Abstract
In this study, the concentration of major and trace elements was measured in rainwater samples to identify their possible sources. From January to April 2014, in Hamedan province, west of Iran, 23 rainwater samples were collected from one urban station and two rural stations. The volume-weighted mean (VWM) was 0.38, 0.88, and 0.52 meq L−1 for calcium and 0.42, 0.35, and 0.44 meq L−1 for magnesium in Hamedan, Kushkabad, and Nematabad stations, respectively, indicating that the above cations were dominant in these areas. Rainwater samples in the Hamedan station showed the lowest pH value with a VWM of 6.38 due to the lowest amount of neutralizing ions. The results indicated that calcium and magnesium were the key contributors to acid rain neutralization in all three stations. Although zinc had the highest concentration in the rainwater samples, the calculated crustal enrichment factor showed that cadmium had maximum enrichment factors in three stations. Because of the more anthropogenic activities, Hamedan station had the highest concentration of trace elements. The crustal enrichment factors showed that cadmium, nickel, and zinc were obtained primarily from anthropogenic activities and that iron, manganese, potassium, calcium, and magnesium may have originated from terrestrial sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent centuries, researchers have paid special attention to rainwater composition studies since knowledge of rainwater chemical composition provides data on rainwater chemistry sources and local and regional dispersion of contaminants (Budhavant et al. 2011; Kumar et al. 2002; Momin et al. 2005; Rao et al. 2016; Saxena et al. 1996; Szép et al. 2019). Rainwater’s chemical composition varies from place to place, from shower to shower, and even from season to season at the same location. This has drawn special attention to precipitation chemistry around the world (Huang et al. 2010; Willey et al. 2006). Owing to urbanization and industrialization in the world, emissions of sulfur, nitrogen oxides, and trace elements have recently increased, thereby disrupting the normal acid–base balance of the atmosphere. As a result, acid rain and atmospheric deposition of trace elements pollute natural resources such as soil and surface water.
Trace elements can easily adsorb on mineral surfaces, and thus, they can be dispersed over large distances (Başak and Alagha 2004; Gao et al. 2016). The trace elements can be transported for long distances by attaching to aerosol and then be inputted into the ecosystem as a function of meteorological change by dry and wet deposition (Gao et al. 2016). Besides, the use of water by animals and plants contributes to the accumulation of trace elements in the food chain and, thus, acid rain containing a high concentration of trace elements presents a significant threat to natural resources and human health. The sources of sulfate (SO42−) ions in rainwater are the combustion of fuels containing sulfur, shallow-water marine environments, fresh-water lakes, salt flats, and ocean waters, while the source of nitrogen oxides is the combustion of fossil fuels. Various ranges of SO42− concentration have been reported by researchers. Nadzir et al. (2017) reported the concentrations of SO42− between 0.51 and 4.23 mg L−1 in Bangi of Malaysia. Although considerable increases in human-made acid oxide have been recorded in some areas, neutralizing compounds such as calcium carbonate and ammonia neutralize the acid rain (Norman et al. 2001; Tang et al. 2005). For example, in the arid and semi-arid regions, soil particles, as an important atmospheric constituent with a high concentration of alkaline salts, are adsorbed by rainwater. So it appears that soil properties influence the chemical composition and pH of precipitation (Niu et al. 2014).
Xu et al. (2015) studied the chemical composition of Beijing rainwater samples in northern China and Chizhou in southern China. They reported that the high concentrations of alkaline ions play an important role in the neutralization of rainwater acidity in Beijing. Wu et al. (2016) studied the effect of terrestrial sources on the chemical composition of rainwater in China. They found that more than 60% of the samples had a pH value higher than 6.0, and calcium (Ca2+) was the most dominant neutralization substance. Calvo et al. (2010) analyzed rainwater samples in Viznar, Spain. They reported that Ca2+, SO42−, and chloride (Cl−) were dominant ions. Kumar et al. (2002) conceived a long-term study to explore rainwater’s chemical composition and the impact of dust on acid rain neutralization. Santos et al. (2011) compared the composition of rainwater in 2008–2009 with those obtained at the same site between 1986 and 1989. They reported an increase in nitrate (NO3−) due to industrialization.
In developing countries, there are many drawbacks to assess rainwater quality, including data quality, accuracy, and accessibility. There is insufficient regional information on air quality and small stations in rural sites. On the other hand, air quality is impacted by building works such as site planning, foundation works, road construction, and maintenance in the evaluation of urban pollution impacts in so-called rural sites. Although some researchers reported useful information on the chemical composition of rainwater in the world, the chemical composition of rainwater in Iran, a vast country with different climates, is rarely studied and is not available in the west of Iran. Thus, in this paper, for the first time, the chemical composition of rainwater was determined at three different areas in Hamedan province, west of Iran. This study aims to: (1) investigate the chemical composition of major and trace elements (2) identify possible sources that contribute to its chemical composition, and (3) compare rainwater composition between urban and rural areas.
Material and methods
Site description
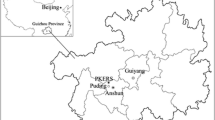
Three sampling sites (Hamedan, Nematabad, and Kushkabad) were selected. These sites are located in three different areas in the Hamedan province in Iran (Fig. 1). Nematabad dam is located about 12 km northwest of Asadabad city in Hamedan province. It has an area of 161 km2 and 500 inhabitants. The annual average temperature and precipitation are 7.9 °C and 457 mm, respectively. Kushkabad is located in Bahar County, Hamadan Province, Iran. It has an area of 4500 km2 and 1620 inhabitants. The annual average temperature and precipitation are, respectively, 11.5 °C and 320 mm. Hamedan has an area of 19,368 km2 and 554,406 inhabitants. The average annual temperature and precipitation are, respectively, 3 °C and 339 mm. These regions have cold winter and dry summer. Precipitation occurs during the spring, autumn, and winter seasons. Snow is the winter’s form of precipitation. Nematabad and Kushkabad regions are appropriate for farming operations, while industrialization has been raised beside the use of private vehicles in Hamedan, and fossil fuels are dominant sources of energy in these areas. Rainwater samples were collected manually using polyethylene which was cleaned with hydrochloric acid then prewashed with distilled water, from January to April 2014. The electrical conductivity (EC) and pH of rainwater samples were measured immediately (after 10 min) after collection with the help of a pH meter (WTW-2029 pH meter) and conductivity meter (Milwaukee MW302), respectively. Samples were divided into two subsamples. One of them was used for acid-bearing ions determination, and another was acidified and sorted into the refrigerator for major ions and trace elements. Major anions including SO42− and NO3− were determined by spectrophotometry method using a Jenway 6105 spectrophotometer. Phosphorous (P) was analyzed by the (Murphy and Riley 1962) method. The titration technique was employed for measuring bicarbonate (HCO3−), Cl−, Ca2+, and magnesium (Mg2+). Sodium (Na+) and potassium (K+) were determined by the flame photometry method. Additionally, cadmium (Cd), iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn) were measured in rainwater samples using atomic absorption spectrophotometry. The blank sample from the laboratory was analyzed along with the rainwater samples. For the blank sample, double-distilled deionized water was used.
Data quality
A total number of 23 events occurred at three sites during the study period. The following equation was used to calculate the volume-weighted mean (VWM) concentrations of measured chemical species in rainwater samples.
where Pi is the amount of rainwater (mm) for each occurrence relating to the ith sample, Xi is the species concentration (meq L−1), and n is the total number of events of rainfall (Sequeira and Lai 1998).
The ionic balance was used to assign the data quality. The equivalent ratio of total anions to that of cations in an individual sample was considered to be an indication of the validity of the key measured ions, and the percent error should be within ± 5% (Gobre et al. 2010). The results of the rainwater sample percentage difference values are shown in Table S1 (supplementary information).
Acid neutralization capacity
The acid neutralization capacity is evaluated by neutralization factors (NF) (Kulshrestha et al. 1996) and calculated by the following equation:
where X is the concentration of alkaline cations, all concentrations are expressed in meq L−1. In hydrogeochemical studies, numerous diagrams have been used to explain water chemistry, including the Piper diagram, Gibbs diagrams, USSL diagram, Wilcox diagram, and PI classification diagram. In this research, the piper diagram is used based on dominant ions to categorize water chemistry. In the Piper diagram, the groundwater samples are plotted according to the milliequivalent percentages in the samples of the eight typical major ions (He and Li 2020; Piper 1944). Several Piper diagram drawing applications are available, including AquChem, Origin, Grapher, etc. We used the Piper plot-QW. XLS developed by the US Geological Survey to draw the piper diagram.
Enrichment factor (EF) with marine and crustal contributions
The relative contribution of seawater source can be determined by calculating seawater enrichment factors (EFseawater), which are determined by the chemical composition of rainwater (Cao et al. 2009). Among major ions, Na+ is chosen as the reference element because its origin is from seawater. This parameter was assessed by comparing the ratio of significant ions to Na+ cation in rainwater with the proportion of significant ions to Na+ in seawater (Keene et al. 1986):
X is the interest ion concentration. Relative to the reference source, an EFseawater value much higher than 1 or much less than 1 is known to be enriched or diluted (Wu et al. 2016). Sea salt fraction (SSF) and non-sea water fractions (NSSF) are calculated to determine the rainwater chemistry percentage of the sea and non-sea sources:
where X is the interest component.
Crustal enrichment factors (EFcrust), the ratio of the element to reference element in crustal material, were calculated to evaluate the contribution of the lithosphere on rainwater chemistry using the following equation. Among components, aluminum (Al), Fe, and Ca2+ in the earth's crust are abundant (Mason 1966). Any of these components can, therefore, be used as a reference item. Calcium was regarded as a reference component in this research:
where X is the component of interest, (X/Ca)rain is the ratio of the concentration of an element X and Ca2+ in the rainwater sample, and (X/Ca)crust is the ratio of the same element and Ca2+ from the crustal composition. The EFc’s were calculated by using average concentrations from crustal elements from Mason (1966) and the VWM concentrations of measured elements in rainwater samples.
An EFcrust < 1 indicates that an element in rainwater has a substantial crustal origin, and EFcrust greater than 1 in rainwater samples represents that a substantial percentage of an element has an anthropogenic origin and demonstrates that components are enriched (Chester et al. 1999). An EFcrust value close to unity shows the crustal is only sources (Igbinosa and Aighewi 2017).
Correlation and cluster analysis
The correlation analysis is a valuable way to find out the origin of ions in the rainwater samples. In this study, using the SPSS software, Pearson correlation analysis was performed. Cluster analysis was used to interpret results and to find the similarities between different measured parameters (Li et al. 2019; Wu et al. 2020) using Minitab software. Single linkage and correlation coefficient distances were used. All mathematical and statistical calculations were carried out using SPSS software.
Backward trajectory
One-day (24 h) back trajectories of each rainfall event were computed using the web HYSPLIT model (https:/ready.arl.noaa.gov/HYSPLITtraj.php) to explore the possible effect of air mass transport on rainwater chemistry and to classify the potential source regions of elements and their transport pathways in the study locations. In this work, the trajectories were determined using the meteorological data of the GDAS (Global Data Assimilation System) with a one-degree archive conducted on the READY web interface by the National Center for Environmental Prediction (NCEP). All the trajectories are started from 500 m above ground level (AGL) over the sampling site.
Results and discussion
pH and electrical conductivity of rainwater
Table 1 presents the descriptive statistical compositions of the rainwater samples. The pH values for individual precipitation occurrences ranged from 5.30 to 7.50 for Hamedan with a volume-weighted mean (VWM) of 6.38, from 6.70 to 7.60 with a VWM of 7.17 for Kushkabad, and from 6.50 to 7.60 with a VWM of 7.01 for Nematabad, respectively (Table 1). Hamedan is urban, while Kushkabad and Nematabad are rural sites. Rainwater verified with a pH equal to 5.6 is called normal pH because carbon dioxide (CO2) reacts with water and a weak carbonic acid is formed (Charlson and Rodhe 1982). Therefore, rainwater with a pH of less than 5.6 is known to be acid rain, and rainfall with a pH of more than 5.6 indicates that an alkaline material is present. Demirak et al. (2006) recorded a pH range of 4.5–7.7 with an average of 6.9, which was in the alkaline range. pH values in a coastal site in India ranged from 4.8 to 6.4 (Prathibha et al. 2010), which were less than the pH value in this study. The average pH value was 6 ± 0.57 in Malaysia (Tay et al. 2014). In rainwater samples in Shenzhen, South China, a varying pH range (3.72–6.77) was observed (Zhou et al. 2019). The pH value of the rainfall relies on the neutralization with an alkaline substance of acid-forming ions (Wang and Han 2011). The current study's pH VWM was contrasted with other researches worldwide (Table 2). As can be seen in this research, the pH VWM is greater than other reported values in Table 2 (except Monterrey, Mexico, which is greater than the Hamedan site). Rainwater obtains its compositions primarily through the dissolution of particulate matter in the atmosphere and, secondarily, through the dissolution of atmospheric gases (Jain et al. 2019). Lower industrial activities and higher loading of alkaline earth cations in our studied areas resulted in a higher pH value in rainfall than in other studies. Soil particles, which are rich in alkaline salts, are a major atmospheric constituent in arid and semi-arid regions. The reaction between these alkaline salts and rainwater determines the final pH of rainwater, resulting in neutralizing acid rain and alkaline rainfall in this study. The VWM of EC was 87.2, 137.1, and 105.0 µS cm−1 for Hamedan, Kushkabad, and Nematabad, respectively (Table 1). The lower EC in Hamedan indicates the dilution effect, which can be due to the relatively abundant rainfall in Hamedan in these months. In Western Poland, the mean EC of rainwater was greater (> 1000 μS cm−1) (Walna 2015) than that recorded in this study. Research conducted in Pakistan's Karachi city found that rainwater EC ranged from 20.0 to 210.0 μS cm−1 (Chughtai et al. 2014). In Ya’an, southwestern China, the EC of rainwater collected at a semi-rural site ranged from 30.0 to 274.0 μS cm−1, with a VWM value of 79.6 μS cm−1 (Zhao et al. 2013). The EC of rainwater in southwestern Nigeria fluctuated between 19.4 and 122.6 μS cm−1 (Adeniyi and Olabanji 2005). Different EC ranges were obtained in different areas due to variations in climate, geographical location, and special rainfall distribution.
Major and trace element composition of rainwater
The average value along with the VWM of the main concentrations of ions in rainwater is shown in Table 1. Based on VWM values, the concentrations of anions in rainwater in three stations had following trend SO42− > C1− > HCO3− > NO3− > P for Hamedan and C1− > SO42− > HCO3− > NO3− > P for Kushkabad and Nematabad stations. The concentration of major cations decreases in the order Ca2+ > Mg2+ > Na+ > K+ > H+ for Kushkabad and Nematabad stations and Mg2+ > Ca2+ > Na+ > K+ > H+ for Hamedan station. In Maharashtra, India, the ionic concentration of rainwater samples showed the order of SO42− > C1− > NO3− for anions and Ca2+ > Na+ > Mg2+ > K+ for cations (Salve et al. 2008). Calcium and SO42− ions were the most abundant cations and anions, respectively, in southwest China (Wang and Han 2011). Momin et al. (2005) reported that the dominant cations in rainwater samples were Ca2+ and Mg2+, and the major anions were SO42− and C1−. Our results are similar to those reported in some areas (Keresztesi et al. 2020; Xu et al. 2015).
The high Ca2+ concentration in rainwater events reflects the presence of calcium-rich dust in the atmosphere. Soil dust contains calcite minerals that are mainly due to the presence of calcium carbonate in soils in these regions. Therefore, the high concentration of SO42− in rainwater is neutralized by aerosols produced from soil (Keresztesi et al. 2020; Liu and Han 2020). The predominant cations were Ca2+ and Mg2+ which accounted for (sum of both cations and based on VMW) 82.5%, 90.4%, and 88.1% of total cations for Hamedan, Kushkabad, and Nematabad stations, respectively. Keresztesi et al. (2020) reported that Ca2+, SO42−, and NH4+ concentrations were notable in desert areas or regions with significant anthropogenic activity. In the same way, the contribution of SO42− and NO3− was 39.8%, 33.8%, and 34.1% of the total anions for Hamedan, Kushkabad, and Nematabad stations, respectively. Figure 2 shows the average percentage distribution (overall rainwater occurrences and stations) of ionic compositions in rainwater. This Figure indicates that ionic species in rainfall had following order Ca2+ > SO42− > Mg2+ > C1− = HCO3− > Na+ > K+ > NO3− > P. The contribution of the concentration of P and H+ in rainwater was less than 1%. Although the VWM pH of the current study was generally contrasted with other research worldwide (Table 2), the comparable pH value was stated in some studies (Demirak et al. 2006; Nadzir et al. 2017). Comparison of our results with other studies has shown that alkaline earth cations such as Ca2+ and Mg2+ have an important effect on acid anion neutralization such as SO42− and NO3−. Besides, in semi-arid and arid areas, these cations dominate, leading to a rise in rainwater pH.
Table 2 shows that the levels of Ca2+, Mg2+, K+, Na+, SO42−, and C1− ions in this study were higher than those reported by other researchers around the world, while decreased levels of NO3− were observed compared to those reported in the literature. The concentration of NO3− in this study ranged from 19 to 30 μeq L−1 which was lower than that in Ahvaz (Iran); Beijing (China), Tirupati (India), Ankara (Turkey), and Mexico reported by (Naimabadi et al. 2016), Tang et al. (2005), (Mouli et al. 2005), (Kaya and Tuncel 1997), respectively, while it was higher than that in Lijiang (China), Juiz deFora (Brazil), Bermuda, Amsterdam, and Pacific ocean reported by (Niu et al. 2014), (Facchini Cerqueira et al. 2014), (Galloway et al. 1989), and (Galloway and Gaudry 1984), respectively.
The order of trace element concentrations in rainwater was Zn > Ni > Fe > Cd > Mn. Oliveira et al. (2012) stated that the VWM concentration of trace elements declined as follows: Zn > Fe > Mn > Ni. The mean concentration of the trace elements in the rainwater was shown to obey the order of Pb > Fe > Zn > chromium (Cr) > Cd = Al (Akoto et al. 2011). Despite our results, Gana’s rainwater samples had a higher Fe concentration than Zn. Different results were observed in rainwater samples collected in Shiraz, southwest of Iran (Mirzaei et al. 2018). Local sources have an important role in trace element concentrations in rainwater (Mirzaei et al. 2018; Tripathee et al. 2014). The most prevalent trace element in the bulk deposition at the three sites was Zn. A similar outcome has been observed in some studies (Avila and Rodrigo 2004; Gao et al. 2016). The construction of the Nematabad Dam began in 2004. Owing to funding limitations, it was postponed and suspended multiple times. In every civil engineering project, it is obvious that the use of machinery is an unavoidable part of construction work. During building work, site planning, foundation work, road construction, and repair, air pollution occurs. Dam building activities and machine traffic are possibly one of the factors for trace element enrichment in rainwater in the Nematabad dam. The high concentration of trace elements in the Hamedan station has also been attributed to the drastic rise in the number of vehicles in the city of Hamedan in recent decades. Dust emissions are the primary explanation for trace element enrichment in all three stations. In the last decade, the west and southwest of Iran were affected by a dust storm that originated from Syria and northern Saudi Arabia (Cao et al. 2015). Dust storms originating in Iraq have been recorded to have high concentrations of airborne trace elements, such as Pb and Cd (Kadhum 2020; Leili et al. 2008). Table 3 shows that the levels of trace elements in this study were higher than those reported by other researchers around the world, except for Fe concentration and Ni and Zn concentrations that were reported by Uygur et al. (2010) and by Başak and Alagha (2010), respectively, in Istanbul, Turkey.
The rainwater sources of trace elements are primarily from combustion factories, non-ferrous metallurgy, iron and steel sectors, waste incineration plants, car traffic, cement or glass manufacturing sectors, ores for metal refining, and waste material burning (Göbel et al. 2007). Trace elements are adsorbed by minerals, and wind erosion can transport them over continents (Gallon et al. 2011). Zinc, Pb, Cu, Ni, and Cr oxides are released by tire wear and brake shoes, while the origin of Fe is due to the worn brake (McKenzie et al. 2009). Researchers reported that among trace elements, Zn was abundant in the precipitation (Al-Momani et al. 1995; Avila and Rodrigo 2004). It is clear that the order of trace element concentrations was affected by sampling sites and a similar trend was reported around the world. Cadmium had the lowest concentration, while Zn had the highest concentration in the rainwater. The elevated atmospheric concentration of Zn is due to a rise in the number of vehicles. Zinc is used in car breaks, and it is released into the air from wearing breaks (Valiulis et al. 2002). Similar high Zn concentrations were observed in highly polluted Mexican cities like Zacatecas and Mexico City (Mugica-Álvarez et al. 2012). Trace elements concentration in rainwater reflects the main type of land uses in watersheds (Herngren et al. 2005).
Neutralization factor in three stations
The NF for Ca2+ ranged from 0.47 to 1.38 in Hamedan, with an average of 0.88, and changed from 0.70 to 3.07 with an average of 2.0 for Kushkabad, and this value fluctuated between 0.70 and 1.89 with an average of 1.11 for Nematabad (Table 4). The mean NF for Mg2+ in precipitation events was 0.96, 0.80, and 0.94 for Hamedan, Kushkabad, and Nematabad, respectively. The NF for Na+ for Hamedan, Kushkabad, and Nematabad was 0.32, 0.25, and 0.17, respectively. The NF for K+ was negligible (Table 4), showing that the dominant ions for neutralization were Ca2+ and Mg2+ (Fig. 3). The elevated concentration of alkaline earth metals in rainwater is associated with soil dust transport with a big proportion of calcite and dolomite due to the calcareous soil in these areas. Salve et al. (2008) found that NF for different cations followed a sequence of Ca2+ > Mg2+ > NH4+ with factors of 1.1, 0.38, and 0.15, respectively. The primary neutralizing agent of rainwater in Romania was Ca2+ (Szép et al. 2019). Similar findings have also been reported in other studies (Xu et al. 2015; Zhang et al. 2012). Although industrial activities cause to release of acid-forming ions into the atmosphere, alkaline substances neutralize the impact of acid-forming ions (Keresztesi et al. 2020; Saxena et al. 1996; Wu et al. 2016). Some researchers reported that NH3 was the dominant compound to neutralize acid rain. The dominant source of NH3 in rainwater is fertilizer applications and livestock farming. The concentration of NH3 was not measured since most of the samples were collected in winter, and NH3 did not play a role in neutralizing, as agricultural activities such as fertilization are negligible in the winter season. The release of NH3 into the atmosphere decreases during the wet season (Başak and Alagha 2004). During the dry season, NH3 volatility increases due to the high temperature and farming. Therefore, according to the above findings, the Ca2+ and Mg2+ ions tend to be the key sources of neutralization, consistent with the results found by Budhavant et al. (2011).
Correlation and cluster analysis
The results of the correlation between measured parameters in rainwater samples are presented in Table 5. There was a significant relation between pH and HCO3− ion, while there was no correlation between pH and acid-bearing anions (SO42−, NO3−). Calcium and K+ ions have a positive correlation with SO42−. The positive correlation between Ca2+ and K+ cations and SO42− indicates that Ca2+ and K+ ions are neutralized in rainwater with SO42− ions and regulate rainwater chemistry (Mouli et al. 2005; Saxena et al. 1996). The existence of dust particles presents in atmosphere derived from gypsum or other evaporite/halite may explain the positive association between Ca2+ and SO42−. In previous studies, a positive association was found between SO42− and Ca2+ (Wang and Han 2011). The atmospheric SO42− originated from natural sources including local weathering, volcanic source, sea salt, and atmosphere deposition (Bao et al. 2004) and anthropogenic activities due to the combustion of fossil fuels and the smelting of metals. Sulfuric acid droplets and other secondary aerosols are formed when SO2 is oxidized in the gas phase (Jain et al. 2019). Although in the present study the dust particles were not analyzed, in some studies the presence of gypsum as aerosol particles was reported (Miyamoto et al. 2020; Rea et al. 2020; Trochkine et al. 2003). Trochkine et al. (2003) studied mineral aerosol particles collected in Dunhuang, China, and indicated that 13–41% of the aerosol particles were Ca-rich particles composed of calcite, dolomite, and gypsum. Since studied areas are located in arid and semi-arid regions, the presence of gypsum particles in aerosol particulates is expected. There has been a poor correlation between Ca2+ and Mg2+ ions, suggesting that crustal sources do not necessarily provide Mg2+ and the sources of these two ions may be different. Moreda-Piñeiro et al. (2014) found a similar very low correlation in rainwater on Spain’s northwest coast. They proposed that the origins of Ca2+ and Mg2+ ions could be different and that Ca2+ originated from terrestrial sources, whereas Mg2+ originated from marine sources. The findings are inconsistent with those of previous results (Kumar et al. 2002; Wang and Han 2011; Zhou et al. 2019). Wang and Han (2011) indicated that the sources of Ca2+ and Mg2+ in the southwest of China are mainly due to the soil dust, while Zeng et al. (2020) indicated that the main sources of Ca2+ and Mg2+ in rainwater in southwest China are calcite dissolution. There was a high positive correlation between rainwater levels of Na+ and Cl−. A similar positive correlation was reported in different studies (Başak and Alagha 2004). The extremely high association stated by (Keresztesi et al. 2020) between Na+ and Cl− since they assume these ions derive primarily from sea spray and marine salts. A significant correlation between Ca2+ and Cl− shows that Cl− was enriched in rainwater samples by NSSF sources. Das et al. (2005) found that in rainwater samples, crustal and anthropogenic sources had an important role in Cl− concentration. Additionally, a positive correlation is found between HCO3− and Ca2+ and Mg2+ cations. Our results were similar to those reported by (Bisht et al. 2017). Phosphate ion showed a positive relation with SO42−, HCO3− and the sum of Ca2+ and Mg2+ cations, indicating that crustal and anthropogenic sources (Das et al. 2005). A positive correlation found between Mn and K+ suggesting the terrestrial source of Mn. A significant relation was observed between Mn and SO42−, implying that Mn-like major cations play an important role in neutralizing rainwaters. The positive correlation between Zn and Ni suggests that under certain physical conditions, such as wind speed, air temperature, and rainfall quantity, they may have similar behavior. Similar results were reported by Báez et al. (2007); Başak and Alagha (2004) and Başak and Alagha (2010). The adverse relationship between Zn and Mg2+ indicates that they came from various sources.
The dendrogram was used to examine the relationships between different measured parameters using water samples (Fig. 4). The first cluster contained pairs of EC-Ca2+, K+-P, and pH, HCO3−, SO42−, Mn, and Mg2+ variables, indicating contribution primarily from natural sources. The second cluster contained a pair of Na+-Cl− variables, indicating an association with sea aerosols ad anthropogenic sources. The third, fourth, fifth, and sixth clusters contained NO3−, Cd, Fe, and a pair of Ni-Zn variables, respectively, indicating anthropogenic sources, but from different sources. Similarly, Zeng et al. (2020) used factor analysis to explore the alkaline rain pattern in southwest China, and found three factors: factor 1 included Ca2+, Mg2+, K+, SO42−, and Cl−, which was related to natural dust, factor 2 included Na+, which was related to the marine, and factor 3 included NO3−, NH4+, and F−, which were related to anthropogenic sources.
Enrichment factor, sea salt fraction, and non-sea water fractions
The primary sources of atmospheric aerosols are sea salts, terrestrial wind erosion dust, and anthropogenic emissions (Guerrot and Millot 2007). The results of the EFseawater are shown in Table 6. Ions with EFseawater higher than 1 reflect the impact of other sources than sea salt. According to Table 6, the EFseawater values for NO3−, SO42, Ca2+, Mg2+, and K+ were higher than 1 in all three stations, indicating a major earthly impact, whereas Cl− had a low EFseawater value, indicating a significant contribution from marine water. The EFseawater of Mg2+, Ca2+, and SO42− was greater than one in a study conducted by (Salve et al. 2008), indicating that they are predominantly affected by soil and anthropogenic sources. In polluted areas, including industrial belts, Cl− can derive from anthropogenic sources in addition to marine sources (Keresztesi et al. (2020). The anthropogenic contribution of Cl− could come from different sources of pollutions, including car exhaust, coal combustion, and fertilizers (Wu et al. 2016). Similar results were observed in previous studies (Bisht et al. 2017; Kumar et al. 2002; Wu et al. 2016).
The SSF and NSSF fractions are presented in Table 7. As can be seen, all element ratios were much higher than those ratios in seawater values (Das et al. 2005). The increase in these ratios showed a close contribution due to evaporites, crustal materials (soil dust), and anthropogenic sources to rainwater chemistry (Zhang et al. 2012). Kumar et al. (2002) stated that the anthropogenic and crustal nature of major components such as K+, Ca2+, SO42, and NO3− are can be shown by SSF and NSSF parameters. Wu et al. (2016) at three non-urban sites in Hebei Province, north China analyzed the chemical compositions of rainwater and found that all major ions derived from non-sea sources. Table 7 showed that SO42− and NO3− ions are brought through anthropogenic activities. The contribution of these anions to the NSSF is more than 95% (Facchini Cerqueira et al. 2014; Jawad Al Obaidy and Joshi 2006). The contribution of the soil source was considerable for Ca2+ and Mg2+ ions (Table 7) (Saxena et al. 1996; Xu et al. 2015). Based on data obtaining by SSF fraction, Cl− ion in precipitation has seawater and anthropogenic sources so that approximately 40.60%, 28.36%, and 18.56% of Cl− originated from the sea in Hamedan, Kushkabad, and Nematabad, respectively. Our findings were consistent with those results that were presented by (Zhang et al. 2012). Although sea salt, anthropogenic, and soil resources had a significant role in K+ in all samples, anthropogenic activities were the dominant source.
Table 8 indicates that Cd, Zn, and Ni in rainwater have EFcrust values higher than 1, while rainwater was not enriched with Fe and Mn. Cadmium had the largest EFcrust value in all stations among trace elements. Mirzaei et al. (2018) found that samples were not enriched with Fe and Mn, but that Zn, Cu, and Ni were fair to highly enriched. Malassa et al. (2014) reported that rainwater used for drinking was polluted with Ni. The EFcrust of Cd, Zn, and Ni was the highest in Hamedan station due to the excessive activities of humans on this site. The EFc’s of trace elements in stations follow the order: Cd > > Ni > Zn > > Mn > Fe. The higher Zn concentration at Hamedan station is due to the contribution of the traffic. The role of traffic congestion on Zn concentration in rainwater samples was reported by (Avila and Rodrigo 2004; Morselli et al. 2003). Besides, high levels of these trace elements can be attributed to storms of dust in the Middle East that have increased dramatically in recent years due to the dry wetlands in Iraq and the western neighbor of Iran (Cao et al. 2015; Pirsaheb et al. 2014). Başak and Alagha (2004) found that the trace element EFc's in rainwater in Büyükçekmece had following the order: Cd > Cu > Pb ∼ Zn > > Ni > Cr > V. The higher EFcrust for Zn in this study compared to other studies may be related to the impact of local sources of Zn. Churc et al. (1984) showed that the EFc’s were more than 1 (except for Mn). The EFc’s value for trace elements in Bermuda was in the following order Cd > Pb > Zn > Cu > Ni > V. Together with distinct wind and precipitation patterns, these differences can be due to variations in industrial operations.
Backward air mass trajectory analysis
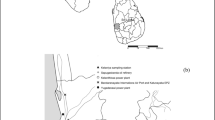
Figure 5 demonstrates the outcomes of the trajectory model. The model showed that two major sources of contaminants affected the quality of rainwater at three stations. The main source was the dust storm of Iraq, Syria, and northern Saudi Arabia during the recent decays. This phenomenon was dominant in Kushkabad station, as can be seen in Fig. 5. The origin of the air masses at the station of Hamedan was divided mainly into two branches, namely the west and the north. The air mass of the west may have transported significant quantities of contaminants from Iraq. The northern pollutant originates in the Caspian Sea, situated in northern Iran. The sources of air pollution in certain rainwater events emerged in the southwest of Iran. Strong storms of dust have been observed in the southwest of Iran in recent years.
Backward 24 h trajectory plot from three study locations at 500 m above ground level using the Global Data Assimilation System meteorological data achieved by the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model (https://www.ready.noaa.gov/HYSPLIT traj.php) Hamedan station (a); Kushkabad station (b); Nematabad station (c)
Conclusion
In Hamedan, western Iran, the chemical composition of rainwater indicated that the rainwater was usually alkaline, especially in rural sites of Kushkabad and Nematabad. Due to the low content of acid-bearing ions, rainwater events at Hamedan station demonstrated the lowest pH. The low EFseawater of Ca2+ in Hamedan caused less SO42− to be neutralized, whereas the EFseawater of SO42− anions in Hamedan was close to that in Kushkabad station. According to the results found in three stations, calcium carbonate had strong neutralizing effects on acids from fossil fuel combustion. Chloride ion originates mostly from sea salt sources in three stations. Cadmium, Ni, and Zn in rainwater were enriched, and Cd had the highest EFcrust. High concentrations of these trace elements can be ascribed to storms of dust in the Middle East. It is suggested that the anthropogenic activities at Hamedan station were more due to the greater number of cars and industrialization than at other stations. The results generally suggest that terrestrial and anthropogenic sources have been important sources of cations and have had a major influence on rainwater chemistry.
References
Adeniyi IF, Olabanji IO (2005) The physico-chemical and bacteriological quality of rainwater collected over different roofing materials in Ile-Ife, southwestern Nigeria. Chem Ecol 21:149–166. https://doi.org/10.1080/02757540500117318
Akoto O, Darko G, Nkansah MA (2011) Chemical composition of rainwater over a mining area in Ghana. Int J Environ Res 5:847–854. https://doi.org/10.22059/ijer.2011.442
Al-Khashman OA (2005) Study of chemical composition in wet atmospheric precipitation in Eshidiya area, Jordan. Atmos Environ 39:6175–6183
Al-Khashman OA (2009) Chemical characteristics of rainwater collected at a western site of Jordan. Atmos Res 91:53–61
Al-Momani IF, Tuncel S, Eler Ü, Örtel E, Sirin G, Tuncel G (1995) Major ion composition of wet and dry deposition in the eastern Mediterranean basin. Sci Total Environ 164:75–85. https://doi.org/10.1016/0048-9697(95)04468-G
Avila A, Alarcón M (1999) Relationship between precipitation chemistry and meteorological situations at a rural site in NE Spain. Atmos Environ 33:1663–1677
Avila A, Rodrigo A (2004) Trace metal fluxes in bulk deposition, throughfall and stemflow at two evergreen oak stands in NE Spain subject to different exposure to the industrial environment. Atmos Environ 38:171–180. https://doi.org/10.1016/j.atmosenv.2003.09.067
Báez A, Belmont R, García R, Padilla H, Torres MC (2007) Chemical composition of rainwater collected at a southwest site of Mexico City, Mexico. Atmos Res 86:61–75. https://doi.org/10.1016/j.atmosres.2007.03.005
Balls P (1989) Trace metal and major ion composition of precipitation at a North Sea coastal site. Atmos Environ 23:2751–2759
Bao H, Jenkins KA, Khachaturyan M, Dı́az GC, (2004) Different sulfate sources and their post-depositional migration in Atacama soils. Earth Planet Sci Lett 224:577–587. https://doi.org/10.1016/j.epsl.2004.05.006
Başak B, Alagha O (2004) The chemical composition of rainwater over Büyükçekmece Lake, Istanbul. Atmos Res 71:275–288. https://doi.org/10.1016/j.atmosres.2004.07.001
Başak B, Alagha O (2010) Trace metals solubility in rainwater: evaluation of rainwater quality at a watershed area, Istanbul. Environ Monit Assess 167:493–503. https://doi.org/10.1007/s10661-009-1066-7
Bisht DS, Srivastava AK, Joshi H, Ram K, Singh N, Naja M, Srivastava MK, Tiwari S (2017) Chemical characterization of rainwater at a high-altitude site “Nainital” in the central Himalayas, India. Environ Sci Pollut Res 24:3959–3969. https://doi.org/10.1007/s11356-016-8093-z
Budhavant KB, Rao PSP, Safai PD, Ali K (2011) Influence of local sources on rainwater chemistry over Pune region, India. Atmos Res 100:121–131. https://doi.org/10.1016/j.atmosres.2011.01.004
Calvo AI, Olmo FJ, Lyamani H, Alados-Arboledas L, Castro A, Fernández-Raga M, Fraile R (2010) Chemical composition of wet precipitation at the background EMEP station in Víznar (Granada, Spain) (2002–2006). Atmos Res 96:408–420. https://doi.org/10.1016/j.atmosres.2010.01.013
Cao Y-Z, Wang S, Zhang G, Luo J, Lu S (2009) Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos Res 94:462–469. https://doi.org/10.1016/j.atmosres.2009.07.004
Cao H, Liu J, Wang G, Yang G, Luo L (2015) Identification of sand and dust storm source areas in Iran. J Arid Land 7:567–578. https://doi.org/10.1007/s40333-015-0127-8
Charlson RJ, Rodhe H (1982) Factors controlling the acidity of natural rainwater. Nature 295:683. https://doi.org/10.1038/295683a0
Chester R, Nimmo M, Preston M (1999) The trace metal chemistry of atmospheric dry deposition samples collected at Cap Ferrat: a coastal site in the Western Mediterranean. Marine Chem 68:15–30
Chughtai M, Mustafa S, Mumtaz M (2014) Study of Physicochemical Parameters of Rainwater: A Case Study of Karachi, Pakistan. Am J Anal Chem 5(4):8. https://doi.org/10.4236/ajac.2014.54029
Churc TM, Tramontano JM, Scudlark JR, Jickells TD, Tokos JJ, Knap AH, Galloway JN (1984) The wet deposition of trace metals to the western atlantic ocean at the mid-atlantic coast and on Bermuda. Atmos Environ 18:2657–2664. https://doi.org/10.1016/0004-6981(84)90330-5
Das R, Das S, Misra V (2005) Chemical composition of rainwater and dustfall at Bhubaneswar in the east coast of India. Atmos Environ 39:5908–5916
Demirak A, Balci A, Karaoğlu H, Tosmur B (2006) Chemical characteristics of rain water at an urban site of South Western Turkey. Environ Monit Assess 123:271–283. https://doi.org/10.1007/s10661-006-9196-7
Facchini Cerqueira MR, Pinto MF, Derossi IN, Esteves WT, Rachid Santos MD, Costa Matos MA, Lowinsohn D, Matos RC (2014) Chemical characteristics of rainwater at a southeastern site of Brazil. Atmos Pollut Res 5:253–261. https://doi.org/10.5094/APR.2014.031
Gallon C, Ranville MA, Conaway CH, Landing WM, Buck CS, Morton PL, Flegal AR (2011) Asian industrial lead inputs to the north pacific evidenced by lead concentrations and isotopic compositions in surface waters and aerosols. Environ Sci Technol 45:9874–9882. https://doi.org/10.1021/es2020428
Galloway J (1967) Gaudry A (1984) The composition of precipitation on Amsterdam island Indian Ocean. Atmos Environ 18:2649–2656
Galloway A, Birkby WH, Jones AM, Henry TE, Parks BO (1989) Decay rates of human remains in an arid environment. J Forensic Sci 34:607–616
Gao Y, Hao Z, Yang T, He N, Tian J, Wen X (2016) Wash effect of atmospheric trace metals wet deposition and its source characteristic in subtropical watershed in China. Environ Sci Pollut Res Int 23:20388–20401. https://doi.org/10.1007/s11356-016-7254-4
Garnaud S, Mouchel J-M, Chebbo G, Thévenot DR (1999) Heavy metal concentrations in dry and wet atmospheric deposits in Paris district: comparison with urban runoff. Sci Total Environ 235:235–245
Göbel P, Dierkes C, Coldewey W (2007) Storm water runoff concentration matrix for urban areas. J Contam Hydrol 91:26–42
Gobre T, Salve P, Krupadam R, Bansiwal A, Shastry S, Wate S (2010) Chemical composition of precipitation in the coastal environment of India. Bull Environ Contam Toxicol 85:48–53
Guerrot C, Millot R (2007) Chemical and strontium isotope characterization of rainwater in France: influence of sources and hydrogeochemical implications AU—Négrel, Philippe. Isot Environ Health Stud 43:179–196. https://doi.org/10.1080/10256010701550773
He S, Li P (2020) A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry 80:125550. https://doi.org/10.1016/j.chemer.2019.125550
Herngren L, Goonetilleke A, Sukpum R, Silva Dd (2005) Rainfall simulation as a tool for urban water quality research. Environ Eng Sci 22:378–383
Hu GP, Balasubramanian R, Wu CD (2003) Chemical characterization of rainwater at Singapore. Chemosphere 51:747–755
Huang K, Zhuang G, Xu C, Wang Y, Tang A (2008) The chemistry of the severe acidic precipitation in Shanghai, China. Atmos Res 89:149–160
Huang X-F, Li X, He L-Y, Feng N, Hu M, Niu Y-W, Zeng L-W (2010) 5-Year study of rainwater chemistry in a coastal mega-city in South China. Atmos Res 97:185–193
Igbinosa IH, Aighewi IT (2017) Assessment of the physicochemical and heavy metal qualities of rooftop harvested rainwater in a rural community. Glob Chall 1:1700011. https://doi.org/10.1002/gch2.201700011
Jain CD, Madhavan BL, Ratnam MV (2019) Source apportionment of rainwater chemical composition to investigate the transport of lower atmospheric pollutants to the UTLS region. Environ Pollut 248:166–174. https://doi.org/10.1016/j.envpol.2019.02.007
Jawad Al Obaidy AHM, Joshi H (2006) Chemical composition of rainwater in a tropical urban area of northern India. Atmos Environ 40:6886–6891. https://doi.org/10.1016/j.atmosenv.2005.01.031
Kadhum SA (2020) A preliminary study of heavy metals pollution in the sandy dust storms and its human risk assessment from middle and south of Iraq. Environ Sci Pollut Res Int 27:8570–8579. https://doi.org/10.1007/s11356-019-07380-4
Kamani H, Hoseini M, Safari GH, Jaafari J, Mahvi AH (2014) Study of trace elements in wet atmospheric precipitation in Tehran, Iran. Environ Monit Assess 186:5059–5067
Kaya G, Tuncel G (1997) Trace element and major ion composition of wet and dry depositon in Ankara, Turkey. Atmos Environ 31:3985–3998. https://doi.org/10.1016/S1352-2310(97)00221-5
Keene WC, Pszenny AA, Galloway JN, Hawley ME (1986) Sea-salt corrections and interpretation of constituent ratios in marine precipitation. J Geophys Res Atmos 91:6647–6658
Keresztesi Á, Nita IA, Boga R, Birsan MV, Bodor Z, Szép R (2020) Spatial and long-term analysis of rainwater chemistry over the conterminous United States. Environ Res 188:109872. https://doi.org/10.1016/j.envres.2020.109872
Khwaja HA, Husain L (1990) Chemical characterization of acid precipitation in Albany, New York. Atmos Environ Part A General Topics 24:1869–1882
Kulshrestha UC, Sarkar AK, Srivastava SS, Parashar DC (1996) Investigation into atmospheric deposition through precipitation studies at New Delhi (India). Atmos Environ 30:4149–4154. https://doi.org/10.1016/1352-2310(96)00034-9
Kumar R, Rani A, Singh SP, Kumari KM, Srivastava SS (2002) A long term study on chemical composition of rainwater at dayalbagh, a suburban site of semiarid region. J Atmos Chem 41:265–279. https://doi.org/10.1023/a:1014955715633
Kyoung Lee B, Hee Hong S, Soo Lee D (2000) Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmos Environ 34:563–575
Leili M, Naddafi K, Nabizadeh R, Yunesian M, Mesdaghinia A (2008) The study of TSP and PM10 concentration and their heavy metal content in central area of Tehran, Iran. Air Qual Atmos Health 1:159–166. https://doi.org/10.1007/s11869-008-0021-z
Li P, Tian R, Liu R (2019) Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo Health 11:81–94. https://doi.org/10.1007/s12403-018-0277-y
Liu J, Han G (2020) Major ions and δ(34)S(SO4) in Jiulongjiang River water: investigating the relationships between natural chemical weathering and human perturbations. Sci Total Environ 724:138208. https://doi.org/10.1016/j.scitotenv.2020.138208
Loye-Pilot MD, Morelli J (1988) Fluctuations of ionic composition of precipitations collected in Corsica related to changes in the origins of incoming aerosols. J Aerosol Sci 19:577–585
Malassa H, Al-Rimawi F, Al-Khatib M, Al-Qutob M (2014) Determination of trace heavy metals in harvested rainwater used for drinking in Hebron (south West Bank, Palestine) by ICP-MS. Environ Monit Assess 186:6985–6992. https://doi.org/10.1007/s10661-014-3904-5
Mason B (1966) Principles of geochemistry, 3rd edn. Wiley, New York, London, p 329
McKenzie ER, Money JE, Green PG, Young TM (2009) Metals associated with stormwater-relevant brake and tire samples. Sci Total Environ 407:5855–5860. https://doi.org/10.1016/j.scitotenv.2009.07.018
Mirzaei S, Hashemi H, Hoseini M (2018) Concentration and potential source identification of trace elements in wet atmospheric precipitation of Shiraz, Iran. J Environ Health Sci Eng 16:229–237. https://doi.org/10.1007/s40201-018-0310-x
Miyamoto C, Sakata K, Yamakawa Y, Takahashi Y (2020) Determination of calcium and sulfate species in aerosols associated with the conversion of its species through reaction processes in the atmosphere and its influence on cloud condensation nuclei activation. Atmos Environ 223:117193. https://doi.org/10.1016/j.atmosenv.2019.117193
Momin GA, Ali K, Rao PSP, Safai PD, Chate DM, Praveen PS, Rodhe H, Granat L (2005) Study of chemical composition of rainwater at an urban (Pune) and a rural (Sinhagad) location in India. J Geophys Res Atmos. https://doi.org/10.1029/2004jd004789
Moreda-Piñeiro J, Alonso-Rodríguez E, Moscoso-Pérez C, Blanco-Heras G, Turnes-Carou I, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D (2014) Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmos Environ 88:30–38. https://doi.org/10.1016/j.atmosenv.2014.01.067
Morselli L, Olivieri P, Brusori B, Passarini F (2003) Soluble and insoluble fractions of heavy metals in wet and dry atmospheric depositions in Bologna, Italy. Environ Pollut 124:457–469. https://doi.org/10.1016/S0269-7491(03)00013-7
Mouli PC, Mohan SV, Reddy SJ (2005) Rainwater chemistry at a regional representative urban site: influence of terrestrial sources on ionic composition. Atmos Environ 39:999–1008
Mugica-Álvarez V, Figueroa-Lara J, Romero-Romo M, Sepúlveda-Sánchez J, López-Moreno T (2012) Concentrations and properties of airborne particles in the Mexico City subway system. Atmos Environ 49:284–293
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nadzir MSM, Lin CY, Khan MF, Latif MT, Dominick D, Hamid HHA, Mohamad N, Maulud KNA, Wahab MIA, Kamaludin NF, Lazim MASM (2017) Characterization of rainwater chemical composition after a Southeast Asia haze event: insight of transboundary pollutant transport during the northeast monsoon. Environ Sci Pollut Res 24:15278–15290. https://doi.org/10.1007/s11356-017-9131-1
Nagamoto C, Parungo F, Kopcewicz B, Zhou MY (1990) Chemical analysis of rain samples collected over the Pacific Ocean. J Geophys Res Atmos 95:22343–22354
Naimabadi A, Ghadiri A, Idani E, Babaei AA, Alavi N, Shirmardi M, Khodadadi A, Marzouni MB, Ankali KA, Rouhizadeh A, Goudarzi G (2016) Chemical composition of PM10 and its in vitro toxicological impacts on lung cells during the Middle Eastern Dust (MED) storms in Ahvaz. Iran Environ Pollut 211:316–324. https://doi.org/10.1016/j.envpol.2016.01.006
Naimabadi A, Shirmardi M, Maleki H, Teymouri P, Goudarzi G, Shahsavani A, Sorooshian A, Babaei AA, Mehrabi N, Baneshi MM, Zarei MR, Lababpour A, Ghozikali MG (2018) On the chemical nature of precipitation in a populated Middle Eastern Region (Ahvaz, Iran) with diverse sources. Ecotoxicol Environ Saf 163:558–566
Niu H, He Y, Lu XX, Shen J, Du J, Zhang T, Pu T, Xin H, Chang L (2014) Chemical composition of rainwater in the Yulong Snow Mountain region, Southwestern China. Atmos Res 144:195–206. https://doi.org/10.1016/j.atmosres.2014.03.010
Norman M, Das S, Pillai A, Granat L, Rodhe H (2001) Influence of air mass trajectories on the chemical composition of precipitation in India. Atmos Environ 35:4223–4235
Oliveira PL, Figueiredo BR, Cardoso AA (2012) Rainwater major and trace element contents in Southeastern Brazil: an assessment of a sugar cane region in dry and wet period. J Braz Chem Soc 23:2258–2265
Özsoy T, Örnektekin S (2009) Trace elements in urban and suburban rainfall, Mersin, Northeastern Mediterranean. Atmos Res 94:203–219
Pirsaheb M, Zinatizadeh A, Khosravi T, Atafar Z, Dezfulinezhad S (2014) Natural airborne dust and heavy metals: a case study for kermanshah, Western Iran (2005–2011). Iran J Public Health 43:460–470
Prathibha P, Kothai P, Saradhi IV, Pandit GG, Puranik VD (2010) Chemical characterization of precipitation at a coastal site in Trombay, Mumbai, India. Environ Monit Assess 168:45–53. https://doi.org/10.1007/s10661-009-1090-7
Ramírez Lara E, Miranda Guardiola R, Gracia Vásquez Y, Balderas Rentería I, Bravo Álvarez H, Sosa Echeverría R, Sánchez Álvarez P, Alarcón Jiménez A, Torres M, Kahl J (2010) Chemical composition of rainwater in northeastern México. Atmósfera 23:213–224
Rao PSP, Tiwari S, Matwale JL, Pervez S, Tunved P, Safai PD, Srivastava AK, Bisht DS, Singh S, Hopke PK (2016) Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos Environ 146:90–99. https://doi.org/10.1016/j.atmosenv.2016.06.069
Rea P, Ma L, Gill TE, Gardea-Torresdey J, Tamez C, Jin L (2020) Tracing gypsiferous White Sands aerosols in the shallow critical zone in the northern Sacramento Mountains, New Mexico using Sr/Ca and 87Sr/86Sr ratios. Geoderma 372:114387. https://doi.org/10.1016/j.geoderma.2020.114387
Roy A, Chatterjee A, Tiwari S, Sarkar C, Das SK, Ghosh SK, Raha S (2016) Precipitation chemistry over urban, rural and high altitude Himalayan stations in eastern India. Atmos Res 181:44–53
Salve P, Maurya A, Wate S, Devotta S (2008) Chemical composition of major ions in rainwater. Bull Environ Contam Toxicol 80:242–246
Santos PSM, Otero M, Santos EBH, Duarte AC (2011) Chemical composition of rainwater at a coastal town on the southwest of Europe: What changes in 20years? Sci Total Environ 409:3548–3553. https://doi.org/10.1016/j.scitotenv.2011.05.031
Saxena A, Kulshrestha UC, Kumar N, Kumari KM, Srivastava SS (1996) Characterization of precipitation at Agra. Atmos Environ 30:3405–3412. https://doi.org/10.1016/1352-2310(96)00049-0
Sequeira R, Lai C (1998) Small-scale spatial variability in the representative ionic composition of rainwater within urban Hong Kong. Atmos Environ 32:133–144
Staelens J, De Schrijver A, Van Avermaet P, Genouw G, Verhoest N (2005) A comparison of bulk and wet-only deposition at two adjacent sites in Melle (Belgium). Atmos Environ 39:7–15
Szép R, Bodor Z, Miklóssy I, Niță I-A, Oprea OA, Keresztesi Á (2019) Influence of peat fires on the rainwater chemistry in intra-mountain basins with specific atmospheric circulations (Eastern Carpathians, Romania). Sci Total Environ 647:275–289. https://doi.org/10.1016/j.scitotenv.2018.07.462
Tanner PA, Wong AYS (2000) Soluble trace metals and major ionic species in the bulk deposition and atmosphere of Hong Kong. Water Air Soil Pollu 122:261–279
Tang A, Zhuang G, Wang Y, Yuan H, Sun Y (2005) The chemistry of precipitation and its relation to aerosol in Beijing. Atmos Environ 39:3397–3406
Tay JH, Jaafar S, Mohd Tahir N (2014) Ionic composition of rainwater at selected sites of Kuantan, Pahang, Malaysia: a preliminary study. Bull Environ Contam Toxicol 92:329–333. https://doi.org/10.1007/s00128-014-1203-z
Tripathee L, Kang S, Huang J, Sharma CM, Sillanpää M, Guo J, Paudyal R (2014) Concentrations of trace elements in wet deposition over the central Himalayas, Nepal. Atmos Environ 95:231–238. https://doi.org/10.1016/j.atmosenv.2014.06.043
Trochkine D, Iwasaka Y, Matsuki A, Yamada M, Kim Y-S, Nagatani T, Zhang D, Shi G-Y, Shen Z (2003) Mineral aerosol particles collected in Dunhuang, China, and their comparison with chemically modified particles collected over Japan. J Geophys Res Atmos. https://doi.org/10.1029/2002JD003268
Uygur N, Karaca F, Alagha O (2010) Prediction of sources of metal pollution in rainwater in Istanbul, Turkey using factor analysis and long-range transport models. Atmos Res 95:55–64
Valiulis D, Čeburnis D, Šakalys J, Kvietkus K (2002) Estimation of atmospheric trace metal emissions in Vilnius City, Lithuania, using vertical concentration gradient and road tunnel measurement data. Atmos Environ 36:6001–6014. https://doi.org/10.1016/S1352-2310(02)00764-1
Walna B (2015) Results of long–term observations of basic physico–chemical data of atmospheric precipitation in a protected area in Western Poland. Atmos Pollut Res 6:651–661. https://doi.org/10.5094/APR.2015.074
Wang H, Han G (2011) Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos Res 99:190–196
Willey JD, Kieber RJ, Avery GB (2006) Changing chemical composition of precipitation in Wilmington, North Carolina, U.S.A.: implications for the continental U.S.A. Environ Sci Technol 40:5675–5680
Wu Y, Xu Z, Liu W, Zhao T, Zhang X, Jiang H, Yu C, Zhou L, Zhou X (2016) Chemical compositions of precipitation at three non-urban sites of Hebei Province, North China: influence of terrestrial sources on ionic composition. Atmos Res 181:115–123. https://doi.org/10.1016/j.atmosres.2016.06.009
Wu J, Li P, Wang D, Ren X, Wei M (2020) Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum Ecol Risk Assess Int J 26:1603–1621. https://doi.org/10.1080/10807039.2019.1594156
Xu Z, Wu Y, Liu W-J, Liang C-S, Ji J, Zhao T, Zhang X (2015) Chemical composition of rainwater and the acid neutralizing effect at Beijing and Chizhou city, China. Atmos Res 164–165:278–285. https://doi.org/10.1016/j.atmosres.2015.05.009
Zeng J, Yue F-J, Li S-L, Wang Z-J, Wu Q, Qin C-Q, Yan Z-L (2020) Determining rainwater chemistry to reveal alkaline rain trend in Southwest China: evidence from a frequent-rainy karst area with extensive agricultural production. Environ Pollut 266:115166. https://doi.org/10.1016/j.envpol.2020.115166
Zhang X, Jiang H, Zhang Q, Zhang X (2012) Chemical characteristics of rainwater in northeast China, a case study of Dalian. Atmos Res 116:151–160. https://doi.org/10.1016/j.atmosres.2012.03.014
Zhao M, Li L, Liu Z, Chen B, Huang J, Cai J, Deng S (2013) Chemical composition and sources of rainwater collected at a Semi-Rural Site in Ya¡¯an, Southwestern China. Atmos Clim Sci 3(4):11. https://doi.org/10.4236/acs.2013.34051
Zhou X, Xu Z, Liu W, Wu Y, Zhao T, Jiang H, Zhang X, Zhang J, Zhou L, Wang Y (2019) Chemical composition of precipitation in Shenzhen, a coastal mega-city in South China: influence of urbanization and anthropogenic activities on acidity and ionic composition. Sci Total Environ 662:218–226. https://doi.org/10.1016/j.scitotenv.2019.01.096
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peikam, E.N., Jalali, M. Chemical composition of rainwater at an urban and two rural stations in the west of Iran, Hamedan. Environ Earth Sci 80, 605 (2021). https://doi.org/10.1007/s12665-021-09865-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09865-3