Abstract
Impact to streams that receive runoffs from the abundant abandoned artisan-scale mines disseminated throughout the middle and upper Sonora river basin in central Sonora state, Northwestern Mexico, was evaluated by determining total concentrations, enrichment factor (EF), geoaccumulation index (Igeo) and geochemical and granulometric fractionations of heavy metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn) in sediment of those streams. Total concentrations of As were also determined. Excluding Fe, the metal found in highest concentrations in all sites was Mn. Findings indicate that sediment enrichment is moderate only for Pb (3.76) and Zn (3.68) in 1 point, and for Cu (3.61) in a different site, out of the 11 points under study. Enrichment is absent or minimal for the remaining elements and sites. Maximum contamination levels as established by Igeo were moderate for Cu (1.14) and lower than moderate for Cu (0.77) and Pb (0.32) in one site each; and absent for all other elements and sites. The granulometric analyses found a close correlation between the percent mass composition of sediment in gravel, sand, clay and silt, and the distributions of metal content in those compartments, with the exception for Cu and, to a lesser extent, Pb. The geochemical fractionation indicates the metal found in the exchangeable fraction in a highest proportion was Mn, while the highest percent in the reducible fraction corresponds to Pb and Mn. At present, sediments of streams in the middle and upper Sonora River basin show a low or null impact on their quality by the sporadic runoffs from abandoned artisan mines they receive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activity has historically been abundant in the middle and upper Sonora River basin, located in the state of Sonora, Northwestern Mexico. As a result, there are a large number of small abandoned mines and tailings whose ephemeral runoffs are captured by short-lived streams that flow into the river. Large-scale mining is also present in the region. Actually, one of the largest active copper extraction operations in the world is located in the City of Cananea, Sonora, near the sources of the Sonora and San Pedro Rivers. Several chemical spills that have adversely affected sediment and water quality in both rivers’ basins have been caused by mining activity in the region (Gٕómez-Álvarez et al. 2007, 2009, 2011). Contamination of the Sonora river waters and sediments, whenever detected, has been solely attributed to the large-scale mining operations located in the Cananea area while a probable contribution from abandoned artisan mines or other sources has been overlooked. The analysis of sediment in streams that receive runoffs from small-scale artisan extraction or processing mining operations in state of abandonment is used in this research to assess a potential contribution of such remnants to the spread of trace metal contamination into the main course of the Sonora River. It is important to mention that 60% of water concessions in the Sonora River basin are dedicated to public urban use, followed by agriculture with a 31% (Pineda et al. 2014).
Leaching processes in abandoned or operating mines and tailings caused acidic runoffs, known as acid mine drainage (AMD). AMD forms through a series of microbially catalyzed oxidation reactions of sulfurous minerals that become exposed to surface weathering conditions by mineral extraction and processing operations (Johnson and Hallberg 2005; Sheoran and Sheoran 2006; Anawar 2013; Simate and Ndlova 2014). AMD can lead to the dissemination of metals and metalloids through surface and groundwater, and to their accumulation in sediments at potentially dangerous levels.
The accumulation of trace metals in sediments by precipitation, coprecipitation and sorption processes (McGregor et al. 1998; Berger et al. 2000) attenuates their mobilization by reducing their levels in the liquid phase. Remobilization of trace metals from sediments, however, can result in water concentration levels potentially toxic to humans and living organisms (Long et al. 1996; Fichet et al. 1998; Cukrov et al. 2008).
In view of this, it is important to evaluate inorganic pollutant concentrations in sediments and to establish the chemical fractions to which they are associated, to determine how susceptible they are to becoming mobilized through redissolution or resuspension processes originated by the natural conditions of the region or anthropogenic factors.
Persistence of metals in the environment allows their concentrations in waterbodies and sediments to become a valuable tool in assessing levels of ecological disturbance and establishing the natural or anthropogenic origin of their presence in temporal and spatial contexts (Leung and Jiao 2006; Ouyang et al. 2006; Cukrov et al. 2008). Nevertheless, the mere quantification of total metal concentrations does not, on its own, offer conclusive indications of the level of contamination existing in superficial layers of soil or sediments. This can be better accomplished by use of parameters such as the enrichment factor (EF) and the geoaccumulation index (Igeo) that take into account the mineralogical characteristics of the native natural context.
Furthermore, it should be emphasized that total concentrations in sediments are also insufficient for estimating the degree of contamination that can be induced in waterbodies by the potential remobilization of metals (Fan et al. 2002). More accurate information on pollutant mobility and availability can be obtained by sequential extraction techniques directed at establishing the distribution of trace elements in the different geochemical fractions of a solid (Lopez and Mandado 2002).
Unfortunately, the lack of a standard technique for conducting partition studies makes the comparison of results complex and difficult. In view of this, a sequential partitioning three-step method known as BCR was designed. The procedure calls for the successive extraction of the metal fraction present in the acid-soluble phase, composed mainly of carbonates; the reducible phase, comprised by Fe and Mn oxides, and the oxidizable phase, which includes organic matter and sulfides (López and Mandado 2002). The sequential extraction method used in this research, described by Rauret et al. (2000), is a modified version of the BCR method that requires an additional final stage for the chemical digestion of the residual material from the three previous extractions.
The objective of this research is to evaluate the potential impact on sediment quality in streams that receive runoffs from small abandoned artisan mines and tailings in the Sonora River basin as a means to determine whether the ubiquitous presence of such vestiges may contribute to the contamination of the Sonora River, located in a semi-arid region of Northwestern Mexico. Metal content distribution in geochemical and granulometric fractions was also established for 5 of the 11 sampling sites under study to obtain information on the likelihood that metals present in sediments become mobilized through dissolution or suspension into the aqueous phase.
Study area
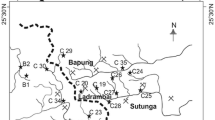
The study was conducted in Mexico, in the northwestern state of Sonora, which by its latitude, is part of an arid strip that encircles the world. The climate in the region is hot semi-arid and watercourses are ephemeral. Sediment samples were collected from streams in the upper and middle Sonora River basin, in the central region of the state (Fig. 1), that potentially receive runoffs from abandoned artisan-scale mining operations located near their banks. Mining has been endemic in this area since the time of the Spanish colonization and today is a fundamental aspect of the state economy.
It is also important to stress that the natural flow of underground waters can potentially lixiviate minerals from unexploited deposits that finally arrive to the watercourses. Actually, the Sonora river is perennial in some sections due to the outcropping of underground water. This implies that the potential of river sediment contamination from natural sources exists. The Mexican Geological Service (SGM 2013) has determined that the primary inputs naturally reaching the riverbed include elements such as barium, cadmium, copper, chromium, iron, manganese, sodium, lead, nickel and antimony. Places particularly vulnerable to this kind of pollution are located in the middle and upper sections of the Sonora River basin.
The Sonora river is the main source of water for human consumption and economical activities in the central valleys of the state of Sonora, where a number of communities, including the state capital with a population greater than 800,000 inhabitants, are located (Gómez-Alvarez et al. 2009).
Materials and methods
Sample collection and analysis
Abandoned mines considered in this project are located at a linear distance of 10 km or less from the Sonora river course. Thirty-three sediment samples were taken from the beds of ten streams. Samples from the most southern stream were taken at two points; one upstream and the other downstream from the abandoned mine “8 Hermanos”. In all other cases, samples were taken downstream from an identified potential source of contamination. Sampling sites are located along 142 km of the Sonora River basin, between the towns of Arizpe to the north and Guadalupe de Ures to the south, in the state of Sonora, Mexico. Streambeds under study were completely dry when samples were taken. Representative samples were collected from sediment surface layer, between 0 and 10 cm in depth; using 10 cm in diameter polyethylene sediment corers (Gómez-Álvarez et al. 2011). Samplers were manually inserted into the sediment by means of a rotational movement; the material surrounding the samplers’ exterior was manually removed and then corers were lifted, taking the appropriate measures to prevent material in their interiors from exiting. Each sample was transferred into a properly labeled 1-L plastic bottle. In total, three separate samples per site were taken; one at the center and one near each edge of the streambeds. Such samples were employed to obtain a composite sample that was later used in chemical analyses.
Composite sediment samples were analyzed to determine: (1) total concentration of arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), lead (Pb) and zinc (Zn); (2) metal concentrations in four granulometric fractions according to the criterion established by Folk (1980); (3) metal concentration in three geochemical fractions by the modified BCR method for sequential extraction described by Rauret et al. (2000); (4) sulfur (S) as sulfate (SO4−2) by the EPA 375.4 method (1978). In addition, hydrogen potential was measured by the method of Mudroch and Azcue (1995) in each of the three individual samples from each site. The granulometric and geoachemical analyses were performed in only 5 out of the 11 samples.
Analysis of total heavy metals and geochemical fractionation (sequential extraction)
Sediment samples were totally digested with an acid mixture (HNO3–HF–HClO4) in Teflon vessels. Residues were then dissolved with HNO3 and boric acid (2%) (Agemian and Chau 1975). Total concentrations of Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb and Zn were analyzed by atomic absorption spectrometry (AAS) using a Perkin Elmer Analyst 400 equipment. Arsenic was determined through chemical digestion using HNO3 and H2SO4 according to the method outlined by Gómez-Álvarez (2004), and extracts were analyzed by inductively coupled plasma spectrometry (ICP) (Detection Limit, 0.004 mg/kg). Chemical analyses were performed in duplicates. The accuracy and precision of the analytical process were evaluated by making use of the certified reference standard NIST 2702 (Inorganic Marine Sediment). The standard was analyzed in triplicates after being subject to the same digestion processes as sediment samples. Percent of metal mass recovery for the NIST reference standard was within 91–106% of the reference concentrations, which is acceptable. Chemical digestion blanks were also used in triplicates. The calibration curves needed in the AAS analyses were obtained using five concentration standards for each metal. Correlation coefficients of 0.9990 or higher were attained.
Sequential extractions were conducted by a series of consecutive chemical digestions on sediment samples in accordance with the modified BCR method from Rauret et al. (2000) to obtain separate extracts for each geochemical fraction. The first extraction is aimed at the removal of exchangeable species using 0.11 mol/L acetic acid. Subsequently, reducible species are extracted with a 0.5 mol/L hydroxylammonium chloride solution. Both of the former processes are performed at room temperature. Oxidable compounds are then separated through a two-step digestion. The first step requires the use of a 8.8 mol/L hydrogen peroxide solution and heating to 85 °C; the second employs 1.0 mol/L ammonium acetate at room temperature. Final residue from all previous extractions was also subject to a chemical digestion with aqua regia for the removal of chemical species bonded to silicates. Digestion extracts of all fractions were analyzed by AAS. Detection Limits (DL) (mg/kg) for each metal fraction were: exchangeable: Fe (0.01), Mn (0.02), Ni (0.002), Pb (0.01), Zn (0.03), Cd (0.01), Cu (0.01), Cr (0.03); Fe–Mn oxides: Fe (0.01), Mn (0.003), Ni (0.004), Pb (0.001), Zn (0.04), Cd (0.02), Cu (0.03), Cr (0.02); organic matter/sulfides: Fe (0.01), Mn (0.03), Ni (0.01), Pb (0.002), Zn (0.08), Cd (0.04), Cu (0.02), Cr (0.01); residual: Fe (0.01), Mn (0.003), Ni (0.01), Pb (0.002), Zn (0.04), Cd (0.03), Cu (0.01), Cr (0.01).
Granulometric analysis
A granulometric analysis was carried out to obtain the absolute concentration of the elements of interest in four texture categories, based on particle size, in which the total mass of each sample was divided (Folk 1980). Samples used were from the same sites for which a metal fractionation analysis in geochemical phases was also performed. These sites are “8 Hermanos Downstream”, “Arizpe”, “Huépac”, “El Lavadero”, and “Sinoquipe”. The texture categories that were analyzed are gravel (with a mesh particle size > 10); sands (> 230 and < 10); silt (> 325 and < 230) and clays (< 325). Sample material in the range of > 325 to < 230 was only recovered for the sites “Arizpe” and “Huépac”. The percentage distribution of their total contents among these categories was also determined.
Normalization studies
The natural or anthropogenic source and the contamination level of metals in sediments were established by calculating the values of the enrichment factor (EF) and the Index of geoaccumulation (Igeo) at all sites where metal concentrations were above the analytical detection limit. Enrichment factor is calculated by the equation:
where the ratio (M/R)sample denotes the relationship of the concentrations of the metal or metalloid of interest (M) and the element designated as reference (R) in a particular sample. The fraction (M/R)background, on the other hand, defines the value of the same ratio, but this time using the values corresponding to the respective background sediment concentrations in the study area. Traditionally, the average contents of the different elements in the earth’s crust have been used as background values. However, some authors believe that subsoil concentrations can be used as background concentrations at a local level in relation to higher layers (Nowrouzi and Pourkhabbaz 2014; Barbieri 2016). The level of enrichment in sediments and soils is categorized, based on the range in which the EF value of a particular metal situates, as absent (EF < 1), minimal (1 < EF < 3), moderate (3 < EF < 5), moderately severe (5 < EF < 10), very severe (10 < EF < 25), or extremely severe (EF > 50) (Chen et al. 2007; Ghrefat 2011; Barbieri 2016).
The Geoaccumulation Index (Igeo) was defined by Muller (1969) to evaluate pollution levels in sediments by comparing current to preindustrial metal concentrations; but has also been applied to the study of soil and dust contamination. The magnitude of Igeo is determined by the following equation:
where M and B denote the sample and background concentrations, respectively, of the metal of interest. The use of factor 1.5 accounts for possible fluctuations in background concentrations originated by lithological variations. Igeo assesses the metal contamination status of a soil or sediment by relating its numerical value to a specific level of contamination as follows: uncontaminated (Igeo < 0), not contaminated to moderately contaminated (0 < Igeo < 1), moderately contaminated (1 < Igeo < 2), moderate to heavily contaminated (2 < Igeo < 3), heavily contaminated (3 < Igeo < 4), from strong to extremely contaminated (4 < Igeo < 5), extremely contaminated (Igeo > 5) (Muller 1969; Ghrefat 2011; Barbieri 2016). The active sediment concentrations reported by the Mexican Geological Service (SGM 2013) in streams located within a radius of 2–5 km from sampling points were used as background concentrations in EF and Igeo calculations.
Results and discussion
Heavy metals
The trace metal or metalloid concentration of water in contact with sediment depends on multiple physicochemical and biological factors which together influence and determine the magnitude of sorption–desorption; precipitation, coprecipitation and dissolution; or oxidation–reduction processes (Benjamin 2002). In particular, the concentrations and chemical characteristics of a metal species present in solid and liquid phases that are in contact with each other, are a fundamental factor underlying the driving force that promotes mobilization processes, and that determine the magnitude and direction of such processes as the system approaches equilibrium. The total concentrations found in the analyzed sediments are shown in Table 1. The element found at a highest concentration was Fe, as it would be expected, with a range of 0.86–4.03%. Mn and Zn followed with concentrations ranging from 98.14 to 638.71 mg/kg, and between 25.20 and 124.60 mg/kg, respectively.
The highest Fe, Co, Cr, and Ni concentrations were found at the site “8 Hermanos Upstream”; as well as some of the highest for Cu, Mn and Pb. This sampling point is located at a short distance upstream of the abandoned mine “8 Hermanos”, as opposed to all the rest, which are downstream of an identified abandoned mine or mineral processing operation. It was included in this project to be used as reference. The abandoned mine “8 Hermanos” and processing plant “El Lavadero” were the only vestiges of mining operations that could be accessed; so that the corresponding samples, downstream and upstream in the case of “8 Hermanos” mine, were taken at a short distance from the mine. This does not exclude the possibility that the streams that capture the eventual runoffs generated from both sites may also receive discharges from other mines, abandoned or in operation, located upstream of the sampling points analyzed in this study.
The highest concentrations of As and Cu were detected at the site “8 Hermanos Downstream”; and this may be related to its proximity to the abandoned mine “8 Hermanos”. The highest concentrations of Zn, Pb and Mn were reported in “Arizpe”, “Sinoquipe” and “El Rebaje”, respectively. “El Lavadero” showed relatively high concentrations of Zn and Pb compared to the rest of the sampling points.
Table 2 shows the sediment concentration ranges for metals and metalloids in the sites under study, and the respective content levels in places around the world exhibiting evident signs of strong contamination. The maximum concentrations obtained in this research in no case exceeded the maximum values in those sites and are substantially lower than those found in certain sites of the Sonora River basin affected by large-scale copper mining activity during periods of high sediment contamination. Particularly, concentration ranges of Cd, Cr, Fe and Mn are lower than those found in sediments from the streams “El Jaralito” and “La Mexicana” in 2012; when such watercourses were receptors of acidic runoffs from mining activity in the region (Aguilar-Hinojosa et al. 2016). Also, the maximum concentrations of Cd, Cr, Cu, Fe, Mn, Pb and Zn are much lower than those reported in sediment of the Abelardo L. Rodríguez dam, in Hermosillo (Gómez-Álvarez et al. 2011), situated in the middle course of the Sonora River.
Total sulfates
Sulfate is found in almost all natural waters and can be produced by the oxidation of sulfurous minerals that are removed from subsoil by mining activities. The maximum sulfate concentration was only 2,500.00 mg/kg (0.25%) in the site “Huepac” (Table 1). This indicates that, at least recently, no appreciable contributions of this oxyanion have occurred. The results obtained were lower than those detected in the San Pedro river sediment (4.51–5.63%) in 2006, a watercourse that has been impacted by the mining activity of the region (Gómez-Alvarez et al. 2011). Low streambed concentrations of sulfate suggest the absence of AMD in the water that has recently flowed through those streams, and with it, a diminished capacity to disperse cationic contaminants.
Hydrogen potential
The average sediment pH at each site is shown in Table 1. In all cases, pH is circumneutral or slightly alkaline, ranging from 7.1 to 8.0. The pH of pure water in contact with air is 5.2 because of the dissolution of atmospheric CO2, and the formation and dissociation of carbonic acid. The normal pH of uncontaminated surface continental waters falls in the range approximately from 6.0 to 9.0. Slightly alkaline pH levels as the ones observed may be indicative of the existence of some acid neutralization capacity in the sediment; probably originated by a significant presence of minerals such as calcite, dolomite or aluminosilacates (Anawar 2013). On the other hand, this highlights the fact that sediments in the sections of the streams that were investigated have not been exposed, at least recently, to acidic flows (Gómez–Álvarez et al. 2007).
Several factors may contribute to mitigate the dispersion and deposition of metal contaminants in sediments. Among these are the magnitude of potentially acid runoffs and the dimension of the mines or tailings that generate them. Thanks to the semi-arid climate of the region, it is expected that rainfall scarcity will contribute to the formation of relatively low volumes of acid mine drainage (AMD) and only if the mineralogical characteristics of exposed subsurface materials in mines and tailings are currently suitable for such process to occur (Anawar 2013). This despite the fact that high temperatures, prevalent in hot semi-arid areas, enhance microbial sulphur-reducing activity (Masson et al. 2007). Furthermore, the torrential character of precipitation during summer, typical of arid zones, would favor the dilution of acid runoffs that might be generated (Chapman et al. 1983; Hudson-Edwards et al. 1996; Gomez-Alvarez 2007).
Another factor likely influencing AMD generation is the exposure time of mining waste to weathering conditions, especially considering that these mining operations are relatively small or very small. It is certainly not easy to determine the antiquity of the abandoned mines in this area; however, it is possible that reiterative leaching processes taking place during long periods have resulted in a poor capacity to emit acid runoffs at present and, consequently, in a decline in metal mobility. In this regard, it would be necessary to perform mineralogical analyses in the accumulations of residual material and pits to determine their current capacity to generate acid mine drainage and to quantify the presence of metals and metalloids susceptible of becoming mobilized by it. Another factor that favors mitigation of AMD adverse effects is the distance between the emission point and the location of the study site. The abandoned mines considered in this project are located at a linear distance of 5 km or less from the river bed. However, only the sampling points “8 Hermanos Upstream”, “8 Hermanos Downstream” and “El Lavadero” were much closer to a potential contamination source than to the river, while the opposite was true for the rest. Nevertheless, the fact that very similar pH levels were observed in all sampling sites suggests that a diminished capacity to produce AMD currently exists in the mining sites near the streams under study at current environmental conditions.
In any case, pH levels in the analyzed sediments indicate limited propensity to induce the mobilization of metallic cations; furthermore, metal retention capacity of soils is expected to achieve its maximum at circumneutral pH (Burgos et al. 2006). The mobilization of oxyanions, on the other hand, such as those formed by arsenic, could potentially be favored. The mobilization or remobilization of arsenic oxyanions from sediments is a function of the solubility of the chemical species to which they are bound. In addition, it is also strongly influenced by the feasibility that a negative charge be generated on the surface of insoluble metal oxides to which oxyanions may be adhered by sorption processes. Metal oxides’ surface charge is controlled by the pH of the liquid phase engulfing them and the chemical structure of the sorbent (Magalhaes 2002; Guzmán et al. 2016). In this sense, it has been established that in general the metallic arsenites are more soluble than their corresponding arsenates and that arsenites tent to be adsorbed in a lower proportion by metal oxides than arsenates at circumneutral pH (Magalhaes 2002).
Granulometric fractionation
The mass content distributions into granulometric fractions of Co, Cr, Cu, Fe, Ni, Mn, Pb and Zn were determined in sediment samples from five sites, namely “Arizpe”, “8 Hermanos Downstream”, “Huépac”, “El Lavadero” and “Sinoquipe”. Cd was excluded from the analyses because its total concentration was below detection limit in all sampling points. This was also the case for Co and Ni in “Huepac”, “El Lavadero”, and “Sinoquipe”. In total, 34 mass partitions were obtained for the individual metals in the sites where their total concentrations were quantifiable. To establish the distribution of a particular metal among sediment particle size categories, it is necessary to take into account its absolute concentration in each granulometric compartment, as well as the amount of sediment material present in those partitions relative to the whole sample. This is why the percent distribution of a metal across particle size categories does not necessarily correspond to the magnitude of absolute concentrations in such fractions.
Sediment material was classified into for particle size fractions shown in Table 3, namely gravel (with a mesh particle size > 10), sands (> 230 and < 10), silt (> 325 and < 230) and clays (< 325). Sediment mass was mostly found to be constituted by sand or gravel. Clays and silt represent less than 1.12 and 0.53% of the sediment material in individual samples, respectively. Collectively, 66.9% of all sample material was comprised of sand and 32% of gravel. These two strata encompass more than 98.9% of the combined mass of samples from all sites. Clays and silt only constitute 0.9 and 0.2%, respectively.
As much as 62.9% of the combined mass of all metals was found to be present in the sand compartment. This is because most of sediment is constituted by sand (Table 3) and because 44.2% of the individual metal mass distributions show their highest absolute concentration in a grain size range within the sand fraction (Table 4). Absolute metal concentrations in the gravel fraction are low compared to those observed in other grain size ranges; however, the significant amount of gravel in relative terms ensues that 35.4% of the overall metal content be contained in it. Clay and silt fractions only represent 1.4 and 0.3% of the total metal content in sediments despite the fact that 26.5% of the individual metal mass distributions show their highest absolute concentration in the clay fraction.
The Pearson correlation coefficient for the percent mass distribution of each metal into the four texture compartments in a particular site (Table 4) with respect to that of the sediment material (Table 3) was higher than 0.96 in 29 out of the 34 metal distributions under study. Interestingly, correlation coefficients for Cu in Huépac, Arizpe, El Lavadero, and 8 Hermanos Downstream were 0.26, 0.34, 0.60, and 0.70, respectively. In these sites, the percent of total Cu present in clays fluctuated between 33.2 and 57.6%; an extraordinarily high proportion compared to those observed for the rest of the metals and in relation to the proportion of clay content in sediments. The correlation obtained for Pb in Arizpe was 0.79, with a high percent of its content in sands (82.3%) that greatly exceeds the apportionment of sand in the sediment for this site (56.4%); and a low percentage (11.38%) in gravel, which constitute 42.4% of the sediment.
These results indicate that, from a granulometric standpoint, only Cu, by virtue of being present at high relative levels in clay in four of the sites, and to a much lesser extent Pb, are potentially susceptible to remobilization into the aqueous phase by dissolution or suspension processes. However, in establishing the likelihood that a metal can become eventually present at high levels in the aqueous phase, the total concentration in the sediment has to be taken into account and not only its partition. In other words, a metal like Mn may have a greater potential for remobilization than Cu and Pb, despite only a small fraction of its total mass is present in clay or silt, due to its total concentrations being relatively high. Percent distribution based on sediment texture of the joint mass of samples from all sites and of the global metal content correlate by a Pearson coefficient of 1. Fe constitutes 97.8% of the total metal mass identified in these analyses; however, since its distribution according to sediment texture in the individual samples, closely matched that of samples’ sediment mass (Table 3), the change in the correlation factor formerly mentioned is negligible when Fe is excluded from calculations.
Geochemical fractionation
The distribution of trace metals in the different geochemical fractions of a sediment may be indicative of the degree to which they are prone to be incorporated into the liquid phase and, thus, become dispersed and bioavailable (Harrison et al. 1981). The percent mass distribution among the different fractions of individual elements in each site is shown in Table 5. The designations I, II, III, and RF in the table denote the metal geochemical fractions that are (1) easily removable via ion-exchange reactions or associated with carbonates, (2) associated to oxide of Fe and Mn (reducible fraction), (3) contained in organic matter and sulfides (oxidizable fraction), and (4) those present in the residual compartment forming silicates, respectively.
Fraction I: exchangeable and carbonate-associated fraction
This geochemical compartment comprises metals weakly bound to mineral surfaces by electrostatic forces, and metals precipitated or coprecipitated with carbonates that can be easily mobilized by pH reduction and variations in solution composition. Percentage contents below 10% of total mass of the individual metals or “below detection limit” were 76.5% of all observations for fraction I. The highest percent levels observed in this fraction fluctuated between 22.6 and 34.8% for Mn. In general, 31.9% of Mn, considering all samples as a whole, is in fraction I, while this proportion is 11.3% for Zn and 6.5% for Cr.
Fraction II: reducible fraction
This fraction constitutes a sink for heavy metals. It comprises metals that form coatings or isolated particles through coprecipitation, adsorption, ion exchange, surface complex formation, and lattice penetration processes in Fe and Mn oxy-hydroxides, which are stable minerals under oxic conditions (Filgueiras et al. 2002). The highest percent for Pb, in relative terms, was found in fraction II considering all samples as a whole. Mn percent in fraction II fluctuated between 21.8 and 43.3%. The relatively high total concentration of Mn and its abundance in fractions I and II seems to be indicative of a significant presence of carbonates and oxi-hydroxides, that may confer the high acid-neutralizing capacity to sediments that accounts for the circumneutral and slightly alkaline pH observed in all sites (Calmano et al. 1993). Percentage contents below 10% or “below detection limit” were 44% of all values for fraction II.
Fraction III: oxidizable fraction
This fraction is not very mobile and comprises trace metals associated with organic matter and sulfides (Filgueiras et al. 2002). Percentage contents below 10% or “below detection limit” were 76.5% of all observations for fraction III. The highest Pb mass distribution percentage is found in fraction III in “Huepac” (58.5%) and in “Sinoquipe” (57.11%). An important proportion of the overall Co (16.24%) and Zn (10.20%) is in this fraction.
Residual fraction
This is the most stable fraction since metals are an integral part of the chemical lattice of silicate minerals and consequently immune to dissolution by pH or ionic strength changes or by oxidation–reduction processes. In general, the highest mass distribution percent, in absolute terms for Co, Cr, Cu, Fe, Ni and Zn, and in relative terms for Mn, was found in residual fraction. The maximum percent for Cr, falling in the range of 44.8–91.9%; for Zn, between 48.5 and 75.4%, and for Co in the two points where its total concentration was not below the detection limit, were also found in this fraction. In total, 30 out of 34 quantifiable mass content distributions showed the highest percentage, either absolute or relative, in RF. Content levels above 50% comprise 70.6% of the results for fraction RF. In fact, Pb and Mn were the only elements for which the highest distribution percentages were not found exclusively in fraction RF; only the site “8 Hermanos Downstream” and “Arizpe” have their highest Pb percentage levels in this fraction. A mass distribution percent for individual metals greater than 50% in fraction RF allows to assume that such elements are unlikely to be appreciably dispersed, even when extraordinary events take place, such as a sudden pH drop in the water column sediments are in contact with. Furthermore, when the weighed concentration of all elements in the different geochemical fractions of the five sampling sites was considered (Table 6), as much as 94.5% of the total metal mass was in the residual fraction. However, these data should be taken with caution since the content of Fe, by representing 98.9% of the total mass of the metals analyzed, introduces an important bias in the calculations.
Excluding Fe, the residual fraction still contains 44.71% of the overall remaining metals mass, while fractions I, II and III constitute 23.5, 25.5 and 6.26%, respectively. The propensity of a metal to become remobilized by pH changes taking place in the water column that is in contact with the sediment increases as the proportion of the total mass present in the exchangeable and reducible phases also increases. Based on this, Mn, Zn, Cr and Pb can be deemed as the most susceptible to being potentially mobilized.
Enrichment factor
Given the lack of official regulations that define sediment quality on the basis of total content of individual elements, it is possible to compare the concentration obtained in a particular area with those observed in similar environments that have been strongly impacted by anthropogenic activities or unusual natural phenomena. It is important to mention that high concentration in sediments may be due to sampling sites being located in mineralized zones and not necessarily to high levels of contamination from either anthropogenic or natural sources. In this research, EF was used to evaluate the potential anthropogenic contribution over natural metal content levels (Table 7). The determination of this parameter was omitted for Fe since the concentrations of this element were taken as reference (Ghrefat 2011). Those cases in which it was not possible to calculate the EF because sample concentration of the respective element was below the detection limit are indicated as “ND” (below detection limit) on Table 7.
In interpreting EF results, it is important to consider that an EF between 0.5 and 1.5 indicates that the concentration of a given metal is mainly the result of geological material weathering, whereas values exceeding 1.5 reveal the presence of anthropogenic inputs or from other sources (Tang et al. 2010). In addition, an EF lower than one shows the absence of enrichment, one between 1 and 3 is indicative of a minimal enrichment level, and those between 3 and 5 of a moderate enrichment (Chen et al. 2007; Ghrefat 2011; Barbieri 2016).
The maximum EFs were found in “El Bagote”, and were 3.76 for Pb and 3.68 for Zn. The EF for Cu in “8 Hermanos Downstream” was 3.61. The aforementioned instances were the only cases in which values higher than 3, revealing of a moderate level of enrichment, were obtained. The lowest EF´s were found at the sites “El Rebaje” and “El Lavadero, where the absence of enrichment was corroborated by EFs lower than one for all the elements. “El Rebaje” is located at the northern end of the study area and it is particularly interesting that their total concentrations, for the most part, are at high or medium level in comparison with the rest of the sites. It is also noteworthy that the low values of EF for “El Lavadero” site were obtained despite its proximity to the abandoned process facility of the same name, where a significant alteration to the natural environment is still visible today. Furthermore, “El Lavadero” exhibits the second highest total concentration of Zn and the third highest of Pb among all sampling sites (Table 1).
In nine sampling sites, at least one element showed an EF higher than 1.5, which is indicative of inputs from likely anthropogenic sources. In five of these sites, two metals had such a characteristic while in the remaining four only one. The elements that exhibited an EF greater than 1.5 are Cu, Ni, Pb and Zn. The EFs for Pb and Zn showed this particularity in five and two sampling points, respectively; furthermore, in the two points in which the EF of Zn is greater than 1.5 so it is that of Pb. Such sites are “El Bagote” and “El Rodeo”. The EF of Pb and Cu in “Huepac” are 2.50 and 2.07, respectively. The other two sampling sites where the EFs of two metals exceeded 1.5 are “Banamichi” and “8 Brothers Downstream”. In both sites, the EFs in question correspond to Cu and Ni. The EFs of Cu in “El Picacho” and “8 Hermanos Upstream” and of Pb in “Arizpe” and “Sinoquipe” exceed 1.5. In last four sites, only one metal showed an EF greater than 1.5.
The EFs obtained make possible to reiterate that total concentration alone is not adequate to evaluate the magnitude of contributions due to causes other than weathering of the parent material. This can be explained by the fact that EF is calculated in the context of the background concentration levels of the elements of interest and not only in terms of their total concentrations.
Geoaccumulation index (Igeo)
The Igeos for all sites and elements of interest, including Fe, are shown in Table 8. These results stand out for their low value; specifically, when the anthropogenic character of the ecological impact that Igeo refers to is considered. Igeos shown as “ND” designate those cases in which the sediment total concentration of the specific element was below the detection limit. Igeos lower than zero or labeled as “ND” are 104 out of the 110 results obtained. They include all data related to As, Cd, Co, Cr, Fe, Mn and Zn, and eight of those related to Cu, Ni and Pb, respectively. Values of a magnitude between 0 and 1 (shown in bold in Table 8) that show a null to moderate degree of contamination were 5; 1 for Cu, 2 for Pb and 2 for Ni. The highest Igeo was for Cu in the site “8 Hermanos Downstream” and fell in the range of 1 to 2. Based on these results, only “8 Hermanos Downstream” shows a moderate level of contamination and only with respect to Cu. The levels of contamination between absent and moderate by Pb are present in “Arizpe” and “Sinoquipe”, by Ni in “Banamichi”, and by Cu and Ni in “8 Hermanos Upstream”. Remarkably, “El Bagote” was found to be ostensibly uncontaminated despite exhibiting the two highest values of EF, one for Pb and the other for Zn, of all sampling points; placing the degree of enrichment for such metals at a moderate level. This seems to suggest that the cause of the observed Pb and Zn enrichment in that particular site is fundamentally mineralogical weathering rather than anthropogenic activities. The site “8 Hermanos Downstream” showed minimal contamination by Cu regardless of an EF for this metal very similar to those for Pb and Zn in “El Bagote”.
Conclusions
Sediments of streams in the middle and upper Sonora River basin show a low or null impact on their quality at present by the sporadic runoffs from abandoned artisan mines that they receive. Metal concentrations are substantially lower than those that have been observed during certain periods in similar watercourses of the same basin due to large-scale mining operations’ questionable wastewater management practices. This is apparent from maximum EFs indicating a moderate level of enrichment only for Cu, Pb, and Zn in only three of the eleven sites under study, while EFs for As, Cd, Co, Cr, Mn and Ni are overwhelmingly lower than one. Additionally, Igeo for As, Cd, Co, Cr, Fe, Mn, and Zn, and most of those for Cu, Ni, and Pb indicate the absence of contamination by these metals in the respective sites. The highest Igeo, indicative of a moderate level of contamination, was for Cu in the site “8 Hermanos Downstream”. The low level of contamination and the circumneutral or slightly alkaline pH found in sediments in spite of the ubiquity of abandoned small artisan mines in the region can be attributed to the scarcity of precipitation due to the arid climate of the region. This condition greatly limits the formation of AMD and, consequently, traces metal mobilization.
The geochemical partition study shows that, when the joint metal mass in the five sites that were studied is considered, most of Co, Cr, Cu, Fe, Ni, and Zn are contained in the residual fraction. The metal found in the exchangeable fraction in a highest proportion was Mn, while the highest percents in the reducible fraction correspond to Pb and Mn. Metals contained at a relatively significant proportion in the oxidizable fraction are Pb, Co, and Zn. In this scenario, Mn, Zn, Cr, and Pb are the most susceptible to become potentially mobilized.
The granulometric study shows that for all metals, except for Cu and, to a much lesser extent, Pb, mass distribution correlates to the proportion of mass distribution of the sediment into the four texture categories of gravel, sand, silt and clays. Since sand and gravel constitute more than 97% of sediment mass in all sites, from a granulometric stance, Cu and Pb are the most susceptible to potentially undergo mobilization into the aqueous phase.
References
Agemian H, Chau ASY (1975) An atomic absorption method for the determination of 20 elements in lake sediments after acid digestion. Anal Chim Acta 80:61–66. https://doi.org/10.1016/S0003-2670(01)84974-6
Aguilar-Hinojosa Y, Meza-Figueroa D, Villalba-Atondo AI, Encinas-Romero MA, Valenzuela-García JL, Gómez-Álvarez A (2016) Mobility and Bioavailability of Metals in Stream Sediments Impacted by Mining Activities: the Jaralito and the Mexicana in Sonora, Mexico. Water Air Soil Pollut 227:345. https://doi.org/10.1007/s11270-016-3046-1
Anawar HM (2013) Impact of climate change on acid mine drainage generation and contaminant transport in water ecosystems of semi-arid and arid mining areas. Phys Chem Earth 58–60:13–21. https://doi.org/10.1016/j.pce.2013.04.002
Barbieri M (2016) The importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to evaluate the soil composition. J Geol Geophys 5:237. https://doi.org/10.4172/2381-8719.1000237
Benjamin M (2002) Water chemistry. International Ed. McGraw-Hill. Singapore
Berger AM, Bethke CM, Krumhansl LK (2000) A process model of natural attenuation in drainage from a historic mining district. Appl Geochem 15:655–666. https://doi.org/10.1016/S0883-2927(99)00074-8
Burgos P, Madejón E, Pérez-de-Mora A, Cabrera F (2006) Spatial variability of the chemical characteristics of a trace-element-contaminated soil before and after remediation. Geoderma 130:157–175. https://doi.org/10.1016/j.geoderma.2005.01.016
Calmano W, Hong J, Förstner U (1993) Binding and mobilization on heavy metals in contaminated sediments affected by pH and redox potential. Water Sci Technol 28:223–235
Chabukdhara M, Nema AK (2012) Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere 87:945–953. https://doi.org/10.1016/j.chemosphere.2012.01.055
Chandra K, Chary NS, Kamala CT, Suman DS, Sreenivasa A (2004) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int 29:1001–1008. https://doi.org/10.1016/S0160-4120(03)00094-1
Chapman BM, Jones DR, Jung RF (1983) Processes controlling metal ion attenuation in acid mine drainage streams. Geochim Cosmoschim Acta 47:1957–1973. https://doi.org/10.1016/0016-7037(83)90213-2
Chen CW, Kao CM, Chen CF, Dong CD (2007) Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 66:1431–1440. https://doi.org/10.1016/j.chemosphere.2006.09.030
Cukrov N, Cmuk P, Mlakar M, Omanivic D (2008) Spatial distribution of trace elements in Krka river, Croatia: An example of the self-purification. Chemosphere 72:1559–1566. https://doi.org/10.1016/j.chemosphere.2008.04.038
Enguix A, Ternero M, Jiménez JC, Fernández AJ, Barragán FJ (2000) Assessment of metals in sediments in a tributary of Guadalquivir river (Spain). Heavy metal partitioning and relation between the water and sediment system. Water Air Soil Pollut 121:11–29
Fan W, Wang WX, Chen J, Li X, Yen YF (2002) Cu, Ni and Pb speciation in surface sediments from a contaminated bay of northern China. Mar Pollut Bull 44:820–826. https://doi.org/10.1016/S0025-326X(02)00069-3
Fichet D, Radenac G, Miramand P (1998) Experimental studies of impacts of harbour sediments resuspension to marine invertebrates larvae: bioavailability of Cd, Cu, Pb and Zn and toxicity. Mar Pollut Bull 36:509–518. https://doi.org/10.1016/S0025-326X(97)00190-2
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857. https://doi.org/10.1039/b207574c
Folk RL (1980) Petrology of sedimentary rocks. Hemphill Publishing Company, Austin
Ghrefat HA, Abu-Rukah Y, Rose MA (2011) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environ Monit Assess 178:95–109. https://doi.org/10.1007/s10661-010-1675-1
Gómez-Alvarez A, Valenzuela-García JL, Meza-Figueroa D, De la O-Villanueva M, Ramírez-Hernández J, Almendáriz-Tapia J, Pérez-Segura E (2011) Impact of mining activities on sediments in a semi-arid environment: San Pedro River, Sonora, Mexico. Appl Geochem 26:2101–2112. https://doi.org/10.1016/j.apgeochem.2011.07.008
Gómez-Álvarez A (2004) Manual de métodos analíticos para rocas y minerales. Universidad de Sonora. Hermosillo, México
Gómez-Álvarez A, Valenzuela-García JL, Aguayo-Salinas S, Meza-Figueroa D, Ramírez-Hernández J, Ochoa-Ortega G (2007) Chemical partitioning of sediment contamination by heavy metals in the San Pedro River, Sonora, Mexico. Chem Speciat Bioavail 19:25–35. https://doi.org/10.3184/095422907X198013
Gómez-Álvarez A, Meza-Figueroa D, Villalba-Atondo AI, Valenzuela-García JL, Ramírez-Hernández J, Almendáriz-Tapia J (2009) Estimation of potential pollution from mine tailings in the San Pedro River (1993–2005), Mexico-US border. Environ Geol 57:1469–1479. https://doi.org/10.1007/s00254-008-1424-8
Guzman HM, Li J, Keshta M, Sáez AE, Ela WP (2016) Release of arsenic from metal oxide sorbents under simulated mature landfill conditions. Chemosphere 151:84–93. https://doi.org/10.1016/j.chemosphere.2016.02.039
Harrison RM, Laxen DPH, Wilson SJ (1981) Chemical Associations of Lead, Cadmium, Copper, and Zinc in Street Dusts and Roadside Soils. Environ Sci Technol 15:1378–1383
Hudson-Edwards KA, Macklin MG, Curtis CD, Vaughan DJ (1995) Processes of formation and distribution of Pb-, Zn-, Cd-, and Cu-bearing minerals in the Tyne basin, Northeast England: Implications for metal contaminated river systems. Environ Sci Technol 30:72–80. https://doi.org/10.1021/es9500724
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14. https://doi.org/10.1016/j.scitotenv.2004.09.002
Leung CM, Jiao JJ (2006) Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in mid-levels area. Hong Kong Water Res 40:753–767. https://doi.org/10.1016/j.watres.2005.12.016
Long ER, Robertson A, Wolfe DA, Hameedi J, Sloane GM (1996) Estimates of the spatial extent of sediment toxicity in major US estuaries. Environ Sci Technol 30:3585–3592. https://doi.org/10.1021/es9602758
Longjiang M, Qiang F, Duowen M, Ke H, Jinghong Y (2011) Contamination assessment of heavy metal in Surface sediments of the Wuding River, northern China. J Radioanal Nucl Chem 290:409–414. https://doi.org/10.1007/s10967-011-1227-3
Lopez DL, Gierlowski-Kordesch E, Hollenkamp C (2010) Geochemical Mobility and Bioavailability of Heavy Metals in a Lake Affected by Acid Mine Drainage: Lake Hope, Vinton County, Ohio. Water Air Soil Pollut 213:27–45. https://doi.org/10.1007/s11270-010-0364-6
López PL, Mandado JM (2002) Extracciones químicas secuenciales de metales pesados. Aplicaciones en ciencias biológicas. Estud Geol 58:133–144
Magalhaes MCF (2002) Arsenic. An environmental problem limited by solubility. Pure Appl Chem 74:1843–1850. https://doi.org/10.1351/pac200274101843
Masson M, Schafer J, Blanc G, Anschutz P (2007) Seasonal variations and annual fluxes of arsenic in the Garonne, Dordogne and Isle Rivers, France. Sci Total Environ 373:196–207. https://doi.org/10.1016/j.scitotenv.2006.10.039
McGregor RG, Blowes DW, Jambor JL, Robertson WD (1998) Mobilization and attenuation of heavy metals within a nickel mine tailings impoundment near Sudbury, Ontario, Canada. Environ Geol 36:305–319
Mudroch A, Azcue JM (1995) Manual of aquatic sediment sampling. Lewis Publishers, USA
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geoj 2:108–118
Nowrouzi M, Pourkhabbaz (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve, Iran. Chem Speciat Bioavail 26(2):99–105. https://doi.org/10.3184/095422914X13951584546986
Ouyang TP, Zhu ZY, Kuang YQ, Huang NS, Tan JJ, Guo GZ, Gu LS, Sun B (2006) Dissolved trace elements in river water: spatial distribution and the influencing factor, a study for the Pearl River Delta Economic Zone, China. Environ Geol 49:733–742. https://doi.org/10.1007/s00254-005-0118-8
Pineda N, Moreno JL, Salazar A, Lutz AN (2014) Derechos de agua y gestión por cuencas en México. El caso del río Sonora. Espiral Estud Estado Soc 61:191–225
Rauret G, López-Sánchez JF, Sahuquillo A et al (2000) Application of a modified BCR sequential extraction (three-step) procedure for a determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J Environ Monit 2:228–233. https://doi.org/10.1039/b001496f
SGM (2013) Resultados de Análisis Geoquímicos de la Carta Cananea H12-5 y la Carta Hermosillo H12-8
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116. https://doi.org/10.1016/j.mineng.2005.08.006
Simate GS, Ndlovu S (2014) Acid mine drainage: Challenges and opportunities. J Environ Chem Eng 3:1785–1803. https://doi.org/10.1016/j.jece.2014.07.021
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments – a tributary of the Ganges, India. J Hidrol 312:14–27. https://doi.org/10.1016/j.jhydrol.2005.01.021
Tang W, Shan B, Zhang H, Mao Z (2010) Heavy metal sources and associated risk in response to agricultural intensification in the estuarine sediments of Chaohu Lake Valley, East China. J Hazard Mater 176:645–951. https://doi.org/10.1016/j.jhazmat.2009.11.131
Acknowledgements
The authors wish to acknowledge the National Council of Science and Technology, Mexico (CONACYT), for its financial support, and the Department of Chemical Engineering and Metallurgy and the Direction of Research and Graduate Studies of the University of Sonora for their technical assistance to the realization of this project. Authors also wish to express their special thanks to Mr. David Schofield from Manchester, UK, for his kind help in editing this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guzmán, H.M., Gómez-Álvarez, A., Valenzuela-García, J.L. et al. Assessment of the impact on sediment quality from abandoned artisanal mine runoffs in a semi-arid environment (the Sonora River basin—Northwestern Mexico). Environ Earth Sci 78, 145 (2019). https://doi.org/10.1007/s12665-019-8131-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8131-5