Abstract
Rice straw biogas residue contains high lignin content, so the decomposition of this material by natural compost is slow. This study investigated the effect of the combination of rice straw addition and microbial inoculation (Aspergillus niger CICIMF0410 and Phanerochaete chrysosporium AF96007) on the physiochemical properties of rice straw biogas residue in the composting process. Four composting treatment groups were designed: rice straw biogas residue (RR; T1), RR + rice straw (RS) (T2), RR + inoculants (T3), and RR + RS + inoculants (T4). The addition of rice straw significantly accelerated the initial temperature rise during composting, and microbial inoculation accelerated lignocellulose decomposition. After composting, the electrical conductivities of samples T1 to T4 were 1.22, 0.87, 0.99, and 0.82 DS ml−1, respectively. Compared with other treatment samples, T4 showed noticeably increased degradation rates of HC, CL, and ADL after decomposition. The total humic acid levels of samples T1 to T4 were 9.5, 12.7, 10.5, and 18.6%, with humification index values of 1.45, 1.80, 1.69, and 2.46, respectively, after maturation. After 12 weeks of composting, T4 showed the highest increase in total nitrogen (37.6%), phosphorus (33.6%), and potassium (29.8%) levels. Furthermore, compared with other treatment samples, T4 showed the best maturity indices, such as morphology, color, and odor. Rice straw addition and biological inoculation for biogas residue composting not only significantly decreases the time required for decomposition but also eliminates the toxicity risk for crops and improves the stability of the biogas residue fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the rapid increase in the global population and boom in economic activity, the biogas industry is witnessing explosive growth [1]. China’s current annual biogas production is approximately 2 billion m3, which corresponds to an annual output of approximately 100 million tons of fresh biogas residue [2]. In Europe, the production from biogas reactors has increased by 2.83 times in the past 8 years (2008–2016), corresponding to the production of approximately 128 million tons of biogas residue [3].
Rice straw is an important component of biogas residue. Returning rice straw biogas residue to the field after decomposition can result in full utilization of its organic components, facilitating resource utilization in the form of fertilizer [4]. The aerobic composting process involves a microbial colony that decomposes [5] and converts various degradable organic components in the composting material and produces CO2, H2O, heat, and humus (Hu) precursor substances [6], thereby converting the organic components into stable humic acids (HAs) [7]. Successful composting requires a variety of microorganisms to decompose the corresponding organic components at different stages to finally produce products with a high maturity index. Carbon and nitrogen are two essential components of composting materials, with the former being the energy base for microbial life activities and the latter being essential for the synthesis of microbial amino acids and nucleic acids [2]. In biogas production, the organic carbon content of rice straw biogas residue significantly decreases, leading to insufficient energy for the survival of microorganisms, so the functional succession of microorganisms cannot be achieved in the composting process, and decomposition cannot be thoroughly completed. Furthermore, rice straw biogas residue contains high lignocellulose content. During anaerobic fermentation, although the unstable organic components of the residue can be converted to biogas [8], the rest is mainly composed of lignocelluloses, particularly lignin, which is difficult to degrade through traditional biochemical reactions [9]. Consequently, the slow and incomplete decomposition of rice straw biogas residue leads to low maturity indices, which is a problem in composting that needs to be urgently addressed.
The addition of a certain amount of rice straw during cocomposting of livestock manure could increase the activity of microorganisms by altering the physiochemical environment during composting [10], thereby promoting the degradation of organic matter (OM) in animal manure materials and accelerating maturation due to the increased content of organic carbon in the materials [11]. For the decomposition of compost materials with high lignocellulose content, researchers have attempted different methods, such as hydrogen peroxide addition [12], steam explosion [13,14,15], and charcoal addition [11]. However, all these methods failed to result in satisfactory lignin decomposition. Recent advances in biology have shown that some fungi and bacteria can have an active effect on lignocellulose decomposition [16,17,18,19]. Nevertheless, the breeding of microorganisms is closely associated with the environment in which they live [20, 21]. For instance, Meng et al. [2] adopted spent mushrooms for biogas residue composting and found that the lignin component was not degraded. Therefore, an appropriate physicochemical environment and the addition of related functional microorganisms during decomposition may have a synergistic effect on the degradation of the biogas residue lignocellulose and may further result in the formation of safe and stable decomposition products. However, studies on this aspect have been rarely reported.
On the other hand, high maturity of the material does not indicate high quality of the fertilizer. Instead, the humic substance (HS) and HA levels of the decomposed fertilizer are the most important indicators for evaluating the quality of compost [22]. Theoretical studies have demonstrated that lignin in lignocellulose plays a crucial role in the formation of HA [23, 24]. However, the degradation of the lignin polymer is strongly impacted by the composting materials and by periodic physiochemical changes in the environment during the composting process, such as changes in temperature, pH, electrical conductivity (EC), and OM content. From the perspective of agronomic use of fertilizers, a comprehensive analysis and evaluation should be conducted by taking the carbon to nitrogen ratio (C/N), NH+ 4/NH− 3 ratio, seed germination index (GI), humification index (HI), and other chemical and agronomic indicators into consideration [25].
Based on the aforementioned considerations, this study conducted a scientific and systematic analysis of the effect of coapplication of rice straw and functional microorganisms in rice straw biogas residue decomposition, attempting to realize rapid decomposition of the residue and thus produce safe and high-quality fertilizer with high HA content.
The novelty of this study includes the following aspects: (1) rice straw that contains high OM content and functional microorganisms that exhibit strong degradation ability toward cellulose (CL) and lignin were combined for rice straw biogas residue composting; (2) variations in physiochemical factors, such as maturity indices, component degradation of lignocelluloses, HA production, and degree of humification, during composting were systematically investigated; and (3) this study focused on a composting method for returning of rice straw to the field as an effective fertilizer and for cycling the utilization of the substance and energy of the residue waste of rice production.
2 Materials and methods
2.1 Rice straw
Rice straw was purchased from the paddy fields in the Lin’an District of Hangzhou city after harvesting. The sample was manually cut to a length of 3–5 cm and dried at 105 °C for 2 h to characterize the relevant physical and chemical properties [26].
2.2 Rice straw biogas residue
The rice straw was first digested by ruminants (at the dairy breeding base in the town of Banqiao, Lin’an District, Hangzhou), and the manure was collected and then put into a biogas pool (a marsh pond in an east Tianmu mountain farmhouse in Lin’an District) for anaerobic fermentation. The fermentation time was 60 days [27]. The chemical properties of the rice straw and rice straw biogas residue are shown in Table 1.
2.3 Microbial culture
After activation, Aspergillus niger CICIMF0410 and Phanerochaete chrysosporium AF 96007 [18, 19] (stored at the Engineering College of Zhejiang Agriculture and Forestry University) were added to potato dextrose agar medium (containing potato (200.0 g), glucose (20.0 g), agar (20.0 g), and distilled water (1000 ml) with a pH of 6.0–6.5) for 3 days of cultivation at 28 °C. Then, an appropriate amount of liquid slanting medium (0.5 g of straw powder, 0.50 g of MgSO4·7H2O, 0.22 g of K2HPO4, 100 ml of distilled water, natural pH) was used for the propagation culture on a 150 r min−1 shaker for 7 days at 30 °C (away from light) [18, 19]. The number of microbial colonies was determined by the spread plate method [7], and sterile water was used to adjust the microbial concentration to 1 × 108 CFU ml−1.
2.4 Composting design
The biogas residue and rice straw (chopped to 3–5 cm long) were treated according to the experimental design shown in Table 2. The C/N ratio of the mixture of biogas residue and rice straw was 32:1, which is the optimal ratio for degradation of materials by microorganisms [28].
The sample was placed in a special aerobic composting container with dimensions of 1.0 × 0.8 × 0.8 m3. The four corners of the container were separately supported by a 1.0 m × 0.06 m × 0.06 m pillar stand, and the four lateral sides were separately fixed onto the pillar stand by 11 0.92 m × 0.05 m × 0.02 m boards through zincified bolts, with parallel intervals of 0.02 cm between the boards. Pure water was added to adjust the humidity to 60%. Nine silica gel tubes from an aerobic pump were used for continuous ventilation in the container box, with 3 evenly distributed in the radial direction (0.05 m in diameter) along the median surface of the container and the surfaces 25 cm above and below the median surface. The ventilatory volume was 0.25 l (kg min)−1 [8]. The prepared microbial suspension was inoculated at 1 cm3 kg−1, and then, the aerobic fermentation composting experiment was performed at room temperature. Four different treatment groups were designed: the rice straw biogas residue (RR, T1) group, the RR + rice straw (RR + RS; T2) group, the RR + 1% microbial suspension (T3) group, and the RR + RS + 1% microbial suspension (T4) group (Table 2).
2.5 Sampling and determination
There were three sampling points, located at depths of 20, 40, and 60 cm from the upper surface of the container. Three repetitions were performed for each sample point.
The temperature was measured using a digital temperature recorder (RC-4, Jiangsu Jingchuang Electric Co., Ltd.), and recording was performed every 4 days. The pH and EC values were determined by a pH and EC meter (a special, solid metal probe-based, digital pH and EC detector, Hangzhou Luheng Biotechnology Co., Ltd.). Prior to measurement, the samples were leached with deionized water at a ratio of 1:10 (w/v, g cm−3) for 1 h. The moisture content was measured by drying at 105 °C for 24 h [29].
Ash was generated by drying at 550 °C for 5 h, and the determination of total OM content was based on the difference between the masses of the starting dry matter and the ash [29]. The total nitrogen (TN) content was determined by the Kjeldahl method [2]. The total carbon (TC) content was 0.58 times the mass of the OM. The C/N was calculated from the mass ratio of TC to TN [26]. Water-soluble amino nitrogen (NH4+-N), nitro nitrogen (NO− 3-N), and available nitrogen levels were determined by the potassium chloride extraction-indophenol blue colorimetric method, an ultraviolet spectrophotometer, and alkali decomposition diffusion, respectively [10]. The total phosphorus (TP) content was determined by a flame photometer [26]. The available phosphorus (AP) was extracted with 0.5 M NaHCO3 (1:10 w/v ratio, g cm−3) as sample extractant [29]. Determination of the total potassium (TK) and available potassium (AK) content was performed by the nitric acid-perchloric acid digestion-based flame photometer method and the ammonium acetate extraction-based flame photometer method [2], respectively.
The biodegradation coefficient was calculated according to Haug’s equation [28]:
where OMi and OMf are the initial and final levels of OM, respectively.
The relative change rates for the nitrogen, phosphorus, and potassium levels were calculated according to the following formula (2) [30]:
where X1 is the final OM content, and X2 is the initial OM content.
The lignocellulose content was determined by the washing method, as described previously [27]. The final absolute levels of hemicellulose (HC), CL, and acid-washed lignin (ADL) were obtained from the difference in mass of the dry matter before and after the reaction and the final ash content. The relative degradation rates of HC, CL, and ADL were calculated by the following formula [30]:
where M1 is the initial content, and M2 is the final content.
The Hu content was determined by the sodium hydroxide extraction method [31]. After acidification, HA was precipitated to separate fulvic acid (FA). The HA content was measured, and the percentages of HA and FA were calculated [8].
The seed GI was determined using the Zucconi method as follows [22, 32]:
The volume shrinkage rate was calculated by the following formula:
where V1 is the volume before composting, and V2 is the volume after composting.
Density was determined according to Ravindran et al. [26]. The Munsell standard colorimetric method was used for color analysis after composting [33].
3 Results and discussion
3.1 Physical properties of the compost samples
3.1.1 Temperature
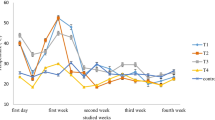
All the samples showed four distinct stages in the composting process, namely, the heating phase, the high-temperature phase, the cooling phase, and the mature phase (Fig. 1a). The addition of rice straw had a strong effect on the increase in temperature in the early stage of biogas residue composting. According to Saludes et al. [34], the reason for this change is that the C/N value of rice straw is high, and the microorganisms have a material source for heat generation in the process of OM decomposition. The T4 heating rate was the highest among all the samples. A comparison between T3 and T1 suggests that the addition of microorganisms also promoted an increase in temperature in the early stage of composting.
The times to reach the high-temperature phase in samples T1 to T4 were 20 days, 18 days, 20 days, and 16 days, with maximum temperatures of 47.4 °C, 62.3 °C, 52.1 °C, and 68.3 °C, respectively. The maximum temperature of composting needs to reach above 55 °C for more than 15 days to kill various harmful pathogens in the sample and achieve contaminant-free treatment [28]. Based on our results, the addition of organic substances may eliminate pathogens from rice straw biogas residue, thereby achieving contaminant-free material.
The durations of the high-temperature phases of T1 and T3 were approximately 34 days and 26 days, respectively, and the durations of the high-temperature phases of T2 and T4 were 34 days and 22 days, respectively. Both rice straw addition and microbial inoculation accelerated the decomposition of the sample; the former exhibited greater acceleration than the latter. The durations of the cooling phases of the T1, T2, T3, and T4 samples were approximately 31, 18, 20, and 16 days, respectively, until the samples cooled to ambient temperature and then entered the mature phase. The cooling phase of the pure biogas residue was longer than that of the other samples, and the addition of straw and microbial inoculation effectively shortened the cooling phase. In contrast, in the experiment by Meng et al. [2], who adopted biogas residue and spent mushroom substrate for cocomposting, the temperature variation during the composting process did not show four typical intervals. It is obvious that the presence of carbon and microbial inoculation are key factors for functional microbial succession and further high-quality composting of biogas residue. According to early-stage composting-related microbial theory, microorganisms are primarily responsible for the initial decomposition of OM and the generation of heat; then, in the high-temperature phase, the microbes contribute to complex material decomposition [6]. This role is particularly noticeable in the composting of biogas residue with high lignocellulose content.
3.1.2 pH
During the fermentation process, the pH values of the four groups of samples all showed a trend of an initial increase followed by a decrease (Fig. 1b). This phenomenon was more obvious for the samples supplemented with rice straw than for the other samples. In contrast, the pH values of the T2 and T4 samples increased at a faster rate than those of the other samples in the early stage, while the pH values of the T1 samples showed the slowest change. As the compost matured, the pH values of the four samples gradually stabilized. After 12 weeks, the pH values of samples T1 to T4 were 7.6, 7.4, 7.4, and 7.3, respectively, indicating weak alkalinity. These results were basically consistent with those reported by Meng et al. [2]. During the heating and high-temperature phase of compost, with the degradation and mineralization of OM, the decomposition of amino acids and volatilization of ammonia gas lead to an increase in the pH of samples; after the reactions are completed, the pH gradually becomes stable [26].
3.1.3 EC
As shown in Fig. 1c, the EC values of all the samples showed a downward trend during the composting process. The EC of all samples decreased slowly in the early stage, and then there was a faster decreasing region. Afterwards, the EC tended to be stable. Among the samples, T4 entered the decreasing zone approximately 1 week earlier than T2 and T3, and the EC of T1 decreased more slowly than that of other samples and presented a stepwise decline. Finally, the EC values of samples T1 to T4 were 1.22, 0.87, 0.99, and 0.82 DS ml−1, respectively. If the soluble salt content of the soil is too high, the growth of crops in the soil ceases. A concentration of 3.0 DS ml−1 is considered the maximum threshold of toxicity [26]. Based on Watson’s research, Ferial showed that when the EC value of the decomposed fertilizer was < 1.5 DS ml−1, it could be used as a soil fertilizer [28]. Therefore, in terms of the EC value, the four samples above meet the requirements after a certain period of decomposition. The decrease in the EC value of the sample was due to a reduction in water-soluble components, ammonia evaporation, and precipitation of mineral salts during the composting decomposition process [27]. Our results show that the abovementioned hypothesis is also applicable to the composting of rice straw biogas residue with high lignocellulose content.
3.2 Maturity parameters during composting
3.2.1 OM
As shown in Fig. 1d, the OM content of all samples showed a gradual decline during composting. According to Haug’s equation [28], the biodegradation coefficient (kb) values of samples T1 to T4 were 0.34, 0.39, 0.38, and 0.50, respectively. As the data show, the combined effect resulting from the addition of rice straw and microbial inoculation can effectively promote the degradation of OM in the samples. Based on the comparison between the outcomes of T1 and T2, although the OM degradation rate of T2 was higher than that of T1, the absolute OM content in T2 remained high because the added rice straw itself contained OM. Compared with T1, T3 showed a decrease in the final OM content by 1.4%, which indicates that the microbial inoculation promoted OM degradation. This finding was consistent with that reported by Tran et al. [35], according to whom inoculation with lactic acid bacteria accelerated OM degradation during composting. A. niger and P. chrysosporium degrade polysaccharides in xylem fibers into monosaccharides, accelerating microbial multiplication and therefore realizing OM utilization [36]. At the same time, the OM content of the four samples after decomposition exceeded the national standard index of 45% stipulated by China’s organic fertilizer industry.
3.2.2 TN
The TN content of the samples showed a gradual upward trend during the composting process, and this upward trend slowed in the late stages of composting (Fig. 2a). After 12 weeks of composting, the final TN levels of the four samples T1 to T4 were 1.77, 1.85, 1.80%, and 2.05%, respectively, and these outcomes were consistent with those reported in the literature [10, 17, 37]. After composting, the nitrogen, phosphorus, and potassium contents of the four samples T1 to T4 increased by 0.09, 25, 10.4, and 37.6%, respectively, because as composting proceeded, the volatile components in the samples were lost, which resulted in a relative increase in their levels [2].
3.2.3 C/N ratio
When the C/N ratio is lower than 20:1, samples can be considered to have reached maturity [26]. As shown in Fig. 2b, the C/N values of all the samples gradually decreased after 12 weeks of composting, and the C/N values of samples T1 to T4 decreased to 19.8, 19.5, 19.0, and 16.3, respectively. After composting, the C/N ratio in sample T1 was at the critical point of maturity, and that of sample T4 was at approximately the theoretical optimal value, that is, 16:1. OM decomposition and mineralization and moisture loss from the compost jointly lead to a decrease in the C/N ratio [17].
3.2.4 GI
The variation in the seed GI of all samples experienced early fluctuations and then rose rapidly and stabilized (Fig. 2c). When the GI value is > 80%, samples are considered to be maturing [26]; thus, in this experiment, the T2, T3, and T4 samples reached maturity. T2, T3, and T4 had GI values of 80% at 63, 72, and 56 days, respectively. However, the GI of the T1 sample did not reach 0.8 by 90 days. Presumably, the mechanisms underlying GI fluctuations in the early stage of composting are ammonia volatilization and organic acid release during the high-temperature phase [30]. Our results indicate that rice straw addition combined with microbial inoculation significantly improved the GI during the composting of rice straw biogas residue.
3.3 Compost quality and stability
3.3.1 Chemical forms
As shown in Fig. 2d, the initial amino nitrogen and nitro nitrogen (nitrification index) levels of T1, T2, T3, and T4 were 440, 320, 436, and 318 ppm and 22.1, 8.65, 15.6, and 6.91 ppm, respectively. With the degradation and mineralization of organic nitrogen in the composting process, the ratio of amino nitrogen to nitro nitrogen (NH+ 4/NH− 3) in the T1, T2, T3, and T4 samples showed a strong initial increase followed by a decrease. The peaks appeared approximately 2 weeks after the start of composting, with values of 30.2, 68.0, 41.5, and 72.4, followed by a gradual decrease. This phenomenon is attributable to the inhibition of nitrification by temperature, pH, and ammonium salt at the high-temperature phase [2]. The addition of organic substances affects the NH+ 4/NH− 3 equilibrium and nitrogen mineralization to favor the retention of NH+ 4, ammonia, and other organic nitrogen materials [37]. Our study showed that microbial inoculation had a weak but consistent influence on the retention of NH+ 4. After 12 weeks, the amino nitrogen to nitro nitrogen ratios of all samples were stable, with final values of 1.2, 0.83, 0.85, and 0.76 for T1, T2, T3, and T4, respectively. According to Rashad et al. [28], the ratio of amino nitrogen to nitro nitrogen should be less than 1.0 after composting. Based on this criterion, the T1 sample was still not fully decomposed after 12 weeks of composting. The amino nitrogen levels of samples T1, T2, T3, and T4 after composting for 12 weeks were 330, 220, 280, and 160 ppm, respectively, and the above data all met the minimum requirement of being less than 400 ppm [32, 38].
The TP, TK, AP, and AK levels of each sample after composting for 12 weeks are shown in Table 3. The relative levels of TP in the T1, T2, T3, and T4 samples increased by 8.70, 21.9, 29.9, and 33.6%, respectively, and the relative levels of TK increased by 17.0, 22.4, 12.1, and 29.8%, respectively. After composting, most of the dissolved phosphorus are fixed onto microbial cells; this phenomenon is different from traditional soil phosphorus fixation, in which water-soluble P is fixed to or precipitates with soil minerals [39]. Microbial organic phosphorus exhibits slow release, providing relatively long-term phosphorus availability and in turn participating in soil improvement.
3.3.2 Decomposition and humification of lignocellulose
The change in the absolute content of lignocelluloses during composting is shown in Table 4 and Fig. 3. Overall, the HC content in the four samples showed a rapid decrease at the early stage followed by a gradual decrease at the late stage. The CL content exhibited a gradual decrease followed by a rapid decrease and then became stable gradually. The ADL content exhibited a gradual increase, with the exception of the T4 sample. The relative degradation rates of HC, CL, and ADL were 59.2, 77.0, 62.0, and 88.6%; 53.0, 51.5, 63.9, and 87.8%; and 0.00, 4.43, 18.5, and 40.5%, respectively, in samples T1 to T4 (Table 4). The data show that the pure biogas residue can play a positive role in the decomposition of HC during composting and decomposes CL, but the ADL is not degraded in the process. The comparison between groups showed that the addition of rice straw increased the relative decomposition levels in HC and CL, and inoculation with microorganisms led to not only the decomposition of HC and CL in the composting process but also the decomposition of lignin. This result is in sharp contrast to the results obtained by Meng et al. [2], who studied cocomposting with only biogas residues and spent mushroom. In their experiment, lignin levels remained unchanged throughout the composting period. Due to the bioresistance of compost to lignin, different microbial species exhibit selective biodegradation [40]. Although some naturally existing microorganism species can degrade HCs and CL during composting by altering cellulosic lattices [18], the degradation and depolymerization of lignin can be achieved only by specific fungi [16, 19]. Therefore, selection of appropriate microbial species is very important for the degradation of lignocellulose during composting [31]. Relevant functional microorganisms, such as white rot fungi, can degrade lignin through their own life activities.
In all samples, the HA abundance showed a gradual increase, whereas the FA abundance showed a gradual decrease, with more noticeable variations in T2 and T4 (Table 4). This finding indicates a role played by microorganisms. As HA has a more stable structure than FA [37], HI (HI = HA/FA) is an important indicator of compost maturity. The minimum acceptable HI of mature compost is generally considered to be 1.6 [25]. The HI values of samples T1 to T4 were 1.45, 1.80, 1.69, and 2.46, respectively, after composting. Based on the HI, the T1 sample could not be regarded as decomposed, while T4 had the highest degree of maturity. In addition, based on the compost temperature change chart, although the T3 sample entered the mature phase 4 days earlier than the T2 sample, its humification rate was lower than that of the T2 sample, which shows that the composting temperature and duration have greater impact than the humification rate of the sample. The underlying reason may be that the transformation from FA into HA can only be completed under long-term exposure to high temperatures [37].
3.3.3 Sample morphology
The volume shrinkage, moisture content, density, color, and odor of all samples after composting are shown in Fig. 4 and Table 4. The table shows that the volume shrinkage of the T2 and T4 samples (samples with added rice straw) was larger than that of the T1 and T3 samples. The reason is that the density of rice straw is low, and the structure is loosely packed, so its volume shrinks markedly after composting. The initial density of the biogas residue was high, and the volume was not very different after composting. Before composting, the color of rice straw was light yellow, and the biogas residue was brown. As the composting progressed, the color of the pile gradually darkened from the inner layer to the outer layer. The color of the T1 and T3 samples was dark brown after the end of composting, and the T2 and T4 samples showed a yellowish brown color (Fig. 4). According to the Munsell Color Standard Card, the color of the samples after maturity is shown in Table 4. This color is quite similar to the color obtained by Meng et al. [2] in the cocomposting of biogas residue and spent mushroom. The odor of all the samples gradually increased in intensity after 5 days of composting fermentation. After 15 days, the odor became thicker, and with the appearance of plaque, the pile smelled strongly until the samples decomposed. After the sample was decomposed, T1 had basically no remaining odor, and the T4 sample exuded the scent of Hu. The underlying mechanism may be that fungi and actinomycetes can produce soil odorants and 2-methylisobornol, leading to the earthy taste [41].
After maturation, the T1 and T3 samples became peatlike and had slightly fluid characteristics, whereas T2 and T4 samples were relatively loosely packed and appeared porous and flocculent (Fig. 4). Compared with the T1 sample, the T3 sample was more obviously in a cotton-like state and filled with porous voids. The same was true for the T4 sample versus the T2 sample. The results indicate that microbial inoculation has a certain swelling and dispersal effect on the morphology of the biogas residue compost.
Composting is a dynamic biochemical transmission process in which microorganisms first decompose easily degradable OM, such as soluble organic carbon and monosaccharides [26]. However, if the raw material contains a large amount of organic polymers, such as CL and ADL (which are difficult to degrade), enzymatic hydrolysis is usually needed first [25]. In this study, the total content of lignocellulosic components of the rice straw biogas residue selected was 69.6%, with a low level of easily hydrolyzable HC but high levels of CL and ADL. The results showed that T1 treatment failed to provide enough microbial heat within a short period for the succession of microbial colonies. Upon adding rice straw, the organic content of the material increased, and the C/N ratio of the material was adjusted, leading further to adjustment of physical parameters (e.g., temperature interval and pH) as well as a series of chemical changes during composting, resulting in acceleration of the decrease in EC [10]. In terms of the final C/N and NH+ 4/NH− 3 ratios, the addition of rice straw with microorganisms led to rapid maturation of the rice straw biogas residue, and after maturation, the material exhibited a high GI, which indicates that this treatment eliminated the chemical toxicity of the biogas residue, thereby increasing the agricultural safety of the fertilizer. Furthermore, from the perspective of quality and efficiency for composting, the abovementioned HA content and HI value indicate that microbial inoculation promotes the stability and efficiency of the composting of biogas residue.
Relevant studies have suggested that the of lignin is achieved by specific microbial colonies that play different roles in each phase of the decomposition process [25]. In the initial period of microbial decomposition, the relevant colonies change the characteristics of lignin, while the main degradation of lignin occurs in the subsequent period [42]. For example, spores and hyphae of fungi can diffuse into lignin, and microorganisms can start a chain reaction of free radicals by secreting laccase, peroxidase, and manganese peroxidase to realize lignin degradation [16]. Furthermore, various products, such as phenols, quinones, and other products produced by lignin degradation, may be subjected to various reactions, such as coupling with amino acids. Small HAs are formed first, and then, large molecules with a high humification rate and stable HAs are formed through condensation and polymerization [27]. In this experiment, xylomycin could effectively degrade the CL in the biogas residue, while white rot fungus had a strong ability to decompose lignin in biogas residue. Therefore, this experiment enhanced the aromatization of the HA sample, and the humification rate after inoculation of the sample was significantly increased [8].
Moreover, the experimental sample supplemented with rice straw exhibited a high C/N ratio, which can result in a high temperature in the composting process and can last for a long period of time, thereby promoting the degradation of lignocellulose. Tomati et al.’s results showed that the presence of the high-temperature region is the main factor underlying lignin degradation [43]. The compost of pure biogas residue, because of its low C/N ratio, has difficulty providing sufficient bioheat, so lignin is hardly degraded during the process. In contrast, the addition of rice straw and microbial inoculation satisfied both the temperature and microbial requirements, thereby achieving different degrees of lignin degradation.
With respect to the HA formation mechanism, researchers have proposed the phenol-protein theory [37] and the synthesis theory [44]. The former states that HA precursors are derived from the condensation of phenol, quinone, and other substances generated from lignin decomposition [37], while the latter considers HA precursors to be the condensation products of polysaccharides and amino acids [44]. These theories are directly or indirectly related to the conversion of lignocellulose. Therefore, the selection of microbial species, the mechanisms underlying the interactions of the microorganisms with lignin and CL, and the environment affecting the interactions strongly influence the degradation of lignocellulose and then affect the Hu content and stability of the final products of composting. Based on the results of this study, coinoculation with rice straw and microorganisms promoted the degradation of xylem fibers and led to an increase in the HA content and HI, facilitating rapid maturation of rice straw biogas residue and stabilization of compost quality. The underlying reason may be that lignocellulose decomposition promotes the formation of HA precursors [45].
4 Future research
Excessively high temperatures in the high-temperature phase during composting are not conducive to the survival of CL-decomposing bacteria [28], and the duration of the low-temperature phase should be appropriately extended to facilitate the decomposition of lignocellulose by fungi [34]. In particular, the theory of HA formation shows that the decomposition products of lignocellulose undergo polycondensation at low temperatures and form HA polymers, which leads to an increase in the humification rate [6]. Therefore, proper control of the maximum temperature to achieve the succession of microbial colonies during composting and moderate extension of the cooling phase to promote the complexation of HA precursors into stable HAs are important regulatory directions for improving the humification rate and HA content of compost in the future [30]. Therefore, in future research, these theories can be applied to composting practice to further enhance the humification rate and HA content, thereby realizing the production of high-quality HA fertilizer from biomass materials with high lignocellulose content. Second, microorganisms need to utilize nitrogen to synthesize essential living matter [25]. Therefore, the existing forms of nitrogen in different materials themselves may affect the humification of compost. This is another key factor that is worth considering in future studies.
5 Conclusion
This study aimed to propose a method for rapid humification of rice straw biogas residue to obtain safe, harmless, high-quality, and stable HA fertilizer, and the results of this study led to the following conclusions:
-
1.
The combination of rice straw supplementation and multifunctional microbial inoculation of rice straw biogas residue for aerobic composting achieved maturation 30 days earlier than composting alone, in terms of temperature, EC, and GI.
-
2.
The combinatorial method accelerated the aerobic decomposition of rice straw biogas residue. Compared with composting alone, the combination method increased the degradation rates of HCs, CL, and lignin by 29.4%, 34.8%, and 40.5%, respectively.
-
3.
The HA content and humification rate of the composted materials increased significantly in the same period. Compared with composting alone, the combination composting method increased the HA and HI by 7.58% and 0.91, respectively.
-
4.
The addition of rice straw and inoculation with lignocellulose-degrading microorganisms can improve the quality and enhance the stability of composted fertilizer made from rice straw biogas residue. The added rice straw improves the physiochemical environment during composting by adjusting the OM content and the C/N ratio and promotes the succession of functional microorganisms, leading to rapid humification of rice straw biogas residue, thereby producing a highly stable product with a high HA content. This product can be applied in actual agricultural practice in the form of organic fertilizer to promote the substance and energy recycling of the rice straw resource.
References
Baetge S, Kaltschmitt M (2018) Rice straw and rice husks as energy sources—comparison of direct combustion and biogas production. Biomass Convers Bior 03:719–737
Meng XY, Liu B, Zhang H, Wu JW, Yuan XF, Cui ZJ (2019) Co-composting of the biogas residues and spent mushroom substrate: physicochemical properties and maturity assessment. Bioresour Technol 276:281–287
Tsachidou B, Scheuren M, Gennen J et al (2019) Biogas residues in substitution for chemical fertilizers: a comparative study on a grassland in the Walloon Region. Sci Total Environ 666:212–225
Odlare M, Abubaker J, Lindmark J, Pell M, Thorin E, Nehrenheim E (2012) Emissions of N2O and CH4 from agricultural soils amended with two types of biogas residues. Biomass Bioenergy 44:112–116
Zhu LJ, Zhao Y, Zhang WS et al (2019) Roles of bacterial community in the transformation of organic nitrogen toward enhanced bioavailability during composting with different wastes. Bioresour Technol 267:378–385
Sánchez ÓJ, Ospina DA, Montoya S (2017) Compost supplementation with nutrients and microorganisms in composting process. Waste Manag 69:136–153
Duan YM, Kumar SK, Awasthi MK et al (2019) Evaluating the impact of bamboo biochar on the fungal community succession during chicken manure composting. Bioresour Technol 272:308–314
Wu JQ, Zhao Y, Yu HM et al (2019) Effects of aeration rates on the structural changes in humic substance during co-composting of digestates and chicken manure. Sci Total Environ 658:510–520
Tsapekos P, Alvarado-Morales M, Panagiotis G et al (2019) Enhancing anaerobic digestion of agricultural residues by microaerobic conditions. Biomass Convers Bior 05:1–9
Qian XY, Shen GX, Wang ZQ, Guo C, Liu Y, Lei Z, Zhang Z (2014) Co-composting of livestock manure with rice straw: characterization and establishment of maturity evaluation system. Waste Manag 34:530–535
Casini D, Barsali T, Rizzo AM (2019) Production and characterization of co-composted biochar and digestate from biomass anaerobic digestion. Biomass Convers Bior 07:1–9
Qiao XL, Zhao C, Shao QJ et al (2017) Application of hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment of energy crops. Fuel 205:184–191
Conrad M, Häring H, Smirnova I (2019) Design of an industrial autohydrolysis pretreatment plant for annual lignocellulose. Biomass Convers Bior 07:1–18
Nielsen F, Galbe M, Zacchi G et al (2019) The effect of mixed agricultural feedstocks on steam pretreatment, enzymatic hydrolysis, and cofermentation in the lignocellulose-to-ethanol process. Biomass Convers Bior 06:1–14
Li B, Chen KJ, Zhao C et al (2015) Influence of steam explosion on rice straw fiber content. J Biobased Mater Bio 9:596–608
Xu R, Zhang K, Liu P et al (2018) Lignin depolymerization and utilization by bacteria. Bioresour Technol 269:557–566
Gou CL, Wang YQ, Zhang XQ, Lou YJ, Gao YH (2017) Inoculation with a psychrotrophic-thermophilic complex microbial agent accelerates onset and promotes maturity of dairy manure-rice straw composting under cold climate conditions. Bioresour Technol 243:339–346
Hastrup ACS, Howell C, Larsen FH, Sathitsuksanoh N, Goodell B, Jellison J (2012) Differences in crystalline cellulose modification due to degradation by brown and white rot fungi. Fungal Biol 116:1052–1063
Asina F, Brzonova I, Voeller K, Kozliak E, Kubátová A, Yao B, Ji Y (2016) Biodegradation of lignin by fungi, bacteria and laccases. Bioresour Technol 220:414–424
Wu J, Zhao Y, Qi HS et al (2017) Identifying the key factors that affect the formation of humic substance during different materials composting. Bioresour Technol 244:1193–1196
Steinbach D, Kruse A, Sauer J (2017) Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production—a review. Biomass Convers Bior 02:247–274
Jiang JS, Kang K, Chen D, Liu NN (2018) Impacts of delayed addition of N-rich and acidic substrates on nitrogen loss and compost quality during pig manure composting. Waste Manag 72:161–167
Sari GL, Trihadiningrum Y, Wulandar DAI, Pandebesie ES, Warmadewanthi IDAA (2018) Compost humic acid-like isolates from composting process as bio-based surfactant: properties and feasibility to solubilize hydrocarbon from crude. J Environ Manag 225:356–363
Spaccini R, Cozzolino V, DiMeo V, Savy D, Drosos M, Piccolo A (2019) Bioactivity of humic substances and water extracts from compost made by ligno-cellulose wastes from biorefinery. Sci Total Environ 646:792–800
Salgado MMM, Blu RO, Janssens M, Fincheira P (2019) Grape pomace compost as a source of organic matter: evolution of quality parameters to evaluate maturity and stability. J Clean Prod 216:56–63
Ravindran B, Nguyen DD, Chaudhary DK et al (2019) Influence of biochar on physico-chemical and microbial community during swine manure composting process. J Environ Manag 232:592–599
Li X, Shi XS, Yang ZM, Xu XH, Guo RB (2019) Effects of recyclable ceramsite as the porous bulking agent during the continuous thermophilic composting of dairy manure. J Clean Prod 217:344–351
Rashad FM, Saleh WD, Moselhy MA (2010) Bioconversion of rice straw and certain agro-industrial wastes to amendments for organic farming systems: 1. Composting, quality, stability and maturity indices. Bioresour Technol 101:5952–5960
Wang XK, Zheng GD, Chen TB, Nie E, Wang Y, Shi X, Liu J (2019) Application of ceramsite and activated alumina balls as recyclable bulking agents for sludge composting. Chemosphere 218:42–51
Guo XX, Liu HT, Wu SB (2019) Humic substances developed during organic waste composting: formation mechanisms, structural properties, and agronomic functions. Sci Total Environ 262:501–510
Zeng GM, Yu M, Chen YN et al (2019) Effects of inoculation with Phanerochaete chrysosporium at various time points on enzyme activities during agricultural waste composting. Bioresour Technol 101:222–227
Zucconi F, Forte M, Monaco A, Bertoldi M (1981) Biological evaluation of compost maturity. Biocycle 22:27–29
Pérez LR, Martínez C, Marcilla P, Boluda R (2009) Composting rice straw with sewage sludge and compost effects on the soil–plant system. Chemosphere 75:781–787
Saludes R, Iwabuchi K, Miyatake F, Abe Y, Honda Y (2008) Characterization of dairy cattle manure/wallboard paper compost mixture. Bioresour Technol 99:7285–7290
Tran QNM, Mimoto H, Nakasaki K (2015) Inoculation of lactic acid bacterium accelerates organic matter degradation during composting. Int Biodeterior Biodegrad 104:377–383
Wang XQ, Cui HY, Shi JH, Zhao X, Zhao Y, Wei Z (2015) Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour Technol 198:395–402
Yu HY, Xie BT, Khan R, Shen GM (2019) The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour Technol 271:228–235
Sharma D, Yadav KD, Kumar S (2018) Role of sawdust and cow dung on compost maturity during rotary drum composting of flower waste. Bioresour Technol 264:285–289
Zayed G, Abdel-Motaal H (2005) Bio-active composts from rice straw enriched with rock phosphate and their effect on the phosphorus nutrition and microbial community in rhizosphere of cowpea. Bioresour Technol 96:929–935
Malherbe S, Cloete TE (2002) Lignocellulose biodegradation: fundamentals and applications. Rev Environ Sci Bio 1:105–114
Keller P (1961) Methods to evaluate maturity of compost. Compost Sci Util 2:20–26
He XS, Yang C, You SH et al (2019) Redox properties of compost-derived organic matter and their association with polarity and molecular weight. Sci Total Environ 665:920–928
Tomati U, Galli E, Pasetti L, Volterra E (1995) Bioremediation of olive-mill wastewaters by composting. Waste Manag Res 13:509–518
Qi HS, Wei ZM, Zhang JM, Zhao Y, Wu J, Gao X, Liu Z, Li Y (2019) Effect of MnO2 on biotic and abiotic pathways of humic-like substance formation during composting of different raw materials. Waste Manag 87:326–334
Wu JQ, Zhao Y, Zhao W et al (2017) Effect of precursors combined with bacteria communities on the formation of humic substances during different materials composting. Bioresour Technol 226:191–199
Funding
This research was financially supported by the Zhejiang Natural Science Foundation (LQ17C130001), National Natural Science Foundation of China (31801317), School Research and Development Fund Project (2014FR046), and Student Research Training Project (101-2013200080).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, X., Li, B., Chen, K. et al. Rice straw addition and biological inoculation promote the maturation of aerobic compost of rice straw biogas residue. Biomass Conv. Bioref. 11, 1885–1896 (2021). https://doi.org/10.1007/s13399-019-00587-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00587-y