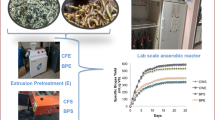
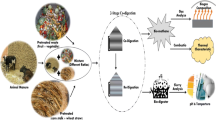
Abstract
Purpose
This study was carried out to explore the potentials for producing bioelectricity, biogas and bio-fertilizer from cassava peels, cassava stem biochar and cow dung using integrated biorefinery approach.
Materials and Methods
Four 5-L capacity bioreactors were constructed using 5-L plastic containers. Each bioreactor was charged with 800 g cassava waste peels (CPL), 200 g cow dung (CD) and 3-L cassava mill effluent (CME). Thereafter, 0, 10, 20 and 30 g of cassava stem biochar were respectively added to each of the bioreactors and sealed for 15-day Hydraulic Retention Time (15-d HRT) analysis. The experiments were carried out in duplicates. The effects of different doses of cassava stem biochar on bioelectricity, biogas and bio-fertilizer production were investigated.
Results
Power density (94.33 ± 2.99 mW/m2) elicited by CPL with 30 g biochar was 7.36-fold higher than the control, while optimum volume (79 ± 0.14 ml) of biogas produced was 102% higher than the control after 15-day HRT. Analysis of the digestate microbial community showed Methanomassiliicoccus and Methanobrevibacter as methanogens present, while Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria were the dominant phyla. Substrate parameters such as organic carbon, volatile solids, and COD were significantly reduced after digestion. The digestate cured with isolated potassium and phosphate solubilizers (biofertilizers) was used to supplement soil for cultivation of Zea mays and resulted in 68.8% total length increase.
Conclusion
The results from this study suggest that the biorefinery approach for waste valorization is sustainable and good for bioeconomic value addition.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
This study was carried out to explore the potential of producing biogas, bioenergy, bioelectricity and bio-fertilizer from cassava waste peels mixed with cow dung and cassava stem-based biochar using the integrated biorefinery approach unlike the single biorefinery approach. The work was designed to deepen our knowledge for the valorization of the huge cassava processing wastes that abound in Africa, especially Nigeria. It explored ways to address the paucity of data in the use of cassava stem-based biochar in biogas, bioenergy and biofertlizer production. The effects of the produced biofertilizers in the supplementation of garden loam soil for cultivation of Zea mays were also studied.
Introduction
In the tropics, cassava is regarded as the third most important source of calories, after rice and maize. About 550 million metric tons (MMT) of cassava are produced each year, resulting in 350 MMT of solid cassava wastes such as bagasse, peel, stem, rhizome, and leaves [53]. Cassava waste water contains as much suspended solids as up to 15,000 mg/L which are many occasions not treated before being released into the environment thus, contaminating nearby land [67] and water resources in the process. These wastes possess serious environmental threat due to acidification via hydrolysis of cassava cyanogenic glucoside, linamarin and lotaustralin (Methyl linamarin) which produces hydrogen cyanide (a compound toxic to household animals, fisheries and other organisms) [68]. According to [69] Adult female catfish, Clarias gariepinus was shown to have signs of gill and liver damage due to exposure to cassava waste water, with histopathological examination carried out on the gill, kidney and liver of fingerlings of the Nile Tilapia (Oreochromis noliticus) also revealing damages to these organs due exposure to cassava waste water. Omotosho and Amori [69] also reported anomalies in cell division process and chromosome aberration induction in Allium cepa root meristem due to cassava waste water heavy metal-cyanide interaction. These wastes can be employed for solid, liquid, and gaseous biofuels production [53]. According to Ekpo [18], one litre of cassava wastewater produces 16 ml and 9 ml of bioethanol and bio-methane respectively, while one kilogram of cassava peelings produces 226 ml of bioethanol and 118 ml of bio-methane. One kilogram of bagasse produces 20 ml of bioethanol and 10 ml of bio-methane. Okunade and Adekalu [, 71, 73] reported that cassava waste water which is five times denser than water contains alcohols, acids, and other organic compounds (Table 1). Whereas, Aripin et al. [, 72, 73] found that cassava solid waste was rich in holocellulose, cellulose, hemicellulose, lignin, and ash content with 1% of sodium hydroxide and hot water solubility (Table 1).
The current over-dependence on crude oil for the synthesis of fuels and chemicals is not sustainable and poses a great challenge due to its negative impact on climate change and other adverse environmental effects [, 17, 35]. This has reawakened interest in the development of greener and cleaner alternatives to fossil fuels [, , 1, 12, 16]. Several researchers have proposed the concept of bioeconomy, which is centred on biomass sustainability, for the diversification of bio-based processes [8]. Many bacteria can catalyze biochemical reactions to produce bioelectricity via electron transfer, and this is what is now classified as Microbial fuel cells (MFCs).
In this bioelectrochemical process, electrons are released in the anode compartment (the negative terminal) via substrate oxidation by bacteria are transferred to the cathode compartment (the positive terminal) through a conductive material, whereas, in the cathode oxygen combines with the electrons causing the protons to diffuse through a proton exchange membrane [65].
Lignocellulosic materials from agricultural wastes are abundant and easily accessible naturally. Thus, they are sustainable bioenergy sources. Anaerobic digestion (AD) for the efficient use of lignocellulosic materials for the production of biogas has been studied extensively [5]. Cassava food crop has been used effectively as raw material for agro-based industries in the production of starch, bioethanol and other bio-based products. Huge quantities of wastes, that are abundant in organic matter and suspended solids, are generated from cassava-based industries. However, these generated wastes constitute weighty environmental concerns. Their multiple biochemical constitutions, as well as appreciable organic contents, avail them of the potential for bioconversion into value-added products via biorefinery, thus, providing environmental and economic sustainability to cassava processing industries [, 18, 64]. To improve the production of biogas from food wastes, various steps such as boosting operational parameters, additives application, and recirculation of digestate, regular feeding, and feedstock pretreatments can be explored [36]. Biochar has proven to be a viable additive in achieving steady and effective methane production during AD amongst other optimization procedures [51]. Biochar, as carbonaceous material, can be produced from several organic waste feedstocks such as municipal sewage sludge and agricultural wastes [56]. This could be done through the combustion of plant and animal materials in oxygen-limiting condition at particular temperatures. This combustion results in increased absorbing capacity for chemicals and compounds onto the surfaces of the biochar [20]. Biochar has been reported to be an extremely versatile material, economical and reliable additive in AD, which aids in microbial enrichment and biogas production boost [, 14, 32]. It is imperative to note that when biochar-amended digestate is augmented with nutrients such as phosphorus, potassium and nitrogen, it subsequently develops huge capability for soil applications [50] and can, thus, be used to improve the soil ecosystem [20].
In most previous studies, the valorization of cassava peels to biofuels and biofertilizers was considered only a single refinery process, thus discarding the useful biomass that could serve as raw materials for further successive biorefinery operations. This study, therefore, employed the integrated biorefinery approach to produce biofuels (biogas and bioelectricity) and bio-fertilizer from cassava peels, cow dung and cassava stem biochar.
Materials and Methods
Waste Collection, Preparation and Charring
Fresh samples of cassava solid (cassava peels (CPL), Cassava stems (CS), and cassava mill effluent (CME) wastes were collected from a local cassava processing factory located in Agbani community while the cow dung used as inoculum was collected from Ikpa market abattoir, all located in Nsukka Local Government Area of Enugu State, Nigeria. The CS and CPL were washed three times in running tap water to remove impurities. The washed CS and CPL were mechanically crushed in bits with a mechanical grinding machine. Thereafter, the crushed CPL was stored in plastic bags prior to use, while the CS was pyrolyzed at 400 °C for 1 h in the absence of oxygen and stored in an airtight bag after cooling prior to use. The CME was sieved with a 600 µm size mesh and thereafter, the filtrate was collected with a clean plastic container and stored prior to use.
Bioreactor Setup for Anaerobic Digestion
Four pilot scale 5 L (L) capacity bioreactors were constructed using 5L plastic containers with airtight lids. For each bioreactor, two graphite electrodes (1.5 cm diameter and 16 cm long) were inserted through two openings on the lid into the anode and cathode chambers of the bioreactor. Thereafter, the electrodes from each chamber were connected using a number 4 copper wires. Three (3) other openings were made on the lid of the cathode chamber, where a thermometer (for temperature reading), a rubber tubing that extends to the base of the chamber with a tight clip to maintain anaerobiosis (for sample collection), and another rubber tubing just above the liquid level (for gas collection) were inserted. The gas collection was by upward delivery and downward displacement of water (Fig. 1). The four bioreactors containing varying doses of biochar (0 g, 10 g, 20 g and 30 g) in the anode chamber were each charged with 800 g of crushed CPL, 200 g of cow dung and 3 L of CME and mixed together to make a 4 L mixture. The controls were bioreactors containing 4 L of water alone in place of the CPL, Cow dung and CME mixture. The bioreactors were then properly sealed using Epoxy glue to prevent air influx. Anaerobic digestion was carried out for 15 days. The experiments were carried out in duplicates. The volume of biogas produced was measured every 24 h by calculating the volume of water displaced into the collection bottle. The power outputs (current and voltage) were recorded every 24 h using a digital multimeter (model NENG™ Q1). The slurries (mixtures) were sampled before and after the 15-day digestion to determine the total solids (TS), volatile solid (VS), chemical oxygen demand (COD), organic carbon (OC), nitrogen content and pH values.
Determination of Bioenergy Generation and Parameter Change in the MFC
Current and Voltage Generation Determination
Current (I) and voltage (V) measurements were done using an auto-range digital multimeter (model NENG™ Q1). The readings were taken and recorded by connecting the multimeter to the anode and cathode terminals of each digester. Power (W) was calculated using formula:
where I and V represents current and voltage respectively.
Determination of Power Density
The quantity of power produced per unit area is known as power density. The anode surface area was utilized to determine power density since all biological reactions, including degradation and anion generation, take place at the anode [31]. The power density (P) based on the surface area of the graphite rod anode (m2) was determined according to the formula of Momoh and Naeyor [37].
where I: current (in mA); V: voltage (V); and A: surface area (m2).
Determination of Current Density
The quantity of current flowing through per unit area is known as the current density. The anode was also utilized because it is where all biological actions occur [39]. The current density (mA/m2) was determined using the formula:
where J is current density (mA/m2), A is area of the anode (m2) and I is flowing current (mA).
Determination of COD Removal Efficiency
The efficiency of COD removal was calculated using the formula of Kumar et al. (2020):
where Ci is initial value of COD and Cf is the final value of the COD
Parameters Change
The changes in the different physicochemical parameters (COD, TS, VS, pH, OC and NC) were calculated using the formula:
Microbial Isolation, Screening and Digestate Curing
Composite soil samples were obtained using a sterile spatula from 4 cm depths at different spots at the cassava processing factory dumpsite. The samples were transported to the laboratory in sterile plastic containers for storage at 4 °C prior to use. Thereafter, bacteria and fungi were isolated from the soil samples by inoculating 1.0 g of each soil sample into different test tubes containing 9 ml of sterile distilled water. Six-fold serial dilution was done, after which a loopful of the diluted sample was used to inoculate freshly prepared nutrient agar (NA: 2.0 g/L yeast extract, 5.0 g/L sodium chloride, 15 g/L agar) and Sabouraud dextrose agar (SDA: 40 g/L dextrose, 10 g/L peptone, and 20 g/L agar) respectively using standard protocol [66]. The NA plates were incubated at 30 °C for 24 to 48 h, while the SDA plates were incubated at 30 °C for 3 to 5 days. Morphologically distinct colonies were purified by step-wise sub-culturing onto the same freshly prepared medium plate. The pure isolates were stored in slants at 4 °C for further use.
Qualitative Isolate Screening for Phosphate and Potassium Solubilizers
For Phosphate solubilization, the Pikovskaya medium used was prepared by dissolving 0.5 g (NH4)2SO4, 0.1 g MgSO4·7H2O, 0.02 g NaCl, 0.003 g FeSO4·7H2O, 0.003 g MnSO4·H2O, 0.02 g KCl, 5 g Ca3 (PO4)2, 0.5 g yeast extract, 10.0 g glucose and 15.0 g agar in 1 L of distilled water. The medium was thereafter autoclaved at 121 °C for 15 min and 20 ml of each of the sterilized molten agar media was poured into different sterile petri dishes and allowed to solidify before inoculation. Spot inoculation of bacterial and fungal isolates was made onto the respective petri dishes and incubated at 30 °C for 72 h. This was done in triplicates. Uninoculated Pikovskaya medium plate served as the control. The formation of clear zones around a colony was indicative of phosphate solubilization potential by the isolate. The solubilization index for the isolates was calculated using the formula [7].
C = Colony diameter in millimeter, Z = Halo zone diameter in millimeter.
For Potassium solubilization, the Aleksandrov medium containing (g/L) 3.0 g potassium aluminum silicate, 0.5 g MgSO4.7H2O, 0.1 g CaCO3, 5.0 g glucose, 0.006 g FeCl3, 2.0 g Ca3(PO4)2, 20.0 g agar in 1 L of distilled water was used [23]. The medium, after sterilization, was transferred into sterile plates to solidify at room temperature. Screening for potassium solubilization was carried out according to the method described by Hu [23]. The isolates (fungal and bacterial) were spot inoculated onto prepared sterile petri dishes in triplicates and incubated at 30 °C for 72 h. The formation of a clear zone around an isolate was indicative of potassium solubilization potential of the isolate. The solubilization indices were determined using the formula:
where C = Colony diameter in millimeter, Z = Halo zone diameter in millimeter.
The isolates with significant solubilization indices for both phosphorous and potassium were selected and stored at 4 °C for further use.
Digestate Curing for Biofertilizer Formulation and Agronomic Test
Bacterial isolates used for digestate curing were standardized according to the method described by Elias et al. [19]. About 10 loop full of 24 h freshly prepared cultures of the selected phosphate and potassium bacterial solubilizers were inoculated into 50 ml nutrient broths, and incubated at 30 °C for 24 h at 120 rpm in an IKA KS 4000i rotary shaker. Thereafter, the bacterial cells were harvested by centrifuging at 5000 rpm for 10 min using a Hettich Rotoflix 11 centrifuge. The supernatant was discarded, and the cells were re-washed and vortexed thrice with normal saline. After washing, the cells were resuspended in normal saline. For the selected fungi, fresh spores of the fungal phosphate and potassium solubilizers were grown on SDA medium at 30 °C for 5 days. Using 5 ml of sterile 0.2% Tween-20 with gentle scraping, the spores were put in suspension and counted using a Neubauer counting chamber. One millilitre (1 ml) of the bacterial suspension (containing 106 CFU of each bacterial isolate) or fungal suspension (containing 108 spores of each fungal isolate) was used to cure 10 g of the digestate for 21 days at 30 °C in an open aluminum pan before use in biofertilizer formulation. The biofertilizer formulation was done according to the method described by Ogbo [41].
Five hundred grams (500 g) of screened loam soil (without debris, broken bottles and stones) was collected from the University of Nigeria Agriculture farm and moistened slightly with water at field capacity before it was packaged into polyethene bags and sterilized thrice at 120 °C for 50 min at 48 h intervals. The sterile loam soil in the polyethene bags was then mixed with the biofertilizers in the ratio of 10:1 and grouped as follows: Pot1-B5CPL; Pot2- B7CPL; Pot3-B24CPL; Pot4-F1CPL; Pot5-F3CPL; Pot6-F15CPL; Pot7- control (un-inoculated). Each group was done in triplicate and seeded with three seeds of maize (Zea mays). The seeded pots were frequently watered uniformly in a greenhouse (tunnel) for 20 days. The germination counts were taken at weekly intervals up to the third week to determine the germination indices.
Measurement of Germination Indices
The Germination percentage (GP) and germination rate indices (GRI) were determined using the formula of Shinde et al. [52]:
Bacterial Community Analysis
The liquid digestate samples at the end of the 15 day digestion in Sect. "Bioreactor Setup for Anaerobic Digestion" were collected and examined for the bacterial community composition. The bacterial communities attached to the anode chamber were analyzed by high-throughput metagenomic sequencing using the 16S rRNA. Digestate samples taken at the 15th day for analysis of bacterial and methanogenic communities were centrifuged at 12,000×g for 15 min. Thereafter, 0.4 g of the residue was used to extract genomic DNA of microbial communities using DNA isolation kits (Mo Bio Laboratories, Inc. Carlsbad, USA). The DNA quality was confirmed using a 1.0% agarose gel electrophoresis, and measured at 260 and 280 nm wavelengths. Amplifications of the V3–V4 region of 16S rRNA gene in bacterial and methanogenic communities were performed via the polymerase chain reaction (PCR). A set of primers ARC787F (5′-ATTAGATACCCSBGTAGTCC-3′) and ARC1059R (5′-GCCATGCACCWCCTCT-3′) were used to target methanogenic communities, while another set of primers 1369F (5′-CGGTGAATACGTTCYCGG-3′) and 1492R (5′-GGWTACCTTGTTACGACTT-3′) were used to target bacterial communities. Subsequently, sequencing of the DNA samples was conducted by Illumina Hiseq 2000 pyrosequencing platform. The phylogenetic relationship was analysed using Graph Pad Prism (version 7.04) and Microsoft excel (2013 version).
Statistical Analysis
All the data obtained were statistically analyzed using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests. The correlation regression analysis was also done to ascertain the effect of biochar dosage on biogas and bioelectricity production as well as the germination index potentials of the biofertilizers at p < 0.05. Evaluations of all graphs in this study were made using GraphPad Prism 7.04.
Results and Discussion
The Effect of Different Doses of Biochar on Current and Voltage Generation
Maximum current and voltage were elicited at different times by different biochar concentrations (Fig. 2). The values of the current and voltage produced by each biochar concentration were significantly higher than the control and increased progressively as the biochar concentrations were increased from 10.0 to 30.0 g. The addition of biochar caused a surge in the power generation in all the digesters with biochar up to the 168th h anaerobic digestion. Thereafter, there was a steady decline in power up to the 360th h. This could be attributed to the gradual depletion of the readily available nutrients and shift in the microbial community present in the batch reactors. On initiation of the AD, the power output was extremely minimal probably because the microbial biofilm had not yet been established on the surface of the anode [22]. Biochar dose of 30 g gave the highest power density (94.33 ± 2.99 mW/m2) which was about 7.36-fold higher than the value of the control (12.81 ± 2.1 mW/m2) (Fig. 3). The increased power density observed with increased biochar concentration suggests that the biochar used in this study may be a good catalyst for microbial fuel cells probably due to its excellent electrocatalytic activity in promoting oxygen reduction reaction (ORR) [62]. ORR is currently reported as a major barrier in up-scaling the generation of bioelectricity in microbial fuel cell [, 27, 28]. The increased bioelectricity generation at higher doses of biochar in this study indicates the possibility of overcoming this limitation in up-scaling bioelectricity generation by using biochar. Furthermore, the use of waste-derived biochar in place of a catalyst to improve bioelectricity generation from CPL is considered cost efficient when compared to carbon, iron and nitrogen-based functionalized electrodes or cathode catalysts [62] at the commercial level for wastewater treatment and generation of power for low-energy devices. It is a welcome development as reported by Chakraborty et al. [11]. Interestingly, biochar dosing in this study demonstrated triple positive effects on commercial values. For instance, it enhanced waste treatment and electricity generation as reported by Hassan et al., [22], and simultaneous biogas production probably via direct interspecies electron transport (DIET) as observed by Onwosi et al. [43].
Effect of Different Doses of Biochar on Biogas Production
The 5-L bioreactor design and set-up for anaerobic digestion are shown in Fig. 1. Biogas production commenced within 24 h of the digestion for all the biochar concentrations. However, the days of optimum production varied at different concentrations of biochar (Fig. 4). This appears to be biochar concentration dependent as the cumulative volume of biogas produced (79.8 ± 0.14 ml) with addition of 30.0 g biochar was expressively higher than the values generated by other concentrations, while the control (without biochar) yielded the least value (Fig. 5). The decline in biogas production over time could be linked to the gradual exhaustion of the readily accessible nutrients for the microorganisms to metabolize in the biodigester. Several workers have reported increased biogas production at increased biochar concentrations [, , 34, 56, 63]. However, in the present study, concentrations above 30.0 g of biochar were not tested. The commencement of biogas production within 24 h of charging the digesters is in agreement with the report of Saravanan and Manikandan [49] on water hyacinth systems. On the contrary, Dahunsi and Oranusi [15], in their work, reported that biogas generation commenced on the ninth day. This may be due to different substrates or biochar sources used in the various studies. According to Pan et al. [45], some important functions of biochar include enhancing and balancing the hydrolysis, acidogenesis-acetogenesis, and methanogenesis stages of biogas production as well as relieving inhibitor stress, support for microbial colonies, and a reinforcer for buffer ability. Being a carbonaceous adsorbent material, biochar has the potential to sorb chemicals onto its surface [20]. It is envisaged that cumulative biogas production from all the digesters would increase over time. Thus, the increased cumulative production of biogas from all digesters during anaerobic digestion was expected, considering that the amendment of the cassava processing wastes CPL with cow dung inoculum introduced a consortium of microorganisms. These microorganisms were synergistically availed of essential nutrients for optimum activities throughout the anaerobic digestion. Digesters with different doses of biochar yielded significant biogas when compared with digesters without biochar (control). This agrees with Luo et al. [33], who reported that the addition of biochar (10 g/L) to mesophilic anaerobic digesters decreased the methanogenic lag phase and upstretched the maximum methane production rate when compared with the controls without biochar. Biochar undoubtedly has proven effective as an additive that enhances methane production via anaerobic digestion [51]. In this study, the optimum biochar dosage range was ascertained to be 20 g to 30 g per 4-L working volume for CPL.
Physicochemical Parameters of the Digestate
There were progressive reductions in the COD, OC and VS as the dose of biochar increased in all the setups except in the digester with 10 g biochar, which had the highest COD reduction (81.0%) after digestion (Table 2). This was consistent with the feasibility study of Rashid et al. [47] on paraboloid graphite-based microbial fuel cells for bioelectrogenesis and pharmaceutical wastewater treatment, which showed a considerable COD reduction of 80.55%. However, in this study, the percentage reduction of the COD was 65.3% for 30 g biochar addition, 58.5% for 20 g biochar addition and 60.0% for no biochar addition (Control).
Reductions of 60.6, 54.4, 53.5, and 51.5% were observed in the organic carbon (OC) after digestion for 0, 10, 20 and 30 g biochar additions respectively. Organic carbon reductions of 69.21% and 74.3% have been reported by Kumar et al., [25] and Owamah et al. [44] respectively. Though their rates of reduction were much higher than the rate in the study, this could be linked to the HRT of their respective studies. The VS percentage change results after digestion showed that CPL with no biochar addition had the highest reduction of 69.5%, followed by 10 g biochar addition (59.5%), 20 g biochar addition (49.7%) and 30 g biochar addition (43.6%). The highest reduction in VS was congruent with the report of Kumar et al. [25] which showed a reduction of 68.69% in volatile solid. The observed reductions in feedstock before digestion may be attributed to the biodegradation of the organic matter present in the substrate due to the actions of mesophilic microorganisms.
It was observed that before the digestion, the addition of biochar increased the concentration of TS, NC, and pH in all the CPL digesters as shown in Table 2. However, there was a corresponding reduction of TS, NC, COD, OC, and NC after digestion at different biochar doses. The percentage change increase for TS was 47.5, 50.0, 45.1 and 56.4% for 0, 10, 20 and 30 g biochar addition respectively. Owamah et al. [44], noted an upsurge of 12.4% in TS after the co-digestion of food wastes and human excreta for biofertilizers production. There were 52.1, 52.2, 52.9 and 60.2% NC increases in the digesters with 0, 10, 20 and 30 g biochar additions respectively.
Throughout the duration of the anaerobic digestion, the pH values fluctuated between 6.25 and 7.15 and this aligns with the report of Shen et al. [51] which showed that pH fluctuated between 6.6 and 7.4 throughout the anaerobic digestion period. For temperature, it was observed to be between 25 and 360C all through the period of the anaerobic digestion process. This agrees with Giwa et al. [21] who studied the performance of anaerobic digestion in reactors with biochar and noted that as biochar addition improved, there was higher methane yield and reactor stability under mesophilic temperatures. The temperatures were between 26 and 36 °C. This also is in accord with the study of Oladejo et al. [42].
The C/N ratio was within the normal range both before and after digestion. This could be ascribed to the substrate which served as both carbon and nitrogen source. Also, the metabolic activities of the diverse microorganisms in the bioreactors could have played a role.
Solubilization Indices and Identification of Microbial Isolates
A total of 55 strains (30 bacterial and 25 fungal) were isolated from the soil samples. Based on the screening potentials on agar plates (Pikovskaya and Alexandrov respectively), nine out of the 30 bacterial isolates (30%) and seven out of the 25 fungal isolates (28%) had halo zones (Fig. 6). However, three each of the bacterial (b5, B7 and B24) and fungal (F1, F3 and F15) isolates were selected based on their high solubilization indices (SI). The selected bacterial and fungal strains had the potential for simultaneous solubilization of both phosphate and potassium as suggested in the study of Muthukumarasamy et al. [38] that urged farmers to employ a single microbe for microbial supplementation of N, P and K rather than a consortium of bacteria in order not to jeopardize the compatibility between various microbes.
The selected bacterial isolates (B5, B7, and B24) had the best solubilization indices of 6.55 ± 0.36 mm, 5.47 ± 0.56 mm, 6.07 ± 1.02 mm and 5.78 ± 0.44 mm, 7.16 ± 1.62 mm, 5.41 ± 0.17 mm for phosphorus and potassium respectively. Again, the selected fungal isolates (F1, F3 and F15) had the best solubilization indices of 6.08 ± 0.42 mm, 5.86 ± 0.32 mm, 5.51 ± 0.17 mm and 5.41 ± 0.66 mm, 7.99 ± 0.44 mm, 5.04 ± 0.25 mm for phosphate and potassium respectively. The phosphate and potassium solubilization indices for both bacterial and fungal isolates in this study are significantly higher than the values of 1.63–3.29 mm reported by Alam et al. [3]. However, findings from this study are in agreement with the range of values reported by Batool and Iqbal [7]. In addition, Kumari et al. [26] reported index values in the range of 8 to 13 mm for phosphate solubilization and 9.4 to 15 mm for potassium solubilization. Also, Aliyat [4] reported a bacterial solubilization index of 4.79 mm. The observed disparities in the ranges of the solubilization indices could be a pointer that different strains of microorganisms have different capacities or potentials for solubilization. Furthermore, different environmental factors, such as temperature and pH, can either promote or retard the solubilization processes.
The six (6) selected microbial strains used in this study (Plate 1) were identified using 16S rRNA gene sequence comparisons. The B5 isolate is Stenotrophomonas rhizophila strain 16YSM- P 39 16S, B7 is Alcaligenes faecalis strain C1FT MFB 14,415 (VRL 20) 16S, B24 is Alcaligenes faecalis strain BT10 16S, F1 is Aspergillus aculeatus strain JO6 18S, F3 is Aspergillus aculeatus strain CLSS 18S and F15 is Aspergillus alliaceus strain IHEM 22,316.
Formulation of Biofertilizers and Agronomic test
Phosphate and potassium solubilizers caused substantial increases (p < 0.05) in seed germination indices analysed (Fig. 7). The Zea mays planted in the soil inoculated with the selected bacterial strains had Zea mays had higher total lengths than Zea mays planted in the soil inoculated with selected fungal strains. When total mean lengths elicited by the biofertilizers were compared with the controls, there were significant increases in pot3-B24CPL (88.7 ± 13 mm), pot2-B7CPL (76 ± 15 mm), pot1-B5CPL (74.6 ± 15 mm), pot5-F3CPL (70.3 ± 17 mm), pot6-F15CPL (65.8 ± 8 mm) and pot4-F1CPL (58.3 ± 4 mm), while the control (uninoculated) had value of 40.55 ± 14 mm. The B24CPL had the highest total length of 88.7 ± 13 mm with a corresponding root length of 24.5 ± 1.2 mm, while F1CPL had the least total length of 58.3 ± 4 mm with a corresponding root length of 12.3 ± 5.5 mm amongst the planted seeds. These results agree with the report of Shinde et al. [52] and support the suggestion that the selected bacterial and fungal isolates selected are good phosphate and potassium solubilizers. Thus, they are good biofertilizers with potentials to improve maize crop production. In addition to improving crop production, the application of biofertilizers has the dual advantage of environmental sustainability and bioeconomy as against the application of chemical fertilizer [, 2, 7]. Table 3 shows the standard specification of biochemical properties of biofertilizer as adapted from Abdullahi et al. [70]. Furthermore, the biofertilizers have potential for commercialization, which is good for bioeconomy although the genetic stability should be verified. Several other authors have reported on the use of biofertilizers for the cultivation of maize seedlings [, 24, 48]. Table 4 shows effects of biofertilizers on Zea mays germination from this study.
Community Analysis of the Microbes
The bacterial and archaeal populations were assessed using Illumina sequencing of 16S rRNA gene amplicons. The sludge/digestate samples were collected at the end of the bioelectricity generation and biogas production phase for community analysis of the microbes. The preferred sequencing primer group for the 16S pyrosequencing allowed direct confirmation to be established for the classification of the microbial communities in the biodigester and also captured their relative abundance. As shown in Fig. 8, the top 5 dominant bacterial phyla in the digesters amended with biochar were Firmicutes (73.99%), Bacteroides (17.90%) Actinobacteria (5.18%), unclassified at the phylum level (2.67%) and Proteobacteria (0.08%), covering 99.82% of total abundance.
The dominant bacterial community in this study was consistent with the work of Sun et al. [55] who reported that acetic acid and hydrogen generation were carried out by Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. They were also found to dominate in the anaerobic digester of beer lees [55]. Firmicutes have the potential for secreting varieties of extracellular enzymes, which contain cellulases, proteases and other enzymes that facilitate complex macromolecules hydrolysis [13]. The dominant phyla in this study were electricigens which are in conformity to previous studies [, , 30, 58, 61]. Electricigens are essential as biocatalysts for MFCs. In MFCs, several isolated electricigens are employed. However, most of these electricigens belong to Proteobacteria and Firmicutes. Diverse types of electricigens have various electron transmission methods and capacities, which directly impact on the performance of MFC and are critical in determining the performance and application of the MFC [60]. The majority class of electricigens, which are the dominating strains in the microbial communities of MFCs, are represented by Proteobacteria. Many are capable of transferring electrons directly to the electrode. In the Proteobacteria phylum, electricigens are widely distributed in α-proteobacteria, β-proteobacteria, δ-proteobacteria and γ-proteobacteria [10]. Short-chain fatty acids and aromatic acids, such as cyclohexane-1-carboxylate, benzoate, or butyrate, are broken down by the metabolic expert proteobacteria into acetate, CO2, formate, and H2. However, to remove H2 and formate and maintain thermodynamically favourable catabolic processes, the system needs a hydrogenotrophic partner [6]. Proteobacteria typically carry out the direct electron transfer to the electrode [57], while Bacteroidetes are associated with the hydrolysis of complex macromolecular carbohydrates [, 13, 54]. There are reports that Phylum Actinobacteria ferment pentoses and metabolize oligosaccharides to yield lactic and acetic acids. These bacteria develop best at optimum temperature and pH ranges of 39–41 °C and 6.4–7.0 respectively. They were first discovered as symbionts in the human intestine [, 9, 59]. These pH and temperature (mesophilic) values are in agreement with this study. Generally, Bacteriodetes, Firmicutes and Proteobacteria are the predominant phyla and comprise mainly of acidogenic bacteria. The hydrolysate monomers are fermented by these bacteria into acetate, alcohol, butyrate, propionate, CO2, H2, and other solvents. They have significant impacts on VFA concentration and distribution, which are also influenced by other AD digester's working parameters [6]. Firmicutes and Proteobacteria, which are the first and fifth most dominant phyla respectively in this study, are sulfate-reducing bacteria (SRBs). To oxidize organic materials and produce H2S, they use sulfate as an electron acceptor [29]. They break down propionate and butyrate which act as carbon sources and electron donors/acceptors to produce acetate, either independently or in syntrophic conjunction with hydrogen-scavenging methanogens [6]. The great efficiency of methane production and organic carbon removal in the biodigesters can be linked to the presence of Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria. However, the results revealed that the electrochemically active bacteria including Proteobacteria, Actinobacteria, Firmicutes, and Acidobacteria are present in higher proportions. Similarly, some previous works have reported the existence of exoelectrogenic bacterial species [, , , 30, 40, 58, 61].
At the class level, Bacilli, Bacteroidia, Actinobacteria, Clostridia, Unclassified at Class level, Erysipelotrichia, Negativicutes and Chloroplast were the most abundant bacterial communities. Furthermore, evaluating the bacterial taxonomy, a total of 206 types of OTUs (operational taxonomic units) were identified thus, revealing the diversities of bacterial microorganisms in the bioreactors. The most abundant genus is the Lactobacillus (Fig. 9), followed by the genus Bacteroides, as was reported by Giwa et al. [21]. These are typically present in anaerobic environments, where they primarily act as fermenters and acidogens. Hydrogenotrophic methanogen (Fig. 10) dominated the archaeal community density. Methanobrevibacter was the most detected methanogen at the genus level with a relative abundance of 75%, out-striving the Methanomassiliicoccus. Methanobrevibacter and Methanomassiliicoccus which exhibited a synergistic effect on methanogenic pathways. This conjointly enhanced the productivity of methane. Firmicutes, as well as Proteobacteria, have shown to have a dual purpose in the context of this study, as they aid both biogas production (hydrolytic, acidogenic and acetoclastic stages of biogas production) and bioelectricity generation as electricigen. This is in agreement with the work of Quashie et al. [46].
Additional studies would be essential in providing unambiguous experimental proof for the positive impact of higher biochar dosage and its effect on microbial dynamics. Furthermore, different kinds of biochar from different feedstock subjected to varying environmental conditions should be investigated in subsequent MFCs operations via an integrated biorefinery approach.
Conclusion
This study demonstrated an innovative bio-refinery process that has the potential for utilizing pyrolyzed cassava stem biochar mixed cassava waste peels (CPL) amended with cow dung in anaerobic digestion (AD) to simultaneously improve wastewater treatment, generate electricity and produce biogas and biofertilizer for agricultural benefits in MFCs. This concept has the potential for wastewater treatment through COD reduction which was observed to be highest (approximately 81%) with the addition of 10 g biochar in CPL slurries. The optimum power density elicited by CPL waste seeded with 30 g biochar (94.33 ± 2.99 mW/m2) was about 7.36-fold higher than the value (12.81 ± 2.1 mW/m2) produced with the control (CPL waste without biochar). The cumulative volume of biogas produced after the 15-day HRT was 79 ± 0.14 ml. Analysis of the microbial community of the slurry digestate showed that Methanomassiliicoccus and Methanobrevibacter were the main dominant methanogens present as methane formers while the bacterial phyla Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria were dominant as electricigens for electricity generation. Some physicochemical parameters of the CPL slurry digestate, such as organic carbon, volatile solids, and COD, were significantly reduced after anaerobic digestion. When the anaerobic digestate was seeded with locally isolated potassium and phosphate solubilizers (biofertilizers) and applied on the loam soil, the total length of the planted Zea mays increased by about 68.8% when compared to the control (without biofertilizer). The preferred optimal dosage range for a 4-L working volume in 5L digester batch AD operation was found to be 20–30 g biochar and not 10 g biochar. This study offers increased knowledge on cassava processing wastes beneficiation/value addition, and further explains the mechanism of biochar action in MFCs. The advocacy of large-scale production and application of this biorefinery approach will ultimately reduce our dependency on fossil fuels and chemical fertilizers which are deleterious to the environment and human.
Data Availability
Our manuscript has data included as electronic materials.
References
Ahmad, B., Yadav, V., Yadav, A., Rahman, M.U., Yuan, W.Z., Li, Z., Wang, X.: Integrated biorefinery approach to valorize winery waste: a review from waste to energy perspectives. Sci. Total Environ. 719, 137315 (2020)
Alam, S., Seth, R.K.: Comparative study on effect of chemical and bio-fertilizer on growth, development and yield production of paddy crop (Oryza sativa). Int. J. Sci. Res. (IJSR) 3(9), 411–414 (2014)
Alam, S., Khall, S., Ayub, N., Rashid, M.: In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganisms (PSM) from maize rhizosphere. Int. J. Agric. Biol. 4(4), 454–458 (2002)
Aliyat, F.Z., Maldani, M., Guilli, M.E., Nassiri, L., Ibijbijen, J.: Isolation and characterization of phosphate solubilizing bacteria from phosphate solid sludge of the moroccan phosphate mines. Open Agric. J. 14, 16–24 (2020). https://doi.org/10.2174/1874331502014010016
Amin, F.R., Khalid, H., Zhang, H., Rahman, S.U., Zhang, R., Liu, G., Chen, C.: Pretreatment methods of lignocellulosic biomass for anaerobic digestion Mini review. AMB Express 7, 72 (2017). https://doi.org/10.1186/s13568-017-0375-4
Amin, F.R., Khalid, H., El-Mashad, H., Chen, C., Liu, G., Zhang, R.: Functions of bacteria and archaea participating in the bioconversion of organic waste for methane production. Sci. Total Environ. (2020). https://doi.org/10.1016/j.scitotenv.2020.143007
Batool, S., Iqbal, A.: Phosphate solubilizing rhizobacteria as alternative of chemical fertilizer for growth and yield of Triticum aestivum (Var. Galaxy 2013). Saudi J. Biol. Sci. 26, 1400–1410 (2019). https://doi.org/10.1016/j.sjbs.2018.05.024
Broring, S., Laibach, N., Wustmans, M.: Innovation types in the bioeconomy. J. Clean. Prod. 266, 121939 (2020)
Bruno, F.A., Lankaputhra, W.E.V., Shah, N.P.: Growth, viability and activity of Bifidobacterium spp. in skim milk containing prebiotics. J. Food Sci. 67(7), 2740–2744 (2002)
Cao, Y., Mu, H., Liu, W., Zhang, R., Guo, J., Xian, M., Liu, H.: Electricigens in the anode of microbial fuel cells: pure cultures versus mixed communities. Microb. Cell Fact. 18(1), 1–14 (2019). https://doi.org/10.1186/s12934-019-1087-z
Chakraborty, I., Sathe, S.M., Dubey, B.K., Ghangrekar, M.M.: Waste-derived biochar: applications and future perspective in microbial fuel cells. Biores. Technol. 312, 123587 (2020)
Chandra, R., Iqbal, H.M.N., Vishal, G., Lee, H.S., Nagra, S.: Algal biorefinery: a sustainable approach to valorize algal-based biomass towards multiple product recovery. Review. Bioresource Technol. 278, 346–359 (2019)
Chen, M., Liu, S., Yuan, X., Li, Q.X., Wang, F., Xin, F., Wen, B.: Methane production and characteristics of the microbial community in the co-digestion of potato pulp waste and dairy manure amended with biochar. Renew. Energy 163, 357–367 (2021)
Chiappero, M., Norouzi, O., Hu, M., Demichelis, F., Berruti, F., Di Maria, F., Masek, O., Fiore, S.: Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 131, 110037 (2020)
Dahunsi, S.O., Oranusi, U.: Co-digestion of food waste and human excreta for biogas production. Br. Biotechnol. J. 3(4), 485–499 (2013)
Dessie, W., Luo, X., Wang, M., Feng, L., Liao, Y., Wang, Z., Yong, Z., Qin, Z.: Current advances on waste biomass transformation into value-added products. Appl. Microbiol. Biotechnol. 104, 4757–4770 (2020). https://doi.org/10.1007/s00253-020-10567-2
Dohaei, M., Karimi, K., Rahimmalek, M., Satari, B.: Integrated biorefinery of aquatic fern Azolla filiculoides for enhanced extraction of phenolics, protein, and lipid and methane production from the residues. J. Clean. Prod. 276, 123175 (2020)
Ekop, I.E., Simonyan, K.J., Evwierhoma, E.T.: Utilization of cassava wastes for value added products: an overview. Int. J. Sci. Eng. Sci. 3(1), 31–39 (2019)
Elias, F., Woyessa, D., Muleta, D.: Phosphate solubilization potential of rhizosphere fungi isolated from plants in Jimma Zone, Southwest Ethiopia. Int. J. Microbiol. (2016). https://doi.org/10.1155/2016/5472601
Fagbohungbe, M.O., Herbert, B.M., Hurst, L., Ibeto, C.N., Li, H., Usmani, S.Q., Semple, K.T.: The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag. 61, 236–249 (2016). https://doi.org/10.1016/j.wasman.2016.11.028
Giwa, A.S., Xu, H., Chan, F., Wu, J., Li, Y., Ali, N., Ding, S., Wang, K.: Effect of biochar on reactor performance and methane generation during the anaerobic digestion of food waste treatment at long-run operations. J. Environ. Chem. Eng. 7, 103067 (2019)
Hassan, S.H., Abd el Nasser, A.Z., Kassim, R.M.: Electricity generation from sugarcane molasses using microbial fuel cell technologies. Energy 178, 538–543 (2019). https://doi.org/10.1016/j.egyr.2019.12.002
Hu, X.F., Chen, J., Guo, J.F.: Two phosphate and potassium solubilising bacteria isolated from Tiannu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 22, 983–990 (2006)
Kifle, M.H., Laing, M.D.: Isolation and screening of bacteria for their diazotrophic potential and their influence on growth promotion of maize seedlings in greenhouses. Front. Plant Sci. 6(1225), 1–8 (2016). https://doi.org/10.3389/fpls.2015.01225
Kumar, V., Kumar, P., Kumar, P., Singh, J.: Anaerobic digestion of Azolla pinnata biomass grown in integrated industrial effluent for enhanced biogas production and COD reduction: optimization and kinetics studies. Environ. Technol. Innov. 17, 100627 (2020). https://doi.org/10.1016/j.eti.2020.100627
Kumari, S., Rajwade, V., Ramteke, P., Lawrence, R., Masih, H.: Isolation and characterization of potassium and phosphorus solubilising bacteria and fungus (KSB, PSB, KSF, PSF) and its effect on cauliflower. Int. J. Curr. Microbiol. Appl. Sci. 6(4), 987–1006 (2017)
Lee, J., Kim, K.H., Kwon, E.E.: Biochar as a catalyst. Renew. Sustain. Energy Rev. 77, 70–79 (2017)
Li, S., Ho, S. H., Hua, T., Zhou, Q., Li, F., Tang, J.: Sustainable biochar as electrocatalysts for the oxygen reduction reaction in microbial fuel cells. Green Energy Environ. (2020).
Liu, Z.H., Yin, H., Lin, Z., Dang, Z.: Sulfate-reducing bacteria in anaerobic bioprocesses: basic properties of pure isolates, molecular quantification, and controlling strategies. Environ. Technol. Rev. 7(1), 46–72 (2018). https://doi.org/10.1080/21622515.2018.1437783
Logan, B.E.: Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 7(5), 375–381 (2009). https://doi.org/10.1038/nrmicro2113
Logan, B.E., Hamelers, B., Rozendal, R., Schroder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W., Rabaey, K.: Microbial fuel cells: methodology and technology. Environ. Sci. Technol. 40, 5181–5192 (2006)
Lu, F., Liu, Y., Shao, L., He, P.: Powdered biochar doubled microbial growth in anaerobic digestion of oil. Appl. Energy 247, 605–614 (2019)
Luo, C., Lü, F., Shao, L., He, P.: Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 68, 710–718 (2015)
Ma, J., Pan, J., Qiu, L., Wang, Q., Zhang, Z.: Biochar triggering multipath methanogenesis and subdued propionic acid accumulation during semi-continuous anaerobic digestion. Biores. Technol. 293, 122026 (2019)
Martins, F., Felgueiras, C., Smitkova, M., Caetano, N.: Analysis of fossil fuel energy consumption and environmental impacts in European Countries. Energies 12(964), 1–11 (2019). https://doi.org/10.3390/en12060964
Mirmohamadsadeghi, S., Karimi, K.M., Aghbashlo, M.: Biogas production from food wastes: a review on recent developments and future perspectives. Bioresour. Technol. Rep. 7, 100202 (2019)
Momoh, O.L., Naeyor, B.A.: A novel electron acceptor for microbial fuel cells: nature of circuit connection on internal resistance. J. Biochem. Technol. 2, 216–220 (2010)
Muthukumarasamy, R., Revathi, G., Vadivelu, M., Arun, K.: Isolation of bacterial strains possessing nitrogen fixation, phosphate and potassium solubilization and their inoculation effect on sugarcane. Indian J. Exp. Biol. 55, 161–170 (2016)
Naik, S., Jujjavarappu, S.E.: Simultaneous bioelectricity generation from cost effective MFC and water treatment using various wastewater samples. Environ. Sci. Pollut. Res. 27(22), 27383–27393 (2019)
Nguyen, D., Khanal, S.K.: A little breath of fresh air into an anaerobic system: how microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 36(7), 1971–1983 (2018). https://doi.org/10.1016/j.biotechadv.2018.08.007
Ogbo, F.C.: Conversion of cassava wastes for biofertilizer production using phosphate solubilizing fungi. Biores. Technol. 101, 4120–4124 (2010)
Oladejo, O.S., Dahunsi, S.O., Adesulu-Dahunsi, A.T., Ojo, S.O., Lawal, A.I., Idowu, E.O., Olanipekun, A.O., Ibikunle, R.A., Osueke, C.O., Ajayi, O.E., Osueke, N., Evbuomwan, I.: Energy generation from anaerobic co-digestion of food waste, cow dung and piggery dung. Bioresource Technol. 313, 123694 (2020). https://doi.org/10.1016/j.biortech.2020.123694
Onwosi, C.O., Eke, I.E., Igbokwe, V.C., Odimba, J.N., Ndukwe, J.K., Chukwu, K.O., Aliyu, G.O., Nwagu, T.N.: Towards effective management of digester dysfunction during anaerobic treatment processes. Renew. Sustain. Energy Rev. 116, 109424 (2019)
Owamah, H.I., Dahunsi, S.O., Oranusi, U.S., Alfa, M.I.: Fertilizer and sanitary quality of digestate biofertilizer from the co-digestion of food waste and human excreta. Waste Manag. 34(4), 747–752 (2014)
Pan, J., Ma, J., Zhai, L., Luo, T., Mei, Z., Liu, H.: Achievements of biochar application for enhanced anaerobic digestion: a review. Biores. Technol. 292, 122058 (2019)
Quashie, F.K., Feng, K., Fang, A., Agorinya, S., Antwi, P., Kabutey, F.T., Xing, D.: Efficiency and key functional genera responsible for simultaneous methanation and bioelectricity generation within a continuous stirred microbial electrolysis cell (CSMEC) treating food waste. Sci. Total Environ. 757, 143746 (2021)
Rashid, T., Sher, F., Hazafa, A., Hashmi, R.Q., Zafar, A., Rasheed, T., Hussain, S.: Design and feasibility study of novel paraboloid graphite based microbial fuel cell for bioelectrogenesis and pharmaceutical wastewater treatment. J. Environ. Chem. Eng. 9(1), 104502 (2021)
Sandanakirouchenane, A., Haque, E., Geetha, T.: Recent studies on N2 fixing Burkholderia isolates as a biofertilizer for the sustainable agriculture. Int. J. Curr. Microbiol. Appl. Sci. 6(11), 2780–2796 (2017)
Saravanan, M., Manikandan, K.: Experimental study on biogas production in batch type digester with different feed stocks. Int. J. Res. Environ. Sci. Technol. 2(4), 132–135 (2012)
Shen, Y., Linville, J.L., Urgun-Demirtas, M., Schoene, R.P., Snyder, S.W.: Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 158, 300–309 (2015)
Shen, Y., Yu, Y., Zhang, Y., Urgun-Demirtas, M., Yuan, H., Zhu, N., Dai, X.: Role of redox-active biochar with distinctive electrochemical properties to promote methane production in anaerobic digestion of waste activated sludge. J. Clean. Prod. 278, 123212 (2021)
Shinde, S., Paralikar, P., Ingle, A.P., Rai, M.: Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger. Arab. J. Chem. 13, 3172–3182 (2020). https://doi.org/10.1016/j.arabjc.2018.10.001
Sivamani, S., Chandrasekaran, A.P., Balajii, M., Shanmugaprakash, M., Hosseini Bandegharaei, A., Baskar, R.: Evaluation of the potential of cassava-based residues for biofuels production. Rev. Environ. Sci. Biotechnol. 17, 553–570 (2018). https://doi.org/10.1007/s11157-018-9475-0
St-Pierre, B., Wright, A.D.G.: Comparative metagenomic analysis of bacterial populations in three full-scale mesophilic anaerobic manure digesters. Appl. Microbiol. Biotechnol. 98(6), 2709–2717 (2014). https://doi.org/10.1007/s00253-013-5220-3
Sun, C., Liu, F., Song, Z., Wang, J., Li, Y., Pan, Y., Sheng, T., Li, L.: Feasibility of dry anaerobic digestion of beer lees for methane production and biochar enhanced performance at mesophilic and thermophilic temperature. Biores. Technol. 276, 65–73 (2019)
Wang, J., Wang, S.: Preparation, modification and environmental application of biochar: a review. J. Clean. Prod. 227, 1002–1022 (2019)
Wang, H., Qu, Y., Li, D., Zhou, X., Feng, Y.: Evaluation of an integrated continuous stirred microbial electrochemical reactor: wastewater treatment, energy recovery and microbial community. Biores. Technol. 195, 89–95 (2015)
Wang, H., Song, H., Yu, R., Cao, X., Fang, Z., Li, X.: New process for copper migration by bioelectricity generation in soil microbial fuel cells. Environ. Sci. Pollut. Res. 23(13), 13147–13154 (2016). https://doi.org/10.1007/s11356-016-6477-8
Wirth, R., Kovács, E., Maróti, G., Bagi, Z., Rákhely, G., Kovács, K.L.: Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 5(1), 1–16 (2012). https://doi.org/10.1186/1754-6834-5-41
Xia, Z., Ying, X., Qiao-hong, Z., Zhen-bin, W.: Research progress on electricigens in microbial fuel cell. J. Biotechnol. Bull 33(10), 64–73 (2017)
Xing, D., Cheng, S., Logan, B.E., Regan, J.M.: Isolation of the exoelectrogenic denitrifying bacterium Comamonas denitrificans based on dilution to extinction. Appl. Microbiol. Biotechnol. 85(5), 1575–1587 (2010)
Yuan, Y., Yuan, T., Wang, D., Tang, J., Zhou, S.: Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell. Biores. Technol. 144, 115–120 (2013). https://doi.org/10.1016/j.biortech.2013.06.075
Zhang, L., Lim, F.Y., Loh, K.C., Ok, Y.S., Lee, J.T.E., Shen, Y., Wang, C.H., Dai, Y., Tong, Y.W.: Biochar enhanced thermophilic anaerobic digestion of food waste: focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers. Manag. 209, 112654 (2020). https://doi.org/10.1016/j.enconman.2020.112654
Zhang, M., Xie, L., Yin, Z., Khanal, S.K., Zhou, Q.: Biorefinery approach for cassavabased industrial wastes: current status and opportunities. Biores. Technol. 215, 50–62 (2016). https://doi.org/10.1016/j.biortech.2016.04.026
Ramanjaneyulu, G., Reddy, B.R.: Emerging Trends of Microorganism in the Production of Alternative Energy. In: Recent Developments in Applied Microbiology and Biochemistry, pp. 275–305 (2019). https://doi.org/10.1016/b978-0-12-816328-3.00021-0
Hungerford, L.L., Campbell, C.L., Smith, A.R.: Veterinary Mycology Laboratory Manual. Iowa State University Press, Ames (1998)
Izonfuo, W., Bariweni, P., George, D.M.C.: Soil contamination from cassava wastewater discharges in a rural community in the Niger Delta, Nigeria. J. Appl. Sci. Environ. Manag. 17(1), 105–110 (2013)
Oladele, P.K.: Cassava processing and the environmental effect. In: The 4th World sustainability Forum 2014. Conference Proceedings Paper (2014).
Omotosho, O., Amori, A.: Caustic hydrogen peroxide treatment of effluent from cassava processing industry: prospects and limitations. Int. J. Eng. Technol. Innov. 5, 75–85 (2015)
Abdullahi, B., Adie, D.B., Abubakar, U.A., Giwa, A., Adamu, Y., Dauda, J.A.: Development of biofertilizer from composted cow-dung. Sci. Forum 16, 1–7 (2019)
Okunade, D., Adekalu, K.: Characterization of cassava-waste effluents contaminated soils in Ile-Ife, Nigeria. Eur. Int. J. Sci. Technol. 3(4), 173–182 (2014)
Aripin, A.M., Mohd-Kassim, A.S., Daud, Z., Mohd-Hatta, M.: Cassava peels for alternative fibre in pulp and paper industry: chemical properties and morphology characterization. Int. J. Integr. Eng. 5(1), 30–33 (2013)
Obonukut, M., Alabi, S., Jock, A.: Biochar from cassava waste: a paradigm shift from waste to wealth. In: Biochar - Productive Technologies, Properties and Applications (2023). https://doi.org/10.5772/intechopen.105965
Acknowledgements
The authors utilized the laboratory facilities of the Department of Microbiology, University of Nigeria, Nsukka. The laboratory team assisted the authors in carrying out the tests. We are indeed very grateful to the Department and laboratory staff. The authors also wish to thank the Centre for Environmental Management and Control, University of Nigeria, Nsukka for their encouragement.
Author information
Authors and Affiliations
Contributions
CCA: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing—original draft, Writing—review and editing, Visualization and Funding. JKN: Software, Validation, Formal analysis, Data curation, Writing—original draft. CKO: Data curation, Writing—original draft, Writing—review and editing. SCO: Writing—review and editing. ANM: Conceptualization, Methodology, Investigation, Resources, Data curation, Writing—original draft, Writing—review and editing, Visualization and Funding.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aduba, C.C., Ndukwe, J.K., Onyejiaka, C.K. et al. Integrated Valorization of Cassava Wastes for Production of Bioelectricity, Biogas and Biofertilizer. Waste Biomass Valor 14, 4003–4019 (2023). https://doi.org/10.1007/s12649-023-02126-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02126-3