Abstract
Extractions of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) were carried out using a shaker and an ultrasound device. A rotational central composite design was applied for each device used. Citric acid concentration and extraction time were the factors investigated. Increased citric acid increased pectin yield obtained with both the shaker and the ultrasound. Increase extraction time had a negative effect on the yield in shaker and it had a positive effect on the yield in ultrasound. Pectin yield obtained with both devices were fitted to second-order polynomial models. The coefficients of determination (R2) were higher than 0.90 and the cross-validated R2 (Q2) were higher than 0.92. Higher yield (about 55 %) was obtained using 0.75 M citric acid concentration and sample submitted to ultrasound for 90 min. This was 81 % higher than the highest obtained from using the shaker. Diet gels containing calcium can be produced from the pectin extracted by ultrasound.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Passion fruit peel is a waste, which contains pectin [25, 27] and it has been receiving increasing attention in research for the development of new food products [6]. Pectin is mainly used in the food processing industry as a gel and thickening agent [4].
The esterification degree (ED), expressed as a percentage of the esterified carboxyl groups, is an important means to classify pectin, and has a major influence on the gel properties of pectin [35]. Depending on the ED, pectin is commercially divided into two groups: high-ester pectin, with ED higher than 50 %, and low-ester pectin, with ED lower than 50 % [29]. Pectin with an ED >50 needs sugar (such as sucrose) and acid (pH 2.0–3.5) to form gels, whereas pectin with an ED <50 needs calcium ions to form gels within a larger pH range (2.0–7.0) whether sugar is present or not [35].
The techniques most frequently used for extraction of pectin are: (1) acid extraction by stirring and heating, (2) heat refluxing extraction and (3) microwave heating extraction [4]. Different acids can be used in the acid extraction by stirring and heating. Nitric, hydrochloric and sulfuric acids are the most used [35]. This generally results in degradation of the arabinan side-chains and therefore in a loss of feruloyl groups, which are the key factors in cross-linking pectin [22]. Furthermore, the use of these strong acids leads to corrosion of equipment and has deleterious effects on the environment [27]. Therefore, organic acids are generally used to reduce the adverse effects of mineral acids.
Studies indicate that citric acid is a natural and safe food additive, and is also advantageous from an economic and environmental point of view [3]. The extraction of pectin from fruit peel using citric acid has been studied recently using passion fruit peel [10, 13, 25] and other vegetable matrices, such as mango peel [7], cacao pod husks [30], sugar beet pulp [12] and banana peel [21].
The use of an ultrasound device for extraction has increased recently, as it is seen to be efficient. This device uses low energy and consumes less solvent [15, 20, 34]. Ultrasound processing is widely used in the food industry due to its capability to induce chemical and physical changes in food components [1, 5, 24]. The increase of emulsifying capacity, the release and diffusion of cell material, and enhanced foaming are some of the improvements in food processing using sonication [28].
In this study, response surface methodology (RSM) was employed to study the extraction of pectin from passion fruit peel using two devices (shaker and ultrasound) to understand the combined effects of variables (citric acid concentration and extraction time) on pectin yield.
Materials and Methods
Yellow Passion Fruit Peel
Yellow Passion fruit (Passiflora edulis f. flavicarpa L.) was obtained from the local market in Igarassu, Pernambuco state, Brazil. The fruit was washed, cut and the pulp and seeds were removed. The peels were washed in tap water to remove the adhering pulp and soluble sugars. The passion fruit peels with mesocarp were dried in a hot air oven (NT514) at 55 °C overnight. The raw material was milled to an average particle size of 20 Mesh, using a commercial mill (Marconi). The fruit peel powder was packaged in a polyethylene bag and stored at −20 °C in a freezer. All the chemicals used in the study were of analytical grade and purchased from Sigma-Aldrich Chemicals, Brazil.
Pectin Extraction
The extraction procedure was based on the methodology reported by Kliemann et al. [9]. A dry mass of passion fruit peel flour (5 g) was subjected to extraction and 250 mL of citric acid was added. The citric acid concentrations and extraction times were according to the experimental design (Table 1). The suspensions were then incubated in a shaker at 50 °C and 150 rpm (New Brunswick Scientific C25 KC) or using an ultrasound without temperature control and without stirring (UltraSonic Cleaner-UNIQUE). For both processes, the same conditions of time and concentration of citric acid were applied. After extraction, the suspension was filtered using filter qualitative paper of 12.5 cm diameter and the filtrate was cooled down to 4 ± 1 °C for 60 min. The filtrate was coagulated using an equal volume of 96 % (V/V) ethanol and left for 1 h. The coagulated pectin was separated by filtration through a nylon cloth, washed first with 70 % (v/v) acidic ethanol (0.5 % HCl), then with 70 % (V/V) ethanol to neutralize the pH and finally with 96 % (V/V) ethanol. After this process, the resulting material was dried overnight at 55 °C in an air-forced oven. Pectin yield (Y) was expressed as the ratio of the amount of extracted pectin (M in grams) obtained after extraction to the initial amount of passion fruit peel powder (5 g) used for extraction (Eq. 1).
Experimental Design
In this study, rotational central composite designs with two factors (citric acid concentration and extraction time) were carried out for passion fruit peel flour pectin extraction process using a shaker and an ultrasound device. The extraction time and citric acid concentration varied according to Table 1.
The experimental ranges for the factors were determined according to the results obtained by Kliemann et al. [9]. The whole design consisted of 10 experimental points, two replicates at the central point of the design, which were used to estimate a pure error sum of squares. All the experiments were randomly carried out in order to minimize the effect of unexplained variability due to systematic errors. The experimental design and data analysis were performed using Statistica software 7.0, for both devices (shaker and ultrasound). The response variables were fitted to two quadratic polynomial models, the general form of which was as follows:
where \( \hat{y} \) is the pectin yield simulated by Eq. (2); X1 and X2 are the factors; b0 is the model intercept coefficient; b1, b2, b11, b22 and b12 are interaction coefficients of linear, quadratic and the second-order terms, respectively [19].
Esterification Degree
Esterification degree (ED), number of esterified carboxyl groups per the total number of carboxyl group (Eq. 3), was determined by potentiometric titration, as described by Bochek et al. [2].
A dry portion of the extracted pectin (0.2 g) was placed in an Erlenmeyer for titration and wet with ethanol. Distilled water heated at 40 °C (20 mL) was added and the mixture was stirred for 2 h. The resulting solution was titrated with 0.1 N NaOH. The number of free carboxyl groups (K f ) was calculated using Eq. (4).
where n = f (free) or e (esterified); a = portion of pectin with absorbed water (g); NNaOH = concentration of NaOH (N); VNaOH = volume (mL) of NaOH spent for titration.
To determinate the number of esterified carboxyl groups (Ke), a 0.1 N NaOH solution (10 mL) was added to a neutralized polygalacturonic acid sample after determination of the free carboxyl groups. The number of the esterified carboxyl groups was calculated from the volume of 0.1 NaOH solution spent for titration (Eq. 4).
Results and Discussion
The values of responses (yield of pectin) under different experimental combinations are given in Table 2. Depending on the experimental conditions, a high yield pectin (more than 40 %) could be achieved. The pH remained between 1.5 and 2.2 for all citric acid concentrations. Low pH stimulated a hydrolysis of protopectin, which is a compound formed by the combination of cellulose with pectin molecules [8]. Therefore, pH can be considered as one of the more crucial parameters affecting the yield of extracted pectin [13].
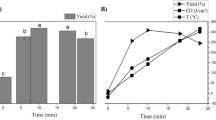
Regardless of the acid concentration (experiments 1, 2 and 9), the relative standard deviation of yield obtained with both devices was less than 10 %, when the extraction time was less or equal to 30 min. This indicates that there was no significant variation in the yield of experiments 1, 2 and 9, for each combination of concentration and time. For these three experiments, the highest yield (about 30 %) was found when 0.75 M and 30 min were used (experiment 2). On the other hand, when the concentrations varied from 0.15 to 0.5 M, the relative standard deviation was higher than 10 % (between 18 and 41 %), for 60 or 90 min (experiments 3, 5, 6 and 7). Given these conditions, the highest yields were obtained with ultrasound. Finally, for extraction time higher or equal to 90 min and concentrations higher or equal to 0.5 M (experiments 4, 8 and 10), the relative standard deviation was higher than 50 %, indicating an even higher difference between the devices used.
Differences in performance of these two devices, can be related to the type of energy used in the extraction process. The increase yield of pectin was observed for extractions conducted under ultrasound and this effect may be due to the changes occurring in plant tissues, for example, a rupture of the parenchymal cells of the plant material [17]. However, this was only observed when the extraction time was higher than 30 min.
Studies have shown that the application of ultrasound can help the extraction of pectin from different vegetable raw materials in addition to the passion fruit. Among these are included grapefruit [32, 34], tomato waste [4], grape pomace [18], sisal waste [15], and pomegranate peel [20].
Equation (2) was fitted to the experimental data. The predicted response Y for the yield of pectin could be obtained by the following regression models for the shaker (Eq. 5) and the ultrasound (Eq. 6) devices.
The coefficients of determination (R2) of the models for both devices were 0.9195 (shaker) and 0.9749 (ultrasound), which further indicated that the models were able to represent the real relationships among the selected variables. The cross-validated R2 (Q2) were 0.9206 and 0.9601, for shaker and ultrasound, respectively. This parameter describes the goodness of prediction, showing how well new experiments can be predicted using this mathematical model. R2 and Q2 values higher than 0.75 and 0.60, respectively, indicate that the model is acceptable [14].
Response surface of the pectin yield as a function of independent variables (citric acid concentration and extraction time) is shown in Fig. 1 for the shaker. Increase citric acid concentration increased the yield. On the other hand, increased extraction time decreased the yield and this effect is shown in the model as the linear coefficient of x2, since it was negative. These effects were significant according to the t test (95 % confidence) calculated using Statistica.
The response surface of the pectin yield obtained in ultrasound is shown in Fig. 2. Increase citric acid concentration or extraction time increased the yield. All the effects of variables on the yield were significant according to a t test (95 % confidence). The higher yield pectin (54.69 %) was found in ultrasound, when using 0.75 M citric acid, for 90 min at 27 °C. This was higher than yields reported in the literature for extraction of pectin from passion fruit peel. Kulkarni and Vijayanand [10] found yield (14.8 %) when using hydrochloric acid and hot water (60–100 °C). Liew et al. [13] found that the pectin yield ranged from 2.25 to 14.60 %, using shaking with water at 70 °C, for 75 min and with citric acid.
Heating extraction has disadvantages for pectin extraction, since it can lead to the degradation of pectin [34]. In the present work, the highest temperature in the shaker in relation to that used in ultrasound may have contributed to a lower yield, when the extraction time was increased. In ultrasound extraction, increased temperature can increase pectin yield due to increased solubility. However, the increase of pectin yield can decrease when the temperature is higher than 80 °C [16, 26].
The ED ranged from 18 % (0.75 M citric acid and 90 min) to 30 % (0.15 M citric acid and 60 min), when the ultrasound device was used. Extracted pectin was only good for the preparation of Ca2+ mediated low-ester pectin gels. ED vary with fruit peels, extraction parameters and extractors used [13]. Low-ester pectin forms gels in a higher pH range than high-ester pectin, without requiring the presence of sugar [33], therefore, may be used as a gelling agent in low sugar products, such as low-calorie jams and jellies, confectionery jelly products, and other foods applications [31]. In general, pectin extracted from vegetables exhibits methoxyl content between 10 and 12 % [23]. The extraction of pectin yellow passion fruit peel with hot water or oxalate acidified with nitric acid resulted in pectin with ED <50 % [36, 37].
Conclusion
Extraction of pectin from passion fruit peel was investigated by RSM. The surfaces response disclosed the relationships between citric acid concentration and extraction time. This generated pectin yields for shaker and ultrasound study. Using these surfaces, a satisfactory condition of 0.75 M citric acid concentration, regardless of the equipment used and extraction time of 30 or 90 min for shaker (30.2 %) or ultrasound (54.7 %), respectively, were established. The extraction of pectin from yellow passion fruit using ultrasound and citric acid results in acceptable pectin that can be used to form dietetic gels containing calcium.
References
Barba, F.J., Grimi, N., Vorobiev, E.: Evaluating the potential of cell disruption technologies for green selective extraction of antioxidant compounds from Stevia rebaudiana Bertoni leaves. Food Eng. 149, 222–228 (2015)
Bochek, A.M., Zabivalova, N.M., Petropavlovskii, G.A.: Determination of the esterification degree of polygalacturonic acid. Russ. J. Appl. Chem. 74(5), 796–799 (2001)
Canteri-Schemin, M.H., Fertonani, H.C.R., Waszczynskyj, N., Wosiacki, G.: Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 48(2), 259–266 (2005)
Grassino, A.N., Halambek, J., Djakovic, S., Brncic, S.R., Dent, M., Grabaric, Z.: Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 52, 265–274 (2016)
Herceg, Z., Brnčić, M., Režek, A.J., Brnčić, S.R., Badanjak, M., Sokolić, I.: Possibility of application high intensity ultrasound in milk industry. Mljekarstvo 59, 65–69 (2009)
Ishimoto, F.Y., Harada, A.I., Branco, I.G., Conceição, W.A.S., Coutinho, M.R.: Alternative use of yellow passionfruit skin (Passiflora edulis f. var. flavicarpa Deg.)for the production of cookies. RECEN 9(2), 279–292 (2007)
Kermani, Z.J., Shpigelman, A., Pham, H.T.T., Loey, A.M.V., Hendrickx, M.E.: Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 44, 424–434 (2015)
Kertesz, A.J.: The Pectin Substance. Interscience, New York (1951)
Kliemann, E., Simas, K.N., Amante, E.R., Prudêncio, E.S., Teófilo, R.F., Ferreira, M.M.C., Amboni, R.D.M.C.: Optimisation of pectin acid extraction from passion fruit peel (Passiflora edulis flavicarpa) using response surface methodology. Int. J. Food Sci. Technol. 44, 476–483 (2009)
Kulkarni, S.G., Vijayanand, P.: Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Sci. Technol. 43, 1026–1031 (2010)
Laufenberg, G., Kunz, B., Nystroem, M.: Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 87, 167–198 (2003)
Li, D., Du, G., Jing, W., Li, J., Yan, J., Liu, Z.: Combined effects of independent variables on yield and protein content of pectin extracted from sugar beet pulp by citric acid. Carbohydr. Polym. 129, 108–114 (2015)
Liew, S.Q., Chin, N.L., Yusof, Y.A.: Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Procedia 2, 231–236 (2014)
Mandenius, C.F., Brundin, A.: Review: biocatalysts and bioreactor design. Biotechnol. Prog. 24, 1191–1203 (2008)
Maran, J.P., Priya, B.: Ultrasound-assisted extraction of pectin from sisal waste. Carbhydr. Polym. 115, 732–738 (2015)
Mason, T.J., Lorimer, J.P.: Applied sonochemistry: the uses of power ultrasound in chemistry and processing. Chem. Technol. Biotechnol. 79, 207–208 (2004)
Mason, T. J., Riera, E., Vercet, A., Lopez-Buesa, P. Application of Ultrasound, Introduction to Food Engineering. pp. 325-350. Oxford (2005)
Minjares-Fuentes, R., Femenia, A., Garau, M.C., Meza-Velazquez, J.A., Simal, S., Rosselló, C.: Ultrasound-assisted extraction of pectins from grape pomace using citric acid: a response surface methodology approach. Carbohydr. Polym. 106, 179–189 (2014)
Moorthy, I.M.G., Baskar, R.: Statistical modeling and optimization of alkaline protease production from a newly isolated alkalophilic Bacillus species BGS using response surface methodology and genetic algorithm. Prep. Biochem. Biotech. 43, 293–314 (2013)
Moorthy, G., Maran, J.P., Surya, S.M., Naganyashree, S., Shivamathi, C.S.: Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int. J. Biol. Macromol. 72, 1323–1328 (2015)
Oliveira, T.Í.S., Rosa, M.F., Cavalcante, F.L., Pereira, P.H.F., Moates, G.K., Wellner, N., Mazzetto, S.E., Waldron, K.W., Azeredo, H.M.C.: Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 198, 113–118 (2016)
Oosterveld, A., Beldman, G., Schols, H.A., Voragen, A.G.: Arabinose and ferulic acid rich pectic polysaccharides extracted from sugar beet pulp. Carbohydr. Res. 288, 143–153 (1996)
Padival, R.A., Ranganna, S., Manjrekar, S.P.: Mechanism of gel formation by low methoxyl pectins. J. Food Technol. 14(3), 277–287 (1979)
Pingret, D., Fabiano-Tixier, A.S., Le Bourvellec, C., Renard, C.M.C.G., Chemat, F.: Lab and pilot-scale ultrasound-assisted water extraction of polyphenols from apple pomace. Food Eng. 11, 73–81 (2012)
Pinheiro, E.R., Silva, I.M.D.A., Gonzaga, L.V., Amante, E.R., Teófilo, R.F., Ferreira, M.M.C., Amboni, R.D.M.C.: Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 99, 5561–5566 (2008)
Raso, J., Manas, P., Pagan, R., Sala, F.J.: Influence of different factors on the output power transferred into medium by ultrasound. Ultrason. Sonochem. 5, 157–162 (1999)
Seixas, F.L., Fukuda, D.L., Turbiani, F.R.B., Garcia, P.S., Petkowicz, C.L.O., Jagadevan, S., Gimenes, M.L.: Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocoll. 38, 186–192 (2014)
Šic Žlabur, J., Voća, S., Dobričević, N., Dujmić, F., Brnčić, M., RimacBrnčić, S.: Optimization of ultrasound assisted extraction of functional ingredients from Stevia rebaudiana bertoni leaves. Int. Agrophys. 29, 231–237 (2015)
Thakur, B.R., Sing, R.K., Handa, A.K.: Chemistry and uses of pectin e a reviews. Crit. Rev. Food Sci. Nutr. 37(1), 47–73 (1997)
Vriesmann, L.C., Teófilo, R.F., de Petkowicz Oliveira, C.L.: Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. Food Sci. Technol. LEB 49, 108–116 (2012)
Wai, W.W., Alkarkhi, A.F.M., Easa, A.M.: Effect of extraction conditions on yield and degree of esterification of durian rind pectin: an experimental design. Food Bioprod. Process. 88, 209–214 (2010)
Wang, W., Ma, X., Xu, Y., Cao, Y., Jiang, Z., Ding, T., Ye, X., Liu, D.: Ultrasound-assisted heating extraction of pectin from grapefruit peel: optimization and comparison with the conventional method. Food Chem. 178, 106–114 (2015)
Wicsenborn, D.P., Wang, J., Chang, K.C., Schwarz, J.G.: Comparison of continuous and batch processes for pectin extraction from sunflower heads. Ind. Crops Prod. 19, 171–181 (1999)
Xu, Y., Zhang, L., Bailina, Y., Ge, Z., Ding, T., Ye, X., Liu, D.: Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 126, 72–81 (2014)
Yapo, B.M.: Lemon juice improves the extractability and quality characteristics of pectin from yellow passion fruit by-product as compared with commercial citric acid extracting. Bioresour. Technol. 100, 3147–3151 (2009)
Yapo, B.M., Koffi, K.L.: Yellow passion fruit rind: a potential source of low-methoxyl pectin. J. Agric. Food Chem. 54, 2738–2744 (2006)
Yapo, B.M., Koffi, K.L.: Dietary fiber components in yellow passion fruit rind: a potential fiber source. J. Agric. Food Chem. 56, 5880–5883 (2008)
Zhang, W., Xub, P., Zhang, H.: Pectin in cancer therapy: a review. Trends Food Sci. Technol. 44, 258–271 (2015)
Acknowledgments
The authors acknowledge the financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasilia DF, Brazil (CNPq). The English text of this paper was revised by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip - TESL (Cambridge University).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dos Santos, E.K.R., Azoubel, P.M. & Gouveia, E.R. Better Pectin Yield From Passion Fruit Peel (Passiflora edulis f. flavicarpa): From Shaker or Ultrasound? A Comparison. Waste Biomass Valor 8, 905–910 (2017). https://doi.org/10.1007/s12649-016-9611-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9611-4