Abstract
Arundo donax is a suitable lignocellulosic feedstock for the production of bio-based chemicals due to its low economic value, rapid growth in low-nutrient soils, and rich structural composition. The present study aimed to selectively extract hemicellulosic and cellulosic sugars from A. donax biomass by using dilute acid and alkali pretreatment as well as utilize those sugars for biological production of xylitol and ethanol by Candida tropicalis. Dilute acid pretreatment with 1.5% sulphuric acid effectively released 22.14 g/L of xylose in the hydrolysate, while, 1 M sodium hydroxide treatment removed 77.9% of the total lignin present in the biomass. The crystallinity index of A. donax biomass was reduced from 65.89 to 61.39% after alkali pretreatment. Dilute acid hydrolysate was detoxified by activated charcoal treatment that completely removed 5-HMF, whereas, 92.5% and 18.99% removal of furfural and acetic acid was achieved. The maximum xylitol yield and volumetric productivity in A. donax hydrolysate were 0.54 g/g and 0.274 g/L/h, respectively after 96 h of incubation. In the parallel process, enzymatic hydrolysis of cellulose-rich fraction of A. donax resulted in a glucan conversion of 85.66%. Subsequent ethanol fermentation in A. donax saccharified broth by C. tropicalis produced a yield and productivity of 0.47 g/g and 0.63 g/L/h, respectively. The present study concludes that sequential dilute acid and alkali pretreatments of A. donax were efficient in extracting most of the xylose and glucose from the biomass. These pretreatment methods can be successfully employed for maximum utilization of this low-value biomass for biological production of value-added chemicals including ethanol and xylitol by C. tropicalis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass is gaining continuous global attention as a preferred source of bio-based chemicals due to its easy and plentiful availability and substantially greater proportions of usable structural polysaccharides [1]. Proper disposal of these low-value biomasses is essential to minimize burden on the environment and maximize the land available for cultivation of food crops. Lignocellulosic feedstocks are primarily composed of biopolymers of cellulose and hemicellulose as well as a macromolecule lignin. These components act as precursors for various industrial chemicals such as ethanol, xylitol, furfural, and acetic acid [2]. Maximum number of studies published on biomass valorization are based on rice straw, sugarcane bagasse, and corncobs due to their widespread production across most parts of the world.
Among the different lignocellulosic biomasses, Arundo donax is a perennial rhizomatous cane belonging to the family Poaceae. It is considered one of the most promising feedstocks for biorefineries due to its ability to grow rapidly in varied climatic conditions including marginal soils, low agronomic input requirements, high structural carbohydrates content (up to 60 wt%), resistance to biotic and abiotic stress as well as high productivity (around 30 tons/hectare/year) [3, 4]. Arundo donax is commonly used in the construction of temporary sheds, fences, carpets, fishing poles, and musical instruments [5]. It has also been explored for other applications including paper and pulp production from the fibers extracted from the stem of this plant, as a reinforcement material in polymer composites, and oil-spill remediation [6, 7]. A. donax was also reported to produce about 11,000 L per hectare of ethanol through fermentative processes, which is 50% and 20% higher than ethanol produced from sugarcane bagasse and Miscanthus respectively [8]. Although it is primarily reported feedstock for ethanol and bioenergy production, it is also considered a good source of other biomolecules such as levulinic acid, γ-valerolactone, biochar, and bio-oils [9, 10].
Xylitol, a naturally occurring pentitol, is regarded as one of the most versatile platform chemicals among the top twelve sustainable value-added chemicals that can be obtained from biomass. Due to its enormous commercial potential, the global market is estimated to reach $1.37 billion by 2025 [11]. Commercially, the xylitol demands are fulfilled by the chemical hydrogenation of xylose at high pressure and temperature in the presence of toxic chemical catalysts like Pl, Pt, Ru, and Ni. However, this chemical process is eco-unfriendly, labour-intensive, and involves high operational costs [12]. Therefore, the bioconversion of xylose to xylitol using microorganisms such as yeasts, fungi, and bacteria serves as an attractive alternative to the chemical hydrogenation process due to several advantages including environment-friendly process, low energy requirement, and high product yield [13]. Xylitol production through biological route has been reported by a number of yeast genera including Candida, Pichia and Debaryomyces with Candida as the most preferred candidate due to the ability to utilize both pentose and hexose sugars and flourish in multiple types of hemicellulosic hydrolysates obtained from biomass [14]. In the past decade, Candida tropicalis has been widely explored for the production of xylitol from lignocellulosic biomass-derived sugars [15]. Various strains of C. tropicalis are known to convert up to 86.8% pure xylose and 79% xylose in hemicellulosic hydrolysate into xylitol [16, 17].
Ethanol is a promising biofuel that is used to supplement gasoline to reduce the burden on crude petroleum resources as well as decrease the carbon emissions from gasoline-powered automobiles. In 2016, ethanol blending in gasoline reduced greenhouse gas emissions by 43.5 million metric tonnes which is equivalent to taking 9.3 million cars off the road for a whole year [18]. The global ethanol market in 2022 is estimated at US$ 89.7 billion and is expected to reach US$105.2 billion by 2025 [19]. Second-generation ethanol production has been identified as a viable path toward a sustainable circular economy. It has been produced at industrial scale from a variety of second-generation feedstocks including corncobs, corn stover, wheat straw, and sugarcane bagasse [2]. Saccharomyces cerevisiae is the most widely explored yeast for bioethanol production due to its larger cell size, high-temperature resistance, and higher ethanol yields; however, it has an inevitable drawback of lacking xylose metabolizing enzymes rendering it unable to utilize xylose as a carbon and energy source. Thus, this yeast is unsuitable for the co-production of ethanol and xylitol [20]. Owing to the easy availability as well as structural polysaccharides-rich composition of A. donax, this study was mainly aimed at the selective extraction of xylose and glucose from hemicellulosic and cellulosic fractions of A. donax biomass by sequential dilute acid and alkali pretreatment. Moreover, the ability of a locally isolated strain of C. tropicalis to effectively ferment glucose and xylose was investigated for the efficient conversion of extracted sugars into ethanol and xylitol.
Materials and Methods
Chemicals and Plant Biomass
All the chemicals, enzymes, and microbiological media used in this work were purchased from Himedia Laboratories, Mumbai, India, and Sigma-Aldrich, Co., Denmark. Arundo donax plants were harvested from Attawa Choa, a seasonal rivulet adjoining the Center of Innovative and Applied Bioprocessing, Mohali campus in July, 2021. The plant biomass including stems and leaves was cut into small pieces (< 1 inch) by a chaff cutter followed by sun-drying for one week. The dried biomass was then stored in a dry and ventilated place for further use.
Microorganism
A previously isolated, in-house strain of Candida tropicalis GS18 (Acc. No. OK165575) was used for ethanol and xylitol production. The yeast was stored on YEPD (yeast extract 1, peptone 2, and dextrose 2 g/L) medium slants at 4 °C. The yeast was subcultured over fresh YEPD and YEPX (dextrose replaced by xylose) agar plates followed by incubation at 30 °C. Loopful of fresh yeast cultures were used for inocula preparation in YEPD and YEPX broth media for ethanol and xylitol production, respectively.
Compositional Analysis of Arundo Donax
The sun-dried biomass was further dried in a hot-air oven at 70 °C for 48 h for estimation of the structural components of the whole biomass. The dried biomass (< 10% moisture content) was further ground in a steel kitchen grinder to obtain small pieces (< 2 mm) suitable for compositional analysis. The compositional analysis was carried out by following the NREL protocol with some minor modifications [21]. The estimated composition of A. donax was compared with that of the reported studies to mark the major differences.
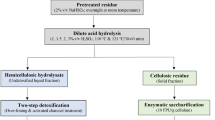
Dilute Acid Pretreatment
The hemicellulosic sugars present in Arundo donax biomass were recovered by using dilute sulphuric acid pretreatment. The biomass was ground to get particle size < 10 mm for efficient solubilization of xylose. The concentration of sulphuric acid and solid: liquid ratio of biomass: acid solution was optimized to achieve maximum sugar yield and minimum generation of toxic inhibitors. Acid concentrations of 0.5, 1.0, 1.5, and 2.0% were used, whereas, the S: L ratios used were 1: 8, 1: 10, 1.5: 8, and 1.5: 10. Pretreatment was carried out in a customized lab scale hydrolyser fitted with spiral agitator and heating-cooling jacket. The dilute acid pretreatment temperature and time were pre-optimized in our laboratory at 140 °C and 30 min. Following pretreatment, the contents in the hydrolyser vessel were cooled with running tap water circulation and removed for the separation of solid and liquid fractions. The liquid fraction containing fermentable sugars and the solid fraction containing cellulose and lignin were stored at room temperature for further processing.
Neutralization and Detoxification of A. Donax Hydrolysate
The dilute acid hydrolysate (liquid fraction) of Arundo donax was neutralized to achieve pH > 5.5 suitable for fermentation experiments. The hydrolysate was filtered through a white-coloured, fine-pore cotton cloth (FPCC) (pore size < 20 μm) followed by the addition of small quantities of calcium oxide with continuous stirring. Calcium oxide was used as a neutralizing agent due to its relatively low cost and minimal loss of fermentable sugars following neutralization. On achieving the desired pH, the hydrolysate was again filtered through FPCC. The use of conventional filter paper was minimized in this study due to the non-recyclability of filter paper, more time consumption and the inability to squeeze the liquid when used without a vacuum pump. On the other hand, the FPCC is cheaper than Whatman filter paper, can be used multiple times, can be squeezed manually to recover the maximum volume of hydrolysate, and is less time-consuming.
The filtered hydrolysate was then detoxified to remove toxic fermentation inhibitors including phenolic compounds, 5-hydroxylmethyl furfural, and furfural. The detoxification was carried out at pH 5.5 and 28 °C in baffled flasks placed in a rotary shaker incubator. The activated charcoal concentration used for detoxification was 4% (w/v) which is slightly above the previously reported concentrations due to the presence of higher amounts of phenolics and colour-contributing compounds present in the Arundo donax hydrolysate. Activated charcoal treatment was performed for one hour followed by filtration of the detoxified hydrolysate. The filtered detoxified hydrolysate was stored at 4 °C until further use.
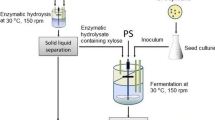
Xylitol Production by C. Tropicalis on Commercial Xylose Medium
The ability of C. tropicalis strain to produce xylitol from the pure xylose-based minimal medium was evaluated to set the known yield target. The inoculum for xylitol fermentation was prepared in 20 ml YEPX broth medium in 100 ml Erlenmeyer flask. The fermentation medium was prepared by adding ammonium sulphate 1 g/L, potassium dihydrogen orthophosphate 0.2 g/L, disodium hydrogen phosphate 0.2 g/L, magnesium sulphate 0.1 g/L, yeast extract 1 g/L and xylose > 50 g/L in 180 ml of demineralized water in 500 ml conical flask. The pH of the defined medium was adjusted to 5.5 by using 1 M HCl followed by autoclaving at 121 °C for 10 min. Reduced sterilization time was used to prevent the darkening of the broth medium by caramelization of xylose in the presence of a nitrogen source. After sterilization, the medium was cooled and poured with 20 ml inoculum of actively growing C. tropicalis cells. The flasks were then placed in a rotary incubator shaker set at 150 rpm and 30 °C. The samples were withdrawn regularly to estimate the sugars consumption, xylitol production, and changes in the dry cell weight of C. tropicalis. The fermentation experiments were continued until at least 90% of the xylose was consumed in each replicate.
Xylitol Production by C. Tropicalis in Concentrated Detoxified A. Donax Hydrolysate
The ability of C. tropicalis to produce xylitol from A. donax hydrolysate was assessed by shake flask fermentation. The detoxified hydrolysate was concentrated 2.5 times by using a rotary vacuum evaporator at 70 °C. The hydrolysate was filtered through Whatman filter paper no. 1 multiple times to remove the precipitates of calcium sulphate formed by the addition of calcium oxide during neutralization. The filtered and concentrated hydrolysate was supplemented with equivalent amounts of inorganic salts and yeast extract as added in the pure xylose-based medium previously. The pH of the medium was adjusted to 5.5 followed by autoclaving at 110 °C for 15 min. The sterilized medium was then inoculated with 20 ml of C. tropicalis inoculum prepared in YEPX broth at 30 °C for 20 h. The inoculated flasks were incubated and monitored in the same way as described in Sect. 2.6.
Alkali Pretreatment of Solid Residue
The solid fraction obtained after dilute acid pretreatment was washed several times with tap water to neutralize the pH followed by drying to achieve < 10% moisture content. This fraction majorly consisted of cellulose and lignin. The aim of alkali pretreatment was to dissolve the lignin in solution to make cellulose freely available for subsequent enzymatic hydrolysis. The pretreatment was carried out by using 1 M sodium hydroxide with a solid: liquid ratio of 1: 10. The contents were mixed in a wide-mouthed glass bottle before autoclaving at 121 °C for 30 min. The alkali-pretreated cellulose-rich fraction (CRF) was repeatedly washed with tap water to remove any traces of sodium hydroxide. On reaching pH > 5, the fraction was squeezed to remove all the water and dried at 90 °C in a hot air oven. The resultant dried CRF was ground in a steel grinder to make it ready for enzymatic saccharification.
Enzymatic Saccharification of A. Donax Cellulosic Fraction
The CRF was further subjected to enzymatic saccharification to obtain glucose which would be used as a substrate for ethanol fermentation. The saccharification was carried out in 500 ml flasks containing 200 ml of 0.05 M sodium acetate buffer and 5% w/v CRF. A cellulose enzyme blend (Sigma-Aldrich, MO, USA) was used to hydrolyse the CRF with a concentration of 25 FPU/g of substrate. The hydrolysis was carried out at 4.5 pH, 50 °C and 170 rpm for 72 h. The enzyme hydrolysis was performed in multiple numbers of flasks to obtain maximum volume of the saccharified broth. The rate of saccharification was monitored by withdrawing small amounts of samples at regular intervals followed by their sugar analysis by HPLC. After achieving the maximum concentration of glucose in the saccharified broth, the remaining solids were removed by vacuum filtration through Whatman filter paper no. 1. The resultant clear to translucent broth was used for subsequent ethanol fermentation.
Ethanol Production in Commercial Glucose and Arundo Donax Saccharified Broth
C. tropicalis was first used to produce ethanol from pure glucose-based medium to set the yield target for subsequent saccharified broth-based experiments. The yeast inoculum was prepared in 20 ml YEPD broth by adding a loopful of yeast cells from the fresh culture plate and incubating the flask at 30 °C and 150 rpm for 16 h. Ethanol fermentation was carried out in 500 ml flasks with a working volume of 200 ml. The fermentation medium contained ammonium sulphate 1 g/L, potassium dihydrogen orthophosphate 0.15 g/L, disodium hydrogen phosphate 0.15 g/L, magnesium sulphate 0.1 g/L, yeast extract 1 g/L and glucose 50 g/L. The pH of the medium was adjusted to 6.0 before autoclaving at 121 °C for 15 min. The inoculum was added to the fermentation medium when > 90% sugar in the former was utilized. After inoculation, the flasks were stored at 30 °C and 150 rpm. Sugar consumption, ethanol production and cell dry weight were regularly monitored. Incubation was continued until the ethanol titre started declining due to exhaustion of available glucose. For ethanol production in A. donax saccharified broth, the filtered broth was concentrated by vacuum rotary evaporator at 60 °C to obtain a glucose concentration around 50 g/L. All the remaining steps of ethanol fermentation were similar to the pure glucose-based fermentation.
Calculations
For compositional analysis of Arundo donax
% Cellulose = [Glucose in filtrate (g/L) x volume of filtrate x 0.9] / [k1 x total weight of biomass] x 100
% Hemicellulose = [xylose in filtrate (g/L) x volume of filtrate x 0.88] / [k2 x total weight of biomass] x 100
+ [arabinose in filtrate (g/L) x volume of filtrate x 0.88] / [k3 x total weight of biomass] x 100
k1, k2 and k3 are the calibration coefficients of glucose, xylose and arabinose for their decomposition in the autoclave and correspond to values 0.97, 0.84 and 0.88 respectively.
% Acid insoluble lignin = (weight of solid residue after filtration ? weight of ash recovered after calcination at 500˚C) / total weight of biomass x 100
For Crystallinity index (CrI)
The crystallinity index of untreated and pretreated biomass was calculated by applying peak intensity method with the following equation [22]:
Where, I(002) represents peak intensity of the crystalline portion of the sample and I(am) is the peak intensity of the amorphous part of the sample. CrI calculations were performed by using OriginPro 2016.
Analytical Techniques and Statistical Analysis
Sugars and inhibitors estimation in the whole study was carried out by using analytical HPLC (Agilent Technologies, 1260 Infinity) with a reverse phase column (BIORAD Aminex HPX-87 H column (300 × 7.8 mm) and a refractive index detector. The column chamber temperature was kept at 60 °C with 0.55 ml/min flow rate. Degassed 5 mM sulphuric acid was used as mobile phase. Fresh standard curves of sugars and inhibitors were made during each HPLC run by using five different concentrations ranging from 0.2 g/L to 1 g/L. All the samples were diluted according to the approximate concentration of analyte present in the sample before analysis. Scanning electron microscopy of biomass at various stages was performed by JCM-6000 benchtop SEM (JEOL Ltd., Japan). X-ray diffraction analysis of untreated and pretreated biomass was performed in a high-resolution X-ray diffractometer (SmartLab SE, Rigaku Corporation, Tokyo, Japan). Thermal gravimetric analysis of biomass samples at various stages of pretreatment was carried out in nitrogen environment by simultaneous thermal analyser (STA 8000, PerkinElmer Inc. CT, USA). The moisture content of biomass before and after drying was measured by MA-35 moisture analyser (Sartorius Weighing Technology, Goettingen, Germany). The total phenolics content was estimated by Folin–Ciocalteu assay in a UV-visible spectrophotometer (UV-1900i, Shimadzu, Japan) [23]. All the experiments in this study were performed in triplicate and the mean of the three values and standard deviation in the values were plotted by using Graphpad prism 5.0.
Results and Discussion
Compositional Analysis of Arundo Donax Biomass
The structural composition of mature terrestrial plants depends on several external abiotic factors such as soil fertility, moisture content of the soil, intensity of sunlight received and time of harvesting. In this study, compositional analysis of ground A. donax biomass was performed by using a modified NREL protocol which revealed that it contained the highest amount of cellulose followed by hemicellulose and lignin (Table 1). The comparison of structural polymers present in the biomass showed that A. donax, irrespective of its origin, contains significantly high amounts of usable polysaccharides (up to 80%) that can be efficiently valorised for the production of bio-based chemicals.
Dilute Acid Pretreatment of Arundo Donax
Acid pretreatment of biomass is the most popular method for achieving high sugar yields. Both concentrated and dilute acid pretreatments have been reported in the literature for efficient recovery of fermentable sugars especially xylose. However, the use of concentrated acids poses certain limitations due to toxicity, need of specialized corrosion resistant reactors, need of acid recycling and excessive production of sugar degradation products [27]. Therefore, dilute acid-based pretreatment, due to less severe conditions has been found to be a better pretreatment option that produces high yields of sugars with minimal production of toxic inhibitors. Though, the concentrations of acids and other factors must be optimized to make the overall process more economically feasible. In the present study, sulphuric acid was chosen for pretreatment owing to its relatively low cost, ready availability, no generation of toxic fumes and high xylose yields. The concentration of sulphuric acid and solid: liquid ratio of A. donax biomass was optimized to find the best combination for high xylose yield (Table 2). The time and temperature of pretreatment were kept constant at 30 min and 140 °C as it was previously optimized in our laboratory (data not shown). It was observed that with an increase in acid concentration, the sugars yields were also increasing, however, high sugar yield was associated with the generation of large amounts of fermentation inhibitors including acetic acid, HMF, and furfural. Although the production of inhibitors could not be escaped, the acid concentration that produced higher yields of sugars and comparatively less generation of inhibitors could be selected for further experiments. Furthermore, the optimization of S: L ratio with 1.5% sulphuric acid revealed that 1: 8 solid to liquid ratio was the best option due to the high yields of both the sugars and relatively less production of inhibitors. Therefore, 1.5% sulphuric acid with 1: 8 solid to liquid ratio was considered the best option for large-scale pretreatment.
In another study, the optimization of dilute sulphuric acid pretreatment of Arundo donax biomass demonstrated that 1.27% acid concentration and 141.6 °C for 36.4 min were the most suitable conditions for maximum xylose recovery [28]. In a similar work, different varieties of sorghum biomass were pretreated with 2% sulphuric acid and 1:10 solid to liquid ratio at 121 °C for 71 min. The yield of recovered xylose was between 16 and 17 g/L and that of glucose was 6–8 g/L for all three tested varieties [29]. The yield of arabinose is usually ignored by most of the authors due to its low concentration and less preference of yeasts to consume arabinose instead of glucose and xylose. A study of the co-production of xylitol and ethanol from hemicellulosic hydrolysates of banana and water hyacinth indicated that 2.5% sulphuric acid with 1: 10 solid to liquid ratio at 121 °C for 30 min yielded 18.32 and 21.95 g/L xylose from banana and water hyacinth respectively [30]. In this study, along with the high concentrations of glucose and xylose in the hydrolysate, fermentation inhibitors were also detected in relatively higher concentrations which might be due to the high pretreatment temperature. Though, these inhibitors could be efficiently removed by activated charcoal-based detoxification.
Neutralization and Detoxification of Arundo Donax Hydrolysate
The dilute acid hydrolysate has always a very low pH value that needs to be neutralized before further use. In the present study, following neutralization with calcium oxide, colour of the A. donax hydrolysate was changed from orange-brown to dark brown. On reaching pH 5.5, large amount of gypsum was formed due to the reaction between sulphuric acid and calcium oxide. The filtered hydrolysate when stored at 4 °C developed more brown coloured precipitates which were again filtered through Whatman filter paper no. 1. The filtered hydrolysate was then detoxified by 4% activated charcoal which completely removed HMF within one hour of treatment. On the other hand, only 92.5% furfural and 18.99% acetic acid were removed post-detoxification (Table 3). Fermentable sugars recovery after activated charcoal treatment was 94.52 and 95.57% for glucose and xylose respectively. Following vacuum concentration, the concentration of sugars, as well as inhibitors, was roughly increased by 2.5 times resulting in glucose to xylose ratio of roughly 1: 5. The major limitations of A. donax biomass as a feedstock for xylitol production were (i) the comparatively higher concentrations of phenolics and other colour contributing compounds which decrease the efficiency of activated charcoal based detoxification that is the most preferred way of inhibitor removal from the hydrolysate, (ii) presence of relatively larger portion of amorphous cellulose in the biomass which contributes high concentrations of glucose in the dilute acid hydrolysate, affecting the suitable glucose to xylose ratio required for xylitol production. Otherwise, A. donax possesses a significant proportion of hemicellulose roughly equivalent to rice and wheat straw that must be effectively utilized for bio-based chemicals production.
Alkali Pretreatment
Dilute acid pretreatment hydrolyses most of the hemicellulosic fraction of the biomass, however, it is not fully effective in lignin removal. Alkali pretreatment is widely used for biomass disintegration by saponification of intermolecular ester bonds between lignin and hemicellulases. More specifically, dilute sodium hydroxide pretreatment increases the internal surface area of lignocellulosic biomass, reduces the degree of polymerization as well as crystallinity of cellulose which makes it easily accessible to the hydrolytic enzymes [31]. Sodium hydroxide is the most preferred alkali for biomass pretreatment due to its high efficiency, less severe process conditions, easy availability, and low cost [32]. In the present study, alkali pretreatment of dilute acid pre-treated solid fraction of A. donax was carried out with 1 M sodium hydroxide to dissolve the residual lignin and make the cellulose accessible for hydrolytic enzymes. Following alkali pretreatment, most of the solid fraction turned into hair-like fibers which were lighter in colour and weaker in strength as compared to the dilute acid-treated fraction. The analysis of dried CRF revealed that it contained negligible amount of hemicellulose due to its prior solubilization in the liquid fraction during dilute acid pretreatment. The estimated mass loss following alkali pretreatment was 46.47% which ascertained significant removal of lignin from the solid fraction. The dual pretreatment of A. donax biomass was effective in removing a total of 77.9% lignin which was the greatest hurdle in the utilization of glucose packed in the biomass. A similar study on the utilization of A. donax reported 84.1% removal of lignin by 5% alkali pretreatment which is slightly higher in comparison to the present study [33]. This variation might be due to the increased concentration of sodium hydroxide which plays a key role in any pretreatment process. In the present work, 93.52% cellulose was recovered for subsequent enzyme hydrolysis which produced a total of 26.64 g glucose per 100 g of A. donax biomass. Similarly, two-stage microwave-based dilute acid and alkali pretreatment of A. donax produced 26.4 g glucose / 100 g biomass which is in agreement with our findings [34]. The role of potassium hydroxide pretreatment of A. donax biomass for enhanced methane yield has been demonstrated, where, the maximum daily rate of methane production and its concentration in biogas was increased by 42% and 23% respectively [35]. Similarly, 0.5 M sodium hydroxide pretreatment of A. donax enhanced the reducing sugar yield by more than 2-fold, which in turn, increased the cumulative CH4 yield by 63% in comparison to the untreated biomass [36]. Most of the literature reports demonstrate the use of direct alkali pretreatment of A. donax for the production of ethanol, biogass and hydrogen. However, the sequential dilute acid and alkali pretreatment of lignocellulosic biomass is believed to be a better option to enhance the hydrolytic effect for recovering higher yields of xylose meant for xylitol production as well as making cellulose more accessible in enzymatic conversion to glucose for ethanol production [37, 38].
SEM Analysis
The structural and morphological changes in A. donax biomass before and after dilute acid and alkali pretreatments were observed by using SEM imaging (Fig. 1a-c). The smooth and intact surface of a small piece of A. donax stem before dilute acid pretreatment can be seen clearly in Fig. 1a. Following dilute acid pretreatment, most of the hemicellulose fraction was broken down to contribute xylose and arabinose in the hydrolysate giving the biomass, rough and porous appearance. The destruction of the surface layer and shrinkage of A. donax biomass components can be seen in Fig. 1b. The solid fraction consisting mainly of lignin and cellulose, when subjected to alkali pretreatment, was further broken down to give rise to easily accessible cellulose (Fig. 1c). Most of the lignin was dissolved in the alkali solution resulting in the liberation of fine cellulose microfibrils which could give rise to higher amounts of glucose following enzymatic hydrolysis. A similar breakdown of A. donax biomass was observed after steam explosion pretreatment at very high temperatures ranging from 180 to 220 °C [39]. The SEM imaging confirmed the effective destruction of recalcitrant structure of A. donax by two stage sequential pretreatment.
Thermal Gravimetric Analysis (TGA)
TGA of A. donax biomass was performed to study changes in the untreated and pretreated biomass overheating up to 800 °C with a temperature rise of 10 °C per minute. Thermal decomposition of different components of biomass occurs at different temperatures beginning with moisture loss at 100 °C. Hemicellulose being amorphous and more delicate to temperature starts decomposing at 180 °C and goes up to 330 °C, while cellulose degrades between 300 and 400 °C. Lignin is a slow degrading tough fraction that starts decomposing at 200 °C and stands up to 600 °C [40]. The TGA plot of A. donax in Fig. 2 shows most significant thermal decomposition between 200 and 600 °C. Due to minimal moisture in the samples subjected to TGA, there is negligible weight loss at 100 °C. Untreated biomass shows significant weight loss up to 350 °C due to the decomposition of hemicellulose, the most heat-sensitive biomass component followed by cellulose and lignin degradation between 350 and 600 °C. Dilute acid-treated solid fraction, due to the absence of hemicellulose in it, displayed steep and continuous mass loss between 300 and 600 °C confirming the degradation of cellulose and lignin component. In the case of alkali-treated CRF (lacking hemicellulose and lignin), the most significant mass loss was observed between 300 and 400 °C when the cellulose component rapidly decomposed. In the final passive pyrolysis stage, slow decomposition of all three samples was observed giving rise to “tails” up to 800 °C which could be due to ash and stable fixed carbon components. The TGA profiles of untreated, dilute acid and alkali pretreated biomass clearly demonstrated the effectiveness of different pretreatments in conserving the selective portions of structural polysaccharides for specific applications.
XRD Analysis
X-Ray diffraction was used to observe the changes in cellulose crystallinity after dilute acid and alkali pretreatment of A. donax (Fig. 3). Untreated powdered biomass showed two characteristic peaks at 2θ angles = 16.29° and 21.89° representing cellulose I lattice planes. Cellulose I, due to its crystalline nature is relatively more resistant to enzymatic hydrolysis than cellulose II [41]. These two peaks amplified in size with each successive pretreatment indicating the removal of lignin and hemicellulose from the biomass and a gradual increase in the quantity of cellulose. Following dilute acid pretreatment, the peak at 21.89° did not shift significantly, however, the peak at 16.29° slightly shifted at 15.96°. Moreover, after alkali pretreatment it further shifted to 15.54° indicating the transition of the crystalline form of cellulose to the amorphous form. The breakdown of hydrogen bonds within the crystalline regions of cellulose causing destruction of glycosidic linkages and ester bonds could be the reason behind intensification and shifting of peaks [42]. A third relatively smaller peak was also observed at 35.18° in untreated A. donax biomass [43]. This peak was slightly increased in area and intensity as well as shifted at 34.68° after dilute acid pretreatment and remained unchanged following alkali pretreatment. The increment of this peak could have occurred due to the overall increase in cellulose content in the biomass after each pretreatment. The calculated CrI for untreated A. donax biomass was 65.89% while in case of dilute acid pretreated biomass it was 64.94%, indicating a negligible reduction in the crystallinity of cellulose. On the other hand, alkali pretreated cellulose-rich fraction showed CrI of 61.39% which could be due to the successful removal of lignin as well as destruction of hydrogen bonds between the cellulose resulting in a reduced degree of crystallinity [44]. The findings of the present study are similar to the already reported work on ultrasound-assisted alkali pretreatment of A. donax, where the CrI of alkali-pretreated biomass was found to be 61.08% [45]. The XRD analysis revealed that alkali pretreatment removed significant amount of lignin and made more amorphous cellulose available for subsequent enzymatic attack. The sequential pretreatment affected A. donax biomass in two separate ways; (i) dilute acid pretreatment removed most of the hemicellulose from the biomass leaving a relatively larger portion of cellulose and lignin in solid fraction (ii) alkali pretreatment removed most of the lignin giving rise to cellulose-rich fraction. Additionally, alkali pretreatment significantly reduced the crystallinity index of cellulose making the substrate easily hydrolysable by cellulases.
Enzymatic Saccharification of CRF
The enzymatic hydrolysis of ground alkali pretreated solids was carried out to release the fermentable sugar in the acetate buffer. The enzyme blend used for saccharification consisted of cellulase, hemicellulase and β-glucosidases from various sources. The conversion of cellulose to glucose was fastest in the first 6 h of hydrolysis releasing 24.57 g/L of glucose. Between 6 and 72 h of incubation at 50 °C the hydrolysis was roughly linear giving rise to a maximum of 42.83 g/L of glucose at 72 h of incubation. When incubated further, the concentration of glucose did not increase indicating the complete conversion of cellulose to glucose. The final glucan conversion efficiency of enzymatic saccharification was found to be 85.66%. Similarly, 85% glucan conversion was previously reported by hydrodynamic cavitation assisted alkali pretreatment of reed grass for bioethanol production [46]. In another study, up to 89% glucan conversion was obtained by microwave irradiation-based pretreatment of wheat and rye stillage [47].
Xylitol Production by C. Tropicalis
The metabolism of glucose and xylose in C. tropicalis is demonstrated in Fig. 4. The intracellular conversion of xylose to xylitol is solely dependent on the presence of the enzyme xylose reductase in the yeast cell. Moreover, the movement of xylose towards xylitol production or cell multiplication depends on oxygen availability in the surrounding medium. In the presence of access of oxygen, NADH formed during xylitol to xylulose conversion is rapidly re-oxidized causing the repetition of this step. The xylulose then enters into pentose phosphate pathway and contributes to normal cell growth and multiplication. This causes less accumulation of xylitol in the cell. On the other hand, in low oxygen availability, intracellular NADH levels would increase, thereby causing an imbalance between xylose reductase and xylose dehydrogenase leading to xylitol accumulation [48, 49]. C. tropicalis was employed to produce xylitol from pure xylose-based medium as well as concentrated detoxified hydrolysate of A. donax at shake flask level. In the former medium, the yeast successfully produced a maximum of 36.44 g/L of xylitol by consuming 51.54 g/L of xylose within 96 h of incubation at 30 °C and 150 RPM (Fig. 5a). The xylitol yield and productivity in pure xylose-based medium was 0.7 g/g of xylose and 0.379 g/L/h. In similar studies, xylitol yields of 0.81 and 0.868 g/g were produced by Candida tropicalis KCTC 7221 and C. tropicalis DSM7524 respectively from pure xylose-based media. These elevated yields could be due to the controlled pH and aeration conditions maintained in different capacity fermenters which efficiently supported the yeast growth and provided appropriate environment for xylitol production [16, 50].
The behaviour of most of the yeasts is typically affected by the presence of minute quantities of inhibitors in the hydrolysate. Sometimes the growth is affected, or else the rate of xylitol production is altered. The A. donax concentrated hydrolysate medium used in this study contained 0.24 g/L furfural and 5.09 g/L of acetic acid. When C. tropicalis was grown in this medium, it displayed relatively slower consumption of xylose as well as decreased production of xylitol (Fig. 5b). In the first 12 h of incubation, complete glucose was consumed, but only 2.4 g/L of xylose was utilized. Thenceforth, xylose consumption increased steadily in subsequent samples, but it did not match the rate of consumption in the commercial xylose-based medium. The maximum xylitol titre obtained at the end of fermentation batch was 26.3 g/L by consuming 48.83 g/L xylose presenting a yield of 0.54 g/g and productivity of 0.274 g/L/h. The growth and multiplication of yeast cells in hydrolysate medium were also lesser than in pure xylose-based medium producing a maximum cell dry weight of 10.21 g/L after 96 h of incubation unlike 12.82 g/L in the pure xylose-based medium. In a similar work on xylitol production, Kluyveromyces marxianus CCA510 produced 0.5 g/g xylitol by using xylose derived from cashew apple bagasse hydrolysate detoxified by 3% activated charcoal [51]. Similarly, Candida guilliermondii FTI was reported to produce 0.55 g/g xylitol yield in sugarcane bagasse hydrolysate with a volumetric productivity of 0.31 g/L/h [52]. Debaryomyces hansenii was reported to produce a xylitol yield of 0.39 g/g in detoxified corncob hydrolysate with 0.236 g/L/h productivity in a 50 L fermenter [53]. In another study, the effect of oxygen transfer volumetric coefficient on xylitol production from sugarcane bagasse hydrolysate in a stirred tank bioreactor was evaluated. It was concluded that a maximum xylitol yield and productivity of 0.58 g/g and 0.69 g/L/h, respectively, was obtained by increasing the oxygen transfer volumetric coefficient from 10 to 20/h [54]. In another investigation, the xylitol yield from detoxified and non-detoxified sugarcane bagasse hydrolysate by Pichia fermentans was 0.67 and 0.54 g/g, respectively [55]. Even though the fermentation was carried out in controlled conditions in a 2.5 L bioreactor, the effect of toxic inhibitors on overall xylitol yield could not be evaded.
The relatively low xylitol yield obtained in A. donax hydrolysate-based medium in comparison with the pure xylose-based medium might be due to the presence of trace quantities of furfural and acetic acid which hampered the yeast growth and interfered in xylitol production [56]. Detoxification of dilute acid hydrolysate with 4% activated charcoal did not completely remove furfural due to the presence of high concentrations of phenolics and coloured phytoconstituents These components occupy the adsorption sites of activated charcoal more quickly and reduce its overall efficiency to remove toxic inhibitors. Moreover, the concentration of acetic acid which is a potent yeast inhibitor is not significantly affected by activated charcoal-based detoxification. Another reason behind the low yield in the hydrolysate medium may be the higher concentration of glucose in the medium. Glucose supports cell growth and is required for generating intermediary metabolites for efficient conversion of xylose to xylitol, however, more than 1: 10 glucose to xylose ratio interferes in xylitol production by obstructing the xylose transport into the cell and inhibits the activity of xylose reductase [55,56,57].
Xylitol production from the hydrolysates of various lignocellulosic materials by different strains of C. tropicalis has been compared in (Table 4). It is clearly evident that the xylitol yield obtained in the detoxified hydrolysate-based medium in the present study is comparable to the yields obtained from different biomasses by using different strains of C. tropicalis. A. donax, although being investigated for several industrial chemicals, has not been widely studied as a substrate for the biological production of xylitol. The findings of the present study provide a foundation to further explore the hemicellulosic fraction of this plant to produce xylitol.
Ethanol Production by C. Tropicalis
The most commonly employed yeast for bioethanol production is S. cerevisiae, which is only capable of utilizing glucose for its growth and multiplication. It lacks the xylose reductase enzyme necessary for the entry of xylose into the metabolic pathway. On the other hand, C. tropicalis due to the presence of xylose reductase enzyme is fully efficient in metabolizing glucose as well as xylose. The second major objective of this study was to evaluate the efficiency of C. tropicalis to produce ethanol from the saccharified broth of A. donax. The maximum possible yield and productivity of ethanol by C. tropicalis was first evaluated in the pure glucose-based medium. It was observed that more than 90% of glucose in the medium was consumed by C. tropicalis within 24 h of incubation at 30 °C and 150 RPM (Fig. 6a). A maximum ethanol titre of 24.4 g/L was observed after 36 h of incubation after which the concentration of ethanol started declining. The cell dry weight of C. tropicalis also reached a maximum value of 9.74 g/L after 36 h of incubation. The calculated yield and productivity of ethanol in pure glucose-based medium were 0.49 g/g of glucose and 0.677 g/L/h. This corresponds to a theoretical ethanol yield of 96.62%.
Subsequently, the concentrated saccharified broth of A. donax was used to study its ability to compete with the pure glucose-based medium. C. tropicalis displayed a similar trend in the saccharified broth with some minor alterations. In the first 24 h of incubation, 86.39% of the initially present glucose was consumed producing an ethanol titre of 18.44 g/L (Fig. 6b). The maximum ethanol concentration of 22.68 g/L was obtained after 36 h of incubation by utilizing 48.06 g/L of glucose giving an ethanol yield and productivity of 0.47 g/g of glucose and 0.63 g/L/h. This corresponded to a theoretical yield of 92.53% by C. tropicalis. The cell dry weight of C. tropicalis reached a maximum concentration of 9.45 g/L after 36 h of incubation and slightly declined in the next 12 h. This decrease could be due to the exhaustion of the sole carbon source in the medium which made actively metabolizing yeast cells to starve. The performance of C. tropicalis in the saccharified broth of A. donax was found to be similar to that in pure glucose medium. C. tropicalis was also previously employed to produce ethanol from A. donax biomass, where under the optimized conditions of fermentation, 24.78 g/L of ethanol was produced within 24 h of incubation presenting a yield of 0.45 g/g [61]. Here, the time taken for fermentation was shorter in comparison with the present study but the final yield is lower. The reason behind the rapid conversion of glucose to ethanol could be the engagement of a bioreactor with controlled conditions of pH and aeration which significantly affect the overall yield of the bioprocess. In a similar study using C. tropicalis, separate hydrolysis and fermentation of pretreated sugarcane bagasse produced 55.64 g/L ethanol titre corresponding to an ethanol yield of 0.48 g/g [62]. C. tropicalis has also been investigated to produce ethanol from water hyacinth hydrolysed by a combination of fungal and sulphuric acid pretreatment. The maximum ethanol titre observed was 14 g/L after 42 h of incubation [63]. The authors suggested that Aspergillus terreus F-98-based hydrolysis followed by dilute acid pretreatment was highly effective for total reducing sugar extraction, however, the concentration of glucose destined for ethanol production was not very high which resulted in lower ethanol yields. In another study, C. tropicalis ATCC 13,803 was used for ethanol production from rice straw. The adapted yeast cells were employed in simultaneous saccharification and co-fermentation that produced 0.36 g/g ethanol yield with a volumetric productivity of 0.57 g/L/h within 36 h of incubation [64]. The ethanol yield by C. tropicalis in A. donax saccharified broth is higher than or comparable to the already reported studies. Although A. donax is an extensively researched energy crop due to its rapid growth and high cellulose content, C. tropicalis is still an under-examined yeast species. The present investigation advocates further experimentation over the use of C. tropicalis in place of conventional yeast for large-scale bioethanol production. This could lay a foundation for simultaneous or separate production of ethanol and xylitol from two different sugar fractions of lignocellulosic materials by a single yeast culture. Since acid hydrolysis of biomass produced side products in addition to sugar, so lower yield of xylitol in comparison to synthetic medium than contained pure sugar. Also, enzymatic hydrolysis produced pure sugar without side products and hence higher xylitol yield.
Conclusion
A. donax has a holocellulose-rich composition, which makes it a suitable candidate for exploration as a substrate for the production of value-added chemicals. In the present study, sequential dilute sulphuric acid and sodium hydroxide pretreatment was employed for the liberation of xylose and maximum removal of lignin from A. donax biomass, respectively. A total of 93.52% cellulose was recovered following alkali pretreatment which was enzymatically hydrolysed to produce high titres of glucose for subsequent ethanol fermentation. C. tropicalis produced a xylitol yield of 0.54 g/g in the detoxified dilute-acid hydrolysate-based medium. The same yeast, when grown in saccharified broth-based medium, produced an ethanol yield of 0.47 g/g within 48 h of incubation. The present study advocates the use of sequential dilute acid and alkali pretreatment of A. donax biomass for the maximum utilization of its holocellulose content as a suitable feedstock for xylitol and ethanol production. C. tropicalis has been characterized for xylitol production from biomass and could be a potential candidate for industrial production of xylitol.
Data availability
All the data is included in the article.
References
Okolie, J.A., Nanda, S., Dalai, A.K., Kozinski, J.: Chemistry and specialty industrial applications of lignocellulosic biomass. Waste Biomass Valor. 12, 2145–2169 (2021)
Dasgupta, D., Sidana, A.: Biorefinery involving terrestrial and marine lignocellulosics: concept, potential, and current status, In: S. Sahay (Ed.), Handbook of Biofuels, Academic Press, pp. 167–188 (2022)
Danelli, T., Laura, M., Savona, M., Landoni, M., Adani, F., Pilu, R.: Genetic improvement of Arundo donax L.: Opportunities and challenges. Plants. 9, 1584 (2000)
Licursi, D., Antonetti, C., Mattonai, M., Pérez-Armada, L., Rivas, S., Ribechini, E., Galletti, A.M.R.: Multi-valorisation of giant reed (Arundo donax L.) to give levulinic acid and valuable phenolic antioxidants. Ind. Crops Prod. 112, 6–17 (2018)
Fiore, V., Piperopoulos, E., Calabrese, L.: Assessment of Arundo donax fibers for oil spill recovery applications. Fibers. 7, 75 (2019)
Suárez, L., Castellano, J., Romero, F., Marrero, M.D., Benítez, A.N., Ortega, Z.: Environmental hazards of giant reed (Arundo donax L.) in the Macaronesia region and its characterisation as a potential source for the production of natural fiber composites. Polym. (Basel). 13(13), 2101 (2021)
Shatalov, A.A., Pereira, H.: High-grade sulfur-free cellulose fibers by pre-hydrolysis and ethanol-alkali delignification of giant reed (Arundo donax L.) stems. Ind. Crops Prod. 43(1), 623–630 (2013)
Qin, Z., Zhuang, Q., Zhu, X., Cai, X., Zhang, X.: Carbon consequences and agricultural implications of growing biofuels crops on marginal agricultural lands in China. Environ. Sci. Technol. 45, 10765–10772 (2011)
Galletti, A.M.R., Antonetti, C., Ribechini, E., Colombini, M.P., Di Nasso, N.N.O., Bonari, E.: From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Appl. Energ. 102, 157–162 (2013)
Bartoli, M., Rosi, L., Giovannelli, A., Frediani, P., Frediani, M.: Production of bio-oils and bio-char from Arundo donax through microwave-assisted pyrolysis in a multimode batch reactor. Anal. Appl. Pyrol. 122, 479–489 (2016)
Hernández-Pérez, A.F., de Arruda, P.V., Sene, L., da Silva, S.S., Chandel, A.K., de Almeida Felipe, M.D.G.: Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Crit. Rev. Biotechnol. 39, 924–943 (2019)
Kumar, V., Krishania, M., Sandhu, P.P., Ahluwalia, V., Gnansounou, E., Sangwan, R.S.: Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by Candida tropicalis MTCC 6192. Bioresour Technol. 251, 416–419 (2018)
Prakash, G., Varma, A.J., Prabhune, A., Shouche, Y., Rao, M.: Microbial production of xylitol from d-xylose and sugarcane bagasse hemicellulose using newly isolated thermotolerant yeast Debaryomyces hansenii. Bioresour Technol. 102(3), 3304–3308 (2011)
Jia, H., Shao, T., Zhong, C., Li, H., Jiang, M., Zhou, H., Wei, P.: Evaluation of xylitol production using corncob hemicellulosic hydrolysate by combining tetrabutylammonium hydroxide extraction with dilute acid hydrolysis. Carbohydr. Polym. 151, 676–683 (2016)
Kaur, S., Guleria, P., Sidana, A., Yadav, S.K.: Efficient process for xylitol production from nitric acid pretreated rice straw derived pentosans by Candida tropicalis GS18. Biomass Bioenerg. 166, 106618 (2022)
Tamburini, E., Costa, S., Marchetti, M.G., Pedrini, P.: Optimized production of xylitol from xylose using a hyper-acidophilic Candida tropicalis. Biomolecules. 5(3), 1979–1989 (2015)
Yewale, T., Panchwagh, S., Sawale, S., Jain, R., Dhamole, P.B.: Xylitol production from non-detoxified and non-sterile lignocellulosic hydrolysate using low-cost industrial media components. 3 Biotech. 7, 68 (2017)
Robak, K., Balcerek, M.: Review of second-generation bioethanol production from residual biomass. Food Technol. Biotechnol. 56(2), 174–187 (2018)
Global ethanol market to reach $105.2: (2025). https://www.prnewswire.com/news-releases/global-ethanol-market-to-reach-105-2-billion-by-2025--301510584.html. (Last accessed on 27-12-2023)
Kim, H., Lee, H.S., Park, H., Lee, D.H., Boles, E., Chung, D., Park, Y.C.: Enhanced production of xylitol from xylose by expression of Bacillus subtilis arabinose: H + symporter and Scheffersomyces stipitis xylose reductase in recombinant Saccharomyces cerevisiae. Enzyme Microb. Technol. 107, 7–14 (2017)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. (2012)
Segal, L., Creely, J.J., Conrad, M. Jr.: An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 29, 786–794 (1959)
Ainsworth, E.A., Gillespie, K.M.: Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2(4), 875–877 (2007)
Silva, C.F.L., Schirmer, M.A., Maeda, R.N., Barcelos, C.A., Pereira, N.: Potential of giant reed (Arundo donax L.) for second generation ethanol production. Electron. J. Biotechnol. 18(1), 10–15 (2015)
Scordia, D., Cosentino, S.L., Jeffries, T.W.: Enzymatic hydrolysis, simultaneous saccharification and ethanol fermentation of oxalic acid pretreated giant reed (Arundo donax L.). Ind. Crops Prod. 49, 392–399 (2013)
Lemões, J.S., e Silva, C.F.L., Avila, S.P.F., Montero, C.R.S., e Silva, S.D.A., Samios, D., Peralba, M.C.R.: Chemical pretreatment of Arundo donax L. for second-generation ethanol production. Electron. J. Biotechnol. 31, 67–74 (2018)
Talebnia, F., Karakashev, D., Angelidaki, I.: Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour Technol. 101, 4744–4753 (2010)
Shatalov, A.A., Pereira, H.: Xylose production from giant reed (Arundo donax L.): Modeling and optimization of dilute acid hydrolysis. Carbohydr. Polym. 87(1), 210–217 (2012)
Camargo, D., Sene, L., Variz, D.I.L.S., Felipe, M.G.A.: Xylitol Bioproduction in Hemicellulosic Hydrolysate obtained from Sorghum Forage Biomass. Appl. Biochem. Biotechnol. 175, 3628–3642 (2015)
Shankar, K., Kulkarni, N.S., Sajjanshetty, R., Jayalakshmi, S.K., Sreeramulu, K.: Co-production of xylitol and ethanol by the fermentation of the lignocellulosic hydrolysates of banana and water hyacinth leaves by individual yeast strains. Ind. Crops Prod. 155, 112809 (2020)
Baruah, J., Nath, B.K., Sharma, R., Kumar, S., Deka, R.C., Baruah, D.C., Kalita, E.: Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 6, 144 (2018)
Keshwani, D.R., Cheng, J.J.: Microwave-based alkali pretreatment of switchgrass and coastal Bermuda grass for bioethanol production. Biotechnol. Progr. 26, 644–652 (2009)
Saynik, P.B., Moholkar, V.S.: Insight into chemical pretreatment of hardwood (Arundo donax) for improvement of pyrolysis. Bioresour Technol. Rep. 11, 100545 (2020)
Komolwanich, T., Tatijarern, P., Prasertwasu, S., Khumsupan, D., Chaisuwan, T., Luengnaruemitchai, A., Sujitra, W.: Comparative potentiality of Kans grass (Saccharum spontaneum) and giant reed (Arundo donax) as lignocellulosic feedstocks for the release of monomeric sugars by microwave/chemical pretreatment. Cellulose. 21, 1327–1340 (2014)
Vasmara, C., Cianchetta, S., Marchetti, R., Ceotto, E., Galletti, S.: Potassium hydroxide pre-treatment enhances methane yield from Giant Reed (Arundo donax L). Energies. 14(3), 630 (2021)
Jiang, D., Ge, X., Zhang, Q., Li, Y.: Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour Technol. 216, 60–68 (2016)
Lee, J.W., Kim, J.Y., Jang, H.M., Lee, M.W., Park, J.M.: Sequential dilute acid and alkali pretreatment of corn stover: Sugar recovery efficiency and structural characterization. Bioresour Technol. 182, 296–301 (2015)
Loow, Y.L., Wu, T.Y., Jahim, J.M., Mohammad, A.W., Teoh, W.H.: Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose. 23, 1491–1520 (2016)
Lizasoain, J., Rincón, M., Theuretzbacher, F., Enguídanos, R., Nielsen, P.J., Potthast, A., Zweckmair, T., Gronauer, A., Bauer, A.: Biogas production from reed biomass: Effect of pretreatment using different steam explosion conditions. Biomass Bioenerg. 95, 84–91 (2016)
Kumabe, K., Hanaoka, T., Fujimoto, S., Minowa, T., Sakanishi, K.: Co-gasification of woody biomass and coal with air and steam. Fuel. 86, 684–689 (2007)
Cheng, G., Varanasi, P., Li, C., Liu, H., Melnichenko, Y.B., Simmons, B.A., Kent, M.S., Singh, S.: Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis. Biomacromolecules. 12(4), 933–941 (2011)
Gao, C., Xiao, W., Ji, G., Zhang, Y., Cao, Y., Han, L.: Regularity and mechanism of wheat straw properties change in ball milling process at cellular scale. Bioresour Technol. 241, 214–219 (2017)
Bano, S., Negi, Y.S.: Studies on cellulose nanocrystals isolated from groundnut shells. Carbohydr. Polym. 157, 1041–1049 (2017)
Peces, M., Astals, S., Mata-Alvarez, J.: Effect of moisture on pretreatment efficiency for anaerobic digestion of lignocellulosic substrates. Waste Manage. 46, 189–196 (2015)
Muthuvelu, K.S., Rajarathinam, R., Kanagaraj, L.P., Ranganathan, R.V., Dhanasekaran, K., Manickam, N.K.: Evaluation and characterization of novel sources of sustainable lignocellulosic residues for bioethanol production using ultrasound-assisted alkaline pre-treatment. Waste Manage. 87, 368–374 (2019)
Kim, I., Lee, I., Jeon, S.H., Hwang, T., Han, J.I.: Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour Technol. 192, 335–339 (2015)
Mikulski, D., Kłosowski, G.: Hydrotropic pretreatment on distillery stillage for efficient cellulosic ethanol production. Bioresour Technol. 300, 12266 (2020)
Mussatto, S.I., Roberto, I.C.: Xylitol production from high xylose concentration: Evaluation of the fermentation in bioreactor under different stirring rates. J. Appl. Microbiol. 95, 331–337 (2003)
Pal, S., Choudhary, V., Kumar, A., Biswas, D., Mondal, A.K., Sahoo, D.K.: Studies on xylitol production by metabolic pathway engineered Debaryomyces hansenii. Bioresour Technol. 147, 449–455 (2013)
Kim, T.B., Lee, Y.J., Kim, P., Kim, C.S., Oh, D.K.: Increased xylitol production rate during long-term cell recycles fermentation of Candida tropicalis. Biotechnol. Lett. 26, 623–627 (2004)
de Albuquerque, T.L., Gomes, S.D.L., Marques, J.E. Jr., da Silva, I.J. Jr., Rocha, M.V.P.: Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal. Today. 255, 33–40 (2015)
de Arruda, P.V., do Santos, J.C., Rodrigues, R.C.L.B., da Silva, D.D.V., Yamakawa, C.K., Rocha, G.J.M., Nolasco, J. Jr., Pradella, J.G.C., Rossell, C.E.V., Felipe, M.G.A.: Scale-up of xylitol production from sugarcane bagasse hemicellulosic hydrolysate by Candida guilliermondii FTI 20037. Ind. Eng. Chem. Res. 47, 297–302 (2017)
Vazquez, G.B., Perez-Rodriguez, N., Salgado, J.M., Oliveira, R.P.S., Dominguez, J.M.: Optimization of salts supplementation on xylitol production by Debaryomyces hansenii using a synthetic medium or corncob hemicellulosic hydrolyzates and further scaled up. Ind. Eng. Chem. Res. 56, 6579–6589 (2017)
Martínez, E.A., Silva, S.S., Felipe, M.G.A.: Effect of the oxygen transfer coefficient on xylitol production from sugarcane bagasse hydrolysate by continuous stirred-tank reactor fermentation. Appl. Biochem. Biotechnol. 84, 633–641 (2000)
Narisetty, V., Castro, E., Durgapal, S., Coulon, F., Jacob, S., Kumar, D., Awasthi, M.K., Pant, K.K., Parameswaran, B., Kumar, V.: High-level of production by Pichia fermentans using non-detoxified xylose-rich sugarcane bagasse and olive pits hydrolysates. Bioresour Technol. 342, 126005 (2021)
Morita, T.A., Silva, S.S.: Inhibition of microbial xylitol production by acetic acid and its relation with fermentative parameters. Appl. Biochem. Biotechnol. 84–86, 801–808 (2000)
López-Linares, J.C., Ruiz, E., Romero, I., Castro, E., Manzanares, P.: Xylitol production from exhausted olive pomace by Candida boidinii. Appl. Sci. 10(19), 6966 (2020)
Rao, R.S., Jyothi, C.P., Prakasham, R.S., Sarma, P.N., Rao, L.V.: Xylitol production from corn fiber and sugarcane bagasse hydrolysates by Candida tropicalis. Bioresour Technol. 97(15), 1974–1978 (2006)
Ko, C.H., Chiang, P.N., Chiu, P.C., Liu, C.C., Yang, C.L., Shiau, I.L.: Integrated xylitol production by fermentation of hardwood wastes. J. Chem. Technol. Biotechnol. 540, 534–540 (2008)
Zahed, O., Jouzani, G.S., Abbasalizadeh, S., Khodaiyan, F., Tabatabaei, M.: Continuous co-production of ethanol and xylitol from rice straw hydrolysate in a membrane bioreactor. Folia Microbiol. (Praha). 61(3), 179–189 (2016)
Madian, H.R., Hamouda, H.I., Hosny, M.: Statistical optimization of bioethanol production from giant reed hydrolysate by Candida tropicalis using Taguchi design. J. Biotechnol. 360, 71–78 (2022)
Raj, K., Krishnan, C.: Improved co-production of ethanol and xylitol from low-temperature aqueous ammonia pretreated sugarcane bagasse using two-stage high solids enzymatic hydrolysis of Candida tropicalis. Renew. Energy. 153, 392–403 (2020)
Madian, H.R., Sidkey, N.M., Abo Elsoud, M.M., Hamouda, H.I., Elazzazy, A.M.: Bioethanol Production from water hyacinth hydrolysate by Candida tropicalis Y-26. Arab. J. Sci. Eng. 44, 33–41 (2019)
Oberoi, H.S., Vadlani, P.V., Brijwani, K., Bhargav, V.K., Patil, R.T.: Enhanced ethanol production via fermentation of rice straw with hydrolysate-adapted Candida tropicalis ATCC 13803. Process. Biochem. 45(8), 1299–1306 (2010)
Acknowledgements
The authors acknowledge the financial support and infrastructure provided by the Department of Biotechnology, Ministry of Science and Technology, India, to conduct this project. We also thank the Chief Executive Officer of CIAB for the continuous motivation and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sidana, A., Guleria, P. & Yadav, S.K. Selective Extraction of Second-Generation Sugars by Sequential Acid and Alkali Pretreatment of Arundo donax for Xylitol and Ethanol Production by Candida tropicalis. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02580-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02580-7