Abstract
One of the major environmental problems is to accumulate heavy metals in the soil, which can be hazardous while transferring the heavy metals to plants or water. The study aims in understanding fractionation, distribution, mobility as well as the toxicity of lead (Pb), nickel(Ni), cadmium(Cd), copper (Cu) and zinc (Zn) in contaminated soils of Tehran province, Iran. The total concentrations of Pb, Ni, Cd, Cu and Zn in the 18 samples were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES). The fractionation of Pb, Ni, Cd, Cu and Zn in these soils have also been ivistigated using a sequential extraction method to assess their possible mobility, bioavailability and toxicity. Accordingly, five chemical fractions of heavy metals under study have been described, including exchangeable (F1), carbonate (F2), organic (F3), Fe–Mn oxide (F4) as well as residual (F5). The highest Pb, Ni, Cd, Cu and Zn concentrations were found in the residual fraction. Actually, the distribution and fractionation of Zn and Cu were same in this study. The distribution pattern of Cd and Pb indicated theses heavy metals are strongly associated with the Fe–Mn oxide fraction in all soils. Our results indicated significant correlations between Eh, pH, clay, silt content, and organic matter and retention of all heavy metals. Then, microbial and enzymatic toxicity experiments have been performed to evaluate heavy metal toxicity in the environment (MetPLATE™); a correlation (r2 = 0.991) was obtained between toxicity and total heavy metal concentration in this research. The recovery percent of the all heavy metals studied were above 90. The highest and lowest mobility factor values were calculated to be 23.8 and 4.4 percent for Cd and Zn, respectively. These findings, therefore, show the highest risk of mobilization of heavy metals and their potential toxicity. They could represent risk of pollution of ground water, influenced food chain and become an environmental risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the key environmental challenges in many countries has been considered to be contaminating the soil by the heavy metals. There is a growing concern about heavy metals toxicological effects on ecosystems, agriculture and, human health; this had led to scientific and public awareness of their environmental issues. Soils receive large quantities of heavy metals from diverse anthropogenic resources like municipal and industrial wastes. Their contamination effect as well as toxicity in soils can be described in terms of their solubility and bioavailability. Bioavailability is the main factor considered in assessing the potential toxicity of heavy metals and their compounds (Huang et al. 2019; Bing et al. 2019). The potent poisonousness of the heavy metals in the soil has been introduced as one of the functions of their bioavailability and mobility, depending on the heavy metals phase and chemical procedures which handle the transformation between these phases. The heavy metals result in damages to internal organs and nervous system and apply carcinogenic impacts (Vongdala et al. 2019; Ahmad et al. 2020). Multiple investigations demonstrated content of heavy metals in the contaminated soils (Wali et al. 2013). Chemical fraction of soil is defined as the identification of the forms in which metals occurs in the soil (Li et al. 2019, 2020). Nonetheless, the total content of the heavy metals would not present adequate outputs regarding their environmental behavior, anthropogenic and natural (rock or soil weathering), mobility, pathways and bioavailability (Davutluogluet al. 2011; Huang et al. 2018). Several chemical speciation and fractionation methods have been developed for heavy metals analysis in the soil. On the contrast, the heavy metals speciation analysis of soil can serve as one of the suitable indicators of the quality of the ecosystem. Heavy metals can have associated with soil with numerous different chemical stages like sinks for soluble or exchangeable heavy metals and amorphous materials (Fe/Mn oxides) linked to the organic materials, sulfide or residual fractionation (Perez-Lopez et al. 2010; Li et al. 2018). The heavy metals in the soil through fraction study can be classified into two major immobile and mobile. The immobile or residual fraction heavy metals are not available, due to strong bound which exists between these metals and crystal lattice of silicate of the soils. Therefore, they are from natural source that is rock or soils weathering. The mobile fraction divided to exchangeable fraction, carbonate fraction, reducible (Fe–Mn oxides) fraction and oxidisable (organic) fraction (Liu et al. 2017: Fang et al. 2019). The four mobile fractions are available for plant uptakes and bioaccumulation in aquatic biota. The availability of mobile fractions depends on pH, redox-potential and temperature (Algul and Beyhan 2020). The changeable ionic form can be removed from a polluted soil by the use of suitable ion exchange extractants. The Fe and Mn oxide as single or jointly contribute importantly to the release of the heavy metals from the contaminated soils. Fe and Mn oxide is stable in the soils at the aerobic condition; however, they are unstable at anaerobic condition provided by oxidizing the organic materials. In addition, the soil organic materials are capable of absorbing the heavy metals. The degradation of the organic matters releases heavy metals. Moreover, organic matter on the anoxic condition may produce H2S and form insoluble sulfides while contacting with the heavy metals. Enhancement of the potentialities of the soil redox will release heavy metals from the sulfides. Heavy metals are capable of reacting with the carbonate; they can be combined in their lattice instead of complete adsorption on their surfaces (Yang et al. 2012).
Determination of their fractionation, therefore, has been regarded to be a major thing because bioavailability and mobility of the heavy metals depend on the respective specific chemical forms or binds to all the soil phases. Experts in the field devised sequential extraction processes for determining heavy metals speciation in the soils. The processes offer greater insight into chemical availability, origin, mobilization, and transfer of heavy metals in the soils (Aiju et al. 2012; Rao et al. 2010). The BCR [‘European Community Bureau of Reference’, that is now called: ‘European Community (EC) Standards Measurement and Testing Programme’] protocol was proposed to measurement of the extractable heavy metals in soils. Sequential extraction procedures involve various sequential extractions and reagents separating the heavy metal forms. However, selecting different extracts in different steps of accommodating variable extraction times and diverse samples have led to some outputs that cannot be directly compared. Unlike the mentioned limitations, the sequential extraction processes are beneficial to understand and anticipate environmental behaviors of the metals. Also, redox potential, organic matter as well as pH may trigger retention and mobilization of heavy metals in soil (Naji et al. 2010; Tessier et al.1979; Gu et al. 2020). Accordingly, researchers presented enzymatic and microbial toxicity experiments for assessing the toxicity of trace metals in the environment (MetPLATE™, MetPAD™) (Kong and Bitton 2003). These experiments have been proven to be helpful devices to evaluate the bioavailability of heavy metals in soils (Elkhalil et al. 2008). The chosen soils are considered as the main source of pollution. To date, no consequent research has been conducted on fractionation of heavy metals in these soils. Unfortunately, the total concentration of heavy metals is not sufficient to evaluate their environmental impact. Therefore, the orientation of this research is to study the fractionation and bioavailability of the Cu, Ni, Cd, Zn and Pb and their toxicity in contaminated soils of Tehran Province, Iran. Distributions of heavy metals in soils were determined using a sequential extraction method which allowed us to describe heavy metals in five different fractions, including; (1) exchangeable metals, (2) metals bound to carbonates, (3) metals bound to iron and manganes oxides, (4) metals bound to organic matter, and (5) metals in residual fraction.
Material and methods
Three contaminated sites were selected, including diverse heavy metals in Tehran Province, Iran. The study areas are located in the: 1. Varamin city (35°6′ 29.37"N and 51°33 59.58ʺE), 2. Mallard city (35°42′ 27.35ʺN and 50°46 42,35ʺE) and 3. Rey city (35°17′ 17.28ʺN and 51°10 59.10ʺE). These areas have been used as landfills for hazardous wastes without environmental standards.
Sample collection and preparation
Eighteen samples were collected from three sites surface in 2019. Theses soils have been selected due to their areal scope (Table 1). To collect the samples, a completely random sampling method has been used. An auger and a spoon were used for the sample collection. The slightly wet soil samples have been brought to the lab, all contents were distributed out on a stainless-steel tray, followed by disaggregation of large pieces and all containers were labeled. In the lab, all trays were placed in an open space at room temperature for about 7 days, until all samples were completely dried and then sieved for removing the particles higher than 2 mm. Then, soil samples have been stored in the plastic bags with suitable labeling for analysis.
Soil characterization
The following soil characteristics were analyzed. Particle size distribution was determined by the hydrometer method for sand, silt and clay (Gee and Bauder 1982). Soil pH was measured and redox potential was gauged by insertion of a platinum electrod (PT-Agcl/Ag) in a 1:2 soil water ratio (Mclean 1982; Shazia et al. 2014); also, organic carbon and cation exchange capacity(CEC) were determined by standard procedures (Nelson and Sommers 1982) and heavy metals were analyzed by ICP-AES (Krishnamurti et al. 1994).
Sequential extraction
Cd, Ni, Cu, Zn and Pb have been extracted by sequential process (Tessiar et al. 1979) to define fractions of carbonate-bound, exchangeable, organic- bound, residual, and Fe–Mn oxide bound.
-
1.
Exchangeable Fraction. 1 g of the soil has been extracted with 8-ml unbuffered 1 M Mgcl2 at pH of 7. Then, the samples have been put in a stirrer for at 25 °C for 1 h prior to centrifugation.
-
2.
The Carbonate-Bound Fraction. Residues remained after removing exchangeable fraction have been extracted with 8 ml of 1 M NaOAc and set to pH of 5 with acetic acid (HOAc). The samples have been put in the shaker for 25 °C 6 h at prior to centrifugation.
-
3.
Fe–Mn Oxide-Bound Fraction. Residues remained following were removed the Carbonate-bound fraction has been extracted with 20 ml of 0.04 M NH2OH–HCl into 25% HOAc (v/v). Then, heating the samples has been done for 6 h in a water bath at 96 ± 3 °C with intermittent agitation prior to centrifugation.
-
4.
The organic-Bound Fraction. Residues remained after removal of Fe–Mn oxide-bound fraction have been extracted with 3 ml of 0.02 M HNO3 + 5 ml of 30% H2O2 set to pH 2with HNO3. Then heating the samples has been done to 85 ± 2 °C for 2 h with intermittent agitations. Then, 3 ml 30% H2O2, has been added of pH 2 and heating the samples has been done at 85 ± 2 °C for 3 h with intermittent agitations. After cooling, 5 ml of 3.2 M NH4OAc in 20% (v/v) of HNO3 added. Sample volume reached 20 ml with the deionized water and put on a shaker for 30 min prior to centrifugation.
-
5.
Residual Fraction. Residues remained following removing the organic-bound fraction have been digested with 4 ml of 50% HNO3 and 10 ml of 20% HCl. Then, the samples have been put in the shaker for 30 min and heating has been done to 95 °C for 30 min with an intermittent mix of residue and acid. Consequently, they have been cooling down to the room temperature prior to centrifugation.
Upon every stage, centrifugation of the samples has been done at 3000 rpm for 30 min. Then, the supernatants have been pipette into 100-ml volumetric flasks and marked to 100 ml with the deionized water. Then, the left residue has been washed two times with 8-ml deionized water after strong hands shaking prior to the next metal extraction.
Quality assurance and quality control
All necessary precautionary measures were adhered to to avoid contamination during drying, sieving and storage. Laboratory glass and plastic wares used for the analysis were first cleaned with de-mineralized water, then with 10% HNO3 and then rinsed with de-mineralized water. All solutions and dilutions were prepared with Mili-Q water. Intermediate standards and reagent solutions were stored in laboratory glass to avoid possible contamination from plastic. During the digestion of the soils, method blanks (65% concentrated HNO3) were included in each set of samples digested for the total metal determination. The samples were digested with acids under heated conditions and the liquid digestive is aspirated into inductively coupled plasma. Light focused on the excited metals further excites the electron states, and they emit spectral light that is passed through a gradient filter and detected by wavelength and intensity. For the sequential extraction steps, the blanks included were the respective reagent(s) recommended for each step without soil samples. The recovery of the sequential extraction procedure was evaluated as follows(Jorg and Sabry 2014):
Statistical analyses
The means heavy metals concentrations and standard deviations in various fractions and numerous linear regression analyses have been carried out in developing a considerable association between heavy metals fraction and the soil properties, using SPSS software.
MetPLATE ™
MetPLATE ™ was utilized to determine toxicity of the heavy metals in the soil samples. The mentioned test has been on the basis of the particular suppression of β-galactosidase of test bacteria (E. coli) by heavy metals. The kit involves a freeze dried E. coli, moderately hard water, phosphate buffered enzyme substrate as well as a 96-well micro-plate. The freeze-dried E.coli is rehydrated into 5.0 ml of moderately hard water and is mixed. A volume of 0.1 ml of E. coli added to 0.9 ml of solution or a dilution thereof. Then the mixture is vortexed and incubated at 35 °C for 60 min. At the end of the 60-min exposure period a 0.2-ml aliquot of the suspension is dispensed in a well of the assay micro-plate. Then, 0.1 ml of the substrate is added to each well. Upon mixing in each of the wells, the micro-plate is incubated an additional 60 to 90 min at 35 °C for color development. The intensity of the resulting purple color gives an indication of enzyme (β-galactosidase) activity and is inversely proportional to the toxicity of the sample. Absorbance is measured at 575 nm using a 96-well micro-plate reader (Kong and Bitton 2003).
Results and discussions
Soil characterization
The chemical and physical characteristics of soils under study are ptesented in Table 2. These soils exhibited diverse properties. The clay, silt and sand contents of the soils varied from 16.8 to 25.4%, 43.9–63.4% and 17.7–39.3%, respectively. The pH values of the soils ranged from 6.6 to 7, indicating that the soils were slightly acidic to neutal. The results show that, Eh value ranged from 27 for site 2–58.5 for site 1. CEC values of the soils varied from 13.6 to 18.9 Cmolc/kg. Organic material content ranged from 2.76 to 3.29%. Table 3 reports the means ± SD of the heavy metals of the soil samples. Petruzzelli et al. (2020) reported soils were mainly sandy with a mean value of 83.6% in a range from 72.3 to 91.9%. Clay content ranged from 1.62 to 14.6% with a mean value of 5.25%. Silt content ranged from 4.35 to 21.4% with a mean value of 11.1%. Soils were characterized by a pH mean value of 6.2, an average organic matter content 7.8% and a mean CEC value of 27.1 Cmolc/kg.
Heavy metals concentrations and their fractionation in soils
Total heavy metals concentrations in the investigated soils varied in a broad range of values. Results showed, concentration of Zn within the ranges of 1038–2955 mg kg−1 while Pb recorded concentrations from 297 to 1029 mg kg−1. Ni, Cu and Cd had concentrations within the ramges of 34.84–470, 519.2–978 and 380–1877 mg kg−1, respectively. These higher heavy metals concentrations within the investigation study areas are indicative of anthropogenic sources for the selected heavy metals in to the environment. Table 4 reports the mean concentration ± standard deviation (mg kg−1) of diverse heavy metals fractions for 18 samples.
The exchangeable fraction of Zn in whole soils ranged from 10.29 to 29 mg kg−1. The distibution of exchangeable fraction of Pb, Ni, Cu and Cd ranged from19 to 65.88, 1.87–25.5, 0.93–14.2 and 56.76–304.1 mg kg−1 at all sampling sites.The highest concentration of Zn, Pb, Ni, Cu and Cd was observed at sampling site 1, site 3, site 1, site 3 and site 2, respectively.The exchangeable fractions of heavy metals studied are weakly adsorbed and retained on soil surfaces by weak electrostatic interaction.This fraction constitutes the mobile forms of heavy metals in soils.The exchangeable heavy metals released into the environment easily and their remobilization can be caused by changes in ionic composition, influence of adsorption–desorption reactions and acidification (Filgueiras et al. 2002). The carbonate fraction of Zn, Pb, Ni, Cu and Cd in all soils ranged from 34.99 to 98.6, 11.4–39.53, 0.4–24.1, 4.63–139.78 and 24.14–129.3 mg kg−1, respectively. The maximum concentration of Zn, Pb, Ni, Cu and Cd recorded at samling site 1, site 3, site 1, site 3 and site 3. In carbonate fraction, heavy metals are adsorbed onto surfaces of carbonate minerals (Wuana et al. 2010). The carbonate form has a loose bound and liable to changes in environmental conditions (Filgueiras et al. 2002). The heavy metals associated in this fraction are released to the environment like exchangeable fraction. Similar levels of zn and Pb bounds to carbonate fraction have been reported by Osakwe and Okolie (2015). Amorphous Fe and Mn oxides have the capacity to adsorb heavy metals and mobility in soils. Our results indicated the occurrence of Zn and Ni in Fe and Man oxides fraction at site 1, Pb and Cu at site 3 and Cd at site 3 are considerable in the all soils.The Fe and Mn oxides fractions in all soils, showed Fe and Mn can be released upon oxidation of oxides of Fe and Mn compounds. The substantial amount of Fe could be attributed to transformation of Fe2+ into Fe3+ under oxidizing conditions and neutral PH values. Fe–Mn-oxide fraction is referred to as resource of heavy metals. These oxides appear as coating on mineral surfaces, can occur as a combination of the precipitation, adsorption, surface complex formation and ion exchange (Okoro et al. 2012). The highest organic fraction of Zn represented at site 1, Pb and Cu at site 2 and Cd at site 3 in current study. The heavy metals bound to organic matter constitute the oxidizable fraction and heavy metals form complexes may be released upon decomposition under oxidation conditions to the environment (Filgueiras et al. 2002). Organic matter and redox potential play important role in heavy metals binding and mobility in soil. The complexation of heavy metals by organic ligands affects their mobility, solubility and uptake by organisms (Okoro et al. 2012). The heavy metal forms associated with organic matter could be considered potentially active bound, depending on the physical characteristics of the soil (Kabirinejad et al. 2014). Tessier et al. (1979), suggested that under oxidizing conditions, organic matter can be degraded, leading to a release of soluble heavy metals. Residual fraction is a major fraction of sequential extraction as it assesses the long-term risk of toxic heavy metals. The heavy metals fractions bound to the crystalline structure of soils are less mobile. In this study, approximately 62.70–63.34 percent of Zn, 37.72–37.78 percent of Pb, 48.67–51.03 percent of Ni, 33.7–72.05 percent of Cu and 32.30–33.34 percent of Cd were recorded at all sites. Similar results has been reported by Sarkar et al. (2014). The residual fraction referred to inert fraction contains heavy metals that are associated with minerals in soil (Caplat et al. 2005). The residual fraction can not be released from soils and therefore, considered to be less hazard to environment. However, the heavy metals can be available from the crystalline structure by weathering and degradation processes (Tessier et al. 1979).
The multiple linear regression relationships between the selected soil characteristics as well as the most heavy metals fractions could explain 100 percent of the variability of different soils, as listed in Table 5.
Analyses showed that the greatest amount of Cd related to Fe–Mn-oxide-bound and residual fraction (Table 4). It has been found that cadmium distribution proceeded in the sequence: residual˃ Fe–Mn-oxide˃ exchangeable˃ organic˃ carbonate. In this study, Cd was mainly correlated to silt and clay properties in all fractions. On the contrary, absorption on the surface of clay and Fe–Mn oxide particles have been a more prominent Cd retention mechanism. Due to lower overall Cd concentration and their strong correlation with Fe–Mn and residual fraction, the toxicity issues ascribed to Cd have been not anticipated to happen, except deterioration in the drainage condition would cause declines in Mn or Fe, resulting in mobilization of Cd. Despite the relatively low exchangeable level, Cd exhibited a closer correlation to exchangeable fraction in comparison with other metals (Shuguang et al. 2018). Notably, exchangeable Cd fraction has been considered as one of the sensitive indicators of the uptake and bioavailability of the plants. The Cu distribution has been in accordance with a sequence of residual˃ organic˃ Fe–Mn-oxide˃ carbonate˃ exchangeable (Table 4). Copper mostly occurs as Cu+2 in the natural environment. Unlike overall idea, the correlation of Cu and the inorganic and organic particle surfaces has been verified (Park et al. 2016). The present study outputs demonstrated the highest amounts of Cu were found in the residual fraction of samples of soil. Therefore, high concentrations of Cu in residual fraction, as shown by Karathanasis and Pils (2005). The high Cu concentration could be ascribed to the Cu-Al substitution in the alumina-silicate mineral’s structure (Park et al. 2016). The organic and Fe–Mn oxide fraction has been also other two factors with the decreased concentration of Cu in comparison with the residual fraction. In addition, the correlation between Cu and Fe–Mn oxide could be ascribed to the Cu+2–Fe+2 competitive sorption or the coprecipitation procedures (Shuguang et al. 2018), organic matter, pH, Eh, Silt and Clay had an association to Cu retention in all fractions. Cu was associated with the organic fractions that focused on the significance of complication as well as chelation reactions for retention of Cu in the soil. Because Cu correlated to residual and Fe–Mn oxide fraction that have been highly insoluble at the natural condition, Cu bound to Fe–Mn oxide fraction may be mobilized in case of deterioration of the soil drainage condition, resulting in the declined Mn and Fe. During organic matter degradation, Cu from organic fraction may be released to the environment (Wuana et al. 2010). The increases in Cu concentration associated with organic fraction may be attributed to its high affinity to organic matter through organo-metal complexes formatio (Ashworth and Alloway 2008).
Ni distribution has been in line with sequence residual˃ organic˃ Fe–Mn-oxide ˃ exchangeable ˃ carbonate. According to Karathanasis and Pils (2005), Ni origin in the soil is detrital where in it commonly occurs as an occlusion in the structure of the weathering silicate minerals.The multiple regression analysis demonstrated organic matter, pH, Eh, silt and clay were controlling Ni retention in all fractions(Table 5). This was compatible to other researchers' outputs, who suggested Ni mobilization in the course of the weathering procedures would be mostly coprecipitated with the Fe–Mn oxide minerals (Karathanasis and Pils 2005). Furthermore, it has been found that the organic matter affected the Fe–Mn oxide surfaces in the presence of organic coating, offering further heavy metals binding sites. It is notable that the Fe–Mn oxide coatings on the surface of the clay particles could justify Ni contribution of clay in Fe–Mn oxide fraction. Multiple regression analysis also showed organic matter, pH and Eh had a positive impact on the Ni association, while silt and clay had a negative one in all fractions(Table 5). The Ni mobility should be regarded as an issue in the soils under study, especially in case of decline of soil pH or exposure of OM-Ni complexes to the induced oxidation condition.
The Pb distribution has been in accordance with sequence residual˃Fe–Mn-xide˃organic˃ exchangeable ˃ carbonate. Residual Pb concentrations could be correlated well with an increase in clay, silt and organic matter (Table 5). Research showed that lead is found as Pb+2 in the environment; however, in case of oxidation with Pb+4, possible incorporation into the clay mineral and Fe–Mn oxide structures could be seen (Karathanasis and Pils 2005). The Fe–Mn oxide fraction has been recognized as the second prominent sink for Pb. Karathanasis and Pils (2005) presented the Fe–Mn oxide fraction played a predominant contributory to the retention of Pb. Multiple regression analysis also suggested the positive impact of clay, silt and organic matter on Fe–Mn oxide association (Table 5). The results of multiple regression analysis, indicated that organic matter, clay and silt percent could serve as the suitable predictors of the organic—Pb bound. Moreover, multiple regression analysis also indicated clay and silt percent had a positive influence on the Pb association in Exchangeable fraction, while silt and clay had a negative one on the carbonate fraction. Carbonate Pb concentrations could be well correlated with an increase in the organic matter. The highest concentration of Pb was observed in a relatively labile exchangeable, organic and carbonate fractions of the soil, indicating greater risks for poisonousness issues due to the acidification from the anthropogenic deposition and natural weathering procedures. Sarkar et al. (2014) found Pb had bound to Fe and Mn oxides. The considerable amount of Pb associated with Fe and Mn oxides can be hazard t after releasing to the environment. The significant of Pb in Fe–Mn oxide fraction has been attributed to sorption of Pb to Fe–Mn-oxides (Ogundiran and Osibanjo 2009). Caplat et al. (2005) reported the distribution of Pb exhibiting strong affinity for organic matter fractions in sediment.
The Zn distribution has been in accordance with sequence residual˃ organic ˃ Fe–Mn-oxide˃ carbonate˃ exchangeable. Beckers et al. (2019) showed the predominantly residual accumulation of Zn in unpolluted soil, in particular, the clay minerals while Zn (OH)2, Zn (PO4) as well as ZnCO3 phases have been mainly observed in the polluted soil. It is notable that Zn retention in Fe–Mn oxide fraction has been described by increased stability constant of the Zn oxide and Zn capability for substitute Mn on the surface of oxide (Zubala et al.2017). Multiple regression analysis revealed the strong effects of organic matter, Eh and pH seemingly through a pH-dependent charge enhances on the Zn retention, while clay and silt percent negatively influenced Zn correlation in all fractions (Table 5). Given the lower level of Zn in no residual fractions of the soils studied, the toxic impacts were to lead to acidification or the organic matter decomposition.
Osakwe and Okolie (2015) presented the heavy metals bound to exchangeable fraction followed an average of 6.46% Ni, 2.64% Pb and 2.55% Zn. The Pb was predominantly associated with carbonate fraction with an average of 52.32%. The Ni and Zn averages in the carbonate fractions were 9.66% and 8.73%, giving the abundance trend of Pb > Ni > Zn. The Zn in the Fe–Mn oxide fraction was 42.49% and the averages Pb and Ni bound to this fraction were 13.24% and 6.46%, respectively. The organic bound heavy metals were in the abundance trend of Zn > Pb > Ni with average of 30.87% for Zn, 8.54% for Pb and 4.94% for Ni. The Ni with an average of 51.58% was predominantly associated with the residual fraction. The other heavy metals bound to this fraction in order of their importance to the fraction had the average of 13.96% for Zn and12.24% for Pb. Okolona et al. (2011) showed that Cd, Ni, Pb, Cu and Zn are predominantly occur in the Fe–Mn oxide, residual, carbonate bound, organic and residual fractions, respectively. It was found that concentrations in the exchangeable fractions were generally low for most of the studied heavy metals. Considering the proportion of heavy metals bound to the exchangeable and the carbonate-bound fractions, the comparative mobility of heavy metals presented the order; Ni > Cd > Pb Cu > Zn. Yusuf (2007) observed Cu was predominantly associated with Fe–Mn oxide and residual fractions, the carbonate fraction of Cd represents more than 70% of the total Cd found in the soils. A main portion (> 60%) of Zn was associated with the Fe–Mn oxide and carbonate fractions, whereas Pb was largely associated with organic matter and Fe–Mn oxide fractions with significant amounts in the carbonate and residual fractions.
Recovery of the sequential extraction
In this step, an internal evaluation has been done on the sequential extraction outputs with a comparison of the total heavy metals concentration extracted by diverse reagents in the course of the sequential extraction process with the outputs observed through total digestion (Jorg and Sabry 2014).
Hence, the sequential extraction recovery has been computed by Eq. (1):
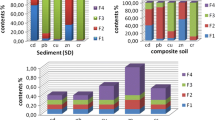
The recovery of heavy metals percent under study was in the following ranges: 98.14–99.13% Zn, 98.4–98.52% Pb, 97.4–97.59% Ni, 91.83–96% Cu and 97% Cd as shown in Fig. 1.
The recovery percentage has been obtained above 90% for all the extracted heavy metals. Therefore, the obtained results for both total metal content and the amounts of extractable metals following BCR validate the applied procedures. Generally, the percentage difference between laboratory duplicates ranged from 0.87 to 8.17% with an average of 2.8%. The accuracy of the BCR method used could be evaluated from the heavy metal recovery after whole extraction procedures. The recoveries of the heavy metals under investigation were all in the range 92.5–98.7%. The incomplete and variable recoveries obtained might be ascribed to errors due to loss and contaminations during the extraction process and uncertainties in measurements which is unavoidable. Therefore the percentage recovery of these heavy metals was of the order: Cr (95.6) > Pb (95.0) > Ni (94.8) > Cd (92.9), ranged between 92.9% and 95.6% (Umoren and Udousoro 2009).
Mobility factor of the heavy metals in soils
Resarchers described sequential extraction fractions of heavy metals in soils for declining solubility. Therefore, mobility factor could be assessed with regard to their potential active or bioavailable forms that have been specified by the sequential extraction. Consequently, exchangeable and carbonate (F1 + F2) fractions are regarded as the most reactive, most mobile and most potentially available/bioavailable fractions (Osakwe 2010). The mobility of heavy metals in soils can be assessed on the basis of their potential bioavailable. A high mobility factor indicates that heavy metals can enter to the biota.
The mobility of heavy metals factor has been computed as MF with regard to Eq. (2) (Vanek et al. 2005; Jwegbue 2013).
Figure 2 represents, of all the heavy metals studied, Cd has the highest mobility in soils, since it presents the highest content in most labile fraction. This is particularly marked in all the sampling sites. Approximately, 16.21% of its total concentration was measured in the first extraction, and about 6.8% of which were found in the second fraction. Moreover, mobility of the heavy metals factor of soils under study has been in the following ranges: 23.8% Cd, 0.6–16.84% Cu, 10.4–11.7% Pb, 6.7–10.83% Ni and 4.4% Zn. Researchers demonstrated heavy metals could be potentially provided for the uptake of the plant in case of a mobility factor greater than 10 percent (Beckers et al. 2019). Moreover, it has been shown that Cd and Pb mobility factor was > 10% except for Zn. Therefore, Cu mobility factor was higher than 10%, except for the soil samples from site 2; Ni mobility factor was above 10% of the soil samples from the site 1, offering that their availability points to increased risk since these heavy metals can accumulate in micro-organisms and plants.The finding of this study report that heavy metals are capable of forming insoluble complex compound with soil organic matter. Several soil properties like pH, form and amount of the carbonate and oxide, organic matter, charge features mineral compositions can impact mobility of heavy metals in soils (Fageria et al. 2002).
The mobility factor was calculated by Okolona et al. (2011). Some of the studied soils had Cu mobility factor below 10%, while others had mobility factor higher 10%. Kabala and singh (2001), reported the mobility factor of Cu is not higher than 10%. The mobility factor less than 10% obtained for cu in the soils of Ojato, Lagos. The Pb mobility factor was very high in the studied soils. This result an indication of anthropogenic source(Yusuf 2007). The range of Cd mobility factor in the studied soils (11.98–48.11%) was lower than the range of Cd (10–48%) reported by Yusuf in soils of waste disposal sites. Osakwe and Okolie (2015), indicated the mobility factor of the heavy metals were high with the abundance trend of Pb > Ni > Zn.
MetPLATE™ for heavy metal toxicity
In this study, Cu, Ni, Cd, Zn and Pb in the samples were measured. Table 6 presents heavy metal toxicity in various soil fractions. The heavy metals toxicity of three sites were obtained on average, 88, 3%, 63% and 72%, respectively. It has been found that heavy metals toxicity distribution proceeded in the sequence: residual˃ Organic˃ Fe–Mn-oxide˃ Carbonate˃ exchangeable. The all fractions have been the major sink of Zn, Pb, Ni, Cu and Cd. The heavy metals can be released from soils in all fractions and therefore, micro-organisms may be adversely affected by the toxicity effect of these heavy metals Fig. 3 shows the correlation between the fraction distribution of total heavy metals and toxicity, as measured via MetPLATE™. As shown in Fig. 1, regression coefficients (r2) were as follows: residual˃ Fe–Mn oxide˃ organic ˃exchangeable˃ carbonate. Such results could be due to the effects of the complexity of the interactions of various heavy metals with the soil components. Some studies have shown a small correlation between heavy metals content and toxicity (Kong and Bitton 2003). However, a significant correlation (r2 = 0.991) was obtained between toxicity and total heavy metals concentration in this study. According to this study, it is difficult to find significant correlations between heavy metals content of each fraction and the toxicity of the contaminated soil samples. However, the exchangeable, organic, Fe–Mn oxide and residual fraction showed a better correlation with toxicity (r2 = 0.99), as compared with carbonate fractions. The results of our study, therefore, explained the possible usage of the MetPLATE ™ toxicity test for the assessment of the impact of ecosystems contaminated with a mixture of heavy metals.
Conclusions
According to this study, the residual fraction has been a major sink of Cu, Cd, Zn, Ni and Pb. The heavy metal distribution of the studied soils followed different patterns, but Zn and Cu had the same distribution, following the sequence residual˃ organic˃ Fe–Mn-oxide˃ Carbonate˃ Exchangeable. It has been found that residual was highest overall fraction for retention of all heavy metals. The carbonate-bound fraction was the lowest fraction for Cd, Ni and Pb.
Significant correlations between Eh, pH, organic matter, clay and silt content and most fractions of the heavy metals indicate that these soil characteristics may control the fate and transport of the heavy metals in the soils.
The recovery of the sequential extraction index, presented the obtained results for both total heavy metals concentration and the amounts of extractable metals following BCR validate the applied procedures.
The mobility factor indicated that the heavy metals had values above 10% and were available for plant and micro- organisms. MetPLATE ™ revealed potential toxicity of the heavy metals in the soils. The toxicity of heavy metals distribution has been determined via a sequential extraction method; the regression coefficients (r2) have been in following order: residual˃ Fe–Mn oxide˃ organic ˃exchangeable˃ carbonate.
These soils also exhibited the greatest risks for mobilization of the heavy metals and potent toxicity because they can be influenced by remarkable redox potential modifications which could de-stabilize the exchangeable, organic, carbonate as well as the Fe–Mn oxide heavy metal retention pools. In this context, more works should be done: (1) to evaluate the behaviour of natural and anthropogenic heavy metals in buried and contaminated soils with depth and their fate; and (2) to quantify hydrodynamic and physico-chemical phenomena occurring in these soils.
Change history
29 November 2021
Reference to be updated.
References
Ahamad MI, Song J, Sun H, Wang X, Mehmood MS, Sajid M, Su P, Khan AJ (2020) Contamination level, ecological risk, and source identification of heavy metals in the hyporheic zone of the Weihe River, China. Int J Environ Res Public Health 17:1070–1080. https://doi.org/10.3390/ijerph17031070
Aiju L, Yanchun G, Honghai W, Gao Peiling G (2012) An assessment of heavy metals contamination in Xiaofu river sediments through chemical speciation study. Int J Earth Sci 5:1235–1240
Algül F, Beyhan M (2020) Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Sci Rep 10:11782–11794. https://doi.org/10.1038/s41598-020-68833-2
Ashworth DJ, Alloway BJ (2008) Influence of dissolved organic matter on the solubility of heavy metals in sewage sludge amended soils. Commun Soil Sci Plant Anal 39:538–550. https://doi.org/10.1080/00103620701826787
Beckers F, Awad YM, Beiyuan J, Abrigata J, Mothes S, Tsang DCW, Ok YS, Rinklebe J (2019) Impact of biochar on mobilization, methylation, and ethylation of mercury under dynamic redox conditions in a contaminated floodplain soil. Environ Int 127:279–290. https://doi.org/10.1016/j.envint.2019.03.040
Bing H, Wu Y, Zhou J, Sun H, Wang X, Zhu H (2019) Spatial variation of heavy metal contamination in the riparian sediments after two-year flow regulation in the Three Gorges Reservoir, China. Sci Total Environ 649:1004–1016. https://doi.org/10.1016/j.scitotenv.2018.08.401
Caplat C, Texier H, Barillier D, Lelievre C (2005) Heavy metals mobility in harbour contaminated sediments: the case of Port-en-Bessin. Mar Pollut Bull 50:504–511. https://doi.org/10.1016/j.marpolbul.2004.08.004
Davutluoglu OI, Seckin G, Ersu CB, Yilmaz T, Sari B (2011) Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J Environ Manag 92:2250–2259. https://doi.org/10.1016/j.jenvman.2011.04.013
Elkhalil H, El Hamiani O, Button G, Ouazzani N, Boularbah A (2008) Heavy metal contamination from mining sites in south Morocco: Monitoring metal content and toxicity of soil runoff and groundwater. Environ Monit Assess 136:147–160. https://doi.org/10.1007/s10661-007-9671-9
Fageria NK, Baliger VC, Clark RB (2002) Micronutrients in crop production. Adv Agron 77:185–268. https://doi.org/10.1016/S0065-2113(02)77015-6
Fang X, Peng B, Wang X, Song Z, Zhou D, Wang Q, Qin Z, Tan C (2019) Distribution, contamination and source identification of heavy metals in bed sediments from the lower reaches of the Xiangjiang River in Hunan province, China. Sci Total Environ 689:557–570. https://doi.org/10.1016/j.scitotenv.2019.06.330
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857. https://doi.org/10.1039/B207574C
Gee GW, Bauder JW (1982) Particle size analysis. In: Klute A (ed) Methods of soil analysis. Part I: physical and mineralogical methods, 2nd edn. American Society of Agronomy, Madison, pp 383–412
Gu C, Zhang Y, Peng Y, Leng P, Zhu N, Qiao Y, Li Z, Li F (2020) Spatial Distribution and health risk assessment of dissolved trace elements in groundwater in southern China. Sci Rep 10:7886–7897. https://doi.org/10.1038/s41598-020-64267-y
Huang X, Li N, Wu Q, Long J, Luo D, Huang X, Li D, Zhao D (2018) Fractional distribution of thallium in paddy soil and its bioavailability to rice. Ecotoxicol Environ Saf 148:311–317. https://doi.org/10.1016/j.ecoenv.2017.10.033
Huang X, Luo D, Zhao D, Li N, Xiao T, Liu J, Wei L, Liu Y, Liu L, Liu G (2019) Distribution, source and risk assessment of heavy metal(oid)s in water, sediments, and Corbicula Fluminea of Xijiang river, China. Int J Environ Res Public Health 16:1823–1830. https://doi.org/10.3390/ijerph16101823
Jorg R, Sabry MSH (2014) Assessing the mobilization of cadmium, lead, and nickel using a seven-step sequential extraction technique in contaminated floodplain soil profiles along the central Elbe river, Germany. Water Air Soil Pollut 225:2039. https://doi.org/10.1007/s11270-014-2039-1
Jwegbue CMA (2013) Chemical fractionation and mobility of heavy metals in soils in the vicinity of Asphalt plants in Delta State, Nigeria. Environ Forensics 14:248–259. https://doi.org/10.1080/15275922.2013.814178
Kabala C, Singh BR (2001) Fractionation and mobility of copper, lead, and zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–492. https://doi.org/10.2134/jeq2001.302485x
Kabirinejad S, Kalbasi M, Khoshgoftarmanesh AH, Hoodaji M, Afyuni M (2014) Chemical forms and phytoavailability of copper in soil as affected by crop residues incorporation. Am J Anal Chem 5:604–612. https://doi.org/10.4236/ajac.2014.59068
Karathanasis AD, Pils JR (2005) Solid- Phase chemical fractionation of selected trace metals in some Northern Kentucky Soils. Soil Sediment Contam 14:293–308. https://doi.org/10.1080/15320380590954033
Kong C, Bitton G (2003) Correlation between heavy matal toxicity and metal fractions of contaminated soil in Korea. Environ Contam Toxicol 70:557–565. https://doi.org/10.1007/s00128-003-0022-4
Krishnamurti GSR, Huang PM, Van Rees KCI, Korak L, Rostead HPW (1994) Microwave digestion technique for the determination of total cadmium in soils. Soil Sci Plant Anal 25:615–625. https://doi.org/10.1080/00103629409369067
Li R, Tang C, Cao Y, Jiang T, Chen J (2018) The distribution and partitioning of trace metals (Pb, Cd, Cu, and Zn) and metalloid (As) in the Beijiang River. Environ Monit Assess 190:399. https://doi.org/10.1007/s10661-018-6789-x
Li H, Chai L, Yang Z, Liao Q, Liu Y, Ouyang B (2019) Seasonal and spatial contamination statuses and ecological risk of sediment cores highly contaminated by heavy metals and metalloids in the Xiang jiang River. Environ Geochem Health J 41:1617–1633. https://doi.org/10.1007/s10653-019
Li K, Cui S, Zhang F, Hough R, Fu Q, Zhang Z, Gao S, An L (2020) Concentrations, possible sources and health risk of heavy metals in multi-media environment of the Songhua River, China. Int J Environ Res Public Health 17:1766–1783. https://doi.org/10.3390/ijerph17051766
Liu J, Xu Y, Cheng Y, Zhao Y, Pan Y, Fu G, Dai Y (2017) Occurrence and risk assessment of heavy metals in sediments of the Xiangjiang River, China. Environ Sci Pollut Res Int 24:2711–2723. https://doi.org/10.1007/s11356-016-8044-8
McLeen EO (1982) Soil pH and lime requirement. In: Page AL, Miller RH, Keeny DR (eds) Methods of soil analysis. Part 2: chemical and microbiological properties, 2nd edn. American Society of Agronomy, Madison, pp 199–224
Naji A, Ismail A, Ismail AR (2010) Chemical speciation and contamination assessment of Zn and Cd by sequential extraction in surface sediment of Klang River, Malaysia. Microchem J 95:285–292. https://doi.org/10.1016/j.microc.2009.12.015
Nelson DW, Somers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeny DR (eds) Methods of soil analysis. Part 2: chemical and microbiological properties, 2nd edn. American Society of Agronomy, Madison, pp 538–580
Ogundiran MB, Osibanjo O (2009) Mobility and speciation of heavy metals in soils impacted by hazardous waste. Chem Speciat Bioavailab 21:59–69. https://doi.org/10.3184/095422909X449481.
Okolona OJ, Uzairu A, Gimba CE, Kagbu JA (2011) Geochemical partitioning of heavy metals in Roadside surface soils of different grain size along major roads in Kano metropolis, Nigeria. Br J Appl Sci Technol 1:94–115
Okoro HK, Fatoki OS, Adekola FA, Bhekumusa JX, Reinette GS (2012) A review of sequential extraction procedures for heavy metals speciation in soil and sediments. J Environ Anal Toxicol. https://doi.org/10.4172/scientificreports.181
Osakwe SA (2010) Chemical speciation and mobility of some heavy metals in soils around automobile waste dumpsites in Northern part of Niger Delta, south central Nigeria. J Appl Sci Environ Manag 4:123–130. https://doi.org/10.4314/jasem.v14i4.63284
Osakwe SA, Okolie LP (2015) Distribution of different fractions of Iron, Zinc, Chromium, lead and Nickel in Soils around Petrol filling stations in selected Areas of Delta State, Nigeria. J Appl Sci Environ 19:706–716
Park JH, Ok YS, Kim SH (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83. https://doi.org/10.1016/j.chemosphere.2015.05.093
Perez-Lopez R, Nieto JM, Lopez-Coto I, Aguado JL, Bolivar JP, Santisteban M (2010) Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): from phosphate rock ore to the environment. Appl Geochem 25:705–715. https://doi.org/10.1016/j.apgeochem.2010.02.003
Petruzzelli G, Pedron F, Rosellini I (2020) Bioavailability and bioaccessibility in soil: a short review and a case study. Environ Sci 7:208–225. https://doi.org/10.3934/environsci.2020013
Rao CRM, Sahuquillo A, Lopez-Sanchez JF (2010) Comparison of single and sequential extraction procedures for the study of rare earth elements remobilization in different types of soils. Anal Chem Acta 662:128–136. https://doi.org/10.1016/j.aca.2010.01.006
Sarkar SK, Favas PJ, Rakshit D, Satpathy K (2014) Geochemical speciation and risk assessment of heavy metals in soils and sediments. InTech, London. https://doi.org/10.5772/57295
Shazia A, Shazia I, Mahmood UH (2014) Effect of chelating agent on heavy metal extraction from contaminated soils. Res J Chem Sci 9:70–87
Shuguang W, Yan X, Namkha N, Zhan W (2018) Remediation of biochar on heavy metal polluted soils. Earth Environ Sci 10:042113. https://doi.org/10.1088/1755-1315/108/4/042113
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. J Anal Chem 51:844–851. https://doi.org/10.1021/ac50043a017
Umoren I, Udousoro I (2009) Fractionation of Cd, Cr, Pb and Ni in roadside soils of Uyo, Niger Delta Region: Nigeria using the optimized BCR sequential extraction technique. Environmentalist 29:280–286. https://doi.org/10.1007/s10669-008-9193-1
Vanek A, Boruvka L, Drabek O, Mihaljevic M, Komarek M (2005) Mobility of lead, Zinc and cadmium in alluvial soils heavily polluted by smelting industry. Plant Soil Environ 51:316–321
Vongdala N, Hoang-Dung T, Tran Dang X, Teschke R, Tran Dang K (2019) Heavy metal accumulation in water, soil, and plants of municipal solid waste landfill in Vientiane, Laos. Int J Environ Res Public Health 16:22–35. https://doi.org/10.3390/ijerph16010022
Wali A, Coinet G, Khadhraoui M, Ksibi M (2013) Trace metals in surface soil contamination by release of Phosphate industry in the surroundings of Sfax-Tunisia. Environ Res Eng Manag 65:20–30. https://doi.org/10.5755/j01.erem.65.3.4865
Wuana RA, Okieimen FE, Imborvungu JA (2010) Removal of heavy metals from a contaminated soil using organic chelating. Int J Environ Sci 7:485–496. https://doi.org/10.1007/BF03326158
Yang Y, Chen F, Zhang L, Liua J, Wu S, Kang M (2012) Comprehensive assessment of heavy metal contamination in sediment of the Pearl River Estuary and adjacent shelf. Mar Pollut Bull 64:1947–1955. https://doi.org/10.1016/j.marpolbul.2012.04.024
Yusuf KA (2007) Sequential extraction of lead, copper, cadmium and zinc in soils near Ojata waste site. J Agron 6:441–337. https://doi.org/10.3923/ja.2007.331.337
Zubala T, Patro M, Boguta P (2017) Variability of Zinc, Copper and Lead contents in sludge of the municipal storm water treatment plant. J Environ Pollut Res 24:17145–17152. https://doi.org/10.1007/s11356-017-9338-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbarpour, F., Gitipour, S., Baghdadi, M. et al. Correlation between chemical fractionation of heavy metals and their toxicity in the contaminated soils. Environ Earth Sci 80, 726 (2021). https://doi.org/10.1007/s12665-021-10024-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-10024-x