Abstract
The pure cultures of microalgae Chlorella vulgaris ATCC 13482 and Scenedesmus obliquus FACHB 417 were grown in municipal wastewater in 7-L airlift bubble column photobioreactor supplied with 5% CO2/air (v/v). Batch experiments were conducted at 25 °C with 14-h light/10-h dark cycle for a period of 10 days. The CO2 capture efficiencies for both the microalgae were monitored in terms of their respective biomass productivities, carbon contents, and CO2 consumption rates. In the present study, the initial concentration of ammonia (43.7 mg L−1) was decreased to 2.9 and 3.7 mg L−1 by C. vulgaris and S. obliquus, respectively. And, the initial concentration of phosphate (18.5 mg L−1) was decreased to 1.1 and 1.6 mg L−1 by C. vulgaris and S. obliquus, respectively. CO2 biofixation rates by C. vulgaris and S. obliquus, cultivated in municipal wastewater, were calculated to be 140.91 and 129.82 mg L−1 day−1, respectively. The findings from the present study highlight the use of microalgae for wastewater treatment along with CO2 uptake and biomass utilization for pilot scale production of biodiesel, biogas, feed supplements for animals, etc., thus minimizing the production costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Energy dependence on fossil fuels and global climate change are the two pertinent issues posing serious environmental threats to the modern society. Hence, there is an alarming call to cut down global emissions of anthropogenic CO2. Until now, major contribution for the electricity generation and transportation has been shared by coal and petroleum, respectively. According to an estimate by Boden et al. (2015), annual global carbon emissions from the use of fossil fuels crossed ~9 billion tons (or 33 Gt CO2) in 2011. CO2 alone contributes to more than two-thirds of total greenhouse gas emissions from human undertakings (Ho et al. 2011). CO2 discharge from the fossil fuel burning in 2014 was 60% above the emissions in 1990 being the reference year for the Kyoto Protocol (Global Carbon Emissions 2016). Atmospheric CO2 concentration even crossed an appalling figure of 407.9 ppm in May 2016 (Scripps Institution of Oceanography 2016) from the pre-industrial level of 280 ppm (Allas et al. 2007).
Researchers have tried with physical and chemical methods of CO2 capture and storage into deep oceans, mineral formations, or enhanced oil recovery (Metz et al. 2005, Leung et al. 2014, Rao and Rubin 2002). But these technologies have inherent shortcomings and trigger further environmental risks too. On the other hand, it has been suggested that biological method of CO2 sequestration causes an overall negative greenhouse gas emissions and thus might prove to be a viable carbon sink. For the sustainable bioenergy options from algae, wastes are converted into methane (Hernandez et al. 2014; Ward et al. 2014), hydrogen (Ullah et al. 2014), biodiesel (Kligerman and Bouwer 2015; Galadima and Muraza 2014), and electricity (Gajda et al. 2015). In yet another novel approach, researchers are harnessing the potential of CO2 sequestration by photosynthetically growing algae or cyanobacteria. Algae-mediated utilization of CO2 is considered as one of the most promising negative emission technologies. Additionally, algae use the wastewater for nutrient supplementation and eventually cause bioremediation of the wastewater. Energy (biodiesel or methane) obtained from the harvested microalgae is comprehensibly carbon-neutral and such projects earn carbon credits too.
Although CO2 emissions from petroleum and biodiesel are nearly equal, the foremost difference lies in carbon consumption during initial stages of the life cycle of these fuels. Edible vegetable oil fuels are more expensive than petroleum and have also continuously raised concern over “food versus fuel” debate. It has also been reported that traditional terrestrial plants (sugarcane and maize for ethanol; palm, groundnut, rapeseed, and sunflower for oil) have slower growth rates; they are seasonal and can contribute to only 3–6% reduction in global CO2 emissions. In order to put forth a remedial measure, Chisti (2007, 2008) supported bioethanol and biodiesel from microalgae over oil crops as a renewable fuel having tremendous potential to replace the current conventional petroleum-based transport fuels. Many experimental findings suggest that there are numerous benefits in using microalgae-based systems for management of wastewater. Microalgae are 10–50 times more efficient in CO2 sequestration than those by conventional terrestrial plants (Costa et al. 2000; Wang et al. 2008). Some microalgae like Chlorella can even consume combustion products such as NOx or SO2 from flue gas (Costa et al. 2000; Cuellar-Bermudez et al. 2015). Algae require comparatively less water than the agricultural crops and they are also cultivated in saline/brackish water on non-arable lands as the case may be.
Several studies (de Morais and Costa 2007; Sydney et al. 2010) reiterate the possibility of microalgae-mediated capture of CO2 from the simulated flue gases composition with CO2 fixation efficacy within 28–53%. Microalgae exhibit faster growth rates than that of the terrestrial green plants, and therefore, they have better rates of CO2 sequestration (Fulke et al. 2010). Chlorella and Scenedesmus species are widely accepted for the very promising potential of carbon sequestration (Fulke et al. 2010; Ho et al. 2010; Tang et al. 2011; Toledo-Cervantes et al. 2013). CO2 is utilized as the carbon source by microalgae for their cellular growth, and hence, they act as micro-biofactories consuming greenhouse gas (CO2). Other than feed supplements for animals, growing microalgae may also yield commercial products of high value like neutraceuticals, cosmetics, and pharmaceuticals (Cardozo et al. 2006; Gong et al. 2011) which can compensate for the capital investment and the operational costs. Therefore, coupling the commercial benefits of microalgae with CO2 fixation and/or recycling process for wastewater treatment or biodiesel production/methane generation will help minimize environmental impacts of energy consumption during biodiesel production, and net energy gain (NEG) will surely be positive.
The objectives of the present work were to cultivate the microalgae, Chlorella vulgaris ATCC 13482 and Scenedesmus obliquus FACHB 417, in municipal wastewater, and to quantify their efficiencies for uptake of inorganic carbon CO2. The wastewater was collected from the primary sedimentation tank of water reclamation plant (Ulu Pandan, Singapore). CO2/air supply was maintained at 5% v/v during the light hours and only air was supplied during the dark hours. The final characteristics of the municipal wastewater treated simultaneously were also determined. Dry algal biomass and its elemental carbon contents during the experimental period were calculated to evaluate CO2 uptake efficiencies of the two microalgae.
Materials and methods
Microalgae culture
The stock cultures of Chlorella vulgaris ATCC 13482 and Scenedesmus obliquus FACHB 417 were maintained in Bold’s basal (BB) medium as recommended for freshwater algae (https://www.ccap.ac.uk/media/documents/BB.pdf) in the Corning cell culture flasks (surface area, 100 cm2) maintained at 25 °C with fluorescent illumination of 90 ± 5 μmol m−2 s−1 operated in 14-h light/10-h dark cycle. The BB medium (per liter of DI water) contained the following macro-nutrients: 0.25 g NaNO3, 0.075 g MgSO4 .7H2O, 0.025 g NaCl, 0.075 g K2HPO4, 0.175 g KH2PO4, 0.025 g CaCl2 .2H2O, and 1-mL trace elements solution. The trace elements solution was autoclaved to be dissolved and contained the following (per liter of DI water): 8.82 g ZnSO4 .7H2O, 1.44 g MnCl2 .4H2O, 0.71 g MoO3, 1.57 g CuSO4 .5H2O, 0.49 g Co(NO3)2 .6H2O, 11.42 g H3BO3, 50 g EDTA, 31 g KOH, 4.98 g FeSO4 .7H2O, and 1 mL H2SO4 (conc.). All the chemicals used for preparing the culture medium were of analytical grade (Sigma-Aldrich, Singapore).
Characterization of wastewater parameters
The municipal wastewater (MW) was collected from the primary sedimentation tank of the Water Reclamation Plant at Ulu Pandan (Singapore) and stored at 4 °C until further characterization. The wastewater sample was filtered through 0.45-μm filter to remove the suspended particles before the estimation of its quality indicators (given in Table 1) as per Standard Methods for the Examination of Water and Wastewater (APHA 2005). The chemical kits and DR900 colorimeter (Hach, USA) were used to estimate chemical oxygen demand (COD), total nitrogen (TN), ammonia, nitrate, total phosphorus (TP), and orthophosphate (PO4 3−). pH of wastewater sample was measured using multi-parameter analyzer (3200M Agilent Technologies, USA).
Microalgae cultivation and acclimatization
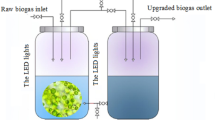
Ready to use inoculum of the two algae strains were cultivated in 2-L cylindrical glass bottles maintained at 25 °C in BB medium. Sterile air passing through 0.2-μm PTFE filter was fed at 0.2 vvm, i.e., 0.2 L min−1 of gas per liter of culture. The microalgae culture was maintained at pH 7.0 using 1-M NaOH solution. C. vulgaris and S. obliquus were cultured for 10 days in BB medium and then acclimatized to municipal wastewater in conical flasks of 3 L working volume (Fig. 1) uniformly mixed at 100 rpm on a magnetic stirrer. The inoculum:wastewater ratio was 1:20, i.e., 5% v/v for starting the experiment of algal CO2 utilization in 7-L airlift bubble column photobioreactors (PBRs).
Photobioreactor setup
The airlift bubble column PBR (Fig. 2) was used for this experiment. The PBRs (diameter 0.1 m, height 1 m, working volume 7 L) were fed with undiluted municipal wastewater and the algae inoculum. An artificial set of cool white fluorescent light (3 tubes ×24 W) was used to maintain 14-h light/10-h dark cycle. The reactors were supplied with gas (air +5% CO2 v/v) at flow rate of 1.4 L min−1 (superficial gas velocity 0.0013 m/s) equal to 0.2 vvm (gas volume per liquid volume per min) through the bottom of the reactor. The input of gas (air + CO2) at flow rate <0.2 vvm is insufficient to provide enough mixing, and sedimentation of the algae was the recurrent problem. Though the gas flow rates >1 vvm have better mixing and mass transfer effects to support higher biomass concentration, they have negative impacts considering greater power consumption and the resultant high shear stress on the algal cells. For adequate mixing of nutrients in the medium thereby disrupting diffusion barriers at the algal cell surfaces, gas flow rate in range 0.2–0.4 vvm has been reported in literature (Guo et al. 2015; Kargupta et al. 2015). Furthermore, proper mixing helps in uniform distribution of light to algae cells averting the dark zones, prevents buildup of the oxygen produced during photosynthesis, and subsequently, checks the potential oxidative stress. All the facilities were set up in temperature-stabilized laboratory at 25 °C throughout the experiment.
Analytical estimation
Dry algae weight
The biomass concentration of algae was calculated as its dry weight (g L−1) as per the method described by Guo et al. (2015). The harvested algal biomass was centrifuged (Eppendorf, Germany) at 5600 rpm for 10 min. After discarding the supernatant, the cell pellets were washed thrice with DI water and dried in an oven at 105 °C for 24 h, and the weight was estimated by gravimetric method. The biomass productivity (g L−1 day−1) denoted by P biomass is given by Eq. (1):
where W t and W 0, respectively, represent the dry algae mass and the initial biomass concentration; and t represents the cultivation period.
Elemental analysis of algae biomass
The dried algal biomass was pulverized into fine powder using a mortar and pestle and then analyzed for their elemental carbon contents (Ccarbon, wt.%) using Elementar Vario Micro Cube (GmbH, Germany) at the Department of Chemistry, National University of Singapore.
CO2 utilization and conversion into biomass
According to the method described by de Morais and Costa (2007), the CO2 biofixation rate (mg CO2/L·day) denoted by F CO2 is given by Eq. (2):
where M C was the molecular weight of carbon, M CO2 was the molecular weight of CO2, and Ccarbon was the carbon content (wt.%) in the algal biomass.
The percentage efficiency (E CO2) of conversion of CO2 into algae biomass is given by Eq. (3):
where V column, V CO2, and ρ CO2 represent the working volume of the PBR, total CO2 consumed (vol.) during the experimental period, and the density of CO2, respectively.
Results and discussion
Microalgae growth measurement
Municipal wastewater (7 L) was inoculated with the above cultured C. vulgaris and S. obliquus into PBRs (triplicates for each algae species). The initial inoculum density of the microalgae was adjusted to be at 0.1 g L−1 for each reactor. The cell growths of the two microalgae grown in municipal wastewater with normal air (0.03% v/v CO2) and 5% v/v CO2 were estimated by cell density measurement using UV/Vis spectrophotometer (Shimadzu, Japan). The relationship between optical density (OD680) and the dry cell weights of C. vulgaris ATCC 13482 and S. obliquus FACHB 417 was established by linear regressions given in the Supplementary Material (Figs. S1 and S2). Biomass productivities and specific growth rates calculated for C. vulgaris and S. obliquus are given in Table 3.
Estimation of CO2 biofixation rate
C. vulgaris and S. obliquus were grown in undiluted MW (filtered and autoclaved MW). Initial inoculum of the algae was obtained from the acclimatization study for 10 days. Suspended solids (78 mg L−1) was determined after filtering MW through 50-μm stainless steel filter mesh sieve (GmbH, Germany), and its value was subtracted from the dried algal biomass to get the net algae dry weight. The amount of CO2 consumed (L/day) during the cultivation period in our study has been compared with similar observations from previously reported studies (Table 2).
The estimation of CO2 biofixation rates for C. vulgaris and S. obliquus was done using Eq. (2). The stepwise calculation of CO2 sequestration (denoted as S CO2) using Eq. (4) is given in the Supplementary Material (Appendix A).
The results from the elemental analysis showed that the carbon contents (C carbon, wt.%) of C. vulgaris and S. obliquus were marginally higher when 5% CO2 was supplied during aeration than that of bubbling only air (Table 3). In our work, CO2 biofixation rates for C. vulgaris and S. obliquus were found to be 140.91 and 129.82 mg L−1 day−1, respectively. The efficiencies of CO2 conversion into biomass (E CO2) were calculated to be 14.9% by C. vulgaris and 13.8% by S. obliquus given in the Supplementary Material (Appendix A).
It is very well documented in literature that dissolution of gaseous CO2 into water induces principally three carbon species: CO2, HCO3 −, and CO3 2−, which are inorganic carbon sources for microalgae. Owing to the low solubility of inorganic carbon species in water, CO2 availability is limiting factor for the microalgal photosynthesis, and hence, microalgae have evolved carbon concentrating mechanisms (CCMs) that augment CO2 concentration by the enzyme RubisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) (Trimborn et al. 2009). However, microalgae vary widely in their preferences to the utilization of carbon sources (Trimborn et al. 2009). For some species, major flux of dissolved inorganic carbon into microalgal cells is the direct CO2 uptake across the plasma membrane (Spalding 2008).
The CO2 input conditions in the present study were manually adjusted as per the light and dark conditions. Instant online control of CO2 input is henceforth necessary based on the feedback from CO2 and light sensors. Moreover, a feedback sensor connected with pH variations of the microalgal culture is needed to regulate on/off for CO2 inlet. When pH exceeds 9.0, there will be inlet of CO2 into the algal culture whereas when pH drops below 6.0, CO2 supply will be cut off. This feedback regulated operation will also save the unnecessary loss of CO2.
The importance of the present study is also relevant for the production of fatty acid methyl esters (FAME) which are starting materials for biodiesel. A study on CO2 input conditions by Guo et al. (2015) has brought forward the point that augmented CO2 concentration with air supply into microalgal culture increases polyunsaturated fatty acids (PUFA). Tang et al. (2011) also highlighted that CO2 concentration higher than the ambient air was favorable for the accumulation of PUFAs in microalgal cells.
Quality indicators of treated wastewater
The removal percentages of ammonia and phosphate were measured over the experimental period of 10 days (Figs. 3 and 4). In the present study, ammonia was decreased by 93.4 and 91.5% and phosphate was decreased by 94.1 and 91.3% by C. vulgaris and S. obliquus, respectively. Lau et al. (1996) reported the removal of 86% inorganic nitrogen and 70% inorganic phosphorus using Chlorella sp. The removal of nitrogen and phosphorus by both the microalgae bubbled with 5% v/v CO2 in our work is comparable with similar studies by Feng et al. (2011), Sydney et al. (2011), McGinn et al. (2012), Ji et al. (2013), and Discart et al. (2014).
The increase in pH with growth of algal biomass induces precipitation of phosphorus as calcium phosphates (Hammouda et al. 1994). Microalgal photosynthesis is associated with increase in pH of the culture medium which further enhances NH3 stripping or P precipitation thereby causing nutrient removal (Nunez et al. 2001; Nurdogan and Oswald 1995; Oswald 2003). Alkaline condition (pH >8) also inhibits coliform bacteria (Lefyedi and Taylor 2006; Nilsson et al. 2013). Alkaline pH is also suitable for the growth of green microalgae (Olaizola et al. 2004; Suryata et al. 2010) allowing better capture of inorganic CO2 (dissolved in liquid) and uptake by algae (Suryata et al. 2010). Alkaline pH (range 8–9) was observed over the experimental duration (data not shown) which meets the effluent discharge standards set by the Environment (Protection) Rules, India (CPCB 1986). Final characteristics of the wastewater treated by the microalgae in our study (Table 4) follow the range for trade effluents discharged into watercourse/controlled watercourse set by Public Utilities Board of Singapore (National Environment Agency 2016).
Conclusion
In the present study, the two species of green microalgae C. vulgaris ATCC 13482 and S. obliquus FACHB 417 were grown in the municipal wastewater. Both of the species effectively treated undiluted municipal wastewater. C. vulgaris proved to be better than S. obliquus in terms of wastewater treatment efficiency, biomass generated, and CO2 fixation rate over the test period. As compared with PUB effluent discharge standards, final wastewater after algal treatment in the present study had significantly lower ammonia and phosphate. The results in our work demonstrated that C. vulgaris ATCC 13482 and S. obliquus FACHB 417 can be potential microalgae species to integrate the approach of wastewater treatment with CO2 fixation thereby scoring positive points over conventional chemical methods of CO2 capture. Harvested algae biomass after treatment could be used for biomethane production under anaerobic digestion and/or be harnessed for lipid/biodiesel extraction. The study presented in our work is environmentally more sustainable as it does not use synthetic culture medium to cultivate microalgae and also takes into account the wastewater treatment.
References
Allas T, Arima J, Bailey P (2007) World energy: China and India insights. International Energy Agency, France
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association
Boden TA, Marland G, Andres RJ (2015) Global, regional and national fossil-fuel CO2 emissions. Carbon dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy. doi:10.3334/CDIAC/00001_V2015
Cardozo KHM, Guaratini T, Barros MP, Falcao VR, Tonon AP, Lopes NP, Campos S, Torres MA, Souza AO, Colepicolo P, Pinto E (2006) Metabolites from algae with economical impact. Comp Biochem Physiol C: Toxicol Pharmacol 146:60–78. doi:10.1016/j.cbpc.2006.05.007
Central Pollution Control Board. The Environment (Protection) Rules, Government of India (1986). Available on http://cpcb.nic.in/GeneralStandards.pdf accessed 14.08.2016
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131. doi:10.1016/j.tibtech.2007.12.002
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. doi:10.1016/j.biotechadv.2007.02.001
Costa JAV, Linde GA, Atala DIP, Mibielli GM, Krüger RT (2000) Modelling of growth conditions for cyanobacterium Spirulina platensis in microcosms. World J Microbiol Biotechnol 16(1):15–18. doi:10.1023/A:1008992826344
Cuellar-Bermudez SP, Garcia-Perez JS, Rittmann BE, Parra-Saldivar R (2015) J Clean Prod 98:53–65. doi:10.1016/j.jclepro.2014.03.034
De Morais MG, Costa JAV (2007) Biofixation of CO2 by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445. doi:10.1016/j.jbiotec.2007.01.009
Discart V, Bilad MR, Marbelia L, Vankelecom IFJ (2014) Impact of changes in broth composition on Chlorella vulgaris cultivation in a membrane photobioreactor (MPBR) with permeate recycle. Bioresour Technol 152:321–328. doi:10.1016/j.biortech.2013.11.019
Feng Y, Li C, Zhang D (2011) Lipid production of Chlorella vulgaris cultured in artificial wastewater. Bioresour Technol 102:101–105. doi:10.1016/j.biortech.2010.06.016
Fulke BA, Mudliar SN, Yadav R, Shekh A, Srinivasan N, Ramanan R, Krishnamurti K, Devi SS, Chakrabarti T (2010) Bio-mitigation of CO2, calcite formation and simultaneous biodiesel precursors production using Chlorella sp. Bioresour Technol 101:8473–8476. doi:10.1016/j.biortech.2010.06.012
Gajda I, Greenman J, Melhuish C, Ieropoulos I (2015) Self-sustainable electricity production from algae grown in microbial fuel cell system. Biomass Bioenergy 82:87–93. doi:10.1016/j.biombioe.2015.05.017
Galadima A, Muraza O (2014) Biodiesel production from algae by using heterogeneous catalysts: a critical review. Energy 78:72–83. doi:10.1016/j.energy.2014.06.018
Global Carbon Emissions (2016) Available at https://www.co2.earth/global-co2-emissions accessed 03.10.2016
Gong YM, Hu HH, Gao Y, Xu XD, Gao H (2011) Microalgae as platform for production of recombinant proteins and valuable compounds: progress and prospects. J Ind Microbiol Biotechnol 38:1879–1890. doi:10.1007/s10295-011-1032-6
Guo Z, Phooi WBA, Lim ZJ, Tong YW (2015) Control of CO2 input conditions during outdoor culture of Chlorella vulgaris in bubble column photobioreactors. Bioresour Technol 186:238–245. doi:10.1016/j.biortech.2015.03.065
Hammouda O, Gaber A, Abdel-Raouf N (1994) Microalgae and wastewater treatment. Ecotoxicol Environ Saf 31:205–210. doi:10.1006/eesa.1995.1064
He PJ, Mao B, Shen CM, Shao LM, Lee DJ, Chang JS (2013) Cultivation of Chlorella vulgaris on wastewater containing high levels of ammonia for biodiesel production. Bioresour Technol 129:177–181. doi:10.1016/j.biortech.2012.10.162
Hernandez D, Solana M, Riano B, Garcia-Gonzalez MC, Bertucco A (2014) Biofuels from microalgae: lipid extraction and methane production from the residual biomass in a biorefinery approach. Bioresour Technol 170:370–378. doi:10.1016/j.biortech.2014.07.109
Ho SH, Chen CY, Lee DJ, Chang JS (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198. doi:10.1016/j.biotechadv.2010.11.001
Ho SH, Chen WM, Chang JS (2010) Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730. doi:10.1016/j.biortech.2010.06.112
Ji MK, Abou-Shanab RAI, Kim SH, Salama E, Lee SH, Kabra AN, Lee YS, Hong S, Jeon BH (2013) Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol Eng 58:142–148. doi:10.1016/j.ecoleng.2013.06.020
Jin HF, Lim BR, Lee K (2006) Influence of nitrate feeding on carbon dioxide fixation by microalgae. J Environ Sci Health A 41:2813–2824. doi:10.1080/10934520600967928
Kao CY, Chiu SY, Huang TT, Dai L, Hsu LK, Lin CS (2012) Ability of a mutant strain of the microalga Chlorella sp. to capture carbon dioxide for biogas upgrading. Appl Energy 93:176–183. doi:10.1016/j.apenergy.2011.12.082
Kargupta W, Ganesh A, Mukherji S (2015) Estimation of carbon dioxide sequestration potential of microalgae grown in a batch photobioreactor. Bioresour Technol 180:370–375. doi:10.1016/j.biortech.2015.01.017
Kligerman DC, Bouwer EJ (2015) Prospects for biodiesel production from algae-based wastewater treatment in Brazil: a review. Renew Sustainable Energy Rev 52:1834–1846. doi:10.1016/j.rser.2015.08.030
Lau PS, Tam NFY, Wong YS (1996) Wastewater nutrients removal by Chlorella vulgaris: optimization through acclimation. Environ Technol 17(2):183–189. doi:10.1080/09593331708616375
Lefyedi ML, Taylor JRN (2006) Effect of dilute alkaline steeping on the microbial contamination, toxicity and diastatic power of Sorghum malt. J Inst Brew 112(2):108–116. doi:10.1002/j.2050-0416.2006.tb00240.x
Leung DYC, Caramanna G, Maroto-Valer MM (2014) An overview of current status of carbon dioxide capture and storage technologies. Renew Sustainable Energy Rev 39:426–443. doi:10.1016/j.rser.2014.07.093
McGinn PJ, Dickinson KE, Park KC, Whitney CG, MacQuarrie SP, Black FJ, Frigon J-C, Guiot SR, O’Leary SJB (2012) Assessment of the bioenergy and bioremediation potentials of the microalga Scenedesmus sp. AMDD cultivated in municipal wastewater effluent in batch and continuous mode. Algal Res 1:155–165. doi:10.1016/j.algal.2012.05.001
Metz B, Davidson O, de Coninck HC, Loos M, Meyer LA (2005) IPCC special report on carbon dioxide capture and storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York. Available from https://www.Ipcc.Ch/Pdf/Special-reports/srccs/srccs_wholereport.Pdf. Accessed 14 Aug 2016
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after as shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283. doi:10.1016/j.biortech.2012.07.057
National Environmental Agency (2016) Allowable limits for trade effluent discharge to watercourse/controlled watercourse. Public Utilities Board, Singapore. Available at http://www.nea.gov.sg/anti-pollution-radiation-protection/water-pollution-control/allowable-limits. Accessed 4 Oct 2016
Nilsson C, Renman G, Westholm LJ, Renman A, Drizo A (2013) Effect of organic load on phosphorus and bacteria removal from wastewater using alkaline filter materials. Water Res 47:6289–6297. doi:10.1016/j.watres.2013.08.001
Nunez VJ, Voltolina D, Nieves M, Pina P, Medina A, Guerrero M (2001) Nitrogen budget in Scenedesmus obliquus cultures with artificial wastewater. Bioresour Technol 78:161–164. doi:10.1016/S0960-8524(00)00183-8
Nurdogan Y, Oswald WJ (1995) Enhanced nutrient removal in high rate ponds. Water Sci Technol 31:33–43. doi:10.1016/0273-1223(95)00490-E
Olaizola M, Bridges T, Flores S, Griswold L, Morency J, Nakamura T (2004) Microalgal removal of CO2 from flue gases: CO2 capture from a coal combustor. U.S. Department of Energy, Colorado
Oswald WJ (2003) My sixty years in applied algology. J Appl Phycol 15:99–106. doi:10.1023/A:1023871903434
Rao AB, Rubin ES (2002) A technical, economic and environmental assessment of amine-based carbon capture technology for power plant greenhouse gas control. Environ Sci Technol 36(20):4467–4475. doi:10.1021/es0158861
Scripps Institution of Oceanography (2016) The Keeling curve. Available https://scripps.ucsd.edu/programs/keelingcurve/ accessed on 03.10.2016
Spalding MH (2008) Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot 59:1463–1473. doi:10.1093/jxb/erm128
Suryata I, Svavarsson HG, Einarsson S (2010) Geothermal CO2 bio-mitigation techniques by utilizing microalgae at the Blue Lagoon, Iceland. Proceedings at 34th Workshop on Geothermal Reservoir Engineering, Stanford, California
Sydney EB, da Silva TE, Tokarski A, Novak AC, de Carvalho JC, Woiciecohwski AL, Larroche C, Soccol CR (2011) Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl Energy 88:3291–3294. doi:10.1016/j.apenergy.2010.11.024
Sydney EB, Sturm W, de Carvalo JC, Thomas-Soccol V, Larroche C, Pandey A, Ricardo-Soccol C (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896. doi:10.1016/j.biortech.2010.02.088
Tang D, Hang W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. doi:10.1016/j.biortech.2010.10.047
Toledo-Cervantes A, Morales M, Novelo E, Revah S (2013) Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus. Bioresour Technol 130:652–658. doi:10.1016/j.biortech.2012.12.081
Trimborn S, Wolf-Galdrow D, Richter K, Rost B (2009) The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J Exp Mar Bio Ecol 376:26–36. doi:10.1016/j.jembe.2009.05.017
Ullah K, Ahmad M, Sofia SVK, Lu P, Harvey A, Zafar M, Sultana S, Anyanwu CN (2014) Algal biomass as a source of transport fuels: overview and development perspectives. Prog Natural Sci Mater Int 24:329–339. doi:10.1016/j.pnsc.2014.06.008
Wang B, Li Y, Wu N, Lan CQ (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79(5):707–718. doi:10.1007/s00253-008-1518-y
Ward AJ, Lewis DM, Green FB (2014) Anaerobic digestion of algae biomass: a review. Algal Res 5:204–214. doi:10.1016/j.algal.2014.02.001
Acknowledgements
The funding for this project work was supported by the National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) program. The first author also extends heartfelt thanks to the National University of Singapore for hosting his doctoral research under Joint PhD program initiated with the Indian Institute of Technology Bombay (India).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Elena Maestri
Electronic supplementary material
.
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Chaudhary, R., Dikshit, A.K. & Tong, Y.W. Carbon-dioxide biofixation and phycoremediation of municipal wastewater using Chlorella vulgaris and Scenedesmus obliquus . Environ Sci Pollut Res 25, 20399–20406 (2018). https://doi.org/10.1007/s11356-017-9575-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9575-3