Abstract
Several groups of bacteria such as Dehalococcoides spp., Dehalobacter spp., Desulfomonile spp., Desulfuromonas spp., or Desulfitobacterium spp. are able to dehalogenate chlorinated pollutants such as chloroethenes, chlorobenzenes, or polychlorinated biphenyls under anaerobic conditions. In order to assess the dechlorination potential in Yangtze sediment samples, the presence and activity of the reductively dechlorinating bacteria were studied in anaerobic batch tests. Eighteen sediment samples were taken in the Three Gorges Reservoir catchment area of the Yangtze River, including the tributaries Jialing River, Daning River, and Xiangxi River. Polymerase chain reaction analysis indicated the presence of dechlorinating bacteria in most samples, with varying dechlorinating microbial community compositions at different sampling locations. Subsequently, anaerobic reductive dechlorination of tetrachloroethene (PCE) was tested after the addition of electron donors. Most cultures dechlorinated PCE completely to ethene via cis-dichloroethene (cis-DCE) or trans-dichloroethene. Dehalogenating activity corresponded to increasing numbers of Dehalobacter spp., Desulfomonile spp., Desulfitobacterium spp., or Dehalococcoides spp. If no bacteria of the genus Dehalococcoides spp. were present in the sediment, reductive dechlorination stopped at cis-DCE. Our results demonstrate the presence of viable dechlorinating bacteria in Yangtze samples, indicating their relevance for pollutant turnover.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Yangtze River is the third largest river in the world, with a mean annual water discharge of 29,400 m3/s and a sediment load of 500 million tons/year (Zhang 1995). In the last decades, pollutant discharge into Chinese rivers has increased because of growing industrial development and the widespread application of fertilizers, pesticides, and herbicides in agriculture (Müller et al. 2008). Additionally, changes in water quantity, water quality, and the aquatic ecosystems have to be expected from large dam constructions, e.g., the Three Gorges Dam (TGD) at the Yangtze. A joint Sino-German research project was initiated to obtain more insight into the environmental processes under these highly dynamic conditions (Bergmann et al. 2012).
In this study, the dehalogenation of chlorinated pollutants and monitoring the growth of dechlorinating bacteria are reported. Chlorinated contaminants such as polychlorinated biphenyls (PCBs) and chlorinated benzenes (CBs) have been detected in the Yangtze River water and sediments in low concentrations (Jiang et al. 2000; Wang et al. 2009). PCBs are anthropogenic environmental pollutants, which originate from the recycling procedures or dumping of electronic wastes (Shen et al. 2009; Liu et al. 2008). CBs, especially hexachlorobenzene, had a lot of applications in industry and agriculture (Barber et al. 2005). In China, it was used as a cheap broad spectrum insecticide (Jiang et al. 2000). Chlorinated ethenes belong to the most commonly found contaminants in the groundwater worldwide, because they had been widely used as cleaning and degreasing agents (Schmidt and Tiehm 2008; Tiehm and Schmidt 2011). There are several groups of bacteria which are able to dehalogenate chlorinated aliphatic and aromatic hydrocarbons under anaerobic conditions. Remarkably, similar groups of bacteria have been reported to be capable of dechlorination of CBs (Field and Sierra-Alvarez 2008; Taş et al. 2011), PCBs (Bedard 2008), and chlorinated ethenes (Löffler et al. 2000; Schmidt et al. 2006). In this study, chloroethenes were used as model compounds. Microorganisms which are known to be able to reductively dechlorinate pollutants are Desulfomonile spp., Desulfitobacterium spp., Dehalobacter spp., Desulfuromonas spp., and Dehalococcoides spp., but only bacteria belonging to Dehalococcoides spp. are capable of complete dehalogenation of tetrachloroethene (PCE) to ethene (Holliger et al. 1993; Smidt and de Vos 2004; Schmidt et al. 2006; Aktaş et al. 2012; Löffler et al. 2012).
Previously, in most laboratory studies on anaerobic dechlorination, only one or two groups of bacteria were investigated and monitored by polymerase chain reaction (PCR) analysis, e.g., Desulfitobacterium sp. and Dehalococcoides spp. (Lohner and Tiehm 2009), Desulfitobacterium dehalogenans and Desulfomonile tiedjei (El Fantroussi et al. 1997), Desulfuromonas spp. and Dehalococcoides mccartyi (Löffler et al. 2000; Löffler et al. 2012), Dehalobacter spp. and Dehalococcoides spp. (Smits et al. 2004; Grostern and Edwards 2006) or Dehalococcoides bacteria (Schmidt et al. 2006; Aktaş et al. 2012). It has been shown recently that in the field, often several dechlorinating bacteria are present simultaneously (Rouzeau-Szynalski et al. 2011). In the environment, the reductively dechlorinating bacteria have been detected in different compartments, mainly in contaminated soil (Löffler et al. 2000; Hendrickson et al. 2002) and polluted groundwater (Schmidt et al. 2006; Schmidt and Tiehm 2008; Vancheeswaran et al. 1999). For river sediments, their occurrence in the Dutch part of the Rhine River (Holliger et al. 1993), the Red Cedar, Père Marquette (Löffler et al. 2000), Tahquamenon and Pine Rivers in the USA, and the Perfume River in Vietnam (Griffin et al. 2004) was reported. However, no reports were available addressing the anaerobic dechlorination potential in the Yangtze River. The objectives of this study are (1) to assess the dechlorination potential in Yangtze sediment samples and (2) to monitor the changes of the dechlorinating microbial community during PCE dechlorination.
Materials and methods
Sampling and sample storage
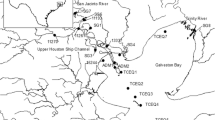
Eighteen sediment samples were taken in the Three Gorges Reservoir (TGR) catchment area (Fig. 1). Seven samples (MP1, MP2, MP3, MP4, MP5, MP6, and MP7) are from a sediment core with a total length of 0.7 m taken 76.5 m below the water level in the Yangtze River near the TGD. The sediment core was collected with a stainless gravity sediment core sampler (100 cm in length and 25 cm in diameter). Three samples (YCQ1, YCQ4, and YEND) originate from the Yangtze River close to and within Chongqing city, and another sample was also taken within Chongqing from the tributary Jialing River (JL1). These four samples are surface sediment samples from the littoral zone and were collected manually. Six samples (XX01, XX02, XX04, XX07, PYK, and WJW) were taken from the tributary Xiangxi River in a depth between 3 and 71 m below the water level, and another sample (DH2) originated from the tributary Daning River (depth 35 m). These samples have been collected with a sediment grabber. All samples were stored in plastic bags, which were put into gastight tins. After sampling, the sediments were stored at 4 °C until usage.
Biodegradation studies
Experiments were performed in 2-L laboratory glass bottles capped with teflon-coated septa, held in place with screw caps. The mineral medium contained the following mineral salts (analytical grade, Merck, Darmstadt, Germany) per liter of demineralized water: 3.17 g of KH2PO4, 14.33 g of Na2HPO4·12H2O, 0.45 g of (NH4)2HPO4, 0.12 g of MgHPO4·3H2O, 5 mL of trace element solution 1 (200 mg of FeSO4·7H2O, 20 mg of MnSO4·5H2O, 4 mg of CoCl2, 20 mg of ZnSO4·7H2O, 20 mg of CuSO4·5H2O, 530 mg of CaCl2, 3 mg of H3BO3, 4 mg of NaMoO4·2H2O, and 1 mL of concentrated H3PO4 per liter), and 100 μL of trace element solution 2 (50 mg of Na2WO4·2H2O, 50 mg of Na2SeO3·5H2O, and 250 mg of NiCl2 per liter). The medium was adjusted to pH 7.2 ± 0.2 and autoclaved at 121 °C for 20 min. Oxygen was removed by repeatedly applying vacuum and flushing with nitrogen. Additionally, all cultures received filter-sterilized 1 mL vitamin solution (2 mg of biotin, 2 mg of folic acid, 10 mg of pyridoxamine, 5 mg of riboflavin, 5 mg of thiamine, 0.1 mg of cyanocobalamin, 5 mg of nicotinamide, 5 mg of p-amino benzoic acid, 5 mg of lipoic acid, 5 mg of pantothenic acid per 100 mL). Sodium acetate (40 mg), 40 mg of sodium pyruvate, 0.5 g of yeast extract, and 10 mL H2 per liter were added in order to provide the electron donors required for reductive dechlorination. Depletion of acetate and pyruvate was followed by ion chromatographic analysis. All handling, except H2 addition, was performed inside the anaerobic gas chamber flushed with nitrogen. All bottles were amended with 50 μM (8.3 mg/L) PCE (99 %, Fluka, Steinheim, Germany). Each batch test was inoculated with 20 g of wet sediment. According to the dehalogenation of chloroethenes and consumption of electron donors, the cultures have been repeatedly spiked with 50 μM (8.3 mg/L) PCE and amended with acetate, pyruvate, and hydrogen. Bottles were kept at room temperature (22–24 °C) in the dark. In order to avoid volatilization of chloroethenes during sampling, the teflon-coated septa were pierced with stainless steel needles. Aqueous phase samples were taken using glass syringes, and septa were quickly changed subsequently. Chlorinated ethenes and ethene were analyzed by gas chromatography. Chloride formation was monitored via ion chromatography. Samples for gas chromatographic analyses were acidified to pH 2 with phosphoric acid in order to stop biological reactions and stored at 4 °C until analysis.
Analytical methods
Chloroethene and ethene concentrations were determined using a gas chromatograph (Series II 5890, Hewlett Packard, Waldbronn, Germany) equipped with headspace sampler, flame ionization detector, and electron capture detector. Separation was accomplished in a capillary column (Hewlet Packard PONA, 50 m, 0.2 mm inside diameter, crosslinked methyl siloxane, 0.5-μm film thickness). The injector and detector temperatures were 180 and 220 °C, respectively, and the following temperature program was used: held at 35 °C for 15 min, heated gradually to 60 °C (1.5 °C/min), heated gradually to 130 °C (15 °C/min), held for 7 min, heated gradually to 200 °C (30 °C/min), and held at 200 °C for 5 min. Concentrations were determined using external standards (R 2 = 0.998). Reproducibility on standard analyses was ± 6 %. Each reported value was the average of at least duplicate samples and represents the aqueous phase concentration.
Chloride concentrations were determined via ion chromatograph (Metrohm 761 compact IC, Metrohm, Filderstadt, Germany) equipped with a Metrohm A-Supp-5 column conductivity detector. Concentrations were determined using external standards (R 2 = 0.996). Reproducibility on standard analyses was ± 1 %.
All samples were taken from the aqueous phase. At each time point, oxidation–reduction potential (ORP) and pH were determined with a multimeter WTW Multiline P4 (Weilheim, Germany), and oxygen was measured using a WTW oximeter Oxi 330.
Polymerase chain reaction
For the analysis of total DNA from the sediment, the gastight tins were opened in the anaerobic gas chamber. Per sample, 1 g of soil was removed and extracted using the Ultra Clean Soil DNA Kit (MoBio Laboratories, Carlsbad, USA) according to the manufacturer's protocol. For PCR analysis of the culture liquid, 50 mL of the culture was transferred to a conical centrifuge tube (VWR, Darmstadt, Germany) and afterwards filtered through a 0.2 μm membrane filter (47 mm diameter) (PALL, Michigan, USA). The membrane was stored at −20 °C until DNA extraction and analysis. The DNA of the frozen filters was extracted using the Fast DNA® Spin Kit for soil (MP Biomedicals, OH, USA).
The pre-screening of sediment samples for the 16S rRNA gene sequences specific for dechlorinating microorganisms Desulfomonile spp., Desulfitobacterium spp., Dehalobacter spp., Desulfuromonas spp., and Dehalococcoides spp. was done with nested PCR containing 2 μL of the template, 13.4 μL of DNase free water, 2 μL of 10× reaction buffer, 0.2 μL of Taq polymerase, 200 μM of each nucleotide, and 500 nM of each primer, using a Biometra thermocycler (Biometra, Goettingen, Germany). First, a PCR with bacterial primers was performed using different dilutions of DNA extracts as templates. Thus, false negative results caused by PCR inhibitors could be precluded. Second, a 1 to 10 dilution of the bacterial PCR product was used as template for a second PCR with primers specific for the five dechlorinating groups. The used primers and temperature programs are listed in Table 1. Amplicons were tested with agarose gel electrophoresis, and gels were documented with a GeneFlash camera (Syngene, Cambridge, UK).
For enumeration of the 16S RNA gene copies in the degradation experiments, quantitative PCR (qPCR) was performed in an Eppendorf Realplex2 qPCR machine (Eppendorf, Hamburg, Germany) with primers and temperature profiles listed in Table 1. The qPCR was done with a Master Mix containing 8.5 μL of RNase and DNase free water (Invitrogen, Carlsbad, USA), 12.5 μL of 2× Sensi Mix including Hot Start Taq Polymerase (Bioline, Luckenwalde, Germany), and 500 nM of each primer. All samples and standards were analyzed in duplicates, and gene concentrations were reported as gene copies per liter of culture liquid. Calibration was performed with serial dilutions of a known quantity of linearized plasmid containing 16S gene fragments. The quantification limits (QL) for all qPCR assays were 1E + 5 16S RNA gene copies/L.
Results
The pre-screening of the sediment samples revealed that dechlorinating bacteria were present in most of the samples. In 16 of the 18 sediment samples, positive PCR signals for at least one of the 5 different dechlorinating groups were detected (see supplementary data Table T1). For subsequent dehalogenation studies, nine sediment samples from different areas and with different dechlorinating microbial communities were selected.
All nine batch cultures were able to reductively dechlorinate PCE, thus demonstrating the viability of the bacteria. During 28 weeks, in all batch cultures, the pH stayed at a constant level between 7.0 and 7.4. The oxygen concentration in the medium never exceeded the limit of detection (0.2 mg/L), and the ORP values decreased at the beginning and afterwards remained between −24 and −124 mV (supplementary Figs. S1 and S2). Thus, the O2, pH, and ORP values confirmed suitable conditions for reductive dechlorination. All batch cultures were spiked at least two times with PCE and showed repeatedly reductive dechlorination. qPCR analysis showed different patterns of dechlorinating microorganisms during the dehalogenation tests (Table 2). The growth of Dehalococcoides spp. was observed in all cultures showing complete dechlorination of PCE to ethene. Three of the batch experiments are shown exemplarily in Figs. 2, 3 and 4. The remaining six batch experiments are shown in the supplementary data Figs. S3–S8.
The culture MP1 transformed PCE after the first addition of PCE mainly to trichloroethene (TCE). Initially, only a small amount of about 5 μM cis-DCE was formed (Fig. 2a). No further dechlorination of TCE to cis-DCE was observed within the first 15 weeks. After addition of PCE and electron donors, the dehalogenation of TCE started also, and TCE was completely dechlorinated to cis-DCE after 22 weeks. Repeated spiking with PCE resulted in an accumulation of cis-DCE. No further dechlorination products such as vinylchloride (VC) or ethene were measured. In this culture, a nearly stoichiometric chloride formation was observed.
The dechlorinating microbial community analysis is shown in Fig. 2b. The main increase in bacterial genes was detected for Desulfitobacterium spp. within the first 16 weeks. During this time, the gene copies per liter of Desulfitobacterium spp. increased about four orders of magnitude. In the case of Dehalobacter spp., the main growth of one order of magnitude occurred in the first 4 weeks. After the second spiking, again a growth of about one order of magnitude to 10E + 7 16S RNA gene copies/L was observed. Desulfomonile spp. showed a weak growth, and Dehalococcoides spp. was below the quantification limit during the whole investigation period.
One culture (YCQ1) which completely dechlorinated PCE via TCE, cis-DCE, and VC to ethene is shown in Fig. 3a. The culture was spiked six times with PCE and showed a complete dehalogenation to ethene each time. The dechlorination of PCE started quickly, and after 3 weeks, PCE was completely transformed to cis-DCE. At the end of the experiment, the time for complete dechlorination of 48 μM PCE via cis-DCE and VC to ethene was less than 2 weeks.
The growth of the dechlorinating microorganisms is shown in Fig. 3b. Within 4 weeks, the number of Dehalococcoides spp. increased by at least three orders of magnitude, and in the following 24 weeks, it increased by another three orders of magnitude. Finally, 10E + 11 gene copies/L was reached. In the case of Desulfitobacterium spp., first, an increase of four orders of magnitude was observed, and then a slow decline to about 10E + 8 16S RNA gene copies/L occurred. Dehalobacter spp. and Desulfomonile spp. showed a small incline of about 1 and two orders of magnitude.
In Fig. 4a, the results for the culture XX07 are shown. In contrast to the other cultures, the dehalogenation of PCE to trans- and cis-DCE in a ratio of 3:1 was observed. After repeated addition of PCE and electron donors, a complete dechlorination of PCE and the lower chlorinated metabolites to ethene and stoichiometric chloride formation were observed.
The growth of Dehalococcoides spp. was observed concomitantly with the reduction of TCE to cis- and trans-DCE (Fig. 4b). The gene copy number per liter increased at least four orders of magnitude within 8 weeks. After 28 weeks, a value of approximately 1E + 10 16S RNA gene copies/L was reached. The gene copy numbers of Desulfitobacterium spp. were already high initially and increased about one order of magnitude. Desulfomonile spp. and Dehalobacter spp. showed growth in the first 4 weeks about one order of magnitude, but afterwards remained constant until the end of the experiment.
The results of the batch studies are summarized in Table 2 (for more detailed information see supplementary data Figs. S3–S8). Obviously, different bacteria known for their organohalide respiring potential developed during the incubation of Yangtze sediment samples. In particular, increase of gene copy numbers over four orders of magnitude was observed for Desulfitobacterium spp. (Figs. 2 and 3, Fig. S5), Dehalobacter spp. (Figs. S4 and S6), and Dehalococcoides spp. (Figs. 3 and 4, Figs. S3–S8). If bacteria of the Dehalococcoides group were not present, the reductive dechlorination stopped at cis-DCE. In the presence of Dehalococcoides spp., in all samples except one, a complete reductive dechlorination to ethene occurred. Only in one sediment sample (MP3), VC was observed as the end product of reductive dechlorination (Fig. S3).
Discussion
In all batch tests incubated with sediments from the TGR catchment area, anaerobic biological dechlorination was demonstrated (Table 2). Although Dehalococcoides spp. were below the limit of detection in the initial nested PCR screening (Table T1), these bacteria were detected in most of the batch dechlorination tests. However, differences in the biochemical pathways and end-products of reductive dechlorination were observed, most probably due to different halorespiring bacteria community compositions. In nearly all sediment samples, Desulfuromonas spp. was detected with the nested PCR approach (Table T1). However, growth of Desulfuromonas spp. was not studied by qPCR in the batch tests because of the lack of suitable primers.
The culture MP1 repeatedly dechlorinated PCE via TCE to cis-DCE (Fig. 2a). In this culture, Dehalococcoides spp. was below the quantification limit during the whole incubation time. In contrast, Dehalococcoides spp. were detected with increasing numbers over investigation time in the YCQ1 (Fig. 3b) and the XX07 cultures (Fig. 4b). These cultures showed complete dechlorination, thus confirming previous reports that bacteria of the Dehalococcoides group are the only known microorganism to completely dehalogenate PCE to ethene (Holliger et al. 1999; Löffler et al. 2000; Maymó-Gatell et al. 2001; Hendrickson et al. 2002; Smidt and de Vos 2004; Schmidt et al. 2006). During 28 weeks and anaerobic transformation of approximately 60 mg/L PCE, the Dehalococcoides spp. gene copies/L increased by five orders of magnitude. Also, Aktaş et al. (2012) reported a several orders of magnitude increase of gene numbers during the reductive dechlorination of approximately 100 mg/L PCE to ethene.
Although in both cultures, YCQ1 and XX07, complete dechlorination to ethene was observed, the temporarily detected metabolites differed. As in most previous studies (Löffler et al. 2000; Schmidt and Tiehm 2008; Aktaş et al. 2012), dechlorination via cis-DCE without any detection of trans-DCE was observed in culture YCQ1 (Fig. 3a). The culture XX07 dechlorinated PCE via TCE to trans-DCE and cis-DCE in a ratio of 3:1. Similar ratios were already reported in some other studies (Griffin et al. 2004; Kittelmann and Friedrich 2008; Cheng and He 2009; Cheng et al. 2009; Marco-Urrea et al. 2011). Chow et al. (2010) and Marco-Urrea et al. (2011) found that the Dehalococcoides strains MB and CBDB1 dechlorinate PCE and TCE to mainly trans-DCE. In our study, mainly the growth of Dehalococcoides spp. and smaller increases of Desulfitobacterium spp., Desulfomonile spp., and Dehalobacter spp. were observed. The D. mccartyi group encompasses the six bacterial isolates of strains 195, BAV1, CBDB1, FL2, GT, and VS (Löffler et al. 2012). Most probably, different strains of the genus Dehalococcoides spp. with different dechlorinating abilities were present in the cultures YCQ1 and XX07. In order to obtain more insight into the dechlorinating strains of Dehalococcoides spp. within the Yangtze samples, further investigation focusing on chloroethene reductive dehalogenase genes such as pceA, tceA, vcrA, and bvcA are promising (Behrens et al. 2008; Maphosa et al. 2010).
In culture MP1, Desulfitobacterium spp. was the predominant growing bacterium in the absence of Dehalococcoides spp. The transformation of approximately 50 mg/L PCE to cis-DCE resulted in an increase of Desulfitobacterium spp. over four orders of magnitude, and an increase of Dehalobacter spp. over two orders of magnitude (Fig. 2b). Obviously, the addition of PCE and the electron donors acetate/pyruvate and H2 could stimulate the growth of the different dechlorinating bacteria. However, a small amount of sulphate and Fe(III) was available in the mineral medium, and it is also possible that fermentation of the yeast extract resulted in the formation of fumarate. As reviewed recently by Maphosa et al. (2010), these compounds might also have been used as electron acceptors by Desulfitobacterium spp. or Desulfomonile spp. in the cultures.
Mass balance calculations revealed that the formation of chloride corresponded to the dechlorination of PCE to DCE or ethene (Figs. 2, 3 and 4). Also, the amount of cis-DCE detected in the aqueous phase samples of the culture MP1 reflected the amount of PCE added to the flask in total, i.e., by several spikings. However, in all cultures with complete dechlorination, less ethene formation was observed than calculated from the total amount of spiked PCE. As discussed previously (Aktaş et al. 2012), the repeated aqueous phase sampling results in an increase of the gas/liquid ratio in the bottles. Therefore, the high volatility, in particular, of ethene leads to a transfer into the gas phase resulting in lower concentrations in the aqueous phase.
In the case of incomplete dechlorination of PCE to the end-product cis-DCE, as observed in culture MP1, bioaugmentation with a Dehalococcoides spp.-containing culture can stimulate further dechlorination to ethene (Ritalahti et al. 2005). On the other hand, sequential anaerobic/aerobic degradation (Tiehm and Schmidt 2011) might be important in the field. Higher chlorinated compounds like PCE and TCE are more easily dechlorinated in an anaerobic reductive environment (Schmidt et al. 2010; Tiehm and Schmidt 2011). Lower chlorinated compounds like DCE and VC also can be degraded by aerobic degradation (Tiehm et al. 2008; Schmidt et al. 2010; Zhao et al. 2010; Zhao et al. 2011). At contaminated field sites, anaerobic dechlorination could stop at cis-DCE or VC, followed by aerobic mineralization of the lower chlorinated metabolites (Schmidt and Tiehm 2008; Lohner et al. 2011; Tiehm and Schmidt 2011). A similar sequential anaerobic/aerobic degradation might be possible also in the Yangtze TGR, taking into consideration the periodical water level fluctuations and expected spatio-temporal dynamics of the hydrochemical parameters (Bergmann et al. 2012; Kranzioch and Tiehm 2012). However, additional studies are required to prove this hypothesis.
Conclusions and outlook
In this study, the presence of viable dechlorinating bacteria in Yangtze sediment samples was demonstrated, thus indicating their relevance for pollutant turnover in the catchment area. The applied PCR methods proved to be suitable for the detection and quantification of the different dechlorinating bacteria. The qPCR analysis showed, in several samples, that the dehalogenation of PCE corresponded to the increasing numbers of Dehalococcoides spp., Desulfitobacterium spp., Desulfomonile spp., or Dehalobacter spp. In this study, the focus was on different bacteria, which are capable growing on chlorinated ethenes as electron acceptor. Studies focusing on the dehalogenase genes and dynamic environmental conditions are in progress.
References
Aktaş Ö, Schmidt KR, Mungenast S, Stoll C, Tiehm A (2012) Effect of chloroethene concentrations and granular activated carbon on reductive dechlorination rates and growth of Dehalococcoides spp. Biores Technol 103:286–292. doi:10.1016/j.biortech.2011.09.119
Barber JL, Sweetman AJ, van Wijk D, Jones KC (2005) Hexachlorobenzene in the global environment: emissions, levels, distribution, trends and processes. Sci Total Environ 349:1–44. doi:10.1016/j.scitotenv.2005.03.014
Bedard DL (2008) A case study for microbial biodegradation: anaerobic bacterial reductive dechlorination of polychlorinated biphenyls-from sediment to defined medium. Annu Rev Microbiol 62:253–270. doi:10.1146/annurev.micro.62.081307.162733
Behrens S, Azizian MF, McMurdie PJ, Sabalowsky A, Dolan M, Semprini L, Spormann AM (2008) Monitoring abundance and expression of Dehalococcoides species chloroethene-reductive dehalogenases in a tetrachloroethene-dechlorinating flow column. Appl Environ Microb 74:5695–5703. doi:10.1128/AEM.00926-08
Bergmann A, Bi Y, Chen L, Floehr T, Henkelmann B, Holbach A et al (2012) The Yangtze-Hydro Project: a Chinese-German environmental program. Environ Sci Poll Res 19:1341–1344. doi:10.1007/s11356-011-0645-7
Cheng D, He J (2009) Isolation and characterization of Dehalococcoides sp. strain MB, which dechlorinates tetrachloroethene to trans-1,2-dichloroethene. Appl Environ Microb 75:5910–5918. doi:10.1128/AEM.00767-09
Cheng D, Chow WL, He J (2009) A Dehalococcoides-containing co-culture that dechlorinates tetrachloroethene to trans-1,2-dichloroethene. ISME J 4:88–97. doi:10.1038/ismej.2009.90
Chow WL, Cheng D, Wang S, He J (2010) Identification and transcriptional analysis of trans-DCE-producing reductive dehalogenases in Dehalococcoides species. ISME J 4:1020–1030. doi:10.1038/ismej.2010.27
El Fantroussi S, Mahillon J, Naveau H, Agathos SN (1997) Introduction of anaerobic dechlorinating bacteria into soil slurry microcosms and nested-PCR monitoring. Appl Environ Microb 63:806–811
Field JA, Sierra-Alvarez R (2008) Microbial degradation of chlorinated benzenes. Biodegradation 19:463–480. doi:10.1007/510532-007-9155-1
Griffin BM, Tiedje JM, Löffler FE (2004) Anaerobic microbial reductive dechlorination of tetrachloroethene to predominately trans-1,2-dichloroethene. Environ Sci Technol 38:4300–4303. doi:10.1021/es035439g
Grostern A, Edwards EA (2006) Growth of Dehalobacter and Dehalococcoides spp. during degradation of chlorinated ethanes. Appl Environ Microb 72:428–436. doi:10.1128/AEM.72.1.428-436.2006
Hendrickson ER, Payne JA, Young RM, Starr MG, Perry MP, Fahnestock S et al (2002) Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl Environ Microb 68:485–495. doi:10.1128/AEM.68.2.485-495.2002
Holliger C, Schraa G, Stams AJ, Zehnder AJ (1993) A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl Environ Microb 59:2991–2997
Holliger C, Wohlfarth G, Diekert G (1999) Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol Rev 22:383–398. doi:10.1111/j.1574-6976.1998.tb00377.x
Jiang X, Martens D, Schramm KW, Kettrup A, Xu SF, Wang LS (2000) Polychlorinated organic compounds (PCOCs) in waters, suspended solids and sediments of the Yangtse River. Chemosphere 41:901–905. doi:10.1016/s00456535(99)00435-x
Kittelmann S, Friedrich MW (2008) Novel uncultured Chloroflexi dechlorinate perchloroethene to trans-dichloroethene in tidal flat sediments. Environ Microbiol 10:1557–1570. doi:10.1111/j.1462-2920.2008.0157.x
Kranzioch I, Tiehm A (2012) Assessment of pollutant biodegradation at the Yangtze Three Gorges Dam, China. Int J Water Management “blue facts” 2012:70–77
Liu H, Zhou Q, Wang Y, Zhang Q, Cai Z, Jiang G (2008) E-waste recycling induced polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and dibenzo-furans pollution in the ambient environment. Environ Int 34:67–72. doi:10.1016/j.envint.2007.07.008
Löffler FE, Sun Q, Li J, Tiedje JM (2000) 16S rRNA gene-based detection of tetrachloroethene-dechlorinating Desulfuromonas and Dehalococcoides species. Appl Environ Microb 66:1369–1374. doi:10.1128/AEM.66.4.1369.1374.2000
Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT et al (2012) Dehalococcoides mccartyi gen. nov., sp. nov., obligate organohalide-respiring anaerobic bacteria, relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidetes classis nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.034926-0
Lohner ST, Tiehm A (2009) Application of electrolysis to stimulate microbial reductive PCE dechlorination and oxidative VC biodegradation. Environ Sci Technol 43:7098–7104. doi:10.1021/es900835d
Lohner ST, Becker D, Mangold K-M, Tiehm A (2011) Sequential reductive and oxidative biodegradation of chloroethenes stimulated in a coupled bioelectro-process. Environ Sci Technol 45:6491–6497. doi:10.1021/es200801r
Maphosa F, de Vos WM, Smidt H (2010) Exploiting the ecogenomics toolbox for environmental diagnostics of organohalide-respiring bacteria. Trends Biotechnol 28:308–316. doi:10.1016/j.tibtech.2010.03.005
Marco-Urrea E, Nijenhuis I, Adrian L (2011) Transformation and carbon isotope fractionation of tetra- and trichloroethene to trans-dichloroethene by Dehalococcoides sp. strain CBDB1. Environ Sci Technol 45:1555–1562. doi:10.1021/es1023459
Maymó-Gatell X, Nijenhuis I, Zinder SH (2001) Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by Dehalococcoides ethenogenes. Environ Sci Technol 35:516–521. doi:10.1021/es001285i
Müller B, Berg M, Yao ZP, Zhang XF, Wang D, Pfluger A (2008) How polluted is the Yangtze river? Water quality downstream from the Three Gorges Dam. Sci Total Environ 402:232–247. doi:10.1016/j.scitotenv.2008.04.049
Ritalahti KM, Löffler FE, Rasch EE, Koenigsberg SS (2005) Bioaugmentation for chlorinated ethene detoxification: bioaugmentation and molecular diagnostics in the bioremediation of chlorinated ethene-contaminated sites. Ind Biot 1:114–118. doi:10.1089/ind.2005.1.114
Rouzeau-Szynalski K, Maillard J, Holliger C (2011) Frequent concomitant presence of Desulfitobacterium spp. and Dehalococcoides spp. in chloroethene-dechlorinating microbial communities. Appl Microbiol Biot 90:361–368. doi:10.1007/s00253-010-3042-0
Schmidt KR, Tiehm A (2008) Natural attenuation of chloroethenes: identification of sequential reductive/oxidative biodegradation by microcosm studies. Water Sci Technol 58:1137–1145. doi:10.2166/wst.2008.729
Schmidt KR, Stoll C, Tiehm A (2006) Evaluation of 16S-PCR detection of Dehalococcoides at two chloroethene-contaminated sites. Water Sci Technol 6:129–136. doi:10.2166/ws.2006.787
Schmidt KR, Augenstein T, Heidinger M, Ertl S, Tiehm A (2010) Aerobic biodegradation of cis-1,2-dichloroethene as sole carbon source: stable carbon isotope fractionation and growth characteristics. Chemosphere 78:527–532. doi:10.1016/j.chemosphere.2009.11.033
Shen C, Tang X, Cheema SA, Zhang C, Khan MI, Liang F et al (2009) Enhanced phytoremediation potential of polychlorinated biphenyl contaminated soil from e-waste recycling area in the presence of randomly methylated-beta-cyclodextrins. J Hazard Mater 172:1671–1676. doi:10.1016/j.jhazmat.2009.08.064
Smidt H, de Vos WM (2004) Anaerobic microbial dehalogenation. Annu Rev Microbiol 58:43–73. doi:10.1146/annurev.micro.58.030603.123600
Smits THM, Devenoges C, Szynalski K, Maillard J, Holliger C (2004) Development of a real-time PCR method for quantification of the three genera Dehalobacter, Dehalococcoides, and Desulfitobacterium in microbial communities. J Microbiol Meth 57:369–378. doi:10.1016/j.mimet.2004.02.003
Taş N, van Eekert MHA, Wagner A, Schraa G, de Vos WM, Smidt H (2011) Role of Dehalococcoides spp. in the anaerobic transformation of hexachlorobenzene in European rivers. Appl Environ Microbiol 77:4437–4445. doi:10.1128/AEM.01940-10
Tiehm A, Schmidt KR (2011) Sequential anaerobic/aerobic biodegradation of chloroethenes-aspects of field application. Curr Opin Biotech 22:415–421. doi:10.1016/j.copbio.2011.02.003
Tiehm A, Schmidt KR, Pfeifer B, Heidinger M, Ertl S (2008) Growth kinetics and stable carbon isotope fractionation during aerobic degradation of cis-1,2-dichloroethene and vinyl chloride. Water Res 42:2431–2438. doi:10.1016/j.watres.2008.01.029
Vancheeswaran S, Hyman MR, Semprini L (1999) Anaerobic biotransformation of trichlorofluoroethene in groundwater microcosms. Environ Sci Technol 33:2040–2045. doi:10.1021/es9811952
Wang J, Bi Y, Pfister G, Henkelmann B, Zhu K, Schramm KW (2009) Determination of PAH, PCB, and OCP in water from the Three Gorges Reservoir accumulated by semipermeable membrane devices (SPMD). Chemosphere 75:1119–1127. doi:10.1016/j.chemosphere.2009.01.016
Zhang J (1995) Geochemistry of trace metals from Chinese river/estuary systems: an overview. Estuar Coast Shelf S 41:631–658. doi:10.1006/ecss.1995.0082
Zhao HP, Schmidt KR, Tiehm A (2010) Inhibition of aerobic metabolic cis-1,2-di-chloroethene biodegradation by other chloroethenes. Water Res 44:2276–2282. doi:10.1016/j.watres.2009.12.023
Zhao HP, Schmidt KR, Lohner S, Tiehm A (2011) Robustness of an aerobic metabolically vinyl chloride degrading bacterial enrichment culture. Water Sci Technol 64:1796–1803. doi:10.2166/wst.2011.752
Acknowledgments
The authors gratefully acknowledge the financial support from the German Ministry of Education and Research (BMBF, grant no.: 02WT1130). This study is part of the Sino-German Yangtze-Hydro Project (www.yangtze-project.de).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Robert Duran
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1616 kb)
Rights and permissions
About this article
Cite this article
Kranzioch, I., Stoll, C., Holbach, A. et al. Dechlorination and organohalide-respiring bacteria dynamics in sediment samples of the Yangtze Three Gorges Reservoir. Environ Sci Pollut Res 20, 7046–7056 (2013). https://doi.org/10.1007/s11356-013-1545-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1545-9