Abstract
This paper focuses on the evaluation of the biomass production of a novel native microalgal strain Chlorella sorokiniana using centrate from municipal wastewater as the sole source of nutrients and six different temperatures. The experiments were performed in semi-continuous cultures using Bold’s Basal Medium and centrate separately, modifying the temperature (25–50 °C). The study aimed to elucidate whether a thermotolerant strain can be used for both the production of biomass and the removal of nutrients from wastewater. It was observed that C. sorokiniana biomass can be produced using centrate as the sole nutrient source. The highest biomass yield, for both culture media, was achieved at 35 °C showing maximum values for centrate (1.0 gbiomass·L−1, 0.20 gbiomass·L−1·day−1 dry weight), surpassing the productivity obtained when using Bold’s Basal Medium (BBM) (0.60 gbiomass·L−1; 0.13 gbiomass·L−1·day−1 dry weight). Variable chlorophyll fluorescence confirmed that the excess of ammonium (601 mgNH4·L−1) did not inhibit the growth of C. sorokiniana, and thus, the biomass productivity was not affected. The maximum nutrient removals reached for BBM and centrate were 96% and 61.5% for nitrogen, 53.2% and 61% for phosphorus, and 93% and 0.18% for total carbon, respectively. Fatty acids accumulating in the microalgal biomass were mainly composed of arachidonic, oleic, and eicosatetraenoic acids. Consequently, it is demonstrated that microalgae native to desertic areas can be used to produce microalgal biomass using centrate from wastewater treatment plants as the exclusive nutrient source, reaching high productivities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgal biomass has excellent potential for biorefinery processes because it can be converted into different co-products such as energy, animal feed, and agricultural products (Rizwan et al. 2018). Its biochemical composition will be determined by various culture parameters such as temperature, presence or absence of light, and the culture medium used, among others (Yeh and Chang 2012; Mutaf et al. 2019).
The production of biomass from microalgae to obtain commodities for animal feed and agricultural products has emerged as a promising and real alternative that competes with the use of synthetic compounds for these purposes (Ruiz et al. 2016). However, the microalgal production process is still expensive. One of the main obstacles to reducing production costs is the energy expenditure required to keep cultures at a constant temperature below 27 °C for optimal growth (Acién et al. 2014). Therefore, it is necessary to search for native strains in areas with desert climates, which resist high temperatures and, at the same time, present high productivity rates to alleviate the need for cooling systems for the cultures, especially in places with very hot climates.
Another problem is the cost of the nutrients. Therefore, in recent years, different sources of nutrients suitable for developing microalgal cultures have been investigated (Chimenos et al. 2003; Singh et al. 2017). Among these sources is wastewater, characterized by containing organic and inorganic compounds, such as nitrogen, phosphorus, and other nutrients that make it an ideal source for use as a growing medium (Loera-Quezada et al. 2010).
Microalgal treatment of wastewater has been widely reported as an efficient process for removing nutrients (Voltolina et al. 2005; Ledda et al. 2016), generating a lower energy demand and, therefore, lower costs than conventional systems (Ruiz et al. 2016). The use of wastewater as the sole source of nutrients for the production of microalgal biomass reduces production costs, representing 40% of the total costs of the process (Gouveia et al. 2016). Also, the cultivation of microalgae in wastewater promotes an attractive and effective treatment to reduce the environmental impact of human activity.
Although the benefits of using wastewater as a culture medium have been widely reported, it is important to consider that many wastewaters contain high concentrations of ammonia, which are toxic to most microalgal species, due to its effect on the processes photosynthetic in chloroplasts (Crofts 1966), and various investigations suggest that a significant dilution is necessary for the proper growth of microalgal cultures (Wang et al. 2012). Therefore, the search for an efficient strain in removing nutrients from a wide variety of wastewaters is a critical requirement for the economically viable operation of the production of microalgal cultures and their application in different industries (Draaisma et al. 2013; Jebali et al. 2015; Chuka-Ogwude et al. 2020).
Single-celled green microalgae are often limited by nutrient depletion; however, Chlorella sorokiniana has significant potential as a future source of different products for agriculture and animal feed due to its resistance to high concentrations of nitrogen (Ge et al. 2013) and its high adaptability to temperatures above 30 °C. Therefore, the objective of this work was to determine the feasibility of producing biomass from the newly isolated strain of C. sorokiniana using leachate from urban wastewater without dilution, with a high concentration of ammonia (601 mgNH4·L−1) as the only source of nutrients and grown at different temperatures (15 °C, 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C). In addition to studying the productivity of the biomass, the quality of the biomass obtained was also analyzed.
Materials and methods
Microorganism and culture media
The freshwater Chlorella sorokiniana was isolated in summer (>40 °C) from swine wastewater from Ciudad Obregon, Sonora, Mexico. Therefore, this microalga has robustness against the high ammonium content (>136 mgNH4·L−1). The inoculum cultures were maintained photoautotrophically in 0.5-L Erlenmeyer flasks containing 350 mL of Bold’s Basal Medium (BBM) (Nichols and Bold 1965). Microalgae were grown aseptically in a thermostated room at 25 °C, with an aeration rate of 0.5 v·v−1·min−1 and under continuous illumination of 100 μmol photons·m−2·s−1.
Batch bioassays were carried out in triplicate using BBM. Macro- and micro-nutrients were prepared in freshwater and sterilized by autoclaving at 126 °C for 15 min. Semi-continuous bioassays were performed in triplicate. “Prepared BBM” and “filtered centrate” (taken from a sewage treatment plant located in Almería, Spain) were used separately. Additionally, centrate was enriched with phosphate to achieve a N:P ratio of 5:1, because it had been demonstrated previously that this is required for the optimal performance of microalgal cultures and for efficient removal of nitrogen (Ledda et al. 2015; Sepúlveda et al. 2015; Romero Villegas et al. 2017). To reduce the content of suspended solids, the centrate was filtered through one cartridge filter (20 μm) before being injected into the cultures. The centrate composition is shown in Table 1.
Photoreactor and culture conditions
Figure 1 shows the schematic process of microalgal biomass production used in this experiment. The assays were performed in triplicate, indoors in 3-L poly(methyl methacrylate) bubble-column photobioreactors of 0.09 m diameter and 0.5 m height. Columns were provided with an inlet valve and an outlet valve (harvest); a pH sensor was situated at the top of the reactor. Air was injected from the base of the reactor at a rate of 0.1 v·v−1·min−1 to agitate the culture. Initially, to study the effect of the temperature, cultures were thermostated using several baths with immersion thermostats at different temperatures (25 °C, 30 °C, 35 °C, 40 °C, and 50 °C). These temperatures were chosen because no productivity was observed at any temperature above or below in batch assays. The pH was kept at 7.5 by injection of pure CO2 on demand at a rate of 0.001 v·v−1·min−1. For this, pH 5330 probes and an R21 pH controller (Crison) were used. The reactors were illuminated with 28-W high-efficiency fluorescent tubes (Philips Daylight T5), simulating the circadian cycle at an irradiance of 320 μmol photons·m−2·s−1. The irradiance mean value was measured at twelve different positions utilizing a quantum sensor (spherical SQS-100; Walz GmbH, Germany). The indoor batch cultivation was performed by inoculating at 0.2 g·L−1, until reaching the stationary phase. To determine the optimum conditions for C. sorokiniana, the influence of dilution rates (D) on the productivity of the biomass (the imposed dilution rate influences the microalgal biomass harvest and thus the final biomass concentration inside the culture at a steady state) was analyzed. D represents the rate of flow of the culture medium over the volume of culture in the bioreactor, and it was determined that the best D value for this strain was 0.2 day−1 (data not shown). The reactors were operated in semi-continuous, chemostat mode by adding a fresh medium daily to the reactors for 4 h at the beginning of the light stage and, at the same time, harvesting an equal volume of culture. For semi-continuous bioassays, a D value of 0.2 day−1 was used for both culture media (BBM and undiluted centrate). Both cultures started at 0.8 gbiomass·L−1 using six different temperatures (15 °C, 20 °C, 25 °C, 30 °C, 35 °C, and 40 °C), maintaining the culture until the stationary state.
Taxonomic and molecular identification
To determine the taxonomic division of the isolated strains, LucidCentral (Stafford Heights, Australia) keys were used to compare morphological characteristics. The samples were then stained with Lugol, cresyl blue, and bright green to observe starch in pyrenoids, cell walls, nuclei, and mucilages under an optical microscope coupled to a digital camera (Tsarenko and John 2011; Camarena-Bernard and Rout 2018). The number of cells per coenobium, cell shape, and cell arrangement were documented.
Partial taxonomic identifications up to genus was performed according to Tsarenko and John (2011) and molecular identification was carried out as per our previous work (Camarena-Bernard and Rout 2018). Genomic DNA from three algal strains was extracted according to Murray and Thompson (1980) with minor modifications. The 18–28S ribosomal RNA internal transcribed spacer (rRNA ITS) region of all three algal strains was amplified using specific primer pairs as follows: ITS1 (5′–>3′-TCCGTAGGTGAACCTGCGG) and ITS4 (5′–>3′-TCCTCCGCTTATTGATATGC) (White et al. 1990). Amplified PCR products were subjected to agarose gel electrophoresis and visualized using a UV transilluminator. The specific products of direct PCR as well as the products of PCR from the agarose gel were purified using kits according to the manufacturer’s instructions (Qiagen, Germany) and sequenced. The DNA sequences were viewed and edited using Sequence Scanner (Applied Bioscience). A global BLAST search was then performed to identify the most closely related sequences from the NCBI database using PubMed Nucleotide BLAST. The AU8 local blast and multiple sequence alignments were compiled and analyzed using BioEdit v7.2.5 (Ibis Biosciences, USA), and the phylogenetic analysis was performed using software MEGA 10 (Tamura and Nei 1993).
Analytical procedures
Biomass concentration was determined daily by measuring absorbance at 750. Spectrophotometric measurements were verified by dry weight determinations twice a week. The dry weight biomass concentration (Cb) was measured by centrifuging 100 mL of culture for 5 min at 9000×g and freeze-drying over 48 h. Volumetric productivity (Pbvol) was calculated by multiplying the biomass concentration (Cb) by the set D using Eq. 1.
The cell status was checked daily by measuring the Fv/Fm ratio; for this, samples were acclimated in the dark for 7 min using an AquaPen AP 100 (PSI, Czech Republic) PAM fluorometer. The outlet culture medium was analyzed using the International Standard Official methods (Ministerio de Agricultura 1998). Ammonium was measured according to the Nessler method (ammonium standard for IC: 59755) (APAT-IRSA/CNR 2003). To measure nitrate content, a spectrophotometer between 220 and 275 nm (APAT-IRSA/CNR 2003) was used. Phosphates were calculated by visible spectrophotometry through the phosphorus vanadate–molybdate complex (APAT-IRSA/CNR 2003). For total carbon (TC) (total inorganic carbon (TIC) + total organic carbon (TOC)), filtered supernatant samples were injected into a Shimadzu 5050A TOC analyzer (provided with an NDIR detector). Freeze-dried biomass samples from each steady state were analyzed to determine the biochemical composition. The total lipid content was determined by the method of Kochert (1978). Proteins were determined by the modified Lowry method (López González et al. 2010). The fatty acid (FA) content was quantified by direct transesterification of microalgal biomass to transform all saponifiable lipids into fatty acid methyl esters (FAMEs), which were then analyzed by gas chromatography (GC) as described by Jiménez Callejón et al. (2014).
Nutrient removal capacity and coefficient yield
The removal capacity of nitrogen and phosphorus was measured. For this, two major variables were calculated: the removal efficiency (Re) and the removal capacity (Rc). The Re is calculated as the ratio between the concentration outlet (C) and inlet (C0) in the reactor, thus representing the reduction in the nutrient concentration outlet (Eq. 2). Rc is calculated as the total amount of nutrient removed per unit volume over time, thus indicating the system’s net capacity for removing whatever nutrient (Eqs. 2 and 3).
Statistical analysis
Three replicates of each sample were used for statistical analysis, and the values were reported as the value ± standard deviation. A multifactorial ANOVA was used to evaluate significant differences (p < 0.05). The relationship between culture medium and temperature was assessed using Statgraphics Centurion XVI (StatPoint Technologies, Inc.).
Results and discussion
Taxonomic and molecular identification
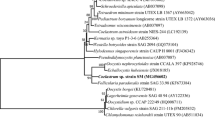
Taxonomic identification based on appearance suggested that this microalga is Chlorella sorokiniana. The 18–28S rRNA ITS gene region was successfully amplified for molecular identification and sequenced. The sequences were deposited in the NCBI database under the GenBank accession Seq1 MN658361 and Seq2 MN658362. They were compared with several reference sequences (including the ITS region of several Chlorella species) obtained from the NCBI database. Phylogenetic tree analysis of Chlorella fell into five distinct clusters and several sub-clusters. The C. sorokiniana was found to be very close sequentially and formed a cluster with them. The four other species were distributed as per the expectation forming independent clusters. The C. sorokiniana of this study was very close to accession KM514858.1 (Nanjing Agricultural University, China) and KJ676109.1 (UTEX 2805) (Fig. 2).
The evolutionary history was inferred by using the maximum likelihood method and Tamura and Nei (1993) model. The tree with the highest log likelihood is shown (Fig. 1). The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial trees for the heuristic search were obtained automatically by applying neighbor-joining and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach and then selecting the topology with a superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 32 nucleotide sequences. The codon positions included were 1st + 2nd + 3rd + noncoding. There was a total of 4649 positions in the final dataset. Evolutionary analyses were conducted in MEGA X (Kumar et al. 2018).
Centrate composition
The centrate was prepared daily by adding phosphorus to balance the nitrogen:phosphorus ratio (N:P; 5:1). The centrate compositional analysis is presented in Table 1 and shows an elevated content of ammonium (601 mg·L−1, the main source of nitrogen), chlorides (511 mg·L−1), and bicarbonate (1574 mg·L−1), while nitrate and phosphorous showed lower values of 13 mgN·L−1 and 15.8 mgP·L−1, respectively. Table 1 also presents other essential elements for microalgal growth: zinc, copper, and boron, among others. Considering the expected composition of microalgal biomass (50% C, 7% N, 1% P), it can be calculated that to produce 1.0 gbiomass of standard microalgal biomass, 2500 mg of HCO3−, 90 mg of NH4+ (310 mg of NO3−), and 10 mg of P are required. Nitrogen content in the centrate exceeds the values required for the efficient uptake of phosphorus and carbon. Moreover, N:P ratio should be close to the optimum nitrogen–phosphorus stoichiometry encountered in phytoplankton (Klausmeier et al. 2004). Thus, using this centrate without any added phosphorous causes an imbalance of these two compounds and results in poor biomass productivity and poor nutrient removal (Ledda et al. 2015; Sepúlveda et al. 2015).
Biomass production
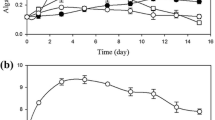
The performance of the selected native strain as a function of culture conditions was evaluated in semi-continuous mode, a dilution rate of 0.2 day−1 being used as optimal. Effects of temperature and centrate on biomass production and Fv/Fm are shown in Fig. 3. Data demonstrated that centrate improves the biomass production (Fig. 3a, b) as compared to BBM (p < 0.05), although it has been reported that an ammonium concentration of more than 100 mgNH4·L−1 could be lethal for some microalgal species (Collos and Harrison 2014). The biomass production, both biomass concentration and productivity, increases as the temperature increases up to 35 °C for both cases, showing maximal values of 0.64 ± 0.15 gbiomass·L−1 (0.13 gbiomass·L−1·day−1) and 1.0 ± 0.04 gbiomass·L−1 (0.20 gbiomass·L−1·day−1) for the BBM and centrate cultures, respectively (p < 0.05). However, biomass production is reduced to 0.6 gbiomass·L−1 (0.12 gbiomass·L−1·day−1) when centrate was used at 40 °C, whereas, when BBM was used, the culture was washed out, indicating that not only temperature influences microalgal growth but also the culture medium affects biomass production. Moreover, Fig. 3c concurs with the behavior shown by biomass productivity. It can be observed that Fm/Fv was always high (≥0.6) for both culture media and for all the temperatures tested, except when the temperature was 40 °C using BBM (Fv/Fm = 0.40). Previous studies have concluded that high ammonium concentration and high temperatures reduce photosynthetic performance (e.g., Yamasaki et al. 2002; Markou et al. 2016).
Influence of the temperature in the culture of Chlorella sorokiniana on the biomass concentration, productivity, and Fv/Fm in the indoor 3-L column photobioreactors with Bold’s Basal Medium and centrate as culture medium. a Biomass concentration. b Volumetric biomass productivity. c Fv/Fm. Mean ± SD values are shown for the independent experiments performed in triplicate (p < 0.05)
Previous studies have reported that percentages of centrate ≥70% decrease the Fv/Fm values in the marine microalga Nannochloropsis gaditana (Sepúlveda et al. 2015). Jebali et al. (2018) observed a similar behavior of the freshwater microalga Scenedesmus sp., showing a decrease in Fv/Fm when a percentage of centrate at 60% was used, which could be due to that Scenedesmus sp. does not tolerate high concentrations of nitrogen (Xin et al. 2010), causing the culture to be stressed, and consequently, the photosynthetic system of the cells is affected. In addition, C. sorokiniana shows a high degree of tolerance to osmotic stress (Li et al. 2018; Lizzul et al. 2018). Thus, the strain of C. sorokiniana used in this study tolerated centrate with any dilution (high nitrogen concentration), without any effect on the photosynthetic system at any condition.
Biochemical composition
The biomass’ (ash-free dry weight) biochemical composition was evaluated at the steady state (Fig. 4). Carbohydrates (Fig. 4a) were the predominant biomass fraction, ranging from 63 to 67% and from 34 to 37% for BBM and centrate, respectively, being constant over all treatments. Protein content (Fig. 4b) ranged from 8 to 14% and from 27 to 32% for BBM and centrate, respectively. However, a constant decrease in lipid content is observed when using BBM (23–16%). With the use of centrate, the percentage of lipids is stable among different temperatures (21–23%), except when the temperature was 30 °C, where a maximum lipid content of up to 29% dry weight (DW) was observed. This can be explained by the stress generated by the adverse conditions produced by using nontreated centrate and its high ammonium content, as compared to the BBM culture medium. Authors like San Pedro et al. (2013) have reported that the total lipid constituents of N. gaditana increase when it was cultured under nitrogen starvation conditions from 12 to 26% wt.
Variation in the a carbohydrate content, b protein content, and c lipid content of the microalgal biomass of Chlorella sorokiniana produced as a function of the temperature indoor 3-L column photobioreactors with Bold’s Basal Medium and centrate as culture medium. Mean ± SD values are shown for the independent experiments performed in triplicate (p < 0.05)
Ruiz et al. (2016) elaborated a cost analysis of microalgal products, showing the prices for proteins (1.0 €·kg−1), carbohydrates (0.3 €·kg−1), and lipids (0.6 €·kg−1). Taking those prices into account, it can be estimated that the maximum biomass cost at the conditions set in this experiment was achieved when centrate was used, resulting in 0.54 €·kg−1 when temperatures from 15 to 25 °C are used. However, the cost derived from the energy used to cool the microalgal cultures located in areas with high temperatures must be discarded. Interestingly, at temperatures of 30 and 35 °C, a similar cost is calculated (0.53 €·kg−1) for the microalgal biomass. This means that costs may be further reduced by eliminating refrigeration in the culture of C. sorokiniana.
The FA profile of C. sorokiniana is shown in Table 2. The percentage of FA varied between 7.0 and 15.5% (DW), decreasing as the temperature is increased for both culture media. The C18:1n9, C20:4n3, and C20:4n6 fatty acids constitute up to 67% of total FA in the best case. C20:4n6 represented the largest proportion of FA, with respect to the total of FA, at all temperatures tested, ranging from 29 to 35% and from 18 to 28% for BBM and centrate, respectively. This result is noteworthy since a rich fraction of FA in the biomass is required for biofuel production (Chisti 2007; Brennan and Owende 2010). At present, studies have focused on the conversion of microalgal biomass to products such as biopesticides, biofertilizers, and biostimulants for agriculture (Acién et al. 2014; Ruiz et al. 2016). In this regard, biomass obtained with BBM had a low protein content that may be beneficial for biofuel production, since a low proportion of nitrogen reduces the undesirable effects of nitrogen compounds in the environment, which usually are produced by the burning of microalgal oil (Biller and Ross 2011).
Carbon removal
Culture media analysis was performed; thus carbon, nitrogen, and phosphorus analysis were carried out in the inlet and the outlet at a steady state to determine nutrient recovery capacity (amount of nutrients recovered by microalgal cultures) and the removal efficiency of nutrients (percentage of nutrients removed from culture medium compared to the inlet).
Regarding carbon (Fig. 5), results show that values of TC (Fig. 5a) are similar in both culture media with values of 200 mgTC·L−1 and 196 mgTC·L−1 for BBM and centrate, respectively. Moreover, TC in the centrate is composed of 55% of TOC and 45% of TIC, while TC contained in BBM contains only TIC. Nevertheless, the values of TC obtained in the outlet at the steady states, when BBM was used, showed a significant decrease (p < 0.05), reaching a minimum value up to 34 mgTC·L−1. In contrast, results obtained with centrate demonstrated that TC in the outlet is higher as than that in the inlet, displaying the minimal value of 196.5 mgTC·L−1 at 35 °C.
Influence of the temperature in the culture medium on the total carbon (organic and inorganic) concentration at the inlet and outlet of the indoor 3-L column photobioreactors with Bold’s Basal Medium and centrate as culture medium. a Total carbon. b Total inorganic carbon. c Total organic carbon. d Total carbon removal capacity. Mean + SD values are shown for the independent experiments performed in triplicate (p < 0.05)
Regarding TIC (Fig. 5b), inlet values of 200 mgTIC·L−1 and 89 mgTIC·L−1 for BBM and centrate, respectively, were observed. Furthermore, there was a substantial reduction in the outlet for BBM (p < 0.05), ranging from 20 to 40 mgTIC·L−1 at ≤35 °C, whereas when centrate was used, TIC shows an increasing trend, ranging from 43 to 64.5 mgTIC·L−1 until a temperature of 40 °C. With respect to TOC (Fig. 5c), values were 108 mgTOC·L−1 for centrate, while BBM did not contain any TOC. In contrast, TOC outlet values show an increment for both culture media, ranging from 4.8 to 18.3 mgTOC·L−1 and from 143 to 191 mgTOC·L−1 for BBM and centrate, respectively, resulting in 76% of TC accumulation in the worst case. Carbon removal capacity and removal efficiency (Fig. 5d) were determined. The microalgal culture in BBM had a better performance in removing TC (p < 0.05), with an average removal capacity of 32 mgTC·L−1·day−1 (Re = 80%) at ≤35 °C. On the other hand, when centrate was used, the Rc was deficient due to TOC accumulation. Martínez and Orús (1991) mentioned that organic carbon utilization might reduce affinity for CO2 and CO2 fixation; thus, microalgal cultures consume TIC. In addition, the microalgal cultures produce exopolysaccharides, which remain in the culture medium (Richert et al. 2005).
Nitrogen removal
With regard to total nitrogen (TN) (Fig. 6) in the inlet and the outlet (Fig. 6a), initial values of 43.3 mgTN·L−1 for BBM and 470.4 mgTN·L−1 for centrate were observed. It is important to note that centrate contained mainly ammonium (≥99%) as the nitrogen source, while BBM contained nitrate. On the other hand, nitrogen at the outlet showed minimal values of 1.8 mgTN·L−1 and 181 mgTN·L−1 for BBM and centrate, respectively.
Influence of the temperature in the culture medium on the nitrogen inlet and outlet concentrations in the indoor 3-L column photobioreactors. a Total nitrogen. b Nitrogen removal efficiency/capacity (CRC centrate removal capacity, BRC BBM removal capacity, CDE centrate depuration efficiency, BDE BBM depuration efficiency). Mean + SD values are shown for the independent experiments performed in triplicate (p < 0.05)
Regarding nitrogen removal capacity and removal efficiency, Fig. 6b shows that microalgal culture which used centrate as culture medium had a better performance, displaying average values of nitrogen Rc up to 56 mgTN·L−1·day−1 (Re = 60%), whereas BBM shows an average of nitrogen Rc of 7.5 mgTN·L−1·day−1 (Re = 88%). However, even though the removal efficiency with the use of BBM is higher, it is important to clarify that the initial concentration of TN in the centrate represents up to 260% of the TN contained in BBM. Lincoln et al. (1996) demonstrated that N–ammonium concentration above 100 mgN-NH4·L−1 is toxic for some microalgal strains, even though other microalgae can stand up to 700 mgN-NH4·L−1 (Collos and Harrison 2014). Sepúlveda et al. (2015) showed that indoor conditions the marine microalgae N. gaditana can depurate up to 35 mgTN·L−1·day−1 of total nitrogen of a mix of 80% centrate–20% seawater at 20 °C, whereas Jebali et al. (2018) showed that the freshwater Scenedesmus sp. could depurate up to 40 mgTN·L−1·day−1 from a centrate–seawater mix (60–40%) at 25 °C. Nevertheless, studies carried out in tubular photobioreactor outdoors showed that a consortium of five microalgae (N. gaditana, Chaetophorales sp., Geitlerinema sp., Chlorella sp., and Limnothrix sp.) can reach up to 73.5 mgTN·L−1·day−1 (97%) from a mix of 40% centrate–60% seawater (Romero Villegas et al. 2017). Given the above, the strain used in this work has a superior capacity in wastewater treatment, tolerating and processing a higher ammonium concentration than other strains previously tested with the same source of nutrients.
Phosphorus
Phosphorus was also analyzed in the inlet and outlet (Fig. 7a). Results showed inlet values of 81.6 mgP·L−1 (BBM) and 112 mgP·L−1 (centrate). Moreover, as a function of temperature increased, the cultures with BBM showed a slight reduction in the outlet ranging from 55.3 mgP·L−1 to 38.2 mgP·L−1, at temperatures from 15 to 35 °C. However, at 40 °C, the phosphorus content reached 59 mgP·L−1, whereas values obtained with centrate had an average of 46.5 mgP·L−1 for all temperatures set. Rc and Re are presented in Fig. 7b. The data show a good performance of all the treatments, achieving the maximum phosphorus Rc of 8.7 mgP·L−1·day−1 (Re = 53.2%) at 30 °C for BBM. Regarding centrate, the best values were obtained at 35 °C, reaching maximal Rc up to 13.6 mgP·L−1·day−1 (Re = 61%). Previous studies have found phosphorus Rc up to 3 mgP·L−1·day−1 for a freshwater Scenedesmus sp. using a mix of 60% centrate–40% freshwater at 25 °C (Jebali et al. 2018) and 5.1 mgTP·L−1·day−1 (80% depuration) for the marine N. gaditana using a mix of 50% centrate–50% seawater at 20 °C (Sepúlveda et al. 2015). For a consortium of five microalgal outdoors, with an average temperature of 35 °C, a maximal Rc value up to 12 mgTP·L−1·day−1 (95%) using 40% centrate–60% seawater as a culture medium has been reported (Romero Villegas et al. 2017).
Influence of the temperature in the culture medium on the phosphorus inlet and outlet concentrations in the indoor 3-L column photobioreactors. a Total phosphorus. b Phosphorus removal efficiency/capacity (CRC centrate removal capacity, BRC BBM removal capacity, CDE centrate depuration efficiency, BDE BBM depuration efficiency). Mean + SD values are shown for the independent experiments performed in triplicate (p < 0.05)
These studies indicate that, despite the excellent performance of the nutrient recovery process by C. sorokiniana, an optimal culture method for this strain has not been established yet. Therefore, it is necessary to study the physiological characteristics of this strain further. Moreover, the research of high temperature and high ammonium tolerant microalgae together with the use of centrate as the only source of nutrients is highly relevant worldwide for the removal of contaminants such as nitrogen, phosphorus, and carbon. This method has great potential in reducing freshwater waste and the costs derived from the use of fertilizers.
Conclusions
The present study demonstrates the relevance of a microalgal strain tolerant to high temperatures and high nitrogen concentration in nutrient recycling. The temperatures set for the experiments strongly influenced biomass productivity. Moreover, the culture shows better performance when centrate is used as a nutrient source. This work presents a novel approach in microalgal cultures regarding the use of strains resistant to high temperatures and high nitrogen concentration while taking advantage of the nutrients contained in wastewater. The biomass productivity suggests that this microalgal culture offers real potential for sub-products with applications in the energy and agricultural industries.
References
Acién FG, Fernández JM, Molina-Grima E (2014) Economics of microalgae biomass production. In: Pandey A, Lee D-j, Chisti Y, Soccol CR (eds) Biofuels from algae. Elsevier, Amsterdam, pp 313–325
APAT-IRSA/CNR (2003) IRSA-CNR Metodi analitici per le acque. Volume Primo. Rome
Biller P, Ross AB (2011) Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour Technol 102:215–225
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energ Rev 14:557–577
Camarena-Bernard C, Rout NP (2018) Native microalgae from eutrophic water: potential for wastewater treatment, low-cost biomass, and lipid production. Ind Biotechnol 14:257–264
Chimenos JM, Fernández AI, Villalba G, Segarra M, Urruticoechea A, Artaza B, Espiell F (2003) Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res 37:1601–1607
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chuka-Ogwude D, Ogbonna J, Borowitzka MA, Moheimani NR (2020) Screening, acclimation and ammonia tolerance of microalgae grown in food waste digestate. J Appl Phycol 32:3775–3785
Collos Y, Harrison PJ (2014) Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar Pollut Bull 80:8–23
Crofts AR (1966) Uptake of ammonium ion by chloroplasts, and the mechanism of amine uncoupling. Biochem Biophys Res Commun 24:127–134
Draaisma RB, Wijffels RH, Slegers PME, Brentner LB, Roy A, Barbosa MJ (2013) Food commodities from microalgae. Curr Opin Biotechnol 24:169–177
Ge Z, Zhang H, Zhang Y, Yan C, Zhao Y (2013) Purifying synthetic high-strength wastewater by microalgae Chlorella vulgaris under various light emitting diode wavelengths and intensities. J Environ Health Sci Eng 11:8
Gouveia L, Graça S, Sousa C, Ambrosano L, Ribeiro B, Botrel EP, Neto PC, Ferreira AF, Silva CM (2016) Microalgae biomass production using wastewater: Treatment and costs. Scale-up considerations. Algal Res 16:167–176
Jebali A, Acién FG, Gómez C, Fernández-Sevilla JM, Mhiri N, Karray F, Dhouib A, Molina-Grima E, Sayadi S (2015) Selection of native Tunisian microalgae for simultaneous wastewater treatment and biofuel production. Bioresour Technol 198:424–430
Jebali A, Acién FG, Sayadi S, Molina-Grima E (2018) Utilization of centrate from urban wastewater plants for the production of Scenedesmus sp. in a raceway-simulating reactor. J Environ Manag 211:112–124
Jiménez Callejón MJ, Robles Medina A, Macías Sánchez MD, Hita Peña E, Esteban Cerdán L, González Moreno PA, Molina Grima E (2014) Extraction of saponifiable lipids from wet microalgal biomass for biodiesel production. Bioresour Technol 169:198–205
Klausmeier CA, Litchman E, Daufreshna T, Levin SA (2004) Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 429:171–174
Kochert G (1978) Quantitation of the macromolecular components of microalgae. In: Hellebust JA, Craigie JS (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 189–195
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549
Ledda C, Romero-Villegas GI, Adani F, Acién-Fernández FG, Molina-Grima E (2015) Utilization of centrate from wastewater treatment for the outdoor production of Nannochloropsis gaditana biomass at pilot-scale. Algal Res 12:17–25
Ledda C, Schievano A, Scaglia B, Rossoni M, Acién Fernández FG, Adani F (2016) Integration of microalgae production with anaerobic digestion of dairy cattle manure: an overall mass and energy balance of the process. J Clean Prod 112:103–112
Li X, Yuan Y, Cheng D, Gao J, Kong L, Zhao Q, Wei W, Sun Y (2018) Exploring stress tolerance mechanism of evolved freshwater strain Chlorella sp. S30 under 30 g/L salt. Bioresour Technol 250:495–504
Lincoln EP, Wilkie AC, French BT (1996) Cyanobacterial process for renovating dairy wastewater. Biomass Bioenergy 10:63–68
Lizzul AM, Lekuona-Amundarain A, Purton S, Campos LC (2018) Characterization of Chlorella sorokiniana, UTEX 1230. Biology (Basel). 7:25
Loera-Quezada MM, Olguín EJ, Manejo R De, Recursos B De, Ecología I De, Carretera a CIK (2010) Las microalgas oleaginosas como fuente de biodiesel: retos y oportunidades. Rev Latinoam Biotecnol Ambient Algal 1:91–116. 00000-00355
López González CV, del Cerón García MC, Acién Fernández FG, Segovia Bustos C, Chisti Y, Fernández Sevilla JM (2010) Protein measurements of microalgal and cyanobacterial biomass. Bioresour Technol 101:7587–7591
Markou G, Depraetere O, Muylaert K (2016) Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: a study on chlorophyll fluorescence and electron transport. Algal Res 16:449–457
Martínez F, Orús MI (1991) Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM 101. Plant Physiol 95:1150–1155
Ministerio de Agricultura P y A (1998) Métodos oficiales de análisis de la Unión Europea, Volume 2. Ministerio de Ministerio de Agricultura, Pesca y Alimentación, Secretaría General Técnica, Madrid, España
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Mutaf T, Oz Y, Kose A, Elibol M, Oncel SS (2019) The effect of medium and light wavelength towards Stichococcus bacillaris fatty acid production and composition. Bioresour Technol 289:121732
Nichols HW, Bold H (1965) Trichosarcina polymorpha gen. et sp. nov. J Phycol 1:34–38
Richert L, Golubic S, Le Guédès R, Ratiskol J, Payri C, Guezennec J (2005) Characterization of exopolysaccharides produced by cyanobacteria isolated from Polynesian microbial mats. Curr Microbiol 51:379–384
Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sust Energ Rev 92:394–404
Romero Villegas GI, Fiamengo M, Acién Fernández FG, Molina Grima E (2017) Outdoor production of microalgae biomass at pilot-scale in seawater using centrate as the nutrient source. Algal Res 25:538–548
Ruiz J, Olivieri G, de Vree J, Bosma R, Willems P, Reith JH, Eppink MHM, Kleinegris DMM, Wijffels RH, Barbosa MJ (2016) Towards industrial products from microalgae. Energy Environ Sci 9:3036–3043
San Pedro A, González-López CV, Acién FG, Molina-Grima E (2013) Marine microalgae selection and culture conditions optimization for biodiesel production. Bioresour Technol 134:353–361
Sepúlveda C, Acién FG, Gómez C, Jiménez-Ruíz N, Riquelme C, Molina-Grima E (2015) Utilization of centrate for the production of the marine microalgae Nannochloropsis gaditana. Algal Res 9:107–116
Singh AK, Sharma N, Farooqi H, Abdin MZ, Mock T, Kumar S (2017) Phycoremediation of municipal wastewater by microalgae to produce biofuel. Int J Phytoremed 19:805–812
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tsarenko P, John D (2011) Phylum Chlorophyta. Order Chlorellales. In: The freshwater algal flora of the British Isles. An identification guide to freshwater and terrestrial algae. 2nd edn. Cambridge University Press, Cambridge, pp. 475–499
Voltolina D, Gómez-Villa H, Correa G (2005) Nitrogen removal and recycling by Scenedesmus obliquus in semicontinuous cultures using artificial wastewater and a simulated light and temperature cycle. Bioresour Technol 96:359–362
Wang H, Xiong H, Hui Z, Zeng X (2012) Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour Technol 104:215–220
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Sninsky JJ, Gelfand DH, White TJ (eds) PCR Protocols. Elsevier, New York, pp 315–322
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Yamasaki T, Yamakawa T, Yamane Y, Koike H, Satoh K, Katoh S (2002) Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol 128:1087–1097
Yeh KL, Chang JS (2012) Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105:120–127
Acknowledgements
This research was carried out in the facilities of the University of Almería. Dr. Ruth Gabriela Ulloa Mercado donated the microalgae for experimental purposes.
Funding
This study received financial support of PROFAPI for carrying out this work. Dr. Gabriel Ivan Romero Villegas and Dr. Vianey Ariadna Charis Burboa contributions were financially supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT, México). Luis Alberto Cira Chavez received support from the SEP-SES, which was provided for conducting a research stay corresponding to the call “Short Stays for Research of Members of Consolidated Academic Bodies 2019.”
Author information
Authors and Affiliations
Contributions
Romero Villegas: conceptualization, methodology, validation, investigation, resources, writing the original draft, visualization, and project administration. Burboa Charis: writing the original draft. Navarro López: investigation and writing the original draft. Cerón Garcia: investigation, resources, and writing the original draft. Acién Fernandez: conceptualization and reviewing and editing supervision. Estrada Alvarado: reviewing and editing supervision. Rout N.P.: investigation and reviewing. Cira Chávez: funding acquisition and reviewing and editing supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Romero-Villegas, G.I., Burboa-Charis, V.A., Navarro-López, E. et al. Biomass production and urban centrate nutrient removal using native microalgae tolerant to high nitrogen concentration and temperature. J Appl Phycol 33, 2921–2931 (2021). https://doi.org/10.1007/s10811-021-02487-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02487-z