Abstract
The main aim of this paper was to investigate the removal efficiency of antimony (Sb) and arsenic (As) from circum-neutral mine drainage in the former Sb mine in Poproč (Slovakia) using a simple field treatment system based on the adsorption onto iron fillings. The treatment system consisted of two batch reactors with a volume of 1 m3: the first was used for settling of spontaneously precipitated ochreous sediments and the second, filled with reactive iron material, was designed to remove Sb and As from mine water. This passively operated treatment system contained 150 kg of low-cost iron fillings and was able to treat approximately 360 l of mine drainage per hour. The average removal efficiency of Sb and As reached 84 and 89% during a period of 2.3 years of the system operation, respectively. On average, dissolved Sb and As concentrations in mine drainage decreased from 175 to 24.3 µg/l and from 452 to 50.6 µg/l, respectively. Based on the electron microprobe (EMP) analyses of corrosion products developed on the surfaces of iron fillings, average Sb and As contents were 0.28 and 0.73 wt%, respectively. The chemical analyses of precipitated HFOs in the settling reactor showed that these ochreous precipitates contained up to 19.3 g/kg Sb and 65.8 g/kg As, indicating their natural role in the removal of the two metalloids from circum-neutral mine drainage. The results of transmission electron microscopy (TEM) and X-ray diffraction (XRD) analyses confirmed the presence of ferrihydrite and goethite in ochreous sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural waters, draining abandoned ore mines, contain high levels of metal elements, such as Al, Cd, Co, Cr, Cu, Fe, Ni, Pb, and Zn, and may also exhibit elevated concentrations of other elements, including As, Sb, Mo, or U. The pH measurements of mine waters often show their strong acidity as a result of iron sulfide weathering. During the oxidative weathering of iron sulfides, mainly pyrite, hydrogen ions (H+), and sulfates (SO42−) are produced, leading to decrease of pH in the aquatic environment. Moreover, ferrous ion (Fe2+) released during pyrite weathering is oxidized to ferric ion (Fe3+), causing further release of H+ ions and concomitant decrease of pH (Jambor 2003; Shim et al. 2015). When mine waters are discharged into streams, lakes, and oceans, serious pollution of water quality and injury to aquatic life can occur (Nordstrom 2011). However, neutral mine waters may be also rich in dissolved metals, but more frequently, they are characterized by elevated concentrations of metalloids, since these chemical elements are highly mobile under circum-neutral pH conditions (Filella et al. 2009; Flores and Rubio 2010; Ritchie et al. 2013; Fawcett et al. 2015; Sprague et al. 2016). The majority of the former antimony mines in Slovakia have neutral mine drainage with high concentrations of Sb and As, reaching hundreds up to thousands µg/l (Majzlan et al. 2007; Flakova et al. 2012; Hiller et al. 2012; Fľaková et al. 2017). Neutral mine drainage at the Slovak abandoned antimony mines is generated mainly through the neutralization reactions with abundant carbonate minerals (calcite, dolomite, and siderite) (Hiller et al. 2012). In addition, elevated concentrations of Sb and As in mine drainages come from the oxidative weathering of stibnite (Sb2S3), the main ore mineral of the former Slovak Sb deposits, and arsenopyrite (FeAsS), respectively. Both minerals undergo rapid dissolution in oxidizing waters and release more than 10,000 µg/l Sb and 1000 µg/l As (Ashley et al. 2003; Corkhill and Vaughan 2009; Asta et al. 2010; Sracek et al. 2014). When carbonate minerals are present, the acidity produced by stibnite and arsenopyrite dissolution is rapidly neutralized, leading to neutral mine drainage formation.
Occurrence of both metalloids in natural waters at levels higher than limit values established for waters is undesirable. In terms of impacts on the human health, Sb and As share similar toxicological properties (Gebel 1997), they induce several diseases. and are classified as carcinogens but Sb may be less toxic than As (Jain and Ali 2000; Alloway 2013; Ungureanu et al. 2015; Herath et al. 2017 and references cited herein). For this reason, the treatment of mine drainages with environmentally elevated concentrations of metalloids is very important task. There are many technological processes used for the removal of As from contaminated waters, such as precipitation, coagulation, ion-exchange, membrane separation, adsorption, and bioremediation (DeMarco et al. 2003; Ungureanu et al. 2015). However, possibilities of Sb removal from mine water are limited and there is still a lack of information on the remediation technologies for Sb (Klimko et al. 2014). Recently, removal of metal(loid)s from waters of different origins reflects the widespread trend of applying natural or synthetic sorbents, as well as wastes from industry and agriculture that may represent cost-effective treatment alternatives to conventional, more expensive removal methods of metals and metalloids (Bailey et al. 1999; Heviánková et al. 2014; Xi et al. 2014; Chmielewská et al. 2017). One of the most promising options for Sb and As removal from mine waters is the application of low-cost iron-based sorption materials, such as waste iron fillings from industry and ferric oxides of diverse structure, size, and chemical composition (Cundy et al. 2008; Ilavský et al. 2015; Kaartinen et al. 2017). Many review papers and individual experimental studies on the retention of Sb and As species from water in Fe oxy-hydroxides and zero-valent iron (ZVI) have been published (Lackovic et al. 2000, Su and Puls 2001; Bang et al. 2005a, b; Leuz et al. 2006; Mohan and Pittman 2007; Zhang et al. 2008; Kolbe et al. 2011; Guo et al. 2014). These studies are focused mainly on the treatment of drinking- and groundwaters using either purely small-scale laboratory experiments or pilot-scale field trials in the form of reactive permeable barriers. However, there is still a lack of peer-reviewed literature sources about the use and performance of these materials in the treatment of circum-neutral, Sb-, As-, or metal-rich mine drainages under field conditions, i.e., large volumes of flowing water and coexistence of several inorganic contaminats (Kaartinen et al. 2017). The majority of existing studies deal with options to treat only acid mine drainage (Vasquez et al. 2016; Skousen et al. 2017).
In this paper, a simple, low-cost treatment system based on the adsorption onto iron fillings was tested for the concomitant removal of Sb and As from circum-neutral mine drainage in the abandoned Sb mine in Poproč (eastern Slovakia). The main objective was to monitor the removal efficiency of Sb and As in the constructed treatment system and to test its performance under field conditions over a period of 2.3 years (from May 2013 to August 2015). The specific objective of this paper was to characterize chemically and mineralogically the corrosion products developed on the surface of iron fillings and ochreous sediments spontaneously precipitated in the settling batch reactor.
Materials and methods
Study site
The study site is an abandoned Sb mine located in the south-eastern part of the Spišsko-gemerské Rudohorie Mts, specifically in the Petrová Valley, 1 km north of the Poproč Village (Fig. 1). The mine started to work in the seventeenth century and was finally closed in 1965. Occurrences of Sb ores at the site were subjected to intense prospecting and mining in the nineteenth century and early twentieth century. Antimony ores were processed by flotation, and remaining tailings were disposed in three tailings ponds in the Petrová Valley above the Poproč Village. The tailings ponds are unmanaged and this caused extensive dispersal of tailings at the study site.
The main exploited Sb mineral was stibnite (Sb2S3) and the other commonly occurring sulfides were pyrite (FeS2) and arsenopyrite (FeAsS). Stibnite veins are hosted in granitic rocks and their position is not related to other stibnite veins in geological units of Gemericum. Within the study site, seven veins are known and linked to steeply sloping and vertical fault structure in the east–west direction. Lenses of quartz with stibnite have a maximum thickness of 20 m, but their average thickness ranges from 1.5 to 2 m. The most significant venous zone of the mine is the Anna-Agneska vein (Grecula et al. 1995). Ore concentrate contained 1.85% Sb, 12.6% Fe, 0.12% Cu, 0.01% Zn, 0.19% As, 0.4% Pb, and also 3–6 g/t Au (Rozložník 1980).
The mine site is drained by the Olšava Creek, which is a left tributary of the Bodva River. Main contamination sources of Sb and As to the Olšava Creek are mine drainages from open adits, heaps, and unsecured tailings ponds (Kaličiaková et al. 1996; Hiller et al. 2012). Total dissolved solids in mine drainages are highly variable, with values ranging from 64 to 2384 mg/l with elevated concentrations of Sb and As (Hiller et al. 2012; Auxt et al. 2015). Among the sources of surface water contamination with Sb and As, uncontrolled discharge of mine water from the Agnes Adit contributes mostly to the total metalloid load to the local stream (Ženišová et al. 2009). Contamination of natural waters mainly with Sb can be traced in household wells in the Poproč Village, where groundwater contains up to 35 µg/l Sb.
Water sampling and analyses
Chemical composition of mine drainage and mine-impacted surface waters at the study site was monitored between 2013 and 2015. The sampling locations are shown in Fig. 1. The Olšava Creek merges with the Bodva River approximately 7 km south of the Poproč Village. Water samples were regularly collected from the Agnes Adit (SP-1) and in the Olšava Creek upstream of the mine workings (SP-2; background water sample), and upstream and downstream of the confluence with mine drainage of the Agnes Adit (SP-3 and SP-4, respectively). Another water samples were taken from the Olšava Creek downstream of three tailings ponds (SP-5) and of the confluence with the Bodva River (SP-6).
Water samples from all sites were collected in 1 l HDPE bottles and filtered in the field using < 0.45 µm membrane filters. An aliquot of water samples was preserved by acidification using concentrated ultrapure HNO3 for major cation and trace metal analyses, while no pre-treatment of the samples was used for anion analysis. Temperature, electrical conductivity (EC), pH, and redox potential (Eh) were measured in situ at the time of sampling using a WTW 350i instrument equipped with TetraCon®325 electrode, SenTix41 electrode, and a WTW 340i instrument with SenTix®ORP electrode, respectively. Sulfates were measured using ion chromatography (Dionex ICS-2000). Total dissolved iron was determined by a Varian Liberty 200 inductively coupled plasma-atomic emission spectrometry (ICP-AES). Arsenic and Sb concentrations were analyzed by hydride generation-atomic absorption spectrometry (HG-AAS) using a Varian Spectr AA 220 instrument equipped with a VGA 76 hydride generation system. In addition, water samples collected from the Agnes Adit at three sampling times were subjected to complete chemical analysis, including major cations and anions (Na, K, Ca, Mg, Fe, Al, Mn, Cl−, NO3−, SO42−, PO43−, HCO3−, and H2SiO3) and trace chemical elements (As, Sb, Cd, Cu, Pb, Ni, Co, Cr, and Zn). The chemistry of water samples is given in Table S1 in Electronic Supplementary Material (ESM). The limit of detection (LOD; mg/l) and the limit of quantification (LOQ; mg/l) were 0.03 and 0.05 for Na, 0.12 and 0.20 for K, 0.006 and 0.01 for Ca, 0.006 and 0.01 for Mg, 0.0012 and 0.002 for Fe, 0.012 and 0.02 for Al, 0.0012 and 0.002 for Mn, 2.0 and 1.5 for Cl−, 0.5 and 0.3 for NO3−, 1.0 and 0.5 for SO42−, 3.0 and 2.0 for HCO3−, 0.167 and 0.278 for H2SiO3, 0.0006 and 0.001 for As and Sb, 0.0012 and 0.002 for Cd, 0.003 and 0.005 for Cu, 0.006 and 0.01 for Pb and Ni, 0.0012 and 0.002 for Co and Cr, and 0.003 and 0.005 for Zn, respectively.
Treatment experiment
Experimental works at the study site were focused on the treatment of mine drainage from the Agnes Adit (Fig. 1). The Agnes Adit is well defined point source of Sb and As contamination to local surface waters with an average flow rate of 3.5 l/s. Iron fillings as a waste from iron and steel processing were selected for the field experiments, since they are readily available and low-cost. First, iron fillings were properly cleaned from unwanted waste and debris, and then left to react for about 16 h in plastic barrels with 5% hydrochloric acid (HCl) to clean and activate them. After the activation process, the material was carefully washed with water to stabilize pH at ∼ 7.
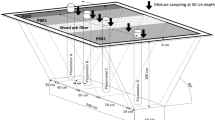
Cleaned and activated iron fillings were transported to the study site (Fig. S1 in ESM). One-hundred fifty kilogram of the reactive material was placed into 1 m3 treatment batch reactor. Since the mine water contains ochreous precipitates, the same reactor was placed behind the treatment batch reactor and used for the settling of the precipitates. Setup of the experimental treatment system is schematically shown in Fig. 2. Mine water from the Agnes Adit flowed in the treatment system through polyvinyl chloride (PVC) tube with a diameter of 5 cm. The actual flow rate in this treatment system was up to 360 l/h. The residence time of mine drainage in each treatment reactor was approximately 2.2 h. The residence time was measured by conductivity measurements with dissolved NaCl prior to treatment experiments. Treatment reactors operated for a period of 2.3 years from May 2013 to August 2015. However, due to overfill of the settling reactor with ochreous precipitates approximately after 1.25 years of the system operation, the reactor had to be cleaned and put again into operation. This implies that the proposed passive treatment for circum-neutral metalloid-rich mine drainage will require certain, at least annual maintenance.
Chemistry and mineralogy of ochreous precipitates
The ochreous precipitates were collected from settling reactor using a plastic scoop and the samples (∼ 1 kg) were stored in plastic bags. Prior to analyses, the samples were air-dried, disaggregated, and homogenized. Dried precipitates were dissolved in aqua regia acid mixture. Flame atomic absorption spectroscopy (FAAS) was used for the determination of Sb, As, Cd, Cu, Pb, and Zn concentrations in the extracts. Iron, Si, Al, Ca, Mg, Na, K, and S were measured using ICP-AES.
X-ray diffraction (XRD) analyses of samples were performed on a Bruker AXS D8 Advance diffractometer equipped with a CuKα radiation source and a diffracted-beam graphite monochromator. Transmission electron microscopy (TEM) study was done on a JEOL 2000FX microscope (Cleome, FNS CU). The TEM method was used to identify mineral phases in ochreous precipitates, their morphology, structure, and size. Copper mesh with a layer of carbon was used as the carrier for sample preparations.
Chemical composition of iron fillings before and after the treatment experiment
Chemical compositions of iron fillings before and after the experiment and backscattered-electron (BSE) images were acquired using a Cameca SX-100 electron microprobe. All quantitative electron microprobe (EMP) analyses were done in wave-length dispersive mode with an accelerating voltage of 15 kV, a constant beam current of 20 nA, and a beam size of 1–5 µm. The following standards and detectors were used: Mg (Kα, forsterite, TAP), Al (Kα, Al2O3, TAP), Si (Kα, SiO2, TAP), S, Fe, Cu (Kα, CuFeS2, PET), P (Kα, GaP, PET), Ca (Kα, wollastonite, PET), Mn (Kα, metallic Mn, LIF), Co (Kα, metallic Co, LIF), Ni (Kα, metallic Ni, LIF), Zn (Kα, ZnS, LIF), Pb (Mα, PbS, PET), Sb (Lβ, Sb2S3, PET), As (Lβ, FeAsS, TAP), and Cr (Kα, metallic Cr, LIF). The measurement period for each component was 20 s with the exception of As (30 s) and Pb (40 s).
Results and discussion
Antimony and arsenic in mine-impacted waters
Selected chemical parameters of water samples are shown in Table 1. Chemical analyses clearly show an influence of mine drainages from abandoned adits and mine wastes on the surface waters of the Olšava Creek, which is the main stream draining the mine site (Fig. 1). The Olšava Creek upstream of any mine workings (sample SP-2) had low concentrations of sulfates, Sb, As, and Fe. However, this uncontaminated stream water was altered by mixing with the mine drainage from the Anna Adit (sample SP-3), containing elevated Sb concentrations at a level of hundreds µg/l (Ženišová et al. 2009; Hiller et al. 2012). Antimony concentration in the Olšava Creek increased significantly after mixing of the two waters. Chemical quality of the creek waters (sample SP-4) was further impaired downstream of the confluence point with the mine drainage of the abandoned Agnes Adit (sample SP-1). As could be seen from Table 1 and Fig. 3, water of the Olšava Creek collected upstream of the mines had a background concentration of Sb and As (sample SP-2). In contrast to this, mine drainage of the Agnes Adit, with an average flow rate of 3.5 l/s, contained up to 406 µg/l Sb and 2053 µg/l As although Sb and As concentrations varied seasonally. Due to its high flow rate and concentrations of Sb and As, mine drainage of the Agnes Adit contributes mostly to the total metalloid load entering the Olšava Creek. There are also other mine waters discharging from adit entrances and tailings ponds at the study site but their flow rate is much lower, and hence, they contribute less to the contamination of the Olšava Creek. Mixing of mine waters from the Agnes Adit with the Olšava Creek (sample SP-4) caused an increase of Sb and As concentrations, on average from 149 to 287 µg/l and from 2.20 to 22.5 µg/l, respectively. Further downstream of the tailings ponds (sample SP-5), Sb and As concentrations in the Olšava Creek increased to 324 and 61.5 µg/l, respectively. The continuous increase is due to water discharge from unmanaged tailings ponds. The tailings waters were shown to have high concentrations of both metalloids, reaching hundreds to thousands µg/l (Ženišová et al. 2009; Hiller et al. 2012). Finally, upon entering the Bodva River, concentrations of Sb and As in the surface water (sample SP-6) were similar to those in the Olšava Creek upstream of the mine workings. Although only a limited number of water samples from the Olšava Creek were collected in this study, the results indicate a difference in the distribution of Sb and As. Previous studies with denser sampling of waters in the Olšava Creek (Ženišová et al. 2009; Hiller et al. 2012) showed that Sb maintained high concentration until the confluence with the Bodva River (almost 8 km far from the main source of Sb to the Olšava Creek). Conversely, As concentration in the Olšava Creek waters is lower despite of its similar or even higher concentrations in source waters during a year. One of the main reasons of the geochemical separation of Sb from As during the riverine transport are likely different sorption–desorption interactions of dissolved metals with sedimentary components, mostly with Fe oxy-hydroxides or clay minerals. It was found that antimony was more prone to desorption from stream sediments of the Olšava Creek than arsenic and the same result was obtained with soils and tailings in this mine area (Hiller et al. 2012). It is, therefore, likely that the transport of Sb further downstream is supported by a higher extent of its release from solid substrates. Similar results provide several studies (Ashley et al. 2003; Casiot et al. 2007; Masson et al. 2009; Milham and Craw 2009; Sharifi et al. 2016) where mass ratio of Sb/As is consistently higher in water than in solid substrates, interacting with the water. Moreover, Ritchie et al. (2013) and Sharifi et al. (2016) found lower sorption affinity of Sb for stream sediments compared with As under oxidizing conditions, causing more conservative behaviour of Sb during the riverine transport, while the concentration of As decreases more quickly with increasing distance from metalloid sources. Recent findings of Qi and Pichler (2017) are interesting in relation to the metalloid distribution in mine-impacted streams. They documented that adsorption of Sb5+ on ferrihydrite at circum-neutral pH values was lower than that of As5+ in binary systems, i.e., under simultaneous presence of both metalloid species. It could be concluded that in addition to water dilution, partitioning in water–sediment system was an important physico-chemical process that influenced the distribution of metalloids in the Olšava Creek.
It should be noted that circum-neutral mine drainage from Agnes Adit is rich in total dissolved Fe. As a consequence, iron staining and sludge are observed in mine drainage of the Agnes Adit. The sludge is dispersed along the stream and accumulates at sites where the water velocity decreases (Fig. S2 in ESM). Thermodynamic calculations using PHREEQC-2 (Parkhurst and Appelo 1999) confirmed that these iron ochreous precipitates would form due to supersaturation of the mine water with respect to common ferric oxy-hydroxides, such as ferrihydrite (Fe2O3·0.5H2O) and goethite (α-FeOOH). Although the precipitates naturally remove Sb and As from mine drainages, dissolved concentrations of the two metalloids remain high in mine drainage of the Agnes Adit.
Removal of Sb and As from mine drainage in field treatment system
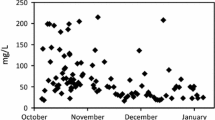
The results of the field experiment are shown in Table 2 and are also presented in Fig. 4 as the removal efficiency in % against the sampling time. As shown in Table 2, the concentrations of Sb (48–406 µg/l) and As (13–2053 µg/l) in influent mine drainage varied significantly at each sampling time. On the other hand, the values of pH (5.8–7.9), EC (464–641 µS/m), sulfates (148–262 mg/l), and Fe (10.8–36.5 mg/l) were much less variable. The highest concentrations of Sb and As were measured after longer dry periods followed by high rainfalls. This phenomenon known as the “flushing effect” was described by several authors (Edwards 1973; Young 1997; Gzyl and Banks 2007) and occurs as a result of sudden washing out of soluble weathering products that accumulated inside the mine during dry periods.
Regarding the pH change, no significant differences between the pH of influent waters and the pH of effluent waters were observed at any sampling time. This is likely due to balance between the corrosion reaction of iron fillings, producing OH− anions (i.e., pH increase) and the oxidation of Fe2+ to Fe3+ that produces H+ ion and decreases pH.
The average removal efficiency of Sb and As from mine drainage was 84% and 89% over a period of 2.3 years, respectively. Comparison of As removal pattern with Sb removal pattern (Fig. 4) showed that As was removed more efficiently than Sb at most sampling dates. In absolute terms, Sb concentrations in treated mine water were lower than 92 µg/l and even they decreased below 25 µg/l in the majority of the treated water samples, that is, they were lower than the limit value of Sb in groundwater (25 µg/l; Anon 2015). With the exception of two samples, all treated mine waters contained As below its limit value for groundwater (50 µg/l). Although there was a significant correlation between the concentrations of Sb and As in influent waters of the Agnes Adit (r = 0.72 at α < 0.001), indicating a common source of these metalloids and similar geochemical behaviour, the correlation between Sb and As concentrations in treated mine water was insignificant (r = 0.28 at α = 0.24). This might be due to competitive behaviour between Sb and As during the adsorption and/or co-precipitation as shown recently by Qi and Pichler (2017), studying the simultaneous adsorption of inorganic Sb and As species on ferrihydrite, rather than due to different removal mechanisms of Sb and As by Fe oxy-hydroxides. Recent studies using different spectroscopic methods have shown that Sb and As are removed from solution by zero-valent Fe through adsorption on the surface of Fe corrosion products, incorporation into their structure or precipitation of insoluble mineral phases (Lackovic et al. 2000; Bang et al. 2005a; Li et al. 2015, 2017). Relatively high efficiency of Sb and As removal could be attributed in part to acid-base and redox conditions prevailing in the mine drainage. The values of pH were in a circum-neutral range (∼ 6–8) and Eh values were positive between ∼ 170 and 230 mV (Table 1). It was shown that sorption of inorganic Sb and As species by zero-valent iron and its corrosion products reached a maximum in a pH range of 5–7 (Su and Puls 2001; Dixit and Hering 2003; Bang et al. 2005a; Leuz et al. 2006; Guo et al. 2014). Literature documented that Sb and As could be also removed from mine drainages through reduction of sulfate S to sulfide S and consequent precipitation of Sb and As sulfides. However, the net sulfate removal in the treatment system was not observed (Table 2), indicating that this process did not play any role in the removal of Sb and As. Concentrations of Sb and As were also measured in effluent water samples from the settling reactor at four sampling times to estimate contributions of the settling reactor and the reactor with iron fillings to the total removal of Sb and As from mine drainage. On average, As (59.1%) was removed more efficiently in the settling reactor than Sb (46.2%) although both metalloids had a high variability of the removal efficiency, ranging between 17.9 and 83.5% for As and 16.2 and 72.0% for Sb (Table S2). This finding agrees well with approximately 4 times higher content of As in ochreous precipitates of the settling reactor compared with Sb (Table 4). The observed variability in the removal efficiency of As and Sb does not allow to quantify accurately contributions of each reactor to the total removal efficiency of the passive treatment system; however, it seems that the settling reactor contributes more to the total removal of As than the reactor with iron fillings. The efficiency of Sb removal from mine drainage is approximately the same in both reactors (Table S2).
Comparison of removal efficiencies for Sb and As obtained in this study with those from previous studies using zero-valent iron materials is only very informative, since the majority of the studies were performed under controlled laboratory conditions using closed systems, small volumes of water samples, only individual inorganic As or Sb species, and most frequently no flow-through batch techniques. However, despite of different removal efficiencies of Sb and As from waters under oxidizing conditions that were obtained in different studies, it can be concluded that zero-valent iron is an efficient material of metalloid removal from natural waters. For example, Bang et al. (2005a, b) documented >99 and 83% removal efficiency of As5+ and As3+ from water at pH ∼ 6–7 in their batch and column studies using zero-valent Fe fillings. Nikolaidis et al. (2003) also found high removal efficiency of As from groundwater (∼ 99%) in a large-scale column study using iron fillings. Regarding Sb removal from waters using zero-valent iron, the data are very limited in the literature but as an example, >90% of the total Sb5+ was removed from water in batch reactors at pH 5 (Li et al. 2015). In our field treatment system, lower removal efficiencies of Sb and As were achieved than those mentioned above. All the possible reasons could not be accounted for but they were likely due to (i) competition between Sb, As, and Si for sorption sites on corrosion products of zero-valent iron fillings, (ii) different dimensions of these fillings (centimeter in this study vs. tens to hundreds of micrometer in previous studies), and (iii) differences in the chemistry of influent waters and setup of removal experiments.
Chemical composition and mineralogy of iron fillings and ochreous precipitates
Fresh iron fillings used in the field treatment test were almost pure iron (Table 3). Based on their chemical composition by EMP analyses, two groups of iron fillings were identified: ZVIF-1, containing higher amount of Cr (> 1 wt%), and ZVIF-2 with low Cr content, generally below 0.1 wt%. Since the iron fillings are a waste from industry, it seems that ZVIF-1 group comes from the processing of low-alloyed steel. The contents of Sb and As in fresh zero-valent iron fillings were low, usually lower than the detection limit of EMP analysis in the case of Sb contents.
Corrosion products developed on the surface of iron fillings differed chemically from fresh iron fillings (Table 3), and formed coatings with a thickness of tens to hundreds of micrometer (Fig. 5). Point EMP analyses showed that these corrosion products were composed mainly of Fe and O with minor abundance of Si, As, and Sb. Compared with fresh iron fillings, contents of Sb and As in the corrosion products were higher (an average of 0.28 and 0.73 wt%, respectively). Recently, it is well established that the removal of Sb and As from water takes place through the adsorption onto corrosion products of zero-valent iron materials. Although the mineralogy of corrosion products was not investigated in this paper, several reports have identified mainly ferric oxy-hydroxides and mixed ferrous-ferric oxides (e.g., akaganeite, ferrihydrite, goethite, lepidocrocite, magnetite, maghemite, and green rust minerals) as the main minerals of zero-valent iron corrosion (Furukawa et al. 2002; Li et al. 2015). These secondary iron minerals serve as a favorable host environment for the sorbed Sb and As species (Manning et al. 2002; Su and Puls 2003; Leuz et al. 2006; Kolbe et al. 2011; Li et al. 2015).
Furthermore, ochreous precipitates from the settling reactor were subjected to chemical and mineralogical analyses. Chemical composition of the precipitates is given in Table 4. The concentration of Fe varied from 45.0 to 67.3 wt%, with an average value of 53.7 wt%. Among the trace elements, high concentrations of Sb and As were found in the ochreous precipitates, reaching up to 19.3 g/kg Sb and 65.8 g/kg As. Mineralogical analyses using TEM and XRD were performed to determine the composition of ochreous precipitates. Two- and six-line ferrihydrites were the main mineral phases in bulk samples, while goethite, quartz, albite, and muscovite were also identified in insoluble residues after the dissolution in ammonium oxalate. XRD patterns and TEM images of ochreous precipitates are shown in Fig. S3 and Fig. S4 in ESM, respectively. Speciation calculations performed in PHREEQC-2 also indicated that the formation of ferrihydrite and goethite from circum-neutral mine drainage of the Agnes Adit was thermodynamically favorable due to supersaturation of the mine water with respect to these Fe oxy-hydroxides (Table S3). Ferrihydrite and goethite are widespread and common constituents of ochreous precipitates in mine drainages with pH values close to neutral (Schwertmann and Taylor 1989; Bigham et al. 1992; Bowell and Bruce 1995; Lintnerová et al. 1999; Majzlan et al. 2007; Lalinská-Voleková et al. 2012; Woo et al. 2013). Substantial amount of Sb and As in the ochreous precipitates is indicative of a high capability of ferrihydrite and goethite to scavenge the metalloids, which is consistent with previous studies. However, their role in the natural attenuation of dissolved Sb and As in mine drainages may be limited, since the metalloids bound to ferrihydrite can be released back into the water during its progressive transformation. Ferrihydrite is a poorly crystalline and metastable phase that transforms to crystalline, more stable phases, e.g., goethite, although the transformation rate is dependent on the species previously adsorbed on the surface of ferrihydrite (Cornell and Schwertmann 2003).
Conclusions
Geochemical analyses of mine-impacted waters at an abandoned Sb mine in Slovakia demonstrate that the waters are rich in total Sb and As. A field treatment system was constructed to remove Sb and As from circum-neutral mine water through the adsorption onto zero-valent iron fillings and settled ohreous sediments that precipitate from the mine water. The treatment system reached high level of metalloid removal over a period of 2.3 years (84% for Sb and 89% for As) at a flow rate of 360 l/h and a residence time of 2.2 h. Influent concentrations of Sb and As varied seasonally between 48 and 406 and 13 and 2053 µg/l; however, almost all effluent concentrations of these metalloids were lower than their respective limit values of 25 and 50 µg/l, respectively. This is an evidence of good performance of the field treatment system in the removal of Sb and As from mine drainage. Point EMP analyses showed that corrosion products of iron fillings had higher contents of Sb, As, and Si than fresh iron fillings. Ochreous sediments settled in the treatment system contributed significantly to the total removal of Sb and As. They contained high amounts of metalloids, reaching up to tens of gram/kilogram, and ferrihydrite was their main mineral constituent. Overall, the treatment system used in this paper was successful in removing Sb and As from mine drainage. However, due to overfill of the settling reactor with precipitated ochreous sediments after 15 months since the start of the field experiment, the treatment system required a maintenance, i.e., the settling reactor had to be cleaned.
References
Alloway BJ (2013) Heavy metals in soils, 3rd edn. Springer, Dordrecht
Anon (2015) Guideline of Ministry of Environment of the Slovak Republic No. 1/2015–7 for the elaboration of risk assessment analysis of contaminated sites. http://www.minzp.sk/files/sekcia-geologie-prirodnych-zdrojov/ar_smernica_final.pdf. Accessed 11 Nov 2017 (in Slovak)
Ashley PM, Craw D, Graham BP, Chappell DA (2003) Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern Zealand. J Geochem Explor 77:1–14. https://doi.org/10.1016/S0375-6742(02)00251-0
Asta MP, Cama J, Ayora C, Acero P, de Giudici G (2010) Arsenopyrite dissolution rates in O2-bearing solutions. Chem Geol 273:272–285. https://doi.org/10.1016/j.chemgeo.2010.03.002
Auxt A, Jurkovič Ľ, Šottník P, Bačik M, Sekula P, Sekula K, Peťková K, Brčeková J, Voleková B (2015) Environmental impact reconnoissance KS (012)/Poproč-Petrová Valley, SK/EZ/KS/353), Final report. Ministry of Environment of the Slovak Republic, Bratislava (in Slovak)
Bailey SE, Olin TJ, Brick RM, Adrian DD (1999) A review of potentially low-costs sorbents for heavy metals. Water Res 33:2469–2479. https://doi.org/10.1016/S0043-1354(98)00475-8
Bang S, Korfiatis GP, Meng X (2005a) Removal of arsenic from water by zero-valent iron. J Hazard Mater 121:61–67. https://doi.org/10.1016/j.jhazmat.2005.01.030
Bang S, Johnson MD, Korfiatis GP, Meng X (2005b) Chemical reactions between arsenic and zero-valent iron in water. Water Res 39:763–770. https://doi.org/10.1016/j.watres.2004.12.022
Bigham JM, Schwertmann U, Carlson L (1992) Mineralogy of precipitates formed by the biogeochemical oxidation of Fe(II) in mine drainage. In: Skinner HCW, Fitzpatrick RW (eds) Biomineralization processes of iron and manganese – modern and ancient environments. Catena Verlag, Berlin, pp 219–232
Bowell RJ, Bruce I (1995) Geochemistry of iron ochres and mine waters from Levant Mine, Cornwall. Appl Geochem 10:237–250. https://doi.org/10.1016/0883-2927(94)00036-6
Casiot C, Ujevic M, Munoz M, Seidel JL, Elbaz-Poulichet F (2007) Antimony and arsenic mobility in a creek draining an antimony mine abandoned 85 years ago (upper Orb basin, France). Appl Geochem 22:788–798. https://doi.org/10.1016/j.apgeochem.2006.11.007
Chmielewská E, Tylus W, Drábik M, Majzlan J, Kravčak J, Williams C, Čaplovičová M, Čaplovič Ľ (2017) Structure investigation of nano-FeO(OH) modified clinoptilolite tuff for antimony removal. Microporous Mesoporous Mater 248:222–233. https://doi.org/10.1016/j.micromeso.2017.04.022
Corkhill CL, Vaughan DJ (2009) Arsenopyrite oxidation—a review. Appl Geochem 24:2342–2361. https://doi.org/10.1016/j.apgeochem.2009.09.008
Cornell RM, Schwertmann U (2003) The iron oxides—structure, properties, reactions, occurrences and uses, 2nd edn. Wiley-VCH, New York
Cundy AB, Hopkinson L, Whitby RLD (2008) Use of iron-based technologies in contaminated land and groundwater remediation: a review. Sci Total Environ 400:42–51. https://doi.org/10.1016/j.scitotenv.2008.07.002
DeMarco MJ, SenGupta AK, Greenleaf JE (2003) Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res 37:164–176. https://doi.org/10.1016/S0043-1354(02)00238-5
Dixit S, Hering JG (2003) Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37:4182–4189. https://doi.org/10.1021/es030309t
Edwards AMC (1973) The variation of dissolved constituents with discharge in some Norfolk rivers. J Hydrol 18:219–242. https://doi.org/10.1016/0022-1694(73)90049-8
Fawcett SE, Jamieson HE, Nordstrom DK, McCleskey RB (2015) Arsenic and antimony geochemistry of mine wastes, associated waters and sediments at the Giant Mine, Yellowknife, Northwest Territories, Canada. Appl Geochem 62:3–17. https://doi.org/10.1016/j.apgeochem.2014.12.012
Filella M, Philippo S, Belzile N, Chen Y, Quentel F (2009) Natural attenuation processes applying to antimony: A study in the abandoned antimony mine in Goesdorf, Luxembourg. Sci Total Environ 407:6205–6216. https://doi.org/10.1016/j.scitotenv.2009.08.027
Flakova R, Zenisova Z, Sracek O, Krcmar D, Ondrejkova I, Chovan M, Lalinská B, Fendekova M (2012) The behavior of arsenic and antimony at Pezinok mining site, southwestern part of the Slovak Republic. Environ Earth Sci 66:1043–1057. https://doi.org/10.1007/s12665-011-1310-7
Fľaková R, Ženišová Z, Krčmář D, Ondrejková I, Sracek O (2017) Occurrence of antimony and arsenic at mining sites in Slovakia: Implications for their mobility. Carpath J Earth Environ Sci 12:41–48
Flores AN, Rubio LMD (2010) Arsenic and metal mobility from Au mine tailings in Rodalquilar (Almería, SE Spain). Environ Earth Sci 60:121–138. https://doi.org/10.1007/s12665-009-0174-6
Furukawa Y, Kim J-W, Watkins J, Wilkin RT (2002) Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ Sci Technol 36:5469–5475. https://doi.org/10.1021/es025533h
Gebel T (1997) Arsenic and antimony: comparative approach on mechanistic toxicology. Chem Biol Interact 107:131–144. https://doi.org/10.1016/S0009-2797(97)00087-2
Grecula P, Abonyi A, Abonyiová M, Antaš J, Bartalský B, Bartalský J, Dianiška I, Drzík E, Ďuďa R, Gargulák M, Gazdačko Ľ, Hudáček J, Kobulský J, Lörincz L, Macko J, Návesňák D, Németh Z, Novotný L, Radvanec M, Rojkovič I, Rozložník L, Rozložník O, Varček C, Zlocha J (1995) Mineral deposits of the Slovak Ore Mountains. Geokomplex, Bratislava (in Slovak)
Guo X, Wu Z, He M, Meng X, Jin X, Qiu N, Zhang J (2014) Adsorption of antimony onto iron oxyhydroxides: adsorption behavior and surface structure. J Hazard Mater 276:339–345. https://doi.org/10.1016/j.jhazmat.2014.05.025
Gzyl G, Banks D (2007) Verification of the “first flush” phenomenon in mine water from coal mines in the Upper Silesian Coal Basin, Poland. J Contam Hydrol 92:66–86. https://doi.org/10.1016/j.jconhyd.2006.12.001
Herath I, Vithanage M, Bundschuh J (2017) Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ Pollut 223:545–559. https://doi.org/10.1016/j.envpol.2017.01.057
Heviánková S, Bestová I, Kyncl M (2014) The application of wood ash as a reagent in acid mine drainage treatment. Miner Eng 56:109–111. https://doi.org/10.1016/j.mineng.2013.10.032
Hiller E, Lalinská B, Chovan M, Jurkovič Ľ, Klimko T, Jankulár M, Hovorič R, Šottník P, Fľaková R, Ženišová Z, Ondrejková I (2012) Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl Geochem 27:598–614. https://doi.org/10.1016/j.apgeochem.2011.12.005
Ilavský J, Barloková D, Munka K (2015) Antimony removal from water by adsorption to iron-based sorption materials. Water Air Soil Pollut 226:2238. https://doi.org/10.1007/s11270-014-2238-9
Jain CK, Ali I (2000) Arsenic: occurrence, toxicity and speciation techniques. Water Res 34:4304–4312. https://doi.org/10.1016/S0043-1354(00)00182-2
Jambor JL (2003) Mine-waste mineralogy and mineralogical perspectives of acid – base accounting. In: Jambor JL, Blowes DW, Ritchie AIM (eds) Environmental aspects of mine wastes, vol 31. Mineralogical Association of Canada, Quebec, pp 117–145
Kaartinen T, Laine-Ylijoki J, Ahoranta S, Korhonen T, Neitola R (2017) Arsenic removal from mine waters with sorption techniques. Mine Water Environ 36:199–208. https://doi.org/10.1007/s10230-017-0450-8
Kaličiaková E, Pacindová N, Repčiak M, Seliga J, Volko P (1996) Poproč – heaps, dumps, tailings ponds – the environment, Final report. State Geological Institute of Dionýz Štúr, Bratislava (in Slovak)
Klimko T, Heviánková S, Šottník P, Jurkovič Ľ, Lacková E, Vozár J (2014) Experimental removal of antimony from mine waters (abandoned Sb deposit Poproč, eastern Slovakia). Acta Geol Slov 6:203–213. (in Slovak with English abstract and summary)
Kolbe F, Weiss H, Morgenstern P, Wennrich R, Lorenz W, Schurk K, Stanjek H, Daus B (2011) Sorption of aqueous antimony and arsenic species onto akaganeite. J Colloid Interface Sci 357:460–465. https://doi.org/10.1016/j.jcis.2011.01.095
Lackovic JA, Nikolaidis NP, Dobbs GM (2000) Inorganic arsenic removal by zero-valent iron. Environ Eng Sci 17:29–39. https://doi.org/10.1089/ees.2000.17.29
Lalinská-Voleková B, Majzlan J, Klimko T, Chovan M, Kučerová G, Michňová J, Hovorič R, Göttlicher J, Steininger R (2012) Mineralogy of weathering products of Fe–As–Sb mine wastes and soils at several Sb deposits in Slovakia. Can Mineral 50:1207–1226. https://doi.org/10.3749/canmin.50.2.481
Leuz A-K, Mönch H, Johnson CA (2006) Sorption of Sb(III) and Sb(V) to goethite: influence on Sb(III) oxidation and mobilization. Environ Sci Technol 40:7277–7282. https://doi.org/10.1021/es061284b
Li J, Bao H, Xiong X, Sun Y, Guan X (2015) Effective Sb(V) immobilization from water by zero-valent iron with weak magnetic field. Sep Purif Technol 151:276–283. https://doi.org/10.1016/j.seppur.2015.07.056
Li S, Wang W, Liang F, Zhang W-X (2017) Heavy metal removal using nanoscale zero-valent iron (nZVI): theory and application. J Hazard Mater 322:163–171. https://doi.org/10.1016/j.jhazmat.2016.01.032
Lintnerová O, Šucha V, Streško V (1999) Mineralogy and geochemistry of acid mine Fe-precipitates from the main Slovak mining regions. Geol Carpath 50:395–404
Majzlan J, Lalinská B, Chovan M, Jurkovič Ľ, Milovská S, Göttlicher J (2007) The formation, structure, and ageing of As-rich hydrous ferric oxide at the abandoned Sb deposit Pezinok (Slovakia). Geochim Cosmochim Acta 71:4206–4220. https://doi.org/10.1016/j.gca.2007.06.053
Manning BA, Hunt ML, Amrhein C, Yarmoff JA (2002) Arsenic(III) and arsenic(V) reactions with zerovalent iron corrosion products. Environ Sci Technol 36:5455–5461. https://doi.org/10.1021/es0206846
Masson M, Schäfer J, Blanc G, Dabrin A, Castelle S, Lavaux G (2009) Behavior of arsenic and antimony in the surface freshwater reaches of a highly turbid estuary, the Gironde Estuary, France. Appl Geochem 24:1747–1756. https://doi.org/10.1016/j.apgeochem.2009.05.004
Milham L, Craw D (2009) Antimony mobilization through two contrasting gold ore processing systems, New Zealand. Min Water Environ 28:136–145. https://doi.org/10.1007/s10230-009-0071-y
Mohan D, Pittman JCU (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53. https://doi.org/10.1016/j.jhazmat.2007.01.006
Nikolaidis NP, Dobbs GM, Lackovic JA (2003) Arsenic removal by zero-valent iron: field, laboratory and modeling studies. Water Res 37:1417–1425. https://doi.org/10.1016/S0043-1354(02)00483-9
Nordstrom DK (2011) Mine waters: acidic to circumneutral. Elements 7:393–398. https://doi.org/10.2113/gselements.7.6.393
Parkhurst DL, Appelo CAJ (1999) PHREEQC-2, A hydrogeochemical computer program. U.S. Geological Survey Water Resources Investigation, pp Report, 99–4259
Qi P, Pichler T (2017) Competitive adsorption of As(III), As(V), Sb(III) and Sb(V) onto ferrihydrite in multi-component systems: implications for mobility and distribution. J Hazard Mater 330:142–148. https://doi.org/10.1016/j.jhazmat.2017.02.016
Ritchie VJ, Ilgen AG, Mueller SH, Trainor TP, Goldfarb RJ (2013) Mobility and chemical fate of antimony and arsenic in historic mining environments of the Kantishna Hills district. Denali National Park Preserve Alaska Chem Geol 335:172–188. https://doi.org/10.1016/j.chemgeo.2012.10.016
Rozložník O (1980) Questions of genesis and prospecting of stibnite deposits in Poproč and Zlatá Idka. In: Ilavský J (ed) Antimony ores of Czechoslovakia. State Geological Institute of Dionýz Štúr, Bratislava, pp 141–146 (in Slovak)
Schwertmann U, Taylor RM (1989)) Iron Oxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. SSSA Book Series 1, 2nd edn. Soil Science Society of America, Madison, pp 380–438
Sharifi R, Moore F, Keshavarzi B (2016) Mobility and chemical fate of arsenic and antimony in water and sediments of Sarouq River catchment, Takab geothermal field, northwest Iran. J Environ Manag 170:136–144. https://doi.org/10.1016/j.jenvman.2016.01.018
Shim MJ, Choi BY, Lee G, Hwang YH, Yang J-S, O’Loughlin EJ, Kwon MJ (2015) Water quality changes in acid mine drainage streams in Gangneung, Korea, 10 years after treatment with limestone. J Geochem Explor 159:234–242. https://doi.org/10.1016/j.gexplo.2015.09.015
Skousen J, Zipper CE, Rose A, Ziemkiewicz PF, Nairn R, McDonald LM, Kleinmann RL (2017) Review of passive systems for acid mine drainage treatment. Mine Water Environ 36:133–153. https://doi.org/10.1007/s10230-016-0417-1
Sprague DD, Michel FA, Vermaire JC (2016) The effects of migration on ca. 100-year-old arsenic-rich mine tailings in Cobalt, Ontario, Canada. Environ Earth Sci 75:405. https://doi.org/10.1007/s12665-015-4898-1
Sracek O, Mihaljevič M, Kříbek B, Majer V, Filip J, Vaněk A, Penížek V, Ettler V, Mapani B (2014) Geochemistry of mine tailings and behavior of arsenic at Kombat, northeastern Namibia. Environ Monit Assess 186:4891–4903. https://doi.org/10.1007/s10661-014-3746-1
Su C, Puls RW (2001) Arsenate and arsenite removal by zerovalent iron: kinetics, redox transformation, and implications for in situ groundwater remediation. Environ Sci Technol 35:1487–1492. https://doi.org/10.1021/es001607i
Su C, Puls RW (2003) In situ remediation of arsenic in simulated groundwater using zerovalent iron: laboratory column tests on combined effects of phosphate and silicate. Environ Sci Technol 37:2582–2587. https://doi.org/10.1021/es026351q
Ungureanu G, Santos S, Boaventura R, Botelho C (2015) Arsenic and antimony in water and wastewater: overview of removal techniques with special reference to latest advances in adsorption. J Environ Manag 151:326–342. https://doi.org/10.1016/j.jenvman.2014.12.051
Vasquez Y, Escobar MC, Neculita CM, Arbeli Z, Roldan F (2016) Biochemical passive reactors for treatment of acid mine drainage: effect of hydraulic retention time on changes in efficiency, composition of reactive mixture, and microbial activity. Chemosphere 153:244–253. https://doi.org/10.1016/j.chemosphere.2016.03.052
Woo ES, Kim JJ, Kim YH, Jeong GC, Jang YD, Dick WA (2013) Mineralogical and geochemical characterization of precipitates on stream receiving acid mine water. Korea Environ Earth Sci 69:2199–2209. https://doi.org/10.1007/s12665-012-2048-6
Xi J, He M, Zhang G (2014) Antimony adsorption on kaolinite in the presence of competitive anions. Environ Earth Sci 71:2989–2997. https://doi.org/10.1007/s12665-013-2673-8
Young PL (1997) The longevity of minewater pollution: a basis for decision-making. Sci Total Environ 194–195:457–466. https://doi.org/10.1016/S0048-9697(96)05383-1
Ženišová Z, Fľaková R, Jašová I, Cicmanová S (2009) Antimony and arsenic in waters influenced by mining activities in selected parts of Slovakia. Podzemná voda 15:100–117. (in Slovak with English abstract and summary)
Zhang R, Sun H, Yin J (2008) Arsenic and chromate removal from water by iron chips—effects of anions. Front Environ Sci Eng China 2:203–208. https://doi.org/10.1007/s11783-008-0036-6
Acknowledgements
We acknowledge the project APVV-0344-11 and the Ministry of Environment of the Slovak Republic that financially supported the research presented in this paper. We wish to thank Dr. Andrew Cundy for editing of the English in the manuscript. We would also like to thank the two anonymous reviewers for their valuable and helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sekula, P., Hiller, E., Šottník, P. et al. Removal of antimony and arsenic from circum-neutral mine drainage in Poproč, Slovakia: a field treatment system using low-cost iron-based material. Environ Earth Sci 77, 518 (2018). https://doi.org/10.1007/s12665-018-7700-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7700-3