Abstract
Bacterial cultures were enriched from sediments in Germany and Vietnam reductively dechlorinating hexachlorobenzene and the highly persistent 1,3,5-trichlorobenzene to monochlorobenzene. The main products of the reductive dechlorination of hexachlorobenzene were monochlorobenzene and dichlorobenzenes (1,2-; 1,3- and 1,4-dichlorobenzene) while no trichlorobenzenes accumulated. For the reductive dechlorination of 1,3,5-trichlorobenzene with the mixed culture from Vietnam sediment, 1,3- dichlorobenzene and monochlorobenzene were produced as intermediate and final end-product, respectively. The pattern of dechlorination did not change when the cultures were repeatedly exposed to oxygen over seven transfers demonstrating oxygen tolerance of the dechlorinating bacteria. However, reductive dechlorination of 1,3,5-trichlorobenzene was inhibited by vancomycin at a concentration of 5 mg L−1. Vancomycin delayed reductive dechlorination of hexachlorobenzene in mixed cultures by about 6 months. When repeatedly applied, vancomycin completely abolished the ability of the mixed culture to transform hexachlorobenzene. Sensitivity to vancomycin and insensitivity to brief exposure of oxygen indicates that the dechlorinating bacteria in the mixed cultures did not belong to the genus Dehalococcoides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorinated benzenes are widely used in chemical processes as solvents and intermediates. Chlorobenzenes have adverse effects on the environment and humans due to the accumulation in the food chain and their carcinogenicity. Among the chlorinated benzenes, hexachlorobenzene was used widely as a pesticide, fungicide and seed protectant (Peters et al. 1987). Hexachlorobenzene is ubiquitously distributed in the environment and the burden in the environment was estimated to be between 10,000 and 26,000 tones in 2005 (Barber et al. 2005), although its production and use was banned in most countries of the world (IPCS 1997). Hexachlorobenzene is a very recalcitrant environmental pollutant with estimated half-life ranging from 2.7 to 22.9 years and is listed as one of the 12 persistent organic pollutants in the UN Stockholm Convention on Persistent Organic Pollutants (UNEP 1997).
Another chlorinated organic compound under concern is 1,3,5-trichlorobenzene. This compound is important for industrial organic syntheses and has been considered to be important as the starting material for explosives and various fine chemicals. Moreover, it is the major final end-product of reductive dechlorination of hexachlorobenzene by specialized anaerobic bacteria (Adrian and Görisch 2002; Chang et al. 1997, 1998; Fathepure et al. 1988; Fennell et al. 2004; Wu et al. 2002). 1,3,5-Trichlorobenzene is persistent under aerobic and anaerobic conditions.
Several studies have reported reductive dechlorination of hexachlorobenzene under anaerobic conditions. For example, reductive dechlorination of hexachlorobenzene to less chlorinated benzenes (1,3,5-trichlorobenzene and dichlorobenzenes) was found in anaerobic sewage sludge and mixed cultures (Chang et al. 1997; Chang et al. 1998; Fathepure et al. 1988). Continuous addition of surfactants as carbon sources to mixed cultures sustained the reductively dechlorination for more than 1 year (Yeh and Pavlostathis 2001). Hexachlorobenzene was used as electron acceptor for growth via organohalide respiration by the pure strain Dehalococcoides mccartyi strain CBDB1. This strain reductively dechlorinated hexachlorobenzene to 1,3-dichlorobenzene, 1,4- dichlorobenzene and 1,3,5-trichlorobenzene at a ratio of about 2:5:3. Strain CBDB1 was able to grow in saturated solutions of hexachlorobenzene so that it was possible to grow the strain by adding hexachlorobenzene in crystalline form. However the strain was not able to use hexachlorobenzene added as a solution in hexadecane (Jayachandran et al. 2003). Chang et al. (1997) investigated the effects of environmental factors such as incubation temperature, pH, substrate concentration, electron donors and acceptors and microbial inhibitors on the dechlorination of hexachlorobenzene by a 1,2,3-trichlorobenzene-adapted mixed culture and 1,3,5-trichlorobenzene was the only final end product under all investigated conditions.
Under aerobic conditions, a pentachloronitrobenzene-degrading bacterium, Nocardioides sp. strain PD653 was able to transform and to mineralize hexachlorobenzene to chloride ions and CO2 (Takagi et al. 2009). Studies on hexachlorobenzene transformation were also carried out in artificial wetlands planted with Phragmites australis or Typha latifolia. The matrix was neutralized peat, enriched with nutrients and amended with hexachlorobenzene in concentrations of up to 300 μg per g (Zhou et al. 2012). The end products in these experiments, where the reaction most likely took place under anaerobic conditions in the wetlands, were 1,2,3-, 1,2,4- and 1,3,5-trichlorobenzene. In contrast to hexachlorobenzene, transformation of 1,3,5-trichlorobenzene has not been studied in detail, yet.
In this paper we describe microbial enrichments from sediments in Vietnam and Germany transforming hexachlorobenzene via a dechlorination sequence preventing the formation of 1,3,5-trichlorobenzene and give evidence for microbial dechlorination of 1,3,5-trichlorobenzene. By using controlled exposition of the cultures to oxygen, which has been described to be toxic for Dehalococcoides strains, and by adding vancomycin, an antibiotic inhibiting the synthesis of a peptidoglycan cell wall which is absent in Dehalococcoides (Adrian et al. 2000; Maymó-Gatell et al. 1997; He et al. 2003), we also show indications that Dehalococcoides are not involved in the described dehalogenation activities.
Materials and methods
Inocula and culture conditions
Inocula were obtained from anaerobic sediments of a town lake in Leipzig, Germany (Arthur-Bretschneider-Park 51°21′59.60″N, 12°22′53.61″E) and Nam Pho Canal, Hue, Vietnam (16°29′39.59″N, 107°35′47.14″E). Samples were collected, transported and cultured under strictly anaerobic conditions in 60-mL serum bottles. A purely synthetic medium was used for cultivation with bicarbonate as pH buffer, vitamins (Pfennig 1978), trace elements (Hölscher et al. 2010), 5 mM acetate and titanium (III) citrate as a carbon source and reducing agent, respectively (Adrian et al. 1998). The flasks were sealed with Teflon-lined butyl-rubber-septa and aluminum crimp caps and the headspace was flushed with N2/CO2 (80:20 %, v/v). Hexachlorobenzene was added directly as crystals to the medium (approximately 10 mg per 30 mL of liquid medium) before the flasks were sealed and autoclaved, whereas 1,3,5-trichlorobenzene was added from a 2 M solution in acetone to the culture to a final concentration of 30 μM. Hydrogen was added as electron donor (0.4 bar to give a total pressure of 1.4 bar). The cultures were incubated at 30 °C in the dark without shaking. All experiments were conducted in triplicate. Control experiments were done in medium containing electron acceptors and substrates but no inoculum or they contained an autoclaved inoculum. Cultures were regularly transferred to fresh medium using 5 % (v/v) inoculum. Cultures were monitored for the generation of transformation products by GC-FID. Each culture was normally sampled seven times.
The most active culture among a triplicate, i.e. the culture producing highest concentration of product, was selected for the next transfer. Hexachlorobenzene cultures were transferred after 2 months, 1,3,5-trichlorobenzene cultures were transferred when 75 % or more of the added electron acceptor was transformed. A total of seven transfers were carried out.
Exposure to oxygen and addition of vancomycin
To briefly expose bacteria to oxygen, an inoculum was taken up with a sterile 3-mL syringe and air bubbles were taken up through the liquid of the culture until the redox indicator resazurin in the sample turned pink. After 20 s of waiting, the air was pressed out and the culture was injected into a culture flasks containing reduced fresh medium.
To test the sensitivity to cell wall antibiotics, vancomycin was applied to the cultures at a concentration of 5 mg L−1. Exposure to oxygen and additions of vancomycin were conducted from the second transfer onwards and stopped if a culture was inactive.
Analytical methods
Chlorobenzene concentrations were measured by headspace gas chromatography and flame ionization detection (GC/FID). Samples were prepared by adding 1 mL of bacterial suspension and 1 mL of 1 M NaCl to a 20 mL GC headspace vial which was then sealed with a Teflon-lined butyl-rubber-septum and an aluminum crimp cap. A 5890 Hewlett Packard gas chromatograph equipped with a capillary column (HP-5, 5 % phenyl methyl siloxan, Agilent, length: 30 m; inner diameter: 320 μm; film thickness: 0.25 μm) was used for analyzing chlorobenzenes. The column temperature was initially set to 55 °C for 1 min. Then the temperature was increased by 10 °C min−1 to 90 °C, then increased by 6 °C min−1 to 130 °C. Finally, the temperature was increased to 220 °C with a rate of 30 °C min−1.
Dehalococcoides-specific PCR
To screen for the presence of D. mccartyi strains, the Dehalococcoides-targeted primers 5′-AAGGCGGTTTTCTAGGTTGTCAC-3′ and 5′-CGTTTCGCGGGGCAGTCT-3′ (Löffler et al. 2000) were used in PCR amplifications of DNA samples extracted from cultures with HCB and 1,3,5-TCB. PCR reactions (final volume of 20 μL) contained 10 μL of 2 × SensiMix™ SYBR Kit PCR Master Mix (Bioline, London, England), 5 μM of each primer, 1 μL of DNA template and deionized water up to 20 μL. PCR cycling conditions included an initial enzyme activation step at 95 °C for 10 min, followed by 30 cycles of 95 °C for 30 s, 58 °C for 30 s and 72 °C for 45 s. A final extension of 72 °C for 5 min was included. Genomic DNA from D. mccartyi strains 195 and CBDB1 were used as positive controls. The PCR products were checked for correct sizes on a 1 % (v/v) agarose gel.
Chemicals
All chemicals used were of analytical grade. Hexachlorobenzene and 1,3,5-trichlorobenzene 99.9 % analytical standards were purchased from Sigma-Aldrich (Steinheim, Germany). Vancomycin hydrochloride was supplied by AppliChem GmbH, Damstadt, Germany. N2 and H2 were obtained in 99.999 % and CO2 in 99.8 % (v/v) quality. Trace oxygen was eliminated by a reduction column (Ochs, Bovenden, Germany).
Results
Dechlorination of hexachlorobenzene and 1,3,5-trichlorobenzene
Culture flasks with synthetic medium were amended with hexachlorobenzene crystals or 1,3,5-trichlorobenzene as electron acceptor and inoculated with sediment samples from Germany or Vietnam. One of the hexachlorobenzene amended cultures formed monochlorobenzene and was selected for further work with hexachlorobenzene. Several cultures from Hue, Vietnam, showed activity against 1,3,5-trichlorobenzene and one of these cultures was selected for further work on this congener. All experiments shown here were obtained from these two initial cultures.
Reductive dechlorination of hexachlorobenzene could be detected after 1 month of incubation (Fig. 1). Products from hexachlorobenzene transformation were monochlorobenzene, 1,3-dichlorobenzene and 1,4-dichlorobenzene. Low concentrations of 1,3,5-trichlorobenzene were found after 100 days of incubation but disappeared later, after about 200 days of incubation. Negative controls containing hexachlorobenzene but no inocula and cultures inoculated with autoclaved samples did not show any products from hexachlorobenzene within the incubation time of 200 days.
Dechlorination products from hexachlorobenzene (HCB) by a mixed culture from Germany. Hexachlorobenzene was added in crystalline form and could not be quantified. Penta- and tetrachlorobenzenes were not detected as intermediates. Symbols: (open triangle) 1,3,5-trichlorobenzene; (open square) 1,4-dichlorobenzene; (filled square) 1,3-dichlorobenzene; (filled circle) monochlorobenzene; (O) negative control without cells and negative control with autoclaved cells
The cultures were transferred seven times each in triplicate with 5 % inocula in the same medium. This resulted in cultures that were completely free of sediment and other undefined components. 1,4-Dichlorobenzene and monochlorobenzene were produced as the main final-end products from hexachlorobenzene transformation in all seven transfers (Fig. 2A). 1,2- and 1,3-dichlorobenzene were also found in low concentrations as products of hexachlorobenzene dechlorination. However, most importantly, 1,3,5-trichlorobenzene, a persistent organic pollutant that was produced from hexachlorobenzene by the initial enrichment cultures, was not found in any of the subcultures. The pathway of reductive dechlorination of hexachlorobenzene remained stable over all seven transfers.
Product formation patterns from hexachlorobenzene in seven consecutive transfers of the mixed culture enriched from Germany. The plot was calculated according to a previously described procedure (Hölscher et al. 2010) and allows comparison of dechlorination pathways independent from the dechlorination rate. Hexachlorobenzene was added in crystalline form and could not be quantified. Penta- and tetrachlorobenzenes were not detected. A Standard cultures; B Cultures in which the inoculum was briefly exposed to oxygen; C Cultures with vancomycin. Symbols: (open triangle) 1,3,5-trichlorobenzene; (open square) 1,4-dichlorobenzene; (filled square) 1,3-dichlorobenzene; (filled circle) monochlorobenzene
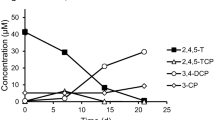
Cultures enriched from Hue in Vietnam dechlorinated 1,3,5-trichlorobenzene via 1,3-dichlorobenzene to monochlorobenzene (Figs. 3, 4). No dechlorination products from 1,3,5-trichlorobenzene were detected in negative controls without cells or with autoclaved inocula. Dechlorinating cultures were maintained over a total of seven transfers in the sediment-free synthetic medium using 5 % inoculum for each transfer without losing dechlorination activity. Also the dechlorination pattern of 1,3,5-trichlorobenzene remained stable over the seven transfers showing always monochlorobenzene as the final product.
Dechlorination of 1,3,5-trichlorobenzene by a mixed culture enriched from sediment in Hue, Vietnam. (open triangle) 1,3,5-trichlorobenzene; (filled square) 1,3-dichlorobenzene; (filled circle) monochlorobenzene; (O) 1,3,5-trichlorobenzene in negative control cultures without cells; (filled diamond) negative control cultures with autoclaved cells; arrows indicate further additions of 30 μM 1,3,5-trichlorobenzene to active cultures. The values of the additions represent calculated amounts
Effects of the exposure to oxygen of the inoculum on the transformation of hexachlorobenzene and 1,3,5-trichlorobenzene
In one series of transfers, inocula were briefly exposed to oxygen before injecting into fresh medium to test the oxygen tolerance of the dehalogenating organisms within the cultures. In such cultures, transformation of hexachlorobenzene and 1,3,5-trichlorobenzene occurred at the same rate as in cultures with inocula without oxygen exposure. Hexachlorobenzene was mainly transformed to monochlorobenzene and 1,4-dichlorobenzene with smaller amounts of other dichlorobenzenes being formed. 1,3,5-Trichlorobenzene was not produced from hexachlorobenzene in cultures with oxygen-exposed inocula (Fig. 2B) as also seen in the positive control cultures without oxygen (Fig. 2A). Similarly as in cultures with non-oxygen-exposed inocula, 1,3,5-trichlorobenzene was transformed via 1,3-dichlorobenzene to monochlorobenzene as the final end product (Fig. 5). The patterns of reductive dechlorination of the two persistent organic compounds remained unchanged over all of the subcultures, each exposed to oxygen. There was no change in the rate of reductive dechlorination of 1,3,5-trichlorobenzene and hexachlorobenzene transformation between cultures exposed to oxygen and those not exposed to oxygen (positive control). However, the rate of the sub-step of 1,3-dichlorobenzene transformation to monochlorobenzene was slower in most of the oxygen-exposed cultures compared to those without oxygen treatment.
The effect of oxygen on the reductive dechlorination of 1,3,5-trichlorobenzene by a mixed culture enriched from freshwater sediment in Vietnam. Symbols: (open triangle) 1,3,5-trichlorobenzene; (filled square) 1,3-dichlorobenzene; (filled circle) monochlorobenzene; the downwards arrows indicate time points of additional amendment with 1,3,5-trichlorobenzene. These values represent calculated, not measured concentrations
Effects of vancomycin on the transformation of hexachlorobenzene and 1,3,5-trichlorobenzene
To investigate the influence of cell wall antibiotics on transformation of hexachlorobenzene and 1,3,5-trichlorobenzene vancomycin was applied at a concentration of 5 mg L−1. The results indicated that dechlorinating activities towards both compounds were strongly inhibited by the presence of vancomycin and that the dechlorination pathway was changed. Only trace amounts of 1,3,5-trichlorobenzene and very low concentrations of 1,3-dichlorobenzene were formed from hexachlorobenzene and 1,3,5-trichlorobenzene, respectively within 6 months of incubation. However, stronger dechlorination started after 6 months of incubation. Then, monochlorobenzene and all of the isomers of dichlorobenzene and 1,3,5-trichlorobenzene were produced from hexachlorobenzene (Fig. 2C). When further subcultures were set up from vancomycin-containing cultures, again containing vancomycin, only 1,3,5-trichlorobenzene was found with very low concentration. This was true also for 1,3,5-trichlorobenzene cultures containing vancomycin, which first were slow in dechlorination to 1,3-dichlorobenzene but in which transformation rates increased significantly after 6 months of incubation (Fig. 6).
The effect of vancomycin on the reductive dechlorination of 1,3,5-trichlorobenzene by the mixed culture enriched from Vietnam. Symbols: (open triangle) 1,3,5-trichlorobenzene; (filled square) 1,3-dichlorobenzene; (filled circle) monochlorobenzene. 1,3,5-Trichlorobenzene loss within the first 150 days represents the rate at which 1,3,5-trichlorobenzene was escaping from the cultures through the injured Teflon liner into the septa and was also seen in negative controls
PCR analysis
The application of PCR with Dehalococcoides-targeted PCR primers did not result in PCR products of the expected size.
Discussion
Detection of monochlorobenzene and all dichlorobenzene isomers but not 1,3,5-trichlorobenzene during the reductive dehalogenation of hexachlorobenzene in most of our cultures demonstrates a new variant of an organohalide respiration pathway with hexachlorobenzene. Most previous reports showed hexachlorobenzene transformation to all three isomers of dichlorobenzene and trichlorobenzene as final products. Especially 1,3,5-trichlorobenzene was often found as the dominant end product by pure and mixed cultures (Adrian and Görisch 2002; Chang et al. 1997, 1998; Fathepure et al. 1988; Fennell et al. 2004; Wu et al. 2002). In these studies, preferentially those chlorine substituents were removed that were flanked by two other chorine substituents (‘doubly flanked’). Previously we described trichlorobenzene dechlorinating cultures which catalyzed a different pattern by preferentially dechlorinating singly-flanked chlorine substituents, e.g. forming 1,2-dichlorobenzene from 1,2,3-trichlorobenzene (Hölscher et al. 2010). Ramanand et al. (1993) described soil slurry cultures that were able to reductively dechlorinate hexachlorobenzene without the formation of 1,3,5-trichlorobenzene. Also in these soil slurries, singly-flanked chlorine substituents were preferred over doubly-flanked chlorine substituents. In our study now we show that organisms catalyzing the dechlorination of hexachlorobenzene to monochlorobenzene can be established in purely synthetic medium and can be transferred many times in such medium and at the same time maintain the 1,3,5-trichlorobenzene-avoiding dechlorination sequence. Monochlorobenzene formation from all three dichlorobenzene isomers was also found by Fung et al. 2009, who found consecutive formation of benzene from monochlorobenzene (Fung et al. 2009), an activity that was not detected in significant amounts in our study. Formation of 1,3,5-trichlorobenzene in parent cultures inoculated with the original sediment and the lack of formation in all subcultures indicates that two types of chlorobenzene-dechlorinating bacteria were present in the cultures: those preferentially removing doubly-flanked chlorines and those preferentially removing singly-flanked chlorines. The more prominent formation of monochlorobenzene in the subcultures supports this hypothesis. While in the original sediment both types of organisms were active, it seems that in the subcultures the organism that removes singly-flanked substituents outcompeted the organism removing doubly-flanked substituents. By applying oxygen to inocula or by adding vancomycin to the culture this competition was influenced in favor of one or the other organism, respectively.
Apart from describing cultures avoiding the formation of 1,3,5-trichlorobenzene during hexachlorobenzene dechlorination we also describe cultures dechlorinating 1,3,5-trichlorobenzene itself. To our knowledge, there are no detailed analyses published on anaerobic microbial 1,3,5-trichlorobenzene dechlorination. We previously reported the preferential reductive dechlorination of singly-flanked substituents from chlorobenzenes by mixed, sediment-free cultures (Hölscher et al. 2010), however, these cultures were not active against 1,3,5-trichlorobenzene. Reductive dechlorination of 1,3,5-trichlorobenzene requires the removal of isolated substituents (not flanked by other chlorine substituents) which are also present in other anaerobically persistent chlorobenzene congeners such as 1,4-dichlorobenzene or monochlorobenzene. The cultures therefore offer the option to establish a mixed culture in which different bacteria are responsible for different dechlorination steps. The fact that no dechlorination of chlorinated benzenes occurred in negative controls without inoculum or in medium with autoclaved inoculum demonstrated that viable microorganisms were the determining factor also for this type of dechlorination.
Vancomycin had a strong inhibitory effect on the chlorobenzene-dechlorinating activity in our cultures. It was shown previously that vancomycin inhibits the synthesis of a peptidoglycan cell wall in bacteria (Williams and Bardsley 1999). Because Dehalococcoides strains do not contain a peptidoglycan cell wall they are generally not sensitive to vancomycin (Adrian et al. 2000; Maymó-Gatell et al. 1997; He et al. 2003). Inhibition by vancomycin therefore indicates that the dechlorinating reactions observed in our cultures were not catalyzed by Dehalococcoides species. Because vancomycin is especially effective against gram positive bacteria, the results might hint to the involvement of Firmicutes in the preferential dechlorination of singly-flanked substituents. However, after 6 months of incubation, monochlorobenzene, dichlorobenzenes and 1,3,5-trichlorobenzene were found in cultures incubated with hexachlorobenzene, and 1,3-dichlorobenzene was found in cultures incubated with 1,3,5-trichlorobenzene. This can be explained by an inactivation, destruction or depletion of the antibiotic after this incubation time allowing bacteria to grow and to dechlorinate. Moreover, the production of 1,3,5-trichlorobenzene in vancomycin-containing-cultures shows that chlorobenzene-dechlorinating-bacteria that preferentially removed doubly-flanked chlorines, such as Dehalococcoides species, were less sensitive to this antibiotic than bacteria which preferential singly-flanked substituents. Therefore, vancomycin can differentially repress dechlorinating populations and favors doubly-flanked chlorine removing bacterial leading to the production of 1,3,5-trichlorobenzene from hexachlorobenzene. The result that we could change the dechlorination pattern by applying vancomycin also supported our hypothesis that a competition between different types of dechlorinating bacteria exists in the cultures.
From the positive dechlorination results from oxygen-exposed inocula, it was apparent that the bacteria preferentially dechlorinating singly-flanked residues in our culture were insensitive to the applied oxygen treatment, indicating that they did not belong to Dehalococcoides species, which are described to be extremely sensitive to the exposure of oxygen (Adrian et al. 2000; Maymó-Gatell et al. 1997; Löffler et al. 2012). However, the decrease in the rate of dechlorination in cultures with oxygen-exposed inoculum indicated a change in the populations, possibly affecting bacteria that were important for syntrophic interactions with the dechlorinating bacteria. The two complementary results from oxygen treatment and from vancomycin additions that Dehalococcoides were not involved in the transformation reactions were further confirmed with the PCR approach which confirmed that no Dehalococcoides were present in the cultures or that they were present only in very low cell numbers.
In conclusion, using chlorobenzene-dehalogenating bacteria which preferentially remove singly-flanked and/or isolated halogen substituents as shown in our study has a great potential for bioremediation of hexachlorobenzene-contaminated sites, because the accumulation of chlorinated and persistent intermediates such as 1,3,5-trichlorobenzene can be avoided. Moreover, the removal of isolated chlorine substituents from 1,3,5-trichlorobenzene or 1,3-dichlorobenzene also has a significant practical value for in situ application at 1,3,5-trichlorobenzene-contaminated sites. Isolation of pure cultures from the mixed cultures catalyzing the dechlorination of singly-flanked or isolated substituents from chlorobenzenes is necessary in the future to enable microbiological and biochemical characterization. This will allow rational improvement of cultivation conditions and the process optimization of bioaugmentation with the responsible bacteria at contaminated sites.
References
Adrian L, Görisch H (2002) Microbial transformation of chlorinated benzenes under anaerobic conditions. Res Microbiol 153:131–137. doi:10.1016/S0923-2508(02)01298-6
Adrian L, Manz W, Szewzyk U, Görisch H (1998) Physiological characterization of a bacterial consortium reductively dechlorinating 1,2,3- and 1,2,4-trichlorobenzene. Appl Environ Microbiol 64:496–503
Adrian L, Szewzyk U, Wecke J, Görisch H (2000) Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580–583. doi:10.1038/35046063
Barber JL, Sweetman AJ, van Wijk D, Jones KC (2005) Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes. Sci Total Environ 349:1–44. doi:10.1016/j.scitotenv.2005.03.014
Chang BV, Chen YM, Yuan SY, Wang YS (1997) Reductive dechlorination of hexachlorobenzene by an anaerobic mixed culture. Water Air Soil Pollut 100:25–32
Chang BV, Su CJ, Yuan SY (1998) Microbial hexachlorobenzene dechlorination under three reducing conditions. Chemosphere 36:2721–2730. doi:10.1016/S0045-6535(97)10231-4
Fathepure BZ, Tiedje JM, Boyd SA (1988) Reductive dechlorination of hexachlorobenzene to tri- and dichlorobenzenes in anaerobic sewage sludge. Appl Environ Microbiol 54:327–330
Fennell DE, Nijenhuis I, Wilson SF, Zinder SH, Häggblom MM (2004) Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ Sci Technol 38:2075–2081. doi:10.1021/es034989b
Fung JM, Weisenstein BP, Mack EE, Vidumsky JE, Ei TA, Zinder SH (2009) Reductive dehalogenation of dichlorobenzenes and monochlorobenzene to benzene in microcosms. Environ Sci Technol 43:2302–2307. doi:10.1021/es802131d
He J, Ritalahti KM, Yang KL, Koenigsberg SS, Löffler FE (2003) Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62–65. doi:10.1038/nature01717
Hölscher T, Lisec J, Baani M, Duan TH, Adrian L (2010) Bacterial cultures preferentially removing singly flanked chlorine substituents from chlorobenzenes. Environ Sci Technol 44:8936–8942. doi:10.1021/es101971m
IPCS (1997) (International Programme for Chemical Safety). Environmental Health Criteria 195. Hexachlorobenzene. ISBN 924157950, ISSN 0250-863X, World Health Organisation, Geneva, Switzerland
Jayachandran G, Görisch H, Adrian L (2003) Dehalorespiration with hexachlorobenzene and pentachlorobenzene by Dehalococcoides sp strain CBDB1. Arch Microbiol 180:411–416. doi:10.1007/s00203-003-0607-7
Löffler FE, Sun Q, Li S, Tiedje JM (2000) 16S rRNA gene-based detection of tetrachloroethene-dechlorinating Desulfuromonas and Dehalococcoides species. Appl Environ Microbiol 66:1369–1374
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, Müller JA, Fullerton H, Zinder SH, Spormann AM (2012) Dehalococcoides mccartyi gen. nov., sp. nov., obligate organohalide-respiring anaerobic bacteria, relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidetes classis nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol Online. doi:10.1099/ijs.0.034926-0
Maymó-Gatell X, Chien YT, Gossett JM, Zinder SH (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568–1571. doi:10.1126/science.276.5318.1568
Peters H, Cripps D, Göcmen A, Bryan G, Ertürk E, Morris C (1987) Turkish epidemic hexachlorobenzene porphyria. A 30-year study. Ann NY Acad Sci 514:183–190. doi:10.1111/j.1749-6632.1987.tb48773.x
Pfennig N (1978) Rhodocyclus purpureus gen. nov. and sp. nov., a ring-shaped, vitamin B12 -requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Ramanand K, Balba MT, Duffy J (1993) Reductive dehalogenation of chlorinated benzenes and toluenes under methanogenic conditions. Appl Environ Microbiol 59:3266–3272
Takagi K, Iwasaki A, Kamei I, Satsuma K, Yoshioka Y, Harada N (2009) Aerobic mineralization of hexachlorobenzene by newly isolated pentachloronitrobenzene-degrading Nocardioides sp. strain PD653. Appl Environ Microbiol 75:4452–4458. doi:10.1128/aem.02329-08
UNEP (1997) Stockholm convention on persistent organic pollutants. United Nations Environment Program, Geneva, Switzerland. http://www.pops.int
Williams DH, Bardsley B (1999) The vancomycin group of antibiotics and the fight against resistant bacteria. Angew Chem Int Ed Engl 38:1172–1193. doi:10.1002/(sici)1521-3773(19990503)38:9<1172:aid-anie1172>3.0.co;2-c
Wu QZ, Milliken CE, Meier GP, Watts JEM, Sowers KR, May HD (2002) Dechlorination of chlorobenzenes by a culture containing bacterium DF-1, a PCB dechlorinating microorganism. Environ Sci Technol 36:3290–3294. doi:10.1021/es0158612
Yeh DH, Pavlostathis SG (2001) Development of hexachlorobenzene-dechlorinating mixed cultures using polysorbate surfactants as a carbon source. Water Sci Technol 43:43–50
Zhou Y, Trestip S, Li X, Truu M, Truu J, Mander Ü (2012) Dechlorination of hexachlorobenzene in treatment microcosm wetlands. Ecol Eng 42:249–255. doi:10.1016/j.ecoleng.2012.02.017
Acknowledgments
The authors thank Benjamin Scheer for excellent technical assistance. This study was supported by the Vietnam International Education Development (VIED) and the Helmholtz Centre for Environmental Research to T.H.D and the Deutsche Forschungsgemeinschaft (DFG-FOR1530) to L.A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duan, T.H., Adrian, L. Enrichment of hexachlorobenzene and 1,3,5-trichlorobenzene transforming bacteria from sediments in Germany and Vietnam. Biodegradation 24, 513–520 (2013). https://doi.org/10.1007/s10532-012-9607-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-012-9607-0