Abstract
Heavy metal (HM) mobility and dynamics are greatly influenced by environmental factors, such as oxidation–reduction potential, pH, and salinity. The present work used an experimental setup to evaluate the influence of oxidation conditions and Eh-dependent parameters on the mobility of HMs in the sediments of Gomishan Wetland. Our findings revealed that the rise in aquatic Eh from 109 to 487 mV enhanced the release of Co, Cd, Cr, V, Mo, and Pb, with their mobility ordered as Mo > Pb > V > Co > Cd > Cr. The Tessier sequential extraction revealed that all elements were closely correlated with their terrestrial fractions. Correspondingly, terrestrial sources accounted for about 97.9%, 97.7%, 96.8%, 95.9%, 85.8%, and 69.2% of the total concentration of V, Cr, Co, Mo, Pb, and Cd in sediments, respectively. Enrichment factor (EF) and pollution index (Ipoll) were used to assess the HMs' contamination of the sediments. EF values revealed the depletion of V, Cr, Co, Pb, and Cd to the minimal enrichment (EF < 2), whereas Mo had a significant enrichment (2 < EF < 5). Ipoll values showed that the sediments were not polluted with Co, Cr, Mo, V, and Pb (Ipoll < 0.50), while they were slightly polluted with Cd (0.50 < Ipoll < 0.89).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HMs) are often deposited in sediments due to metal hydrolysis, adsorption, and accumulation (Geng et al. 2022). Although a small portion of HMs is remained dissolved in the water column. As a result, sediments in aquatic media can either retain metals or move them into the water column via various mechanisms (El Nemr et al. 2007; Bastami et al. 2015). Because of their toxicity, non-biodegradability/persistence, and ability to bioaccumulate in the food chain, HMs constitute a significant hazard to the aquatic environment (Gopinath et al. 2010; Zhang et al. 2017). Metals have different environmental behaviors depending on their chemical speciation, as various fractions of HMs are critical to assessing their mobility and bioavailability (Nagajyoti et al. 2010). Sediments' HMs may return to the aquatic environment through biological activity or changes in temperature, salinity, redox potential (Eh), and pH (Yang et al. 2012; Zhou et al. 2020; Madadi et al. 2022). Various indices have been implemented in recent decades to determine the ecological risk or contamination severity of HMs in sediments (Yang et al. 2012).
The redox reaction is a vital quality in sediment and soil systems. For instance, observation of Woolston Canal sediments showed an acceleration in the dissolved concentrations of Zn, Ni, Cu, Pb, and Cr under oxidation conditions, albeit their concentrations were reduced under redox conditions (Hartley and Dickinson 2010). In wetlands, soil qualities change from aerobic and oxidative conditions on the surface to anaerobic and reductive conditions at the sub-layers (Caple 1996). At different depths, the structure of the sediments is governed by the water level, pH, temperature, and oxidation potential (Bundt et al. 2001; Agnelli et al. 2004). The Eh–pH diagrams can estimate the solubility of different chemical species in aqueous environments (Karbassi and Heidari 2015). Oxidation–reduction reactions regulate several biochemical processes in coastal wetlands (Zhao et al. 2020).
Geochemists quantify total metals involved in petrogenesis and their remobilization potential during early diagenesis or resuspension of deposited sediments using several sequential extraction techniques, i.e., Tessier Five-step chemical separation (Yoo et al. 2013; Ranjbar Jafarabadi et al. 2018; Madadi et al. 2021). The sequential partitioning of HMs in Pacific pelagic sediments could also determine the source rock of the sediments(Chakraborty et al. 2016). Sediments have five different fractions of HMs, including F1 (soluble and exchangeable), F2 (carbonate-bound), F3 (bound to Fe–Mn oxides, reducible), F4 (bound to organic matter and sulfides, oxidizable), and F5 (residual fractions) (Zhang et al. 2017). F1 may be immediately absorbed by biota, whereas F2, F3, and F4 may be converted to active forms by changing the sediment environment, i.e., redox conditions, pH, salinity, and total organic matter (Li et al. 2016). Nevertheless, F5 is inactive due to its attachment to the crystalline lattices of sediments; upon the arrival of anthropogenic HMs, they mainly accumulate in F1, whereas lithogenic metals accumulate in F5 (Palleiro et al. 2016).
Up to now, a few studies have addressed the effect of Eh–pH on the behavior of HMs in sediments. Besides, the mobility and bioavailability of HMs in Gomishan Wetland sediments under dynamic redox conditions have remained unexplored. Therefore, this study was primarily aimed to evaluate the quality of water and sediments via pH, Eh, electrical conductivity (EC), dissolved oxygen (DO), and chemical oxygen demand (COD). In addition, we assessed the effects of oxidation conditions and Eh-dependent factors on the release and association of HMs (Co, Cd, Cr, V, Mo, and Pb) with different sedimentary phases. Finally, the geochemical factions/sources of the sediments' HMs were identified using Tessier sequential extraction procedure.
Materials and methods
Sampling
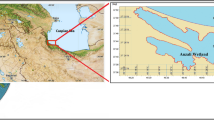
Sediment and water samples were taken from the Gomishan Wetland in Golestan Province, northern Iran, on November 30th, 2020. About 20 kg of sediment samples were collected using Peterson grabs. 60 L of the wetland water was also collected. Water and sediment samples were transferred to the laboratory in previously N2 purified PET containers and stored at 4 °C.
Electro-photocatalytic decomposition experiment
There has been increasing interest in photo-assisted electrochemical process (PEC) for the degradation of organic matter. This method utilizes photocatalysis and electrocatalysis in synergistic cooperation resulting in enhanced degradation performance (Yu et al. 2021). A photoanode (Ti/TiO2) and cathode (graphite) were used for the electro-photocatalysis tests with the dimensions of 1 × 20 × 100 mm and 10 × 50 × 100 mm, respectively (BaojiQi Xin dioxide Titanium Co., Ltd). The electrodes were placed in the aquarium at a distance of 7 cm in a parallel arrangement. Then, a DC-regulated power supply (UTP3704S UNI-T) was used with five positive and negative outputs. Anode was connected to the positive and the cathode was connected to the negative terminal to apply the voltages of 5 and 7 V. The UV lamp (8 W power; wavelength 380 nm; Philips Actinic BL TL) was set at a distance of 3 cm from the anode. Figure 1 schematically illustrates the PEC reactor system.
Before each test, the electrodes were rinsed with distilled water and 90% alcohol and oven-dried at 110 °C. To simulate the wetland condition, approximately 1 cm of sediments and 7 cm of water were added to each aquarium illuminated with a 250 W tungsten lamp (visible irradiation). The experiments were carried out at room temperature (25 °C), although the solution temperature increased by 10 °C after the PEC process due to the heat generated by the UV lamp.
The experiments were conducted under five experimental conditions, including 5 V, 7 V, 7 V/lamp/UV, 7 V/lamp, and 7 V/UV. Two electrodes were set at a 7 cm distance in all experiments.
Analytical methods
Temperature (T) was measured by a mercury thermometer; a portable multi-parameter probe was used to measure pH, Eh, DO, and EC. These measurements were all in situ. COD was determined using a spectrophotometer (DR 5000-Hach). Inductively coupled plasma mass spectrometry (ICP-MS) was employed to measure the concentrations of Co, Cd, Cr, V, Mo, and Pb (PerkinElmer, America). To calculate the concentrations of HMs and COD, 400 cc of water (100 cc for HMs and 300 cc for COD) was collected from each aquarium after 6 h and filtered through a 0.45 µm Whatman filter. To reduce and stabilize the solution pH < 2, H2SO4 (98%) and HNO3 (65%) were added to COD and HMs samples, respectively.
Tessier sequential extraction method (Tessier et al. 1979) was applied to determine the different fractions of HMs as follows: exchangeable (F1), acid-soluble (carbonates) (F2), reducible (Fe–Mn oxide bound) (F3), oxidizable (organically bound + sulphide bound) (F4), and residual (F5). The suspension was centrifuged at 3000 rpm for 15 min after each step to separate the obtained extract from the sediment. The supernatant was filtered and acidified with HNO3 to pH 2. The preceding phase's residue was washed with 10 mL of deionized water, waggled for 10 min, and centrifuged at 3000 rpm for 15 min, with the washing being thrown away in each step. Pre-dried sediment subsamples were digested using procedure 3050B to measure the residual and total concentrations of HMs. Finally, the concentration of HMs was measured by ICP-MS (PerkinElmer, America).
Statistical analysis
The present research used HSC Chemistry 6 software package. Inserting the Eh and pH values into HSC Chemistry 6 yields the predominant elements' temperature and concentration, chemical forms, and oxidation state in the aqueous system. Further statistical processing was made by making use of Excel 2019.
Sediment assessment
To determine the environmental pollution intensity, the geo-accumulation index (Igeo) was used as follows:
where Igeo refers to the soil pollution intensity index, Cn is the HM concentration (soil or sediment), Bn is the HM concentration in the shale, and 1.5 is the shale correction factor. Chemical partitioning can accurately determine Bn value through the separation of anthropogenic and natural sources of HMs.
Since the initial concentration of the element is not acquired through complete partitioning, the concentration of shale, a kind of sedimentary rock, should be utilized in this calculation. The average element concentration in shale is lower than the average element concentration in unpolluted sediments. Hence, it should be multiplied by 1.5 to achieve a balance. Based on this, (Karbassi et al. 2008) optimized the Müller formula as follows:
where Ipoll is the pollution intensity, An is the HM concentration (soil or sediment), and Dn is the background concentration of the HM within the study area.
The accumulation factor for each element was calculated via the enrichment formula of Buat-Menard and Chesselet (1979) as follows:
where EF is the enrichment factor, Cnx is the concentration of sediment HM, Bnx is the Fe concentration of the sediment sample. Cny and Bny are the background concentrations of HM and Fe, respectively. This method uses Fe as a normalizing element to subdue changes arising from sediment heterogeneity (Abrahim and Parker 2008).The pollution index and the enrichment factor classes are shown in Table S1.
Water assessment
In this study, the heavy metal evaluation index (HEI) was used to assess the extent of HMs' pollution and water quality. The HEI index gives the total water quality with regard to the HMs using the following equation:
where Hc and Hmac represent the measured and the maximum permissible concentrations of the ith parameter, respectively (Prasanna et al. 2012). The saltwater quality requirements for aquatic life were used to calculate Hmac values.
Results and discussion
Physicochemical properties of water samples
Table 1 shows the mean values of the physicochemical properties of water samples.
Shrimp breeding is the dominant farming practice in the sampling area and this can be addressed as the main reason for the relatively high salinity (12.77 PSU) and alkalinity of Gomishan Wetland water samples. Basins with high agricultural or urban intensities have higher salinity and pH than the adjacent areas with different land use (Chessman and Townsend 2010). The Eh mean value revealed an aerobic condition in the studied wetland (Goncharuk et al. 2010).
Heavy metals concentrations
Table 2 shows the mean heavy metal concentrations in surface sediment samples. The mean contents of Cd, Cr, Co, Pb, Ni, Zn, V, and Mn were 0.2,36,5.5,5,23,44,53, and 357 mg·kg−1, respectively. The mean concentrations of Cd, Co, Pb, V, and Zn in Gomishan Wetland sediments were lower than in similar studies. The concentration of Mn in the current study was significantly higher than previously published levels for Queensland (Qiu et al. 2011). Cr and Ni also showed relatively lower concentrations in comparison with similar studies (see Table 2). Based on Table 2, the mean metal concentrations in this study are lower than those in the earth's crust. Overall, it is possible to conclude that the amount of studied metals in the surface sediments of the Gomishan Wetland is almost less than that of the other studies mentioned in Table 2.
The effect of voltage variations on the PEC process
Table 3 presents the effects of the PEC processes on pH, Eh, EC, and DO under different conditions. pH, Eh, EC, and DO ranged between 7.20–7.42, 208–487 mV, 4.70–6.24 mS/cm, and 8.30–9.88 mg/l, respectively.
Eh, DO and COD degradation increased with the increasing voltage in samples. This can be justified, because oxygen is an electron acceptor and plays an essential role in separating electron–hole pairs. Oxygen reacts with two electrons and two hydrogen atoms, reducing up to H2O2. The latter produces a hydroxide radical. Accordingly, Oxygen increases the oxidation rate and COD degradation(Wang et al. 2017). The oxidation efficiency could result from rising the redox potential (Ebraheim et al. 2021).
The photocatalytic process decreased COD values significantly by 82% from 1986 mg/l for a blank sample to 350 mg/l at the base potential of 7 V (Table 3), which is consistent with the results of (Upadhyay et al. 2019) and (Fernandes et al. 2019). Our results also demonstrated that the increase of anodic potential intensified the COD reduction, which is consistent with the findings of (Chimonyo et al. 2017). These authors found a linear relationship between redox potential and the rate of COD removal. The increase of the anodic potential accelerated the movement of electrons, separation of electron–hole pairs, production of oxidant on the electrodes, and the oxidizing potential of PEC (Ghimire et al. 2019) (see Table 3).
pH change affects the PEC process through the metal oxide's surface charge, adjusting the pollutant's adsorption capacity on the surface, electron–hole separation, and oxidation potential of the valence band (Tayyebi et al. 2019). As depicted in Table 3, after oxidation, pH decreased compared with its initial value (7.9), ranging from 7.20 to 7.42. Eh and EC ranged from 487 to 208 (mV) and 4.70 to 6.42 (mS/cm), respectively, indicating a linear relationship between pH and EC and a nonlinear relationship between pH and Eh (Ustaoğlu and Tepe 2019). The decrease in solution pH increased with the mobility of HMs (Hartley and Dickinson 2010).
Heavy metals' mobility under different Eh and pH values
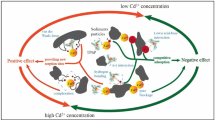
Figure 2 shows the release of selected HMs concerning Eh. As can be seen, the initial dissolved concentrations of Co, Cd, Cr, V, Mo, and Pb (Eh = 109 mV) were 3.2, < 1, 161.6, 44.9, 62.3, and 20 µg/l, respectively (Table 4). The increase in Eh value after PEC released the HMs from the sediment into the water (Fig. 2). This is in line with the result of (Hartley and Dickinson 2010). These authors found the increased mobility of trace metals and the decreased solution pH after drying and aeration of the canal sediment. However, our findings contradict the results of some others (Frémion et al. 2017; Ranjbar Jafarabadi et al. 2018; Madadi et al. 2021). The increase in mobility and release of metals from sediments can be due to the decrease in pH; the negative surface charge of colloids decreases with decreasing pH; thus, HMs desorb from organic matter and other sorbent surfaces, such as clay mineral surfaces (Shaheen and Rinklebe 2017). According to Fig. 2, the highest mobility of Co, Cd, Cr, and V occurred at Eh 369 mV with concentrations of 6.3, 2, 192.7, and 60.8 µg/l, respectively. The concentration of Pb increased after the oxidation, but it remained constant (40µg/l) at Eh values of 208, 235, and 487 mV. Mo had the highest dissolved concentrations (163.3 µg/l); before the Eh rise, it was 62.3 µg/L. The rise in the concentrations of dissolved HMs might attribute to the impact of Eh on metal species (Du Laing et al. 2009). The oxidation state of HMs species is a significant regulating factor in their behavior and bioavailability, since it directly affects the mobility, adsorption, and precipitation of HMs (Chakraborty et al. 2019).
Geochemical fractions of heavy metals
The chemical partitioning results showed that HMs exist in large quantities in the terrestrial phase (Fig. 3). Anthropogenic intrusions are responsible for the exchangeable and carbonate-bound portions. Contaminants from these fractions can quickly enter the water column, posing a severe threat to aquatic life (Saleem et al. 2018). The sulfide and organic matter (F4) were the dominant fractions of Cr (77.5%), V (77%), Mo and Co (76%), Pb (68.8%), and Cd (57%). A change in Eh might discharge the materials from F4 into the water, threatening water quality (Frémion et al. 2017). This reflects the metals' high affinity for sulfide and organic matter. Furthermore, the oxidizable fraction consists primarily of organic bind metals and sulfide-bind metals, with spatial differences in the main components of oxidizable fractions of different metals (Zhao et al. 2021). Metal components in F1, F2, F3, and F4 have a higher potential for mobilization from sediment and bioavailability to benthic organisms (Gao et al. 2010). Toxicity occurs when the rate of metal intake exceeds the rates of metal detoxification and excretion (Zaaboub et al. 2015).
The mean residual (F5) percentages of HMs’ in the Gomishan Wetland sediment were as follows: V (19.6%) > Cr (19.5%) > Co (19.3%) > Mo (19%) > Pb > (17%) > Cd (13.5%). In residual form, HMs are substantially less hazardous to aquatic organisms due to their chemical stability and biological inactivity (Wang et al. 2010). Mineralogy and weathering control the concentration of HMs in this phase (Shaila and Naotatsu 2010).
Indices of contamination
Pollution index (I poll)
The intensities of HMs' pollution index of Co, Cd, Cr, V, Mo, and Pb are shown in Table 5. To determine the contamination grades of HMs in the sediments of Gomishan Wetland, the findings were assessed based on the classification system presented in Table S1.
Ipoll values of HMs followed the order of Cd > Pb > Co > Mo > V > Cr before, and Cd > Pb > Mo > Co > Cr > V after oxidation. The Ipoll index values of all HMs were less than 0.5, indicating that the HMs were within the 'No pollution' range. Except for Cd, the amount of Ipoll of HMs decreased with increasing the Eh, which is consistent with the results of (Mohiuddin et al. 2012). According to their findings, Ipoll rose with decreasing Oxidation Reduction Potential (ORP) in winter as temperature decreased. Correspondingly, an increase in Eh from 109 to 487 mV increased Ipoll of Cd from 0.486 to 0.667, suggesting 'Low Cd pollution' (Table 5).
Enrichment factor
Table 6 shows the severity of sediments pollution in Gomishan Wetland based on the enrichment factor (EF). The EF values suggested depletion to the minimal enrichment for Cd, V, Co, Pb, Cr (< 2), and significant enrichment for Mo (5 < EF = 6.88 < 20). Mining, fertilization, the manufacture of alloys, biosolids, catalysts, coal, and petroleum combustion are all causes of excessive Mo emissions into the environment (McGrath et al. 2010; Zaborowska et al. 2016). Based on this, we concluded that the agricultural activities around the study area are the main causes of high Mo concentration.
Heavy metals evaluation index
Heavy metal evaluation index (HEI) calculates the mean concentrations of HMs in different water samples based on their concentration in aquatic water. The HEI values > 20 indicate poor water quality. In Gomishan Wetland, the minimum and maximum HEI values were 27.23 and 40.25, respectively, occurring at Eh 208 and 487 mV. Figure 4 depicts the dispersion of HEI values. The initial HEI value was 38.59, and it reached 27.23 with rising Eh from 109 to 208 mV. Furthermore, the HEI values were 33.76, 33.11, 31.30, and 40.25 at Eh values of 235, 369, 381, and 487mV, respectively. Although the behavior of HMs in aquatic environment depends on many factors, Eh might be considered as a major one (Fig. 4). Changes in HEI values attribute to the adsorption–desorption of HMs by the sediments. Absorption of HMs into the sediment can decrease the HEI value, whereas the release of HMs from the sediment into the water environment can increase the HEI value (Madadi et al. 2021).
HSC chemistry
The Eh–pH diagram displays the thermodynamic stability of HMs in the aqueous environment. The stability range often appears between two dotted lines. Values above this range indicate that the element is potentially toxic to the aqueous environment. Figure 5 reveals that Co, Cd, Cr, V, Mo, and Pb were all between the stability lines, posing no contamination or toxicity to the aqueous environment.
Figure 5 shows that, at low solution pH (0–4), the main existing forms of HMs were Co+2, Cd+2, Cr+2, V+2, and Pb+2. The positive TiO2 surface found it difficult to absorb these cations. As a consequence, it is unable to produce sufficient electrons and OH. While the pH changed from 6 to 12, TiOH was created, and the TiO2 surface became negatively charged, allowing for further adsorption. Co +2, Cr+2, Mo+2, and Pb+2 ions were oxidized to Co3O4, Co(OH)2, MoO2, MoO4(-2), Cr2O3, CrO4(-2), Cr (OH)4(-1), PbO, PbO2, Pb12O17, Pb4 (OH)4 (+ 4), and Pb6(OH)8(+ 4), respectively, at solution pH between 6 and 12. The predominant species of Cr, Cd, Mo, V, and Co were Cr2O3, Cd +2 ions, MoO4−2, HV10O23−5, Co +2, and Co3O4 in the aquatic environment. Black and colored dots demonstrate the HMs' species before and after the PEC process, respectively.
Conclusion
The behavior of HMs under dynamic redox circumstances could be used to assess the potential risk, determine the destiny of HMs, and develop effective environmental management methods for the restoration of contaminated sites. Our findings indicated that an increase in solution Eh will augment the release of sediments' HMs into the water, which might contribute to the pH reduction. The highest mobility of Co, Cd, Cr, and V occurred at Eh 369 mV. The concentration of Pb increased in water after the oxidation, but it remained constant (40µg/l) at Eh values of 208, 235, and 487 mV. The highest release of Mo occurred at Eh 487 mV.
Sediment samples of Gomishan Wetland were analyzed to evaluate the contamination level of five heavy metals (Cd, Co, Cr, Mo, V and Pb). The findings showed that EF and Ipoll values of the studied wetland were within no pollution range, meaning this wetland receives medium levels of anthropogenic contamination compared with background values. Although for Mo, the EF value 5 < EF < 20, indicating anthropogenic sources. Moreover, Tessier sequential extraction method revealed that terrestrial fractions were the main sources of sediments' HMs. Future research should document the factors (sediment size, microbial activity, organic matter, temperature, and salinity) influencing the release of sediments' HMs. In addition, further studies should include the role of radical OH under various electrical conductivity (EC) ranges along with the other voltages. In addition, the kind of organic matters in sediments should be analyzed.
Data availability
All data generated or used during the study are applicable if requested.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238. https://doi.org/10.1007/s10661-007-9678-2
Agnelli A, Ascher J, Corti G et al (2004) Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol Biochem 36:859–868. https://doi.org/10.1016/j.soilbio.2004.02.004
Bastami KD, Neyestani MR, Shemirani F et al (2015) Heavy metal pollution assessment in relation to sediment properties in the coastal sediments of the southern Caspian Sea. Mar Pollut Bull 92:237–243. https://doi.org/10.1016/j.marpolbul.2014.12.035
Bastami KD, Neyestani MR, Molamohyedin N et al (2018) Bioavailability, mobility, and origination of metals in sediments from Anzali Wetland, Caspian Sea. Mar Pollut Bull 136:22–32. https://doi.org/10.1016/j.marpolbul.2018.08.059
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:399–411. https://doi.org/10.1016/0012-821X(79)90049-9
Bundt M, Widmer F, Pesaro M et al (2001) Preferential flow paths: Biological “hot spots” in soils. Soil Biol Biochem 33:729–738. https://doi.org/10.1016/S0038-0717(00)00218-2
Calijuri ML, Da Fonseca SA, Moreira Neto RF, De Castro CI (2011) Evaluation of the ability of a natural wetland to remove heavy metals generated by runways and other paved areas from an airport complex in Brazil. Water Air Soil Pollut 219:319–327. https://doi.org/10.1007/s11270-010-0709-1
Caple C (1996) Parameters for monitoring anoxic environments. In: Preserving archaeological remains ‘in situ 113–122, proceedings of the conference of the 1st–3rd April, Museum of London Archaeology Service, London
Chakraborty P, Chakraborty S, Vudamala K et al (2016) Partitioning of metals in different binding phases of tropical estuarine sediments: importance of metal chemistry. Environ Sci Pollut Res 23:3450–3462. https://doi.org/10.1007/s11356-015-5475-6
Chakraborty P, Jayachandran S, Chakraborty S (2019) Chromium speciation in the sediments across the oxygen minimum zone, western continental margin of India. Geol J 54:1132–1140. https://doi.org/10.1002/gj.3214
Chessman BC, Townsend SA (2010) Differing effects of catchment land use on water chemistry explain contrasting behaviour of a diatom index in tropical northern and temperate southern Australia. Ecol Ind 10:620–626. https://doi.org/10.1016/j.ecolind.2009.10.006
Chimonyo W, Corin KC, Wiese JG, O’Connor CT (2017) Redox potential control during flotation of a sulphide mineral ore. Miner Eng 110:57–64. https://doi.org/10.1016/j.mineng.2017.04.011
Du Laing G, Rinklebe J, Vandecasteele B et al (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985. https://doi.org/10.1016/j.scitotenv.2008.07.025
Ebraheim G, Karbassi A, Mehrdadi N (2021) Chemical reducing conditions through the photo-assisted electrochemical process in the treatment of the urban rainwater. Int J Human Capital Urban Manag 6:209–224
El Nemr AM, El Sikaily A, Khaled A (2007) Total and leachable heavy metals in muddy and sandy sediments of Egyptian coast along Mediterranean Sea. Environ Monit Assess 129:151–168. https://doi.org/10.1007/s10661-006-9349-8
Fernandes A, Gągol M, Makoś P et al (2019) Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2)for degradation of volatile organic compounds. Sep Purif Technol 224:1–14. https://doi.org/10.1016/j.seppur.2019.05.012
Frémion F, Mourier B, Courtin-Nomade A et al (2017) Key parameters influencing metallic element mobility associated with sediments in a daily-managed reservoir. Sci Total Environ 605–606:666–676. https://doi.org/10.1016/j.scitotenv.2017.06.236
Gao Y, Baeyens W, De Galan S et al (2010) Mobility of radium and trace metals in sediments of the Winterbeek: Application of sequential extraction and DGT techniques. Environ Pollut 158:2439–2445. https://doi.org/10.1016/j.envpol.2010.03.022
Gao C, Lin Q, Bao K et al (2014) Historical variation and recent ecological risk of heavy metals in wetland sediments along Wusuli River, Northeast China. Environ Earth Sci 72:4345–4355. https://doi.org/10.1007/s12665-014-3334-2
Geng N, Bai Y, Pan S (2022) Research on heavy metal release with suspended sediment in Taihu Lake under hydrodynamic condition. Environ Sci Pollut Res 29:28588–28597. https://doi.org/10.1007/s11356-021-17666-1
Ghimire U, Jang M, Jung SP et al (2019) Electrochemical removal of ammonium nitrogen and cod of domestic wastewater using platinum coated titanium as an anode electrode. Energies 12:883. https://doi.org/10.3390/en12050883
Goncharuk VV, Bagrii VA, Mel’nik LA et al (2010) The use of redox potential in water treatment processes. J Water Chem Technol 32:1–9. https://doi.org/10.3103/S1063455X10010017
Gopinath A, Nair SM, Kumar NC et al (2010) A baseline study of trace metals in a coral reef sedimentary environment, Lakshadweep Archipelago. Environ Earth Sci 59:1245–1266. https://doi.org/10.1007/s12665-009-0113-6
Harikumar PS, Nasir UP, Mujeebu Rahman MP (2009) Distribution of heavy metals in the core sediments of a tropical wetland system. Int J Environ Sci Technol 6:225–232. https://doi.org/10.1007/BF03327626
Hartley W, Dickinson NM (2010) Exposure of an anoxic and contaminated canal sediment: mobility of metal(loid)s. Environ Pollut 158:649–657. https://doi.org/10.1016/j.envpol.2009.10.030
Karbassi AR, Heidari M (2015) An investigation on role of salinity, pH and DO on heavy metals elimination throughout estuarial mixture. Glob J Environ Sci Manag 1:41–46. https://doi.org/10.7508/gjesm.2015.01.004
Karbassi AR, Monavari SM, Nabi Bidhendi GR et al (2008) Metal pollution assessment of sediment and water in the Shur River. Environ Monit Assess 147:107–116
Li H, Wang Q, Shao M et al (2016) Fractionation of airborne particulate-bound elements in haze-fog episode and associated health risks in a megacity of southeast China. Environ Pollut 208:655–662. https://doi.org/10.1016/j.envpol.2015.10.042
Liang J, Liu J, Yuan X et al (2015) Spatial and temporal variation of heavy metal risk and source in sediments of Dongting Lake wetland, mid-south China. J Environ Sci Health Part A Toxic/hazard Substances and Environ Eng 50:100–108. https://doi.org/10.1080/10934529.2015.964636
Madadi R, Karbassi A, Saeedi M (2021) Release of heavy metals under pre-set redox potentials in Musa estuary sediments, northwestern of Persian Gulf. Mar Pollut Bull 168:112390. https://doi.org/10.1016/j.marpolbul.2021.112390
Madadi R, Saeedi M, Karbassi A (2022) Redox-induced mobilization of rare earth elements in sediments of the northwestern part of the Persian Gulf. Int J Environ Sci Technol 19:11037–11050. https://doi.org/10.1007/s13762-022-04322-5
McGrath SP, Micó C, Curdy R, Zhao FJ (2010) Predicting molybdenum toxicity to higher plants: Influence of soil properties. Environ Pollut 158:3085–3094. https://doi.org/10.1016/j.envpol.2010.06.030
Mohiuddin KM, Otomo K, Ogawa Y, Shikazono N (2012) Seasonal and spatial distribution of trace elements in the water and sediments of the Tsurumi River in Japan. Environ Monit Assess 184:265–279. https://doi.org/10.1007/s10661-011-1966-1
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Palleiro L, Patinha C, Rodríguez-Blanco ML et al (2016) Metal fractionation in topsoils and bed sediments in the Mero River rural basin: Bioavailability and relationship with soil and sediment properties. CATENA 144:34–44. https://doi.org/10.1016/j.catena.2016.04.019
Prasanna MV, Praveena SM, Chidambaram S, Nagarajan R, Elayaraja A (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67:1987–2001
Qiu Y-W, Yu K-F, Zhang G, Wang W-X (2011) Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of Hainan Island, China. J Hazard Mater 190:631–638
Ramachandra TV, Sudarshan PB, Mahesh MK, Vinay S (2018) Spatial patterns of heavy metal accumulation in sediments and macrophytes of Bellandur wetland, Bangalore. J Environ Manag 206:1204–1210
Ranjbar Jafarabadi A, Riyahi Bakhtiari A, Spanò N, Cappello T (2018) First report of geochemical fractionation distribution, bioavailability and risk assessment of potentially toxic inorganic elements in sediments of coral reef Islands of the Persian Gulf, Iran. Mar Pollut Bull 137:185–197. https://doi.org/10.1016/j.marpolbul.2018.09.052
Saleem M, Iqbal J, Akhter G, Shah MH (2018) Fractionation, bioavailability, contamination and environmental risk of heavy metals in the sediments from a freshwater reservoir, Pakistan. J Geochem Explor 184:199–208
Shaheen SM, Rinklebe J (2017) Sugar beet factory lime affects the mobilization of Cd Co, Cr, Cu, Mo, Ni, Pb, and Zn under dynamic redox conditions in a contaminated floodplain soil. J Environ Manag 186:253–260
Shaila S, Naotatsu S (2010) Fractionation profile and mobility pattern of trace metals in sediments of Nomi River, Tokyo, Japan. J Soil Sci Environ Management 1:1–14
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285
Tayyebi A, Soltani T, Lee B-K (2019) Effect of pH on photocatalytic and photoelectrochemical (PEC) properties of monoclinic bismuth vanadate. J Colloid Interface Sci 534:37–46
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Upadhyay GK, Rajput JK, Pathak TK et al (2019) Synthesis of ZnO: TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 160:154–163
Ustaoğlu F, Tepe Y (2019) Water quality and sediment contamination assessment of Pazarsuyu Stream, Turkey using multivariate statistical methods and pollution indicators. Int Soil Water Conserv Res 7:47–56
Wang S, Jia Y, Wang S et al (2010) Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, China. J Environ Sci 22:23–31
Wang D, Li Y, Puma GL et al (2017) Photoelectrochemical cell for simultaneous electricity generation and heavy metals recovery from wastewater. J Hazard Mater 323:681–689
Xia P, Ma L, Sun R et al (2020) Evaluation of potential ecological risk, possible sources and controlling factors of heavy metals in surface sediment of Caohai Wetland, China. Sci Total Environ 740:140231
Xiao H, Shahab A, Li J et al (2019) Distribution, ecological risk assessment and source identification of heavy metals in surface sediments of Huixian karst wetland, China. Ecotoxicol Environ Saf 185:109700
Yang Y, Chen F, Zhang L et al (2012) Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent shelf. Mar Pollut Bull 64:1947–1955
Yoo J-C, Lee C-D, Yang J-S, Baek K (2013) Extraction characteristics of heavy metals from marine sediments. Chem Eng J 228:688–699
Yu H, Zhang Z, Zhang L et al (2021) Improved Norfloxacin degradation by urea precipitation Ti/SnO2–Sb anode under photo-electro catalysis and kinetics investigation by BP-neural-network-physical modeling. J Clean Prod 280:124412
Zaaboub N, Martins MVA, Dhib A et al (2015) Accumulation of trace metals in sediments in a Mediterranean Lagoon: usefulness of metal sediment fractionation and elutriate toxicity assessment. Environ Pollut 207:226–237
Zaborowska M, Kucharski J, Wyszkowska J (2016) Biological activity of soil contaminated with cobalt, tin, and molybdenum. Environ Monit Assess 188:1–10
Zhang C, Shan B, Tang W et al (2017) Heavy metal concentrations and speciation in riverine sediments and the risks posed in three urban belts in the Haihe Basin. Ecotoxicol Environ Saf 139:263–271
Zhao S, Zhang B, Zhou N (2020) Effects of redox potential on the environmental behavior of nitrogen in riparian zones of West Dongting Lake Wetlands, China. Wetlands 40:1307–1316
Zhao M, Zhang S, Han H, Pan D (2021) Heavy metals in sediments of Yellow Sea and East China Sea: Chemical speciation, distribution, influence factor, and contamination. J Oceanol Limnol 39:1277–1292
Zhou J, Zhang M, Ji M et al (2020) Evaluation of heavy metals stability and phosphate mobility in the remediation of sediment by calcium nitrate. Water Environ Res 92:1017–1026
Acknowledgements
We would express our gratitude to the Graduate Faculty of Environment, University of Tehran, for providing us with the electro-flocculation laboratory to accomplish the experimental analyses of this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
FK, ARK, and TN investigation; FK and ARK data collection and calculations. FK initial draft preparation, TN and ARK review, and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
We wish to confirm that no known competing financial interests or personal relationships could have influenced this work and its outcome.
Ethical approval
All authors agree to participate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kachoueiyan, F., Karbassi, A. & Nasrabadi, T. Effect of electro-photocatalytic decomposition (mainly Eh–pH) on association of metals with sedimentary phases and their mobility in the wetland ecosystem. Environ Earth Sci 82, 437 (2023). https://doi.org/10.1007/s12665-023-11127-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-023-11127-3