Abstract
Lignocellulosic biomasses are extensively used by researchers to produce a variety of renewable bioproducts. This research described an environment-friendly technique of xylitol production by an adapted strain of Candida tropicalis from areca nut hemicellulosic hydrolysate, produced through enzymatic hydrolysis. To enhance the activity of xylanase enzymes, lime and acid pretreatment was conducted to make biomass more amenable for saccharification. To improve the efficiency of enzymatic hydrolysis, saccharification parameters like xylanase enzyme loading were varied. Results exposed that the highest yield (g/g) of reducing sugar, about 90%, 83%, and 15%, were achieved for acid-treated husk (ATH), lime-treated husk (LTH), and raw husk (RH) at an enzyme loading of 15.0 IU/g. Hydrolysis was conducted at a substrate loading of 2% (w/V) at 30 °C, 100 rpm agitation, for 12 h hydrolysis time at pH 4.5 to 5.0. Subsequently, fermentation of xylose-rich hemicellulose hydrolysate was conducted with pentose utilizing the yeast Candida tropicalis to produce xylitol. The optimum concentration of xylitol was obtained at about 2.47 g/L, 3.83 g/L, and 5.88 g/L, with yields of approximately 71.02%, 76.78%, and 79.68% for raw fermentative hydrolysate (RFH), acid-treated fermentative hydrolysate (ATFH), and lime-treated fermentative gydrolysate (LTFH), respectively. Purification and crystallization were also conducted to separate xylitol crystals, followed by characterization like X-ray diffraction (XRD) and scanning electron microscopy (SEM) analysis. Results obtained from crystallization were auspicious, and about 85% pure xylitol crystal was obtained.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing sign demand for sugar substitutes throughout the world motivated researchers to quest for an alternative, sustainable, renewable way of sugar production. Xylitol is a renewal and eco-friendly bioproduct produced from lignocellulosic material [1, 2]. It is a poly-alcoholic sugar with the potential to replace the sugar needed for sweetening purposes partially. Because of its properties (such as insulin-dependent metabolism), this five-carbon sugar alcohol is mainly used in medicine, food, and other industries as a sugar substitute. Currently, xylitol is primarily produced through the chemical hydrogenation of xylose at high temperatures (150 °C) and pressure (5.5 MPa) in the presence of metal catalysts such as Pl, Ru, Pt, and Raney nickel. Separation and purification of xylitol are costly through this process. However, compared to this method, xylitol production through bioconversion of hemicellulosic hydrolysate by microorganisms is an environment-friendly, renewable, less energy-intensive, and overall cost-effective process. This process ensures high safety, low production cost, and high product selectivity [3].
Lignocellulosic biomass is one of the most abundantly available feedstocks for producing biochemical and bioenergy. Its unique property (such as renewal, economical and eco-friendly) makes these feedstocks different from others. The methodology involved in producing xylitol from these biomasses is very economical compared to technologies involved in chemical means. Arecanut husk is one of the lignocellulosic materials that have attracted scientists in India as potential sources for lignocellulosic xylose production for bioconversion into xylitol. India is one of the world’s largest producers of areca nut husk; according to the 2015–2016 statistical report, the total production of areca nut was about 735,860 tons in India, with a corresponding yield of 1558 kg/hectare. Between 2017 and 2018, India exported 5045.6 tons of betel nut, worth 110.8 crores (about US $17.2 million), to other countries [4, 5]. However, accumulating these husks can cause serious environmental problems for producers. The potential use of these residues to produce value-added products such as enzymes, alcohol, and xylitol can overcome these problems [3, 6]. The husk is mainly the outer part (fiber part) of the fruit (nuts) and accounts for about 60–80% volume of the nut and about 15 to 30% of the weight of the nut. The concentration of hemicellulose depends on the maturity of the fruit.
As it is well known, the production of xylitol from lignocellulosic consists of four steps: biomass pretreatment, enzymatic hydrolysis (saccharification), fermentation, and product purification [7]. Bio-waste, such as areca nut husk, is a lignocellulosic material composed of three main components: cellulose, hemicellulose, and lignin. These constituents are arranged in a complex way and have a compact structure, which resists digestion and decomposition by cellulolytic and hemicellulose-degrading enzymes. Therefore, pretreatment is the prerequisite step before the enzymatic hydrolysis to produce sugars that can be used for xylitol fermentation [7]. In any biological conversion of lignocellulosic material, pretreatment is one of the most vital and challenging steps. An ideal pretreatment technique results in minimum loss of fermentable sugar with higher elimination of lignin content [8].
Enzymatic hydrolysis is the second step in producing xylitol from lignocellulosic feedstocks (biomass). Enzymatic hydrolysis mainly de-polymerizes hemicellulose and cellulose into pentose and hexose sugars [9]. Generally, enzymatic hydrolysis is carried out in a temperature range of 30 to 50 °C at low pressure and a long retention time in connection with hemicellulose hydrolysis. The saccharification efficiency of lignocellulosic biomass can be improved by operating the hydrolysis at optimized conditions [10]. Operation of enzymatic hydrolysis at optimized conditions results from the minimal formation of fermentation inhibitors such as furfural and hydroxymethyl furfural.
Various fungal, bacterial, and yeast strains are commonly used for xylitol production, most of which come from the Candida genus. These include Candida boidinii, Candida tropicalis, Candida guilliermondii, Debaryomyces hansenii, and Pachysolen tannophilus, which have the efficient capability to produce xylitol by fermentation. Among all the strains of yeast strains, C. tropicalis is considered inhibitor tolerant. Pentose sugar can be utilized by yeast strains (C. tropicalis) to produce xylitol from hemicellulosic hydrolysate of agricultural wastes [11, 12]. It has been observed that C. tropicalis is a widely acceptable microorganism for the production of xylitol with good volumetric productivity and high yield from xylose-containing hydrolysate [13]. Xylitol production through natural pentose-fermenting microorganisms, primarily yeast (biotechnological routes), is mainly based on xylose metabolism. Firstly, the reduction of xylose into xylitol takes place by NAD (P) H-dependent XR and xylitol oxidation to xylulose catalyzed by NADþ (nicotinamide_adenine-dinucleotide)-depending XDH [14]. Separation and product purification are the final steps in xylitol production. Xylitol recovery techniques such as membrane separation, chromatographic methods, and crystallization were mainly applied to xylitol solutions obtained from fermented media or chemical means.
In the present research work, agricultural residue such as Arecanut husk was used as a lignocellulosic biomass source and was pretreated using lime and dilute acid. The fate of the husk after two different modes of pretreatment was then used for enzymatic hydrolysis for the production of xylose. Finally, xylitol production was conducted through a biotechnological route by the yeast strain Candida tropicalis, followed by product purification.
Materials and Method
Materials
Areca nuts were procured from the local market of Guwahati city, India. Analytical grade chemicals were procured from Sigma Aldrich for the analysis in the present study. Accuracy was maintained in the present study by performing the experiments in duplicate.
Pretreatment Method
Areca nuts were initially washed with running water to remove extraneous matter, followed by sun drying. The straw was oven dried at 60 °C overnight, and the dried husk was crushed using a grinder and sieved (mesh size-BSS 30) to obtain husk powder with a maximal size of 1 − 5 mm. Lime and dilute acid (H2SO4) pretreatment was conducted per the previously discussed protocol [15]. The processed raw husk (RH), lime-treated husk (LTH), and acid-treated husk (ATH) were stored at room temperature in an air-tight plastic bag for further use. The cellulose, hemicellulose, and lignin contents of RH, LTH, and ATH feedstock were determined per the methodology (standard laboratory protocols) described previously [9].
Enzymes and Enzyme Activity Assays
Commercial xylanase enzyme (Trichoderma viride) was used for the present study and procured from Sigma Aldrich, India. Commercially available xylanase enzyme activity was measured using CMC (carboxy methyl cellulose) or birch-wood xylan as substrates for xylanase and cellulase activity, respectively. The di-nitro salicylic acid (DNS) method was used to monitor the rate of polymer hydrolysis, which was measured by estimating the reducing sugars produced by enzymatic activity at 540 nm [11].
Enzymatic Hydrolysis.
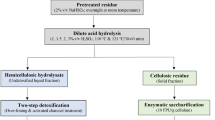
Hydrolysis was conducted as per the diagram depicted in Fig. 1 by mixing 2% (w/v) raw (RH) and treated (LTH and ATH) solid biomass sample with sodium citrate buffer (pH about 4.8) solution. Varying concentration of xylanase enzyme was loaded (2.5 IU/g, 5.0 IU/g, 10.0 IU/g, and 15.0 IU/g). All reactions were performed between pH 4.5 and 5.0 at 30 °C for 12 h at 150 rpm. At every desired period, the sample solution was taken and kept at 80 °C for 10 min before use for further analysis. REH (raw enzymatic hydrolysate), LTEH (lime-treated enzymatic hydrolysate), and ATEH (acid-treated enzymatic hydrolysate) were obtained after saccharification. This hydrolysis analysis was performed in duplicate. The xylose yield was calculated with the help of Eq. (1) as follows:
Microorganism and Inoculum Preparation
Candida tropicalis (MTCC 6192) culture was procured from Microbial Type Culture Collection and GeneBank (MTCC), India, and maintained at 4 °C on YPD (yeast extract, peptone, and dextrose) agar medium. Microorganisms were subcultured every 15 days. The composition of the media used in this study was as follows: yeast extract 10.0 (g/L), peptone 20.0 (g/L), and dextrose anhydrous (glucose) 20.0 (g/L); 1N HCL solution was used to maintain the pH of the medium at about 5.0. The cell culture process was conducted in a 250 mL Erlenmeyer flask (50 mL of medium) on a rotary platform shaker for 48 h at 30 °C with 150 rpm.
Cell Dry Weight Measurements
Dry weight measurements were made by pipetting 5 mL of a well-mixed broth sample into a dry centrifuge tube. Cells from the broth were separated by performing centrifugation for 5 min at 10,000 g. Separate the cell paste from the broth by carefully scrapping the clear broth. Subsequently, separate the cell pastes from the centrifuge tube and keep them in a weighing pan. Dry the cell paste overnight in an oven at 60 °C. Calculation of dry cell weight was performed by using the following Eq. (2).
where CW3 is the weight of the sample including the blank tube, CW2 is the weight of the blank tube, and V1 is the volume of the culture sample.
Microbial Fermentation
Xylitol production was carried out by preparing fermentative media (as shown in Fig. 1) from REH, LTEH, ATEH, and SSFH (synthetic solution fermentative hydrolysate) having an initial xylose concentration (g/L) of about 2.5, 5.9, 3.8, and 7.0, respectively. Xylose concentration acted as the main sugar substrate in the hydrolysate and was supplemented with yeast extract, 10 g/L; (NH4)2SO4, 5 g/L; KH2PO4, 15 g/L; and MgSO4·7H2O, 1 g/L. The pH of the fermentative medium was kept between 4.5 to 5.0. About 4.0% (v/v) yeast strain cultures were transferred (inoculated) into the fermentation medium prepared from different enzymatic hydrolysates [15]. Fermentation was carried out at 30 ± 1 °C for 90 h at 200 rpm. Samples were withdrawn every 6 h of time and stored in a cold place after centrifugation for 15 min, 10,000 rpm.
Fermented Broth Processing and Crystallization
After completion of the fermentation process, the xylitol-rich-fermented solution was centrifuged at 2000 X g for 15 min using a centrifuge (model number: 216P, manufacturer: Sigma, Germany). The fermented broth was collected, treated with 3N NaOH to pH 7.00, and filtered through a 0.5 µm filter (Whatman no. 41). The xylitol-rich solution was further processed with AmberLite™ IRC120 cationic exchange resin and AmberLite™ IRA410 anionic exchange resins and followed by a concentration of xylitol level of about 150 g/L at 65 °C by using Rotavapor (Model No.: R 300, Make: Buchi, Switzerland).
Crystallization of Xylitol Syrup
The xylitol crystallization experiment was conducted, as shown in Fig. 18 and Fig. 19. Initially, the temperature of the xylitol-rich solution was increased above the saturation temperature by 10 °C and kept at this temperature for 5 min to allow the crystals to dissolve completely. The aliquot of the concentrated solution was then transferred to a glass petri dish (Fig. 18a and Fig. 19a), followed by the addition of cotton thread to favor the nucleation of crystals. Petri dishes were kept at room temperature for 3 days for crystal formation. Once crystallization was completed (Fig. 18b and Fig. 19b), the precipitated crystals were separated from the solution (mother liquor) by filtration using a 0.5 μm filter (Whatman No. 41) and washed with 100 mL of distilled water, followed by vacuum drying at room temperature for 24 h (Fig. 18c and Fig. 19c). Pre-weighed crystals were dissolved in water for measuring xylitol purity by using HPLC.
Fluorescence Microscopy
Candida cells were visualized under an Inverted Microscope with Fluorescence (Nikon, Ti-S with a camera, model: DS-Fi2-U3). The stock solution of methylene blue (MB) was prepared for fluorescent labeling. The prepared solution was diluted twofold, followed by filtration by passing through a 0.22 µm membrane filter (MILLEX GP 0.22 µm; Merck Millipore Ltd., India). A 2.5 µL sample volume was pipetted onto a glass slide (VWR, India) and mixed with a 2.5 µL filtered MB solution to a final concentration of 25 µg/mL. Imaging was performed for RFH (raw fermentative hydrolysate), LTFH (lime-treated fermentative hydrolysate), ATFH (acid-treated fermentative hydrolysate), and SSFH (synthetic solution fermentative hydrolysate) samples under constant laser irradiation.
High-performance Liquid Chromatography Analysis
Identification and quantitative measurement of various components such as glucose, xylose, and xylitol were performed by high-performance liquid chromatography (HPLC) using an AMINEX ion exchange column (BioRad, HPX87C, Richmond) and a refractometer (BioRad, 1770, Richmond). A 5 mM sulfuric acid (H2SO4) aqueous solution was used for the analysis as a mobile phase media. The analysis was performed on 0.6 mL min−1 flow rate at 60 °C.
FTIR Analysis
FTIR spectrum of REH, LTE, and ATEH was examined using an FTIR analyzer (model: Perkin Elmer Spectrum). Samples were sonicated before the analysis by a 2 mm probe coupled to a VC 505 ultrasonic Sonicator (model no.: 3.5 L/00H/DTC, Make: PCi, Mumbai) for 10 min. Sonicated samples containing 10 μL of the sample suspension were placed on IR-light transparent silicon plates of the FTIR instrument. All the samples were analyzed in the scanning range of 4000 to 500 cm−1.
X-ray Diffraction Analysis
X-ray diffraction (XRD) studies of xylitol crystal were performed in broad angle X-ray diffractometer (9KW Powder X-Ray Diffraction System, Make: Rigaku Technologies, JAPAN, Model: Smartlab). Samples FXC and XCCX were examined under plateau conditions by placing samples in a sample holder. X-ray radiation was generated by passing 40 mA current and 40 kV voltage through the instrument. The scanning spectrum was fixed in the range of 7–40° with a residence time of 1 s/0.02° and step size of 0.02°. Figure 20 shows the XRD analysis of FXC and XCCX, and the crystallinity index (CrI) of the crystals was determined by using the following Eq. (3).
where Iam is the area of all amorphous peaks and Icr is the area of all crystalline peaks.
Field Emission Scanning Electron Microscopy Analysis
The shape and surface topography of raw, pretreated, and saccharified biomass were analyzed by field emission scanning electron microscope (Gemini 500 FESEM). The device was equipped with a LaB6 field emission electron gun and three types of detectors: InLens, SE2, and ESB. Analysis was conducted by fixing the sample on an aluminum stub (G301, Agar Scientific, UK) through two-way carbon tape (G3347N, Agar Scientific, UK). The electrical conductivity of the sample surface was increased by spraying the chromium under an argon vacuum with the help of a sputter coater (Edwards S 150B sputter coater, layer thickness 12 nm). This analysis was conducted with a probe current of 10 mA and an acceleration voltage of 5 kV. Different magnifications were used for taking the photographs under the utmost conditions.
Results and Discussion
Biomass Pretreatment and Compositional Analysis
The structure of lignocellulosic biomass is very compact and dense, which is difficult to hydrolyze. Hemicellulose molecules are covalently bonded with lignin-cellulose [12]. The hydrolysis process gets intervened due to the formation of a mesh-like structure. Pretreatment of lignocellulosic biomass greatly affects the efficiency of saccharification, and the efficiency of the saccharification process indirectly depends on the constituents of areca nut husk such as cellulose, hemicellulose, and lignin content. Therefore, compositional analysis of raw and pretreated feedstock husk was performed, and the respective composition was reported in Table 1. The initial composition of raw husk is about 43.28% of cellulose, 29.17% of hemicellulose, and 12.64% of lignin. But, pretreatment causes to change in the concentration of cellulose, hemicellulose, and lignin. A common pattern in compositional changes was observed that there is a decrease in hemicellulose content while an increase in the concentration of cellulose and lignin. Alkaline (lime) pretreatment causes about 4.5% and 12.82% increase in the concentration of cellulose and lignin while a 19.43% decrease in the concentration of hemicellulose as compared to the raw husk. Whereas, in the case of acid pretreatment, about a 16.28% and 36.16% increase in the concentration of cellulose and lignin was observed, while a 61.6% decrease in the concentration of hemicellulose was observed as compared to the raw husk. The change in the composition of feedstock during pretreatment is most probably due to the removal of nonstructural aromatic compounds such as chlorophyll, volatile oils, fatty acids, and their esters (tannins, terpenes, waxes, and resins) [16]. The occurrence of about 29.17% and 23.50% hemicellulose fraction (calculated on the dry weight basis of the substrate) in RH and LTH makes this biomass a renewable and potential substrate for xylose production which was further used for the production of xylitol and other value-added chemicals. But, in the present study, the content of hemicellulose is less (about 11.2%) in ATH as compared to RH and LTH. Although, it was used as a substrate for enzymatic hydrolysis because of to investigate the change of structural properties of the areca nut husk in order to clarify how any enhancement in the xylose yield after enzymatic hydrolysis is affected by the various pretreatment techniques. A compositional analysis reported in the present study agrees with other researcher investigation reports [17]. However, the contents of cellulose, hemicellulose, and lignin were different in the present study. The compositional discrepancy of feedstock may be due to variations in geographic location, season, and processing methodology used for biomass [2].
Enzymatic Hydrolysis of Husk
After completion of the deliinification process, the pretreated husk was further used for enzymatic hydrolysis, which is intended to release monosugars after the completion of the saccharification process. Reducing sugars obtained during enzymatic hydrolysis of RH, LTH, and ATH feedstocks exposed to various doses of xylanase enzyme load were exposed in Fig. 2, Fig. 3, Fig. 4, and Fig. 5. LTH and ATH were more efficient to enzymatic hydrolysis as compared to RH when exposed to various doses of enzyme loading. From Fig. 2, it was observed that the concentrations of xylose (g/g) for RH, LTH, and ATH were 0.034, 0.089, and 0.049, respectively, for 2.0 IU/g of biomass loading. Xylose concentration is directly proportional to xylanase enzyme loading; therefore, an increase in xylose concentration was observed with enzyme loading from 2.0 to 15.0 IU/g. The maximum xylose (g/g) release was found to be 0.052, 0.212, and 0.110 for RH, LTH, and ATH, respectively, for 15.0 IU/g of biomass loading at 30 °C, 12 h. However, corresponding yields of RH, LTH, and ATH were shown in Fig. 6, Fig. 7, Fig. 8, and Fig. 9 for enzyme loading of 2.0 IU/g, 5.0 IU/g, 10.0 IU/g, and 15.0 IU/g, respectively. From Fig. 6, it was observed that yields of reducing sugar for RW, LTH, and ATH were 10.11%, 35.31%, and 39.87%, respectively, for 2 IU/g of biomass loading. But, with the increase in enzyme loading from 2.0 to 15.0 IU/g, it was observed that yield of corresponding feedstocks also increases, which is about 15.43%, 83.16%, and 90.08%, respectively, for RW, LTH, and ATH. This analysis revealed that yield was significantly increased by increasing the concentration of enzyme during saccharification and it also highlights the role of pretreatment before enzymatic hydrolysis.
Enzymatic hydrolysis was positively affected by the acidic and alkaline pretreatment of biomass. This may be as a result of structural modification in the lignocellulosic polymer lattice due to huge energy irradiation. In this analysis, saccharification results revealed that pretreated biomass showed a high xylose yield for ATH as compared to LTH and RH. This concludes that ATH has higher xylose production efficiency compared to LTH and RH. This might be because, during diluted H2SO4 pretreatment, structural changes of the areca nut husk occurred due to complete disruption of the crystalline hemicellulose. This causes enzymes to easily hydrolyzed the feedstocks [5]. Similar results have been previously reported by other researchers for other cellulosic materials [18]. Chosdu et al. reported that enzymatic hydrolysis of corn stalk biomass treated with alkaline solution yields 20% higher xylose in comparison with the untreated, after hydrolysis 48 h [19]. However, Bak et al. performed a similar type of work and reported that about a 30% increase in xylose yield was absorbed when alkaline pretreated rice straw feedstocks were hydrolyzed enzymatically after 132 h of hydrolysis [20].
However, compared to conventional methods, acid hydrolysis is mainly useful for the conversion of xylan to xylose which can be further used for xylitol production by fermentation methodology. Moreover, xylose production through this technique causes the formation of many fermentation inhibitors such as furfural, phenolic compounds acetic acid, and hydroxymethylfurfural (HMF), which creates serious fermentation problems [21]. In comparison to this technique, saccharification of lignocellulosic biomass causes the lesser formation of fermentation inhibitors, chemical hazards, and lower environmental impact. Therefore, enzymatic hydrolysis could be a cost-effective and environment-friendly substitute for xylose-rich hemicellulosic hydrolysate production.
FTIR Analysis
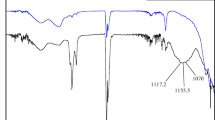
FTIR spectroscopy was performed in the region of 500–4000 cm−1 to compare the molecular conformational changes in the enzymatically hydrolyzed RH, LTH, and ATH hemicellulosic hydrolysate. FTIR spectra were demonstrated in Fig. 10, and the respective peak of spectra was reported in Table 2. The absorbance at 3356 cm−1 was due to the hydroxyl group. This usually results due to the hydroxyl group of the cellulose forming intermolecular hydrogen bonds in the solution [17]. Moreover, a band was detected at 2338 cm−1, indicating CH expansion and contraction vibrations because of the presence of CH3 and CH2 functional groups. In contrast, the extracted hemicellulose signal appeared at 1757 cm−1. This was due to the hemicellulose fraction dissolved during the water treatment and containing a small amount of acetyl and ester bonds of the carboxyl groups [19]. An absorbance signal around 1630 cm−1 was observed due to water absorbed by xylan-type polysaccharides [20]. A reduction at the 1554 cm–1 due to vibrations and stretching of the lignin aromatic ring, this attributed to lignin removal [22]. In addition, the lower absorbance at 1529 cm−1 appeared due to aromatic skeletal oscillations of the associated lignin [17]. In contrast, the band at 1358 cm−1 was due to the presence of CH bending vibration in hemicelluloses and cellulose chemical structures [19]. The sharp absorption peak around 1143 cm−1 shows the COC stretching in the cellulose and hemicellulose content. The sharp absorption peak around at 1051 cm−1 is depicted typically for xylan which mainly arises due to the CO and CC stretching oscillations having glycosidic bonds. FTIR spectral analysis confirmed that RH, LTH, and ATH hemicellulosic hydrolysate contain almost similar molecular compounds which have similar chemical compositions and properties.
Cell Growth Analysis of C. tropicalis
The growth curve of C. tropicalis in YPD media was shown in Fig. 11. This analysis observed that the maximum growth of C. tropicalis in YPD is about 10.16 g/L after 36 h of incubation. Further extending the time of growth, it became stationary. However, the rate of growth was not the same for the obtained hydrolysate after saccharification. Maximum growths of C. tropicalis in RFH, ATFH, LTFH, and SSFH were 3.28 g/L, 3.9 g/L, 4.3 g/L, and 5.1 g/L, respectively. This analysis revealed that the growth of C. tropicalis mainly depends on the concentration of carbon source (xylose). As the concentration of xylose increases from 2.5 to 7.0 g/L for RFH to SSFH, the concentration of dry cells increases from 3.28 g/L to 5.1 g/L, respectively. However, the erratic growth of C. tropicalis in the case of LTFH and ATFH was most probably due to the presence of a small amount of impurities (such as enzymes and traces of chemicals used for pretreatment) in the LTFH and ATFH [23]. These impurities hindered the growth of microbes which results in a reduction in dry cell biomass. However, in the case of RFH, LTFH, and ATFH, the concencentation of the cell was about 35.68%, 23.52%, and 15.68% lower than the concentration of SSFH. In spite of that, the stationary phase was reached by all the samples at the same time.
Xylitol Production from Enzymatic Hydrolysate
Arecanut husk hemicellulosic hydrolysate obtained after enzymatic saccharification may be potentially used for xylitol production by C. tropicalis. As shown in Fig. 12, the fermentation process was carried out for RFH, LTFH, ATFH, and SSFH. The optimum xylitol concentration obtained was about 1.80 g/L, 2.97 g/L, 4.60 g/L, and 6.08 g/L for RFH, ATFH, LTFH, and SSFH, respectively, after 90 h of fermentation. The yield of xylitol was about 71.02%, 76.78%, 79.68%, and 85.67% for RFH, ATFH, LTFH, and SSFH, respectively. In addition to that, the initial concentration of xylose in the fermentative medium is about 2.47 g/L, 3.83 g/L, 5.88 g/L, and 7.09 g/L, respectively, for RFH, ATFH, LTFH, and SSFH. From this analysis, it can be noted that a low concentration of xylose in the hydrolysate could affect xylitol production and cause a lower yield of xylitol [24]. According to the present results, the xylitol yield was increased by 9% from RFH to LTFH when there was an increase in xylose concentration from 2.47 to 5.88 g/L. But, the yield of xylitol is about 17.10%, 10.37%, and 6.98% lower for RFH, ATFH, and LTHF as compared to the yield of SSFH. The most probable reason for the lower yield of xylitol was the concentration of xylose in the fermentative medium and also might be the chance of the presence of some toxic components in the fermentative medium.
Some literature reported the production of xylitol by fermentation process from lignocellulosic hydrolysate obtained from various biomass sources, as shown in Table 3. From the literature, it was observed that mainly dilute sulfuric acid hydrolysis was used to get xylose-rich hydrolysate, followed by detoxification and fermentation for the production of xylitol [25,26,27,28,29,30]. However, very less studies are available on the production of xylitol from enzymatic hydrolysate by biotechnological route [31, 32]. The maximum xylitol yield reported was in the range of 0.3–0.8 g/g xylose [25, 26, 33]. But, this study was able to obtain the optimum yield of xylitol 0.79 g/g by the fermentation of hydrolysate from enzymatic hydrolysis instead of the sulfuric acid process. However, obtained yield can be further improved. It was noted from the literature that D.hansenii can catabolize glucose to ethanol under the anaerobic condition with a glucose/xylose ratio above 30%. Higher glucose concentration in the hydrolysate diverts the process to ethanol production instead of xylitol [34]. In order to avoid byproduct formation (such as ethanol), higher xylanase specificity and lower cellulase activity enzyme would be needed for maximum production of xylose-rich hydrolysate, followed by xylitol production by the fermentation process.
Presently, the production of xylitol in the industry is mainly based on the chemical hydrogenation process in the presence of a nickel-supported catalyst [4]. The major drawback of this process is that xylitol yield is relatively low due to the chemical reduction of produced by-products. Therefore, xylitol production by fermentation of xylose-rich hydrolysate obtained after saccharification of lignocellulosic biomass appears to be a promising alternative. In this research, yeast strain C.tropicalis was used for xylitol production from enzymatic hydrolysate, and the optimum yield achieved was 79.68% which was near about the same as reported by other researchers. Moreover, the process used in this study was simpler than other methodologies because of the dispensable use of the detoxification process. Therefore, the production of xylitol by this methodology makes the process more economical and environmentally friendly.
Viability analysis of C. tropicalis in fermentation media
The proposed analysis can provide an effective tool for research and development in the food, brewery, and pharmaceutical industries. This analysis may be incorporated into product manufacturing to monitor the microorganism growth (yeast cell) and viability analysis during the fermentation process. Monitoring C. tropicalis during fermentation and propagation in an industry is a very important and crucial task [35]. By using the fluorescence microscopy analysis of fermented broth, the number of viable cells in the culture (fermentation) medium can be easily estimated. In Fig. 13, 14, 15, 16, and 17, the dotted point shows the intensity of living and viable cells. Cell viability analysis was conducted by using methylene blue staining (MBS). However, Fig. 17 shows the growth of C. tropicalis in the YPD medium, and almost all cells are living in this analysis. Cell viability test by MBS revealed that the different concentrations of xylose in REH, LTEH, ATEH, and SSEH show different viabilities. Moreover, one can clearly observe a decrease in the number of viable cells with a decrease in the concentration of xylose in the fermentation medium. But, as the concentration of xylose increases, the proportion of living cells get increased which causes to enhancement in xylitol production during fermentation [24, 36].
Downstream Process of Fermented Broth
Fermented xylitol crystal (FXC) from fermentation broth contained yeast and fragments of yeast along with some other contaminants (such as culture medium components, fermentation by-products, and residual substrates) were shown in Fig. 18. Whereas, Fig. 19 shows the separation of xylitol crystal from commercial xylitol (XCCX) solution. To discard precipitating impurities, the fermentation solution was centrifuged, followed by the addition of 3N NaOH to maintain a pH of 7.00. Centrifugation was performed after the neutralization of the fermented solution. This results in a 3% removal of the cell biomass from the fermented broth. The further fermented broth was treated with Amber Lite ™ IRC120 cation exchange resin and Amber Lite™ IRA410 anion exchange resin to purify the fermented product. This resulted in about a 20% reduction in arabinose, a decrease in the color of fermented broth solution (visual), and liquid xylitol content of 9.23% was observed. Mart´ınez et al. reported a huge loss of xylitol (about 15.23%) during the treatment of fermented hydrolysates with ion exchange resins [37]. To obtain suitable concentration conditions for xylitol crystallization, the liquid was concentrated under a vacuum to give a syrup containing about 150 g/L. After the crystallization process, the xylitol crystals were thus obtained (Fig. 18C), collected, and stored in a dry place. The crystal thus obtained was pale yellow in color, whereas commercially available xylitol crystals (Fig. 19D) are colorless.
XRD Analysis
An X-ray diffraction pattern of FXC and XCCX was shown in Fig. 20. This analysis determines the crystallinity of the xylitol crystal. Peaks of the crystals obtained after analysis of XCCX were used as a reference, and the analysis report of FXC showed the characteristic of produced xylitol peaks. Analysis revealed that FXC peaks were very similar to the peaks of CXXC at 21.60°, 26.41°, 28.76°, 36.6°, 41.40°, and 44.78° [38]. The XRD analysis demonstrated the crystalline behavior of FXC and XCCX, which shows the processes that occurred with complete crystallization under the present experimental conditions. The xylitol obtained from FXC in the present study had a crystallinity index (CI) of about 76.85% which is more similar to the crystallinity index of CXXC, having a CI of about 81.44%. Moreover, this process results from the good recovery of xylitol crystal from fermented broth with a level of purity of around 85%. In contrast with other methodologies for the purification of xylitol from fermented media, this process has the advantage of being carried out in a single step with lower production costs [39,40,41]. The present study also reported the use of non-toxic anti-solvents, unlike the methodology reported by other researchers, who used an anti-solvent like methanol [21].
SEM Analysis
SEM analysis revealed that the crystallization process greatly affected the surface morphology and shape of the xylitol crystal (particles). Micrographs of FXC and XCCX were shown in Fig. 21d–f and Fig. 21a–c, respectively, and were obtained after the second crystallization step of the fermentation pathway. From these micrographs, it can be observed that xylitol crystals mainly form in aggregates, and the shape of the crystal was hexagonal in nature. These agglomerates mainly formed due to the random grouping of xylitol crystals, which, after growing together, interpenetrated and adhered to each other, creating irregular forms. Another researcher also reported similar results [37, 42, 43].
Conclusions
In this research work, areca nut husk was used as a potential biomass source for xylose and xylitol production. To increase the biomass digestibility, areca nut husk was exposed to pretreatment (such as dilute acid (H2SO4) and alkali (lime)), which, on subsequent enzymatic saccharification, results in the production of xylose-rich hemicellulosic hydrolysate. Furthermore, enzymatic saccharification of RH, LTH, and ATH resulted in the production of xylose sugar having concentrations of about 0.034 g/g, 0.089 g/g, and 0.049 g/g of feedstocks, respectively. Xylose-rich hemicellulosic hydrolysate was subjected to fermentation with C. tropicalis, which results in a yield of xylitol about 0.71 g/g, 0.76 g/g, and 0.79 g/g for RFH, ATFH, and LTFH, respectively. Moreover, crystallization was performed for FXC and XCCX, and the results obtained were very promising. When using a fairly concentrated solution (150 g/L) at a relatively high temperature, it results in good recovery of xylitol with a level of purity of around 85%. From this study, it can be concluded that the crystallization rate is a function of total solute concentration and supersaturation. The present study confirmed that areca nut husk is a potential and renewable hemicellulosic substrate, which could be an ideal biomass source for lignocellulosic bio-refinery for the production of value-added biochemicals and bioproducts such as xylitol.
Data Availability
The data that support the findings of this study are available from the corresponding authors, upon reasonable request.
Abbreviations
- ATEH:
-
Acid-treated enzymatic hydrolysate
- ATFH:
-
Acid-treated fermentative hydrolysate
- ATH:
-
Acid-treated husk
- CMC:
-
Carboxy methyl cellulose
- CrI:
-
Crystallinity index
- DNS:
-
Di-nitrosalicylic acid
- FESEM:
-
Field emission scanning electron microscopy
- FXC:
-
Fermented xylitol crystal
- HMF:
-
Hydroxymethylfurfural
- LTEH:
-
Lime-treated enzymatic hydrolysate
- LTFH:
-
Lime-treated fermentative hydrolysate
- LTH:
-
Lime-treated husk
- MB:
-
Methylene blue
- MBS:
-
Methylene blue staining
- MTCC:
-
Microbial Type Culture Collection
- OPEFB:
-
Oil palm empty fruit bunch
- REH:
-
Raw enzymatic hydrolysate
- RFH:
-
Raw fermentative hydrolysate
- RH:
-
Raw husk
- SSFH:
-
Synthetic solution fermentative hydrolysate
- XCCX:
-
Xylitol crystal from commercial xylitol
- XRD:
-
X-ray diffraction
- YPD :
-
Yeast extract, peptone, and dextrose
References
Ranjithkumar, M., Ravikumar, R., Sankar, M. K., Kumar, M. N., & Thanabal, V. (2017). An effective conversion of cotton waste biomass to ethanol: A critical review on pretreatment processes. Waste and Biomass Valorization, 8(1), 57–68. https://doi.org/10.1007/S12649-016-9563-8/TABLES/2
Keshav, P. K., Naseeruddin, S., & Rao, L. V. (2016). Improved enzymatic saccharification of steam exploded cotton stalk using alkaline extraction and fermentation of cellulosic sugars into ethanol. Bioresource Technology, 214, 363–370. https://doi.org/10.1016/J.BIORTECH.2016.04.108
Da Silva, A. S. A., Inoue, H., Endo, T., Yano, S., & Bon, E. P. S. (2010). Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation. Bioresource Technology, 101(19), 7402–7409. https://doi.org/10.1016/J.BIORTECH.2010.05.008
de Araújo, C. K. C., de Oliveira Campos, A., de Araújo Padilha, C. E., de Sousa Júnior, F. C., do Nascimento, R. J. A., de Macedo, G. R., & dos Santos, E. S. (2017). Enhancing enzymatic hydrolysis of coconut husk through Pseudomonas aeruginosa AP 029/GLVIIA rhamnolipid preparation. Bioresource Technology, 237, 20–26. https://doi.org/10.1016/J.BIORTECH.2017.03.178
Vardhan, H., Mahato, R. B., Sasmal, S., & Mohanty, K. (2022). Production of xylose from pre-treated husk of areca nut. Journal of Natural Fibers, 19(1), 131–144. https://doi.org/10.1080/15440478.2020.1731905
Goli, J. K., & Hameeda, B. (2021). Production of xylitol and ethanol from acid and enzymatic hydrolysates of Typha latifolia by Candida tropicalis JFH5 and Saccharomyces cerevisiae VS3. Biomass Conversion and Biorefinery, 1–11. https://doi.org/10.1007/S13399-021-01868-1/FIGURES/6
Felipe Hernández-Pérez, A., de Arruda, P. V., Sene, L., da Silva, S. S., Kumar Chandel, A., de AFelipi Almeida, M., & Das, G. (2019). Xylitol bioproduction: State-of-the-art, industrial paradigm shift, and opportunities for integrated biorefineries. Critical Reviews in Biotechnology, 39(7), 924–943. https://doi.org/10.1080/07388551.2019.1640658
Unrean, P., & Ketsub, N. (2018). Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Industrial Crops and Products, 123, 238–246. https://doi.org/10.1016/J.INDCROP.2018.06.071
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2008). Determination of structural carbohydrates and lignin in biomass laboratory analytical procedure (LAP) issue date: 7/17/2005. Retrieved from www.nrel.gov
Meddeb-Mouelhi, F., Moisan, J. K., & Beauregard, M. (2014). A comparison of plate assay methods for detecting extracellular cellulase and xylanase activity. Enzyme and Microbial Technology, 66, 16–19. https://doi.org/10.1016/J.ENZMICTEC.2014.07.004
Martins, M., Henriques, M., Azeredo, J., Rocha, S. M., Coimbra, M. A., & Oliveira, R. (2007). Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryotic Cell, 6(12), 2429–2436. https://doi.org/10.1128/EC.00252-07/ASSET/372A4F99-9812-40E2-9A8A-D1DB245418CE/ASSETS/GRAPHIC/ZEK0120730350003.JPEG
Martínez, E. A., Canettieri, E. V. C., Bispo, J. A., Giulietti, M., de Almeida Silva, J. B., Converti, A., & de Almeida, J. B. (2015). Strategies for xylitol purification and crystallization: A review. Separation Science and Technology, 50, 2087–2098. https://doi.org/10.1080/01496395.2015.1009115
Goli, J. K., & Hameeda, B. (2021). Production of xylitol and ethanol from acid and enzymatic hydrolysates of Typha latifolia by Candida tropicalis JFH5 and Saccharomyces cerevisiae VS3. Biomass Conversion and Biorefinery, 1, 1–11. https://doi.org/10.1007/S13399-021-01868-1/FIGURES/6
Martín, C., De Moraes Rocha, G. J., Dos Santos, J. R. A., De Albuquerque Wanderley, M. C., & Gouveia, E. R. (2012). Enzyme loading dependence of cellulose hydrolysis of sugarcane bagasse. Química Nova, 35(10), 1927–1930. https://doi.org/10.1590/S0100-40422012001000007
Vardhan, H., Sasamal, S., & Mohanty, K. (2022). Fermentation process optimisation based on ANN and RSM for xylitol production from areca nut husk followed by xylitol crystal characterisation. Process Biochemistry, 122(P2), 146–159. https://doi.org/10.1016/j.procbio.2022.10.005
Sun, X. F., Xu, F., Sun, R. C., Fowler, P., & Baird, M. S. (2005). Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydrate Research, 340(1), 97–106. https://doi.org/10.1016/J.CARRES.2004.10.022
Julie Chandra, C. S., George, N., & Narayanankutty, S. K. (2016). Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydrate Polymers, 142, 158–166. https://doi.org/10.1016/J.CARBPOL.2016.01.015
Li, Z., Guo, X., Feng, X., & Li, C. (2015). An environment friendly and efficient process for xylitol bioconversion from enzymatic corncob hydrolysate by adapted Candida tropicalis. Chemical Engineering Journal, 263, 249–256. https://doi.org/10.1016/J.CEJ.2014.11.013
Chosdu, R., Hilmy, N., ErizalErlinda, T. B., & Abbas, B. (1993). Radiation and chemical pretreatment of cellulosic waste. Radiation Physics and Chemistry, 42(4–6), 695–698. https://doi.org/10.1016/0969-806X(93)90354-W
Bak, J. S., Ko, J. K., Han, Y. H., Lee, B. C., Choi, I. G., & Kim, K. H. (2009). Improved enzymatic hydrolysis yield of rice straw using electron beam irradiation pretreatment. Bioresource Technology, 100(3), 1285–1290. https://doi.org/10.1016/J.BIORTECH.2008.09.010
Zhao, X., Zhang, L., & Liu, D. (2012). Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioproducts and Biorefining, 6(4), 465–482. https://doi.org/10.1002/BBB.1331
Cai, C., Bao, Y., Li, F., Pang, Y., Lou, H., Qian, Y., & Qiu, X. (2020). Using highly recyclable sodium caseinate to enhance lignocellulosic hydrolysis and cellulase recovery. Bioresource Technology, 304, 122974. https://doi.org/10.1016/J.BIORTECH.2020.122974
Rafał Łukajtis, Piotr Rybarczyk *, Karolina Kucharska, D. K.-Ł., & Edyta Słupek, K. W. and M. K. ´nski. (2018). Optimization of saccharification conditions of lignocellulosic biomass under alkaline. https://doi.org/10.3390/en11040886
Tavares, J. M., Duarte, L. C., Amaral-Collaço, M. T., & Gírio, F. M. (2000). The influence of hexoses addition on the fermentation of d-xylose in Debaryomyces hansenii under continuous cultivation. Enzyme and Microbial Technology, 26(9–10), 743–747. https://doi.org/10.1016/S0141-0229(00)00166-6
Rivas, B., Domínguez, J. M., Domínguez, H., & Parajó, J. C. (2002). Bioconversion of posthydrolysed autohydrolysis liquors: An alternative for xylitol production from corn cobs. Enzyme and Microbial Technology, 31(4), 431–438. https://doi.org/10.1016/S0141-0229(02)00098-4
Tada, K., Horiuchi, J. I., Kanno, T., & Kobayashi, M. (2004). Microbial xylitol production from corn cobs using Candida magnoliae. Journal of bioscience and bioengineering, 98(3), 228–230. https://doi.org/10.1016/S1389-1723(04)00273-7
de Albuquerque, T. L., Gomes, S. D. L., Marques, J. E., da Silva, I. J., & Rocha, M. V. P. (2015). Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catalysis Today, 255, 33–40. https://doi.org/10.1016/J.CATTOD.2014.10.054
Li, M., Meng, X., Diao, E., & Du, F. (2012). Xylitol production by Candida tropicalis from corn cob hemicellulose hydrolysate in a two-stage fed-batch fermentation process. Journal of Chemical Technology & Biotechnology, 87(3), 387–392. https://doi.org/10.1002/JCTB.2732
Carvalheiro, F., Duarte, L. C., Medeiros, R., & Gírio, F. M. (2007). Xylitol production by Debaryomyces hansenii in brewery spent grain dilute-acid hydrolysate: Effect of supplementation. Biotechnology letters, 29(12), 1887–1891. https://doi.org/10.1007/S10529-007-9468-5
Baek, S. C., & Kwon, Y. J. (2007). Optimization of the pretreatment of rice straw hemicellulosic hydrolyzates for microbial production of xylitol. Biotechnology and Bioprocess Engineering, 12(4), 404–409. https://doi.org/10.1007/BF02931063
Mardawati, E., Maharani, N., Wira, D. W., Harahap, B. M., Yuliana, T., & Sukarminah, E. (2020). Xylitol production from oil palm empty fruit bunches (OPEFB) via simultaneous enzymatic hydrolysis and fermentation process. Journal of Industrial and Information Technology in Agriculture, 2(1), 29–36. https://doi.org/10.24198/JIITA.V2I1.25064
Harahap, B. M., & Kresnowati, M. T. A. P. (2018). Moderate pretreatment of oil palm empty fruit bunches for optimal production of xylitol via enzymatic hydrolysis and fermentation. Biomass Conversion and Biorefinery, 8(2), 255–263. https://doi.org/10.1007/S13399-017-0299-X/TABLES/3
Wang, L., Yang, M., Fan, X., Zhu, X., Xu, T., & Yuan, Q. (2011). An environmentally friendly and efficient method for xylitol bioconversion with high-temperature-steaming corncob hydrolysate by adapted Candida tropicalis. Process Biochemistry, 46(8), 1619–1626. https://doi.org/10.1016/J.PROCBIO.2011.05.004
Cao, N. J., Tang, R., Gong, C. S., & Chen, L. F. (1994). The effect of cell density on the production of xylitol fromd-xylose by yeast. Applied Biochemistry and Biotechnology, 45(1), 515–519. https://doi.org/10.1007/BF02941826
Vandeska, E., Amartey, S., Kuzmanova, S., & Jeffries, T. (1995). Effects of environmental conditions on production of xylitol by Candida boidinii. World Journal of Microbiology and Biotechnology, 11(2), 213–218. https://doi.org/10.1007/BF00704652
Parajó, J. C., Domínguez, H., & Domínguez, J. M. (1998). Biotechnological production of xylitol. Part 1: Interest of xylitol and fundamentals of its biosynthesis. Bioresource Technology, 65(3), 191–201. https://doi.org/10.1016/S0960-8524(98)00038-8
Martínez, E. A., de Almeida e Silva, J. B., Giulietti, M., & Solenzal, A. I. N. (2007). Downstream process for xylitol produced from fermented hydrolysate. Enzyme and Microbial Technology, 40(5), 1193–1198. https://doi.org/10.1016/J.ENZMICTEC.2006.09.003
Marques Júnior, J. E., & Rocha, M. V. P. (2021). Development of a purification process via crystallization of xylitol produced for bioprocess using a hemicellulosic hydrolysate from the cashew apple bagasse as feedstock. Bioprocess and Biosystems Engineering, 44(4), 713–725. https://doi.org/10.1007/S00449-020-02480-9/FIGURES/9
Kresnowati, M. T. A. P., Regina, D., Bella, C., Wardani, A. K., & Wenten, I. G. (2019). Combined ultrafiltration and electrodeionization techniques for microbial xylitol purification. Food and Bioproducts Processing, 114, 245–252. https://doi.org/10.1016/J.FBP.2019.01.005
Wei, J., Yuan, Q., Wang, T., & Wang, L. (2010). Purification and crystallization of xylitol from fermentation broth of corncob hydrolysates. Frontiers of Chemical Engineering in China, 4(1), 57–64. https://doi.org/10.1007/S11705-009-0295-1
Sampaio, F. C., Passos, F. M. L., Passos, F. J. V., De Faveri, D., Perego, P., & Converti, A. (2006). Xylitol crystallization from culture media fermented by yeasts. Chemical Engineering and Processing: Process Intensification, 45(12), 1041–1046. https://doi.org/10.1016/J.CEP.2006.03.012
Deng, L. H., Tang, Y., & Liu, Y. (2014). Detoxification of corncob acid hydrolysate with SAA pretreatment and xylitol production by immobilized Candida tropicalis. Scientific World Journal. https://doi.org/10.1155/2014/214632
Misra, S., Raghuwanshi, S., & Saxena, R. K. (2013). Evaluation of corncob hemicellulosic hydrolysate for xylitol production by adapted strain of Candida tropicalis. Carbohydrate Polymers, 92(2), 1596–1601. https://doi.org/10.1016/J.CARBPOL.2012.11.033
Funding
Present research work vides grant no. (BT/PR16747/NER/95/271/2015) was financially supported by the Department of Biotechnology (DBT), Government of India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Harsha Vardhan, Soumya Sasmal, and Kaustubha Mohanty. The first draft of the manuscript was written by Harsha Vardhan, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vardhan, H., Sasamal, S. & Mohanty, K. Xylitol Production by Candida tropicalis from Areca Nut Husk Enzymatic Hydrolysate and Crystallization. Appl Biochem Biotechnol 195, 7298–7321 (2023). https://doi.org/10.1007/s12010-023-04469-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04469-y