Abstract
Highly chlorinated pollutants are often found in river sediments, such as in the Yangtze River. In this study, the transformation of hexachlorobenzene, pentachlorophenol, polychlorinated biphenyl (PCB), hexachlorocyclohexane and perchloroethene was demonstrated in a mixed microbial culture enriched from Xiangxi River sediment [a tributary of Yangtze River belonging to the Three Gorges Reservoir (TGR)] which contained halo-respiring bacteria. Reductive dehalogenation by the Yangtze bacteria resulted in the formation of lower chlorinated metabolites, such as tri- and dichlorobenzenes, tri- and dichlorophenols, vinyl chloride and ethene. In case of PCB180, the lesser chlorinated metabolite PCB146 was detected and is recommended for future monitoring programs. Increased gene copy numbers of dechlorinating bacteria, e.g. Dehalococcoides spp. and Desulfitobacterium spp., were observed after the transformation of the chlorinated pollutants. In conclusion, the study demonstrates the capability of Yangtze River bacteria to dechlorinate several important chlorinated pollutants, indicating efficient pollutant turnover in the TGR area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorinated organic compounds have been released to the environment through their extensive use in agriculture and chemistry over the past 50 years. Chlorinated substances served as biocides [e.g. hexachlorocyclohexanes (HCHs), hexachlorobenzene (HCB) and pentachlorophenol (PCP)], electrical fluids [e.g. polychlorinated biphenyls (PCBs)], intermediates for chemical synthesis [e.g. HCB and vinyl chloride (VC)], solvents and degreasing agents [e.g. tetrachloroethene/trichloroethene (PCE/TCE)] and have functions in many other applications (Abramowicz 1995; Adrian and Görisch 2002; Kaufhold et al. 2013; Lal et al. 2010; Olaniran and Igbinosa 2011; Schmidt et al. 2010; Wu et al. 2015). The extensive industrial and agricultural applications resulted in the deposition of chlorinated organic compounds in various environments, especially in soils, groundwater aquifers and sediments of lakes and rivers (Dohmann et al. 2016; Liu et al. 2010; Taş et al. 2010a; Xi et al. 2015). Due to their physicochemical properties, exposure to these compounds can have carcinogenic or toxic effects. Therefore, the elimination of chlorinated pollutants from water resources is highly relevant all over the world (Olaniran and Igbinosa 2011; Wang and He 2013; van Doesburg et al. 2005; Taş et al. 2010b; Schmidt and Tiehm 2008).
The Yangtze River, with a length of 6300 km, is one of the biggest rivers in the world. It has been a source of life for the Chinese people for centuries and is a habitat for a remarkable variety of aquatic species (Floehr et al. 2013). In the past decade, rapid industrial and economic growth in China resulted in increasing discharge of pollutants into the environment (Dohmann et al. 2016; Tao et al. 2013). In the Yangtze River, with the impoundment of the 630-km-long Three Gorges Reservoir (TGR), upstream of the Three Gorges Dam, huge industrial, residential and agricultural areas were flooded. As a consequence, notable amounts of organic and inorganic pollutants were released to the reservoir (Bergmann et al. 2012; Wolf et al. 2013). The polychlorinated pollutants of HCB (Jiang et al. 2000; Wang et al. 2009), PCP (Tang et al. 2007), PCB180 (Jiang et al. 2000; Wang et al. 2009) and α-, β-, γ- and δ-HCH (Liu et al. 2015; Wang et al. 2009) have been detected in the Yangtze River.
Anaerobic dechlorination of higher chlorinated organic compounds has been observed at many contaminated sites, in particular in Europe and the USA (Aulenta et al. 2005; Carreón-Diazconti et al. 2009; Demarest 2013; Köber et al. 2014; Schmidt and Tiehm 2008; Semprini 1995). Dehalococcoides spp. represent the only bacteria capable of complete dechlorination of chloroethenes and can also transform other chlorinated compounds (Demarest 2013; Kaufhold et al. 2013; Löffler et al. 2013; Taş et al. 2010a; Wang and He 2013). However, several other microorganisms, such as Dehalobacter spp., Desulfomonile spp., Desulfuromonas spp. and Desulfitobacterium spp., are also able to degrade chlorinated organic contaminants through organohalide respiration (Maphosa et al. 2010; Tiehm and Schmidt 2011; Wang and He 2013). Studies into the capability of halo-respiring bacterial communities to use several different chlorinated pollutants are limited. Only Kaufhold et al. (2013) and Wang and He (2013) studied the substrate spectrum of a dechlorinating culture, and other studies are restricted to only one of the chlorinated pollutants (Adrian et al. 2007; Field and Sierra-Alvarez 2008; Middeldorp et al. 2005; Schmidt and Tiehm 2008). Kaufhold et al. (2013) studied dehalogenation of halogenated substrates using a highly enriched Dehalococcoides-containing culture from the contaminated site in Bitterfeld, Germany. Wang and He (2013) reported dechlorination of multiple halogenated compounds using a culture containing Dehalococcoides spp. and Dehalobacter spp. enriched from a wastewater treatment plant in Gehua, P. R. China. Recent studies with dechlorinating bacteria enriched from Yangtze River sediments focused on chloroethene degradation. The Yangtze River cultures were shown to contain several dechlorinating bacteria, such as Dehalococcoides spp., Desulfitobacterium spp., Dehalobacter spp. and Desulfomonile spp. (Kranzioch et al. 2013, 2015).
The aim of this work was (a) to examine the capability of a mixed microbial culture enriched from Three Gorges Reservoir sediment to dehalogenate HCB, PCP, PCB180 and HCHs and (b) to identify dominating transformation pathways.
Materials and methods
Biodegradation studies
All experiments were performed in 2-L laboratory glass bottles capped with Teflon-coated septa, held in place with screw caps. Cultivation was performed in duplicate in a mineral salt medium amended with electron donors (40 mg of sodium acetate, 40 mg of sodium pyruvate, 0.5 g of yeast extract and 10 mL H2/L) as described previously (Kranzioch et al. 2013). All handling, except H2 addition, was performed inside an anaerobic gas chamber flushed with nitrogen. The bottles were amended with 0.35 µM (0.1 mg/L) HCB (99.9 %), 6 µM (1.5 mg/L) PCP (99.9 %), 0.25 µM (0.1 mg/L) PCB180 (99.5 %), 3.44 µM (1.0 mg/L) HCH (α:β:γ:δ = 1:1:1:1, 99.5 % purity) and 45 µM (7.5 mg/L) PCE (99.9 %) (Sigma-Aldrich, Steinheim, Germany). Each batch test was inoculated with 20 mL of the culture XX01 enriched from Xiangxi River (tributary of Yangtze River) sediment (Kranzioch et al. 2013). Further one bottle was prepared as sterile control with all primary substances and was poisoned with 1 g/L sodium azide. Bottles were stored at room temperature (22–24 °C) in the dark. The aqueous phase samples were taken using glass syringes.
Analytical methods
HCHs and PCBs were determined using a gas chromatograph (PerkinElmer Autosys XL AUSXA 53006, Massachusetts, USA) equipped with an electron capture detector, according to the German standards of DIN 38407, part F2: 02/1993. Other dechlorination products not included in DIN 38407 (such as PCB146) were identified and determined using a gas chromatograph (Agilent 7890A, USA) equipped with a mass spectrometer (Agilent 5975C, USA) and a gas chromatograph (Agilent 7890A, USA) equipped with an electron capture detector, according to the PCB single congener or mixtures of Aroclor from the AccuStandard. CBs were analysed with a gas chromatograph (PerkinElmer Autosys XL, Massachusetts, USA) equipped with a TurboMass Gold spectrometer, according to the German standards of DIN 34807, part F17:1999-02. CPs were analysed using a gas chromatograph (Agilent 6890N, Waldbronn, Germany) equipped with a mass spectrometer. A 500 mL diluted sample volume was preconcentrated with 200 mg of Strata X material (Phenomenex, Aschaffenburg, Germany); elution was conducted with 5 mL of acetone. After reducing the volume of the organic solvent to 200 µL, an aliquot of 2 µL was injected into the GC/MS system. Chloroethenes and ethene concentrations were determined using a gas chromatograph (Series II 5890, Hewlett-Packard, Waldbronn, Germany) equipped with a headspace sampler, flame ionisation detector and electron capture detector. Samples for gas chromatographic analyses were acidified to a pH of 2 with phosphoric acid and stored at 4 °C until analysis. The standard deviation coefficients of the analysis were 30 % in case of the CPs and 25 % in case of HCHs, PCBs and CBs. For chloroethenes, reproducibility on standard analysis was ±6 %. Chloride concentrations were determined using ion chromatography (Metrohm 761 Compact IC, Metrohm, Filderstadt, Germany) with ±1 % reproducibility on standard analysis.
Polymerase chain reaction (PCR)
For PCR analysis, 50 mL of the culture was filtered through a 0.2-µm membrane filter (47 mm diameter) (PALL, MI, USA). The membrane was stored at −20 °C until DNA extraction and analysis. The DNA of the frozen filters was extracted using the Fast DNA® Spin Kit for soil (MP Biomedicals, OH, USA).
The end-point PCR and qPCR of the culture samples for the detection of the dechlorinating bacteria Desulfomonile spp., Desulfitobacterium spp., Dehalobacter spp. and Dehalococcoides spp. were conducted as described previously (Kranzioch et al. 2013). The qPCR for Desulfuromonas spp. was performed with DeSuF205 (AACCTTCGGGTCCTACTGTC) and DeSuR1033 (GCCGAACTGACCCCTATGTT) primers with the following qPCR protocol: 95 °C for 15 min; 45 cycles of 95 °C for 15 s, 63 °C for 30 s and 72 °C for 50 s; and after 45 cycles, a melting curve from 63 to 99 °C. The end-point PCR was previously described in Kranzioch et al. (2013).
Results and discussion
The dechlorination of HCB by the Yangtze culture is shown in Fig. 1a (first flask) and Fig. S1a (second flask). In the two duplicate active cultures, the metabolites PeCB and tetrachlorobenzenes (TeCBs) were detected after 4 weeks of incubation time. After 10 weeks, higher concentrations of dissolved TeCBs and trichlorobenzenes (TCBs) were detected. 1,2,4-Trichlorobenzene (1,2,4-TCB) and 1,3,5-TCB reached maximum concentrations of 0.01 and 0.14 µmol/L after 45 weeks, respectively. After 45 and 56 weeks, two different DCBs (1,3- and 1,4-DCB) were detected at concentrations of 0.01 µmol/L. Due to limited water solubility (Table S6), the aqueous phase concentrations of HCB were below 0.02 µmol/L during the whole incubation time. The less hydrophobic metabolites reached higher dissolved concentrations (Figs. 1a and S1a). The maximum concentration of the detected dissolved chlorobenzenes was approx. 0.25 µmol/L in both flasks. Dechlorination kinetics were most likely triggered by the dissolution rates, and solid-phase HCB was still present at the end of the experiments. Additionally, co-metabolic processes might have affected pollutant transformation but were not examined in this study. Although experimental conditions differ, similar time frames of HCB dechlorination were reported in previous studies (Duan and Adrian 2013; Kaufhold et al. 2013; Taş et al. 2010b, 2011). No dechlorination products were observed in the abiotic control (data not shown).
The observed dechlorination pathway for HCB is illustrated in Fig. 1b. 1,3,5-TCB and 1,2,4-TCB were detected, followed by 1,3- and 1,4-DCB after the next dechlorination step. The degradation pathway from HCB over PeCB and 1,2,3,5-TeCB to mainly 1,3,5-TCB was also observed previously (Kaufhold et al. 2013; Duan and Adrian 2013).
The degradation pattern of PCP is shown in Figs. 2a and S2a. After 4 weeks, 2,3,4,5-tetrachlorophenol (2,3,4,5-TeCP) was detected. Obviously, elimination of the chlorine atom occurred at the ortho-position. The next chlorine was again preferentially removed at the ortho-position, resulting in the formation of 3,4,5-TCP. However, small amounts of 2,4,5-TCP were also detected in the duplicate degradation test (Fig. S2a), resulting from dechlorination at the meta-position.
The observed dechlorination pathway (Fig. 2b) of this study is consistent with the pathway proposed previously by Adrian et al. (2007). However, formation of a specific TeCP isomer was not demonstrated in the previous study. Here, dechlorination of PCP via 2,3,4,5-TeCP, mainly 3,4,5-TCP to 3,4-DCP and 3,5-DCP, is clearly shown. In the sterile control, no metabolites were formed within 56 weeks (data not shown).
The results for PCB180 are shown in Figs. 3a and S3a. Due to the low water solubility and slow dissolution kinetics, the concentration of dissolved PCB180 was increased with increasing incubation time, from starting values of approx. 4 nmol/L to the highest values of 150 nmol/L after 25 weeks. In the sterile control, the concentration of PCB180 also varied during the experiment, from values of 6 nmol/L to approximately 300 nmol/L. In the active cultures, the formation of PCB146, PCB153 and PCB138 was observed. For PCB146, an increase from 19 nmol/L to more than 200 nmol/L could be detected between weeks 4 and 36. For PCB153, a small increase could be detected between weeks 15 and 56, from 0.5 to 1 nmol/L. These results are in accordance with the transformation pathways observed previously, i.e. dechlorination in the para- or meta-position (Field and Sierra-Alvarez 2008). Meta- and para-dechlorination was also observed during the dechlorination of PCB180 through the XX01 culture. The preferred dechlorination position was the double-flanked para-position, resulting in PCB146 as the main dechlorination product. Furthermore, the double-flanked meta-position was preferred over the single-flanked because higher amounts of PCB153 compared to PCB138 were detected. None of the lower chlorinated PCBs were detected in the sterile control (data not shown).
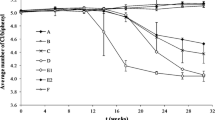
The pattern of α-, β-, γ- and δ-HCH transformation was followed for 56 weeks for the two cultures and the abiotic control. For the HCHs, only analysis of the original compounds was performed, and detection of catabolites was beyond the scope of this study. Therefore, transformation processes were evaluated by comparison of the active cultures with sterile controls. The active cultures showed a fast decrease in γ-HCH (also known as lindane) in the first 4 weeks to 20 % of the concentration in the sterile control (Figs. 4 a and S4a). After 10 weeks, the amount of γ-HCH decreased to below the detection limit. Badea et al. (2009), Cui et al. (2012), van Doesburg et al. (2005) and Middeldorp et al. (2005) reported similar dechlorination rates for γ-HCH. Additionally, a rapid decrease in concentration was found for α-HCH. The concentration of α-HCH after 10 weeks in the active cultures was 5 % of the concentration in the sterile control. After 15 weeks, α-HCH was below the detection limit. The results confirm previous reports that α- and γ-HCH are transformed rapidly under anaerobic conditions (Lal et al. 2010). However, β- and δ-HCH degradation was also reported (Quintero et al. 2005). In this study, a decrease in β- and δ-HCH in the active cultures was observed compared to the sterile control.
To proof the activity of the dechlorinating culture, a PCE control was prepared. The dechlorination pattern of PCE for the duplicate active cultures is shown in Fig. S5. In the active cultures (I) and (II) amended with PCE, a fast dechlorination of approximately 40 µmol/L PCE to 40 µmol/L cDCE occurred. From week 15 to week 36, a complete dechlorination from 40 µmol/L cDCE over VC to ethene was observed. The dechlorination potential of the Yangtze River culture XX01 for PCE was already investigated in the study of Kranzioch et al. (2013) and confirmed for comparison purposes in this study. Approximately 180 µmol/L of chloride formation corresponded to the complete dechlorination of PCE to ethene. Because ethene is volatile (Kranzioch et al. 2013), only low concentrations were found in the aqueous phase. The chloride balance was only possible in the case of PCE, which shows a high water solubility compared to HCB, HCHs, PCP or PCB 180 (Table S6). Because the bioavailable dissolved concentrations and therefore the transformation rates of the more hydrophobic chlorinated pollutants were low, increases in chloride concentrations could not be detected.
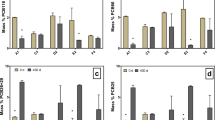
The halo-respiring bacteria Dehalococcoides spp., Desulfitobacterium spp., Dehalobacter spp., Desulfomonile spp. and Desulfuromonas spp. were analysed by quantitative PCR at the end of the incubation period of 56 weeks (Table 1). The results confirmed previous reports (Kranzioch et al. 2013, 2015) that PCE dechlorination in the Yangtze River cultures resulted predominantly in increased gene copy numbers of Dehalococcoides spp. and Desulfitobacterium spp. In this study, growth of Dehalococcoides spp. was also observed with PCP and HCB. Several previous studies (Adrian and Görisch 2002; Adrian et al. 2007; Duan and Adrian 2013; Kaufhold et al. 2013) have shown that Dehalococcoides spp. hold the ability to dechlorinate PCP and HCB.
Thus far, only transformation of CPs has been demonstrated for pure cultures of Desulfitobacterium spp. (Utkin et al. 1994; Gerritse et al. 1996). In this study, an increase in Desulfitobacterium spp. was observed with all tested compounds. The most pronounced increase in Desulfitobacterium spp. copy numbers was found with PCP. Desulfitobacterium spp. also showed the highest increase in gene copy numbers with PCB180, thus indicating a capability to dehalogenate PCBs. However, Desulfitobacterium spp. can use a variety of electron acceptors for growth (Maphosa et al. 2010), and Desulfitobacterium dehalogenans can grow fermentatively on pyruvate (van de Pas et al. 2001). Therefore, additional experiments are required to provide clear evidence for specific dechlorination capabilities.
An increase in the bacterial gene copies of Dehalobacter spp. was found with HCH, PCP and PCB180 (Table 1). Lal et al. 2010 reported that Dehalobacter spp. is capable of HCH dechlorination. Additionally, a correlation between the dechlorination of the PCB mixture Aroclor 1260 and the increase in Dehalobacter spp. gene copies was reported (Wang and He 2013).
Slightly increased gene copy numbers of Desulfuromonas spp. were observed with HCB, HCH, PCB180 and PCE. Similar to Desulfitobacterium spp., Desulfuromonas spp. are known for their versatile metabolism and as having the capability to use a wide range of electron acceptors such as sulphate, sulphite or nitrate or to grow by fermentation of pyruvate (Maphosa et al. 2010).
In summary, for HCB, PCP and PCE, a removal of up to four chlorine substituents was demonstrated for the Yangtze River enrichment culture (Table S1). In all cultures, the oxidative reductive potential was between +92 and −150 mV, which is a suitable environment for reductive dechlorination (Figure S7). For PCB180 carrying 7 chlorine substituents, a transformation to metabolites with 6 substituents was shown. In case of the HCHs, a preferential transformation of α- and γ-HCH was observed. The detected dechlorination products of PeCB, PCB138 and PCB153 have already been analysed and detected in the Yangtze River water column (Wang et al. 2009). The main PCB180 transformation product of the Yangtze River culture, i.e. PCB146, has not been studied in the field. However, this study suggests the inclusion of PCB146 in future monitoring programmes.
Conclusions
The Yangtze enrichment culture XX01, enriched from TGR sediment, was able to dechlorinate HCB, PCP, PCB180, α-, β-, γ- and δ-HCH and PCE. The formation of specific metabolites, such as 1,3- and 1,4-DCBs, 3,4- and 3,5-DCPs and PCB146, was demonstrated. The results suggest that indigenous Yangtze TGR microorganisms play an important role in the transformation of critical environmental compounds in the field and can transform a variety of different chlorinated pollutants.
References
Abramowicz DA (1995) Aerobic and anaerobic PCB biodegradation in the environment. Environ Health Perspect 103:97–99
Adrian L, Görisch H (2002) Microbial transformation of chlorinated benzenes under anaerobic conditions. Res Microbiol 153:131–137. doi:10.1016/S0923-2508(02)01298-6
Adrian L, Hansen SK, Fung JM, Görisch H, Zinder SH (2007) Growth of Dehalococcoides strains with chlorophenols as electron acceptors. Environ Sci Technol 41:2318–2323. doi:10.1021/es062076m
Aulenta F, Bianchi A, Majone M, Petrangeli Papini M, Potalivo M, Tandoi V (2005) Assessment of natural or enhanced in situ bioremediation at a chlorinated solvent-contaminated aquifer in Italy: a microcosm study. Environ Int 31:185–190. doi:10.1016/j.envint.2004.09.014
Badea SL, Vogt C, Weber S, Danet AF, Richnow H-H (2009) Stable isotope fractionation of γ-hexachlorocyclohexane (lindane) during reductive dechlorination by two strains of sulfate-reducing bacteria. Environ Sci Technol 43:3155–3161. doi:10.1021/es801284m
Bergmann A, Bi Y, Chen L, Floehr T, Henkelmann B, Holbach A, Hollert H, Hu W, Kranzioch I, Klumpp E, Küppers S, Norra S, Ottermanns R, Pfister G, Roß-Nickoll M, Schäffer A, Schleicher N, Schmidt B, Scholz-Starke B, Schramm KW, Subklew G, Tiehm A, Temoka C, Wang J, Westrich B, Wilken RD, Wolf A, Xiang X, Yuan Y (2012) The Yangtze-Hydro Project: a Chinese–German environmental program. Environ Sci Pollut Res Int 19(4):1341–1344. doi:10.1007/s11356-011-0645-7
Carreón-Diazconti C, Santamaría J, Berkompas J, Field JA, Brusseau ML (2009) Assessment of in situ reductive dechlorination using compound-specific stable isotopes, functional gene PCR, and geochemical data. Environ Sci Technol 43:4301–4307. doi:10.1021/es803308q
Cui Z, Meng F, Hong J, Li X, Ren X (2012) Effects of electron donors on the microbial reductive dechlorination of hexachlorocyclohexane and on the environment. J Biosci Bioeng 113:765–770. doi:10.1016/j.jbiosc.2012.01.006
Demarest L (2013) Soil composition at the aquifer level, groundwater quality and the presence of Dehalococcoides ethenogenes at Dover AFB. Environ Earth Sci 71:4157–4164. doi:10.1007/s12665-013-2806-0
Dohmann M, Chen C, Grambow M, Kolditz O, Krebs P, Schmidt K, Subklew G, Tiehm A, Wermter P, Dai X, Song Y, Zheng B (2016) German contributions to the major-water program in China: innovation cluster “clean water”. Environ Earth Sci. doi:10.1007/s12665-016-5504-x
Duan TH, Adrian L (2013) Enrichment of hexachlorobenzene and 1,3,5-trichlorobenzene transforming bacteria from sediments in Germany and Vietnam. Biodegradation 24:513–520. doi:10.1007/s10532-012-9607-0
Field JA, Sierra-Alvarez R (2008) Microbial transformation and degradation of polychlorinated biphenyls. Environ Pollut 155:1–12. doi:10.1016/j.envpol.2007.10.016
Floehr T, Xiao H, Scholz-Starke B, Wu L, Hou J, Yin D, Zhang X, Ji R, Yuan X, Ottermanns R, Roß-Nickoll M, Schäffer A, Hollert H (2013) Solution by dilution?—A review on the pollution status of the Yangtze River. Environ Sci Pollut Res Int 20:6934–6971. doi:10.1007/s11356-013-1666-1
Gerritse J, Renard V, Gomes PTM, Lawson PA, Collins MD, Gottschal JC (1996) Desulfitobacterium sp. strain PCE1, an anaerobic bacterium that can grow by reductive dechlorination of tetrachloroethene or ortho-chlorinated phenols. Arch Microbiol 165(2):132–140. doi:10.1007/s002030050308
Jiang X, Martens D, Schramm KW, Kettrup A, Xu SF, Wang LS (2000) Polychlorinated organic compounds (PCOCs) in waters, suspended solids and sediments of the Yangtse River. Chemosphere 41:901–905
Kaufhold T, Schmidt M, Cichocka D, Nikolausz M, Nijenhuis I (2013) Dehalogenation of diverse halogenated substrates by a highly enriched Dehalococcoides-containing culture derived from the contaminated mega-site in Bitterfeld. FEMS Microbiol Ecol 83:176–188. doi:10.1111/j.1574-6941.2012.01462.x
Köber R, Hollert H, Hornbruch G, Jekel M, Kamptner A, Klaas N, Maes H, Mangold K-M, Martac E, Matheis A, Paar H, Schäffer A, Schell H, Schiwy A, Schmidt KR, Strutz TJ, Thümmler S, Tiehm A, Braun J (2014) Nanoscale zero-valent iron flakes for groundwater treatment. Environ Earth Sci 72:3339–3352. doi:10.1007/s12665-014-3239-0
Kranzioch I, Stoll C, Holbach A, Chen H, Wang L, Zheng B, Norra S, Bi Y, Schramm KW, Tiehm A (2013) Dechlorination and organohalide-respiring bacteria dynamics in sediment samples of the Yangtze Three Gorges Reservoir. Environ Sci Pollut Res 20:7046–7056. doi:10.1007/s11356-013-1545-9
Kranzioch I, Ganz S, Tiehm A (2015) Chloroethene degradation and expression of Dehalococcoides dehalogenase genes in cultures originating from Yangtze sediments. Environ Sci Pollut Res 22(4):3138–3148. doi:10.1007/s11356-014-3574-4
Lal R, Pandey G, Sharma P, Kumari K, Malhotra S, Pandey R, Raina V, Kohler HPE, Holliger C, Jackson C, Oakeshott JG (2010) Biochemistry of microbial degradation of hexachlorocyclohexane and prospects for bioremediation. Microbiol Mol Biol Rev MMBR 74:58–80. doi:10.1128/MMBR.00029-09
Liu C, Yuan GL, Yang ZF, Yu T, Xia XQ, Hou QY, Chen L (2010) Levels of organochlorine pesticides in natural water along the Yangtze River, from headstream to estuary, and factors determining these levels. Environ Earth Sci 62:953–960. doi:10.1007/s12665-010-0580-9
Liu M, Yang Y, Yun X, Zhang M, Wang J (2015) Occurrence and assessment of organochlorine pesticides in the agricultural topsoil of Three Gorges Dam region, China. Environ Earth Sci 74:5001–5008. doi:10.1007/s12665-015-4512-6
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Müller JA, Fullerton H, Zinder SH, Spormann AM (2013) Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol 63:625–635. doi:10.1099/ijs.0.034926-0
Maphosa F, de Vos WM, Smidt H (2010) Exploiting the ecogenomics toolbox for environmental diagnostics of organohalide-respiring bacteria. Trends Biotechnol 28:308–316. doi:10.1016/j.tibtech.2010.03.005
Middeldorp PJM, van Doesburg W, Schraa G, Stams AJM (2005) Reductive dechlorination of hexachlorocyclohexane (HCH) isomers in soil under anaerobic conditions. Biodegradation 16:283–290. doi:10.1007/s10532-004-1573-8
Olaniran AO, Igbinosa EO (2011) Chlorophenols and other related derivatives of environmental concern: properties, distribution and microbial degradation processes. Chemosphere 83:1297–1306. doi:10.1016/j.chemosphere.2011.04.009
Quintero JC, Moreira MT, Feijoo G, Lema JM (2005) Anaerobic degradation of hexachlorocyclohexane isomers in liquid and soil slurry systems. Chemosphere 61(4):528–536. doi:10.1016/j.chemosphere.2005.02.010
Schmidt KR, Tiehm A (2008) Natural attenuation of chloroethenes: identification of sequential reductive/oxidative biodegradation by microcosm studies. Water Sci Technol 58:1137–1145. doi:10.2166/wst.2008.729
Schmidt KR, Augenstein T, Heidinger M, Ertl S, Tiehm A (2010) Aerobic biodegradation of cis-1,2-dichloroethene as sole carbon source: stable carbon isotope fractionation and growth characteristics. Chemosphere 78:527–532. doi:10.1016/j.chemosphere.2009.11.033
Semprini L (1995) In situ bioremediation of chlorinated solvents. Environ Health Perspect 103:101–105
Tang ZW, Yang ZF, Shen ZY, Niu JF (2007) Pentachlorophenol residues in suspended particulate matter and sediments from the Yangtze River catchment of Wuhan, China. Bull Environ Contam Toxicol 78:158–162. doi:10.1007/s00128-007-9017-x
Tao Y, Yuan Z, Fengchang W, Wei M (2013) Six-decade change in water chemistry of large freshwater Lake Taihu, China. Environ Sci Technol 47(16):9093–9101. doi:10.1021/es401517h
Taş N, Van Eekert MHA, De Vos WM, Smidt H (2010a) The little bacteria that can—diversity, genomics and ecophysiology of “Dehalococcoides” spp. in contaminated environments. Microb Biotechnol 3:389–402. doi:10.1111/j.1751-7915.2009.00147.x
Taş N, Heilig HGHJ, van Eekert MHA, Schraa G, de Vos WM, Smidt H (2010b) Concurrent hexachlorobenzene and chloroethene transformation by endogenous dechlorinating microorganisms in the Ebro River sediment. FEMS Microbiol Ecol 74:682–692. doi:10.1111/j.1574-6941.2010.00972.x
Taş N, van Eekert MHA, Wagner A, Schraa G, de Vos WM, Smidt H (2011) Role of “Dehalococcoides” spp. in the anaerobic transformation of hexachlorobenzene in European rivers. Appl Environ Microbiol 77:4437–4445. doi:10.1128/AEM.01940-10
Tiehm A, Schmidt KR (2011) Sequential anaerobic/aerobic biodegradation of chloroethenes-aspects of field application. Curr Opin Biotechnol 22:415–421. doi:10.1016/j.copbio.2011.02.003
Utkin I, Woese C, Wiegel J (1994) Isolation and characterization of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol 44(4):612–619. doi:10.1099/00207713-44-4-612
van de Pas BA, Jansen S, Dijkema C, Schraa G, de Vos WM, Stams AJM (2001) Energy yield of respiration on chloroaromatic compounds in desulfitobacterium dehalogenans. Appl Environ Microbiol 67:3958–3963. doi:10.1128/AEM.67.9.3958-3963.2001
van Doesburg W, van Eekert MHA, Middeldorp PJM, Balk M, Schraa G, Stams AJM (2005) Reductive dechlorination of beta-hexachlorocyclohexane (beta-HCH) by a Dehalobacter species in coculture with a Sedimentibacter sp. FEMS Microbiol Ecol 54:87–95. doi:10.1016/j.femsec.2005.03.003
Wang S, He J (2013) Dechlorination of commercial PCBs and other multiple halogenated compounds by a sediment-free culture containing Dehalococcoides and Dehalobacter. Environ Sci Technol 47:10526–10534. doi:10.1021/es4017624
Wang J, Bi Y, Pfister G, Henkelmann B, Zhu K, Schramm K-W (2009) Determination of PAH, PCB, and OCP in water from the Three Gorges Reservoir accumulated by semipermeable membrane devices (SPMD). Chemosphere 75:1119–1127. doi:10.1016/j.chemosphere.2009.01.016
Wolf A, Bergmann A, Wilken R-D, Gao X, Bi Y, Chen H, Schüth C (2013) Occurrence and distribution of organic trace substances in waters from the Three Gorges Reservoir, China. Environ Sci Pollut Res 20:7124–7139. doi:10.1007/s11356-013-1929-x
Wu C, Zhu H, Luo Y, Teng Y, Song J, Chen M (2015) Levels and potential health hazards of PCBs in shallow groundwater of an e-waste recycling area, China. Environ Earth Sci 74:4431–4438. doi:10.1007/s12665-015-4427-2
Xi B, Su J, Sun Y, Huo S, Zheng B, Tiehm A, Kolditz O (2015) Thematic issue: water of the Taihu Lake. Environ Earth Sci 74:3929–3933. doi:10.1007/s12665-015-4732-9
Acknowledgments
The authors gratefully acknowledge the financial support of the German Ministry of Education and Research (BMBF, Grant No. 02WT1130). This study was part of the Sino-German Yangtze-Hydro Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of a Topical Collection in Environmental Earth Sciences on “Environmental Research of the Three Gorges Reservoir”, guest edited by Binghui Zheng, Shengrui Wang, Yanwen Qin, Stefan Norra, and Xiafu Liu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kranzioch-Seipel, I., Beckert, U., Shen, C. et al. Microbial dechlorination of HCB, PCP, PCB180, HCH and PCE in a Yangtze Three Gorges Reservoir enrichment culture, China. Environ Earth Sci 75, 928 (2016). https://doi.org/10.1007/s12665-016-5653-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5653-y