Abstract
The aim of this study was to assess the particulate metal pollution status and the evolution of particulate metal contamination after the implementation of sewage treatment systems in the Deba River catchment during flood events. Concentrations of suspended particulate matter (SPM), dissolved (DOC) and particulate (POC) organic carbon and metals (Fe, Mn, Zn, Ni, Cu, Cr and Pb) were monitored in the urban watershed over four hydrological years. Geoaccumulation index was used to determine the pollution degree and a number of statistical analyses were performed to elucidate temporal changes. 14 flood events were analyzed from October 2011 to September 2015. The content of metals (Fe, Mn, Zn, Ni, Cr, Cu and Pb) in suspended solids showed a high degree of temporal variability which depended on number of factors being the hydrodynamic process the main one controlling the behaviour of particulate metals. Zn, Ni Cr and Cu appear to be mainly related to anthropogenic sources, whereas Pb appears to be lithogenic in character (like Fe) possibly related to an ageing process. The decrease of metal pollution degree and the homogenisation of organic and metal sources could be a consequence of the wastewater treatment, despite it seems likely that this will be a long-term process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rivers constitute a very important link between continents and oceans (Ollivier et al. 2006; Liu et al. 2017). River sediment play an important role in the transportation of pollutants downriver into seas and oceans (Orkun et al. 2011a), mainly during flood events (Chen et al. 2014). Pollutants are adsorbed into and onto particulate matter which can be accumulated at the bottom of the riverbed along the time. Therefore, sediments act as a pollutant vector and reservoir, and can be a historical witness of river pollution (Sekabira et al. 2010). In addition, sediment constitute a paramount important source of pollutants as a result of small environmental changes caused by natural and anthropogenic disturbances.
Many catchments have suffered from the pollution associated with industrial and urban development. As a result of that development, large quantities of different pollutants have found their way into river systems. Trace metals are considered priority pollutants due to their toxicity, persistence and bioaccumulative nature (Akin and Kirmizigül 2017; Sakan et al. 2009). As a result, the intake of metal fluxes in river basins is now a constant concern (Li et al. 2009).
Trace metals can enter river systems from natural or anthropogenic sources. In an urban environment, numerous different anthropogenic sources may exist: industrial and urban effluents, motor vehicle traffic, runoff from roofs and drainpipes, dry deposition, etc. (Wong et al. 2006). Many authors (Chen et al. 2014; Ma et al. 2016; Sakan et al. 2009; Yang et al. 2014) have reported that in aquatic systems, metals are mainly associated with the particulate phase and are mainly stored in the fine-grained sediment (Martínez-Santos et al. 2015; Sakan et al. 2009).
During periods of low flow, rivers accumulate particulate matter as sediment. These are subsequently resuspended and transported downstream during flood events (Coynel et al. 2007). Flood events also lead to changes in the physicochemical parameters of the water and sediment which could increase the metal associated toxicity (Orkun et al. 2011b; Yang et al. 2009). It is, therefore, very important to study the variability of pollutants transported during flood events (Chen et al. 2014).
This study was conducted in the Deba River urban catchment (Basque Country, Northern Spain). Its aims were: (i) to assess the metal pollution status of the suspended particulate matter (SPM) and (ii) to study the effect of a wastewater treatment plant (WWTP) on particulate metal contamination during flood events.
Study area
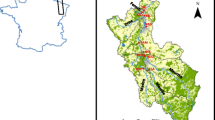
The study area is the Deba River Catchment (538 km2), located in the province of Gipuzkoa, Basque Country (NE of Spain, Fig. 1). The Deba River, the main channel in the catchment, runs across the study area from south to north and into the Cantabrian Sea. It also receives inflows from a number of tributaries. The most important, in terms of discharge and pollutant load, are the Ego and Oñati. The study area covers 424.25 km2 from the catchment headwaters to the Altzola gauging station (Fig. 1), which for the purposes of this study has been taken as the outlet of the catchment to avoid the tidal effect in the lower part of the catchment (Fig. 2).

(modified from Martínez-Santos et al. 2015)
Study area map. Location of Altzola gauging station, urban wastewater plants and urban areas
The maximum elevation in the catchment is 1320 m a.s.l. (summit of Botreaitz mountain) and there is a maximum slope of 40% (in the headwaters). The main bedrock in the southern part of this catchment is an alternation of sandy limestones and lutites with some anhydrite and gypsum deposits; in the middle section, marls and basaltic rocks from the Upper Cretaceous (28%) and in the north, mainly carbonate rocks (EVE 1989). Soils in the catchment are predominantly Cambisols (66%) and Acrisols (25%). This head catchment has been reforested with Pinus spp. (37%) for industrial purposes. Autochthonous woodland occupies around 27% of the land area and farmlands and pastures just 11%.
The Deba River Catchment has suffered industrial development since the mid-nineteenth century, resulting in a high urban and industrial effluent pressure. It is considered to be one of the most polluted catchments in the Basque Country (Borja et al. 2006). The main industrial activities (electrolytic and chemical surface treatment and iron and steel-related metallurgy) are especially concentrated in Arrasate and Bergara, and to a lesser extent in Oñati (Fig. 1). The most important urban centres are Eibar, Arrasate and Ermua, which contain half of the population of the basin (Fig. 1).
During the study period, the Epele (Fig. 2) Waste Water Treatment Plant (WWTP) began operating (May 2012) and two water traps (Fig. 1) were built to carry wastewaters from Oñati to Epele WWTP and from Eibar and Ermua to Apraitz WWTP (June 2014).
At the Altzola gauging station, hydrological and water physiochemical data have been measured and recorded every 10 min since October 1985 (http://www4.gipuzkoa.net/oohh/web/esp/02.asp). Rain is unevenly distributed throughout the year. The wettest period is from November to March and the driest and hottest months are usually during summer (June–September). Mean annual discharge, precipitation and temperature between 1996 and 2015 were 11.1 m3 s−1, 1467.02 mm and 13.8 °C, respectively.
Methodology
Field methodology
Discharge (Q, m3 s−1), precipitation (P, mm), turbidity (NTU) and Suspended Solid Concentration (SSCF, mg L−1), among others, were continuously monitored at the Altzola crump-type gauging station (“F” and “L” subscripts were used after SSC to differentiate between the parameter measured in the field and in the laboratory). These variables are electronically logged at 10-min intervals. SSCF was directly measured in the river course using SOLITAX infrared backscattering probes (namely Dr Lange devices). This SSCF probe is located next to the Altzola gauging station and it is connected to the data collecting and transmitting equipment located inside the station. It is an autonomous probe which is continuously submerged in the water. It is placed inside a rigid protective cylinder, which allows the flow of water, to prevent damages due to blows received by the solid material transported by the river.
An automatic autosampler (SIGMA SD900) was used to take samples automatically during flood events. When the turbidity and SSCF rose to 100 NTU and 100 mg L−1, respectively, the automatic autosampler started pumping 24 water samples of approximately 800 mL into polyethylene bottles every 2 h, making a 46-h time-lapse during which each flood event was sampled. Each flood event is different and it is difficult to predict how it is going to be and how much time it is going to take on the rising limb and on the falling limb. Therefore, the used sampling program was selected after studying the available information of other catchments around the study area and its own characteristics (Martínez-Santos et al. 2013; Peraza-Castro et al. 2016; Zabaleta et al. 2007), with the aim of obtaining samples on the rising and falling limb of the hydrograph to ensure that samples were representative of the flood event. Data used for this study covered 4 hydrological years from 1 October 2011 to 30 September 2015. This period was further divided into 3 different subperiods, during which 14 flood events were analysed (6, 4 and 4, respectively). After eliminating the samples that were considered non-representative, 88, 77 and 62 samples were analyzed in each sub-period, respectively, distributed among the studied flood events. The three subperiods (Fig. 3) were distinguished on the basis of the start-up of the Epele WWTP (May 2012) and construction of the two water traps (June 2014). The first, second and third subperiods cover 232 days (from 1 October 2011 to 20 May 2012), 761 days (from 21 May 2012 to 20 June 2014) and 468 days (from 21 June 2014 to 30 September 2015) respectively, making a total of 1461 days.
After the end of the sampling programme; samples were brought immediately to the Chemical and Environmental Engineering Laboratory (University of the Basque Country) to determine ECL, pHL, SSCL, particulate organic carbon (POC), dissolved organic carbon (DOC) and particulate metal content (Fe, Mn, Zn, Ni, Cu, Cr and Pb). These samples were treated immediately upon arrival at the laboratory to avoid potential alterations, as per the protocol set out in APHA–AWWA–WPCF (1998).
Laboratory methodology
The pHL and CEL were measured at the laboratory using a Crison micro pH2000 and a Crison EC-Meter Basic 30+, respectively. The water samples were then filtered through preweighed and precleaned 0.45 µm Millipore nitrocellulose filters. SSCL was determined using the preweighed filters. Total organic carbon (TOC) and dissolved organic carbon (DOC) were analysed using a total organic carbon analyzer (TOC-L Shimadzu). Particulate organic carbon (POC) was calculated based on the difference between TOC and DOC.
A microwave digestion system (ETHOS 1, Milestone) was used to digest the filters with the suspended matter in Teflon vessels with a mixture of concentrated HNO3:HClO4 (3:1.5), to determine the pseudo total particulate metal content. Samples were heated by increasing the temperature to 180 °C for 10 min and kept at that temperature for an additional 25 min (USEPA 3015A, 2007). After digestion, the samples were filtered through precleaned 0.45 µm Millipore nitrocellulose filters and diluted to 50 mL with Mili-Q water. Additionally, an NBS sediment sample (Buffalo River sediment, USA) was used to control analytical methods. All metals were also measured using this technique, with mean values close to the certified contents and variation coefficients of less than 8%, with the exception of Pb (17% with 0.5 g of sample) (Ruiz et al. 1991). Metals in the pseudo-total digestion (Fe, Mn, Zn, Ni, Cu, Cr and Pb) were determined by ICP-OES (Perkin Elmer Optima 2000). The detection limits (recalculated to relate to SPM in solution) for these metals were: Pb (1 µg g−1), Zn (0.5 µg g−1), Fe and Mn (0.4 µg g−1) and Cr, Cu and Ni (0.1 µg g−1).
Clean procedures were employed to avoid contamination. After washing with tap water and phosphate-free soup and rinsing with distilled water, all material used was acid-washed (10% HNO3 for 24 h) and rinsed with MiliQ water four times. This procedure was repeated for each sampling series.
Data analysis
As variables were not normally distributed, non-parametric statistical analysis have been applied. A Kruskal–Wallis test was carried out to assess the stationarity of time series, and then a Mann–Whitney test was used to determine between which subperiods were the non-stationarity significant. Thus, the results were considered statistically significant when p < 0.05 and not significant otherwise. Spearman correlation analyses (one for each subperiod) were also performed to establish the existence of relationships between analysed parameters and to elucidate if such relationships were temporary stable or not. Finally, three PCA tests (one for each subperiod) were carried out to elucidate the effect of the sewage treatment onto particulate metal relations. All statistical analyses were performed using the IBM SPSS 22 software.
The geoaccumulation index (Igeo) was calculated using Eq. (1) to assess the degree of pollution of each metal. Igeo has been used by many authors (Islam et al. 2015; Nazeer et al. 2014; Yang et al. 2014; Orkun et al. 2011a) as a measure of pollution degree in aquatic environments.
where Cn is the content of metal n in suspended solids (SS) and Bn is the background value of metal n. Table 1 shows the pollution categories according to Igeo (Islam et al. 2015).
The background values used to calculate Cn and Igeo were taken from available data of non-polluted areas of the catchment (Martínez-Santos et al. 2015): 26.4 mg g−1 (Fe), 642.3 µg g−1 (Mn), 230.0 µg g−1 (Zn), 32.9 µg g−1 (Ni), 40.9 µg g−1 (Cr), 28.4 µg g−1 (Cu) and 50.2 µg g−1 (Pb).
Results and discussion
Hydrological and meteorological context during the study period
Taking the last 20 years into account, the study area shows an average discharge of 14.5 m3 s−1. During that period, the minimum discharge (0.5 m3 s−1) was recorded in September 2010 and the maximum (438.2 m3 s−1) in November 2011. The average temperature was 13.8 °C and the mean daily precipitation (m.d.p.) was 4.02 m days−1. The study period covered four hydrological years and it was divided into three subperiods on the basis of the introduction of corrective measures ("Field methodology" section).
The hydrological and meteorological situation during the study period was also similar to the characteristics recorded in the last 20 years (Table 2). However, the first and third subperiods displayed some differences which appear to be related to the fact that they do not include all the periods in a hydrological year. The first subperiod had a lower temperature (11.5 °C) and a higher m.d.p. (4.40 mmdays−1) than the period 1996–2015. This may be because it does not include summer. Rainfalls were very intense and occurred mainly in a short period of time during the first subperiod, which was preceded by a very long dry period (spring and summer 2011). The confluence of these two factors may, therefore, be the reason for the low average discharge. In the case of the third subperiod, it includes the dry seasons for two hydrological years (summer 2014 and summer 2015). This may be the reason for the lower average discharge (12.6 m3 s−1) and m.d.p. (3.54 mmdays−1) and the higher average temperature (17.4 °C). The second subperiod, on the other hand, showed similar values to those for the period from 1996 to 2015 (Table 2). This may be related to the duration of this subperiod, which included all the different stages of two hydrological years.
The term “flood event” is used here to refer to a complete hydrological event with rising and falling limb. During the study period, a total of 14 flood events were analysed (Fig. 4): Event 1 (November 2011), Event 2 (December 2011), Event 3 (January 2012), Event 4 (February 2012), Event 5 (April 2012) and Event 6 (May 2012) for the first subperiod; Event 7 (December 2012), Event 8 (January 2013), Event 9 (March 2013) and Event 10 (May 2014) for the second subperiod; Event 11 and Event 12 (November 2014), Event 13 (January 2015) and Event 14 (March 2015) for the third period. 6 out of 14 of these flood events (Events 3, 4, 8, 9, 13 and 14) occurred in winter (from December to March), 5 out of 14 (1, 2, 7, 11 and 12) in autumn and 3 of 14 (5, 6 and 10) in spring.
A high-intensity flood event occurred from 5 to 9 November 2011 (first subperiod). This flood event (Fig. 4a, number 1) had the maximum discharge (438.2 m3 s−1) recorded at the Altzola gauging station, with a return period of 50 years. In the third subperiod, from 28 January to 2 February 2015, another flood event (Fig. 4c, Number 13) attained a similar maximum discharge (429.1 m3 s−1). The maximum discharge during the second subperiod was lower (334.4 m3 s−1) than those during the other subperiods. On the other hand, a special event was sampled from 1 March to 5 March 2013 (Fig. 4b, number 9), corresponding to the melting of snow which had fallen previously.
As noted above, at each flood event, 24 samples were taken with a frequency of 2 h. However, there were two exceptions: Event number 1 and Event number 9 (Fig. 4). When the first flood event started, the autosampler was not well programmed, with the result that the entire event was not sampled. For Event number 9, the autosampler was programmed with a frequency of 3 h to sample the entire snow-melt event.
Q, SSC, CE, pH, Organic matter and particulate metal during flood events
Table 3 summarizes the Q, pH, EC, SSC, DOC and POC data measured in the samples from the flood events studied. Subperiods showed similar discharge ("Hydrological and meteorological context during the study period") and the statistical test showed an absence of significant differences (p > 0.05), but it was very different for each flood event. Turning to the figures for instantaneous discharge (Fig. 4), three flood events stand out: Number 1 (QMAX = 438.2 m3 s−1), Number 8 (QMAX = 256.9 m3 s−1) and Number 13 (QMAX = 429.1 m3 s−1), which occurred during the first, second and third subperiods, respectively. The discharge in these three events also showed a high rate of variability. In Table 3, the first flood discharge magnitude is shown masked (Fig. 4) because the event was not fully sampled (as noted above in "Hydrological and meteorological context during the study period"). The second and fifth events also showed high discharge and variability compared to the other flood events studied (10–100 m3 s− 1). Flood Event 10 had the minimum average discharge (9–18 m3 s−1) with low variability. However, the minimum spot discharge (6.4 m3 s−1) was seen in Flood Event 11, the first flood of the 2015–2016 hydrologic year. Event number 9 (snow melt) also showed low discharge values and low variability (24–35 m3 s−1). Finally, 6 out of 14 studied events (Events 3, 4, 6, 7, 12 and 14) showed similar discharge ranges (20–80 m3 s−1).
Comparing the three subperiods (Table 3), the pH was slightly basic (around 7.9), with a slight temporal increase; however, it varied between flood events. In contrast, the EC showed a temporal decrease (Table 3) and also seemed to be very dependent on discharge (dilution effect) and the previous hydrological status (after a dry period the EC appeared to be higher due to the dissolved material concentration). Only the pH and EC changes from the first subperiod to the second and third subperiods were statistically significant (p < 0.05), suggesting different physicochemical water characteristics which may affect metal behaviour (Du Laing et al. 2009).
SSC and POC showed the same temporal trend (Table 3) and the same behaviour during floods (Fig. 5). A significant temporal decrease with a p value of < 0.05 was observed in both: from the first subperiod to the third subperiod, SSC decreased by a factor of 2.5, whereas POC fell sevenfold, indicating a decrease in the organic character of particulate matter. The greatest reduction in SSC and POC was observed after the first and second flood events. DOC also showed a statistically significant (p < 0.05) temporal decrease but appeared to be more dependent on hydrology and flood type. A DOC increase was found in the first floods (Events 11 and 12) of the third subperiod. These flood events (Events 11 and 12) had low discharge (median 13.2 m3 s−1 and 26.6 m3 s−1, respectively) and were the first floods after the summer, in which a concentration of dissolved organic matter may occur due to the lower discharge of the river. This temporal trend in SSC, POC and DOC suggests a reduction in inputs of organic pollution into the Deba river catchment. This may be related to the introduction of urban and industrial wastewater treatment systems throughout the catchment.
Boxplot representations of SSC (mg L−1), POC (mg L−1) and particulate metal content: Fe (mg g−1), Mn, Zn, Ni, Cu, Cr and Pb (µg g−1) for each flood event. Dashed lines are used to differentiate the 3 subperiods. N = number of samples per flood event: (N1 = 14, N2 = 24, N3 = 15, N4 = 17, N5 = 13, N6 = 19; N7 = 18, N8 = 15, N9 = 22, N10 = 22; N11 = 14, N12 = 20, N13 = 12 and N14 = 16)
Table 4 summarizes the particulate metal content (Fe, Mn, Zn, Ni, Cr, Cu and Pb) data measured in the flood events. Metal content in SS showed high variability between flood events and also during each flood event (Fig. 5). Several factors may be responsible for this variability, such as different source of particles; different particle size; effect of dilution from soil erosion and different types and locations of active sediment sources (Coynel et al. 2007; Ollivier et al. 2011; Palleiro et al. 2014). The order of abundance of particulate metal content was Fe > Mn > Zn > Cr > Cu > Ni > Pb for the first and third subperiods. In the second subperiod, Ni content increased, changing this order: Fe > Mn > Zn > Cr > Ni > Cu > Pb.
By particulate metal content (Table 4), Fe showed a statistically significant (p < 0.05) increase between the first and the second subperiods. In the study area, Fe is strongly retained in the mineral lattice and is, therefore, considered to be a lithogenic element (Martínez-Santos et al. 2015). The first flood event might, therefore, have exhausted the previously accumulated particulate matter (rich in anthropogenic elements). As a result of that depletion, the next flood events might then have mobilized particulate material from deeper layers with a more lithogenic character.
Like Fe, Mn also showed an increase which was statistically significant (p < 0.05) between the second and third subperiods. The highest Mn content occurred during low discharge floods (Events 9, 10 and 11 events). Both Fe and Mn are commonly related to a lithogenic source (Bibby and Webster-Brown 2005; Li et al. 2009; Violintzis et al. 2009) but in the study area Mn might also be anthropogenic, given the presence of a battery factory (producing 12,000 tonnes of electrolytic manganese dioxide per year) and the heavy vehicle traffic in the study area (Li et al. 2009; Sekabira et al. 2010; Wong et al. 2006), despite a reduction in the permissible concentration of Mn as a gasoline additive (Royal Decree 61/2006; 2006). Mn was also mainly associated with the exchangeable fraction in the bottom sediments (Martínez-Santos et al. 2015) reflecting the possible anthropogenic character of this element in the study area.
In contrast to Fe and Mn, Zn showed a continuous temporal decrease which was statistically significant (p < 0.05) between the second and the third subperiods. Some authors have noted that untreated inputs can be a significant source of metals (Alonso et al. 2006; Carter et al. 2006; Chon et al. 2012; Pereira et al. 2016). Martínez-Santos et al. (2015) reported a Zn hotspot in the Ego tributary due to the input of untreated or partially treated wastewaters. Hargreaves et al. (2017) reported that the particulate Zn concentration of wastewaters diminished significantly after treatment in a WWTP; the decrease in Zn content may, therefore, be related to the treatment of wastewaters from the Ego area.
Ni, Cr and Cu showed a similar pattern: a decrease from the first flood to the middle part of the second subperiod, followed by an increase in the last part of the second subperiod and finally, a decrease during the third subperiod. The changes in Ni and Cr content were statistically significant (p < 0.05) while Cu variations were insignificant. During the first two floods, the suspended solids showed a high Zn, Ni, Cr and Cu content (Fig. 5). This was presumably because of the long dry season after these two flood events in which the study area had accumulated a large quantity of polluted particulate matter. The heavy and intense precipitation and consequent large flow during the first and second flood events may have led to the exhaustion of particulate matter from upper layers. The main reasons for the increase in Ni, Cr and Cu content in Flood Events 9, 10, 11 and 12 could be the low SSC (for Events 9 and 10) and the accumulation of polluted material during a period without rainfall (for Events 11 and 12). Several studies have indicated that metals tend to accumulate within the fine fraction of sediments (Martínez-Santos et al. 2015; Pereira et al. 2016; Sakan et al. 2009). In general, metal content tends to decrease if the discharge increases because of the erosion of less polluted layers, dilution related to the erosion from other areas of the catchment and a higher percentage of relatively larger material (Myangan et al. 2017; Ollivier et al. 2011; Radakovitch et al. 2008). Therefore, low discharge implies low flow energy and consequently smaller grain size of the particulate matter (Bibby and Webster-Brown 2005; Yang et al. 2014). In contrast, before Flood Events 11 and 12, a dry period with no rainfall occurred (summer). During this period, polluted particulate material would have accumulated throughout the study area. The first rainfalls after summer would then have mobilized this metal-rich particulate matter, as in the first and the second flood events but to a lesser extent.
Finally, no clear temporal trend was observed for Pb, with no significant changes between subperiods. It may be related to a permanent and stable source. A main road with heavy vehicle traffic runs through the catchment, parallel to the mainstream. There is also a factory next to the Oñati stream, which in the past produced lead batteries. Despite the prohibition on using Pb as a gasoline additive (in 2001) and the termination of production of lead batteries at the factory, most of the lead deposited in the last century might be still stored in the deep particulate matter (Radakovitch et al. 2008; Sekabira et al. 2010) and constitute a source when particles are detached and transported.
Comparing the metal content with other catchments around the world (Table 5), Zn and Ni content in the Deba river catchment was higher, whereas Fe, Mn, Cr, Cu and Pb were in the same range. However, it is difficult to compare metal content during flood events because each study area has its own characteristics, and each flood event is different.
Influence of Q, SSC, EC, pH and organic matter in metals and their temporal evolution
A Spearman correlation analysis was performed (Table 6) to elucidate the relationships between particulate metals and assess how the parameters measured (Q, SSC, EC, pH, DOC and POC) affect particulate metal content. The test was carried out three times (one for each subperiod) to determine whether the relationships varied from one subperiod to another. Due to the special characteristics of Flood Event 1, it was eliminated from the statistical test to avoid possible discrepancies. However, it is useful in providing a view of the situation of the study area before the study started.
During the three subperiods, discharge was positively correlated with SSC (r = 0.58, 0.50 and 0.44, respectively; p value of Spearman correlation test < 0.01) indicating that flood events export high quantities of particulate matter, reflecting the substantial role of flood events on sediment transport, as it has been previously pointed by other researchers (Nicolau et al. 2012; Oursel et al. 2014; Zabaleta et al. 2016). This might also be an important factor affecting particulate metal behaviour during flood events (Chen et al. 2014; Coynel et al. 2007; Yang et al. 2014).
The relationship between Q and SSC was weaker than in other catchments (Martínez-Santos et al. 2013; Peraza-Castro et al. 2016; Roussiez et al. 2013) and became weaker as the study period progressed. This may be related to high Q and SSC variability and to the fact that for the same discharge value, SSC varied at any given time (hysteresis). This behaviour was also reported by Zabaleta et al. (2016) for the Deba river catchment and for other nearby catchments, which could be related to variations in sediment availability and changes in sediment sources during flood events and during the study period. Precipitation intensity and quantity, previous conditions, event length and sediment availability, among others, are aspects which could affect the quantity of suspended solid an event is able to transport (Nicolau et al. 2012; Ollivier et al. 2011; Zabaleta et al. 2016).
A positive correlation between SSC and POC became stronger over time (r = 0.79, 0.81 and 0.88; p value of Spearman correlation test < 0.01 for the first, second and third subperiods, respectively) suggesting similar behaviour during flood events. Whereas SSC and POC were not correlated with DOC during the first subperiod, they were positive correlated during the second subperiod (r = 0.62 and 0.45; p value of Spearman correlation test < 0.01). This degree of correlation also increased during the third subperiod (r = 0.79 and 0.85; p value of Spearman correlation test < 0.01). The lack of correlation between POC and DOC suggested different source of them. On the other hand, the positive correlation suggests the presence of a common source. In addition, the positive correlation between POC, DOC and SSC suggests the partial dissolution of POC as a DOC source (Nicolau et al. 2012) during the second and the third subperiods. The sources of organic matter, therefore, appear to have changed during the study period suggesting homogenization. This change may be related to the removal of organic matter from the wastewaters.
As noted above ("Q, SSC, CE, pH, Organic matter and particulate metal during flood events"), only changes in pH and EC from the first subperiod to the second and third subperiods were statistically significant (p value of Mann Whitney test < 0.05), suggesting different physicochemical water characteristics from the first subperiod to the second and third subperiods.
According to several authors (Du Laing et al. 2009; Myangan et al. 2017; Orkun et al. 2011b; Palleiro et al. 2014; Yang et al. 2014), a drop in pH increases metal mobility; whereas a pH increase could result in metal transfer from dissolved to particulate phase. However, in this study pH and particulate metal content were not always positively correlated; indeed, in some cases the correlation was negative (Table 6). These correlations changed over time (Table 6), and it, therefore, appears that in this study the pH variation range (7.4–8.5) was too narrow to demonstrate such relationships between pH and particulate metal (Mellor 2001), and/or that the effect of other parameters (Q, SSC, organic matter, etc.) on particulate metal behaviour masked the pH effect due to their wider variability (Table 3).
On the other hand, EC and some metals showed a positive correlation (Table 6). For the first subperiod, EC was positively correlated with Fe, Cr, Cu and Pb (0.28 ≤ r ≤ 0.36; p value of Spearman correlation test < 0.01), while EC showed a positive relationship with Mn, Ni, Cr and Cu for the second subperiod (0.44 ≤ r ≤ 0.54; p < 0.01), which improved for the third subperiod (0.69 ≤ r ≤ 0.80; p value of Spearman correlation test < 0.01). This contrasts with the observations of other researchers who reported a negative correlation between particulate metal and salinity due to the fact that major cations (Ca2+, Mg2+, Na+, K+) compete with heavy metals for SPM binding-sites and because of the formation of metal soluble complexes (Dojlido and Best 1993; Oursel et al. 2014; Wang et al. 2016). However, these studies were performed under steady flow conditions. It, therefore, appears that the hydrology, and its consequent dilution effect, was a more important factor in the control of particulate metal than EC in the study area during flood events.
In most cases, particulate metals were negatively correlated with Q (− 0.72 ≤ r ≤ − 0.42; p value of Spearman correlation test < 0.01) during the entire study period (except Fe and Pb in the first subperiod, and Fe, Zn and Pb in the second subperiod). The decrease in particulate metal content during flood events could be related to a dilution effect by metal-poor soil-borne particles (Nicolau et al. 2012) which could be due to different particle sources and/or changes in particle size (Coynel et al. 2007; Peraza-Castro et al. 2016; Wang et al. 2016). That would, therefore, be related to SSC because as noted in this section, higher SSC means larger particle size. In addition, Martínez-Santos et al. (2015) reported that in the Deba River catchment the fine fraction of sediment showed higher metal content than coarser particles, as in other areas (Owens and Xu 2011; Sakan et al. 2009; Yang et al. 2014). Thus, SSC and particulate metals were negatively correlated (-0.31 ≤ r ≤-0.56; p value of Spearman correlation test < 0.01) during the first and second subperiods (except Zn and Cr for the first subperiod, and Cr and Pb for the second subperiod). However, during the third subperiod only Fe, Zn and Pb were negatively correlated to SSC (− 0.53 ≤ r ≤ − 0.87; p value of Spearman correlation test < 0.01). The changes in correlations may indicate a change in the type and/or source of the particulate matter. Moreover, the loss of correlation between SSC and metals (Mn, Ni, Cr and Cu) during the third subperiod suggests that metal content in SPM was more closely related to land use than to the hydrologic regime (Bibby and Webster-Brown 2005).
Despite the widely studied and reported relationship between organic matter and metals (Banas et al. 2010; Chen et al. 2014; Du Laing et al. 2009), in this study organic matter (DOC and POC) and metals showed little (r < 0.38; p value of Spearman correlation test < 0.01) or no correlation (Table 6) for the first and second subperiods, suggesting different sources or different behaviour during flood events. However, it seems difficult to draw any firm conclusions in this regard; it may perhaps be a result of the hydrology effect as a principal factor in controlling the behaviour of the metals under the conditions in which this study was carried out, as noted above ("Q, SSC, CE, pH, Organic matter and particulate metal during flood events"). On the other hand, DOC and POC were positively correlated to Mn and Cu (0.36 < r < 0.46; p value of Spearman correlation test < 0.01) and negatively to Fe and Zn (− 0.72 < r − 0.56; p value of Spearman correlation test < 0.01) during the third subperiod (Table 6). Such clearer correlations could evince a pollution homogenization that may be related to the channelling and treatment of the wastewater. Martínez-Santos et al. (2015) reported a high affinity of Cu for organic matter in the Deba river catchment, as various researchers did for other study areas (Banas et al. 2010; Chen et al. 2014; Li et al. 2009). The negative correlation between organic matter and Fe could be due to their different origin—anthropogenic for organic matter (urban and industrial wastewaters) and lithogenic for Fe. Zn has high affinity for organic matter (Chen et al. 2014; Dojlido and Best 1993; Radakovitch et al. 2008), but in this case it was negatively correlated with organic matter, which could be related to different origins. A large quantity of Zn was previously accumulated in the bottom sediments (Martínez-Santos et al. 2015), and although initially it was predominantly anthropogenic, Zn could be suffering an ageing process, increasing the degree of mineralisation (Alloway 2013; Kumar 2016). Another reason could be their different sources, as the Ego tributary is the main Zn hotspot in the area (Martínez-Santos et al. 2015). The absence of any correlation between organic matter and Ni, Cr and Pb could be due to their different sources and/or behaviour during flood events.
In the first subperiod, all metals (except Fe) were positively correlated (Table 6), which could indicate that they had the same behaviour and/or origin (Cheng et al. 2017; Liu et al. 2017; Wen et al. 2013). However, it seems unlikely that all of them (Mn, Zn, Ni, Cr, Cu and Pb) to have shared the same origin in light of all the different sources of metal pollution throughout the catchment, especially given that samples were taken under flood event conditions at the outlet of the catchment and were thus affected by all the inputs throughout the study area. Not all metals were correlated to the same degree. Ni, Cr and Cu were highly correlated (r > 0.75, p value of Spearman correlation test < 0.01) suggesting a common source (Oursel et al. 2014), whereas correlations with other metals were weaker (Table 6). It may, therefore, be more plausible that Mn, Zn, Ni, Cr, Cu and Pb showed similar behaviour, possibly due to the existence of a common source of particulate metal. The large quantity of highly polluted particulate matter previously accumulated in the catchment (Martínez-Santos et al. 2015) may have been the principal source of particulate metal before it was exhausted.
After the first subperiod, correlations between certain metals changed (Table 6), suggesting changes in their main source and/or different behaviours. As the study period progressed, a positive correlation of Zn and Pb with Fe appeared (r = 0.53 and 0.40, respectively; p value of Spearman correlation test < 0.01), whereas the positive correlation with the other metals disappeared or became weaker. These might suggest that the Zn and Pb were initially anthropogenic in character and then changed into a more lithogenic behaviour. Several authors (Bibby and Webster-Brown 2006; Eggleton and Thomas 2004; Hu et al. 2017) have reported that Fe-oxy-hydroxides are important scavengers for some metals, including Zn and Pb, while others have pointed to the mainly lithogenic character of Fe (Ma et al. 2016; Myangan et al. 2017; Sekabira et al. 2010), also in the study area (Martínez-Santos et al. 2015). This could be because Zn and Pb had their origin in old particulate matter. Although initially such material was predominantly anthropogenic, it would have suffered an ageing process, increasing the degree of mineralisation (Alloway 2013; Kumar 2016). That old material, coming from deeper layers, might behave like Fe-rich material with a higher lithogenic character, despite the original Zn and Pb anthropogenic source.
Ni, Cr and Cu showed a positive correlation (r > 0.58; p value of Spearman correlation test < 0.01) during the whole study period. Moreover, they were positively correlated with Mn (r > 0.47; p value of Spearman correlation test < 0.01), suggesting a tendency to be adsorbed into Mn-oxy-hydroxides. Several authors have reported that manganese oxy-hydroxides are important ligands to metals (Chon et al. 2012; Sekabira et al. 2010). Despite the fact that Ni, Cr and Cu showed a positive correlation throughout the study, the relationships grew stronger during the third subperiod (r > 0.85, p value of Spearman correlation test < 0.01), suggesting the same source and behaviour, which could be related to source homogenization.
Effect of sewage treatment plant on particulate metal pollution
Three PCA tests (one for each subperiod) were carried out to elucidate temporal changes in particulate metal relations (Fig. 6). Due to the special characteristics of Flood Event 1, it was eliminated from the statistical test to avoid possible discrepancies.
For the first subperiod, the PCA analysis showed four factors: Factor I (29.5% of the variance) related to metal pollution (Mn, Zn, Cr, Cu and Pb to a lesser extent); Factor II (24.3% of the variance), positively characterised by hydrodynamic effect (Q, SSC and POC) and negatively by pH and EC; Factor III (13.2% of the variance) defined by Fe and Ni; and Factor IV (10.6% of the variance) characterised by DOC and Pb. The factorial planes I–III (Fig. 6a) thus differentiated the metals in two main groups, the first composed of Mn, Zn, Cr, Cu and Pb (Factor I) and the second composed of Fe and Ni (Factor III), although Ni shows some trend towards the group of other metals (Factor I). This could, therefore, evince two metal behaviours and/or sources: one anthropogenic (Mn, Zn, Cr, Cu and Pb) and another lithogenic (Fe). In the study area Ni was defined as an anthropogenic metal (Martínez-Santos et al. 2015), and therefore, the appearance of Ni in Factor III might be related to its affinity to precipitate onto Fe oxy-hydroxides (Sakan et al. 2009; Gonelli and Renella 2013).
In the second subperiod, the PCA analysis showed four factors: Factor I (29.5 of the variance) characterised by hydrodynamic effect; positively defined by Q, SSC and POC and negatively by EC; Factor II (21.1% of the variance) positively characterised by pH, Ni and Cu and negatively by Pb; Factor III (16.5% of the variance) defined by DOC, Mn and Cr; and Factor IV (13.4% of the variance) characterised by Fe, Zn and Cu. In this second subperiod, differentiation of metals by PCA was not clear. This could be related to the absence of a principal common source and/or different behaviours.
For the last subperiod, the PCA analysis showed three factors: Factor I (33.8% of the variance) positively defined by EC, Mn, Ni, Cr and Cu; Factor II (32.0% of the variance) positively characterised by SSC, DOC and POC, and negatively by Fe, Zn and Pb; and Factor III (10.8% of the variance) defined by Q and pH. Thus, as in the first subperiod, the factorial planes I–II (Fig. 6b) differentiated two groups of metals: the first composed of Mn, Ni, Cr and Cu positively related to EC and negatively to Q, highlighting the importance of hydrology on these metals; and the second formed by Fe, Zn and Pb, negatively related to SSC, POC and DOC, suggesting that these metals and organic matter had different sources and/or behaviour during the last subperiod, and highlighting the effect of particle size (as explained in "Influence of Q, SSC, EC, pH and organic matter in metals and their temporal evolution").
Based on the results of the PCA results and their development over time, there appear to have initially been one principal common anthropogenic source for the particulate metal (except Fe). This common source of particulate material would have suffered an exhaustion process due to successive rain and drag events (Coynel et al. 2007; Zabaleta et al. 2016), until it was finally depleted in the second subperiod, showing the existence of different sources of metal. Finally, in the third subperiod, two groups of metals can be distinguished, a lithogenic one formed by Fe, Zn and Pb, and an anthropogenic one formed by Mn, Ni, Cr and Cu. There, therefore, seems to have been a homogenisation of anthropogenic metal sources, which could be related to the introduction of corrective measures. Although Zn and Pb are mainly reported as anthropogenic elements (Barać et al. 2016; Coates-Marnane et al. 2016; Li et al. 2009), in this study they appear to be more lithogenic in character during the third subperiod. That could be due to past storage of these metals in the bottom sediments, and the subsequent ageing process (Alloway 2013; Kumar 2016).
I geo was calculated to determine the effect of corrective measures on metal pollution (Fig. 7). Fe and Mn showed Igeo values of below 1, indicating that particulate matter was moderately contaminated or uncontaminated. Except for the first flood event, the Igeo value for Fe was constant, which could be explained by its lithogenic character (Martínez-Santos et al. 2015). In the case of Mn, the behaviour was somewhat erratic during flood events. Zn Igeo values (Fig. 7) showed a temporal decrease from heavily contaminated to uncontaminated/moderately contaminated. This change was statistically significant (p value of Mann Whitney test < 0.05) in the third subperiod. Martínez-Santos et al. (2015) reported the Ego tributary as a Zn hotspot. It, therefore, appears that the construction of the sewage collector may have been influential in this reduction.
I geo representation of Fe, Mn, Zn, Ni, Cu, Cr and Pb for each flood event. Horizontal coloured dashed lines indicate the degree of metal pollution (4 > Igeo ≥ 3, above red line, heavily contaminated; 3 > Igeo ≥ 2, between red and orange lines, moderately to heavily contaminated; 1 > Igeo ≥ 2, between orange and yellow lines, moderately contaminated; 0 > Igeo ≥ 1, between yellow and green lines, uncontaminated to moderately contaminated; Igeo < 0, below green line, practically uncontaminated). Vertical dashed lines are used to differentiate the 3 subperiods
The Igeo values for Ni, Cr and Cu displayed the same trend. They show a temporal decrease but with Igeo peaks at Flood Events 9, 10 and 11 (for Ni, Cr and Cu, respectively). The unclear evolution of Ni, Cr and Cu Igeo values could be due to the fact that they had no main source or hotspot (unlike Zn) and had multiple and different sources throughout the catchment. Cu Igeo changes were not statistically significant, whereas in the case of Cr and Ni, they were (p value of Mann Whitney test < 0.05). Nonetheless, in the last part of the study period, they all showed a decrease in Igeo until reaching uncontaminated/moderately contaminated status. This may be related to the introduction of pollution correction measures in the catchment.
I geo indicated that particulate matter was uncontaminated by Pb, suggesting a lithogenic character. This may be due to the fact that the available particulate Pb had its origin in previous industrial activities in the area. Over time, Pb experienced an ageing process which changed its chemical association with particulate matter, becoming more associated to less labile forms (Alloway 2013) and therefore, showing a behaviour similar to a lithogenic element.
Conclusions
The content of metals (Fe, Mn, Zn, Ni, Cr, Cu and Pb) in suspended solids showed a high degree of temporal variability and depended on the water discharge, the SSC and the source, and also on the previous situation and on the characteristics of each flood event. The hydrodynamic process appears to be the main factor controlling the behaviour of particulate metals. This complicated the study of the way in which other variables affected particulate metal pollution. Hydrology, therefore, appears to be the main factor controlling the particulate metal content in suspended solids.
Despite the fact that the study area suffered Pb pollution pressure in the past, Pb appears to be lithogenic in character, possibly related to an ageing process. This reflects the ability of the sediments to act as a witness of historical pollution. On the other hand, Zn, Ni Cr and Cu appear to be mainly related to anthropogenic sources.
The supposed homogenisation of organic and metal sources could be a consequence of the construction of sewage traps and the introduction of a WWTP. In addition, the collection and subsequent treatment of urban and industrial wastewaters in the WWTP appears to have decreased metal pollution in suspended solids during flood events. Nonetheless, it seems likely that this will be a long-term process, particularly as the area suffered a large degree of industrial and urban pressure in the past.
References
Akin BS, Kirmizigül O (2017) Heavy metal contamination in surface sediments of Gökçekaya Dam Lake, Eskişehir, Turkey. Environ Earth Sci 76:402
Alloway BJ (2013) Sources of heavy metals and metalloids in soils. In: Alloway BJ (ed) Heavy metals in soils. Trace metals and metalloids in soils and their bioavailability, environmental pollution, vol 22, 3rd edn. Springer, Dordrecht, pp 12–50. Print ISBN: 978-94-007-4469-1. https://doi.org/10.1007/978-94-007-4470-7_2
Alonso E, Villar P, Santos A, Aparicio I (2006) Fractionation of heavy metals in sludge from anaerobic wastewater stabilization ponds in Southern Spain. Waste Manag 26:1270–1276
American Public Health Association, American Water Works Association & Water Pollution Control Federation (APHA-AWWA-WPCF) (1998) Standard methods for the examination of water and wastewater. American Public Association, Washington, DC
Banas D, Marin B, Skraber S, Chopin EIB, Zanella A (2010) Copper mobilization affected by weather conditions in a stormwater detention system receiving runoff waters from vineyard soils (Champagne, France). Environ Pollut 158:476–482
Barać N, Ŝkrivanj S, Bukumirić Z, Živojinović D, Manojlović D, Barać M, Petrović R, Ćorac A (2016) Distribution and mobility of heavy elements in floodplain agricultural soils along the Ibar River (Southern Serbia and Northern Kosovo). Chemometric investigation of pollutant sources and ecological risk assessment. Environ Sci Pollut Res 23:9000–9011
Bibby RL, Webster-Brown JG (2005) Characterisation of urban catchment suspended particulate matter (Auckland region, New Zealand); a comparison with non-urban SPM. Sci Total Environ 343:177–197
Bibby RL, Webster-Brown JG (2006) Trace metal adsorption onto urban stream suspended particulate matter (Auckland region, New Zealand). Appl Geochem 21:1135–1151
Borja A, Galparsoro I, Solaun O, Muxika I, Tello EM, Uriarte A, Valencia V (2006) The European Water Framework Directive and the DPSIR, a methodological approach to assess the risk of failing to achieve good ecological status. Estuar Coast Shelf Sci 66:84–96
Carter J, Walling DE, Owens PN, Leeks GJL (2006) Spatial and temporal variability in the concentration and speciation of metals in suspended sediment transported by the River Aire, Yorkshire, UK. Hydrol Process 20:3007–3027
Chen J, Bouchez J, Gaillardet J, Louvat P (2014) Behaviors of major and trace elements during single flood event in the Seine River, France. Proc Earth Planet Sci 10:343–348
Cheng H, Liang A, Zhi Z (2017) Heavy metals sedimentation risk assessment and sources analysis accompanied by typical rural water level fluctuating zone in the Three Gorges Reservoir Area. Environ Earth Sci 76:418
Chon HS, Ohandja DG, Voulvoulis N (2012) The role of sediments as a source of metals in river catchments. Chemosphere 88:1250–1256
Coates-Marnane J, Olley J, Burton J, Grinham A (2016) The impact of a high magnitude flood on metal pollution in a shallow subtropical estuarine embayment. Sci Total Environ 569–570:716–731
Coynel A, Schäfer J, Blanc G, Bossy C (2007) Scenario of particulate trace metal and metalloid transport during a major flood event inferred from transient geochemical signals. Appl Geochem 22:821–836
Dojlido J, Best A (1993) Chemistry of water and water pollution. Ellis Horwood Limited, Chichester, ISBN: 0138789193
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
Eggleton J, Thomas KV (2004) A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ Int 30:973–980
EVE (Ente Vasco de la Energía), (1989) Mapa geológico del País Vasco a escala 1:25.000: 63-I, 63-III, 88-I y 88-III, planos
Gonelli C, Renella G (2013) Chromium and Nickel. In: Alloway BJ (ed) Heavy metals in soils. Trace metals and metalloids in soils and their bioavailability, environmental pollution, vol 22, 3rd edn. Springer, Dordrecht, pp 313–334. Print ISBN: 978-94-007-4469-1. https://doi.org/10.1007/978-94-007-4470-7_2
Hargreaves AJ, Vale P, Whelan J, Constantino C, Dotro G, Campo P, Cartmell E (2017) Distribution of trace metals (Cu, Pb, Ni, Zn) between particulate, colloidal and truly dissolved fractions in wastewater treatment. Chemosphere 175:239–246
Hu N, Liu J, Huang P, Yan S, Shi X, Ma D (2017) Sources, geochemical speciation, and risk assessment of metals in coastal sediments: a case study in the Bohai Sea, China. Environ Earth Sci 76:309
Islam Md S, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Islam MK (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Ind 48:282–291
Kumar M (2016) Understanding the remobilization of copper, zinc, cadmium and lead due to ageing through sequential extraction and isotopic exchangeability. Environ Monit Assess 188:381
Li LY, Hall K, Yuan Y, Mattu G, MnCallum D, Chen M (2009) Mobility and bioavailability of trace metals in the water-sediment system of the highly urbanized brunette watershed. Water Air Soil Pollut 197:249–266
Liu J, Li S-L, Chen J-B, Zhong J, Yue F-J, Lang Y, Ding H (2017) Temporal transport of major and trace elements in the upper reaches of the Xijiang River, SW China. Environ Earth Sci 76:299
Ma Y, Qin Y, Zheng B, Zhao Y, Zhang L, Yang C, Shi Y, Wen Q (2016) Three Gorges Reservoir: metal pollution in surface water and suspended particulate matter on different reservoir operation periods. Environ Earth Sci 75:1413
Martínez-Santos M, Antigüedad I, Ruiz-Romera E (2013) Hydrochemical variability during flood events within a small forested catchment in Basque Country (Northern Spain). Hydrol Process 28:5367–5381. https://doi.org/10.1002/hyp.10011
Martínez-Santos M, Probst A, García-García J, Ruiz-Romera E (2015) Influence of anthropogenic inputs and a high-magnitude flood event on metal contamination pattern in surface bottom sediments from the Deba River urban catchment. Sci Total Environ 514:10–25
Mellor A (2001) Lead and zinc in the Wallsend Burn, an urban catchment in Tyneside, UK. Sci Total Environ 269:49–63
Myangan O, Kawahigashi M, Oyuntsetseg B, Fujitake N (2017) Impact of land uses on heavy metal distribution in the Selenga River system in Mongolia. Environ Earth Sci 76:346
Nazeer S, Hashmi MZ, Malik RF (2014) Heavy metals distribution, risk assessment and water quality characterization by water quality index of the River Soan, Pakistan. Ecol Ind 43:262–270
Nicolau R, Lucas Y, Merdy P, Raynaud M (2012) Base flow and stormwater net fluxes of carbon and trace metals to the Mediterranean sea by an urbanized small river. Water Res 46:6625–6637
Ollivier P, Radakovitch O, Hamelin B (2006) Unusual variations of dissolved As, Sb and Ni in the Rhône River during flood events. J Geochem Explor 88:394–398
Ollivier P, Radakovitch O, Hamelin B (2011) Major and trace element partition and fluxes in the Rhône River. Chem Geol 285:15–31
Orkun ID, Galip S, Cagatayhay BE, Turan Y, Bulent S (2011a) Assessment of metal pollution in water and surface sediments of the Seyhan River, Turkey, Using Different Indexes. Clean Soil Air Water 39(2):185–194
Orkun ID, Galip S, Cagatayhay BE, Turan Y, Bulent S (2011b) Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J Environ Manag 92:2250–2259
Oursel B, Garnier C, Zebracki M, Durrieu G, Pairaud I, Omanović D, Cossa D, Lucas Y (2014) Flood inputs in a Mediterranean coastal zone impacted by a large urban area: dynamic and fate of trace metals. Mar Chem 167:44–56
Owens PN, Xu Z (2011) Recent advances and future directions in soils and sediments research. J Soils Sediments 11:875–888
Palleiro L, Rodríguez-Blanco ML, Taboada-Castro MM, Taboada-Castro MT (2014) Hydroclimatic control of sediment and metal export from a rural catchment in Northwest Spain. Hydrol Earth Syst Sci Discuss 11:3757–3786
Peraza-Castro M, Sauvage S, Sánchez-Pérez JM, Ruiz-Romera E (2016) Effect of flood events on transport of suspended sediments, organic matter and particulate metals in a forest watershed in the Basque Country (Northern Spain). Sci Total Environ 569–570:784–797
Pereira P, Ferreira AJD, Sarah P et al. (2016) Preface. J Soils Sediments 16: 2493. https://doi.org/10.1007/s11368-016-1566-3
Radakovitch O, Roussiez V, Ollivier P, Ludwig W, Grenz C, Probst JL (2008) Input of particulate heavy metals from rivers and associated sedimentary deposits on the Gulf of Lion continental shelf. Estuar Coast Shelf Sci 77:285–295
Roussiez V, Probst A, Probst JL (2013) Significance of floods in metal dynamics and export in a small agricultural catchment. J Hydrol 499:71–81
Ruiz E, Echeandía A, Romero F (1991) Microanalytical determination of metallic constituents of river sediments. J Anal Chem 340:223–229
Sakan S, Dordević D, Manojlović DD, Predrag PS (2009) Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manag 90:3382–3390
Sekabira K, Origa HO, Basamba TA, Mutumba G, Kakudidi E (2010) Assessment of heavy metal pollution in the urban stream sediments and its tributaries. Int J Environ Sci Tech 7(3):435–446
USEPA (2007) Sediment quality guideline. Method 3051A: microwave assisted acid digestion of sediments, sludges, soils and oils. US Environmental Protection Agency, Washington DC. http://www.epa.gov/wastes/hazard/testmethods/%20sw846/online/3_series.htm. Accessed Apr 2013
Violintzts A, Arditsoglou A, Voutsa D (2009) Elemental composition of suspended particulate matter and sediments in the coastal environment of Thermaikos Bay, Greece: delineating the impact of inland waters and wastewaters. J Hazard Mater 166:1250–1260
Wang Y, Liu RH, Zhang YQ, Cue ZQ, Tang AK, Zhang LJ (2016) Transport of heavy metals in the Huanghe River estuary, China. Environ Earth Sci 75:288
Wen Y, Yang Z, Xia X (2013) Dissolved and particulate zinc and nickel in the Yangtze River (China): distribution, sources and fluxes. Appl Geochem 31:199–208
Wong CSC, Li X, Thornton I (2006) Urban environmental geochemistry of trace metals. Environ Pollut 142:1–16
Yang Z, Wang Y, Shen Z, Niu J, Tang Z (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194
Yang Z, Xia X, Wang Y, Ji J, Wang D, Hou Q, Yu T (2014) Dissolved and particulate partitioning of trace elements and their spatial–temporal distribution in the Changjiang River. J Geochem Explor 145:114–123
Zabaleta A, Martínez M, Uriarte JA, Antigüedad I (2007) Factors controlling suspended sediment yield during runoff events in small headwater catchments of the Basque Country. Catena 71:179–190
Zabaleta A, Antigüedad I, Barrio I, Probst JL (2016) Suspended sediment delivery from small catchments to the Bay of Biscay. What are the controlling factors? Earth Surf Process Landforms 41:1894–1910
Acknowledgements
The authors wish to thank the Ministry of Science and Innovation (project CGl2011-465 26236), Ministry of Economy and Competitiveness (project CTM2014-55270-R), Basque Government through the Consolidated Research Group Project ref. IT-742-13 and Consolidated Research Group Project ref. IT-1029-16 for supporting this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
García-García, J., Ruiz-Romera, E., Martínez-Santos, M. et al. Temporal variability of metallic properties during flood events in the Deba River urban catchment (Basque Country, Northern Spain) after the introduction of sewage treatment systems. Environ Earth Sci 78, 23 (2019). https://doi.org/10.1007/s12665-018-8014-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-8014-1