Abstract
Stabilization of expansive soils using lime and cement additives have been used by practitioners over the years. However, recent heaving and premature pavement failures in lime and cement-treated subgrades containing sulfates led to questioning the use of calcium-based stabilization methods for these soils. Annually, millions of dollars are spent to repair pavements distressed by this Ettringite induced heaving. Based on the past studies, researchers and practitioners have proposed various methods to treat sulfate soils. Applicability of these methods is mostly limited to soils with sulfate levels below 8000 ppm. Soils with sulfate content above 8000 ppm are termed as “high sulfate” soils, and chemical treatment of such soils is currently not considered. A research study was designed to aid in understanding the heaving phenomenon in soils with sulfate contents above 8000 ppm and to develop practical techniques to stabilize such soils. High sulfate soils were sampled and treated with lime at varying mellowing periods and treated soils were then subjected to the engineering and chemical tests. Tests results were analyzed to understand the effectiveness of mellowing period on the heaving phenomenon of these soils. Treated soils at higher mellowing periods showed reduced sulfate-induced heaving when sulfate levels are lower than 30,000 ppm. Sulfate levels in excess of 30,000 ppm did not result in effective treatment of soils. It was also observed that compaction void ratios and soil clay mineralogy have a significant impact on the swell behavior of chemically treated high sulfate soils. An innovative method comprising of aerial technologies is used to monitor the pavement heaving. This keynote paper provides a comprehensive review of stabilization of high sulfate soils and methods studied to mitigate sulfate heaving.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and background
Soils of expansive nature have been found around different regions of the world [4]. These soils undergo large volume changes comprising of shrink and swell with the moisture variations, leading to distress in the structures built above them [4, 21]. Calcium-based stabilizers such as lime and cement have been widely used to treat expansive soils [11] mainly for pavement subgrade stabilization applications. Soil stabilization with lime/cement elevates the system pH to 12.4 or above leading to the release of calcium and dissolution of clay alumina and silica and resulting formation of pozzolanic compounds. Formation of the pozzolanic compounds improves the volume stability, durability and stiffness characteristics of the expansive soils. Due to its availability and reasonable cost, lime has been used as the subgrade stabilizer in most of the transportation infrastructure projects.
In the last two decades, many cases of severe pavement heaving and distress were reported when the expansive soils containing sulfates were treated with traditional calcium-based stabilizers [13, 20, 24, 28, 32]. Initially, it was assumed that the heave is a result of expansive soil swelling due to wetting. Later studies by researchers confirmed that the swell originated from the lime-/cement-treated subgrades [13, 14, 18, 23, 25]. Several pavements and infrastructure facilities with design service life of 20–30 years have experienced severe heaving issues shortly after construction. The heave and pavement distress were attributed to the complex reactions occurring in a moist environment between the calcium from the stabilizer, alumina, silica, and soil sulfates. This phenomenon is termed as “Sulfate-Induced Heave” in the literature.
When soils containing sulfate minerals (such as gypsum, CaSO4.2H2O and sodium sulfate, Na2SO4) in natural formation are treated with calcium-based stabilizers, the system pH is elevated at which the calcium from the stabilizer reacts with soil sulfates, alumina and silica to form the highly expansive mineral, “Ettringite” (Ca6.[Al(OH)6]2.(SO4)3.26H2O). As the chemical composition indicates, the mineral Ettringite contains 26 molecules of water and is capable of swelling more than 137% of its volume [16]. Figure 1 depicts the mineral structure of Ettringite. At ideal temperature, humidity and elevated pH conditions mineral Ettringite expand in volume due to crystal growth and hydration [17, 22]. The formation and growth of Ettringite cause expansive stresses in the soils. These expansive stresses often exceed the existing overburden pressure resulting in heaving and pavement failures.
Figure 2 illustrates sulfate-induced pavement failure reported along US 67 near Midlothian, Texas, USA. When the temperature of the system falls below 15 °C and carbonates are present in the system, Ettringite is transformed into “Thaumasite” (Ca6.[Si(OH)6]2.(SO4).(CO3)2.24H2O) through a series of reactions. Both Ettringite and Thaumasite are highly expansive minerals. Thaumasite has been reported as the cause of severe heaving of pavements and parking lots in Las Vegas, Nevada [13]. Ettringite formation and subsequent heave are a complex phenomenon occurring in soils. It was reported that the formation of Ettringite is dependent on the availability of free alumina in soils, free access to temperature, humidity and site drainage conditions.
In addition to these factors, studies by Hunter [12] attributed the observed swell to an increase in void ratio from the initial compacted state due to the mineral growth. Similar observations of increased void ratio were made by recent studies conducted by Talluri et al. [36]. It is interesting to note that under similar chemistry and environmental conditions, sulfate-induced heave is of greater concern in clays than in sands [26].
Based on the previous sulfate heave case studies, researchers recommended that when significant amounts of sulfates are present in the native soil, lime/cement treatment must either be carried out with caution or be completely avoided [20]. Based on the case histories and research conducted on the sulfate heave, there is a need to establish threshold sulfate level above which calcium-based stabilization is not recommended due to the formation of expansive compounds and associated swelling. Studies conducted by Puppala et al. [28] confirmed that at sulfate levels around 1000 ppm, lime stabilization plays an important role in reducing swelling of natural soils. At sulfate levels ranging from 0 to 2500 ppm, the lime stabilization and sulfate reactions occur simultaneously, but the magnitude and extent of heave depend on the lime concentration. At higher lime dosages, swell magnitudes were suppressed, indicating the dominance of stabilizing reactions.
Also, when the sulfate concentrations exceeded 2500 ppm, the increase in lime dosage resulted in increased heaving due to increased amounts of Ettringite formed. Petry and Little [24] stated that if the soluble sulfate content in the native subgrade material is below 2000 ppm or 0.2%, the sulfate heave risk is minimal. Based on the studies conducted on Texas soils, Berger et al. [1] reported that soluble sulfates below 3000 ppm are of little concern, sulfates between 3000 and 5000 ppm are moderate concern, 5000–8000 ppm are moderate to high risk and greater than 8000 ppm sulfate concentrations are of severe concern for stabilization using lime. Transportation agencies across the USA use lime in most of the subgrade stabilization projects due to its cost and availability compared to cement.
According to the guidelines developed by the Texas Department of Transportation (TxDOT), sulfate concentrations up to 3000 ppm can be stabilized by traditional lime with 1 day of mellowing [10]. Soils with sulfate concentrations up to 8000 ppm can be stabilized by providing additional moisture, along with other chemical treatments including combined lime and fly ash treatments. When sulfate concentrations exceed 8000 ppm, alternative treatment such as remove and replace or blend in non-plastic soils is recommended. Solutions such as remove and replace or blend in non-plastic soils are not preferred by the DOTs over the years due to economy as well as sustainability since the transportation of foreign materials involves increased fuel emissions and costs associated with transporting them.
Overall there had been no conclusive threshold sulfate levels that researchers agree. This can be attributed to sulfate levels varied for lime and cement treatments, depending on the source of sulfate and site conditions such as drainage around a project site. It is interesting to note that the sulfate levels at which heaving occurred varied from as low as 320 ppm to as high as 43,500 ppm [26, 31]. The time for sulfate heaving appearance after chemical stabilization ranged from a few days to 18 months. Also, soils that experienced this sulfate heave included sands to silts and clays containing significant amounts of clay fraction.
Given the uncertainty over the sulfate levels and limited literature available, the guidelines developed so far have limited the threshold sulfate level for lime stabilization as 8000 ppm. In addition to this, failures are particularly evident at sites where soil sulfates are at or above 8000 ppm level, which needs attention of the research community. Also, the soils with sulfate concentrations above 8000 ppm are called as “High Sulfate Soils” and are deemed ineffective for lime stabilization. To evaluate options to stabilize soils with sulfate levels above 8000 ppm, a research program was undertaken at the University of Texas at Arlington with the support of Texas Department of Transportation. Advent of the advanced sensor technologies that are compatible with aerial platforms such as unmanned aerial vehicle (UAV) systems has paved way for remotely collecting data using close-range photogrammetry (CRP). UAV–CRP technology has been used for monitoring various civil engineering assets like pavement, bridges, railways, and construction material stockpiles [33]. This innovative technology is used to monitor heaving on the pavement overlain on sulfate-rich soils and the results are provided in the later sections of the paper. This is the primary objective of this research and the results from this study are presented in the following sections.
Testing program
The testing program consisted of two phases. Phase 1 comprised of engineering tests on treated high sulfate soil samples. Phase 2 comprised of chemical and mineralogical tests on the treated high sulfate soils to understand the clay mineralogy and composition. Six different soils from the state of Texas were chosen for this task. Selection of these soils was done based on the sulfate levels and PI values. Based on the measured sulfate contents, the soils were categorized as soils with sulfate contents greater than 8000 ppm, soils with sulfate contents less than 8000 ppm and soils with negligible sulfate contents. Among the six soils, three soils showed sulfate contents above 8000 ppm (Austin, Childress and Sherman). Two of the six soils have sulfate levels ranging from 5200 to 7000 ppm (Dallas and US82). One of the six soils is a control soil with negligible sulfate contents (Riverside). Additional sulfate in the form of gypsum was added to the soils to be considered as high sulfate soils (> 8000 ppm). Based on the plasticity indices (PI), soils were categorized as soils with PI < 30, 30 < PI < 40, 40 < PI < 50 and PI > 50. Sulfate content and PI values for the test soils are presented in Table 1.
The aim of the current research program was to study heave mechanisms as well as develop techniques for stabilization of “high sulfate” soils. The testing program was divided into Phase 1 (basic and engineering tests) and Phase 2 (chemical and mineralogical tests). Basic tests include Atterberg limits, hydrometer, specific gravity and Proctor compaction tests. Engineering tests include 3-D volumetric swell/shrinkage, 1-D swell pressure and UCS tests. These tests were conducted on untreated and treated soil samples. Due to the limitation of space, only the 3-D volumetric swell and shrinkage results and the discussion are presented in the current publication.
Phase 2 tests included sulfate content (before and after treatment), lime dosage, reactive alumina and silica, cation-exchange capacity (CEC), specific surface area (SSA) and total potassium (TP). Cation-exchange capacity, specific surface area and total potassium tests were conducted to determine the basic clay mineralogy as in the procedures outlined by Chittoori and Puppala [5]. The purpose of determining the clay mineralogy is that certain clay minerals release more alumina at elevated pH conditions compared to others, which is the critical component of both pozzolanic and Ettringite formation reactions [6, 30]. Test soils were also subjected to reactive alumina and silica tests, using ICP-MS (inductively coupled plasma mass spectroscopy) to determine the amount of alumina and silica in the natural formation which upon chemical stabilization using lime leads to the formation of both pozzolanic and deleterious compounds.
In the current research study, lime was used as the stabilizer with “Pre-compaction mellowing” technique. Two mellowing periods (0 and 3 days) were considered in the current study. Test soils were treated with lime and allowed to mellow in a moisture-controlled environment. Following the mellowing, the samples were mixed thoroughly and compacted. Engineering tests were conducted on the compacted soil samples. To study the effect of higher mellowing periods, an additional set of 3-D swell tests were conducted using a 7-day mellowing period. After the samples were subjected to swell tests, reactive alumina and silica measurements were conducted to determine the loss of alumina and silica during stabilizing and sulfate reactions. Testing variables are presented in Table 2. Table 3 shows the Proctor compaction results for natural and 6% lime-treated soils at optimum moisture content.
Engineering tests on natural and treated soil samples
Three-dimensional volumetric (3-D) swell tests were conducted in the current study using “double inundation” technique. Double inundation represents the worst possible scenario in field where 100% saturation of the soil is achieved after a continuous rainfall event leading to maximum expansive heave. Oven-dried soil samples were mixed with corresponding moisture content from the proctor tests and stabilizer dosage and compacted by using Gyratory Compactor Machine at two moisture content levels (optimum and we of optimum moisture content). The compacted soil samples were placed in the water bath with moisture access from top and bottom, and the corresponding swell is measured over a period of 10 days. Final swell magnitudes for the natural and treated soil samples (at different mellowing periods) are presented in Figs. 3, 4 and 5.
Volumetric shrinkage tests were conducted to measure the decrease in the total volume of soil specimens due to loss of moisture content from predetermined initial moisture content to a completely dry state. In the current study, 3-D volumetric shrinkage tests were conducted on natural and treated soil samples as per the modified methodology developed by Puppala et al. [29]. Compared to the linear shrinkage bar test, this test offers several advantages such as reduced interference of boundary conditions on shrinkage, larger amount of soil being tested, and simulation of compacted state. Table 4 summarizes the findings of the 3-D shrinkage tests.
Chemical tests on natural and treated soil samples
Phase 2 testing comprised of various chemical tests to determine the optimum lime dosage, sulfate level (before and after 3-D swells), clay mineralogy determination and reactive alumina and silica measurements. Lime content is determined as per the “Eades–Grim” test [7] in accordance with ASTM D 6276 standard. In this method, different dosages of lime are added to the soil and the soil pH is measured. The minimum amount of lime dosage that ensures a sustained pH of 12.4 is considered as the optimal lime dosage. A sustained pH of 12.4 ensures strength-producing lime–soil pozzolanic reactions in the treated soil samples. The optimum lime dosage is determined as 6% for the soils considered in the current study.
Soluble sulfate content for the soils was measured using modified UTA method [27]. Modified UTA method is a gravimetric sulfate measurement technique which provided consistent and accurate results irrespective of the sulfate source and dilution ratio considered as reported by Talluri et al. [35] and Freese et al. [9]. Sulfate measurements were conducted on natural soils as well as treated soils. For the treated soils, sulfate tests were conducted on the compacted samples monitored for 3-D volumetric swell after the swell monitoring period where some portion of the initial sulfate is consumed in the sulfate reactions. Table 5 represents the initial and final sulfate contents for the soils considered in the current study.
Clay mineralogy determination consisted of determining the cation-exchange capacity (CEC), specific surface area (SSA) and total potassium (TP) measurements. CEC of a soil can be defined as the capacity or the ability of the soil to exchange free cations that are available in the exchange locations. The method developed by [3] is the most commonly used method in the field and is used in the current research. The method involves the addition of a saturating solution and then removal of the adsorbed cations, using an extracting solution. Specific surface area or SSA of a soil sample is the total surface area contained in a unit mass of soil. The soils with high specific surface area have high water holding capacity and greater swell potential. SSA is determined in the current study using adsorption by ethylene glycol monoethyl ether (EGME) [2]. Potassium is the inter-layer cation in the clay mineral illite [19]. Hence, measuring the amount of potassium ion in the soil gives a direct indication of the presence of the mineral illite.
In the current study, the methodology formulated by [15] was followed for determining the specific surface area of the soils. Following determination of the CEC, SSA and TP, the clay mineralogy of the soils is obtained as per the procedure outlined by Chittoori and Puppala [5]. Table 6 summarizes the mineralogical composition of the six soils considered in the current study. Reactive alumina and silica are the aluminum and silica present in amorphous or poorly crystalline Al/Si phases, including amorphous aluminosilicate, organically complex alumina and hydroxyl-Al polymers present in montmorillonite inter-layers. Measurement of reactive alumina and silica is important as they constitute the composition of Ettringite and Thaumasite, respectively.
Reactive alumina and silica measurements were conducted in the current study using inductively coupled plasma mass spectroscopy (ICP-MS) analysis as per the procedure outlined by Foster [8]. Table 7 presents the results of the reactive alumina and silica measurements at optimum moisture content on natural and treated soils. In the following sections, analyses of the engineering and chemical tests are presented.
Analysis of the engineering and chemical tests
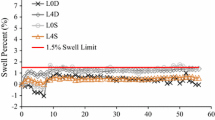
In this section, the authors tried to elucidate the behavior of chemically treated high sulfate soils (based on the engineering and mineralogical tests conducted) as well as the applicability of pre-compaction mellowing (for a particular soil type) based on the swell and mineralogy studies conducted. Figures 3, 4 and 5 present the 3-D volumetric swell for the high sulfate soils considered in the current study. It can be observed that in untreated condition, Austin, Dallas, Sherman and US-82 soils have high swelling potential with the volumetric swell in excess of 10% which characterizes them as expansive soils [4]. With 0-day mellowing, the highest swell magnitude was observed in the US-82 soil (26%), whereas Austin soil showed the lowest swell (8.8%). The volumetric swell increased due to lime treatment at 0-day mellowing in all the soils, except for the Austin soil, where the volumetric swell strain value reduced from 16.6% for natural soil to 8.8% after lime treatment.
In 3-day mellowed samples, the volumetric swell reduced below the natural level for four of the soils tested. Swell magnitudes in these soils were less than the natural swell, showing the effects of stabilization. All the four soils which responded positive to 3-day mellowing have sulfate contents below 30,000 ppm. The two soils which could not be stabilized by 3-day mellowing are Austin and Childress, both with sulfate levels higher than 30,000 ppm. With 7-day mellowing, further reduction in swell was observed in the Riverside, Dallas, US-82 and Sherman soils. The observed volumetric swells in these soils were below 10%, which is considered non-problematic and indicating that mellowing was effective in these soils. In Austin and Childress soils (sulfate contents greater than 30,000 ppm), swell further increased beyond the natural swell with 7-day mellowing, showing the dominance of sulfate reactions with increased mellowing periods. Further discussion and analysis on ineffectiveness of mellowing in two high sulfate soils (Austin and Childress soils) are presented below.
Three-dimensional volumetric shrinkage tests on lime-treated high sulfate soils (at different periods of mellowing) showed reduction in shrinkage behavior compared to those of natural soils. In four of the six soils, there was a slight increase in shrinkage strain with 3-day mellowing compared to the 0-day mellowing. The reason for this behavior could be due to higher moisture provided. An additional 3% to cater for moisture loss during mellowing was added to the treated soils in the case of 3-day mellowed soils. Overall, it can be concluded that the presence of sulfates does not have any impact on the shrinkage strain behavior of the treated high sulfate soils.
From Table 7, Austin and Childress soils have the lowest reactive alumina and silica contents among the six soils considered in the current study. It is reported in the literature that Ettringite formation depends on the amount of reactive alumina present in the system. Low alumina contents favor the trisulfate hydrate (Ettringite) formation, whereas high alumina contents lead to simultaneous formations of pozzolanic and Ettringite reactions. Low alumina contents coupled with high sulfate levels in Austin and Childress soils (sulfate levels in excess of 30,000 ppm) resulted in the Ettringite formation/hydration reactions dominating the pozzolanic reactions which occur in chemically treated soils. This could be the reason for the increased swell volumes in both soils with mellowing.
From Table 6, one can observe that both Austin and Childress soils have mineral Kaolinite dominance. Dermatas [6] reported that Kaolinite mineral releases more alumina during the hydration reactions compared to other clay minerals. Austin soil is high plasticity clay, whereas Childress soil is high plasticity silt, both with mineral Kaolinite dominance over other minerals. High sulfate content and readily available alumina from mineral Kaolinite could be the reason for ineffectiveness of mellowing in Austin and Childress soils. In addition to the mineralogical composition of the soils, an attempt was made to study the effect of compaction void ratios on the swell behavior of soils. The void ratios of the test soils in compacted state were calculated using specific gravity and maximum dry density from proctor curve. The void ratios of the test soils at optimum moisture content (OMC) are presented in Table 8. One can observe that both Austin and Childress soils have low void ratios compared to other soils. Due to the low void ratio, the Ettringite formation and growth could not be accommodated in the dense soil matrix leading to more heaving. Also, Austin and Childress soils have sulfate contents above 30,000 ppm and the reactive alumina and silica contents in these soils were lower than 100 ppm. Combination of low void ratios, high sulfate contents and low reactive alumina and silica could be the reasons why mellowing was ineffective in mitigating heaving in these two soils.
Long-term performance of pavement sections
Three test sections comprising of one control and two treated test sections were constructed on US 82 highway near Bells in Grayson County, Texas. These test sections aided in screening and evaluation of stabilizers for sulfate soil conditions. Control Section (Test Section 3) consisted of a lime treatment subgrade section with a 3-day mellowing period, which is a commonly practiced stabilization technique by TxDOT. Test sections 1 and 2 are composed of lime–fly ash-treated and lime-treated sections, respectively, with varying mellowing time durations. Layout of these test sections is presented in Fig. 6. Figure 7 presents swell strain test results from soil samples taken from three test sections. These results showed that the control soils experienced swelling, whereas soil samples taken from the present test sections 1 and 2 experienced minimal heaving.
Pavement site inspection
Unmanned aerial vehicle monitoring
UAV–CRP technology has been used for various civil engineering applications in the last decade. Visualization of the data obtained from a close-scale inspection of pavement by this technology can provide multiple attributes of the pavement like distress information and geometric information [34]. If this technology can be utilized effectively, thousands of miles of pavements laid over these sulfate soils can be monitored efficiently and safely. This research had adopted the UAV–CRP technology by mounting a visible range camera on the UAV and collected the pavement profile data remotely by flying away from the pavement and inclined camera focusing on the pavement as shown in Fig. 8.
The collected images were processed to generate high quality three-dimensional dense point cloud model, contour map, orthomosaic and digital elevation model (DEM). Heaving can be identified by continuous alternating colors indicating frequent change in elevation along with the stretch. Hence, the perception of the elevation profile of pavement and surroundings offered by the color-coded elevations in DEM helps in identifying heaving, as shown in Fig. 9. The DEM and corresponding orthomosaic shown in Figs. 9 and 10, respectively, do not indicate any abrupt change in the elevation of the pavement profile. DEM also indicates that the pavement profile is sloping downwards along the longitudinal direction, indicated by the black arrow pointing toward the vehicular movement direction in Figs. 9 and 10. The UAV–CRP data collected and processed have indicated that there is no heaving.
Summary and conclusions
The following conclusions were made from the study presented in this paper:
-
The formation of expansive Ettringite mineral was attributed to the presence of calcium from lime, reactive alumina from soil, soluble sulfates and moisture availability in the treated soils.
-
Based on the tests conducted on lime-treated high sulfate soils, it was concluded that it is the swell behavior that is of importance than the shrinkage in chemically treated high sulfate soils.
-
Sulfate content has a significant effect on the stabilization of high sulfate soils. The purpose of mellowing is to solubilize the entire available sulfates during mellowing period so that no sulfate is available later to form Ettringite/Thaumasite. Two of the six soils in the current study have high sulfate contents and the entire sulfate could not be dissolved during mellowing period. These are some of the reasons that have resulted in mellowing not being effective on these two soils.
-
Four of the six soils in the current study were successfully stabilized using “pre-compaction” mellowing technique. These soils have sulfate levels below 30,000 ppm.
-
The current study confirmed past research findings that soils with Kaolinite mineral dominance are more prone to Ettringite induced heaving when compared to the soils with other mineral dominance provided all other factors (sulfate content, reactive alumina and silica contents) remain the same. Since reactive alumina and silica are major constituents of the strength related pozzolanic reactions, soils with low reactive alumina, silica and high sulfate contents showed dominance of Ettringite reactions over the pozzolanic reactions which are manifested in the form of excessive heaving.
-
The compaction void ratios also have significant influence on the Ettringite growth and subsequent heaving since soils with higher void ratios can accommodate the initial Ettringite growth. In the current study, it was observed that the two soils that could not be stabilized effectively with mellowing have lower void ratios that could not possibly accommodate any Ettringite formation and growth leading to higher observed swells.
-
Field sections were constructed using lime–fly ash-treated soils and lime-treated soils, both with 3-day mellowing periods. Both laboratory tests on field samples and field data monitoring using UAVs showed minimal heaving indicating that both methods provided effective treatment of mitigating heaving in high sulfate soils.
References
Berger E, Little DN, Graves R (2001) Technical memorandum: guidelines for stabilization of soils containing sulfate. http://www.lime.org/publications.html. Accessed September 2010
Carter DL, Heilman MD, Gonzales CL (1965) Ethylene glycol monoethyl ether for determining surface area of silicate minerals. Soil Sci 100:356–360
Chapman HD (1965) Cation-exchange capacity 1. In: Black CA et al. (ed) Methods of soil analysis. Part 2—Chemical and microbiological properties. American Society of Agronomy, Madison, WI, pp 891–901
Chen FH (1988) Foundations on expansive soils. Dev Geotech Eng 12:12
Chittoori B, Puppala AJ (2011) Quantitative estimation of clay mineralogy in fine-grained soils. J Geotech Geoenviron Eng 137(11):997–1008
Dermatas D (1995) Ettringite-induced swelling in soils: state-of-the-art. Appl Mech Rev 48:659–673
Eades JL, Grim RE (1966) A quick test to determine lime requirements for lime stabilization. Highw Res Rec 139:61–72
Foster HM (1953) Geochemical studies of clay minerals III. The determination of free silica and free alumina in Montmorillonites. Geochem Cosmochim Acta 3:143–154
Freese K, Abbas A, Senko J, Cutright TJ (2015) Assessment of process variables that can impact results of soluble sulfate evaluated by the Tex-145E method. J Test Eval 43(6):1472–1478
Harris JP, Von Holdt J, Sebesta S, Scullion T (2006) Recommendations for stabilization of high-sulfate soils in Texas. Transportation Research Record No. 1952. TRB, National Research Council, Washington, DC, pp 71–79
Hausmann MR (1990) Engineering principles of ground modification. McGraw–Hill, New York
Hunter D (1989) The geochemistry of lime-induced heave in sulfate bearing clay soils. In: Ph. D. Dissertation, University of Nevada, Reno
Hunter D (1988) Lime-induced heave in sulfate-bearing clay soils. J Geotech Eng ASCE 114:150–167
Kota BVS (1996) Sulfate bearing soils: problems with calcium based stabilizers, vol 1546. Transportation Research Record. National Research Council, Washington, DC, pp 62–69
Knudsen D, Peterson GA, Pratt PF (1982) Lithium, sodium, and potassium. In: Page AL (ed) Methods of soil analysis. Part 2—Chemical and microbiological properties. American Society of Agronomy, Madison, WI, pp 225–246
Little DN, Nair S, Herbert B (2010) Addressing sulfate-induced heave in lime treated soils. J Geotech Geoenviron Eng ASCE 136:110–118
Mehta PK (1973) Effect of lime on hydration of pastes containing gypsum and calcium aluminate or calcium sulfoaluminate. J Am Cer Soc 56:315–319
Mitchell JK, Dermatas D (1992) Clay soil heave caused by lime-sulfate reactions. ASTM Spec Publ 1135:41–64
Mitchell JK, Soga K (2005) Fundamentals of soil behavior. Wiley, New York
Mitchell JK (1986) Practical problems from surprising soil behavior. J Geotech Eng Div ASCE 112(3):259–289
Nelson DJ, Miller JD (1992) Expansive soils: problems and practice in foundation and pavement engineering. Wiley, Hoboken, p 259
Ogawa K, Roy DM (1982) C4A3S hydration, ettringite formation and its expansion mechanism: part II, microstructural observation of expansion. Cem Concr Res 12:101–109
Perrin L (1992) Expansion of lime-treated clays containing sulfates. In: Proceedings of 7th international conference on expansive soils, ASCE Expansive Soil Research Council, New York, vol 1, pp 409–414
Petry TM, Little DN (1992) Update on sulfate induced heave in treated clays: problematic sulfate levels. Transportation Research Board, TRR 1362, Washington, DC
Puppala A, Musenda C (2000) Effects of fiber reinforcement on strength and volume change in expansive soils. Transp Res Rec J Transp Res board 1736(1):134–140
Puppala AJ, Hanchanloet S, Jadeja M, Burkart B (1999) Sulfate induced heave distress: a case study. In: Proceedings, transportation research board annual meeting, Washington DC, USA
Puppala AJ, Viyanant C, Kruzic AP, Perrin L (2002) Evaluation of a modified soluble sulfate determination method for fine-grained cohesive soils. Geotech Test J GTJODJ 25(1):85–94
Puppala AJ, Wattanasanticharoen E, Punthutaecha K (2003) Experimental evaluations of stabilization methods for sulphate-rich expansive soils. Ground Improv 7(1):25–35
Puppala AJ, Katha B, Hoyos LR (2004) Volumetric shrinkage strain measurements in expansive soils using digital imaging technology. ASTM Geotech Test J 27(6):547–556
Puppala AJ, Intharasombat N, Vempati R (2005) Experimental studies on ettringite induced heaving in soils. ASCE J Geotech Geoenviron Eng 31(3):325–337
Puppala AJ, Wattanasanticharoen E, Punthutaecha K (2005) Experimental evaluations of stabilisation methods for sulphate-rich expansive soils. Proc Inst Civ Eng Ground Improv 9(2):89–90
Puppala AJ, Talluri NS, Chittoori BS, Gaily A (2012) Lessons learned from sulfate induced heaving studies in chemically treated soils. In: Proceedings of the international conference on ground improvement and ground control. Research Publishing, pp 85–98
Puppala AJ, Congress SSC, Bheemasetti TV, Caballero S (2018) Visualization of civil infrastructure emphasizing geomaterial characterization and performance. ASCE J Mater. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002434
Puppala AJ, Congress SSC, Bheemasetti TV, Caballero S (2018) geotechnical data visualization and modeling of civil infrastructure projects. In: GeoShanghai international conference, pp 1–12, Springer, Singapore
Talluri N, Gaily A, Puppala AJ, Chittoori B (2012) Comparative study of soluble sulfate measurement technqiues. In: GeoCongress 2012, Oakland CA March 2–29, GSP 225, pp 3372–3381
Talluri N et al (2013) Stabilization of high sulfate soils by extended mellowing. Transportation Research Board Annual Meeting, Washington, DC
Acknowledgements
Authors would like to thank Joe Adams of TxDOT, Jimmy Si of TxDOT, Wade Blackmon of TxDOT Paris District and Richard Williammee of Fort Worth District, for their guidance, support and supervision through the project time period. We acknowledge the funding support from TxDOT for the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puppala, A.J., Talluri, N., Congress, S.S.C. et al. Ettringite induced heaving in stabilized high sulfate soils. Innov. Infrastruct. Solut. 3, 72 (2018). https://doi.org/10.1007/s41062-018-0179-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41062-018-0179-7