Abstract
Purpose
The treatment and management of gentamicin mycelial residue (GMR) is challenging due to the high residual antibiotic content in GMR. The objectives of this study were to investigate the viability of recycling GMR by co-composting with rice chaff, describe the dynamics of the physicochemical and biological parameters and the microbial community, and evaluate the maturity of the composting products.
Methods
The treatment and management of gentamicin mycelial residue (GMR) is challenging due to the high residual antibiotic content in GMR. Three 1-tonne piles of fresh GMR were composted with each treatment; the test treatments contained rice chaff, and the control treatment did not contain rice chaff. Dried GMR was prepared by drying fresh GMR. Composting was performed in cones with a diameter of 2 m and a height of 1.5 m using different ratios of fresh GMR to rice chaff (8:1 and 4:1) or in the absence of rice chaff (control).
Results
The optimal fresh GMR: rice chaff ratio (w/w) was 4:1. Characteristic temperature profiles consisting of a very brief mesophilic phase, a 4–57-day thermophilic phase (maximum of 50–70 ℃) and a cooling phase (45–35 ℃) after 58–73 days were observed. After 73 days of co-composting, 99.66% of gentamicin was degraded. All key parameters of the final products, such as the pH (8.12), EC (2.43 mS/cm), carbon/nitrogen ratio (8.6), germination index (96.06%) and crop growth indexes, met the national standards for compost maturity indicators. Compared with those of fungi, the number of OTUs, Chao1 index and Shannon index of the bacteria in the T2 treatment clearly increased from 78, 105.14 and 0.97 to 149, 161 and 3.30, respectively, during the whole co-composting. A canonical correlation analysis (CCA) revealed that the bacterial community dynamics were closely correlated with the amount of residual gentamicin. Micromonospora and Enterococcus may have been the key microorganisms responsible for the degradation of gentamicin.
Conclusion
The addition of rice chaff improved the decomposition of gentamicin residue in the GMR and made the GMR usable in fertilizer; this result could help antibiotic production factories recycle more of their waste products. The results provide new insight into the potential of applying co-composting with rice chaff to achieve sustainable GMR management.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The biowastes of antibiotic production factories contain high levels of antibiotic residues and thus constitute a potential threat to ecological environmental systems and eventually human health. However, because antibiotic mycelial residue contains high levels of organic nutrients such as protein and crude fat, it can potentially be used as a valuable organic fertilizer. The aims of our study were to explore a feasible method for the disposal of antibiotics mycelial residue to reduce its environmental impact, and concurrently recycle nutrients.
Introduction
Gentamicin is a crucial aminoglycoside antibiotic reliably used to cure serious and life-threatening infections mainly caused by gram-negative bacteria in humans and animals [1]. Gentamicin mycelial residue (GMR), containing gentamicin and its precursors, is a major byproduct, with tens of thousands of tons produced per year in China. High concentrations of antibiotic residue have made GMR treatment an urgent environmental challenge. Inappropriate treatment of GMR may make gentamicin spread into the natural environment, which will increase drug-resistant bacteria and be a potential threat to ecological environmental systems and eventually human health. In 2008, GMR was classified as hazardous waste, which is treated with approved methods, including incineration, biomass pyrolysis, removal to landfills and other treatment technologies [2]. However, the costs of incineration and biomass pyrolysis are high. In landfills, a large amount of GMR will not only affect a large land area but also potentially leach toxic chemicals to soils, surface water and even groundwater. Furthermore, some studies have shown that the application of manure containing antibiotics could influence the collective antibiotic resistance of soil and increase the relative abundances of total antibiotic resistance genes (ARGs) [3, 4]. The acquisition and dissemination of ARGs among pathogens have severely curtailed the effectiveness of many of antibiotics [5] and thus harm human health and public safety. Antibiotics can also affect plant growth, potentially leading to crop losses or even food shortages. Zhang et al. [6] showed that sulfadimidine could reduce folic acid synthesis and ultimately inhibit Arabidopsis growth by inhibiting deoxyribonucleic acid synthesis. Boxall et al. [7] found that enoxacin significantly inhibited the growth of carrots and lettuce. Because GMR contains high levels of organic nutrients such as protein and crude fat, it may have potential to become a valuable organic fertilizer. Therefore, it is urgent to find a sustainable method to degrade residual gentamicin in GMR and concurrently recycle nutrients.
Composting is an effective and suitable technology for transforming organic residues such as sludge and agricultural waste into fertilizer or conditioner [8, 9]. At present, increasing attention has been paid to the removing antibiotics from livestock manure or antibiotic mycelial residue by composting to stabilize the remaining organic matter (OM) and inhibit pathogens. In addition, the final compost product may be used for the fertilization of farmland to ensure that crops absorb most of the essential nutrients needed for growth and development. Ezzariai et al. [10] investigated whether the composting of sludge or manure could be effective in reducing the levels of antibiotics and ARGs, and their results showed that the antibiotic removal ranged from 17 to 100%. Zhang et al. [11] also demonstrated that aerobic composting could be a practicable programme for remove 64.7% of the detected antibiotics in swine manure. For antibiotic mycelial residue, Liu et al. [12] suggested that laboratory aerobic co-composting could be a practicable program for dealing with gentamicin fermentation residues (GFRs). Liao et al. [13] also explored the efficacy of hyperthermophilic composting in removing tylosin antibiotic fermentation residues (TFRs) and found that 95.0% of TFRs were removed.
Mature compost is used as a fertilizer that can increase soil organic matter, stimulate soil microbial communities and help restore degraded soil [14]; thus, this compost must be nontoxic and needs to supply nutrients and structure to soil and plants [15]. Hence, the maturity parameter is often used to evaluate the stability and safety of compost. Researchers generally believe that confirming the chemical and biological stability of compost using a single parameter is difficult, and several parameters should be used to estimate the stability [15, 16]. The use of physicochemical methods, biological activity assays and plant toxicity analyses can more credibly evaluate the maturation and stability of compost. In these studies, the moisture content, temperature, pH, electrical conductivity (EC), carbon/nitrogen (C/N) ratio and germination index (GI) were measured to assess maturity [17, 18]. Furthermore, the time period of composting and the overall content of the compost are highly correlated with the microbial composition and diversity [19]; composting is fundamentally a dynamic process in which microbial flora play a key role, and the composting quality can be measured by analyzing the activity and diversity of microorganisms [16]. Therefore, the analysis of microbial communities may help researchers uncover the mechanisms of antibiotic degradation during composting. However, few studies have investigated the treatment and assessment of GMR as a bioresource, e.g., a fertilizer, using pilot-scale co-composting experiments. Hence, a pilot-scale experiment on the co-composting of GMR and rice chaff was performed in this study. Moreover, analyses of the gentamicin degradation process, physicochemical characteristics, biological parameters and microbial community evolution were performed to evaluate the maturity of the GMR compost product and provide more information on the full-scale composting treatment of GMR. All experiments involved pilot-scale composting under the conditions found outside the laboratory.
Materials and Methods
Feedstock and Sample Collection
Fresh GMR and dried GMR were provided by Henan RenHua Biotechnology Co., Ltd. Dried GMR was prepared by drying fresh GMR. Rice chaff, which was used as a bulking agents to increase the porosity and adjust the C/N ration, were obtained from a local farm. The main physicochemical properties of the feedstocks are listed in Table 1.
Experimental Procedure and Sampling
The experiment was conducted in a plant of Henan RenHua Biotechnology Co., Ltd, from July to September, over a period of 73 days. The feedstocks were mixed for the three composting treatments, all of which included 1000 kg of fresh GMR. The compost treatments were as follows: (1) no rice chaff, fresh GMR-to-dried GMR ratio of 8:1 (weight/weight) (CK); (2) fresh GMR-to-rice chaff ratio of 8:1 (weight/weight) (T1); and (3) fresh GMR-to-rice chaff ratio of 4:1 (weight/weight) (T2). After being manually mixed, the raw materials were prepared into conical piles as illustrated in Table 2, and each pile contained approximately 1.57 m3 each (diameter × height: 2 m × 1.5 m). These treatments included artificial turning every three days to increase the oxygen intake of the materials. Moreover, the moisture content was tested and adjusted to 50–60% with tap water.
Approximately 500 g of composting material was randomly obtained from each pile at a depth of 50 cm and mixed to obtain a cross-sectional and homogeneous sample. One part of each sample was preserved at − 20 °C for molecular assessment of microbial diversity and determination of gentamicin concentrations. Another part of the three samples was used to measure other physicochemical properties and the GI.
Physicochemical Parameters
During composting, the temperatures at five locations (the midline of north–south-east–west and the top, 25–30 cm) in the compost pile and ambient temperature were measured twice per day. The moisture contents of the composting samples were measured by drying the samples to reach a constant weight at 105 °C in an oven. Ten grams of fresh samples was extracted with 1:10 (w/v) deionized water for 5 h. The pH value and EC of the suspensions were measured with a pH meter (Mettler Toledo Co., Shanghai, China) and an EC meter (Leici Chuangyi Instrument Co., Shanghai, China), respectively. The sample stored at 4 °C was used for the determination of total nitrogen (TN) using the Kjeldahl digestion method [20]. The quantitative organic fertilizer samples were mixed with concentrated sulfuric acid, and H2O2 was added to the mixture. Subsequently, the mixture was placed into a graphite digester and digested at 380 °C, and the organic nitrogen was converted into ammonium nitrogen. After digestion, the mixture was distilled with an automatic Kjeldahl apparatus and titrated with HCl. According to the China national organic fertilizer standard (NY 525-2012), OM in the sample was determined using the potassium dichromate method. According to the consumption of potassium dichromate and sulfuric acid before and after oxidation, the organic carbon content was calculated, and the organic carbon content multiplied by the coefficient 1.724 was the OM content of mycelial residue compost. Therefore, the C/N ratio was calculated with the following formula: \(\frac{C}{N}\, = \,\frac{OM}{{1.724\, \times \,TN}}\) [21, 22]. All the aforementioned analyses were performed with three replicates.
Biological Parameters
The phytotoxicity analysis includes the germination test and the plant growth, which can give an intuitive indication of the maturation of compost. The germination test was performed using cabbage seeds. The GI was measured as described previously [23]. Ten milliliters of compost filtrate from each sample was placed in a sterilized dish containing two pieces of filter paper. Ten cabbage seeds were cultured in each dish for 48 h at 25 ± 1 °C. Each treatment was repeated three times with distilled water as a blank control. The seed germination percentage and root lengthening were determined, and the GI (%) was calculated with the following formula: GI (%) = (seed germination percentage (%) × mean of root lengthening during the treatment) × 100%/(seed germination percentage (%) × mean of root lengthening during the control treatment).
Chinese cabbage has a short growth period and is easy to cultivate and sensitive to pollutants; thus, this cabbage is often used in experiments to evaluate pollutant absorption and toxicity effects. The Chinese cabbage seeds were purchased from a seed store in Zhengzhou. This study was designed to include four treatment groups: cow manure/soil (2 g/kg, CM treatment), gentamicin compost product/soil (2 g/kg, GL treatment), gentamicin compost product/soil (4 g/kg, GH treatment) and soil (SCK treatment). Moist soil was obtained from bare soil near the south gate of Zhengzhou University Plastic pots were each filled with 3 kg of soil, and the soil moisture content was kept at the maximum soil water capacity. Ten seeds were planted in each pot. The cabbages were harvested after 75 days of growth. Ten Chinese cabbages with uniform growth from different pots were selected and cleaned with deionized water. Their emergence rate (ER), height from roots to top (Height), fresh weight (Weight), gentamicin residue (GR) and soluble sugar (SS) level were measured. Each processing group consisted of four independent replicates.
Determination of Gentamicin Concentrations
Gentamicin is easily dissolved in water and adsorbed by resin in acidic environments and desorbed in alkaline environments. By changing the pH value of the extract, the GR in the compost samples was extracted. Five grams of freeze-dried samples were diluted 1:40 in distilled water. The solution pH was first adjusted to 4.5 with oxalic acid and then 1.5 with dilute sulfuric acid. The suspensions were incubated for 1 h at 60 °C and 120 rpm. After the incubation period, the liquids were left, and the solids were centrifuged. The solids were cleaned once with 10% methanol solution (water: methanol = 90:10), and the resulting suspensions were collected. Two grams of resin was added to the supernatant (adjusted to pH 4.0 using 40% sodium hydroxide) and incubated for 1 h at 45 °C and 120 rpm. Then, the shaker was run for 1 h at 120 rpm and 20 °C. The suspensions were discarded, and the resin was collected in a conical flask. One hundred milliliters of 5% ammonia water was poured into the conical flask, and it was placed in the shaker at 30 °C and 120 rpm for 3 h. Finally, the liquid was evaporated and collected at 60 °C at 80 rpm. The resulting extracts were fully dissolved with ultrapure water to 10 ml and filtered through a membrane with a 0.22-μm pore size. The gentamicin concentrations were measured by HPLC according to the Chinese Pharmacopoeia (2015).
The gentamicin degradation percentage (GDP) was calculated by the following formula:
where C0 is the initial gentamicin concentration in compost (mg/kg) and Ct is the gentamicin concentration in compost on day t (mg/kg).
Dynamic Model of Gentamicin Biodegradation During Composting
A pseudo-first-order kinetics reaction equation was used to reflect the dynamics of antibiotic biodegradation [24, 25]. Thus, in this study, the biodegradation dynamics of gentamicin were fitted with the following formula: \(C{}_{t} = C0e^{ - kt}\), where \(C_{0}\) and \(C_{t}\) were the same as in the above formula, and \(k\) was the rate constant of antibiotic degradation (day−1). Using this equation, the half-life (\({\text{t}}_{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}\)) of gentamicin was calculated as follows: \(t_{1/2} = - {\text{ln}}2/k\)
Microbial Community Diversity and Structure
To determine the bacterial and fungal diversity during the composting process, microbial community genomic DNA was extracted from each pile in the initial phase (day 0), mesophilic phase (day 3), thermophilic phase (day 12 and 36), and cooling phase (day 73) using the E.Z.N.A.® soil DNA Kit (Omega Biotek, Norcross, GA, USA) depending based on the changes in the pile temperature. The DNA extract was checked on 1% agarose gels, and the DNA concentration and purity were determined with a NanoDrop 2000 UV–Vis spectrophotometer (Thermo Scientific, Wilmington, NC, USA). High-throughput sequencing of the 16S and 18S rRNA genes was performed using the Illumina MiSeq PE300 platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The V3-V4 hypervariable region of the bacterial 16S rDNA was amplified with the primers 338F (ACTCCTACGGGAGGCAGCAG') and 806R (GGACTACHVGGGTWTCTAAT'), purified, quantified, and sequenced. Primers SSU0817F (TTAGCATGGAATAATRRAATAGGA) and 1196R (TCTGGACCTGGTGAGTTTCC), targeting the V5–V7 hypervariable region of the fungal 18S rDNA, were chosen for amplification and subsequent sequencing of the polymerase chain reaction (PCR) products.
Coupled reads in the raw sequences were merged into a sequence, and quality control and filtration were performed to adjust for read quality and splicing effects. Sequences with a quality score < 20 in the tail were removed, and the sequences longer than 50 bp were retained. Operational taxonomic units (OTUs) with a similarity cutoff of 97% were clustered using UPARSE (version 7.1, http://drive5.com/uparse), and the chimeric sequences were identified and removed.
Statistical Analysis
The data obtained during the different composting treatments were analyzed by one-way analysis of variance (ANOVA) and the least significant difference (LSD) of mean values at a probability level of p < 0.05. The abundance and diversity of the microbial community was evaluated based on the alpha diversity. Based on the relative abundances of OTUs, the Chao1 index (which reflects the community richness), the Shannon index (which reflects the community diversity), and the community coverage were calculated. The correlations between bacterial communities and physicochemical properties were assessed by canonical correlation analysis (CCA). The correlations between the ten most abundant bacteria and environmental factors were revealed by Spearman’s correlation coefficients. All statistical calculations were performed using SPSS 19.0 software, and Origin 9.0 and CANOCO 5.0 were used to generate graphs in the study.
Results and Discussion
Changes in Gentamicin Residue
Composting has generally been studied as an economic and environment-friendly method for the treatment of antibiotic residues [17, 26]. Figure 1 shows the evolution of gentamicin residues during 73 days of co-composting. In the initial compost samples, the CK, T1 and T2 exhibited gentamicin concentrations of 1257 mg/kg, 1085 mg/kg and 875 mg/kg, respectively. During the first 15 days of composting, the gentamicin concentrations decreased sharply with all three treatments, and the degradation percentages were all higher than 63%. At the end of the composting period, the residual concentrations of gentamicin in the CK, T1 and T2 groups were 13.71 mg/kg, 9.31 mg/kg and 3.0 mg/kg, respectively. These findings demonstrate the effectiveness and operability of the co-composting of GMR and rice chaff in the removal of residual gentamicin. The T1 and T2 treatments resulted in GDPs of 99.14% and 99.66%, which were higher than that obtained with the CK treatment, and the differences in the GDPs between the T1 and T2 treatments were not significant. As such, the addition of rich chaff likely affects the composting process and gentamicin degradation. Furthermore, a GMR-to-rice chaff ratio of 4:1 (T2) was more conducive to gentamicin degradation. Similarly, the co-composting of gentamicin fermentation residues (GFRs) and lovastatin fermentation residues (LFRs) removed 96.7% of gentamicin residues, and the half-life of gentamicin was shorter (\({\text{t}}_{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}\) = 4.4 days) [12], which may be due to the prefermentation of GFRs and LFRs and the refractory substances being decomposed to small molecules. Many studies have also suggested that the antibiotics degradation is closely related to microbial catabolism. Ezzariai et al. [10] reported that antibiotic degradation could be performed by microorganisms. In this case, sufficient nutrition ensured the growth and metabolism of microorganisms, which had a positive impact on the composting process and gentamicin degradation [27].
The pseudo-first-order kinetic model was used to fit the experimental data. The first-order kinetic equations for the CK, T1 and T2 treatments were fitted as shown in Table 3. The correlation coefficients (R2) of the three equations were all greater than 0.9, indicating that the first-order model was suitable to well fit the degradation of gentamicin during the composting of GMR and rice chaff. K was the degradation rate constant of gentamicin during composting, and a higher value indicates a higher degradation rate. The half-life is the time it takes for the gentamicin concentration to decrease to half of the initial concentration dur to degradation. The half-lives of gentamicin were calculated according to the kinetic equations. A shorter half-life indicates faster gentamicin degradation. Among all treatments, T2 exhibited the highest reaction constant (k = 0.0260 day−1) and the shortest half-life (\({\text{t}}_{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}\) = 22.0 days), demonstrating that gentamicin was degraded most rapidly with the T2 treatment. In contrast, the CK treatment had the lowest reaction constant (k = 0.0233 day−1) and the longest half-life (\({\text{t}}_{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}}\) = 26.1 days), demonstrating that this treatment resulted in the slowest degradation of gentamicin. Considering the degradation percentages and rates, a GMR-to-rice chaff ratio of 4:1 was optimal for gentamicin degradation, and this ratio yielded the highest values for the rice chaff content and C/N ratio.
Many studies have reported that the degradation half-lives of different antibiotics during composting vary greatly, which may be related to the extensive differences in substrates and conditions [25, 28, 29]. Wu et al. [30] reported that the half-life of tetracycline was 11.75 days during the composting of pig manure with mushroom residues (1:2 v/v). However, after 42 days of the composting of pig manure with sawdust (1:1 w/w, dw) the tetracycline degradation is 91.6%, and the half-life is 10.02 days [31]. Compared with these antibiotics, gentamicin is more structurally stable and more difficult to decomposed by microorganisms. Therefore, the microbial communities can be modulated by changing the types of raw materials and physicochemical conditions of the composting process, which has the potential to shorten the composting cycle and improve the composting quality [32].
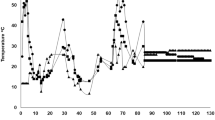
Changes in Temperature
Temperature is considered one of the key factors for successful composting operations because temperature changes are closely tied to the biotransformation of OM and the dynamic changes in the microbial community [33]. This factor is usually used as a parameter to indicate the good initiation and end of the composting progress. During the composting process, the evolution of the temperature of the material provides an indication of the efficiency of the composting process. The changes in temperature during the three treatments were recorded, and the developmental trends of the different phases were similar (Fig. 2). The three types of composting treatments included a mesophilic phase (1–8 days) followed by a thermophilic phase (9–57 days) and then a cooling/mature phase (58–73 days). The ambient temperature ranged from 18 °C to 39 °C.
The first phase was characterized by an increase in the temperature from 40 to 50 °C over 4 days, showing a rapid colonization of mesophilic microbial populations. After the 4th day of composting, the temperature of the T2 treatment increased rapidly to values above 50 °C, and the high-temperature period lasted 53 days. Compared with that of the T2 treatment, the temperatures of the T1 and CK treatments slowly increased, and the high-temperature period was delayed by 4 days. This rapid progress from the mesophilic to the thermophilic phase can be attributed to the high microbial activity generated by the presence of easily degradable organic compounds. During the first 42 days of composting, the average temperatures of the three treatments decreased in the order T2 > T1 > CK (P < 0.05). After 42 days, the temperatures of the T2 and CK treatments decreased sharply, and the samples entered a cooling period; moreover, the average temperature of T1 remained higher than 50 °C until 70 days, when the material entered the cooling period. Indeed, the conversion and biodegradation of OM during the two previous phases (mesophilic and thermophilic) enriched the windrow of stable and hygienic mineral matter, which greatly reduces the microbiological activity and consequently decreases the temperature of the pile. However, on the 61st day of the CK treatment, the temperature increased rapidly from 37 °C to 60 °C, and the temperature remained 60 °C for 8 days before the sample entered the cooling period. These differences in behavior probably occurred because the content of gentamicin in the CK, T1 and T2 treatments decreased at different times, and significant differences were detected among the treatments (P < 0.05). Initially, high levels of residual gentamicin strongly inhibited microbial growth. However, during the composting process, gentamicin was gradually degraded, and its inhibitory effect also gradually weakened, making more microorganisms grow rapidly. Another reason might be that the porosity in the CK, T1 and T2 treatments increased at different times, which was beneficial to the growth and metabolism of aerobic microbes [34]. Therefore, T2 entered the thermophilic stage and completed the aging stage fastest. Microorganisms in T1 were active and continued to decompose OM. In CK, most OM was decomposed in the thermophilic period. The material became loose, and the oxygen content increased, which caused thermophilic microorganisms to become active and the sample to re-enter a high-temperature period to decompose OM. In this study, the numbers of days above 50 °C in the CK, T1 and T2 treatments were 46, 63 and 53, respectively. According to the Chinese national standard for sanitation [35], the temperature of the composting pile must be maintained at more than 50 °C for 7 days to prevent the free breeding of weed seeds and pathogens. All three treatments met this standard.
Evolution of Physicochemical Parameters and Biological Parameters
Changes in the pH, EC and C/N ratio during Composting
The changes in physicochemical parameters in different piles during composting are shown in Fig. 3a–c. Attaining and maintaining the correct pH are important for effective composting and a pH range of 6.5–9.0 supports good microbial activity during the composting process. During CK, T1 and T2 treatment, the pH value changed gradually, and no significant differences were found among the treatments (Fig. 3a). In the medium-temperature phase, the pH values of CK, T1 and T2 increased rapidly until the 30th day and reached maximum values of 8.89, 9.07 and 8.94, respectively. Subsequently, from the high-temperature period to the cooling period, the pH values remained at approximately 8.0. In the medium-temperature phase, the mineralization of OM generally releases volatile ammonia and ammonium, leading to an increase in pH [36]. As the composting continues, organic acids are synthesized and decomposed, causing the pH to fluctuate around approximately 8.0 [37]. According to the China national organic fertilizer standard (NY 525-2012), the optimal pH value of compost is 5.5–8.5. During the mature/cooling periods, the pH values of CK, T1 and T2 were 8.1, 8.14 and 8.12, respectively, which was in line with the national standard.
During the composting process, EC reflects the content of soluble salt in compost extract, which affects the plant toxicity of compost. Thus, to a certain extent, EC demonstrates the maturity degree of compost products and high EC in compost is undesirable. In the medium-temperature phase, the EC values of the CK, T1 and T2 treatments increased rapidly (Fig. 3b). The EC value of T2 peaked at 4.62 mS/cm on the 9th day, and the EC values of the CK and T1 treatments reached their highest values of 7.19 mS/cm and 6.64 mS/cm on the 12th day, respectively, which were significantly lower and occurred earlier than the maximum values obtained with the other treatments (P < 0.05). The increase in EC may be related to the accelerated release of various mineral salts as microorganisms decompose OM [38]. From the high-temperature period to the low-temperature period, the EC values of the CK, T1 and T2 treatments gradually decreased to 2.49 mS/cm, 2.48 mS/cm and 2.43 mS/cm, respectively, and remained stable, indicating that they did not inhibit seed germination [39]. The EC decreased slightly, possibly due to either the volatilization of ammonia or the precipitation of mineral salts [15].
In composting, carbon and nitrogen sources provide the energy and nutrients needed by microorganisms, and the C/N ratio is considered an important indicator of the maturity of compost [40]. The changes in the C/N ratio during CK, T1 and T2 treatments were similar (Fig. 3c). As composting progressed, the C/N ratio gradually decreased. The initial C/N ratios of the CK, T1 and T2 treatments were 15.66, 21.57 and 23.43; all of the treatments with the exception of CK had C/N ratio ranging from 20 to 25:1, which could provide balanced nutrition for microorganismal growth. During composting, the gradual C/N ratio decline occurs mainly due to the decomposition of OM. At the end of the composting period, the C/N ratios of the CK, T1 and T2 treatments ultimately decreased to 7.13, 7.48 and 8.6, respectively, and no significant differences were detected among the treatments. Studies have shown that compost is mature and safe when its solid-phase C/N ratio decreases from an initial of (20–30):1 to less (15–20):1. The T1 and T2 treatments met these conditions, suggesting that these treatments produced mature compost. The initial C/N ratio of the CK sample was less than 20, which was not suitable for the composting treatment. Another criterion for evaluating maturity is T = (endpoint C/N ratio)/(initial C/N ratio); when T < 0.7, compost is deemed to be mature [41]. The T value of the CK treatment was 0.46, indicating that the CK compost also reached the criterion of maturity.
Changes in GI During Composting
If the final compost product is unstable and immature, it would be harmful for seed germination, plant growth, and the soil environment due to the phytotoxic compounds was created by lacks of oxygen exchange and bioavailable N. The GI is relevant to the plant toxicity of compost products and is a quick and efficient indicator of compost maturity. The GI values of the CK, T1, and T2 treatment were 0% at the beginning of the composting period (Fig. 3d), which indicated that these samples were highly toxic to plants. The GI values gradually increased during composting until reaching values of 78.4%, 80.2% and 96.06% at the end of the CK, T1 and T2 composting treatments, respectively. According to Luo et al. [42], a GI value is higher than 80% indicates that the compost is usually not toxic to plants. Therefore, the T1 and T2 composts were believed to be nonphytotoxic and mature, and no significant differences were found between the two treatments. In summary, the results demonstrated that a fresh GMR-to-rice chaff ratio of 4:1 (weight/weight) (T2) yields better physicochemical and biological performance.
Correlations Between Parameters
Composting is actually an aerobic biodegradation of organic matter by microorganisms, with the microorganisms playing a key role [40]. However, the growth and metabolic activities of microorganisms are also affected by environmental factors. The high residual gentamicin in GMR may influence microbial activity and even inhibit composting. Correlations among the parameters were analyzed, and they are shown in Table 4. In the CK, T1 and T2 treatments, the GR was significantly correlated with the pH, C/N ratio and GI, indicating that the degradation of gentamicin was related to the properties and proportions of the compost raw materials [43]. The GDP might be improved by further changing the raw materials, their proportions or the compost conditions.
Crop Growth Indexes
To further determine the plant toxicity and maturity of the composting products, pot experiments were conducted with Chinese cabbage. The gentamicin compost product was prepared by co-composting GMR with rice chaff (4:1) for 73 days. The final compost product had the following basic physical and chemical properties: pH, 8.12; moisture content, 24%; OM content, 58.41%; total C, 33.88%; TN, 3.94%; C/N ratio, 8.60; and residual concentration of gentamicin, 3.0 mg/kg. The ER and quality of Chinese cabbage during the different treatments are shown in Table 5. Compared with the SCK treatment, no significant difference in ER was found among the CM, GL and GH treatments; however, significant differences in the plant height and fresh weight were found among the treatments (P < 0.05), and the finding of GH > GL > CM > SCK indicated that the gentamicin composting product promotes crop growth better than ordinary cow manure and that high fertilizer application is beneficial to crop growth. The SSs in vegetables mainly include glucose, fructose, sucrose, and others. The SS content reflects the taste of vegetables to a certain extent and can be used as an important indicator of vegetable quality. The different treatments were ranked as GL > CM > GH > SCK based on the SS level. The SS content in the GL treatment was significantly higher than that in the SCK treatment (P < 0.05), which demonstrated that the application of a low amount of composting product was beneficial for SS accumulation. No GR was detected by HPLC in the stems and leaves of Chinese cabbage on the 75th day of growth. The comprehensive analysis showed that gentamicin composting not only promoted the growth of the potted crops but also improved their quality; these results further indicate that the composting product was mature.
Microbial Community Diversity
The types of compost substrates have a crucial influence on microbial community composition during composting [44]. To determine the relationships between different compost substrates, GR and microbial community diversity, CK (without rice chaff) and T2 (250 kg of rice chaff) samples were analyzed.
16S rRNA HiSeq sequencing identified 56–149 OTUs for each compost sample, for a total of 1113 OTUs. As shown in Fig. 4a, the coverage indexes of all samples were above 0.999, indicating that the sequencing had good species coverage and reflected the actual communities. As seen from the observed richness (Sobs) of OTUs (Fig. 4b) and the Chao1 index (Fig. 4d), the taxonomic richness in T2 was generally higher than that in CK. The lowest richness value appeared in CK_0, while the highest richness value appeared in T2_73. Alpha diversity indexes can reflect community diversity. The Shannon index of CK was smaller than that of T2 (Fig. 4c). As composting progressed, GR gradually decreased, and the Shannon indexes of T2 and CK both showed an upward trend until the 73rd day, which was consistent with the findings by Chen et al. [45]. Their Shannon indexes peaked at 3.295 and 3.224, respectively. These results showed that GR had a strong inhibitory effect on bacterial abundance and diversity and that co-composting had a significant effect on increasing bacterial richness and diversity. The Chao1 and Shannon indexes showed that the abundance and diversity of the bacterial community in T2 was higher than that in CK. The high microbial diversity in T2 might be attributed to the compost materials, which was beneficial to the growth of microorganisms [46, 47].
18S rRNA HiSeq sequencing identified only 227 OTUs for fungi, much fewer than for bacteria. The coverage indexes (Fig. 4a) of the fungi were also above 0.999, indicating that the obtained sequences reflected the actual communities. According to the Sobs of OTUs (Fig. 4b) and the Chao1 index (Fig. 4d), the change trend of fungi was similar to that of bacteria, but the richness and Chao1 index of fungi were much lower than those of bacteria. Meanwhile, the Shannon index (Fig. 4c) indicated that the fungal diversity was lower than the bacterial diversity, with values of 1.127, 2.004, 1.894, 1.206, and 0.365 in T2 and 0.914, 1.250, 0.402, 1.700, and 2.134 in CK at different stages. In this study, the number of OTUs (Fig. 4b) and the Shannon index (Fig. 4c) of fungi were significantly lower than those of bacteria, suggesting that fungi grew poorly during high-temperature aerobic composting.
Microbial Composition
Figure 5a and b shows the compositions of bacterial and fungal phyla after filtering the high-sequencing results based on an abundance of 0.5%. Globally, the most representative bacterial phyla in the T2 and CK treatments were Firmicutes, Actinobacteria and Proteobacteria. These phyla were present in all types of samples but at different proportions. The two most abundant phylum were the same in the T2 and CK composting treatments (Firmicutes and Actinobacteria). The dynamics of the compost microflora indicated that these phyla were key players in the decomposition of OM throughout the composting process [40]. During the entire composting process, the changes in the bacterial communities at different phases during the T2 and CK treatments was similar. At the thermophilic phase, Firmicutes were the most predominant component, contributing 95.72% and 96.66% of the total bacteria in the T2 and CK treatments, respectively. The possible reason was that GMR was rich in cellulose. Firmicutes was the dominant microflora of cellulose degradation [48]. Some studies have demonstrated that Firmicutes dominates the thermophilic phases and is widely distributed in compost, where it plays a key role in promoting cellulose degradation and utilization [49] As composting continued, the abundance of Actinobacteria first decreased and then increased, accounting for 17.52% and 14.97% of the total abundance in the T2 and CK treatments, respectively, during the cooling period. Zhong et al. [32] also demonstrated that Actinobacteria had a higher relative abundance at the cooling stage during dairy manure composting. Furthermore, Huerta et al. [50] found that ARGs were possibly carried and disseminated by Firmicutes and Actinobacteria. Therefore, more studies that focus on the environmental safety assessment risks of GMR composting are needed. At the maturation stage, the relative abundance of Proteobacteria increased during the T2 and CK treatments, which was consistent with the results reported by Liu et al. [51].
However, the compositions of the fungal communities were not similar to those of the bacteria. Ascomycota and Basidiomycota were the main phyla, accounting for more than 98% of the fungi in the two piles (Fig. 5b). Neher et al. [52] found that in composting, the main fungal phyla were Ascomycota and Basidiomycota under heat stress and different compost recipes. The high percentages of Ascomycota and Basidiomycota may be due to the persistent continuous heat and the addition of rice chaff.
Relationship between Physicochemical Parameters and the Bacterial Community Composition
Due to the higher diversity and abundance of the bacterial community and its dominant position over the fungal community in composting [53], the relationships between environmental factors and the bacterial microbial community were further determined to explore the degradation mechanism of gentamicin. During composting, environmental factors can affect microbial community structure by affecting the decomposition of OM [54]. To better reveal the dynamic correlations between bacterial communities and environmental parameters during composting, CCA (Fig. 6) was used to analyze the bacterial community and physicochemical indicators (GR, temperature, pH, EC and C/N ratio). As shown in Fig. 6, CCA1 and CCA2 explained 40.63% and 18.72% of the variation in bacterial communities, respectively. Among the environmental factors, GR played a key role in differentiating the bacteria between T2 and CK. The CCA values of the two groups in the mesophilic period were not particularly far apart (Fig. 6); however, the values at the thermophilic periods were far apart (Fig. 6), indicating that the bacterial community differences were positively influenced by the physicochemical factors during composting. With gentamicin degradation, the bacterial community compositions between T2 and CK composts gradually stabilized, showing little difference in the mature period. To further determine the correlations between bacteria and physicochemical parameters, Spearman’s correlation coefficient analysis was used to analyze the environmental factors and the ten most abundant bacteria. As shown in Fig. 7, Micromonospora and Enterococcus had conspicuous positive correlations (p < 0.05) with GR, which indicated that gentamicin was probably degraded by these bacteria. Norank_f_Bacillaceae, Bacillus, Oceanobacillus and unclassified_f_Bacillaceae, which belong to Bacillaceae and Firmicutes, were notably positively correlated with pH at the thermophilic and cooling periods (p < 0.05). They could form very thick spore walls to resist high temperatures and high pH values. This could explain the increase in Firmicutes in the community composition of bacteria during the thermophilic and cooling periods (Fig. 5a).
CCA of the relationships between physicochemical parameters and the bacteria of the top 10 genera (explanatory variables of the top 10). The blue arrows indicate the physicochemical parameters. The red triangles represent genera. The green triangles and black squares represent samples of the CK and T2 treatments, respectively, at different composting phases. CK: fresh GMR and dried GMR (8:1, w:w); T2: fresh GMR and rice chaff (4:1, w:w)Fig. 7 Heatmap of correlations between the top ten genera in composting samples and physicochemical factors. *P < 0.05, **P < 0.01, ***P < 0.001
Conclusions
In this study, parameters such as GR, temperature, pH, C/N ratio EC, GI, and crop growth indexes as well as assessments of microbial communities were used to estimate the effectiveness of co-composting GMR with rice chaff. The results demonstrated that co-composting is a practical technological measure for treating and managing GMR. CCA showed that bacterial communities were greatly influenced by residual gentamicin. Micromonospora and Enterococcus were the bacterial genera that might degrade gentamicin. Nevertheless, gentamicin-resistant bacteria, resistant genes and degradation products were not considered; thus, the effects of the application of GMR to soil and crops were hardly predicted. Therefore, in future work, this study could be extended to the dynamic changes in gentamicin-resistant bacteria and resistant genes during composting and the toxicity of gentamicin-degraded products to further determine the feasibility of the aerobic composting of GMR.
Data Availability
The datasets generated and analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- GMR:
-
Gentamicin mycelial residue
- ARGs:
-
Antibiotic resistance genes
- DNA:
-
Deoxyribonucleic acid
- EC:
-
Electrical conductivity
- C/N:
-
Carbon/Nitrogen
- GI:
-
Germination index
- OM:
-
Organic matter
- TN:
-
Total nitrogen
- ER:
-
Emergence rate
- GR:
-
Gentamicin residue
- SS:
-
Soluble sugar
- \(\mathrm{GDP}\) :
-
Gentamicin degradation percentage, %
- \({C}_{0}\) :
-
The initial gentamicin concentration in compost, mg/kg
- \({C}_{t}\) :
-
Gentamicin concentration in compost on day t, mg/kg
- \(k\) :
-
The rate constant of antibiotic degradation, day−1
- t1/2 :
-
The half-life day
- PCR:
-
Polymerase chain reaction
- OTUs:
-
Operational taxonomic units
- ANOVA:
-
One-way analysis of variance
- LSD:
-
Least significant difference
- CCA:
-
Canonical correlation analysis
- R2 :
-
The correlation coefficients
- Sobs :
-
Observed richness
References
Govindappa, P.K., Gautam, V., Tripathi, S.M., Sahni, Y.P., Raghavendra, H.L.S.: Effect of Withania somnifera on gentamicin induced renal lesions in rats. Rev Bras Farmacogn. 29(2), 234–240 (2019). https://doi.org/10.1016/j.bjp.2018.12.005
The People’s Republic of China Ministry of Environmental Protection and National Development and Reform Commission: National Catalogue of Hazardous wastes; ED-89. China Ministry of Environmental Protection and National Development and Reform Commission, Beijing (2008)
Chen, C., Pankow, C.A., Oh, M., Heath, L.S., Zhang, L.Q., Du, P., Xia, K., Pruden, A.: Effect of antibiotic use and composting on antibiotic resistance gene abundance and resistome risks of soils receiving manure-derived amendments. Environ. Int. 128, 233–243 (2019). https://doi.org/10.1016/j.envint.2019.04.043
Mei, Z., Xiang, L.L., Wang, F., Xu, M., Fu, Y.H., Wang, Z.Q., Hashsham, S.A., Jiang, X., Tiedje, J.M.: Bioaccumulation of Manure-borne antibiotic resistance genes in carrot and its exposure assessment. Environ Int. 157, 106830 (2021). https://doi.org/10.1016/j.envint.2021.106830
Jiang, X.L., Ellabaan, M.M.H., Charusanti, P., Munck, C., Blin, K., Tong, Y.J., Weber, T., Sommer, M.O.A., Lee, S.Y.: Dissemination of antibiotic resistance genes from antibiotic producers to pathogens. Nat. Commun. 8, 15784 (2017). https://doi.org/10.1038/ncomms15784
Zhang, H.M., Deng, X.Y., Miki, D., Cutler, S., La, H.G., Hou, Y.J., Oh, J.E., Zhu, J.K.: Sulfamethazine suppresses epigenetic silencing in arabidopsis by impairing folate synthesis. Plant Cell 24(3), 1230–1241 (2012). https://doi.org/10.1105/tpc.112.096149
Boxall, A.B.A., Johnson, P., Smith, E.J., Sinclair, C.J., Stutt, E., Levy, L.S.: Uptake of veterinary medicines from soils into plants. J Agr Food Chem. 54(6), 2288–2297 (2006). https://doi.org/10.1021/jf053041t
Cheng, D.M., Liu, Y.W., Shehata, E., Feng, Y., Lin, H., Xue, J.M., Li, Z.J.: In-feed antibiotic use changed the behaviors of oxytetracycline, sulfamerazine, and ciprofloxacin and related antibiotic resistance genes during swine manure composting. J Hazard. Mater. 402, 123710 (2021). https://doi.org/10.1016/j.jhazmat.2020.123710
Wang, Q., Wang, Z., Awasthi, M.K., Jiang, Y.H., Li, R.H., Ren, X.N., Zhao, J.C., Shen, F., Wang, M.J., Zhang, Z.Q.: Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresour. Technol. 220, 297–304 (2016). https://doi.org/10.1016/j.biortech.2016.08.081
Ezzariai, A., Hafidi, M., Khadra, A., Aemig, Q., Fels, L.E., Barret, M., Merlina, G., Patureau, D., Pinelli, E.: Human and veterinary antibiotics during composting of sludge or manure: Global perspectives on persistence, degradation, and resistance genes. J. Hazard. Mater. 359, 465–481 (2018). https://doi.org/10.1016/j.jhazmat.2018.07.092
Zhang, M., He, L.Y., Liu, Y.S., Zhao, J.L., Liu, W.R., Zhang, J.N., Chen, J., He, L.K., Zhang, Q.Q., Ying, G.G.: Fate of veterinary antibiotics during animal manure composting. Sci. Total Environ. 650, 1363–1370 (2019). https://doi.org/10.1016/j.scitotenv.2018.09.147
Liu, Y.W., Feng, Y., Cheng, D.M., Xue, J.M., Wakelin, S.A., Hu, H.Y., Li, Z.J.: Gentamicin and changes in fungal diversity and physicochemical properties during composting of gentamicin production residue. Bioresour. Technol. 244, 905–912 (2017). https://doi.org/10.1016/j.biortech.2017.08.057
Liao, H.P., Zhao, Q., Cui, P., Chen, Z., Yu, Z., Geisen, S., Friman, V.P., Zhou, S.G.: Efficient reduction of antibiotic residues and associated resistance genes in tylosin antibiotic fermentation waste using hyperthermophilic composting. Environ Int. 133, 105203 (2019). https://doi.org/10.1016/j.envint.2019.105203
Bargougui, L., Guergueb, Z., Chaieb, M., Mekki, A.: Co-composting of olive industry wastes with poultry manure and evaluation of the obtained compost maturity. Waste Biomass Valorization 11(11), 6235–6247 (2018). https://doi.org/10.1007/s12649-019-00901-9
Meng, X.Y., Yan, J., Zuo, B., Wang, Y.H., Yuan, X.F., Cui, Z.J.: Full-scale of composting process of biogas residues from corn stover anaerobic digestion: Physical-chemical, biology parameters and maturity indexes during whole process. Bioresour. Technol. 302, 122742 (2020). https://doi.org/10.1016/j.biortech.2020.122742
Meng, X.Y., Liu, B., Xi, C., Luo, X.S., Yuan, X.F., Wang, X.F., Zhu, W.B., Wang, H.L., Cui, Z.J.: Effect of pig manure on the chemical composition and microbial diversity during co-composting with spent mushroom substrate and rice husks. Bioresour. Technol. 251, 22–30 (2018). https://doi.org/10.1016/j.biortech.2017.09.077
Zhang, D.F., Luo, W.H., Yuan, J., Li, G.X., Luo, Y.: Effects of woody peat and superphosphate on compost maturity and gaseous emissions during pig manure composting. Waste Manage. 68, 56–63 (2017). https://doi.org/10.1016/j.wasman.2017.05.042
Duan, M.L., Gu, J., Wang, X.J., Li, Y., Zhang, S.Q., Yin, Y.N., Zhang, R.R.: Effects of genetically modified cotton stalks on antibiotic resistance genes, intI1, and intI2 during pig manure composting. Ecotoxicol. Environ. Saf. 147, 637–642 (2018). https://doi.org/10.1016/j.ecoenv.2017.09.023
Ren, G.M., Xu, X.H., Qu, J.J., Zhu, L.P., Wang, T.T.: Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J. Microbiol. Biotechnol. 32, 101–111 (2016). https://doi.org/10.1007/s11274-016-2059-7
Li, R.H., Wang, J.J., Zhang, Z.Q., Shen, F., Zhang, G., Qin, R., Li, X., Xiao, R.: Nutrient transformations during composting of pig manure with bentonite. Bioresour. Technol. 121, 362–368 (2012). https://doi.org/10.1016/j.biortech.2012.06.065
Chen, W., Liao, X.D., Wu, Y.B., Liang, J.B., Mi, J.D., Huang, J.J., Zhang, H., Wu, Y., Qiao, Z.F., Li, X., Wang, Y.: Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manage. 61, 506–515 (2017). https://doi.org/10.1016/j.wasman.2017.01.014
Pribyl, D.W.: A critical review of the conventional SOC to SOM conversion factor. Geoderma 156, 75–83 (2010). https://doi.org/10.1016/j.geoderma.2010.02.003
Gu, W.J., Zhang, F.B., Xu, P.Z., Tang, S.H., Xie, K.Z., Huang, X., Huang, Q.Y.: Effects of sulphur and Thiobacillus thioparus on cow manure aerobic composting. Bioresour. Technol. 102, 6529–6535 (2011). https://doi.org/10.1016/j.biortech.2011.03.049
Gupta, A., Garg, A.: Utilisation of sewage sludge derived adsorbents for the removal of recalcitrant compounds from wastewater: mechanistic aspects, isotherms. Kinetics and Thermodyn. Bioresour. Technol. 194, 214–224 (2015). https://doi.org/10.1016/j.biortech.2015.07.005
Yang, B., Meng, L., Xue, N.D.: Removal of five fluoroquinolone antibiotics during broiler manure composting. Environ. Technol. 39, 373–381 (2018). https://doi.org/10.1080/09593330.2017.1301568
Selvam, A., Zhao, Z.Y., Wong, J.W.C.: Composting of swine manure spiked with sulfadiazine, chlortetracycline and ciprofloxacin. Bioresour. Technol. 126, 412–417 (2012). https://doi.org/10.1016/j.biortech.2011.12.073
Zhu, N.W.: Effect of low initial C/N ratio on aerobic composting of swine manure with rice straw. Bioresour Technol. 98(1), 9–13 (2007). https://doi.org/10.1016/j.biortech.2005.12.003
Ho, Y.B., Zakaria, M.P., Latif, P.A., Saari, N.: Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 131, 476–484 (2013). https://doi.org/10.1016/j.biortech.2012.12.194
Yu, Y.S., Chen, L.J., Fang, Y., Jia, X.B., Chen, J.C.: High temperatures can effectively degrade residual tetracyclines in chicken manure through composting. J Hazard. Mater. 380, 120862 (2019). https://doi.org/10.1016/j.jhazmat.2019.120862
Wu, X.F., Wei, Y.S., Zheng, J.X., Zhao, X., Zhong, W.K.: The behavior of tetracyclines and their degradation products during swine manure composting. Bioresour. Technol. 102(10), 5924–5931 (2011). https://doi.org/10.1016/j.biortech.2011.03.007
Selvam, A., Zhao, Z.Y., Li, Y.C., Chen, Y.M., Leung, K., Wong, J.: Degradation of tetracycline and sulfadiazine during continuous thermophilic composting of pig manure and sawdust. Environ. Technol. 34, 2433–2441 (2013). https://doi.org/10.1080/09593330.2013.772644
Zhong, X.Z., Li, X.X., Zeng, Y., Wang, S.P., Sun, Z.Y., Tang, Y.Q.: Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 306, 123091 (2020). https://doi.org/10.1016/j.biortech.2020.123091
Ye, S.J., Zeng, G.M., Wu, H.P., Liang, J., Zhang, C., Dai, J., Xiong, W.P., Song, B., Wu, S.H., Yu, J.F.: The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour. Conserv. Recyl. 140, 278–285 (2019). https://doi.org/10.1016/j.resconrec.2018.10.004
Mitchell, S.M., Ullman, J.L., Bary, A., Cogger, C.G., Teel, A.L., Watts, R.J.: Antibiotic degradation during thermophilic composting. Water Air Soil Poll. 226(2), 13–24 (2015). https://doi.org/10.1007/s11270-014-2288-z
GB 7959-2012.: Hygienic requirem Hygienic requirements for harmless disposal of night soil (2012).
Jiang, J.S., Huang, H., Huang, Y.M., Liu, X.L., Liu, D.: Relationship between maturity and microbial communities during pig manure composting by phospholipid fatty acid (PLFA) and correlation analysis. J. Environ. Manage. 206, 532–539 (2018). https://doi.org/10.1016/j.jenvman.2017.10.067
Awasthi, M.K., Pandey, A.K., Khan, J., Bundela, P.S., Wong, J.W.C., Selvam, A.: Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour. Technol. 168, 214–221 (2014). https://doi.org/10.1016/j.biortech.2014.01.048
Chan, M.T., Selvam, A., Wong, J.W.C.: Reducing nitrogen loss and salinity during ‘struvite’food waste composting by zeolite amendment. Bioresour. Technol. 200, 838–844 (2016). https://doi.org/10.1016/j.biortech.2015.10.093
Garcia-Gomez, A., Bernal, M.P., Roig, A.: Organic matter fractions involved in degradation and humification processes during composting. Compost Sci. Util. 13, 127–135 (2005). https://doi.org/10.1080/1065657X.2005.10702229
Liu, Y.W., Cheng, D.M., Xue, J.M., Weaver, L., Wakelin, S.A., Feng, Y., Li, Z.J.: Changes in microbial community structure during pig manure composting and its relationship to the fate of antibiotics and antibiotic resistance genes. J Hazard. Mater. 389, 122082 (2020). https://doi.org/10.1016/j.jhazmat.2020.122082
Mathur, S.P., Owen, G., Dinel, H., Schnitzer, M.: Determination of compost biomaturity. I. Lit. rev. Biol Agric Hortic. 10, 65–85 (1993). https://doi.org/10.1080/01448765.1993.9754655
Luo, Y., Liang, J., Zeng, G.M., Chen, M., Mo, D., Li, G.X., Zhang, D.F.: Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manage. 71, 109–114 (2018). https://doi.org/10.1016/j.wasman.2017.09.023
Tian, W., Li, L.Z., Liu, F., Zhang, Z.H., Yu, G.H., Shen, Q.R., Shen, B.: Assessment of the maturity and biological parameters of compost produced from dairy manure and rice chaff by excitation-emission matrix fluorescence spectroscopy. Bioresour. Technol. 110, 330–337 (2012). https://doi.org/10.1016/j.biortech.2012.01.067
Khalil, A.I., Beheary, M.S., Salem, E.M.: Monitoring of microbial populations and their cellulolytic activities during the composting of municipal solid wastes. World J Microb Biot. 17, 155–161 (2001). https://doi.org/10.1023/A:1016682329925
Chen, Z., Li, Y.Z., Peng, Y.Y., Ye, C.S., Zhang, S.H.: Effects of antibiotics on hydrolase activity and structure of microbial community during aerobic co-composting of food waste with sewage sludge. Bioresour. Technol. 321, 124506 (2021). https://doi.org/10.1016/j.biortech.2020.124506
De Gannes, V., Eudoxie, G., Hickey, W.J.: Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour. Technol. 133, 573–580 (2013). https://doi.org/10.1016/j.biortech.2013.01.138
Zhang, L.L., Jia, Y.Y., Zhang, X.M., Feng, X.H., Wu, J.J., Wang, L.S., Chen, G.J.: Wheat straw: An inefficient substrate for rapid natural lignocellulosic composting. Bioresour. Technol. 209, 402–406 (2016). https://doi.org/10.1016/j.biortech.2016.03.004
Hu, T., Wang, X.J., Zhen, L.S., Gu, J., Zhang, K.Y., Wang, Q.Z., Ma, J.Y., Peng, H.L.: Effects of inoculation with lignocellulose-degrading microorganisms on antibiotic resistance genes and the bacterial community during co-composting of swine manure with spent mushroom substrate. Environ. Pollut. 252, 110–118 (2019). https://doi.org/10.1016/j.envpol.2019.05.078
Yin, Y.N., Gu, J., Wang, X.J., Song, W., Zhang, K.Y., Sun, W., Zhang, X., Zhang, Y.J., Li, H.C.: Effects of copper addition on copper resistance, antibiotic resistance genes, and intl1 during swine manure composting. Front. Microbiol. 8, 344 (2017). https://doi.org/10.3389/fmicb.2017.00344
Huerta, B., Marti, E., Gros, M., López, P., Pompêo, M., Armengol, J., Barceló, D., Balcázar, J.L., Rodríguez-Mozaz, S., Marcé, R.: Exploring the links between antibiotic occurrence, antibiotic resistance, and bacterial communities in water supply reservoirs. Sci. Total Environ. 456–457, 161–170 (2013). https://doi.org/10.1016/j.scitotenv.2013.03.071
Liu, N., Hou, T., Yin, H.J., Han, L.J., Huang, G.Q.: Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J. Hazard. Mater. 362, 258–265 (2018). https://doi.org/10.1016/j.jhazmat.2018.09.028
Neher, D.A., Weicht, T.R., Bates, S.T., Leff, J.W., Fierer, N.: Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS One 8(11), 79512 (2013). https://doi.org/10.1371/journal.pone.0079512
Tiquia, S.M.: Microbial community dynamics in manure composts based on 16S and 18S rDNA T-RELP profiles. Environ. Technol. 26, 1101–1114 (2005). https://doi.org/10.1080/09593332608618482
Zhang, J.C., Zeng, G.M., Chen, Y.N., Yu, M., Yu, Z., Li, H., Yu, Y., Huang, H.L.: Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 102, 2950–2956 (2011). https://doi.org/10.1016/j.biortech.2010.11.089
Acknowledgements
The authors are grateful to all of the employees of the composting sites that participated in this study. The authors also thank Huimin Zhang, Nan Liu and Ke Wang for their great practical assistance. This work was financed by the National Natural Science Foundation of China (No. 21107100) and the Outstanding Young Talent Research Fund of Zhengzhou University (No. 1421324067).
Funding
This work was financed by the National Natural Science Foundation of China (Grant Number 21107100) and the Outstanding Young Talent Research Fund of Zhengzhou University (Grant Number 1421324067).
Author information
Authors and Affiliations
Contributions
Investigation, Data curation, Writing-Original: WB; Writing—Review & Editing: JW; Resources: HZ; Data curation, Validation: NL; Methodology, Software: KW; Conceptualization, Supervision: YW.
Corresponding author
Ethics declarations
Conflict of Interest
We declare that we have no financial and personal relationships with other people or organizations that could inappropriately influence our work and that there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, this manuscript.
Ethics Approval
This is our original work and we certify that we have participated sufficiently in the work to take public responsibility for the appropriateness of the experimental design and method and for the collection, analysis, and interpretation of the data. This manuscript has not been published previously.
Consent to Participate
All the authors have reviewed the final version of the manuscript and mutually agreed that it should be submitted to “Waste and Biomass Valorization”.
Consent for Publication
If “Waste and Biomass Valorization” accepts our manuscript, all the authors will agree to its publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bu, W., Wan, J., Zhang, H. et al. Effects of Pilot-Scale Co-composting of Gentamicin Mycelial Residue with Rice Chaff on Gentamicin Degradation, Compost Maturity and Microbial Community Dynamics. Waste Biomass Valor 13, 4797–4812 (2022). https://doi.org/10.1007/s12649-022-01805-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01805-x