Abstract
An imbalance in the environment’s composition leads to significant effect on human activities such as farming. Of importance are heavy metals which are introduced anthropogenically or naturally. This calls for environmental monitoring and subsequent remediation if needed. An environmental monitoring exercise was conducted on Ikwo soils of Ebonyi State, eastern Nigeria with the aim of determining concentration levels for possible remediation. A total of 18 soil composite samples taken at 0–50 cm below soil surface from fallowed and cultivated soils not fertilized were subjected to heavy metal analyses and fertility indices like: organic matter (OM), cation exchange capacity (CEC), % total nitrogen (%TN), organic carbon (OC), and salinity. A correlation at 95% confidence level between geo-accumulations (Igeo) of the various heavy metals with salinity, OM, and CEC of the sampled soils reveals that Igeo could be a contributing factor to the fertility status of the soils. With the aid of inductively coupled plasma atomic emission spectrophotometer (ICP-AES), the distribution pattern was determined as Mn> Fe> Zn>Cu> Mo> Cd> V>Hg>Ti> Ni>Bi> Pb> Co>Ag>Au> Cr>Pd>Pt. The Igeo of the heavy metals in the study area varied from heavily to extremely contaminated levels. A remediation exercise was recommended on Ikwo soils due to their high salinity level and low CEC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The earth’s soil is a reservoir of abundant chemical elements. These elements find their way into the soil anthropogenically or are found in it through natural processes. There are concerns by various scientists over the importance of heavy metals in the soil ecosystem. Soil pollution occurs when there is observed imbalance in geoaccummulation of metals beyond the natural cycling and control. This manifests in unhealthy plants growth with consequential deleterious effects on human. Heavy metals which occur naturally in rocks but find a greater introduction into the soil through anthropogenic activities like mining, fertilization, farming, automobile repairs, waste dump sites, and heavy vehicular traffic. These heavy metals exist both in organic and inorganic forms, and are normally affected by pH. The inorganic forms are very toxic and reactive. Their concentrations in the soil and their mobility and subsequent bioaccumulation in biological tissues are a source of concern. This is because heavy metals are non-biodegradable. The long term use of fertilizers and other chemicals on farm lands is an important anthropogenic activity that increases heavy metals such as Cu, Ni, Zn, and Cd in the soil. Other anthropogenic activities that increase the threat of heavy metals to humans include emission of metallic elements from industrial plants, electricity boards, and areas of heavy vehicular traffic (Arifin et al. 2017; Singh and Kumar 2006; Oje et al. 2010; Rafie 2012; Ololade 2014; Zhenwu et al. 2017; Chukwuji et al. 2006).
The rate of introduction of heavy metals into the soil is in direct proportion to the activities carried out on the soil. It is succinct to say that man is responsible for his environment. The natural release from rocks is in levels several fold lower than the releases from anthropogenic sources. Their biodegradability makes them of increasing threat due to their persistence which makes them have a high tendency to accumulate in the food chain. Associated with heavy metal accumulation in tissues includes cancer, kidney failure, dementia, autism, lymphoma, etc. (Gilbert-Diamond et al. 2012; Wang et al. 2016; Zhang et al. 2017; Pastor and Hernandez 2012; Hernandez et al. 2003).
The heavy metal load of soils is usually monitored via chemical analysis. The problem usually encountered with using chemical analysis is that it uses maximum limits to tell the extent of availability but does not reveal the real threat heavy metals poses in the environment. There is an increasing application of supplementary methods to interpret the toxicity levels of heavy metals in the environment (Boularbah et al. 2005). Pollution load index (PLI) provides summation of the overall level of heavy metal toxicity in a particular soil sample. It estimates the metal contamination status in a sample (Angulo 1996). Enrichment Factor (EF) has been used to classify the sources of heavy metal inputs into soils sampled. It is applied in assessing degree of pollution or contamination of a soil by heavy metals (Ololade 2014; Ryszard et al. 2017). Majority of studies on polluted soils are usually limited to few soil samples with little or no consideration to their random or systematic variation. These variations are taken care of by Igeo or geochemical load index (GLI).
Physical observations like yellow coloration of leaves in wet season suggests that nutrient levels of Ikwo soils are low and this raises concern about the fertility of Ikwo soil. Preliminary investigation reveals that farmers in Ikwo depend heavily on inorganic fertilizers, but this is beyond the affordability of most farmers in the area; hence, they remain at subsistence level farming. Interactive sessions with community members revealed that the soil infertility is attributed to high salinity. Saline soils contain high levels of soluble chlorides or sulfates of sodium which impacts negatively on crops growth through reduction of water availability to the plant or toxic effects of ions such as H2BO3 and barium ions under hyper saline conditions. High salinity is observed to reduce CEC (Abdel-Dayem 2005; Francisco et al. 2016); therefore, we hypothesized that the salinity levels of Ikwo soil has a direct impact on its fertility. It is believed that because of the high level of mineral deposition around Ikwo communities, buttressed by the conspicuous mining and quarrying activities, there may be a preponderance of heavy metals even in the common farm soils which can be absorbed by plants, and consumed by human resulting from energy usage across the trophic level.
The aim of this monitoring exercise was to determine the extent of pollution of identified heavy metals using Igeo over the study area. Furthermore the monitoring exercise aims to determine the salinity level, CEC, organic matter (OM), pH, and other ions for soil fertility. This work will provide data for further soil quality monitoring and soil remediation studies.
Materials and methods
Study area
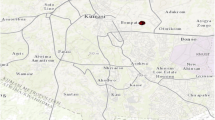
The research was conducted in Ikwo local government area of Ebonyi State, south eastern Nigeria (Fig. 1). Ikwo is located 14 km south of Abakaliki, the capital had quarters of Ebonyi state. An unpublished document reported that Ikwo has a land mass of approximately 5000 km2. The sampled space within Ikwo is 313.22 km2 located within 800,815E and 605, 615 N. Ikwo lies within the mineralized Pb-Zn deposits of the river Benue trough which is within the sub-humid agro ecological zone of Nigeria’s savanna belt. The Pb-Zn deposits are the largest and richest in Nigeria. They include those at Enyigba and Ameka. These mines have been active since 1925. These mines are mined by open cast and underground mining techniques. Long before colonization of Nigeria by Britain, natives dug for galena (PbS) which was used as a cosmetic. Before 1967, the open cast mining and the galena and sphalerite (ZnS) where beneficiated at the site by differential floatation using Xanthate collector. However, in the 70s, there was a shift to salt production from the brine pools associated with Pb-Zn deposits. In recent years, mining has continued intermittently and many firms have renewed interests in operations within Ikwo area. This has resulted to abandoned open pits and several heaps of Pb-Zn mines waste rocks which have altered the original topography of Ikwo. The mining activities have increased surface area of broken rocks and set the stage for erosion and leaching of metals to the environment (Wilberforce et al. 2012; Chukwuma 1993). The sampled area has important land marks like the Federal University Ndufu-Alike, Ikwo (FUNAI), college of Education, Ikwo and Ebony gold Rice Company, and Royal salt company near the salt and Pb-Zn mine at Echara. The features of the study area are ridges and flat lands which are underlain by shales. These ridges and flat lands host the Pb-Zn veins and form watersheds from which many springs and streams are generated. Some of the springs are saline and are found along stream valleys and are consequently flooded during the rainy season. These flooded areas support the cultivation of rice on large scale. The state is richly known as the hub of rice cultivation in Nigeria. Other crops grown in the area include: yam, cassava, maize, ground nut, cocoa yam, sweet potato, etc. The forest zones that once stood are replaced around by farm lands. The organic matter of the soil varies with natural and density of the vegetation cover as well as agric waste disposal practices like tilling under during construction of farm ridges and heaps for tuber crops. The study area has lateritic clay soil, sand soil from mudstone and clayey shaly soil. The clayey shaly soil is seasonally water logged and this occurs in pockets within the valleys and flood plains. Clay soils are soft and can be dug easily from the soil profile so long as it remains moist, but when dry, it hardens into an iron stone that is useless for cultivation.
Soil samples
Field studies were carried out in January, 2017. This is the peak of dry season experienced in the study area. A total of 18 soil samples were obtained in no defined pattern. Heavy metals escape from surface to deeper levels and so at the collection spots, soil samples were collected from 0 to 50 cm down the soil profile using soil screw auger. These samples were taken from farms that were not fertilized and grass/fallow lands. The soil samples were homogenized and sample size was obtained through cone and quartering method. The samples were preserved for further analysis. Table 1 and Fig. 1 represent sampling spots in the studied area. The coordinates shown in Table 1 were obtained with Garmin GPSMAP 76CX.
Physicochemical analyses
Collected soil samples were air dried and sieved through a 2 mm mesh. The soil materials of < 2 mm were preserved for heavy metal determination, salinity and pH analysis, OM and OC, and CEC. %TN, nitrate, sulphate, and phosphorus were also determined. The salinity and pH were measured in a 1:1 (w/v) ratio of soil and water suspension. %TN, nitrate, sulphate, phosphorus, OM, and OC were determined as outlined in IITA manual series No.7 (1981).
Heavy metal determination
For the determination of total heavy metal content of the soil materials, 0.5 g of < 2 mm air dried samples were cold digested overnight using 2 mls of concentrated redistilled nitric acid (HNO3). After which, the digest was dried at various temperatures using a digestion block with a thermal regulator starting with 120 °C to remove carbon. Each time, the solution dried leaving a black residue, further, 2 mls of conc. redistilled HNO3 was added and dried. This step was repeated until the solution was clear. The clear solution was diluted using 50/50 (v/v) nitric acid and perchloric acid and heated to dryness and later allowed to cool to room temperature. The cooled ash was re-dissolved with 1 ml conc. Hydrochloric acid and 10 mls 5% HNO3 and transferred into a 15 mls centrifuge tube and preserved. The heavy metals were determined using ICP-AES (Questron Technologies Corp. TL 6000).
Quality control/statistical analysis of results
The analysis was done in replicates and replicates with more than ± 5% difference were rejected and fresh analysis was done. The quality of the analyses was checked by calibration using known standards after every 40 samples. Various statistical methods were performed with a 95% confidence interval (p < 0.05) using statistical package for the social sciences software, 21.0, USA.
Heavy metal contamination evaluation
The levels of pollution of the soils by the heavy metals were evaluated using Igeo and pollution index (PI) as used by Smart et al. (2016)
Where CHm and BHm are the concentrations of analyzed heavy metal and the heavy metal concentration in reference soil material, respectively. The values of BHm were as used by Ololade (2014).
Igeo is classified into seven groups (0–6) with the seventh group reflecting soil enrichment above background levels. A PI above 1.0 shows that the metal concentration in the soil is above healthy level or may be introduced into the soil anthropogenically. Tables 2 and 3 shows the classification of Igeo and PI.
Results and discussion
Properties of Ikwo soils with their mean ± standard deviation levels are shown in Table 4. The range of pH in the study area ranged from 7.00 in site F to 7.63 in site N. The study area is characterized by statistically significant values for salinity. The salinity ranged from 185.88–362.83 μS with the highest value recorded in site F 362.83 ± 5.08 μS. A careful study of Table 4 reveals that site F had highest values in all properties but less sandy than the other areas of the study area. The mean concentrations of heavy metals in the soils are shown in Table 5. The Table shows Mn having the highest concentration in all the sampled areas and Pt having the least concentration. The level of contamination of the study area (Fig. 1) by the heavy metals is in Tables 6 and 7 as Igeo and PI. The correlation between the Igeo of the heavy metals in the soil samples and the fertility indices of the same soil samples is shown in Table 8. The graphical description of Igeo and PI are shown in Fig. 2.
Physicochemical properties of Ikwo soils
The pH values were all basic and varied from 7.0 ± 0.14 in site F to 7.63 ± 0.66 in site N. The pH reduced towards F which is at the center of the study area (Fig. 1). The salinity in site N was the least 185.88 ± 8.24 and increased in site F. This correlates with the levels of Cl−, SO42−, NO3−, and PO42− with the highest values in site F compared to N, S, E, or W. pH showed an inverse relationship with salinity. The highest pH in site N had a corresponding lowest salinity and the lowest pH the highest salinity. Obviously, it is suggested that making Ikwo soils alkaline could reduce the impact of salinity on farm yield in the area.
%TN in the area ranged from 2.79 ± 0.12% in site N to 5.44 ± 0.08% in site F. The tillage method which involves tilling under of crop residues and weeds during land preparation could be the cause of the high total nitrogen in the soil samples. This implies that at the time of sampling the soil, there was no need for nitrogen fertilizer application for crop production. This trend was similar to that of OC and OM. A study of the behavior of both OC and OM with pH revealed that an increase in pH caused a decrease in either or both OC and OM. It is important to note that OM was quite high in site F (74.22 ± 1.04%) and lowest in N (38.02 ± 1.69%). This may be due to the presence of organic residues which decay and supply OM to the soil. OC is a component of OM; it is obvious that OC will display similar trends.
The CEC has a direct relation with concentrations of Ca, Mg, K, and Na. It increases as they also increase. The CEC in site N was quite low (4.61 ± 0.20) as compared to other sampled areas. The study area had the highest value of in F (8.99 ± 0.13 cmol/kg). The variation between CEC and pH is usually not a linear one. This is because as pH increases, the available cations in soil solution decrease as they get adsorbed to the soil colloid. The non-linear variation between pH and CEC in this study could be as a result of lyotrophic effect (Yang 2009). The concentrations of the cations: Ca, Mg, K, and Na increased with decrease in pH. Increase in OM increases CEC and this promotes plants ability to take up nutrients.
Available P ranged from 369.19 ± 16.37 ppm to 720.65 ± 10.09 ppm. It is stated that P availability increases in submerged soils (Bernhard et al. 2004). Its availability may be influenced by CEC and OM.
Soil texture
Soil texture is known to play a very vital role in plant development. It also influences physical properties of the soil. The soil textures of the various sampling sites were evaluated. The soils from all the sites had high sand content with W having the lowest value (66.75 ± 1.26%) and S the highest (70.5 ± 1.71%) while silt was highest at F (18.50 ± 0.71%) and lowest in N (3.25 ± 0.50%). The sample site in N had least clay content (7.25 ± 1.71%) and the highest in W (19.25 ± 1.17%). These soil textures are shown in Fig. 2.
Contamination levels of heavy metals
The mean level of the heavy metals is shown in Table 4. Site F had the highest concentrations of the heavy metals tested. This site had a moderately strong to strong contamination of all the heavy metals tested (Tables 6 and 7). This was the trend observed in all the sample sites as shown in Fig. 3. All the sample sites had their chief heavy metal (mg/kg) as Mn (2199.77–4293.90). Next to Mn was Fe (1949.34–3805.05), Zn (812.22–1585.44), Cu(216.59–422.78), Mo (60.52–118..13), Cd (31.85–62.17), V (20.67–40.34), Hg (14.63–28.57), Ti (10.24–19.99), Pb (3.48–6.79), Bi (4.63–9.03), Ni (6.61–12.90), Co (2.95–5.75), Cr (0.48–0.95), Ag (1.24–2.42), Au (1.38–2.16), Pt (0.09–0.19), then Pd (0.46–0.72). Pb in these areas could be attributed to atmospheric deposition owing to the accommodation of Pb-Zn mining sites in the area. The highest values found in site F shows a likelihood of mineralization going on in site F and the values vary quite significantly from the other sites (p < 0.05). The distribution of the heavy metals shows the mobility of the ions across the study area. With the strong contamination of the area with Heavy metals, there could be bioaccumulation of the heavy metals in bio tissues across the food chain. A different study elsewhere revealed Fe and Zn having high concentrations in plants used for biomonitoring (Franzaring et al. 2017). These heavy metals are of environmental and health concerns.
Correlation analysis
The correlation analyzed in Table 8 (see appendix) showed the relationship between the Igeo of selected metals and fertility indices, i.e., %TN, OM, OC, and CEC. It was noted that all the metals strongly correlated with each other and were statistically significant (p < 0.05). As shown in Table 5, it could be attributed to the levels of heavy metals following the same trend in distribution in all sample sites. The strong correlation could also be attributed to the similar topography of the study area. There was reduced rate of increase between the Igeo of the heavy metals and salinity, %TN, OM, and CEC. Though statistically significant, it still gives a clue that heavy metal levels and salinity are strong contributors to the fertility status of the study area. Worthy of note is the reduction in rate of increase between CEC and salinity. The rate of increase between them was not as strongly significant as observed in others. It can be inferred from their correlation that increasing CEC will reduce salinity. Since CEC can be increased by increasing OM, it is possible to reduce salinity through OM improvement but this is yet to be verified with a laboratory experiment. However, increase in OM through the use of agricultural waste could be achievable. The correlation between CEC and salinity is useful in the soil remediation of the study area in Ikwo, eastern Nigeria.
Conclusions
This study has exposed the heavy metal distribution pattern and with the combined interpretation of Igeo and PI, we found heavy metal contamination as moderately strong to strong or in terms of PI, severe pollution. We established a strong correlation between the Igeo of the heavy metals and salinity, OM, OC, and CEC. The strong correlation between these parameters shows that the levels of the heavy metals are a contributor to the CEC, OM, and salinity of the soil. However a weak significance between correlation of CEC and salinity shows that the salinity level can be remedied through an improvement in the soils CEC. For the first time, our study provided a data for soil quality of the study area which is helpful in making remediation plan for heavy metal and salinity affected soils. A remediation of the soils from the study area is recommended through CEC improvement.
References
Abdel-Dayem, S. (2005). Understanding the social and economic dimensions of salinity. Proceedings of the International Salinity Forum, Riverside, CA. April 25-27, 2005,1–4.
Angulo, E. (1996). The Tomllinson pollution load index applied to heavy metals, mussel watch data; a useful index to assess coastal pollution. Science of the Total Environment, 187(1), 19–56. https://doi.org/10.1016/0048-9697(96)05128-5.
Arifin, S., Maria, G., Tommy, L., Gunnar, J., & Prosun, B. (2017). Arsenic concentrations in local aromatic and high-yielding hybrid rice cultivars and the potential health rice: a study in an arsenic hotspot. Environment Monitoring Assessment, 189, 184.
Bernhard, A. Z., Pichit, P., Mike, J. M., & Gill, C. (2004). Heavy metals in soils and crops in southeast Asia. 2. Thailand. Environmental Geochemistry and Health, 26, 359–371.
Boularbah, A., Schwartz, C., Bitton, G., & Boulabah, J. L. M. (2005). Heavy metals contamination from mining sites in south Morocco: use of a biotest to assess metal toxicity of tailings and soils. Chemosphere, 63, 802–810.
Chukwuji, M. A., Iwegbue, G. A., Nwajei, G. E., & Isirimah, N. O. (2006). Characteristic of levels of heavy metals in sediments and dredged sediments of a municipal creek in the Niger delta, Nigeria. Environmentalist, 26, 129–133.
Chukwuma, C. (1993). Cadmium, lead and zinc from terrestrial plants in Enyigba lead and zinc mine: search for a monitoring plant specie in trace element distribution. Bulletin of Environmental Contamination and Toxicology, 51(5), 665–671.
Francisco, J.V., Luciano, M., Miguel, Q., Antonio, D. and Elias, F. (2016). Control of salinity: principle of agronomy for sustainable agriculture. Springer International Publishing, 295–320.
Franzaring, J., Mbaka, G. E., Amebe, T. F., Nkengafac, J. N., Schlosser, S., & Fanmeier, A. (2017). Foliar nutrient and metal levels of crops in the mount Cameroon area—reference values for plant nutrition and environmental monitoring. Environmental Monitoring Assessment, 189(4), 186. https://doi.org/10.1007/s10661-017-5896-4.
Gilbert-Diamond, D., Cottingham, K. L., Gruber, J. F., Punshon, T., Sayarath, V., Gandolfi, A. J., Baker, E. R., Jackson, B. P., Folt, C. L., & Karagas, M. R. (2012). Rice consumption contributes to arsenic exposure in US women. Proceedings of the National Academy of Sciences of USA, 108(51), 20656–20660.
Hernandez, L., Probsta, A., Probsta, J. L., & Ulrich, E. (2003). Heavy metal distribution in french forest soils: evidence for atmospheric contamination. Science of the Total Environment, 312(1-3), 195–219. https://doi.org/10.1016/S0048-9697(03)00223-7.
International Institute of Tropical Agriculture (IITA) (1981) Automated and semi automated methods for soil and plant analysis. Manual series No.7.
Oje, A. O., Peter, N. U., Ikechukwu, N. E. I., & Uchechukwu, U. N. (2010). Environmental pollution levels of lead and zinc in Ishiagu and Uburu communities of Ebonyi State, Nigeria. Bulletin of Environmental Contamination and Toxicology, 85(3), 313–317. https://doi.org/10.1007/s00128-010-0082-1.
Ololade, I. A. (2014). An assessment of heavy metals contamination soils within auto mechanic workshops using enrichment and contamination factors with geoaccumulation indexes. Journal of Environmental Protection, 5(11), 970–982. https://doi.org/10.4236/jep.2014.511098.
Oti Wilberforce, J.O., Nwabue, F.I. and Afiukwa, J.N. (2012). Analysis of heavy metals in soils of Enyigba and Abakaliki using proton induced X-ray emission spectroscopy. Environment and Pollution,1(2), 183-193
Pastor, J., & Hernandez, A. J. (2012). Heavy metals salts and organic residues in old solid urban waste landfills and surface waters in their discharge areas: determinants for restoring their impact. Journal of Environmental Management, 95, 42–49.
Rafie, R. M. (2012). Removal of heavy metals from waste water using black teawaste. Arab Journal of Science and Engineering, 37, 1505–1520.
Ryszard, M., Joanna, K., Michal, G., Pawal, Z., Agnieska, J., Tomasz, Z., Wojciech, K., Maryla, T., & Kalina, O. (2017). Assessment of heavy metals contamination in surface layers of Roztocze national park forest soils (SE Poland) by indices of pollution. Chemosphere, 168, 839–850.
Singh, S., & Kumar, M. (2006). Heavy metal load of soil, water and vegetables in Peru urban Delhi. Environmental Monitoring and Assessment, 120(1-3), 79–91. https://doi.org/10.1007/s10661-005-9050-3.
Smart, C. O., Anthony, C., Sadrack, F. T., & Theophilus, C. D. (2016). Assessment of heavy metal contamination in soils around lead-zinc mining areas in Enyigba, southeastern Nigeria. Journal Geological Society of India, 87, 453–462.
Wang, W., Lai, Y., Yuanyuan, M., Liu, Z., Wang, S., & Hong, C. (2016). Heavy metal contamination of urban topsoil in petrochemical industry city in Xinjiang, China. Journal of Arid Land, 8(6), 871–880. https://doi.org/10.1007/s40333-016-0057-0.
Yang, Z. (2009). Hofmeister effects: an explanation for the impact of ionic liquids on biocatalysis. Journal of Biotechnology, 144(1), 12–22. https://doi.org/10.1016/j.jbiotec.2009.04.011.
Zhang, J. S., De, C. X., Yan, S. C., & Zhen, Z. (2017). Heavy metals translocation and accumulation from the rhizosphere soild to the edible parts of the medicinal plant fengdan (paeonia ostii) grown on a metal mining area, China. Ecotoxicology and Environmental Safety, 143, 19–27.
Zhenwu, T., Miao, C., Jiali, C., Jing, J., Yufei, Y., Zhiqiang, N., Qifei, H., & Yanhua, L. (2017). Contamination and health risks of heavy metals instreet dust from a coal mining city in eastern China. Ecotoxicology and Environmental Safety, 138, 83–91.
Acknowledgements
The authors appreciate Oje Obinna of Environmental Toxicology and Gregory Osikakwe Ph.d of the Department of Geology, Federal University Ndufu-Alike, Ikwo, Nigeria, for their contribution to the success of this work.
Funding
This research work is financed by Nigeria Tertiary Education Trust Fund for year 2015 through the collaboration of Federal University Ndufu-Alike, Ikwo, Nigeria.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Tyopine, A.A., Jayeoye, T.J. & Okoye, C.O.B. Geoaccumulation assessment of heavy metal pollution in Ikwo soils, eastern Nigeria. Environ Monit Assess 190, 58 (2018). https://doi.org/10.1007/s10661-017-6423-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6423-3