Abstract
Purpose
The research aimed to assess and compare the effect of bacterial inoculants, chemical additive and their combinations on the fermentation process of sugarcane tops silages along with variations in pH, yeast and mould count after aerobic exposure.
Methods
In harvest season, sugarcane tops were collected from local farmer fields (27.80% DM). The treatments were, control (no additive), LP (Lactiplantibacillus plantarum), LF (Limosilactobacillus fermentum), PA (Propionic acid), a combination of LP + LF, PA + LP, PA + LF, and PA + LP + LF. After 30 days of ensiling, silage fermentation parameters and pH, yeast and mould were accessed after aerobic exposure.
Results
The treatment with additives reduced pH (P < 0.05) and increased lactic acid, acetic acid and dry matter recovery significantly (p < 0.05). In the PA + LP + LF treatment, the oxalate content substantially reduced by 51.36% after ensiling (p < 0.05). NDF content reduced in all treatments as compared with control (p < 0.05). LAB count was higher in PA + LF treatment (8.58 log10 CFU/g) (p < 0.05). Yeast and Mould counts were lower in treatments PA + LP + LF and PA, 2.22 and 3.01 (log10 CFU/g) respectively (p < 0.05). Among the treatments, combinations of PA + LP + LF and PA + LF were the most effective to improve the fermentation quality of silage and the combination of PA + LF has shown more potential to reduce the yeast and moulds after aerobic exposure.
Conclusion
The additives were effective to improve the fermentation quality and reduced oxalates content of the sugarcane tops silage. As the exposure time increased the pH values and the yeast-moulds count remained more stable in silage treated with bacterial inoculants and chemical additive which reduced aerobic spoilage in sugarcane tops silage.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
-
Sugarcanetops (SCT) is a low quality roughage source for ruminants. Co-ensiling sugarcane tops with suitable additive and further inoculatig with suitable bacterialinoculants and enzymes coverts SCT into a superior quality roughage source for ruminants.
Highlights
-
Sugarcane tops after ensiling with suitable additives can be used as quality fodder for livestock
-
The additive treatment, including Lactic acid bacteria (L. plantarum and L. fermentum) and propionic acid (PA) along with urea (0.5%) and molasses (1.5%) reduced pH, increased lactic acid, acetic acid and dry matter recovery in sugarcane tops silage (SCT)
-
NDF content reduced in all treatments as compared with control (p < 0.05)
-
In the PA + LP + LF treatment, the oxalate content substantially reduced by 51.36% and yeast count were also lower in PA + LP + LF treatment
-
The combinations of PA + LP + LF and PA + LF were the most effective to improve the fermentation quality of silage
-
The combination of PA + LF showed a higher ability to inhibit yeast and moulds after aerobic exposure, which are the main contributors of aerobic deterioration of silage
Introduction
Sugarcane (Saccharum spp.) is one of the most agronomical and economically suitable forage that is produced in more than 100 countries worldwide in tropical and sub-tropical regions and India is the second-largest sugarcane producer globally. In India, 21% of the agricultural area is used for sugarcane production and its green leaves considered to be medium-quality forage due to less protein (6.84% CP), minerals, energy and high oxalate content [1,2,3]. Sugarcane tops are a by-product of sugarcane harvesting, comprising green leaves. A significant by-product of the sugarcane industry, the sugarcane tops (SCT) make up around 20% of the entire plant [3]. Even though sugarcane tops are easily accessible after a harvest, still it’s a low quality roughage to be utilised for ruminant feeding Sugarcane tops exhibits a substantial quality loss over time, becoming rough and less desirable to animals during drying and storage. Therefore, ensiling of sugarcane top can not only benefit the sugarcane industry but also support ruminants who are struggling with a lack of green fodder during the lean seasons [4, 5]. Ensiling of sugarcane tops as a feed alternative avoids higher losses of quality and dry matter (DM), reduces oxalate content and could extend the storage time [6,7,8]. Ensiling of silage is a forage conservation method that is based on spontaneous lactic acid production of forage under anaerobic conditions. Lactic acid bacteria and water soluble carbohydrates (WSC) are essential components for optimal silage quality throughout the ensiling process [6, 9]. Because during ensiling process, SCT silage losses their quality with time and high DM losses also observed (up to 15.9% of DM through gaseous loss and an average loss of 250 g/kg), it is a serious issue for silage industry [10,11,12,13].
Applying additives during preparing silage is one of the management strategies during the ensiling, storage, and feed-out phases to reduce a nutrient loss (yeasts cause a significant loss of dry matter), enhance its nutritional value, and prevent nutrient loss. Thus, various biological additives and chemicals have been used to achieve an optimal fermentation process [14, 15]. The two most feasible additives are urea, which gives the silage's microbes fermentable nitrogen and significantly lowers the oxalate level of sugarcane tops [16], and molasses, which is a source of fermentable carbohydrates. Among the various additives, homofermentative bacteria inoculants have been suggested as a potent stimulant to boost the initial lactobacilli load and fermentable substrate in silage [17, 18], but it is more unstable when exposed to air because of the production of less antifungal compounds (such as acetic acid) [19, 20]. The other problem is, after opening the silo the silages are perforated by air, which encourages the growth of aerobic, acid-tolerant microorganisms (Lactate-assimilating yeast such as Saccharomyces, Candida, Cryptococcus, Pichia, and mould) and produce potentially toxic substances which lead to aerobic spoilage and reduced nutritional value [13, 21]. In addition, the quality and nutritional richness of sugarcane silage directly influence aerobic deterioration [22]. The aerobic stability of silages has been enhanced using a variety of methods. For example, applying heterofermentative lactobacilli (L. Buchneri, L. fermentum, etc.) and propionic acid-based additives have enhanced the aerobic stability of silage [23]. However, acetic acid, which is a more effective antimycotic agent than lactic acid, is produced during heterolactic fermentation, which is less effective than homolactic fermentation at conserving nutrients [13, 24, 25]. Recently, the addition of homofermentative and heterofermentative bacteria has shown better results especially in exhibiting higher fermentation quality, and inhibition of yeast and fungal proliferation. Therefore, the studies on sugarcane silage have mainly focused on indenting additives associated with silage fermentation that inhibit forage deterioration to reduce losses. Furthermore, limited information is available regarding the effect of Lactiplantibacillus plantarum, Limosilactobacillus fermentum), and propionic acid or in combination on fermentation profile and changes in pH, yeast and mould count after aerobic exposure of sugarcane tops silage. The findings of this study may provide l guidance and support for future sugarcane top silage production, using appropriate LAB inoculants and chemical additives. Therefore, this study aimed to evaluate the effects of bacterial inoculants, chemical additive and their combinations on the fermentation process of sugarcane tops silage in laboratory silos, along with variations in pH, yeast and mould count after aerobic exposure.
Materials and Methods
Materials and Silage Preparation
The research was conducted at the Animal Nutrition Division of the National Dairy Research Institute (NDRI), Karnal, Haryana, India. NDRI is situated 250 m above sea level, with latitude and longitude of 29° 42″ N and 79° 54″ E, respectively. With a diurnal fluctuation of 15–20 °C. The district typically receives 582 mm of precipitation per year. It is an alluvial plain-like region that is a part of the Indo-Gangetic plain. Sugarcane tops were obtained from a nearby farmer's field during harvest season (late November to early December). Whole sugarcane tops were chopped into 2–4 cm segments using an electrical chaff cutter. When the tops were ensiled, the DM content was 278 g/kg. In all treatment groups, urea (0.5%) and molasses (1.5%) were added on a fresh basis. To avoid fungus growth, molasses was also given a heat treatment by being autoclaved at 121 °C for 15 min. The following additions were used in fresh material to prepare silage: bacterial inoculants (ampoules were obtained from the National Collection of Dairy Cultures), namely Lactiplantibacillus plantarum (2 × 106 CFU g−1) (NCDC No. 344) and Limosilactobacillus fermentum (1 × 106 CFU g−1) (NCDC No. 412) after growing in MRS broth (Himedia Laboratories Pvt. Ltd, Mumbai, India), and chemical additive propionic acid @ 0.1% on fresh matter basis. Treatments were control (no additive), LP (Lactiplantibacillus plantarum), LF (Limosilactobacillus fermentum), LP + LF, PA (Propionic acid), PA + LP, PA + LF, and PA + LP + LF. Three containers were filled with sugarcane tops for each treatment after proper mixing with additives. The containers were weighed and then sealed tightly before being kept at room temperature (Weight of sample 2.80 kg). After 30 days of ensiling [26], the samples were analyzed in triplicate for fermentation parameters, microbial composition, and checked pH, yeast, and mould count after aerobic exposure. Pre-ensiled samples were taken for chemical analysis.
Chemical Composition and Silage Quality Estimation
The chemical composition (DM, CP, OM, and EE) of fresh sugarcane tops was determined as per the method described by AOAC [27]. The pH of fresh fodder and silage was determined using the Eutech pH meter from the aqueous extract. The water-soluble carbohydrate (WSC) content of fresh sugarcane tops was determined by a spectrophotometer after a reaction with an anthrone reagent [28]. The fermentation coefficient (FC) of sugarcane tops was calculated according to the formula given by [29]. Oxalate was estimated according to [30] method. Van Soest et al. [31] methods were used for analysing the acid detergent fibre (ADF) and neutral detergent fibre (NDF) contents. Lactic acid estimation was done per [32] method or modified method as given by [33]. Volatile fatty acids were estimated with the help of Nucon’s gas–liquid chromatography. The supernatant was injected into a Gas chromatograph (Nucon 5700, Nucon Engineers, New Delhi) equipped with flame ionization detector and stainless steel column packed with Chromosorb–101 (length 4’; o.d ¼”; i.d. 3 mm; mesh range 80–100) to serve as a stationary phase. The following were the analytical conditions for VFA fractionation: Injection port temperature, 250 °C; column temperature, 190 °C, and detector temperature, 260 °C. The flow rate of carrier gas (nitrogen) was 40 ml/min; hydrogen 30 ml/min; air 300 ml/min. The injection volume was 3 μl. The injection was carried out using a 10 μl Hamilton syringe (Hamilton, Nevada, USA). Dry matter loss was determined by the disappearance of organic matter after keeping it in a muffle furnace [34]. NRC, 2001 was used to compute the true digestible NFC (tdNFC) and energy content. Numbers of LAB were determined by pour-plating tenfold serial dilutions on de Man, Rogosa, and Sharpe agar [35] from Himedia Laboratories of Pvt Ltd, Mumbai, India. The Petri plates were incubated at 37 °C for 36 h to enumerate LAB in fresh sugarcane tops and silages. Total number of yeast and moulds were determined by pour-plating tenfold serial dilutions on potato dextrose agar that was acidified with 0.5% (vol/vol) of 85% lactic acid after autoclaving. Clostridia spore concentration in fresh silage samples was determined by the most probable number (MPN) procedure described by Tabacco et al. [36].
Assessment of pH, Yeast and Moulds Count After Aerobic Exposure
After exposure to air, the silage's pH level was measured to determine its stability in air [37]. 2 kg of silage was packed in plastic bags and kept in a confined space at room temperature (27 °C). During the aerobic exposure (0, 2, 4, 6, and 8 days), the silage was sampled to determine pH values and yeast and moulds count. 25 grams of sample (silage) from each replicate were homogenized with 225 mL of sterile water. The counting of yeast and moulds were done on a plate of potato dextrose agar acidified with lactic acid (85%). The plates were incubated at 37 °C, for counting of yeast and moulds at 48 and 96 h, respectively based on morphology.
Statistical Design
Data of the fermentation parameters were analyzed by one-way analysis of variance (ANOVA). Using the general linear model process of SPSS (26.0), the pH, yeast and moulds count (after aerobic exposure) were subjected to a two-way analysis of variance with the fixed effects of additives, ensilage period, and additives × ensilage period. For LAB, yeast and moulds used log10 transformed data. Pairwise comparisons of the mean values were tested by Tukey’s test at the significance level (p < 0.05). ClustVis (https:/biit.cs.ut.ee/clustvis/), a web platform for visualizing multivariate data clustering, was used to produce heat-maps and principal component analysis (PCA). PCA was used to obtain statistical differences and relationship among the treatments.
Results and Discussion
Chemical Composition of Fresh Sugarcane Tops
The chemical composition and microbial counts of fresh sugarcane tops are already presented and discussed in our previous publications [38]. The chemical properties of the fresh sugarcane top were analyzed prior to ensiling, and results revealed that DM content in fresh sugarcane top was 278.0 g/kg. Moreover, the crude protein, ether extract, organic matter and total ash content was 63.0, 24.8, 934.1, and 75.9 (g/kg DM) respectively. Additionally, sugarcane tops also had high structural carbohydrate content, with NDF and ADF accounting for about 771.0 and 420.1 g/kg of DM, respectively. The hemicellulose, NDICP, and ADICP content of sugarcane tops was 350.9, 16.2, and 8.10 g/kg of DM, respectively. The TDN, DE (MJ/Kg DM), and ME (MJ/Kg DM) of sugarcane tops fodder were 51.94, 9.40, and 7.61, respectively The pH, Buffering capacity (mE /100 g DM), WSC (%), LAB, and yeast-moulds count of sugarcane tops (log10 CFU/g) fodder was 6.69, 17.00, 13.14, 5.23, and 5.01, respectively [38]. The oxalate content of fresh sugarcane tops was 1.46 (g/ 100 g DM) and the fermentation coefficient (FC) of fresh sugarcane tops was less than 35. According to a study [29], If FC < 35 = bad ensilable between 35 and 45 i.e. 35 < FC < 45 = middle ensilable and if FC > 45 = good ensilable, It might be seen as being low quality, difficult to ensile, and in need of the application of appropriate additions. Consequently, molasses was used in the current study to enhance the FC of sugarcane tops. Molasses directly provide soluble sugar during the initial stage of fermentation, and also improved the N–C synchronization when used with urea. The substantial energy contained in molasses serves as additional fuel for the production of lactic acid and raises the silage's overall energy content. For optimal ensiling, fresh material should contain a dry matter content of 25–30 g/100 g DM, 6–7 g/100 g DM of water-soluble carbohydrates, and a likely amount of LAB (> 106 CFU/g FM) [9].
Chemical Composition of SCT Silages Ensiled with Different Additives
The chemical composition of SCT silage treated with different additives is presented in Table 1. DM is the main factor in deciding the fate of ensiling. Dry matter (%) in different treatments ranges from 24.87 to 27.43%. The PA + LP + LF silage had the highest (p < 0.05) dry matter content (27.43%), followed by the LP silage (27.39%), and control silage (24.87%). However, the addition of the additives might have an impact on the DM content of the ensiled materials, in contrast, to control silage because they improved fermentation, decreased dry matter loss, and boosted dry matter recovery [39, 40].
The highest concentration (p < 0.05) of CP was found in PA + LP + LF (8.66%), followed by PA + LF (8.16), PA + LP (7.67), and LF (7.02) respectively. According to the current research, sugarcane tops ensiled with urea-based additives had a much greater concentration of CP than the fresh sugarcane tops, which was consistent with the previous studies [40]. This was also in agreement with that an increase in CP from 5.6 to 7.15% after ensiling of sugarcane tops. In the current study, it was found that lactic and acetic acid, two silage organic acids, had favourable correlations (0.71 and 0.55) with the silage's CP content (Fig. 1). In relation to the current findings, a study revealed that urease (bacterial origin), an enzyme that hydrolyzes urea to ammonia and carbon dioxide, reacted with silage acids (Lactic, acetic acid, etc.) to generate ammonium salts (non-volatile) and improved the crude protein. It implies that increasing the silage acids would increase the CP in silage [41, 42].
The effects of the additive treatments on DM loss of silages treated with various additives ranged from 12.48 to 8.75%. In contrast to other treatments, the silage treated with L. plantarum had the least (p < 0.05) dry matter loss (8.75%) and the control group experienced the highest dry matter loss (12.48%). According to a study, urea-ensiled SCT silage had the lowest dry matter recovery and the greatest proportion of effluent losses [43]. In the present study, it was observed that L. fermentum had lower DM recovery or more dry matter loss as compared to L. plantarum mean value might be due to L. fermentum producing gas, CO2, and acetic acid. The present study was in agreement with Borreani et al. [44] that, the initial ensiling fermentation carbon dioxide losses were minimised to the greatest extent by the homofermentative LAB. Overall, results of the present study revealed that additives treatments of SCT silage had lower dry matter loss as compared to control, it could be supported by the lower unwanted microbes like yeast and moulds count, which are responsible for lower DM recovery or more losses. Similar results were analysed in an investigation that initial reduction in pH by additives addition favours the lactic acid bacteria and reduces the activity of enterobacteria and clostridia [45]. These results were similar to the findings of Muck and Kung [46]: DM recovery significantly increased by 6% in additives treated silage than in untreated silage. The truly digestible non-fibrous carbohydrates (tdNFC) of SCT silage ranged from 12.33 to 15.91%. The highest (p < 0.05) tdNFC was observed in LF (15.91) and PA + LF (15.75) respectively as compared to others treatments. The reason for the increase in tdNFC could be due to a lower NDF content in the LF and PA + LF treatments (Table 1).Values obtained for tdNFC content of the silage are a clear indication of well-fermented and preserved silage as supported by the findings of Ferreira [47].
The oxalate content of fresh sugarcane tops was 1.46 g/100gm DM reduced by 44.52% in control (0.81 g/100gm DM) after the ensiling with urea and molasses. All of the treatments had lower oxalate contents than fresh sugarcane tops, and PA + LP + LF treatment had the largest reduction (51.36%) in comparison with others treatments (p < 0.05). According to a study, urea treatment of sugarcane tops reduced the oxalate level of SCT by 52.28% significantly [48]. Oxalates are degraded into carbonates and then turned into CO2 by anaerobic bacteria, which may be the cause of the drop in oxalate during ensiling [49].
Effect of Additives on Fibre, Energy and Nitrogen Content of SCT Silage
Table 2 depicts the fibre, energy and nitrogen content of sugarcane tops silage. The NDF, ADF, and hemicellulose (g/100 g DM) content were lower (p < 0.05) in all treatments as compared to fresh sugarcane tops. All treatments significantly affected the NDF contents (p < 0.05) and higher NDF content was reported in control silage (71.03%). While no significant difference (P > 0.05) was observed in ADF content of silages. However, highest hemicellulose content was observed in control (29.64 g/100 g DM) as compared to others treatments (p < 0.05). The NDF contents in the treatment group with LP and LF were lower than control group (P < 0.05), which could be due to the LAB that secrete lingo-cellulase enzymes which stimulate conversion of fibre to WSC, thereby making soluble sugars ready to use for successive conversion to lactate, leading to a decrease in cell wall carbohydrates associated with the effective degradation of NDF contents [50]. A similar observation was made by Wang et al. [51], who reported that lactobacillus treated silage had lower NDF as compared to untreated silage.
The energy content of silage with and without the addition of chemical and biological additives, varied significantly (P < 0.05). All the treated SCT silages had higher TDN%, DE (MJ/Kg DM), and ME (MJ/Kg DM) as compared to fresh sugarcane tops (51.94, 9.40, 7.6) respectively. This could be supported by the higher NFC content and lower NDICP in all the treated silage as compared to fresh sugarcane tops. Our results are in agreement with Akinbode et al. [52], found that high energy of SCT silage compared to fresh SCT, which was attributed to the increased NFC content. In present study, among LAB inoculants, LF treatment had increased energy content than the LP, which could be supported by the improved fermentation. The present findings showed similarities with the reports of Kebede et al. [43] in that ME for sugarcane tops silage was 7.74 MJ/Kg DM however, the ME of SCT silage in present study was 7.76 MJ/Kg DM (Control). The NDICP and ADICP content of SCT silage was significantly reduced in all treatments as compared to fresh SCT (1.62 and 0.81% DM) but stayed similar (p > 0.05) among treatments.
Effect of Additives on Quality Parameters of SCT Silage
Table 3 displays the quality parameters of sugarcane tops silage. The pH of SCT silage ranged between 4.15 to 5.69. The chemical and biological additives (Propionic acid and Lactobacilli) lowered the pH of the SCT silage significantly (p < 0.05) as compared to control (5.69). These results are in line with the observations of the majority of researchers, who reported that adding bacterial inoculants induces the quick drop in pH of silage compared to control [14, 53]. The control treatment had higher pH; this could be because of urea addition. Similarly, Kebede et al. [43] reported sugarcane tops silage treated with urea had a higher pH than molasses treatment or with their combination. Likewise, during the ensiling period, the decrease in pH is delayed because of the alkaline character of ammonia [54]. Silage quality is not adversely impacted by the use of urea and molasses, except for an increase in pH level of silage [55]. In the current investigation, LAB-treated silages had lower pH as compared to the control could be because of OM (Hemicellulose, glucose, starch) converted into organic acid (Lactic, acetic acid) during ensiling by the LAB. LAB used the OM as a substrate and produces more amount of organic acid. These finding coincide with previous investigations, that the pH value of SCT silage decreased as a result of the loss of organic matter, which supports a pH drop [43]. In the current study, it was found that silage treated with propionic acid had lower pH values (4.28) than control (5.69) values, which may be related to its pKa value or acidic character. Similarly, researchers found that propionic acid used as an additive (PA;10 g /kg fresh matter) for high moisture corn (30%) before ensiling leads to a decrease in pH of silage to 4.94 as compared to 5.88 in control silage [56].
In the present study, the pH value was negatively correlated with the LAB, LA: AA, lactic acid, acetic acid, and propionic acid, but positively correlated with DM loss, yeast-mould, and butyric acid (Fig. 1). It indicates that treatments with lower pH values had elevated numbers of LAB, lactic acid, acetic acid, and propionic acid, and lower concentrations of DM loss, yeast-mould, and butyric acid.
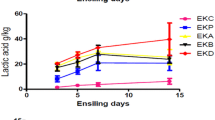
The concentration of lactic acid was higher in all treatments as compared to control silage, which ranged from 4.89 (control) to 8.18 (PA + LP + LF) g/100 g DM (Table 3) and the highest was in PA + LP + LF followed by PA + LF (7.82) and LP + LF (7.40) % DM respectively. When L. plantarum and L. fermentum were used as LAB inoculants, the lactic acid concentration increased as compared to using LP or LF alone. The L. fermentum produced more lactic acid (7.23) compared to L. plantarum (6.56), which might be because the addition of molasses encourages the growth of heterofermentative rather than homofermentative lactic acid bacteria. Therefore, the more bacteria possess the more lactic acid in silage, another reason may be due to the nature of bacteria, LF is less fastidious and grows easily [26]. Lactic acid is the major acid in high quality silage. Therefore, 4 to 12 g/100 g DM lactic acid should be present in high-quality silage [9]. In present study, a sign of well-fermented silage is that, the LA content in SCT silage was within this acceptable range. It appeared that on the addition of molasses, enzymes, and bacterial inoculants, lactic acid content was enhanced. Lactic acid is the main acid, which lowers the pH of silage more effectively than other fermentation products, which produce high-quality silage [57, 58]. During the initial stage of fermentation, molasses directly supplies soluble sugar. A study also reported that the addition of propionic acid slightly reduces the lactic acid contents of silage, because of inhibition of microbial activity [59].
PA (6.17) and PA + LP + LF (5.94) had the highest LA: AA ratio whereas lowest (p < 0.05) was observed in control (3.73. The present study revealed that in all the treatments the LA: AA ratio was above 3, which suggest the good fermentation. So, present experiment results demonstrate that the addition of additives during the ensiling of SCT silage improved the LA: AA ratio and also silage quality [60]. Comparable results were obtained by Borreani et al. [44] which remarked that a higher lactate to acetate ratio in the silage suggests a more homofermentative fermentation process.
The ammonia- nitrogen (%DM) content varied from 0.12 (PA) to 0.21 (PA + LF) and ammonia nitrogen content stayed significantly lower (p < 0.05) in LP and PA treatment. In the present study, NH3-N (% DM) content did not follow any specific trend when ensiled with different additives. A higher NH3-N content observed in present study might be due to addition of urea during the ensiling. In the similar pattern, Singh et al. [26] also reported that urea treated silage had higher ammonia–nitrogen content.
Effect of LAB and Chemical Additive on Microbial Count of Sugarcane Tops Silage
The variations of the lactobacilli and yeast- moulds count in the sugarcane tops silage are depicted in Fig. 2 and Table 3 respectively. After ensiling, LAB colonies increased in all the silage treatments compared to fresh sugarcane tops. The LAB count under this study ranged from 7.60 to 8.58 (log10 CFU/g), with treatment PA + LF having the highest LAB (8.58) as compared to control (7.60) and difference was significant (p < 0.05). The inoculated groups had more Lactobacillus counts than the control. These findings were similar with the analysis that the LAB count was enhanced along with the inhibition of yeast and moulds during ensiling due to and increase lactic acid and decreased pH on adding bacterial inoculants [61]. On individual effect, Lactobacillus fermentum had higher LAB count than Lactobacillus plantarum and this might be due to the addition of molasses, which may favour the growth of heterofermentative lactic acid bacteria as compared to homofermentative. A similar observation was recorded by Singh et al. [26] in sugarcane tops silage.
Effect of additives on lactobacillus count (log10CFU/g) of sugarcane tops silage. LP Lactobacillus plantarum, LF Lactobacillus fermentum, PA propionic acid, PA + LP propionic acid and Lactobacillus plantarum, PA + LF propionic acid and Lactobacillus fermentum, PA + LP + LF combination of propionic acid, Lactobacillus plantarum and Lactobacillus fermentum. The error bars represent standard error of the mean (n = 3). Different letters (a-d) indicate a significant difference (P < 0.05)
Clostridia spores were absent in all the treatment groups. Yeast and moulds count (Table 3) were higher in control (2.81 and 1.53 log10CFU/g) and lower (p < 0.05) in PA + LP + LF treated silage (1.22 and 1.01) respectively. There was a significant trend (p < 0.05) towards reduction in yeast and moulds count on additives treatment as compared to control. PA + LP + LF had the lowest yeast and moulds count could be because of synergistic action of acetic acid (which produced by L. fermentum) and propionic acid, and both are antimycotic agent. Similarly, Borreani et al. [44] reported that acetic and propionic acid reduced aerobic spoilage through the inhibition of yeast and moulds. Corn silage treatment with L. buchneri resulted in lower yeast and moulds counts due to acetic acid and simultaneous improvement in aerobic stability of silage [42]. On the contrary researchers also observed that propionic acid did not affect yeast count and aerobic stability [62]. Control had the highest amount of yeast and mould and this might be due to the highest pH value. This observation was similar to what McAllister et al. [63] who reported that the yeast and mould proliferation was associated with higher pH and low lactic acid concentration [64].
Effect of LAB and Chemical Additive on Volatile Fatty Acid of Sugarcane Tops Silage
Figure 3 displays the volatile fatty acids of the of SCT silages. The acetate concentration varied from 1.45 to 1.12% DM, highest (p < 0.05) acetate concentration was observed in treatment LP + LF (1.45% DM) and lowest in PA treated silage. Some studies reported that an increase in the acetic acid concentration of silage inhibits yeast and moulds growth hence it could lead to increase the aerobic stability of silage [65, 66]. Furthermore, investigations suggested that an increase in acetic acid was associated with the inhibition of spoilage by micro-organisms [38, 67].
Propionate concentration varied from 0.38 to 0.14% DM in all the treatments and highest (p < 0.05) was found in PA and PA + LF (0.38 and 0.35 respectively) treated silage. This result could be due to exogenous addition of propionic acid in these treatments. Some results demonstrated that treatment with propionic acid-based preservatives had no effect on acetic acid concentration but it increased the propionate concentration in the treated silage when compared with the control [68].
Butyric acid concentration ranged from 0.002 to 0.076% DM in all silages. Control had the highest (p < 0.05) butyric acid content (0.076) as compared with other treatments. But no significant difference (p > 0.05) was observed within the propionic acid treated silages like PA, PA + LP, PA + LF and PA + LP + LF. The present study revealed that additives reduce butyric acid concentration significantly. Butyric acid levels below 0.5% of DM in the current study illustrate that silage has not undergone clostridial fermentation [69, 70]. Butyric acid is linked to clostridial fermentation and typically occurs when there is a lot of moisture [9]. The acceptable limit of butyric acid in grass silage, should be less than 0.5–1% [70]. Butyric acid concentrations that are lower suggest good fermentation conditions.
Effect of Additives on pH, Yeast and Moulds Count After Aerobic Exposure of Silage
Variations in pH of SCT Silage After the Aerobic Exposure
Table 4 depicts the pH of sugarcane silage after aerobic exposure. Adding additives had a major impact on pH, aerobic exposure days, and their interaction (p < 0.05). The pH levels of all the treated silages (Table 4) were lower on the zero-day aerobic exposure when the silage was first exposed to the air and then gradually increased (p < 0.05) over the subsequent days. It was observed that the respective lowest and highest pH values in silages were observed at the zero-day and the eighth day of aerobic exposure.. The overall different treatments mean of pH at different time intervals was 4.38, 4.73, 5.91, 6.79, and 6.82 at 0, 2nd, 4th, 6th, and 8th days respectively. When the containers were opened, the pH values of all treatments were significantly lower than the control. The mean pH values of silage from zero to eighth day ranged from 5.58 to 6.28, with the control having the highest mean pH value (6.28) and PA + LF having the lowest (p < 0.05) pH value (5.58). In the present research, silage treated with L. plantarum (5.67) had a higher pH value (p < 0.05) than silage treated with L. fermentum (5.63).
The pH increased with an increase in the days of aerobic exposure, this might be due to the fact that many yeast species convert lactic acid into CO2 and water. lactic acid breakdown raises the pH of the silage in aerobic environment,, which in turn encourages the growth of numerous additional spoilage organisms [9]. This observation was similar to what Liu et al. [71] who opined that the increase in pH could be due to a reduction in the total acid content in the treatment groups after aerobic exposure. The increased number of lactate-assimilating yeasts can cause a decrease in lactic acid content. A similar observation was made by Wang et al. [72] in sugarcane tops silage.
A study also hypothesised that the pH is an indicator of aerobic deterioration of the silage because yeasts use lactic acid during oxygen exposure and the silage becomes favourable substrate for the growth of other undesirable microorganisms like moulds and bacteria, the increase in pH is a sign of aerobic deterioration of the silage [73]. Likewise, the pH of sugarcane tops silage up surged from 3.88 to 4.04 after aerobic exposure, and the amount of yeast grew significantly, reaching 6.12, 7.14, and 7.51 log10 CFU/g FM after 1, 2, and 3 days of exposure to the air [8]. Higher pH is more favourable for yeast and moulds growth. Researchers reported that the pH of the silages rose to a pH value of 7 along with the breakdown of organic acids [74]. L. plantarum treated silage had a higher pH value as compared to L. fermentum might be due to L. fermentum producing comparatively more acetic acid whereas, acetic acid discourages the growth of yeast and mould [14, 72]. Propionic acid-treated silage exhibited a lower pH than the control because propionic acid is an effective antifungal agent [66]. According to the studies, yeast and moulds are responsible for the aerobic deterioration of silage, and propionic acid is potent against them [75].
Change in Yeast Count of Different SCT Silages After the Aerobic Exposure
The yeast count of sugarcane tops silage after aerobic exposure is presented in Fig. 4. All the treated silages had lower yeast count (log10CFU/g) at the zero-day of aerobic exposure after that it increased with the days of aerobic exposure (p < 0.05). Aerobic exposure days, additives, and their interaction all had a substantial impact on the yeast counts (log10 CFU/g) (p < 0.05). The lowest yeast count (p < 0.05) was observed at the zero days (2.04 log10CFU/g) and highest on the 8th day (7.39 log10CFU/g) of aerobic exposure (Fig. 4a). The mean yeast counts of all treatments were lower as compared to the control (Fig. 4b). It was observed that the lowest yeast count was in PA, PA + LF, and PA + LP + LF treated silages and the highest was in control (6.03 log10cfu/g) (p < 0.05). Among the bacterial inoculants, L. fermentum (LF; 5.15 log10CFU/g) had a lower yeast count (p < 0.05) as compared to L. plantarum (LP; 5.55 log10CFU/g) (Fig. 4b).
At the zero-day of aerobic exposure all the treated silages had lower yeast count after that, it increased with the days of aerobic exposure may be due to increasing the pH with the day of aerobic exposure and microbial oxidation of organic acids could be another factor. Yeasts break down the WSC to produce carbon dioxide and water in aerobic environment [76].
A similar report suggests that yeast and mould proliferation might be successfully inhibited by the antimycotic characteristics of propionic acid [45]. The L. Fermentum had a lower yeast count as compared to L. plantarum due to the production of more acetic acid compared to L. plantarum which is an antifungal agent. Homofermentative lactic acid bacterial inoculants decreased the stability of silage due to production of the lesser amount of acetic acid [67]. Acetic acid acts as an inhibitor of the growth of spoilage organisms. The lower yeast count is associated with higher aerobic stability [62]. One study found a negative relationship between yeast and aerobic stability. The stability of silage in air can be zero if yeast count exceeds more than > 6 log10 CFU/g of silage [23]. Good silage should contain less than above count of yeasts on fresh basis.
Mould Count of Different SCT Silages After the Aerobic Exposure
Figure 5 displays the moulds count of the of sugarcane top silages. The lowest moulds count (Fig. 5a) was observed at the zero days (1.33 log10CFU/g) and highest (p < 0.05) at the 8th day (7.57 log10CFU/g) after the aerobic exposure of sugarcane tops silage. Highest mould counts (Fig. 5b) was observed in control (4.91 log10 CFU/g) while the lowest (p < 0.05) was maintained in PA + LF (3.80) respectively.
Initially, (at zero days) moulds count (1.33 log10CFU/g) was lower as compared to yeast count (2.04 log10CFU/g). Usually, Yeasts typically start aerobic degradation by devouring sugars and fermentation acids and increasing the pH and temperature of the silage [77]. Bacilli and other aerobic bacteria flourish at higher pH levels; if the temperature rises further, moulds eventually cause the silage to deteriorate completely. In current study, the lowest mould was observed in PA + LF due to the synergistic effect of antimycotic properties of propionic acid and L. fermentum which was more effective to reduce the moulds growth as compared to other treatments (Fig. 5b). Some researchers also reported that undissociated acetic acid (a product of heterofermentative bacteria), is known to prevent the growth of yeasts and moulds, along with other short-chain fatty acids, but lactic acid (sole fermentative product of homofermentative bacteria) is mainly useless against these catalysts of the aerobic degradation process [67, 76, 77]. When the moulds level in the silage reached greater than 5 log10 CFU/g, moulds become visible on the silage [78, 79]. Some researchers have found that after 5 days of aerobic exposure, the relative abundance of Acetobacter climbed to 90% and Acetobacter replaced Lactobacillus as the main community of bacteria, which led to the aerobic deterioration of silage [72, 80]. Pahlow et al. [77] also reported that, after five days of exposure, the yeast group Candida, which assimilates lactic acid, gradually grew to dominate the fungal population. If moulds count reaches up to 7 log10CFU/g of silage then the nutritional quality of the silage is reduced and the silage can be considered as aerobically deteriorated [44]. Therefore, based on the lower pH, yeast and moulds count, the stability of the sugarcane tops silage in the current experiment could be in between 4 to 6 days.
Cluster Analysis of Sugarcane Tops Silage Treated with Various Additives
Additionally, SCT silage with different treatments was examined for similarities and differences between various parameters and to categorize them. For simpler categorization and comprehension, hierarchical clustering analysis of the different treatments with various parameters in heat maps was performed using a Euclidean distance matrix of quantitative values (Fig. 6). A larger concentration of particular parameters value in the silage sample is indicated by the cell's increased colour intensity. Overall, the clustering of the value of the different parameters in heat maps showed that the findings were in line with those that had already discussed in present work. Principle component analysis (PCA) showed the dynamic variance of the different treatments of silages on the basis of microbial population and fermentation quality parameters. Components 1 and 2 may explain 52.4% and 17.4% of the total variance on the plots' X- and Y-axes, respectively and the group fall inside the ellipse (Fig. 7). The control treatment of silage grouped closely and did not overlap with others treatments of silage. The latter was rather distant from one another, thus demonstrating a higher variability of the considered characteristics in between control and other treatments, it means control had significantly different values than the other seven treatments and this corroborates well with the results of the present study related to chemical and quality profiling of silage samples. The observations indicated that PA + LP + LF and PA + LF had higher scores along with F1. In our study, PA + LP + LF and PA + LF were the most promising additive combination for improving the fermentation quality of the SCT silage. Similarly, a study also applied PCA to selecting the most promising combination of additives for silage [81].
Heat map of hierarchal clustering analysis of quality parameters of SCT silage for different treatments. Coloured cells correspond to concentration value (larger concentration of particular parameters value in the silage sample is indicated by the cell's increased colour intensity); samples in column and quality parameters in row
Distribution of sugarcane tops silage samples according to PCA analysis using pH, DM loss, microbial population, ammonia–nitrogen, and organic acids. Principle component analysis (PCA) showed the dynamic variance of the different treatments of silages on the basis of microbial population and fermentation quality parameters. Components 1 and 2 may explain 52.4% and 17.4% of the total variance on the plots' x- and y-axes, respectively and the group fall inside the ellipse.
Conclusion
The results indicate that the additives were effective to improve the fermentation quality and reduced oxalates content of the sugarcane by-product (sugarcane tops) silage. As the exposure time increased the pH values and the yeast-moulds count remained more stable in silage treated with bacterial inoculants and chemical additive. Among the treatments, combinations of PA + LP + LF and PA + LF were the most effective to improve the fermentation quality of silage and the combination of PA + LF has shown more potential to reduce the yeast and moulds after aerobic exposure. In totality, bacterial inoculants and chemical treated silage positively impacted the lactobacilli, yeast, and moulds. This improved the quality of fermentation and reduced aerobic spoilage in sugarcane tops silage.
E-supplementary data of this word can be found in online version of the paper.
Data availability
Data can be made available on demand.
References
Lagos-Burbano, E., Castro-Rincón, E.: Sugar cane and by-products of the sugar agro-industry in ruminant feeding: a review. Agron. mesoam. 30, 917–934 (2019)
Wang, Y., Wang, C., Zhou, W., Yang, F.Y., Chen, X.Y., Zhang, Q.: Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of Moringa oleifera leaf silage. Front. Microbiol. 6, 9–1817 (2018)
Chen, X., Li, W., Gao, C., Zhang, X., Weng, B., Cai, Y.: Silage preparation and fermentation quality of kudzu, sugarcane top and their mixture treated with lactic acid bacteria, molasses and cellulase. Anim. Sci. J. 88, 1715–1721 (2017)
Ren, F., He, R., Zhou, X., Gu, Q., Xia, Z., Liang, M., Zhou, J., Lin, B., Zou, C.: Dynamic changes in fermentation profiles and bacterial community composition during sugarcane top silage fermentation: a preliminary study. Bioresour. Technol. 1, 285–121315 (2019)
Salinas-Chavira, J., Almaguer, L.J., Aguilera-Aceves, C.E., Zinn, R.A., Mellado, M., Ruiz-Barrera, O.: Effect of substitution of sorghum stover with sugarcane top silage on ruminal dry matter degradability of diets and growth performance of feedlot hair lambs. Small Rumin. Res. 1, 73–77 (2013)
Cai, Y., Benno, Y., Ogawa, M., Kumai, S.: Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 82, 520–526 (1999)
Roth, A.P., Reis, R.A., Siqueira, G.R., Roth, M.D., Resende, F.D., Monteiro, R.R.: Sugarcane silage production treated with additives at different times post burning. Rev. Bras 39, 88–96 (2010)
Zhang, L., Zhou, X., Gu, Q., Liang, M., Mu, S., Zhou, B., Huang, F., Lin, B., Zou, C.: Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure. Bioresour. technol. 1, 291–121835 (2019)
McDonald, P., Henderson, A.R., Heron, S.J.E.: The Biochemistry of Silage, 2nd edn. Chalcombe Publication, London (1991)
Kung, L., Jr., Stanley, R.W.: Effect of stage of maturity on the nutritive value of whole-plant sugarcane preserved as silage. J. Anim. Sci. 54, 689–696 (1982)
Rabelo, C.H., Mari, L.J., Reis, R.A.: Survey about the use of bacterial inoculants in Brazil: effects on silage quality and animal performance. In Advances in silage production and utilization. Intech Open. (2016)
Ferreira, G., Mertens, D.R.: Measuring detergent fibre and insoluble protein in corn silage using crucibles or filter bags. Anim. Feed Sci. Technol. 133, 335–340 (2007)
Siqueira, G.R., Schocken-Iturrino, R.P., Roth, A.P., Domingues, F.N., Ferraudo, A.S., Reis, R.A.: Calcium oxide and Lactobacillus buchneri NCIMB 40788 in the ensiling of in natura or burned sugar cane. Rev. Bras. Zootec. 40, 2347–2358 (2011)
Muck, R.E.: Silage microbiology and its control through additives. Rev. Bras. Zootec. 39, 183–191 (2010)
Daniel, J.L., Jacovaci, F.A., Junges, D., Santos, M.C., Lima, J.R., Anjos, I.A., Landell, M.G., Huhtanen, P., Nussio, L.G.: Fibre digestibility and its relationships with chemical and morphological traits in thirty-two sugarcane varieties. Grass Forage Sci. 72, 545–555 (2017)
Tadesse, A., Fulpagare, Y.G., Gangwar, S.K.: Effect of urea treatment on chemical composition and oxalate content of sugarcane top. Int. J. Nat. Sci. 5, 15–18 (2014)
Ni, K., Wang, Y., Li, D., Cai, Y., Pang, H.: Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. PLoS ONE 10, 0121967 (2015). https://doi.org/10.1371/journal.pone.0121967
Li, M., Zi, X., Zhou, H., Hou, G., Cai, Y.: Effects of sucrose, glucose, molasses and cellulase on fermentation quality and in vitro gas production of king grass silage. Anim. Feed Sci. Technol. 1, 206–212 (2014)
Kung, L., Jr., Tung, R.S., Maciorowski, K.: Effect of a microbial inoculant (Ecosyl™ and/or a glycopeptide antibiotic (vancomycin) on fermentation and aerobic stability of wilted alfalfa silage. Anim. Feed Sci. Technol. 35, 37–48 (1991)
Rust, S.R., Kim, H.S., Enders, G.L.: Effects of a microbial inoculant on fermentation characteristics and nutritional value of corn silage. J. Prod. Agric. 2, 235–241 (1989)
Pedroso, A.D., Nussio, L.G., Loures, D.R., Paziani, S.D., Ribeiro, J.L., Mari, L.J., Zopollatto, M., Schmidt, P., Mattos, W.R., Horii, J.: Fermentation, losses, and aerobic stability of sugarcane silages treated with chemical or bacterial additives. Sci. Agri. 65, 589–594 (2008)
Ávila, C.L., Pinto, J.C., Oliveira, D.P., Schwan, R.F.: Aerobic stability of sugar cane silages with a novel strain of Lactobacillus sp. isolated from sugar cane. Rev. Bras. Zootec. 41, 249–255 (2012)
Kung, L., Jr., Sheperd, A.C., Smagala, A.M., Endres, K.M., Bessett, C.A., Ranjit, N.K., Glancey, J.L.: The effect of preservatives based on propionic acid on the fermentation and aerobic stability of corn silage and a total mixed ration. J. Dairy Sci. 81, 1322–1330 (1998)
Kung, L., Jr., Ranjit, N.K.: The effect of Lactobacillus buchneri and other additives on the fermentation and aerobic stability of barley silage. J. Dairy Sci. 84, 1149–1155 (2001)
Blajman, J.E., Paez, R.B., Vinderola, C.G., Lingua, M.S., Signorini, M.L.: A metaanalysis on the effectiveness of homofermentative and heterofermentative lactic acid bacteria for corn silage. J. Appl. Microbiol. (2018). https://doi.org/10.1111/jam.14084
Singh, D., Johnson, T.A., Tyagi, N., Malhotra, R., Behare, P.V., Kumar, S., Tyagi, A.K.: Synergistic effect of LAB strains (Lb. fermentum and Pediococcus acidilactisci) with exogenous fibrolytic enzymes on quality and fermentation characteristics of sugarcane tops silage. Sugar Tech. 25, 141–153 (2023)
AOAC: Official methods of analysis, 18th edn. J. Assoc. Off. Anal. Chem, Washington DC (2007)
Yemm, E.W., Willis, A.: The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57, 508 (1954)
Weissbach, F., Honig, H.: About the prediction and control of course of fermentation in the ensilage of green fodder from extensive cultivation. Landbauforsch. Volkenrode. 1, 10–17 (1996)
Abaza, R.H., Blake, J.T., Fisher, E.J.: Oxalate determination: Analytical problems encountered with certain plant species. J. Assoc. Off. Anal. Chem. 51, 963–967 (1968)
Van Soest, P.J., Robertson, J.B., Lewis, B.A.: Methods for dietary fiber, neutral-detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597 (1991)
Barker, S.B., Summerson, W.H.: The colorimetric determination of lactic acid in biological material. J. Biol. Chem. 138, 535–554 (1941)
Barnett, A.: The colorimetric determination of lactic acid in silage. Biochem. J. 49, 527 (1951)
Addah, W., Baah, J., Okine, E.K., McAllister, T.A.: Use of thermal imaging and the in situ technique to assess the impact of an inoculant with feruloyl esterase activity on the aerobic stability and digestibility of barley silage. Can. J. Anim. Sci. 92, 381–394 (2012)
De Man, J.C., Rogosa, D., Sharpe, M.E.: A medium for the cultivation of lactobacilli. J. Appl. Microbiol. 23, 130–135 (1960)
Tabacco, E., Piano, S., Cavallarin, L., Bernardes, T.F., Borreani, G.: Clostridia spore formation during aerobic deterioration of maize and sorghum silages as influenced by Lactobacillus buchneri and Lactobacillus plantarum inoculants. J. Appl. Microbiol. 107, 1632–1641 (2009)
Basso, F.C., Bernardes, T.F., Roth, A.P., Lodo, B.N., Berchielli, T.T., Reis, R.A.: Fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri. Rev. Bras. Zootec. 41, 1789–1794 (2012)
Chauhan, N., Kumari, N., Mishra, D.B., Mani, V., Tyagi, N.: Dynamic changes in microbial succession and fermentation profiles of sugarcane tops silage treated with exogenous enzymes and lactic acid bacteria following various duration of ensiling. Sugar Tech. 15, 1–1 (2022)
Alemzadeh, B., Noroozy, S.: Effect of different levels of sugarcane top silage on milk production of dairy cattle. Buffalo Bull. 25, 65–67 (2006)
Pedroso, A.D., Rodrigues, A.D., Barioni- Júnior, W., Souza, G.B.: Fermentation parameters, quality and losses in sugarcane silages treated with chemical additives and a bacterial inoculant. Rev. Bras. Zootec. 40, 2318–2322 (2011)
Bentley, O.G., Klosterman, E.W., Engle, P.: The use of urea to increase the crude protein content of corn silage for fattening steers (1955)
Silva, J.D., Winckler, J.P., Pasetti, M.H., Salvo, P.A., Kristensen, N.B., Daniel, J.L., Nussio, L.G.: Effects of Lactobacillus buchneri inoculation or 1-propanol supplementation to corn silage on the performance of lactating Holstein cows. Rev. Bras. Zootec. 46, 591–598 (2017)
Kebede, G., Mengistu, A., Assefa, G., Animut, G.: Nutritional and fermentative quality of sugarcane (Saccharum officinarum) top ensiled with or without urea and molasses. Afr. J. Agric. Res. 13, 1010–1017 (2018)
Borreani, G.I., Tabacco, E.R., Schmidt, R.J., Holmes, B.J., Muck, R.E.: Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 101, 3952–3979 (2018)
Kung, L., Jr., Stokes, M.R.: Silage additives. Silage Sci. Techno. 42, 305–360 (2003)
Muck, R.E., Kung, Jr L.: Effects of silage additives on ensiling. Silage: Field to feedbunk (1997)
Ferreira, A.V.: Nutritive value of red vine husks and pips for sheep. S. Afr. J. Anim. Sci. 34, 23–25 (2004)
Singh, D., Tyagi, N., Yadav, S., Sharma, B., Chauhan, N.: Growth performance, nitrogen balance and blood biochemical parameters on feeding tmr diet containing sugarcane tops silage supplemented with lactic acid bacteria inoculants and exogenous fibrolytic enzymes in crossbred calves. Indian J. Anim. Res. 56, 1119–1125 (2022)
Ahuja, A.K., Gupta, B.K., Multani, K.K.: Seasonal variation in oxalate content of Hybrid Napier Bajra and its amelioration through ensiling. Indian J. Anim. Res. Nutriti. 15, 147–149 (1998)
Ebrahimi, M., Rajion, M.A., Goh, Y.M., Farjam, A.S., Sazili, A.Q., Schonewille, J.T.: The effects of adding lactic acid bacteria and cellulase in oil palm (Elais Guineensis Jacq.) frond silages on fermentation quality. Chem. Compos. Vitr. Dig. 13, 557–562 (2016)
Wang, Q., Wang, R., Wang, C., Dong, W., Zhang, Z., Zhao, L., Zhang, X.: Effects of cellulase and Lactobacillus plantarum on fermentation quality, chemical composition, and microbial community of mixed silage of whole-plant corn and peanut vines. Appl. Biochem. Biotechn. 194, 2465–2480 (2022)
Akinbode, R.M., Isah, O.A., Oni, A.O., Arigbede, O.M., Ojo, V.O.A.: Nutritional evaluation of sugarcane top ensiled with varying proportion of broiler litter. Livest. Res. Rural Dev. 29 (2017)
Wardynski, F.A., Rust, S.R., Yokoyama, M.T.: Effect of microbial inoculation of high-moisture corn on fermentation characteristics, aerobic stability, and cattle performance. J. Anim. Sci. 71, 2246–2252 (1993)
Filya, I., Ashbell, G., Hen, Y., Weinberg, Z.G.: The effect of bacterial inoculants on the fermentation and aerobic stability of whole crop wheat silage. Anim. Feed Sci. Technol. 88, 39–46 (2000)
Demirel, M., Yilmaz, İ, Deniz, S., Kaplan, O., Akdeniz, H.: Effect of addition of urea or urea plus molasses to different corn silages harvested at dough stage on silage quality and digestible dry matter yield. J. Appl. Anim. Res. 24, 7–16 (2003)
Fellner, V., Phillip, L.E., Sebastian, S., Idziak, E.S.: Effects of a bacterial inoculant and propionic acid on preservation of high-moisture ear corn, and on rumen fermentation, digestion and growth performance of beef cattle. Can. J. Anim. Sci. 81, 273–280 (2001)
McDonald, P.: Animal nutrition, 6th edn. Longman scientific and technical Copublished in the USA, John Wiley and Sons (2002)
Chauhan, N., Singh, D., Kumari, N., Tyagi, N.: Impact of lactic acid bacteria and enzymes on the fermentation processes of sugarcane tops silage at a particular time interval. J. Pharm. Innov. 10, 332–336 (2021)
Britt, D.G., Huber, J.T., Rogers, A.L.: Fungal growth and acid production during fermentation and refermentation of organic acid treated corn silages. J. Dairy Sci. 58, 532–539 (1975)
Kung, Jr, L., Stokes, M.R.: Analyzing silages for fermentation end products. Univ Del Coll Agric Nat Resour, (2001)
Filya, I.: The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages. J. Appl. Microbiol. 95, 1080–1086 (2003)
Weiss, K., Kroschewski, B., Auerbach, H.: Effects of air exposure, temperature and additives on fermentation characteristics, yeast count, aerobic stability and volatile organic compounds in corn silage. J. Dairy Sci. 99, 8053–8069 (2016)
McAllister, T.A., Selinger, L.B., McMahon, L.R., Bae, H.D., Lysyk, T.J., Oosting, S.J., Cheng, K.J.: Intake, digestibility and aerobic stability of barley silage inoculated with mixtures of Lactobacillus plantarum and Enterococcus faecium. Can. J. Anim. Sci. 75, 425–432 (1995)
Kung, L., Jr., Smith, M.L., da Silva, E.B., Windle, M.C., da Silva, T.C., Polukis, S.A.: An evaluation of the effectiveness of a chemical additive based on sodium benzoate, potassium sorbate, and sodium nitrite on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 101, 5949–5960 (2018)
Neureiter, M., dos Santos J.T., Lopez, C.P., Pichler, H., Kirchmayr, R., Braun, R.: Effect of silage preparation on methane yields from whole crop maize silages. In Proceedings of the 4th international symposium on anaerobic digestion of solid waste. Copenhagen: BioCentrum-DTU 1, 109–115 (2005)
Woolford, M.K.: Microbiological screening of the straight chain fatty acids (C1–C12) as potential silage additives. J. Sci. Food Agric. 26, 219–228 (1975)
Danner, H., Holzer, M., Mayrhuber, E., Braun, R.: Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 69, 562–567 (2003)
Ranjit, N.K., Kung, L.I., Jr.: The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 83(3), 526–535 (2000)
Kung Jr, L.: Understanding the biology of silage preservation to maximize quality and protect the environment. InProceedings, California Alfalfa & Forage Symposium and Corn/Cereal Silage Conference, isalia, CA: University of California, Davis, CA. 1–2 (2010)
Kung, L., Jr., Shaver, R.D., Grant, R.J., Schmidt, R.J.: Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 101, 4020–4033 (2018)
Liu, B., Huan, H., Gu, H., Xu, N., Shen, Q., Ding, C.: Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219 (2019)
Wang, T., Teng, K., Cao, Y., Shi, W., Xuan, Z., Zhou, J., Zhang, J., Zhong, J.: Effects of Lactobacillus hilgardii 60TS-2, with or without homofermentative Lactobacillus plantarum B90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 312, 123600 (2020)
Basso, F.C., Adesogan, A.T., Lara, E.C., Rabelo, C.H., Berchielli, T.T., Teixeira, I.A., Siqueira, G.R., Reis, R.A.: Effects of feeding corn silage inoculated with microbial additives on the ruminal fermentation, microbial protein yield, and growth performance of lambs. J. Animal Sci. 92, 5640–5650 (2014)
Herrmann, C., Idler, C., Heiermann, M.: Improving aerobic stability and biogas production of maize silage using silage additives. Bioresour. Technol. 197, 393–403 (2015)
Additives, S.: Bezabih, M., B.J.O.J.A.S. Tamir. Review 4, 258–274 (2014)
Wilkinson, J.M., Davies, D.R.: The aerobic stability of silage: key findings and recent developments. Grass forage Sci. 68, 1–9 (2013)
Pahlow, G., Muck, R.E., Driehuis, F., Elferink, S.J., Spoelstra, S.F.: Microbiology of ensiling. Silage Sci. Technol. 42, 31–93 (2003)
Borreani, G., Tabacco, E., Cavallarin, L.: A new oxygen barrier film reduces aerobic deterioration in farm-scale corn silage. J. Dairy Sci. 90, 4701–4706 (2007)
Lima, L.M., Dos Santos, J.P., Casagrande, D.R., Ávila, C.L., Lara, M.S., Bernardes, T.F.: Lining bunker walls with oxygen barrier film reduces nutrient losses in corn silages. J. Dairy Sci. 100, 4565–4573 (2017)
Akhtar, MF., Ahmad, AU., Ibni Zamir MS., Khalid F., Mohsin AU., Afzal M.: Agro-qualitative studies on forage sorghum (Sorghum bicolor L.) sown alone and in mixture with forage legumes. Pak. J. Sci. Jun. 65, (2013)
Rossi, F., Dellaglio, F.: Quality of silages from Italian farms as attested by number and identity of microbial indicators. J. Appl. Microbiol. 103, 1707–1715 (2007)
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Nutan Chauhan], [Neelam Kumari], [Veena Mani], [Diwas Pradhan], [Gopal R. Gowane], [Sachin Kumar], and [Nitin Tyagi]. The first draft of the manuscript was written by [Nutan Chauhan] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chauhan, N., Kumari, N., Mani, V. et al. Effects of Lactiplantibacillus plantarum, Limosilactobacillus fermentum, and Propionic Acid on the Fermentation Process Of Sugarcane Tops Silages Along with Variations in pH, Yeast and Mould Count After Aerobic Exposure. Waste Biomass Valor 15, 2215–2230 (2024). https://doi.org/10.1007/s12649-023-02280-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02280-8