Abstract
Concentrations of metals along with pH, potential redox (Eh), total dissolved solid (TDS), electrical conductivity (EC) and salinity were measured for sediments of different layers, pore and surface water in the Anzali wetland. Also, the diffusive flux of metals was calculated ad compared with the values in other aquatic bodies. The abundance of metals in sediments was Al > Fe > Ca > Ti > Mn > Zn > V > Cr > Ni > Cu > Pb > Co > As > Mo > Ag > Cd, while the pore-water results indicated a different concentration profile: Ca > Mn > Fe > Al > V > Mo > As > Ni > Cu > Co. Anzali wetland is classified within a high ecological risk. Pore-water toxicity test showed it is in non-polluted condition. Eh–pH diagrams showed that all species of elements are in a stable state in surface and pore-waters. However, Mn and Co (in some layers) are in soluble form and free ions and thus are bioavailable to the organisms. V and As are also present in the form of hydrogen compounds and Ni in the form of hydroxides, therefore, they are toxic to aquatic animals due to their solubility and ionic forms. Diffusive fluxes for As, Al, Fe, Ca, Cu, Mn, Mo, Ni and V are 0.43, 1.80, 48.52, 43,147.50, 0.12, 1.47, 0.15, 1.28, 0.21 µg/m2/day, respectively. Cluster analysis showed that in a solid phase of sediments, elements such as Cr, As and Ti are of petroleum and terrestrial origin. In pore-waters, Cu is of petroleum and biological origin, and Mo, As, Cu and Co are controlled by pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The industrial wastes and wastewaters which include metals in some countries are directly discharged into the river bodies. They eventually find their way into the wetlands, lakes, gulfs, and seas (Pourang et al. 2010; El Houssainy et al. 2020). These metals are either soluble in water or stick to suspended particles or gradually precipitate in the bed (Bastami et al. 2018). This has increased the probability of endangering animal and plant ecosystems (Li et al. 2007; Baramaki Yazdi et al. 2012; Pal and Maiti 2018; Volz et al. 2020; Sahu and Basti 2021), especially aquatic environments such as wetlands and lakes which don’t have any connection with open seas (Zhang et al. 2010).
It is vital to investigate and analysis of sediment properties (Bai et al. 2011; al Naggar et al. 2018; Ahn et al. 2020) and their relationship with surface waters to know about the health of aquatic environment (Cohen 2003; Laxmi Mohanta et al. 2020). It would be very informative to study data related to different periods if the sedimentation rate is known (Vaalgamaa and Conley 2008). The accumulation of contaminants, especially trace metals in sediments and the possibility of their release into the surface water column, brings out the need for detailed water–sediment interactions. Sediments in aquatic environments can act as a source through decomposition, dissolution and desorption and sink through precipitation and adsorption (Lerman 1979; Carling et al. 2013).
In geochemical studies of water zones, the role of pore-water cannot be ignored because diagenesis does not only occur in the solid phase, thus the fluid phase must also be considered (Schroeder et al. 2017). In this regard, measuring and determining the characteristics of pore-water and analyzing the results can provide a better view of the state of diagenesis and pollution of aquatic environment (Hammond 2001). For example, it must be determined at what depth and under what conditions metals are released from the solid phase into the pore-water, what are the predominant species of metal released into the pore-water, how much of them are transferred to the overlying waters and finally what effect do the released metals on the aquatic ecosystem?
Today, many wetlands have dried up and, in addition to disrupting plant and animal life, have damaged local employment (Jafari 2009). As a result of the drying up of the wetlands, their use has changed in some places, and these water bodies, which were once a place for waste disposal, are used for agriculture, and since its products are consumed by humans, it is necessary to evaluate the behavior of trace metals and pollution which they may take up.
Tang et al. (2016) investigated the metals in pore-water of Fuyang River sediments and found that the average concentrations of Cr, Ni, Cu, As, Zn and Pb were 17.06, 15.97, 20.93, 19.08, 43.72 and 0.56 μg/L, respectively. Also, the diffusive fluxes for Cr, Ni, Cu, As, Zn and Pb are − 0.37 to 3.17, 1.37 to 2.63, − 4.61 to 3.44, 0.17 to 6.02, − 180.26 to 7.51 and − 0.92 to − 0.29 μg/m2/day, respectively. The Nemerow index used in the pore-water toxicity test was found to range between 0.11 to 2.06 at different stations. Zhu et al. (2016) measured the concentrations of Cd, Cr, Cu, Ni, Pb and Zn in the pore-water of Ziya river sediments as 0.373, 57.1, 37.7, 20.4, 14.0 and 9.6 μg/L, respectively. The diffusion fluxes of Cd, Cr, Cu, Ni, Pb and Zn were estimated to be − 0.427 to 0.469, − 71.8 to 42.5, 3.16 to 86.6, 5.29 to 14, 7.24 to 19 and − 20.4 to 21.9 μg/m2/day, respectively. The Nemerow index for the pore-water toxicity test ranges from 0.759 to 1.05. Louriño-Cabana et al. (2011) used geo accumulation index and enrichment factor to investigate sediment contamination with metals. The geo accumulation index showed that Cd, Pb and Zn were not heavily contaminated, while Cu and Ni fall within moderate to severe contamination category. They also examined the toxicity of pore-water and examined the Nemerow index in different layers of the sediment core and obtained values ranging from 0.15 to 19.52. El Houssainy et al. (2020) investigated the trace metals in the coastal sediments of the Mediterranean sea and based on enrichment factor found the pattern of Cr < Cd < Hg < Cu < Pb < Ag. They also examined the relationship between sediment and pore-water and found that Fe and Mn are important in the mobility of metals from the solid to the fluid phase.
Anzali wetland is registered in Ramsar Convention. This wetland is very important from an ecological point of view because it is a spawning ground for many fishes and birds. Despite the importance of this marine body, in order to assess pollution state, several research through analysis of solid phase of sediment and benthic community (Pourang et al. 2010; Esmaeilzadeh et al. 2016a, b) have been done but the role of sediment pore-water is ignored. Therefore, the main goal of present research is to bring out the toxicity and diffusive flux of metals in the sediments and pore-water of Anzali wetland. The results of present work help to know the ecological risk within the wetland.
2 Materials and methods
2.1 Study area
Anzali wetland is located in the southwestern part of the Caspian Sea with an area of 193 km2, which dates back to the Pliocene or Holocene period (Rasta et al. 2021). This wetland was registered in 1975 in Ramsar Convention as an important international wetland. Anzali wetland is a eutrophic brackish wetland with shallow ponds and seasonal flood levels (Esmaeilzadeh et al. 2016b; Panahandeh et al. 2018). This wetland is very important from an ecological point of view because it is a spawning ground for many fishes and birds. This wetland can be divided into 4 parts: eastern, central, southern (Siahkeshim) and western or Abkenar. Discharging sewage from surrounding cities, various industries and agricultural wastes directly or indirectly through the rivers leading to the wetland has disrupted the animal and plant life of the wetland (Hassanzadeh et al. 2014; Panahandeh et al. 2018). Parts of Anzali wetland have now dried up and have been used as agricultural land for planting crops such as rice, etc., and other parts of the wetland are experiencing a decrease in depth and are drying up.
2.2 Sampling
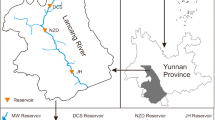
Sampling was carried out from the eastern part of Anzali wetland (Fig. 1) since it has the lowest sedimentation rate (which is 0.9 mm/year, utilizing Lead-210 method; Karbassi 2016) and thus such sediment core may provide useful information from the older years. For core sediment sampling, PVC pipe with an inner diameter of approximately 5 cm was used and a clean polypropylene container was utilized for sampling of surface water. The important point in sampling is to prevent contaminants from entering the core sediment, so the pipe was cut through the middle of the section and reconnected with Adhesive tape. So that the sample could be removed by peeling off the glue without cutting the pipe. Figure 2, depicts the steps of the research work.
Sampling station located in the eastern part of Anzali wetland (with the help of website: www.maphill.com) (2022)
The length of the core sediment was 60 cm that was divided into 5 equal parts of 12 cm. The pieces were labeled as the first to the fifth layers from the surface to the depth and were transferred to the marked containers. To preserve any probable change in sample parameters, the samples were kept in containers immediately inside the icebox without any access to oxygen and sunlight until the time of analysis in the laboratory.
2.3 Sediment analysis
The sediment samples were centrifuged for 30 min at 10,000 rpm (US Environmental Protection Agency 2001) and therefore the pore-water was separated from the sediment. Subsequently, 10 g of sediment samples were mixed with 20 g of surface water of Anzali wetland and kept for 24 h. Finally, the parameters such as Eh, pH, TDS, EC and salinity of the sediments were measured by a portable multi-parameter probe.
The rest of the sediment samples were dried and passed through a 63-micron sieve and then pulverized and prepared for bulk concentration analysis using strong acids such as HF, aqua regia and HClO4. For this purpose, acetic acid (Simpson et al. 2005) was used at pH = 7 (which is one unit lower than the pH of Anzali wetland water) to release the very loose bonded metals from sedimentary phase. Concentrations of major metals (Fe, Al, Ca, Mn, and Ti) and trace elements (Cr, Co, Ni, Cu, Zn, As, Pb, Cd, V and Ag) were measured by inductively coupled plasma mass spectrometry (ICP-MS 7500ce Zarazma Co., possessing ISO-17025). A standard sediment sample (MESS-1) was used to check the accuracy (better than ± 5%) of the analysis.
2.4 Water analysis
Immediately after separating the water from the sediments, the parameters Eh, pH, TDS, EC and salinity of the pore and surface water samples were measured by a portable multi-parameter probe. The water samples then passed through a 45 µm filter and reduced to less than 2 mL by extra pure nitric acid to prevent the changes in the form and distribution of metals through different phases. Subsequently, ICP-MS 7500ce device was used to determine the concentrations of major metals (Fe, Al, Ca, Mn, Ti) and trace elements (Cr, Co, Ni, Cu, Zn, As, Pb, Cd, V, and Ag). It should be noted that the total hardness of the samples was measured by ion chromatography (model Metrohm 930 compact Flex).
2.5 Sediment toxicity tests
2.5.1 Geo accumulation index (Igeo)
The geo accumulation index was introduced to compare the current concentration of metals with the concentration before industrialization and to evaluate the severity of pollution, which was calculated according to Eq. (1):
where Cn is the present concentration of metal in sediments, Bn is the concentration of metal in shale and 1.5 is the correction factor for the concentration of metals in shale (Müller 1981). The concentration of metals in shale is listed in Table 1. Sediment quality status according to the modified geo accumulation index (introduced by Qingjie et al. 2008) for values less than 0.42, between 0.42 to 1.42, between 1.42 to 3.42, between 3.42 to 4.42 and for values greater than 4.42, classified as unpolluted, low pollution, moderate pollution, strongly pollution and extremely pollution, respectively.
2.5.2 Enrichment factor (EF)
The enrichment factor is a simple but effective tool for evaluating anthropogenic effects based on comparing the ratio of the current concentration of metal in the Earth's crust to its background level. The enrichment factor is calculated through Eq. (2):
where (Cn/CFe)sample is the ratio of the present concentration of the metal to the concentration of iron, (Cn/CFe)crust is the ratio of the concentration of the metal to the element iron in the earth's crust. The concentration of metals in the earth's crust is listed in Table 1. Enrichment factor classification is as follows: for values less than 2, between 2 to 5, between 5 to 20, between 20 to 40 and for values greater than 40, respectively, Depletion to minimal enrichment, moderate enrichment, significant enrichment, very high enrichment and Extremely enrichment is considered (Sutherland 2000).
2.5.3 Modified hazard quotient (mHQ)
This index is a tool to assess the degree of danger of each metal to the aquatic environment and living organisms, which is based on the distribution of adverse ecological effects for the threshold levels and was calculated according to Eq. (3):
where Ci is the concentration of the target metal, TEL is the threshold effect level, PEL is the probable effect level, and SEL is the severe effect level. Threshold values are listed in Table 2. Degree of risk according to the modified risk index for values less than 0.5, between 0.5 to 1, between 1 to 1.5, between 1.5 to 2, between 2 to 2.5, between 2.5 to 3, between 3 to 3.5 and for larger values greater than 3.5, respectively, nil to very low, low, moderate, considerable, high, very high and extreme are considered (Benson et al. 2018).
2.5.4 Toxic risk index (TRI)
Toxicity risk index is a multi-element index that has been developed based on the threshold effect level and the probable effect level. To use this index, Eq. (4) was used:
In the classification of TRI index, values less than 5, between 5 to 10, between 10 to 15, between 15 to 20 and values greater than 20 are considered as no toxic risk, low toxic risk, moderate toxic risk, considerable toxic risk and very high toxic risk, respectively (Zhang et al. 2016).
2.5.5 Mean ERM quotient (MERMQ)
This multi-element index was used to express the pollution status of several metals using Eq. (5):
where Ci is the metal concentration, ERM is the average effect range for each metal and n is the number of elements. ERM values for each metal are listed in Table 3. According to the classification of this index, risk levels are divided into four categories: for values less than 0.1, between 0.1 to 0.5, between 0.5 to1.5, And is greater than 1.5 low, medium, high and very high risk level, as well as 9%, 21%, 49% and 76% probability of toxicity, respectively were considered (Carr et al. 1996; Long and MacDonald 1998).
2.5.6 Potential ecological risk index (RI)
The potential ecological risk index was introduced by Hakanson to assess metal pollution and to determine the ecological risk that metals in sediments pose to aquatic. This index calculated using Eqs. (6) and (7):
In these equations, Tr is the toxicity factor for each metal, C0 is the current concentration of each metal and Cn is the concentration of the natural part of the metal. Toxicity factor values for each metal are listed in Table 4 and the background of the studied metal is also listed in Table 5. According to the classification of this index, values less than 150, between 150 to 300, between 300 to 600 and above 600 were considered low, moderate, considerable and very high risk, respectively (Hakanson 1980).
2.6 Pore waters toxicity test
To evaluate the level of pore-water quality, the interstitial water criteria toxic unit was used, which described as:
where [Me]i.w. The concentration of total metal and FCVMe is the final critical value depending on the hardness of each metal (Louriño-Cabana et al. 2011), which was calculated for each element according to Table 6. The total water quality of the pore-water was estimated using Nemerow index (NI), which is calculated according to Eq. (9). The classification of this index is as follows: for values less than 1, between 1 to 2, between 2 to 3, between 3 to 5 and greater than 5, respectively no impact, slight impact, moderate impact, strong impact and serious impact were considered (Liu et al. 2003).
2.7 Diffusive fluxes
The quantitative diffusive fluxes of the metals from sediments to the water column were calculated by using the concentration gradient based on Fick's first law (Schulz and Zabel 2006):
where J is the diffusive flux (µg/m2/day), φ is the porosity, Dsed is the diffusion coefficient (cm2/s) and (∂C/∂X)x=0 the concentration gradient in the boundary between water and sediment (µg/L/cm). The diffusion coefficient was obtained using Eq. (11) (Schulz and Zabel 2006):
where θ is the degree of deviation around the particles (tortuosity) and Dsw is the diffusion coefficient of the solution in seawater, the values of which for each metal are taken from the research of Yuan-Hui and Gregory (1974). Torsion was also calculated in terms of porosity from Eq. (12) (Boudreau 1997) and porosity from Eq. (13) (Berner 1980).
where Viw is the volume of interstitial water and Vs is the total volume of dry sediment.
2.8 Speciation of metals
Chemically trace metals are found in different species in the aquatic environment and the effects they have on the environment vary depending on their chemical forms (Alabdeh et al. 2020; Ebraheim et al. 2021). Therefore, to understand the geochemical and toxicological effects of the environment, it is not only necessary to measure the concentration of metals but also their species must be identified. One way to identify metal species in the aquatic environment is to use Eh–pH diagrams. These diagrams can display the area of thermodynamic stability of water and provide conditions for the study of metal hazards (Huang 2016). The stability zone of the metals in these diagrams is shown between the two lines, and outside this range, the metals are unstable and may be toxic in the aquatic environment.
In present investigation, HSC Chemistry software version 9.5.1 was used to prepare Eh–pH diagrams. The diagrams were plotted by entering data on temperature, redox potential, pH, atmospheric pressure, and concentration within the HSC program.
2.9 Cluster analysis
Pearson coefficient and cluster analysis were used to find correlations between metals and parameters, as well as similarities in the behavior and origin of metals in sediments and pore-water. The software used to calculate the Pearson coefficient was SPSS version 26 and cluster analysis was performed by multivariate statistical package (MVSP) software by a weighted pair group (WPG) clustering technique. In this technique, a linear correlation coefficient is used to measure the similarity and its ability to define clustering tendencies between different samples.
3 Results and discussion
3.1 Distribution of metals and diagenetic processes
The profiles of metals concentration in core sediment were not the same for all metals, but it can be stated that the average abundance of metals in sediments of different layers was Al > Fe > Ca > Ti > Mn > Zn > V > Cr > Ni > Cu > Pb > Co > As > Mo > Ag > Cd. As shown in Figs. 3, 4, 5 and 6, the order of abundance of metals on average in the pore-waters of different layers, illustrated a different trend from metal concentrations in sediments. Generally, the concentrations of Ti, Zn, Cr, Pb and Cd are almost negligible in all pore-waters' layers, indicating no release of sediment in the liquid phase. The other metals in pore-waters follow the trend of Ca > Mn > Fe > Al > V > Mo > As > Ni > Cu > Co. In other words, the trend of metal concentration in pore-water is not dependent on the concentration of metals in sediment core. The color of each layer of the core sediment is demonstrated in Figs. 3 and 4 (the cylinder which is located next to the vertical axis is the schematic form of the core which its color is altered with depth). The down sediment core color change from surface to depth (dark brown, black, and gray, respectively) confirms the prevailing anoxic conditions at the bottom of sediment core.
Ca in sediments and pore-water had a non-uniform downward trend and its highest (27,056 mg/kg in sediments and 531 mg/L in pore-water) and lowest (18,410 mg/kg in sediments and 112 mg/L in pore-water) concentrations belonged to the first and third layers, respectively. Mn in sediments had a uniform decreasing trend in depth and declined significantly, especially from the second layer onwards. But in water, its trend was completely different so that in the first layer its concentration was 0.02 mg/L but in the second layer, it reached 12.67 mg/L and then diminished with depth. Fe had a non-uniform increasing trend in sediments and pore-water with depth and its highest concentration was in sediments and pore-water in the fifth layer (54,826 mg/kg in sediments and 0.97 mg/L in pore-water) and the lowest (49,257 mg/kg in sediments and 0.79 mg/L in pore-water) concentration was in the sediments of the first layer and in the pore-water of the third layer. Al increased irregularly in sediments with depth and had the lowest (79,588 mg/kg) and highest (93,699 mg/kg) concentrations in the first and fifth layers, respectively. In pore-water, the concentration of Al in each layer enhanced compared to the upper layers except in the fifth layer and the highest release volume was observed in the fourth layer where whose concentration in sediment decreased. V increased in sediments unevenly with deep and grows uniformly in pore-water. The lowest and highest concentrations of V belonged to the first (116 mg/kg in sediments and 4.03 µg/L in pore-water) and fifth (140 mg/kg in sediments and 89.98 µg/L in pore-water) layers in both sediments and pore-water, respectively. The concentration of Mo in all sediment layers except in the fourth layer was the same and was equal to about 1 mg/kg. However, in pore-water, it had a different trend and its amount grew from the surface to the third layer, but then diminished. The fact that the concentration of Mo in some layers of pore-water was different, while its concentration was the same in sediments, indicates that factors other than increasing or decreasing the concentration in sediment were involved in the release of Mo in pore-water. As enhanced in sediments with depth except in the fourth layer, but in pore-water its trend was different, so that in the first layer it was 7.57 µg/L, but in the second layer it was so small that the ICP device could not measure it. But in the third layer, its concentration reached 30 µg/L. In the fourth layer, 10 units have been reduced compared to the previous layer, but in the fifth layer, the concentration again reached 30 µg/L. The trend of Ni concentration in sediments and pore-water was completely opposite to each other, so that in sediments the concentration grew from the surface to the third layer, reduced in the fourth layer and enhanced in the fifth layer compared to the fourth layer. The Ni concentration was constantly declining up to the third layer, increasing in the fourth layer and decreasing in the fifth layer compared to the fourth layer. Cu in sediments fluctuates in depth between 54 and 63 mg/kg and it did not show a definite trend, but in pore-water, its general trend could be described as downward increase. Co in sediments had an upward trend from the surface to the third layer, but in the fourth layer it dropped significantly, but it grows in the fifth layer where highest amount of Co is observed. Also, Co concentration rises non-uniformly in pore-water with depth and the lowest (1.11 µg/L) and highest (1.74 µg/L) concentrations belonged to the first and fifth layers, respectively. Ti concentration in sediments has an increasing trend in depth except for the fourth layer. Zn in sediments had a uniform downward decreasing trend from the surface to the fourth layer, but it suddenly increases in the fifth layer. The highest (160 mg/kg) and lowest (114 mg/kg) Zn concentrations belonged to the first and fourth layers, respectively. The lowest (113 mg/kg) and highest (131 mg/kg) concentrations of Cr in sediments were observed in the first and second layers, respectively, and in other layers the amount of concentration fluctuated between the above cited ranges. Pb in sediments had a decreasing trend from the surface to the third layer and had constant values from the third layer to the bottom of core. Cd in the first layer of core sediment had the highest value at 0.5 mg/kg and then decrease. The concentration of Ag in the first two layers was 0.7 mg/kg, in the next two layers it was 0.4 mg/kg and in the last layer, it slightly rose to 0.5 mg/kg.
3.2 Sediment toxicity assessment
The results of geo accumulation indices and enrichment factor are presented in Table 7. According to the results of geo accumulation index, Mn in the first two layers and Ag in all layers caused moderate pollution. Pb in all core sediment layers except the third layer falls within low pollution class and other metals did not have a detrimental effect on the wetland environment. According to the results of the enrichment factor, Cd in the first layer, Mn and Pb and As in all layers except the surface layer had little enrichment and the share of other metals was negligible. In Table 8, the results of the modified hazard quotient in different layers showed that Cd in the surface layer falls within low risk, in the second layer the risk was very low and then up to the fifth layer is risk free. As caused a moderate risk in the first, second and fourth layers and a considerable risk in the third and fifth layers. Ni had a considerable risk in the first, second and fourth layers and a high severity risk in the third and fifth layers. Pb exhibits moderate risk in the surface layer, and low risk in other layers. Cu had a moderate risk in all layers. Cr caused a low risk in the first layer but a high severity risk in the other layers. Zn had a low risk in all layers.
Table 9 lists the results of multi-element indices, which indicate that in terms of toxicity risk index, the fourth layer had a low risk and other layers had a moderate risk. The mean ERM quotient showed the medium risk level for all layers. The potential ecological risk index for the first and second layers was moderate, but for the other layers it was considerable.
3.3 Pore-water toxicity assessment
The IWCTU of Cd, Cu, Ni, Pb and Zn in the pore-water of all sediment layers are computed (Table 10). It is evident that the results of Nemerow index for all layers are less than 1 and thus they don’t impose adverse impact on the eastern part of Anzali wetland.
3.4 Diffusive fluxes from sediments to overlying water
The diffusive fluxes to calculate the release of metals and their transfer from sediments to the water column in Anzali wetland have been calculated and the results are listed in Table 11. The highest diffusion rate was 43,147.5 μg/m2/day and the fluxes of Fe, Al, Mn, Ni, As, V, Mo, Cu and Co were 48.52, 1.8, 1.47, 1.28, 0.43, 0.21, 0.15 and 0.08 μg/m2/day, respectively. Comparing the obtained values with other studies, it can be seen that the diffusive fluxes of Mn, V and As were relatively low and Fe, Ni, Cu and Co were not high compared to other aquatic bodies.
3.5 Speciation of dissolved metals
The parameters of water and sediment samples are listed in Tables 12 and 13, respectively. The chemical forms of metals for various pore-water layers and surface water were identified using Eh, pH, temperature, concentration and pressure values As displayed in the Figs. 7, 8, 9 and 10, all the marked points were between the two dotted lines and indicate that all metals were in a stable range.
Al in the water of the wetland was in the form of hydroxide and soluble (Al3(OH)4+5), which is of considerable biological importance and is toxic to organisms (ALabdeh et al. 2020). Fe is present in the water of the wetland in the form of magnetite (Fe3O4), which is in the oxidized form of Fe and in a colloidal or suspended state, and thus causes less peril to aquatic species than the free ion form. In Anzali wetland, Ca appeared in the form of hydroxides and ionic (CaOH+), which due to its solution property is toxic and available to organisms. Mn existed in surface water and the first and second layers of pore-water in the form of free ion (Mn+2), and because it was highly concentrated in the second layer of pore-water, it can be very toxic with high bioavailability. This form of Mn is available to plants and can be easily transported into root and branches cells, where it eventually accumulates (Marschner 1995). In the lower layers, Mn was present in the form of hydroxide (Mn(OH)2) in lower concentrations and in a colloidal or suspended state, which causes less damage to aquatic life. V appeared in combination with hydrogen (HV10O23−5) in the water of the wetland and, due to its ionic and soluble nature, its toxicity was considered high. Cu existed in the wetland in elemental form (Cu), sediment and insoluble, so its toxicity was relatively low. Ni in Anzali wetland was in the form of hydroxide (Ni2OH+3) and soluble that has high bioavailability and is toxic to organisms. Co in the pore-water of the first layer of wetland sediments was present in the form of free ions (Co+2), so it might be toxic with high bioavailability. But in other samples, it was found in the form of hydroxide (Co4(OH)4+4), which is less dangerous than its free ion, but is still available to organisms because it is soluble and ionic. As species in surface water and pore-water, except the first layer, was arsenate (HAsO4−2), which has less mobility and toxicity than the arsenite (As+3). However, in the first layer of pore-water, As existed in the form of an insoluble oxide (As2O3) and has appeared in the form of colloid or suspended, which has less toxicity for aquatic life. Mo is seen as soluble and ionic oxide (Mo7O24−6, MoO4−2) ones in all layers, thus it might be bioavailable and toxic to aquatic animals.
3.6 Cluster analysis
Correlation coefficient analysis (Table 14) along with cluster analysis (Fig. 11) after normalizing the data by SPSS for different layers of core sediments were done and as dendrogram depicts As, Ti, V, Ni, Fe, Al, and Cr are placed in one cluster (A), plus, there is a strong correlation between them. If V and Ni were considered as an indicator of oil pollution (Esmaeilzadeh et al. 2016a) and on the other hand Fe and Al as a lithogenic indicator (Vaezi et al. 2015), it might be concluded that Cr, As and Ti were partially derived from oil pollution and partly originated from the Earth’s crust. Zn, Cd, Pb, Ag and Mn are placed in cluster “B” and there is a high correlation coefficient between them so they have similar behavior in the marine environment of the Anzali wetland. As cluster “C”, shows there is a significant correlation between pH, Co, and Cu, it may be concluded that the behavior of mentioned elements is affected by pH. pH plays a crucial role in desorption and adsorption of the metals in sediment and in this research it has the non-uniform trend same as most of the metals in the core. However, as its lowest value is more than 6.5, so it seems other factors are effective too on release of metals and it does not depend only on pH value (Peng et al. 2009). It should be noted that pH itself is under control of the geology of area of study (Karbassi et al. 2018). Since there is not any considerable correlation between other parameters such as salinity, EC, TDS, Eh, Mo, and Ca which form clusters “D” and “E” it can not be found any significant relationship with other clusters.
Pursuant to Table 15, Pearson coefficient and dendrogram drawn in Fig. 12 which were drown after normalizing the data by SPSS, for the pore-water at different core sediment layers, Cu, Ca and Ni and Eh are placed in cluster “A” and there is a high similarity coefficient between them. Since Ca might be considered as a biological index, so Cu in pore-waters of Anzali wetland could be originated from oil and biological origin. In cluster "B", there is a considerable correlation between pH, Mo, As, and Co so it might be concluded that the pH has effect on behavior of mentioned elements. Cluster “C”, shows that behavior of some elements such as V and Fe is influenced by EC, TDS and salinity. As cluster “D” indicates that Mn doesn’t have any significant correlation with other metals or parameters it is not possible to get any valuable information about its behavior.
4 Conclusions
In the present research, behavior of trace metals in different core sediment layers in solid and liquid phases were investigated. For this purposed by performing various analysis was carried out on sediment core, pore-water and bottom water of Anzali wetland. The results of TRI multi-element toxicity test, showed that in all layers except the fourth layer, the rest of the layers were classified in the moderate pollution class. The MERMQ index, which is based on the range of effects, indicated all layers in the medium pollution class. However, the RI depicted that the first and second layers were moderate but 3rd to 5th layers had a high ecological risk. The results of pore-water toxicity test illustrated that all layers had no pollution impact.
Moreover, the status and properties of the metals were also studied separately. The results indicated that Al had the largest share in sediments but was released in relatively small amounts in pore-water and also its diffusive flux was insignificant. Al in the water of Anzali wetland was in the form of hydroxide, soluble and bioavailable, which is hazardous for organisms. Fe in the pore and surface water of Anzali wetland was in the form of magnetite, which is the oxidized state of Fe and was present in a colloidal or suspended state, which is not dangerous for aquatic animals when compared with free ions forms. Also, its diffusive flux was not very large compared to other water zones. Ca was present in the water of Anzali wetland in the form of hydroxides and ionic so it is available to organisms due to its solubility and had toxic properties. In addition, its diffusive flux was significant compared to other elements. Though Ti is present in high concentration in the sediments, but according to EF and Igeo indices, it does not cause any contamination. The rate of release of Ti in surface and pore-waters was almost zero and its concentration in water samples was too small that the ICP device was not able to detect it. Igeo and EF indices indicated that Mn in some layers caused pollution. Mn is significantly released in the second and third layers of the pore-water. Mn is present in the form of soluble free ion in surface water and the first and second layers of pore-water, which is indicative high bioavailability and high toxicity to animals and plants. Igeo and EF indices depicted that Zn did not cause any contamination and enrichment and, in terms of mHQ index in all layers, had low risk. Zn in the water had no release and its concentration was negligible. V in sediments had no contamination and enrichment effect. V was in the form of a hydrogen compound in water and had high toxicity due to its ionic and soluble nature, but its risk is reduced due to its low diffusive flux. Cr was evaluated pursuant to Igeo and EF indices without contamination and enrichment, and mHQ described this metal as low risk for the first layer and high risk for the other layers. Cr had no release in water and was of terrestrial and oil pollution origin in sediments. Igeo and EF indices showed the Ni did not cause any contamination and enrichment, but according to the mHQ index, it had a significant risk in the first, second and fourth layers and a high risk for the third and fifth layers. The diffusive flux of Ni in Anzali wetland was 1.276 μg/m2/day. Ni in the water of Anzali wetland was in the form of hydroxide and soluble with high bioavailability and toxicity to aquatic animals. Cu in the water of the wetland was in elemental form, and is not considered a danger to Anzali wetland because it has low toxicity. Pb caused low pollution according to the Igeo index in all layers except in the third layer and in terms of EF index in all layers had low enrichment. Also, in terms of mHQ index in the first layer had a moderate risk and in other layers had a low risk. The results of Igeo and EF indices illustrated that Co did not cause any contamination in the sediments and its diffusive flux was 0.084 μg/m2/day and in the first layer of pore-water it was in the form of free ion which is the most dangerous form for aquatic life and also it has high bioavailability. Co is governed by pH changes in sediments (not completely) and pore-water. As was released in small amounts from the solid phase in the second layer of pore-water and the concentration in this layer was negligible. As, pursuant to the EF index in the second to fifth layers, had moderate enrichment. Additionally, according to the mHQ index, in the first, second and fourth layers, there was a moderate risk and in the third and fifth layers, there was a significant risk. As in surface water and pore-waters of the second to fifth layers was in the form of arsenate (As+5), which has low mobility and toxicity. In the first layer of pore-water was also appeared in the form of insoluble oxide, which is less toxic to aquatic life. Mo in the second to fifth layers of pore-water had a concentration of more than 10 μg/L. Its diffusive flux was 0.148 μg/m2/day and in the water of Anzali wetland existed in the form of soluble oxide and ionic, which is toxic and bioavailable to aquatic life. Ag shows moderate pollution in all sediment layers based on Igeo. According to the Igeo index, Cd did not cause any pollution and EF showed low enrichment only in the first layer. Also mHQ index indicated that Cd had a risk in the first two layers.
References
Ahn JM, Kim S, Kim YS (2020) Selection of priority management of rivers by assessing heavy metal pollution and ecological risk of surface sediments. Environ Geochem Health 42:1657–1669. https://doi.org/10.1007/s10653-019-00284-9
al Naggar Y, Khalil MS, Ghorab MA (2018) Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Access J Toxicol. https://doi.org/10.19080/oajt.2018.03.555603
Alabdeh D, Karbassi AR, Omidvar B, Sarang A (2020) Speciation of metals and metalloids in Anzali wetland, Iran. Int J Environ Sci Technol 17:1411–1424. https://doi.org/10.1007/s13762-019-02471-8
Bai J, Cui B, Chen B et al (2011) Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol Model 222:301–306. https://doi.org/10.1016/j.ecolmodel.2009.12.002
Baramaki Yazdi R, Ebrahimpour M, Mansouri B et al (2012) Contamination of metals in tissues of Ctenopharyngodon idella and Perca fluviatilis, from Anzali wetland, Iran. Bull Environ Contam Toxicol 89:831–835. https://doi.org/10.1007/s00128-012-0795-4
Bastami KD, Neyestani MR, Molamohyedin N et al (2018) Bioavailability, mobility, and origination of metals in sediments from Anzali wetland, Caspian Sea. Mar Pollut Bull 136:22–32. https://doi.org/10.1016/j.marpolbul.2018.08.059
Benson NU, Adedapo AE, Fred-Ahmadu OH et al (2018) New ecological risk indices for evaluating heavy metals contamination in aquatic sediment: a case study of the Gulf of Guinea. Reg Stud Mar Sci 18:44–56. https://doi.org/10.1016/j.rsma.2018.01.004
Berelson W, McManus J, Coale K et al (2003) A time series of benthic flux measurements from Monterey Bay, CA. Cont Shelf Res 23:457–481. https://doi.org/10.1016/S0278-4343(03)00009-8
Boudreau BP (1997) Diagenetic models and their implementation: modelling transport and reactions in aquatic sediments. Springer, Berlin
Bowen H (1979) Environmental chemistry of the elements. Academic Press, London
Carling GT, Richards DC, Hoven H et al (2013) Relationships of surface water, pore water, and sediment chemistry in wetlands adjacent to Great Salt Lake, Utah, and potential impacts on plant community health. Sci Total Environ 443:798–811. https://doi.org/10.1016/j.scitotenv.2012.11.063
Carr RS, Long ER, Windom HL et al (1996) Sediment quality assessment studies of Tampa Bay, Florida. Environ Toxicol Chem 15:1218–1231. https://doi.org/10.1002/etc.5620150730
Cohen AS (2003) Paleolimnology: the history and evolution of lake systems. Oxford University Press Inc, New York
Ebraheim G, Karbassi A, Mehrdadi N (2021) The thermodynamic stability, potential toxicity, and speciation of metals and metalloids in Tehran runoff, Iran. Environ Geochem Health 43:4719–4740. https://doi.org/10.1007/s10653-021-00966-3
El Houssainy A, Abi-Ghanem C, Dang DH et al (2020) Distribution and diagenesis of trace metals in marine sediments of a coastal Mediterranean area: St-Georges Bay (Lebanon). Mar Pollut Bull 155:111066
Esmaeilzadeh M, Karbassi A, Moattar F (2016a) Assessment of metal pollution in the Anzali wetland sediments using chemical partitioning method and pollution indices. Acta Oceanol Sin 35:28–36
Esmaeilzadeh M, Karbassi A, Moattar F (2016b) Heavy metals in sediments and their bioaccumulation in Phragmites australis in the Anzali wetland of Iran. Chin J Oceanol Limnol 34:810–820. https://doi.org/10.1007/s00343-016-5128-8
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hammond D (2001) Pore water chemistry. Encycl Ocean Sci 2263–2271
Hansen DJ, Ditoro DM, Berry WJ et al (2005) Procedures for the derivation of equilibrium partitioning sediment benchmarks (ESBs) for the protection of benthic organisms: metal mixtures (cadmium, copper, lead, nickel, silver and zinc)
Hassanzadeh N, Esmaili Sari A, Khodabandeh S, Bahramifar N (2014) Occurrence and distribution of two phthalate esters in the sediments of the Anzali wetlands on the coast of the Caspian Sea (Iran). Mar Pollut Bull 89:128–135. https://doi.org/10.1016/j.marpolbul.2014.10.017
Huang HH (2016) The Eh–pH diagram and its advances. Metals (basel). https://doi.org/10.3390/met6010023
Jafari N (2009) Ecological integrity of wetland, their functions and sustainable use. J Ecol Nat Environ 1(3):045–054
Karbassi A (2016) Investigation of sediment composition at standard surface and depth. Rasht
Karbassi AR, Tajziehchi S, Khoshghalb H (2018) Speciation of heavy metals in coastal water of Qeshm Island in the Persian Gulf. Glob J Environ Sci Manag 4:91–98. https://doi.org/10.22034/gjesm.2018.04.01.009
Laxmi Mohanta V, Naz A, Kumar Mishra B (2020) Distribution of heavy metals in the water, sediments, and fishes from Damodar river basin at steel city, India: a probabilistic risk assessment. Hum Ecol Risk Assess 26:406–429. https://doi.org/10.1080/10807039.2018.1511968
Lerman A (1979) Geochemical processes. Water and sediment environments. John Wiley and Sons, Inc., New York
Li QS, Wu ZF, Chu B et al (2007) Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ Pollut 149:158–164. https://doi.org/10.1016/j.envpol.2007.01.006
Liu WX, Coveney RM, Chen JL (2003) Environmental quality assessment on a river system polluted by mining activities. Appl Geochem 18:749–764
Long ER, MacDonald DD (1998) Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Hum Ecol Risk Assess 4:1019–1039. https://doi.org/10.1080/10807039891284956
Long ER, MacDonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97
Louriño-Cabana B, Lesven L, Charriau A et al (2011) Potential risks of metal toxicity in contaminated sediments of Deûle river in Northern France. J Hazard Mater 186:2129–2137. https://doi.org/10.1016/j.jhazmat.2010.12.124
Marschner H (1995) Mineral nutrition of higher plants, second
Masoumi F (2018) Effect of Eh/pH on heavy metals behavior in core sediments of Anzali wetland. University of Tehran
Müller G (1981) Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflusse: eine Bestandsaufnahme. Chem Ztg 105:157–164
Ni Z, Zhang L, Yu S et al (2017) The porewater nutrient and heavy metal characteristics in sediment cores and their benthic fluxes in Daya Bay, South China. Mar Pollut Bull 124:547–554. https://doi.org/10.1016/j.marpolbul.2017.07.069
Pal D, Maiti SK (2018) Seasonal variation of heavy metals in water, sediment, and highly consumed cultured fish (Labeo rohita and Labeo bata) and potential health risk assessment in aquaculture pond of the coal city, Dhanbad (India). Environ Sci Pollut Res 25:12464–12480. https://doi.org/10.1007/s11356-018-1424-5
Panahandeh M, Mansouri N, Khorasani N et al (2018) A study of pollution in sediments from Anzali wetland with Geo-accumulation index and ecological risk assessment. Environ Eng Manag J 17:2255–2262
Peng J-F, Song Y-H, Yuan P et al (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640. https://doi.org/10.1016/j.jhazmat.2008.04.061
Persaud D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Ministry of Environment and Energy
Pourang N, Richardson CA, Mortazavi MS (2010) Heavy metal concentrations in the soft tissues of swan mussel (Anodonta cygnea) and surficial sediments from Anzali wetland, Iran. Environ Monit Assess 163:195–213. https://doi.org/10.1007/s10661-009-0827-7
Qingjie G, Jun D, Yunchuan X et al (2008) Calculating pollution indices by heavy metals in ecological geochemistry assessment and a case study in parks of Beijing. J China Univ Geosci 19:230–241. https://doi.org/10.1016/S1002-0705(08)60042-4
Rasta M, Sattari M, Taleshi MS, Namin JI (2021) Microplastics in different tissues of some commercially important fish species from Anzali wetland in the Southwest Caspian Sea, Northern Iran. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2021.112479
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton University Press, New Jersey
Sahu C, Basti S (2021) Trace metal pollution in the environment: a review. Int J Environ Sci Technol 18:211–224
Santos-Echeandia J, Prego R, Cobelo-García A, Millward GE (2009) Porewater geochemistry in a Galician Ria (NW Iberian Peninsula): implications for benthic fluxes of dissolved trace elements (Co, Cu, Ni, Pb, V, Zn). Mar Chem 117:77–87. https://doi.org/10.1016/j.marchem.2009.05.001
Schroeder H, Fabricius AL, Ecker D et al (2017) Metal(loid) speciation and size fractionation in sediment pore water depth profiles examined with a new meso profiling system. Chemosphere 179:185–193. https://doi.org/10.1016/j.chemosphere.2017.03.080
Schulz HD, Zabel M (2006) Marine geochemistry. Springer, Berlin
Simpson SL, Batley GE, Chariton AA et al (2005) Handbook for sediment quality assessment. Centre for Environmental Contaminants Research
Smith SL, MacDonald DD, Keenleyside KA et al (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638. https://doi.org/10.1016/S0380-1330(96)70985-1
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Cases Solut Environ Geol. https://doi.org/10.1007/s002540050473
Tang W, Duan S, Shan B et al (2016) Concentrations, diffusive fluxes and toxicity of heavy metals in pore water of the Fuyang River, Haihe Basin. Ecotoxicol Environ Saf 127:80–86. https://doi.org/10.1016/j.ecoenv.2016.01.013
Telfeyan K, Breaux A, Kim J et al (2017) Arsenic, vanadium, iron, and manganese biogeochemistry in a deltaic wetland, southern Louisiana, USA. Mar Chem 192:32–48. https://doi.org/10.1016/j.marchem.2017.03.010
Turetta C, Capodaglio G, Cairns W et al (2005) Benthic fluxes of trace metals in the lagoon of Venice. Microchem J 149–158
US Environmental Protection Agency (2001) Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses: technical manual. Washington, DC
Vaalgamaa S, Conley DJ (2008) Detecting environmental change in estuaries: nutrient and heavy metal distributions in sediment cores in estuaries from the Gulf of Finland, Baltic Sea. Estuar Coast Shelf Sci 76:45–56. https://doi.org/10.1016/j.ecss.2007.06.007
Vaezi AR, Karbassi AR, Fakhraee M (2015) Assessing the trace metal pollution in the sediments of Mahshahr Bay, Persian Gulf, via a novel pollution index. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4833-7
Volz SN, Hausen J, Nachev M et al (2020) Short exposure to cadmium disrupts the olfactory system of zebrafish (Danio rerio)—relating altered gene expression in the olfactory organ to behavioral deficits. Aquat Toxicol. https://doi.org/10.1016/j.aquatox.2020.105555
Yuan-Hui L, Gregory S (1974) Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38(5):703–714
Zhang H, Cui B, Xiao R, Zhao H (2010) Heavy metals in water, soils and plants in riparian wetlands in the Pearl River Estuary, South China. Procedia Environ Sci 2:1344–1354
Zhang G, Bai J, Zhao Q et al (2016) Heavy metals in wetland soils along a wetland-forming chronosequence in the Yellow River Delta of China: levels, sources and toxic risks. Ecol Indic 69:331–339. https://doi.org/10.1016/j.ecolind.2016.04.042
Zhengqi X, Shijun N, Xianguo T, Cheng-Jiang Z (2008) Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ Sci Technol 31:112–115
Zhu X, Shan B, Tang W et al (2016) Distributions, fluxes, and toxicities of heavy metals in sediment pore water from tributaries of the Ziya River system, northern China. Environ Sci Pollut Res 23:5516–5526. https://doi.org/10.1007/s11356-015-5709-7
Funding
The present research did not receive any financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is not any conflict of interests regarding the publication of this manuscript. In addition, the ethical issues, including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancy has been completely observed by the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehdizadeh, Y., Karbassi, A.R., Nasrabadi, T. et al. Behavior, toxicity and diffusive flux of metals in a sediment core and pore-water from Anzali wetland. Acta Geochim 42, 309–331 (2023). https://doi.org/10.1007/s11631-022-00578-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-022-00578-3