Abstract
The present work aims to assess the efficiency of heavy metal accumulation of native species growing in contaminated soils in the mining district of Taxco, Mexico. Soil and tailing sampling was conducted in three study sites: La Concha, El Fraile, and a control site. The study localities present diverse metal concentrations with significant differences in their proportion in the geochemical fractions. Results show that species Cupressus lindleyi and Juniperus deppeana accumulate Zn and Mn in anomalous concentrations at La Concha, where Zn is present in soluble fractions. Manganese, despite not being present mostly in the soluble fraction in soils and tailings, seems to have been increased in the soluble fraction after the plant growth. In contrast, samples of the same species taken at El Fraile and in the control site, where Zn and Mn are mainly contained in the residual fraction, do not show an anomalous enrichment. Other analyzed species growing under the same contamination conditions in La Concha (Jacaranda mimosifolia and Psidium guajava) do not show anomalous concentrations. These facts confirm the Zn and Mn accumulation capacity of C. lindleyi and Ju. deppeana, which depends on their accumulation ability and on the concentration of these elements in the soluble fraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities have a considerable impact on the environment since they generate large amounts of waste rocks and tailings, which may become sources of heavy metals to the environment (Armienta et al. 2003; Romero et al. 2007). Highly contaminated sites can support the growth of specific plant species called metallophytes, which can potentially be used to control the fluxes of trace elements in the environment (Robinson et al. 2009). Phytoremediation refers to the use of plants to remove, contain or render harmless environmental pollutants (Robinson et al. 2009). However, a successful phytoextraction procedure in heavily contaminated soils would take a long period of time. Taking into account this limitation, some authors recommended applying phytostabilization procedures, which reduce the bioavailability of contaminants in the soil, preventing their leaching and absorption by plants (Dickinson et al. 2009). In spite of the speculation related to the effectiveness of phytoextraction of metals and metalloids of contaminated soils, the use of accumulator plants has been reported in many recent works as a cost-effective tool for phytoremediation (Conesa et al. 2006, 2009; Kidd and Monterroso 2004).
Frequently, wild plant species occurring in contaminated areas have been identified as accumulators. This ability is generally developed by these species because these plants are often better adapted to local conditions. The accumulation and distribution of metals in the plant tissues are important aspects to evaluate their role on the remediation at contaminated sites. Considerable uncertainties often remain regarding metal uptake mechanisms in the plant (Dickinson et al. 2009). The accumulation feasibility depends more on the nutrient function of the element than on the heavy metal concentration in the soil. However, total content of metals in soil is not a good measure for assessing their bioavailability or their potential risks from soil and sediment contamination. Sequential extraction procedures have been used in these cases to evaluate the fraction of the solid phase which is the potential source of metal available to the environment (Moral et al. 2004; Menzies et al. 2007).
Metal uptake through the plant depends, in great part, on the nutritional role of the metals. Roots take metals from the rhizosphere to the plant root tissues and then via the stems to the leaves. The transportation of metals to the aerial parts of the plant requires its entry into the root xylem. Most of them must penetrate a cell. Other metals can only traverse the membrane via embedded protein transporters. On the other side, “ion channels” permit ions with a specific size and charge to move across the cell membrane down the concentration gradient (Robinson et al. 2009). Transporters are known for Mn, Zn, Cu, Fe, Ni, Co and Cd (Reid and Hayes 2003). Nonessential elements with a similar size to nutrients may be taken up into the symplast and be translocated to the shoots (Robinson et al. 2009).
Gupta and Sinha (2007), in a study related to the phytoremediation potential of plants growing in a contaminated site in India, found that the accumulation of essential micronutrients was higher in upper parts of the tested plants, whereas toxic metal accumulation was restricted in lower parts of the plants. Commonly, metal accumulation ability of plants is frequently been assessed in leaves (Unterbrunner et al. 2007; Gupta and Sinha 2007). In Mexico, the presence of metal tolerant species that grow directly on mine tailings in the semiarid region of Chihuahua was reported by Carrillo-González and González-Chavez (2006). They identified the species Polygonum aviculare and Jatropha dioica as Zn accumulators.
Prosopis laevigata and Acacia farnesiana have also been identified to tolerate high As concentrations at Zimapán, Central México (Armienta et al. 2008). Taxco, in the center of Mexico, is one of the main silver mining zones since prehispanic times. This mining district is located in the northern part of Guerrero State in southern Mexico. Large volumes of mining wastes have been produced and accumulated mainly in two important tailing dumpsites: El Fraile and La Concha tailing ponds. For the El Fraile tailings, Romero et al. (2004) reported Pb concentrations of 3,340–7,760 mg/kg. The objective of the present study was to identify the efficiency of the native wild species (C. lindleyi, Ja. mimosifolia, Ju. deppeana, Ps. guajava) to accumulate heavy metals to evaluate their possible use to reduce the environmental impact of mining wastes in Taxco, Mexico.
Experimental procedure
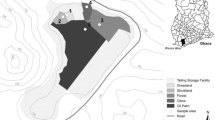
Sampling was conducted in three study sites in the Taxco Mining District: La Concha (LC), El Fraile (EF), and a control site (CS), located about 10 km away from tailing dumpsites (Fig. 1). At sites La Concha and El Fraile, nine soil and tailing samples were collected, as well as three soil samples at the control site. In addition, leave samples were collected from three healthy species of C. lindleyi, Ja. mimosifolia, Ju. deppeana, and Ps. guajava growing in the selected sites. As was mentioned above, metal translocation from roots throughout the plant commonly lead to achieve the highest concentration in the leaves. This fact and the possibility of comparing the data obtained in this research with results of similar work were decisive to conduct this study on plant leaves. Collected species were identified by the Departamento of Biología, Universidad Autónoma Metropolitana. Soil and tailing samples were air dried, sieved to 2 mm, homogenized and stored in plastic bags prior to laboratory analysis. Plants were washed with tap and distilled water, dried at 45 °C for 48 h. All the cleaned and dried plant samples were ground and homogenized using a plastic mill.
Total metal concentrations (Cu, Fe, Mn, Pb and Zn) in plants were analyzed using X-ray fluorescence spectrometry. About 5 g of each sample was mixed with wax-C using an agate mortar, and pressed to form a pellet to be introduced to Siemens SRS-3000 X-ray fluorescence spectrometer equipped with a Rh anode tube as X-ray source at the Instituto de Geología, UNAM. The accuracy of the procedure was determined by analyzing the certified reference material NIST 2586 (National Institute of Standards and Technology, USA). Results were in excellent agreement with certified concentrations (better than 2.6 % RSD). Precision was better than 1.8 % RSD (N = 6). To determine the amount of metals in different chemical fractions in soils and tailing samples, a metal fractionation scheme was carried out following the sequential extraction procedure reported by Mossop and Davison (2003). Sequential extraction procedures were applied for the fractionation of metal content in soils, sediments, tailing and other solid materials. Metals were extracted using reagents possessing diverse chemical properties (acidity, redox potential, or complexing properties). With such procedures, metals and metalloids are divided into water soluble and acid (Fraction 1), reducible (Fraction 2), oxidizable (Fraction 3) and residual (Fraction 4) fractions. In this case, the extraction procedure was applied as described briefly below: Fraction 1: 1 g of air-dried soil sampled and 40 ml acetic acid (0.11 mol l−1) were shaken overnight. Mixture was centrifuged to separate the extract from the residue. Fraction 2: the residue from 1 was leached with 0.5 mol l−1 hydroxyl ammonium and adjusted to pH 1.5 with HNO3. The extract separation procedure was performed as for Fraction 1. Fraction 3: to the residue from step 2, 8.8 mol−1 l H2O2 was added twice and the mixture taken near to dryness. Then 50 ml of ammonium acetate was added, adjusted to pH 2 with HNO3. The separation procedure was performed as above. Residual fraction: material remaining from the extraction of Fraction 3 was digested in 20 ml aqua regia with microwave assistance. Concentrations in each fraction were measured using flame AAS (Perkin-Elmer AAnalyst 100) at the Instituto de Geofísica UNAM.
Results and discussion
Total concentrations of Cu, Fe, Mn, Pb and Zn of the analyzed soil and tailing samples as well as relative amounts of each metal obtained by sequential extraction are reported in Table 1 and Fig. 2. An overall comparison between the mean concentrations of all metals, as well metals as min–max 25–75 %, is shown in Fig. 3. It can be seen that total metal concentrations in both study localities varied widely and that concentration of all analyzed elements is higher at La Concha than at El Fraile. Zinc presents a significant proportion in Fraction 1 (water and acid soluble) in soil and tailing samples from La Concha (up to 39 %). Manganese presents low proportion of water and soluble fraction (until 11 %). Particularly noticeable is the fact that, despite the high Fe concentration reported in soil and in tailings, the majority of its concentration is found in the residual fraction (up to 99 %). Copper concentrations are lower (Table 1; Figs. 2, 3), and in both localities residual fractions reach up to 90.4 %. Lead concentration is higher in La Concha than in El Fraile. In both locations, a high proportion of Pb is contained in the residual fraction. Table 2 shows the mean metal concentration in leaves of the analyzed plants. C. lindleyi and Ju. deppeana leaf samples taken at La Concha showed much higher concentrations of Zn (454 and 469 ppm, respectively) with respect to the other analyzed species (Ja. mimosifolia and Ps. guajava). This fact could make us consider C. lindleyi and Ju. deppeana as Zn phytoaccumulator; however, leaves of this species taken at El Fraile and in the control site, where Zn is not always in the soluble fraction, do not show an enrichment of this element. Table 2 shows high concentrations of Zn in the leaves of C. lindleyi and in Ju. deppeana taken at La Concha, where soils and tailing samples present a high proportion of it in the residual fraction (see Table 1). On the other hand, leaves from other analyzed species which grow under contamination conditions at La Concha do not show Zn enrichment properties. Manganese behaves differently; in spite that the analyzed soils and tailing samples at La Concha show a low proportion in the soluble fraction (between 5.1 and 11.3 %), the leaves of C. lindleyi and Ju. deppeana present high concentration of this metal. Unlike Zn, whose accumulation can be related to its high proportion in the soluble fraction, Mn accumulation might be related to changes in the metal distribution among different soil fractions after plant growth, when acid extractable and reducible fractions of metals often increase. Robinson et al. (2009) reported that plant roots influence soil in the immediate vicinity (rhizosphere). The solubility and speciation of toxic elements in this zone may be distinct from the bulk soil. Plant roots excrete H+ ions that exchange with nutrients base cations. Generated soil acidification increases the solubility of nonessential elements enhancing their accumulation in plants. However, no Mn accumulation is observed in leaves of these two species (C. lindleyi and Ju. deppeana) at El Fraile. This fact can be associated to the low Mn concentrations in soils and tailing samples in this location, compared with the higher concentrations found in La Concha. This data verified the Zn and Mn accumulation capacity of C. lindleyi and Ju. deppeana in contaminated areas, which depends on the availability of these metals in the substrate.
The notional criterium for Mn and Zn hyperaccumulation is 10,000 mg/kg, (Susarla et al. 2002); therefore, the analyzed species cannot be considered as hyperaccumulators. However, Mn and Zn concentrations found in this study are comparable with data of accumulator species reported previously. Massa et al. (2010) reported concentrations up to 754.6 mg kg−1 (Mn) and 333. 2 mg kg−1 (Zn) in native plants growing in a polluted site in Italy. On the other hand, Sainger et al. (2011) investigated heavy metal tolerance in native plants from a contaminated site in an urban area in India. They found Zn concentration between 236 and 958 mg kg−1. Wang et al. (2008) found high Mn concentrations (up to 6,330 mg kg−1) in roots of plants growing in southern China, while Zn concentrations in the same species range between 140 and 647 mg kg−1.
Conclusion
This work was performed with the aim to identify the efficiency of heavy metal accumulation of native wild species (C. lindleyi, Ja. mimosifolia, Ju. deppeana, Ps. guajava) to evaluate their possible use to reduce the environmental impact of mining wastes in Taxco, Mexico. Data obtained in this study showed the variability of metal accumulation capacity between four natural species growing in the tailing zones of Taxco, Mexico. Total metal concentration in soil and tailings varied among location with significant differences in their available proportion. None of the analyzed species was recognized as metal accumulators; however, results show that species C. lindleyi and Ju. deppeana accumulate Zn and Mn in anomalous concentrations at La Concha, where Zn is present in soluble fractions in the substrates. Even though Mn concentration is low in the soluble proportion in the substrate, this seems to be increased after plant growth. In contrast, samples of the same species taken at El Fraile and in the control site, where Zn and Mn are mainly contained in the residual fraction and the concentration of both elements is lower, do not show an anomalous enrichment. From these data, it will be possible to deduce that metal enrichment in plants depends on metal availability in the substrate. Nevertheless, other analyzed species growing under the same contamination conditions in La Concha (Ja. mimosifolia and Ps. guajava) do not show anomalous concentrations. The assessment of the behavior of metals analyzed in this research allows reaching interesting conclusions about the uptake mechanism concerning their nutrient features. Copper and Fe are present in high concentrations in both contaminated sites in soils as well as in tailings. These elements are contained mainly in the residual fraction and are not available to be taken up by plants, despite being essential to plants. Lead is present mainly in the soluble fraction in the substrate (soils and tailings) at both contaminated sites; however, Pb do not play an essential function in plants. Plants tolerate nonessential elements at low concentrations, but higher concentrations are phytotoxic. The fact that Zn and Mn are essential leads to transportation mechanisms being activated in plants with accumulation ability, facilitating the plants phytoremediation function.
Results of this screening study indicate that, in spite of the relatively low accumulated concentrations of Zn and Mn, C. lindleyi and Ju. deppeana can be considered as accumulators. Even so, to be considered as a source of feasible phytoextraction technology, it is important to determine if the metals are concentrated additionally in roots, wood or bark in such a manner that the contaminating concentration can be reduced to levels that comply with environmental regulations. It is planned to conduct this experiment over a period of several years to provide evidences of their annual behavior, as well as to verify the metal distribution among different chemical forms in the substrate after plant growth. Presence of high concentration of Zn and Mn in adult healthy C. lindleyi and Ju. deppeana plants shows them as promising phytostabilization species in the Taxco mining zone.
References
Armienta MA, Talavera O, Morton O, Barrera M (2003) Geochemistry of metals from mine tailings in Taxco, Mexico. Bull Environ Contam Toxicol 71:387–393
Armienta MA, Ongley LK, Cruz O, Mango H, Villaseñor G (2008) Arsenic distribution in mesquite (Prosopis laevigata) and huizache (Acacia farnesiana) in the Zimapán mining area, México. Geochem Explor Environ Anal 8(191):197
Carrillo-González R, González-Chavez MCA (2006) Metal accumulation in wild plants surrounding mining wastes. Environ Pollut 144:84–92
Conesa H, Faz A, Arnaldos R (2006) Heavy metal accumulation and tolerance in plants from mine tailings of the semiarid Cartagena–La Unión mining district (SE) Spain. Sci Total Environ 366:1–11
Conesa HA, Moradi AB, Robinson BH, Kühne G, Lehmann E, Schulin R (2009) Response of native grasses and Cicer arietinum to soil polluted with mining wastes: implications for the management of land adjacent to mine sites. Environ Exp Bot 65:198–204
Dickinson MN, Baker AJM, Doronila A, Laidlaw S, Roger D, Reeves RD (2009) Phytoremediation of inorganics: realism and Synergies. Int J Phytorem 11:97–114
Gupta AK, Sinha S (2007) Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour Technol 98:1788–1794
Kidd PS, Monterroso C (2004) Metal extraction by Alyssum serpyllifolium ssp. lusitanicum on mine-spoil soils from Spain. Sci Total Environ 336:1–11
Massa N, Andreucci F, Poli M, Aceto M, Barbato R, Berta G (2010) Screening for heavy metal accumulators amongst autochthonous plants in a polluted site in Italy. Ecotoxicol Environ Saf 73:1988–1997
Menzies NW, Donn MJ, Koppiteke PM (2007) Evaluation of extractants for estimation of the phyto available trace metals in soils. Environ Pollut 145:121–130
Moral R, Gilkes RJ, Jordán MM (2004) Distribution of heavy metals in calcareous and non-calcareous in Spain water. Air Soil Pollut 162:127–142
Mossop K, Davison CHM (2003) Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal Chim Acta 478:111–118
Reid R, Hayes J (2003) Mechanisms and control of nutrient uptake in plants. Int Rev Cytol 229:73–114
Robinson BH, Bañuelos G, Conesa HM, Evangelou MWH, Schulin R (2009) The Phyto management of trace elements in soil. Crit Rev Plant Sci 28:240–266
Romero FM, Armienta MA, Carrillo A (2004) Arsenic sorption by carbonate-rich aquifer material, a control on arsenic mobility at Zimapan, Mexico. Arch Environ Contam Toxicol 47:1–13
Romero FM, Armienta MA, González-Hernández G (2007) Solid-phase control on the mobility of potentially toxic elements in an abandoned lead/zinc mine tailings impoundment, Taxco, Mexico. Appl Geochem 22:109–127
Sainger PA, Dhankhar R, Sainger M, Kaushik A, Singh RP (2011) Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotoxicol Environ Saf 74:2284–2291
Susarla S, Medina VF, McCutcheon SC (2002) Phytoremediation: an ecological solution to organic chemical contamination. Ecol Eng 18:647–665
Unterbrunner R, Puschenreiter M, Sommer P, Wieshammer G, Tlustos P, Zupan M, Wenzel WW (2007) Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ Pollut 148:07–114
Wang X, Liu Y, Zeng G, Chai L, Xiao X, Song X, Min Z (2008) Pedological characteristics of Mn mine tailings and metal accumulation by native plant. Chemosphere 72:1260–1266
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morton-Bermea, O., Gómez-Bernal, J.M., Armienta, M.A. et al. Metal accumulation by plant species growing on a mine contaminated site in Mexico. Environ Earth Sci 71, 5207–5213 (2014). https://doi.org/10.1007/s12665-013-2923-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2923-9