Abstract
There remains an urgent need for low-cost and effective additives to allow the simultaneous passivation of trace elements and removal of antibiotics during composting. Here, we investigated the effects of the addition of additives, white-rot fungi (WF), wood vinegar (PA), biochar (BC), and a mixture of the three (OAC), on trace element passivation, antibiotic degradation, composting enhancement, and compost quality improvement during the aerobic composting of pig manure. We found that the passivation rate of Cu and Zn, in addition to the removal efficiency of sulfadiazine (SDZ) and norfloxacin (NFC), was enhanced with WF, PA, BC, and OAC treatment in comparison with the control. Moreover, the co-addition of BC, PA, and WF (OAC) allowed more efficient passivation of Cu and Zn (88.31% and 91.38%, respectively), better elimination of antibiotics (SDZ: 100%; NFC: 90.32%), and increased compost quality as compared with the addition of each component alone. Furthermore, the thermophilic stage duration and the germination index (GI) were also enhanced. In conclusion, the use of pyrolytic by-products and white-rot fungi as additives in composting is a promising low-cost, efficient detoxification method to improve compost quality and reduce environmental risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The current farming system relies heavily on chemical fertilizers to improve crop yields as the human population continues to grow; however, repeated use of synthetic fertilizers is not sustainable due to negative effects on soil health and the ecosystem. In contrast, organic fertilizers are important for sustainable agricultural production and integrated nutrient management [1, 2]. It has been demonstrated that using predominantly organic fertilizer in conjunction with a small amount of chemical fertilizer has the most positive effects on soil properties and microbial community [3]. Manure amendment effectively prevents acidification of red soil (Ferralic Cambisol); however, it significantly increases available Cu and Zn as well as antibiotic residues [4, 5]. Cu and Zinc are important trace elements for plant growth; nevertheless, in excessive amounts, they slow plant growth, promote leaf chlorosis [6], and exert toxicity by disturbing nutrient balance and modifying the activity of enzymes that induce damage in plants [7]. In addition to the concentration of elements, their chemical form in soil also affects uptake by plants, especially exchangeable and carbonate forms [8]. Moreover, animals metabolize antibiotics poorly; therefore, there exist high concentrations in manure and urine [9], potentially contaminating soil and water. The presence of antibiotics causes the development and horizontal transfer of antibiotic resistance genes, which increases the level of antibiotic resistance [10, 11].
Composting is widely adopted to convert organic matter into stabilized materials and represents the most economically feasible manure treatment process, combining material recirculation and waste disposal to reduce environmental pollution. Certain additives, such as humic acid, lime, and montmorillonite, affect the distribution and stability of trace elements and antibiotics [12,13,14]; however, these are limited natural mineral resources, which prevent their large-scale utilization.
Biochar serves as a low-cost, porous material for the removal of As(III) and As(V) elements from polluted water [15] and phosphorus from aqueous media [16], in addition to mitigating NH3 and N2O emission and N conservation during composting [17]. Furthermore, the addition of biochar during sediment and agricultural waste co-composting decreases the bioavailability of trace elements and significantly influences bacterial community succession and diversity [18].
Wood vinegar (WV), also called pyroligneous acid (PA), is an acidic liquid containing many kinds of organic matter, such as organic acid [19], and is usually obtained following the condensation of pyrolytic vapors [20]. Currently, wood vinegar serves as an additive for compost, and its effects on the adsorption of heavy metals and aerobic/anaerobic microbial activity have been investigated [19, 21]. Diluted wood vinegar promotes the microbial activity and pollutant removal efficiency of activated sludge [22]. Additionally, wood vinegar enhances the seed germination index (GI) and the degradation of organic matter during cow manure composting [23] and also possesses the ability to increase rice grain yield and NH4+-N concentration in rice paddy soil [24].
Exogenous agents consisting of functional microorganisms have been introduced to shorten the composting period, reduce heavy metal toxicity, and improve compost quality [25,26,27]. White-rot fungi affects the transformation of organic matter, mobility of heavy metals, and degradation of antibiotics. To date, many microbial additives, such as Phanerochaete chrysosporium, Pleurotus ostreatus, and Irpex lacteus, have been applied to reduce environmental risks [28,29,30].
In addition, the integrated use of different additives is considered more effective in enhancing compost than the utilization of a simple additive. For example, according to the seed GI, biochar addition and microbial inoculation have a synergistic effect as compared with each additive alone [31]. Wood vinegar combined with biochar can reduce NH3 volatilization, while wood vinegar alone has been found to increase NH3 volatilization [24]. There is, however, a paucity of studies focusing on the synergistic effects of more than two additives on the degradation of antibiotics and the bioavailability of trace elements during pig manure composting. We hypothesized that three additives, biochar (BA), wood vinegar (PA), and white-rot fungi (WF), may exert a synergistic effect during the pig manure composting process to reduce the bioavailability of trace elements and increase the degradation of antibiotics, thus improving compost quality. Generally, biochar and wood vinegar are renewable residues of the pyrolytic gas production process and are obtained in large amounts, representing 75–88% weight of the total biomass [32]. These additives are low-cost and rich but are required to be used on an industrial scale with technology appropriate for the treatment of pig manure.
In the present study, pig manure and corn stalk were composted together as raw materials with three additives (WF, PA, and BA) to evaluate the effects of different additive concentrations on the bioavailability of trace elements (Zn and Cu) and the degradation of antibiotics.
2 Materials and methods
2.1 Experimental materials
Pig manure (fresh samples) was collected from a pig farm in Harbin, China. The corn stalk was obtained from the experimental field of Northeast Agricultural University, dried naturally, and crushed into pieces (1–2 cm in length). Wood vinegar (PA) and powdered biochar (BC) were purchased from Shijiazhuang Hongsen Activated Carbon Co. Ltd. (Hebei province, China). White-rot fungi (WF) was provided by the China Microbiological Culture Collection Center (http://junzhong.vip) and cultured on potato dextrose agar (PDA) (glucose 20 g L−1, potato extract 6 g L−1, agar 20 g L−1) (Coolaber, Beijing). The effect of WF on the color of PDA medium containing 0.4% o-methoxy-phenol is shown in Fig. S1, which illustrates that white-rot fungi possesses the ability to produce laccase. The main physicochemical properties of the raw materials and additives are shown in Table 1.
2.2 Experimental design and sampling
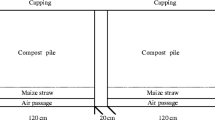
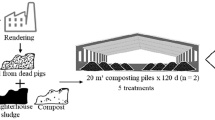
Based on previous studies, the biochar content was set at 2.5%, 3.5%, and 4.5%, referred to as B1, B2, and B3, respectively; the wood vinegar content was set at 0.4%, 0.6%, and 0.8%, referred to P1, P2, and P3, respectively; and the white-rot fungi content was set at 3%, 6%, and 9%, referred to as W1, W2, and W3, respectively. To investigate the synergistic effect of BA, PA, and WF, B2, P2, and W3 were mixed with pig manure and corn stalk, which was named OAC; the absence of additives was used as the control, which was referred to as CK. The composting experimental design is shown in Table 2. The composting experiments were carried out in a 65-L foam box (60 × 53 × 31 cm, length × width × height) in the laboratory. Ventilation holes were arranged around the body of the box and the top cover to facilitate the inflow of ambient air. The fresh weight ratio of pig manure to corn stalk was 2:1, which were mixed thoroughly to obtain a moisture content of 65% and a C/N ratio of 25:1. BC, PA, and WF were subsequently added at different ratios. The composting period lasted 32 days, with the piles being turned and thoroughly mixed every 4 days during the mesophilic and thermophilic stages, and every 7 days during the curing stage. After each artificial turning, approximately 200 g each compost sample was collected from 5 random points in each pile using the centered quarter method and then homogenized. The sampling was conducted on days 1, 4, 8, 12, 16, 24, and 32 [27]. Each sample was divided in half; one half was stored at 4 °C, and the other half was air dried.
2.3 Determination of physicochemical properties
A thermal sensor was used to detect the temperature at a depth of 25 cm, 50 cm, and 75 cm in each pile of compost at 9:00, 14:00, and 20:00 every day to obtain an average daily temperature. Ambient temperature was also recorded. The pH and EC values of the compost were measured according to previously described methods [26]. The fresh compost samples were mixed with distilled water (1:10 w/w ratio), shaken at 150 rpm for 0.5 h, and centrifuged at 2000 × g for 10 min.
The germination index (GI) was determined as previously reported [33]. The water extract of the compost was centrifuged at 2000 × g for 10 min, and the supernatant was passed through a 0.45-μm filter membrane. The number of germinating Pakchoi (Brassica rapa L.) seeds and root length were measured following treatment with compost water extract or distilled water as the control. The following formula was used to calculate the GI:
2.4 Extraction and quantitation of trace elements
A modified Community Bureau of Reference (BCR) sequential extraction procedure was used to determine Cu and Zn content [34]. Five metal species were defined as the water-soluble fraction (WAF), acid-soluble fraction (ASF), reducible fraction (REF), oxidizable fraction (OXF), and residual fraction (RSEF). The water-soluble, acid-soluble, reducible, and oxidizable fractions were extracted with deionized water, 0.1 M CH3COOH, 0.5 M NH2OH·HCl and 2 M HNO3, and 8.8 M H2O2 and 1 M CH3COONH4, respectively. The residual fraction was obtained by digesting the residue from the oxidizable fraction in 10 mL HNO3-HClO4 (v/v 6:1) solution for 2 h at 300 °C. The bioavailability of these fractions was ordered as follows: WAF > ASF > REF > OXF > RSEF [28]. The digested solution was passed through a 0.45-μm filter membrane and subjected to flame atomic absorption spectrometry (DUO AA, China). All extraction and digestion procedures were performed in triplicate. The distribution ratio and passivation rate were calculated using formulas (2) and (3), respectively:
where Cb is the distribution ratio of heavy metals before composting and Ca is the ratio of heavy metals after composting.
2.5 Extraction and quantitation of antibiotics
Extraction of fluoroquinolones (norfloxacin (NFC)) and sulfonamides (sulfadiazine (SDZ)) was performed according to methods previously described by Garcia-Galan, Rodriguez-Rodriguez [35] and Ho, Zakaria [5]. In brief, 5 g each freeze-dried sample was extracted three times with EDTA-sodium phosphate buffer, and the supernatants were combined and loaded at 10 mL/min into cartridges preconditioned with 10 mL methanol and 10 mL ultrapure water. Subsequently, the cartridges were flushed with 6 mL ultrapure water and 10 mL methanol (supplemented with 0.1% formic acid). The eluents were evaporated to dryness under N2 flow, dissolved in 1 mL solution (25% methanol and 0.1% formic acid in ultrapure water), and passed through a 0.22-μm nylon syringe filter. The solutions were stored in brown glass bottles until analysis. High-performance liquid chromatography (Waters-600, USA) was used to determine the presence of antibiotics, as previously described [36]. Each sample was measured in triplicate. The antibiotic removal efficiency was calculated using formula (4):
where Rb is the initial antibiotic concentration of the mixed manure samples before composting and Ra is the antibiotic concentration of the mixed manure samples after composting.
2.6 Statistical analysis
All tests were performed in triplicate (n = 3). Statistical analysis was performed using the SPSS Statistics software 25. Differences were assessed by one-way analysis of variance (ANOVA) followed by Duncan’s test and considered significant at p < 0.05. All figures were created using the Origin 2021 software.
3 Results and discussion
3.1 Effect of additives on physicochemical properties during composting
Composting temperature is known to affect microbial activity and composting efficiency [37]. There typically exist four phases during aerobic composting: a mesophilic phase, a thermophilic phase, a cooling phase, and a maturation phase. The mesophilic phase is present in the first few days of composting, during which mesophilic microbes rapidly decompose soluble degradable compounds, and the temperature of the pile increases quickly. As the temperature rises above 40 °C, the thermophilic stage begins and complex biomacromolecules, such as proteins, fats, and hemicellulose, are degraded by thermophilic microbes. Over time, the temperature of the pile gradually decreases to ambient temperature due to the exhaustion of compounds. During the final maturation phase, organic matter is stable and safe for plants [38]. As shown in Fig. 1a, all the treated piles of compost went through these four phases of composting. The B2 and P2 compost piles reached peak temperature values rapidly during the first 6 and 7 days, respectively, and remained above 50 °C for more than 10 days; however, the temperature of the control compost pile did not peak until day 10, illustrating that biochar and wood vinegar can shorten the mesophilic phase and lengthen the thermophilic phase. Similar results have been obtained previously [39]. Maintenance of the thermophilic phase at 50–60 °C for longer than 5 days meets the requirements for hygienic compost specified by the national standard in China (GB7959-2012). By contrast, the temperature in the OAC compost pile increased again during days 18–23 and remained higher than 50℃ for 12 days. The secondary temperature rise was due to further degradation of refractory organic compounds such as lignin, indicating that white-rot fungi have already adapted to the composting environment and began to exert their advantages on the degradation of lignocellulosic materials [40]. The duration of the thermophilic phase in the OAC compost pile was longer than that in the other piles, illustrating that white-rot fungi, biochar, and wood vinegar have synergistic effects on the degradation of organic matter.
pH is a critical factor influencing microbial activity and community during composting [41]. As shown in Fig. 1b, the pH value decreased and subsequently increased. Firstly, the pH decreased during the thermophilic phase and reached a similar trough on day 8 since the organic matter had been transformed into small molecular organic acids such as oxalic, lactic, citric, succinic, acetic, and formic acids, resulting in a gradual decline in pH [42]. During the cooling phase, the pH of all the compost piles increased significantly, which is likely due to the decomposition of organic acids and production of volatile ammonia as the temperature decreased [43]. Consistent with previous reports [23], the pH in the P compost piles was significantly lower than that in the other piles prior to day 14 because acetic acid accounts for roughly 30–70% of the organic component of wood vinegar [21]. The pH in the OAC compost pile (8.53) increased by a further 2.27% than that in the P3 compost pile (8.34) during the maturation phase, indicating that the addition of alkaline biochar serves as a buffer for acid–base balance in compost, which is more conducive to improvements in soil fertility and the mitigation of NH3 volatilization [24]. Additionally, the pH of compost also affects the stability and bioavailability of trace elements [6].
The EC reflects the degree of compost salinity and organic mineralization [44], which displayed a similar trend in all the piles during composting (Fig. 1c). During the thermophilic phase, the EC of all the compost piles increased sharply, which may be attributed to the conversion of complex organic matter to soluble components, including metallic salts [43]. Subsequently, the EC showed a rapid reduction in stability, which may be due to nitrification, heavy metal immobilization, and/or volatilization of ammonia [26, 45]. At the end of composting, there was a significant difference between the OAC and the P2, B2, and WA compost piles (p < 0.05). The EC values of all the compost piles at the end of composting were lower than that of the control and did not exceed 4.0 mS·cm−1, which is considered safe for plant growth [46]. In addition, there was a difference between the EC of the OAC compost pile and that of the WF, PA, and BA compost piles during the maturation phase, indicating that WF, BA, and PA exert a synergistic effect on the degradation of organic matter.
The GI is an effective biological method for evaluating the quality and maturity of composting products; and with a GI greater than 80%, compost can be considered mature and non-toxic [46]. Figure 1d shows the GI values for all the piles of compost at the end of composting. The GI of the OAC compost was the highest, and there was a significant difference between the OAC and the P2, B2, and WA compost (p < 0.05) (Fig. 1d), demonstrating that WF, BA, and PA synergistically increased the conversion of ammonia and decomposition of toxic substances [27].
The results are presented as the mean ± SD of three replicates. Different letters above the columns indicate a significant difference as determined by Duncan’s multiple comparisons test (p < 0.05).
3.2 Effect of additives on the transformation of Cu and Zn speciation during composting
The total concentrations and speciation of Cu and Zn in the presence of different additives are shown in Tables 3 and 4, respectively. The total concentration of each metal increased during the composting process due to the loss of organic matter [47]. As shown in Fig. 2, Cu and Zn associated with the WAF, ASF, and REF fractions during the initial composting period accounted for 63–67% and 63–74%, respectively, whereas the OXF and RSEF fractions contained a higher percentage of Cu and Zn at the end of composting (35–48% and 49–56%, respectively). These results are in accordance with previous observations [14] and verify that composting is an effective way to reduce the bioavailability of trace elements in livestock manure. Assuming that mobility and bioavailability are related to the solubility of the chemical form of metals [8], Cu displays more apparent mobility and potential bioavailability than Zn (Fig. 2); therefore, Cu is the major trace element in pig manure [48], and greater attention should be paid to its speciation and level during composting.
The passivation rate of Cu was ordered as follows: OAC (88.19%) > B2 (72.01%) > P2 (64.40%) > W3 (66.46%) (Table 3), and that of Zn was OAC (91.38%) > B2 (81.40%) > P2 (73.29%) > W3 (70.22%) > CK (64.82%) (Table 4). The additives significantly influenced the mobility of Cu and Zn (p < 0.05). Furthermore, the co-addition of BC, PA, and WF resulted in more efficient passivation of Cu and Zn than the individual addition. The concentration of Cu and Zn was significantly decreased in the WAF and ASF and significantly increased in the OXF and RSEF, from all compost piles. These results are in accordance with those previously reported [28]. The residual fraction contributes little to heavy metal bioavailability since it is permanently bound to the crystal lattice of the mineral components of the compost [47].
In comparison with Cu, the distribution rate of the oxidizable fraction increased to a greater extent prior to and after composting, indicating that Zn transformed from a more active state to an oxide-binding state, which reduces the risk of toxicity.
Cu and Zn are involved in many essential biological processes. These elements participate in the synthesis of chlorophyll, are components of electron transport during photosynthesis, or form an integral constituent of superoxide dismutase (Cu/Zn-SOD). An imbalance in nutrient elements may cause toxicity under excess Cu and Zn conditions; however, speciation ultimately determines the levels of trace elements in the environment and regulates the transfer of metals from the soil to roots/shoots/grains and finally to humans/animals [7]. Cu and Zn mobility is associated with humus formation during the composting process, and microbes play an important role in enhancing the transformation of organic materials. White-rot fungi can degrade lignin and form stable humic substances possessing functional groups with the ability to immobile heavy metals as a residual fraction, accelerating the formation of organo-metallic complexes and causing accumulation of metal ions in cells [28, 40]. Furthermore, wood vinegar affects the adsorption capabilities of trace elements due to the presence of acetic acid and other organic acids, which possess many carboxyl groups [49]. In the present study, biochar was utilized as an additive for composting to improve the surface roughness of the adsorbents, which increased the pore volume, specific surface area, and number of oxygen-containing functional groups on its surface, favoring the removal of trace elements [50] Zhou, Meng [12, 51]. Lower concentrations of wood vinegar can enhance microbial growth [21] and balance the alkalinity of biochar, which is conducive to the passivation of heavy metals. It has been suggested that modification of the structure of biochar with wood vinegar improves the adsorption capacity for heavy metals [49], which we intend to study in the future.
3.3 Effect of additives on the degradation of antibiotics during composting
Seven antibiotics were detected in all compost samples, which included 4 sulfonamides [sulfadiazine (SDZ, 16.42 µg kg−1), sulfamethoxydiazine (SM, 12.97 µg kg−1), sulfamonomethoxine (SMM, 4.65 µg kg−1), and sulfamethoxazole (SMX, 3.62 µg kg−1)] and 3 fluoroquinolones [norfloxacin (NFC) (1103.67 µg kg−1), enrofloxacin (EFC) (323.52 µg kg−1), and ciprofloxacin (CFC) (17.62 µg kg−1)]. To investigate the influence of different concentrations of additives on the degradation of antibiotics during pig manure composting, we evaluated changes in the levels of the antibiotic in each class that had the highest initial concentration (NFC and SDZ).
The concentration changes and removal efficiencies of SDZ and NFC are shown in Fig. 3. The presence of additives increased the removal efficiency of both SDZ and NFC from pig manure, which is in accordance with a previous report [52]. The removal efficiency of SDZ and NFC from OAC compost was significantly higher than that from the other composts (p < 0.05). Interestingly, the total removal efficiency of NFC was significantly lower than that of SDZ during the entire composting period, which may be due to the azabicyclo structure in the norfloxacin molecule being harder to degrade than the sulfonamide structure in the sulfamethoxazole molecule [53].
The degradation rate of NFC in this study is lower than that reported by Ho et al. (99.8%), which is likely due to the fact that the initial concentrations of NFC were significantly different [5]. The fate of antibiotics during composting is mainly associated with adsorption and degradation. The bioavailability of antibiotics is also significantly influenced by the presence of heavy metals [54]. In the present study, the concentrations of Cu and Zn were high, which may have slowed down the degradation of the antibiotics since NFC has been shown to be adsorbed on the particulate fraction or organic macromolecules, rendering it resistant to biodegradation [55]. Here, white-rot fungi enhanced the degradation of organic matter, which is attributed to the non-specific nature of their ligninolytic enzymes, such as peroxidases and laccases, and to the intracellular activity of the cytochrome P450 system [35]. Moreover, powdered biochar and acidic wood vinegar decreased the adsorption of NFC, which led to increased NFC elimination. The level of SDZ rapidly decreased during the first 16 days of composting in all the piles, and the removal of SDZ reached 100% by the maturation phase. These results are in good agreement with those previously published [5]. SDZ was efficiently degraded by temperature-dependent abiotic processes, since the temperature within the OAC compost remained higher than 50 °C for 12 days. Further, SDZ is often transformed into hydroxyl-sulfadiazine and the non-bioactive conjugate acetyl-sulfadiazine, strongly reducing antibiotic toxicity [5].
In the present study, biochar, wood vinegar, and white-rot fungi synergistically changed the physicochemical properties of the compost and increased the bioavailability of trace elements and degradation of antibiotics during the composting process. Both wood vinegar and biochar are easily obtained in large quantities as waste from pyrolytic gas production and can also be combined with renewable energy; therefore, these additives could be widely applied to composting. Moreover, wood vinegar and biochar have been utilized to enhance plant growth and tolerance to drought stress, improving soil health and carbon sequestration [56, 57], and would likely provide significant economic and environmental benefits if properly applied to agricultural production. Our work describes a new approach for the application of wood vinegar and biochar to increase their added value and commercialization as much as possible. Overall, we developed a new method to remove pollutants from the environment using sustainably produced agricultural and industrial residues.
Nevertheless, since several pyrolysis conditions, such as biomass type and operating temperature, have a significant effect on the characteristics of wood vinegar and biochar [58], it is difficult to determine which factor should be improved to allow these additives to more greatly reduce the bioavailability of Cu and Zn and increase the degradation of antibiotics, and further experimental study is required. To fully evaluate the long-term fate of passivated heavy metals (Cu and Zn) and NFC in soil, the compost needs to be applied to a range of different soils and plants. Furthermore, composting is a complex biological process. In addition to additives, there exist many aspects, such as composting scale, composting process, and composting factors, that influence organic matter transformation, removal of antibiotics, and passivation of trace elements; therefore, industrial-scale composting using OAC still needs further investigation.
4 Conclusions
White-rot fungi and the pyrolytic products derived from corn straw were used as additives for the simultaneous reduction of trace element and antibiotic concentrations. The passivation rate of copper (88.31%) and zinc (91.38%) and the degradation rate of sulfadiazine (100%) and norfloxacin (90.32%) in OAC compost [WF (9%), PA (0.6%), and BC (3.5%)] were increased by 36.27% and 26.48% and by 18.88% and 36.97%, respectively, in comparison with the control. After 32 days of composting, the products satisfy biofertilizer requirements for agricultural production. Our study provides a useful reference for the low-cost, efficient detoxification and utilization of pig manure, but further experiments should be conducted to evaluate the long-term effects of compost products on soil quality.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Chen H, Zheng C, Qiao Y, Du S, Li W, Zhang X et al (2021) Long-term organic and inorganic fertilization alters the diazotrophic abundance, community structure, and co-occurrence patterns in a vertisol. Sci Total Environ 766:142441. https://doi.org/10.1016/j.scitotenv.2020.142441
Atieno M, Herrmann L, Huong Thu N, HoanThi P, Nghia Khoi N, Srean P et al (2020) Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J Environ Manage 275:111300. https://doi.org/10.1016/j.jenvman.2020.111300
Wu L, Jiang Y, Zhao F, He X, Liu H, Yu K (2020) Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci Rep 10:9568. https://doi.org/10.1038/s41598-020-66648-9
Cai Z, Wang B, Zhang L, Wen S, Xu M, Misselbrook TH et al (2021) Striking a balance between N sources: Mitigating soil acidification and accumulation of phosphorous and heavy metals from manure. Sci Total Environ 754:142189. https://doi.org/10.1016/j.scitotenv.2020.142189
Ho YB, Zakaria MP, Latif PA, Saari N (2013) Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour Technol 131:476–484. https://doi.org/10.1016/j.biortech.2012.12.194
Kumar V, Pandita S, Sidhu GPS, Sharma A, Khanna K, Kaur P et al (2021) Copper bioavailability, uptake, toxicity and tolerance in plants: a comprehensive review. Chemosphere 262:127810. https://doi.org/10.1016/j.chemosphere.2020.127810
Zhao H, Wu L, Chai T, Zhang Y, Tan J, Ma S (2012) The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J Plant Physiol 169:1243–1252. https://doi.org/10.1016/j.jplph.2012.04.016
Angelova V, Ivanov K, Ivanova R (2004) Effect of chemical forms of lead, cadmium, and zinc in polluted soils on their uptake by tobacco. J Plant Nutr 27:757–773. https://doi.org/10.1081/pln-120030609
Deng W, Zhang A, Chen S, He X, Jin L, Yu X et al (2020) Heavy metals, antibiotics and nutrients affect the bacterial community and resistance genes in chicken manure composting and fertilized soil. J Environ Manage 257:109980. https://doi.org/10.1016/j.jenvman.2019.109980
Wang J, Ben W, Zhang Y, Yang M, Qiang Z (2015) Effects of thermophilic composting on oxytetracycline, sulfamethazine, and their corresponding resistance genes in swine manure. Environ Sci- Pro Imp 17:1654–1660. https://doi.org/10.1039/c5em00132c
Xie W, Yang X, Li Q, Wu L, Shen Q, Zhao F (2016) Changes in antibiotic concentrations and antibiotic resistome during commercial composting of animal manures. Environ Pollut 219:182–190. https://doi.org/10.1016/j.envpol.2016.10.044
Zhou H, Meng H, Zhao L, Shen Y, Hou Y, Cheng H et al (2018) Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour Technol 258:279–286. https://doi.org/10.1016/j.biortech.2018.02.086
Chen Z, Fu Q, Cao Y, Wen Q, Wu Y (2021) Effects of lime amendment on the organic substances changes, antibiotics removal, and heavy metals speciation transformation during swine manure composting. Chemosphere 262:128342. https://doi.org/10.1016/j.chemosphere.2020.128342
Hao J, Wei Z, Wei D, Ahmed Mohamed T, Yu H, Xie X et al (2019) Roles of adding biochar and montmorillonite alone on reducing the bioavailability of heavy metals during chicken manure composting. Bioresour Technol 294:122199. https://doi.org/10.1016/j.biortech.2019.122199
Peng Y, Azeem M, Li R, Xing L, Li Y, Zhang Y et al (2022) Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J Hazard Mater 423:127081. https://doi.org/10.1016/j.jhazmat.2021.127081
Fang Y, Ali A, Gao Y, Zhao P, Li R, Li X et al (2022) Preparation and characterization of MgO hybrid biochar and its mechanism for high efficient recovery of phosphorus from aqueous media. Biochar 4:40. https://doi.org/10.1007/s42773-022-00171-0
Wang J, Pan J, Ma X, Li S, Chen X, Liu T et al (2022) Solid digestate biochar amendment on pig manure composting: nitrogen cycle and balance. Bioresour Technol 349:126848. https://doi.org/10.1016/j.biortech.2022.126848
Chen Y, Liu Y, Li Y, Wu Y, Chen Y, Zeng G et al (2017) Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Bioresour Technol 243:347–355. https://doi.org/10.1016/j.biortech.2017.06.100
Zhang F, Yang H, Guo D, Zhang S, Chen H, Shao J (2019) Effects of biomass pyrolysis derived wood vinegar (WVG) on extracellular polymeric substances and performances of activated sludge. Bioresour Technol 274:25–32. https://doi.org/10.1016/j.biortech.2018.11.064
Quan X, Shan J, Xing Y, Peng C, Wang H, Ju Y et al (2021) New horizons in the application of a neglected biomass pyrolysis byproduct: a marked simultaneous decrease in ammonia and carbon dioxide emissions. J Clean Prod 297:126626. https://doi.org/10.1016/j.jclepro.2021.126626
Hua D, Fan Q, Zhao Y, Xu H, Chen L, Si H et al (2020) Continuous anaerobic digestion of wood vinegar wastewater from pyrolysis: microbial diversity and functional genes prediction. Front Bioeng Biotechnol 8:923. https://doi.org/10.3389/fbioe.2020.00923
Zhang F, Shao J, Yang H, Guo D, Chen Z, Zhang S et al (2019) Effects of biomass pyrolysis derived wood vinegar on microbial activity and communities of activated sludge. Bioresour Technol 279:252–261. https://doi.org/10.1016/j.biortech.2019.01.133
Xu C, Yuan Q, Qin C, Xie G, He T, Song N (2020) Effect of wood vinegar on physicochemical properties and seeding capability of cow manure aerobic composting. Trans Chin Soc Agric Mach 51:353–360
Sun H, Feng Y, Xue L, Mandal S, Wang H, Shi W et al (2020) Responses of ammonia volatilization from rice paddy soil to application of wood vinegar alone or combined with biochar. Chemosphere 242:125247. https://doi.org/10.1016/j.chemosphere.2019.125247
Wang C, Wu M, Peng C, Yan F, Jia Y, Li X et al (2022) Bacterial dynamics and functions driven by a novel microbial agent to promote kitchen waste composting and reduce environmental burden. J Clean Prod 337:130491. https://doi.org/10.1016/j.jclepro.2022.130491
Wang C, Jia Y, Li J, Li P, Wang Y, Yan F et al (2023) Influence of microbial augmentation on contaminated manure composting: metal immobilization, matter transformation, and bacterial response. J Hazard Mater 441:129762. https://doi.org/10.1016/j.jhazmat.2022.129762
Li MX, He XS, Tang J, Li X, Zhao R, Tao Y et al (2021) Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 264:128549. https://doi.org/10.1016/j.chemosphere.2020.128549
Zhang C, Xu Y, Zhao M, Rong H, Zhang K (2018) Influence of inoculating white-rot fungi on organic matter transformations and mobility of heavy metals in sewage sludge based composting. J Hazard Mater 344:163–168. https://doi.org/10.1016/j.jhazmat.2017.10.017
Tortella G, Duran N, Rubilar O, Parada M, Diez M (2015) Are white-rot fungi a real biotechnological option for the improvement of environmental health? Crit Rev Biotechnol 35:165–172. https://doi.org/10.3109/07388551.2013.823597
Stella T, Covino S, Cvancarova M, Filipova A, Petruccioli M, D’Annibale A et al (2017) Bioremediation of long-term PCB-contaminated soil by white-rot fungi. J Hazard Mater 324:701–710. https://doi.org/10.1016/j.jhazmat.2016.11.044
Tu Z, Ren X, Zhao J, Awasthi S, Wang Q, Awasthi M et al (2019) Synergistic effects of biochar/microbial inoculation on the enhancement of pig manure composting. Biochar 1:127–137. https://doi.org/10.1007/s42773-019-00003-8
Kan T, Strezov V, Evans T (2016) Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew Sust Energ Rev 57:1126–1140. https://doi.org/10.1016/j.rser.2015.12.185
Wang X, Zhang W, Gu J, Gao H, Qin Q (2016) Effects of different bulking agents on the maturity, enzymatic activity, and microbial community functional diversity of kitchen waste compost. Environ Technol 37:2555–2563. https://doi.org/10.1080/09593330.2016.1155650
Horváth M, Boková V, Heltai G, Flórián K, Fekete I (2010) Study of application of BCR sequential extraction procedure for fractionation of heavy metal content of soils, sediments, and gravitation dusts. Toxicol Environ Chem 92:429–441. https://doi.org/10.1080/02772240903036147
Garcia-Galan MJ, Rodriguez-Rodriguez CE, Vicent T, Caminal G, Diaz-Cruz MS, Barcelo D (2011) Biodegradation of sulfamethazine by Trametes versicolor: removal from sewage sludge and identification of intermediate products by UPLC-QqTOF-MS. Sci Total Environ 409:5505–5512. https://doi.org/10.1016/j.scitotenv.2011.08.022
Huang Y, Cheng M, Li W, Wu L, Chen Y, Luo Y et al (2013) Simultaneous extraction of four classes of antibiotics in soil, manure and sewage sludge and analysis by liquid chromatography-tandem mass spectrometry with the isotope-labelled internal standard method. Anal Methods 5:3721–3731. https://doi.org/10.1039/C3AY40220G
Zhang M, He LY, Liu YS, Zhao JL, Liu WR, Zhang JN et al (2019) Fate of veterinary antibiotics during animal manure composting. Sci Total Environ 650:1363–1370. https://doi.org/10.1016/j.scitotenv.2018.09.147
Govindaraju M, Sathasivam KV, Marimuthu K (2021) Waste to wealth: value recovery from bakery wastes. Sustainability 13:2835. https://doi.org/10.3390/su13052835
Wei L, Shutao W, Jin Z, Tong X (2014) Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour Technol 154:148–154. https://doi.org/10.1016/j.biortech.2013.12.022
Chen Y, Chen Y, Li Y, Wu Y, Zeng Z, Xu R et al (2019) Changes of heavy metal fractions during co-composting of agricultural waste and river sediment with inoculation of Phanerochaete chrysosporium. J Hazard Mater 378:120757. https://doi.org/10.1016/j.jhazmat.2019.120757
Awasthi MK, Wang Q, Huang H, Ren X, Lahori AH, Mahar A et al (2016) Influence of zeolite and lime as additives on greenhouse gas emissions and maturity evolution during sewage sludge composting. Bioresour Technol 216:172–181. https://doi.org/10.1016/j.biortech.2016.05.065
Wei Y, Zhao Y, Shi M, Cao Z, Lu Q, Yang T et al (2018) Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour Technol 247:190–199. https://doi.org/10.1016/j.biortech.2017.09.092
Li HH, Zhang T, Tsang DCW, Li GX (2020) Effects of external additives: biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 248:125927. https://doi.org/10.1016/j.chemosphere.2020.125927
Chen W, Liao X, Wu Y, Liang JB, Mi J, Huang J et al (2017) Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manage 61:506–515. https://doi.org/10.1016/j.wasman.2017.01.014
Huang GF, Wong JW, Wu QT, Nagar BB (2004) Effect of C/N on composting of pig manure with sawdust. Waste Manage 24:805–813. https://doi.org/10.1016/j.wasman.2004.03.011
Yuan J, Li Y, Chen S, Li D, Tang H, Chadwick D et al (2018) Effects of phosphogypsum, superphosphate, and dicyandiamide on gaseous emission and compost quality during sewage sludge composting. Bioresour Technol 270:368–376. https://doi.org/10.1016/j.biortech.2018.09.023
Singh J, Kalamdhad AS (2012) Concentration and speciation of heavy metals during water hyacinth composting. Bioresour Technol 124:169–179. https://doi.org/10.1016/j.biortech.2012.08.043
Lu XM, Lu PZ, Chen JJ, Zhang H, Fu J (2015) Effect of passivator on Cu form transformation in pig manure aerobic composting and application in soil. Environ Sci Pollut Res 22:14727–14737. https://doi.org/10.1007/s11356-015-4680-7
Sun S, Gao ZT, Li ZC, Li Y, Gao JL, Chen YJ et al (2020) Effect of wood vinegar on adsorption and desorption of four kinds of heavy (loid) metals adsorbents. Chinese J Anal Chem 48:e20013–e20020. https://doi.org/10.1016/s1872-2040(19)61217-x
Agegnehu G, Srivastava AK, Bird MI (2017) The role of biochar and biochar-compost in improving soil quality and crop performance: a review. Appl Soil Ecol 119:156–170. https://doi.org/10.1016/j.apsoil.2017.06.008
Liu W, Huo R, Xu J, Liang S, Li J, Zhao T et al (2017) Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour Technol 235:43–49. https://doi.org/10.1016/j.biortech.2017.03.052
Chen Z, Wu Y, Wen Q, Bao H, Fu Q (2020) Insight into the effects of sulfamethoxazole and norfloxacin on nitrogen transformation functional genes during swine manure composting. Bioresour Technol 297:122463. https://doi.org/10.1016/j.biortech.2019.122463
Shi H, Wang XC, Li Q, Jiang S (2016) Degradation of typical antibiotics during human feces aerobic composting under different temperatures. Environ Sci Pollut Res 23:15076–15087. https://doi.org/10.1007/s11356-016-6664-7
Riaz L, Wang Q, Yang Q, Li X, Yuan W (2020) Potential of industrial composting and anaerobic digestion for the removal of antibiotics, antibiotic resistance genes and heavy metals from chicken manure. Sci Total Environ 718:137414. https://doi.org/10.1016/j.scitotenv.2020.137414
Khadra A, Ezzariai A, Merlina G, Capdeville MJ, Budzinski H, Hamdi H et al (2019) Fate of antibiotics present in a primary sludge of WWTP during their co-composting with palm wastes. Waste Manage 84:13–19. https://doi.org/10.1016/j.wasman.2018.11.009
Wang Y, Qiu L, Song Q, Wang S, Wang Y, Ge Y (2019) Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int J Mol Sci 20:943. https://doi.org/10.3390/ijms20040943
Luo X, Wang Z, Meki K, Wang X, Liu B, Zheng H et al (2019) Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J Soil Sediment 19:3934–3944. https://doi.org/10.1007/s11368-019-02365-9
Lu X, Jiang J, He J, Sun K, Sun Y (2019) Effect of pyrolysis temperature on the characteristics of wood vinegar derived from Chinese fir waste: a comprehensive study on its growth regulation performance and mechanism. ACS Omega 4:19054–19062. https://doi.org/10.1021/acsomega.9b02240
Funding
This study is funded by the Scientific and Technological Project of Heilongjiang Province (2021ZXJ03B05), Heilongjiang Provincial Postdoctoral Science Foundation (LBH-Q17021), and Key Research and Development projects of Heilongjiang Province (GA21D009).
Author information
Authors and Affiliations
Contributions
Jinxia Fan: Writing, original draft; formal analysis; validation; investigation; visualization. Shuang Ai: Conceptualization, methodology, project administration. Ting Yin: Writing, review and editing; visualization; conceptualization; methodology. Hongqiong Zhang: Writing, review and editing; visualization; conceptualization; methodology. Dongxu Tao: Conceptualization, methodology. Siyu Wang: Conceptualization, methodology, data curation. Guoxiang Zheng: Conceptualization; methodology; writing, review and editing; supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, J., Ai, S., Zheng, G. et al. Insight into effects of pyrolysis products and white-rot fungi on co-composting of pig manure and corn stalk. Biomass Conv. Bioref. 14, 15937–15947 (2024). https://doi.org/10.1007/s13399-023-03797-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03797-7