320850
Harris P, von Holdt J, Sebesta S, Scullion T (2006) Recommendations for stabilization of high-sulfate soils in Texas. Transp Res Rec 1952:71–79. https://doi.org/10.1177/0361198106195200108
He H, Shuang E, Qiao H, Yang J, Lin C, He C, Xu P (2024) A general and simple method to disperse 2D nanomaterials for promoting cement hydration. Constr Build Mater 427:136217. https://doi.org/10.1016/j.conbuildmat.2024.136217
Hekal EE, Kishar E, Mostafa H (2002) Magnesium sulfate attack on hardened blended cement pastes under different circumstances. Cem Concrete Res 32:1421–1427. https://doi.org/10.1016/S0008-8846(02)00801-3
Higgins D (2005) Soil stabilisation with ground granulated blastfurnace slag. UK cementitious slag makers association (CSMA). Metall Mater Transact 1:15. https://doi.org/10.1007/BF02915610
Hobbs D, Taylor M (2000) Nature of the thaumasite sulfate attack mechanism in field concrete. Cem Concr Res 30:529–533. https://doi.org/10.1016/S0008-8846(99)00255-0
Hossain K, Lachemi M, Easa S (2007) Stabilized soils for construction applications incorporating natural resources of Papua New Guinea. Resour Conserv Recycl 51:711–731. https://doi.org/10.1016/j.resconrec.2006.12.003
Hozatlıoğlu DT, Yılmaz I (2021) Shallow mixing and column performances of lime, fly ash and gypsum on the stabilization of swelling soils. Eng Geol 280:105931. https://doi.org/10.1016/j.enggeo.2020.105931
Huang H, Yuan Y, Zhang W, Zhu L (2021) Property assessment of high-performance concrete containing three types of fibers. Int J Concrete Struct Mater 15:1–17. https://doi.org/10.1186/s40069-021-00476-7
Hunter D (1988) Lime-induced heave in sulfate-bearing clay soils. J Geotech Eng 114:150–167. https://doi.org/10.1061/(ASCE)0733-9410
Jha AK, Sivapullaiah P (2015) Susceptibility of strength development by lime in gypsiferous soil—a micro mechanistic study. Appl Clay Sci 115:39–50. https://doi.org/10.1016/j.clay.2015.07.017
Jha AK, Sivapullaiah P (2018) Potential of fly ash to suppress the susceptible behavior of lime-treated gypseous soil. Soils Found -Tokyo- 58:654–665. https://doi.org/10.1016/j.sandf.2018.02.024
Kalipcilar I, Mardani-Aghabaglou A, Sezer A, Altun S, Inan Sezer G (2016) Sustainability of cement-stabilised clay: sulfate resistance. Proceed Institut Civil Eng Eng Sustain 171:254–274. https://doi.org/10.1680/jensu.16.00005
Kinuthia J, Wild S (2001) Effects of some metal sulfates on the strength and swelling properties of lime-stabilised kaolinite. Int J Pavement Eng 2:103–120. https://doi.org/10.1080/10298430108901720
Kinuthia J, Wild S, Jones G (1999) Effects of monovalent and divalent metal sulphates on consistency and compaction of lime-stabilised kaolinite. Appl Clay Sci 14:27–45. https://doi.org/10.1016/S0169-1317(98)00046-5
Knopp J, Moormann C (2016) Ettringite swelling in the treatment of sulfate-containing soils used as subgrade for road constructions. Procedia Eng 143:128–137. https://doi.org/10.1016/j.proeng.2016.06.017
Kota PB, Hazlett D, Perrin L (1996) Sulfate-bearing soils: problems with calcium-based stabilizers. Transp Res Rec 1546:62–69. https://doi.org/10.1177/0361198196154600107
Kowalska M, Grzesik B, Adamczyk Z, Nowak J, Konsek A (2023) Swelling of sulfate-bearing soil: a case study of A1 highway pavement failure. Case Stud Constr Mater 18:e02081. https://doi.org/10.1016/j.cscm.2023.e02081
Li J, Yu Q, Huang H, Yin S (2019a) Difference in the reaction process of slag activated by waterglass solution and NaOH solution. Struct Concrete 20:1528–1540. https://doi.org/10.1002/suco.201900130
Li W, Yi Y, Puppala AJ (2019b) Utilization of carbide slag-activated ground granulated blastfurnace slag to treat gypseous soil. Soils Found 59:1496–1507. https://doi.org/10.1016/j.sandf.2019.06.002
Li W, Yi Y, Puppala AJ (2020) Suppressing ettringite-induced swelling of gypseous soil by using magnesia-activated ground granulated blast-furnace slag. J Geotech GeoEnviron Eng 146:06020008. https://doi.org/10.1061/(ASCE)GT.1943-5606.0002292
Li W, Yi Y, Puppala AJ (2022) Effects of curing environment and period on performance of lime-GGBS-treated gypseous soil. Transp Geotech 37:100848. https://doi.org/10.1016/j.trgeo.2022.100848
Li W, Li R, Chen Y, Xiao H (2023a) Comparison of two sulfate-bearing soils stabilized with reactive magnesia-activated ground granulated blast furnace slag: swelling, strength, and mechanism. Buildings 13:230. https://doi.org/10.3390/buildings13010230
Li Z, Gao M, Lei Z, Tong L, Sun J, Wang Y, Wang X, Jiang X (2023b) Ternary cementless composite based on red mud, ultra-fine fly ash, and GGBS: synergistic utilization and geopolymerization mechanism. Case Stud Constr Mater 19:e02410. https://doi.org/10.1016/j.cscm.2023.e02410
Lin D-F, Lin K-L, Hung M-J, Luo H-L (2007) Sludge ash/hydrated lime on the geotechnical properties of soft soil. J Hazard Mater 145:58–64. https://doi.org/10.1016/j.jhazmat.2006.10.087
Little DN, Nair S, Herbert B (2009) Addressing sulfate-induced heave in lime treated soils. J Geotech GeoEnviron Eng 136:110–118. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000185
Lu D, Ma C, Du X, Jin L, Gong Q (2017) Development of a new nonlinear unified strength theory for geomaterials based on the characteristic stress concept. Int J Geomech 17:04016058. https://doi.org/10.1061/(ASCE)GM.1943-5622.0000729
Lu D, Liang J, Du X, Ma C, Gao Z (2019a) Fractional elastoplastic constitutive model for soils based on a novel 3D fractional plastic flow rule. Comput Geotech 105:277–290. https://doi.org/10.1016/j.compgeo.2018.10.004
Lu D, Zhou X, Du X, Wang G (2019b) A 3D fractional elastoplastic constitutive model for concrete material. Int J Solids Struct 165:160–175. https://doi.org/10.1016/j.ijsolstr.2019.02.004
Massazza F (1993) Pozzolanic cements. Cem Concr Compos 15:185–214. https://doi.org/10.1016/0958-9465(93)90023-3
Mehta PK (1973) Mechanism of expansion associated with ettringite formation. Cem Concr Res 3:1–6. https://doi.org/10.1016/0008-8846(73)90056-2
Mehta PK (1983) Mechanism of sulfate attack on Portland cement concrete—another look. Cem Concr Res 13:401–406. https://doi.org/10.1016/0008-8846(83)90040-6
Mitchell JK (1986) Practical problems from surprising soil behaviour. J Geotech GeoEnviron Eng 112:259–289. https://doi.org/10.1061/(ASCE)0733-9410
Myneni SC, Traina SJ, Logan TJ (1998a) Ettringite solubility and geochemistry of the ca (OH) 2–Al2 (SO4) 3–H2O system at 1 atm pressure and 298 K. Chem Geol 148:1–19. https://doi.org/10.1016/S0009-2541(97)00128-9
Myneni SC, Traina SJ, Logan TJ (1998b) Ettringite solubility and geochemistry of the ca(OH)2–Al2(SO4) 3–H2O system at 1 atm pressure and 298 K. Chem Geol 148:1–19. https://doi.org/10.1016/S0009-2541(97)00128-9
Prusinski JR, Bhattacharja S (1999) Effectiveness of Portland cement and lime in stabilizing clay soils. Transp Res Rec 1652:215–227. https://doi.org/10.3141/1652-28
Puppala AJ, Griffin JA, Hoyos LR, Chomtid S (2004) Studies on sulfate-resistant cement stabilization methods to address sulfate-induced soil heave. J Geotech Geoenviron Eng 130:391–402. https://doi.org/10.1061/(ASCE)1090-0241
Puppala AJ, Intharasombat N, Vempati RK (2005) Experimental studies on ettringite-induced heaving in soils. J Geotech GeoEnviron Eng 131:325–337. https://doi.org/10.1061/(ASCE)1090-0241(2005)131:3(325)
Puppala AJ, Chittoori B, Saride S (2012) Sulfate induced heaving of a taxiway: a case study. Indian Geotech J 42:257–266. https://doi.org/10.1007/s40098-012-0026-2
Puppala A, Talluri N, Congress S, Gaily A (2018) Ettringite induced heaving in stabilized high sulfate soils. Innov Infrastruct Solut 3:72. https://doi.org/10.1007/s41062-018-0179-7
Puppala AJ, Congress SS, Talluri N, Wattanasanthicharoen E (2019) Sulfate-heaving studies on chemically treated sulfate-rich geomaterials. J Mater Civ Eng 31:04019076. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002729
Rahman MA (1986) The potentials of some stabilizers for the use of lateritic soil in construction. Build Environ 21:57–61. https://doi.org/10.1016/0360-1323(86)90008-9
Rajasekaran G (2005) Sulphate attack and ettringite formation in the lime and cement stabilized marine clays. Ocean Eng 32:1133–1159. https://doi.org/10.1016/j.oceaneng.2004.08.012
Shivanshi, Jha AK, Akhtar MP (2022) Influence of soluble sodium sulphate contamination on physical and strength behavior of untreated and lime treated soil. KSCE J Civ Eng 26:3815–3830. https://doi.org/10.1007/s12205-022-1964-6
Shivanshi, Jha AK, Akhtar MP (2023) Physicochemical and strength behavior in lime-treated soil submerged under gypsum and sodium sulfate contamination. J Hazard Toxic Radioactive Waste 27:04023004. https://doi.org/10.1061/JHTRBP.HZENG-1185
Sivapullaiah P, Sridharan A, Ramesh H (2000) Strength behaviour of lime-treated soils in the presence of sulphate. Can Geotech J 37:1358–1367. https://doi.org/10.1139/t00-052
Sol-Sánchez M, Castro J, Ureña C, Azañón J (2016) Stabilisation of clayey and marly soils using industrial wastes: pH and laser granulometry indicators. Eng Geol 200:10–17. https://doi.org/10.1016/j.enggeo.2015.11.008
Solis R, Zhang J (2008) Gypsiferous soils: an engineering problem. Sinkholes and the engineering and environmental impacts of karst. pp 742–749
Song S, Sohn D, Jennings H, Mason TO (2000) Hydration of alkali-activated ground granulated blast furnace slag. J Mater Sci 35:249–257. https://doi.org/10.1023/A:1004742027117
Sridharan A, Sivapullaiah P, Ramesh H (1995) Consolidation behaviour of lime treated sulphatic soils. Compression and consolidation of clayey soils. pp 183–188
Su Y, Wang J, Li D, Wang X, Hu L, Yao Y, Kang Y (2023) End-to-end deep learning model for underground utilities localization using GPR. Autom Constr 149:104776. https://doi.org/10.1016/j.autcon.2023.104776
Sun Z, Zhou J, Qi Q, Li H, Zhang N, Mu R (2020) Influence of fly ash on mechanical properties and hydration of calcium sulfoaluminate-activated supersulfated cement. Materials 13:2514. https://doi.org/10.3390/ma13112514
Sun L, Wang C, Zhang C, Yang Z, Li C, Qiao P (2023) Experimental investigation on the bond performance of sea sand coral concrete with FRP Bar reinforcement for marine environments. Adv Struct Eng 26:533–546. https://doi.org/10.1177/13694332221131153
Tsatsos N, Dermatas D (1998) Correlation between mineralogy and swelling of lime-treated contaminated soil mixes. Environmental geotechnics. pp 473–478
Wang L, Liu J, Yu X, Li X, Puppala AJ, Wang Q (2021) Experimental study on the correlation between the partial and total salt content in saline gravel using ion chromatography. Transp Geotechnics 26:100424. https://doi.org/10.1016/j.trgeo.2020.100424
Wei J, Ying H, Yang Y, Zhang W, Yuan H, Zhou J (2023) Seismic performance of concrete-filled steel tubular composite columns with ultra high performance concrete plates. Eng Struct 278:115500. https://doi.org/10.1016/j.engstruct.2022.115500
Wild S, Abdi M, Leng-Ward G (1993) Sulphate expansion of lime-stabilized kaolinite: II. Reaction products and expansion. Clay Miner 28:569–583. https://doi.org/10.1180/claymin.1993.028.4.07
Wild S, Kinuthia J, Jones G, Higgins D (1999) Suppression of swelling associated with ettringite formation in lime stabilized sulphate bearing clay soils by partial substitution of lime with ground granulated blastfurnace slag (GGBS). Eng Geol 51:257–277. https://doi.org/10.1016/S0013-7952(98)00069-6
Wu X, Jiang W, Roy DM (1990) Early activation and properties of slag cement. Cem Concr Res 20:961–974. https://doi.org/10.1016/0008-8846(90)90060-B
Xing H, Yang X, Xu C, Ye G (2009) Strength characteristics and mechanisms of salt-rich soil–cement. Eng Geol 103:33–38. https://doi.org/10.1016/j.enggeo.2008.07.011
Yi Y, Liska M, Al-Tabbaa A (2014) Properties and microstructure of GGBS–magnesia pastes. Adv Cem Res 26:114–122. https://doi.org/10.1680/adcr.13.00005
Yilmaz I, Civelekoglu B (2009b) Gypsum: an additive for stabilization of swelling clay soils. Appl Clay Sci 44:166–172. https://doi.org/10.1016/j.clay.2009.01.020
Zhang T, Vandeperre LJ, Cheeseman CR (2014) Formation of magnesium silicate hydrate (MSH) cement pastes using sodium hexametaphosphate. Cem Concr Res 65:8–14. https://doi.org/10.1016/j.cemconres.2014.07.001
Zhang G, Wu C, Hou D, Yang J, Sun D, Zhang X (2021) Effect of environmental pH values on phase composition and microstructure of Portland cement paste under sulfate attack. Compos Part B: Eng 216:108862. https://doi.org/10.1016/j.compositesb.2021.108862
Zhu G, Yong L, Zhao X, Liu Y, Zhang Z, Xu Y, Sun Z, Sang L, Wang L (2022) Evaporation, infiltration and storage of soil water in different vegetation zones in the Qilian Mountains: a stable isotope perspective. Hydrol Earth Syst Sci 26:3771–3784. https://doi.org/10.5194/hess-26-3771-2022
Acknowledgements
The authors appreciate the start-up grant (430100319) supported by Hubei University of Technology, Hubei, China, the grant supported by Open Project Funding of Key Laboratory of Intelligent Health Perception and Ecological Restoration of Rivers and Lakes, Ministry of Education, Hubei University of Technology (HGKFZP008), Joint Funds of the Natural Science Foundation of Hubei Province (No. 2022CFD130), and The Key Research and Development Program of Hubei Province (No. 2023BAB024).
Author information
Authors and Affiliations
Contributions
Wentao Li: data curation, funding acquisition, writing-reviewing and editing, methodology, formal Analysis. Li Zhou: supervision, writing-reviewing and editing. Henglin Xiao: funding acquisition, resources. Kang Yang: data curation, writing-original draft. Qipei Han: supervision. Xing Li: supervision, writing-reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare there are no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Zhou, L., Xiao, H. et al. Effect of sulfate types on strength and swelling properties of sulfate-bearing soils stabilized with cement. Environ Earth Sci 83, 516 (2024). https://doi.org/10.1007/s12665-024-11825-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-024-11825-6