Abstract
Schistosomiasis is a neglected tropical disease with considerable morbidity. The lone effective drug, praziquantel (PZQ), is showing emergence of drug resistance hence, searching for new supportive treatment is crucial. This study aimed to evaluate the efficacy of mucus and nucleoproteins (NPs) extracted from Biomphalaria alexandrina (B. alexandrina) snails on miracidia, cercariae and Schistosoma mansoni (S. mansoni) adults in vitro and assess their experimental in vivo effect through parasitological, histopathological, and biochemical parameters. The in vivo study included 90 male Swiss albino mice. Mice were grouped into 9 groups; G1-G5 were infected and treated with; GI: PZQ, GII: mucus, GIII: combined PZQ and mucus, GIV: NPs, GV: combined PZQ and NPs. Control groups; C1: Non infected non treated (negative control), C2: Infected non treated (positive control), C3: Non infected mucus treated and C4: Non infected NPs treated. The in vitro study proved that the mucus had a better lethal effect on cercariae than miracidia, while NPs had better lethal effect on miracidia. The mucus lethal effect on adults surpassed the NPs as 100% and 60%, respectively. The in vivo study proved that the combined NPs or mucus with PZQ added to the effect of individual PZQ resulting in 100% total worm burden (TWB) reduction. As regard oxidative stress markers, the lowest level of nitric oxide (NO) was shown with combined PZQ and NPs. While, the highest glutathione (GSH) level was produced by individual PZQ. The study concluded that mucus and NPs of B. alexandrina had cercaricidal, miracidicidal and anti-schistosomal effect in vitro and that their combination could be considered a contribution to PZQ potentiality in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a prevalent neglected parasitic disease in tropical and sub-tropical areas accounting for the second place in terms of socioeconomic and public health burden (Cardoso et al. 2013). The disease afflicts up to 600 million people in 74 tropical and sub-tropical countries, predominantly in the developing world (El Ridi et al. 2014). Morbidity is associated with many complications; the most important of these is liver damage (WHO 2010). Among the different schistosome species, S. mansoni is the most abundant in Egypt (Helmy et al. 2009).
Pathology associated with S. mansoni results primarily from the accumulation of parasite eggs, giving rise to hepatomegaly that may be superseded by extensive liver fibrosis (Gryseels et al. 2006).. Several medications are used in the treatment of schistosomiasis including praziquantel (PZQ), oxamniquine, metrifonate, antimonials, hycanthone and niridazole. PZQ is the treatment of choice and is effective against adult worms but much less so against juvenile stages like schistosomula, pre-adults and juvenile adults (Keiser et al. 2009). So, in areas of constant reinfection, PZQ might effectively kill adult worms but immature worms would then develop and present as adults, implying drug failure. In such settings, repeated PZQ treatment 3–6 weeks apart kills initially resistant juvenile worms and improves drug treatment (King et al. 2011). In addition, many lines of evidence indicate the emergence of strains of S. mansoni resistant to PZQ, so for controlling schistosomiasis, there is an urgent need to develop a new supportive drug (Zhang and Coultas 2013).
Artemisinin derivatives (such as artemether and artesunate) which were developed as antimalarial drugs, also kill immature larval forms of developing schistosomes (Utzinger et al. 2000). However, because the time of cercarial exposure is normally unknown, the drug's use is limited. In areas of continuous transmission, artemisinin derivatives could be used in conjunction with PZQ to improve overall cure rates and infection control (Del Villar et al. 2012). Oxamniquine is a tetrahydroquinolone compound that was used effectively against S. mansoni, but it had side effects of heightened seizure activity in patients with underlying epilepsy (Keystone 1987).
Cell-based therapy is emerging as a promising therapeutic approach for a wide range of liver diseases. A study performed by El-Shennawy et al. (2015) investigate the regenerative and antifibrotic therapeutic potential of bone marrow derived mesenchymal stem cells (BM-MSCs) in early and late experimental hepatic schistosomiasis model. The regenerative and antifibrotic potential of BM-MSCs was evaluated by histopathological examination, morphometric analysis, electron microscopy as well as liver function tests. Schistosoma-infected mice, which were treated with BM-MSCs, showed decrease in the granuloma size, percentage and density of the fibrotic area, formation of new hepatocytes and improvement of the liver function tests. Immunohistochemical examination of alpha-smooth muscle actin showed a significant decrease in the immunoreactive hepatic stellate cells in mice treated with MSCs. Moreover, an experimental study performed by Bebars et al. (2020) investigated the regenerative and antifibrotic potential of Human Umbilical Cord Blood derived Mesenchymal Stem Cells (HUCB-MSCs) in hepatic granuloma due to schistosomiasis mansoni in experimental murine model, which showed an improvement in the histopathological picture of the liver with diminution in the size of granulomas and the overall fibrotic content. Hyperplasic changes in intrahepatic biliary radicals were decreased in test groups revealing that human umbilical cord blood-derived MSCs are promising and effective therapy for the treatment of diseased or damaged liver tissues. Another experimental study performed by Abou Rayia et al. (2023) assessed the effect of HUCB-MSCs transplantation on schistosomal hepatic fibrosis in mice in comparison to PZQ. It was found that the treated PZQ and HUCB-MSCs groups showed a substantial improvement, with a significant difference regarding the histopathological evaluation of liver fibrosis in the MSCs-treated group, which denote that MSCs could be a promising and efficient cell therapy for liver fibrosis. On the other hand, the diversity of MSCs and their traits with their different clinical applications have not been thoroughly investigated, with controversial opinions about these cells (Musiał-Wysocka et al. 2019). However, recent experience has accumulated numerous reports of adverse events and side effects associated with MSCs therapy. The risks caused by weaknesses in cell processing, including isolation, culturing, and storage. Cell processing and cell culture could dramatically influence cell population profile, change protein expression and cell differentiation paving the way for future negative effects. Long-term cell culture led to accumulation of chromosomal abnormalities. Overdosed antibiotics in culture media enhanced the risk of mycoplasma contamination. Clinical trials reported thromboembolism and fibrosis as the most common adverse events of MSCs therapy (Baranovskii et al. 2022).
A study performed by Mohamed et al. (2023) evaluated the possible additive effect of ursodeoxycholic acid (UDCA) as a cholagogue with PZQ on experimental schistosomiasis mansoni. revealed that UDCA has an auspicious additive effect to PZQ to decrease the worm burden, and the load of ova in both the intestinal wall and other tissues, and to decrease the number and diameter of granulomas due to infection with S. mansoni. Although UDCA is reported to have hepato-protective properties, yet UDCA has unanticipated toxicity as toxicity profile includes fever, hepatitis, cholangitis, vanishing bile duct syndrome, liver cell failure, death, severe watery diarrhea, pneumonia, interstitial lung disease, convulsions and mutagenic effects (Kotb 2012).
The epidermal mucus covering the surface of a snail represents an important barrier to trematode larvae attempting to penetrate the snail and may play a role in mediating snail-trematode compatibility. Facioloides magna miracidia were exposed to mucus harvested from a compatible snail host, Lymnaea elodes (L. elodes) and from an incompatible snail, Helisoma trivolvis (H. trivolvis). In vitro treatment of freshly hatched miracidia with snail-derived mucus exerted dramatically different effects on larvae depending on snail species. At the lowest dilution of mucus tested (1:3) mean damage rates (tegumental damage and/or larval lysis and death) were as high as 100% for miracidia exposed to H. trivolvis mucus, while none of F. magna miracidia were damaged in L. elodes mucus. This indicated the presence of a potent cytotoxic protein-like factor in the mucus of F. magna–incompatible H. trivolvis, and its absence in the mucus of the compatible snail, L. elodes. This finding supported the notion that the epidermal mucus layer may be serving as an important determinant of larval trematode-snail compatibility (Coyne et al. 2015).
Another study described the effective antileishmanial activity of crude cutaneous secretion from the giant African snail, Achatina fulica, and its mammalian cells cytotoxicity and also its potential mode of action against Leishmania promastigotes. The crude secretion showed a 50% Effective Concentration (EC50) of 98.37 µg/mL against Leishmania chagasi promastigotes. By means of enzymatic assays, L-amino acid oxidase (L-AAO) activity was detected in crude secretion, and the hydrogen peroxide produced by this enzyme revealed to be one of the compounds responsible for the antileishmanial effect (Tempone 2007).
The snail mucus of B. alexandrina has been of worldwide increase, in cosmetic products and in the management of wounds and treatment of chronic bronchitis (Tsoutsos et al. 2009). It possesses important biological properties such as antimicrobial activity and protective effects in wound repair (Bortolotti et al. 2016). It plays a fundamental role in homeostasis and protection of the gastric mucosa. Some specific components are mostly present in the snail secretion filtrate such as collagen, elastin, glycolic acid and allantoin (Da Silva 2018).
Snail NPs are conjugated with nucleic acids (either DNA or RNA). Typical NPs include ribosomes and nucleosomes. These complexes play an integral part in several important biological functions that include transcription, translation, regulating gene expression and regulating the metabolism of RNA (Lukong et al. 2008; Hogan et al. 2008). Dietary nucleotides have a therapeutic effect on human liver cirrhosis. Consequently, the combination of susceptible snails’ nucleotide with one of the anti-schistosome drugs may be of future impact (El-Rigal et al. 2011).
The NPs extracted from either susceptible or resistant B. alexandrina snails were evaluated for protection against schistosomiasis. The NP of susceptible snails showed reduction in worm and ova counts by 70.96% and 51.31%, respectively, whereas the NP of resistant snails showed reductions of 9.67% and 16.77%, respectively. It was found that the NPs of susceptible snails were more effective in protecting against schistosomiasis (Hamed et al. 2010).
Hence, it is worth evaluating mucus and NPs of B. alexandrina snails as antischistosomal agents.
Materials and methods
Preparation of mucus and NPs for the in vitro study
Extraction of mucus from B. alexandrina snails (Abd-ElAzeem et al. 2020)
The 200 ml pooled mucus extracted from 200 snails was lyophilized into a powder (Akers et al. 1987), where every 200 ml corresponded to 60 mg powder. The powder content was chemically analysed by Gas Chromatography-Mass Spectrometry (GC/MS) (Jones 2019) revealing; Indole, Trimethylsilyl derivative (TMS), Lactic Acid, 2TMS derivative, Diphenyl phosphine, Silanol, trimethyl-carbonate (2:1), Oxalic acid, 4-Hydroxybutanoic acid, Urea, Glycerol, 3TMS derivative, Myo-Inositol, 6TMS derivative in first used sample using dH2O and N-benzyliden-4-nitrobenzenesulphenamide Urea, 2 TMS derivative, Silanol, trimethyl-, phosphate (3:1) in second sample using PBS.
Extraction of tissue NPs from B. alexandrina (Lee et al. 1988; Zerivitz and Akusjärvi 1989)
Snail tissue of a 5-gm weight was cut into small pieces then washed with cold phosphate buffered saline (PBS) and centrifuged at 500 g at 4 °C for 5 min. The supernatant was discarded to leave the pellet as dry as possible, where the tissue was homogenized using a homogenizer. The tube was vortexed vigorously for 15 s to fully suspend the cell pellet. The tube was incubated on ice for 10 min and then vortexed again for 5 s. The tube was centrifuged at 16,000 g at 4 °C for 5 min, and immediately the supernatant was transferred into a sterile 1 ml Eppendorf tube. The pellet was suspended with 0.75 ml cold PBS and the tube was vortexed for 15 s. The tube was incubated on ice for 40 min and was vortexed for 15 s every 10 min. The tube was centrifuged at 16,000 g at 4 °C for 10 min and the supernatant was transferred immediately into a sterile Eppendorf tube. The residual pellet was re-extracted with 0.25 ml cold PBS.
Determination of the protein content for mucus and NPs (Bradford 1976)
Bovine serum albumin (BSA) (0.1 gm) was dissolved in 10 ml 1 M Guanidinium Chloride (GdmCl) to achieve final concentration of 1 mg/ml. Serial dilutions were performed. From the curve, the concentration of the extracted sample was calculated to be 7000 µg/ml. That was equivalent to 7 µg/µl. Since the best concentration from the curve was corresponding to 150 µg/ml. Calculation was done that resulted in an optimum concentration of 150 µg in 21 µl.
The in vitro study
Snail and parasite collection
B. alexandrina snails were obtained from the laboratory-bred stock in Medical Malacology Laboratory at Theodor Bilharz Research Institute (TBRI). S. mansoni miracidia were obtained from cleaned eggs extracted from the intestines of infected mice by hatching them in dechlorinated tap water (25 ± 1 °C) (Eissa et al. 2011). S. mansoni cercariae were recovered from laboratory-bred infected B. alexandrina snails 20 to 30 days after exposure to miracidia. Infected snails were collected maintained and furtherly processed (Pellegrino et al. 1962). S. mansoni adults were collected from infected hamsters (80–100 g) infected percutaneously with 350–400 cercariae and worms were cleared from the blood of maturely infected hamsters by perfusion technique (Yoles et al. 1947; Smithers and Terry 1965).
Detection of miracidial death rate
Multi-well plates were used, where 100 miracidia were picked using a 100 μl pipette (Aly et al. 2020). Each chosen concentration of mucus was taken from the lyophilized extract and was dissolved optimally in 50 ml distilled water (Onyema 2019). The concentration of NPs (7 µg/µl) was adjusted to a final concentration of 150 µg /100 µl PBS. A titer of concentrations (1, 2, 5, 15 mg/50 ml DW) were used for mucus and (0.01, 0.04, 0.05, 0.08, 0.1, 1, 2 ml) for NPs. The miracidia were observed under a dissecting microscope for survival and mortality at successive intervals of (2, 5, 10, 13, 15, 20, 25, 30, 45, 60, 75 min). Negative control was set up using de-chlorinated water (Aly et al. 2020). LC50 and LC90 were calculated.
Detection of cercaricidal rate
One hundred freshly shed cercariae, were transferred into each petri-dish using a micropipette. The same number of cercariae were placed in a petri-dish containing de-chlorinated water as a control group (Holtfreter et al. 2011). Doses of mucus and NPs were used as previously mentioned in lethal miracidial rate detection (Aly et al. 2020). The cercariae were observed under a dissecting microscope for survival and mortality at successive intervals of (2, 5, 10, 13, 15, 20, 25, 30, 45, 60, 75 min). They were considered dead when they stopped movement, sank down or detached their tail. At the end of each experiment, the total number of cercariae were counted (Holtfreter et al. 2011). LC50 and LC90 were calculated.
Detection of effect on S. mansoni adults (Yousif et al. 2007)
A stock solution of the mucus and NPs were added to 10% dimethyl sulfoxide (DMSO) on a weight/volume basis, by dissolving 1 mg of the compound in 1 ml of solvent to produce 10,000 µg/ml. The worms were exposed to this concentration in sterilized tissue 24 wells culture plates. The culture medium used for washing the worms as well as during the tests was (RPMI 1640) to which 20% fetal calf serum and 300 µg Streptomycin, 300 units Penicillin, and 160 µg Gentamycin/ml of medium were added. Three pairs of Schistosoma worms; males and females equally represented were placed in each well. Positive and negative controls were concurrently used. In the negative controls, 3 wells containing similar numbers of worms in pure medium containing the corresponding concentration of DMSO were used. Test and control wells were examined for 24 h. for worm viability using a stereomicroscope. Worms that did not show any sign of motility for one minute were considered dead. The activity of mucus and NPs were measured by calculating the number of dead worms relative to the total number of worms and compared with the negative control for the determination of LC50 and LC90.
In vitro selective action of mucus and NPs
The same experiment was repeated several times using descending concentrations of mucus (100, 80, 60, 40, 20 ug/ml DMSO) and NPs (100, 80, 60, 40, 20 ul/ml DMSO) of B. alexandrina and the viability of worms was followed up for four days. The worm mortality was recorded in each case.
The in vivo study
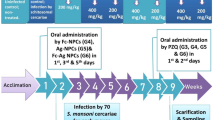
Animal grouping and study design
The study included 90 male Swiss albino mice around 6 to 8 weeks old, weighing 18 to 22 g. Mice were divided into 9 groups; 10 mice each:
Group I: Infected and treated with PZQ.
Group II: Infected and treated with mucus.
Group III: Infected and treated with combined PZQ and mucus.
Group IV: Infected and treated with NPs.
Group V: Infected and treated with combined PZQ and NPs.
Group C1: Non infected non treated (negative control).
Group C2: Infected non treated (positive control).
Group C3: Non infected mucus treated.
Group C4: Non infected NPs treated.
Animals were caged separately according to their group, housed in a controlled temperature and light environment, and were given water and commercial chow ad libitum. Then mice were experimental infected (Liang et al. 1987) after cercariae collection (Pellegrino et al. 1962; Kiros et al. 2014). All groups received treatment 6 weeks post-infection (wpi). Mice were given subcutaneous injection of the selected NPs or mucus (150 µg /100 µl PBS/mouse) (Mathaes et al. 2016). All mice were sacrificed 8 wpi to recover worms for counting and distribution.
Drugs preparations
-
PZQ tablet was ground into powder and suspended in 2% Cremophore-El (Sigma Chemical Co., St. Louis, MO, USA), freshly prepared before administration. It was administered by oral gavage using a stainless-steel oral cannula to each mouse in doses related to individual host body weights (500 mg/kg) for 2 days (Sabry et al. 2004).
-
Mucus and NPs amount of 100 µl was prepared for each (the maximum amount to be injected in vivo per mouse) (Turner et al. 2011). Considering that the average weight of each mouse was 20 g, the volume of fluids administrated subcutaneously per mouse was calculated as (5 ml/kg = 100 µl /mouse). The lyophilized mucus extract (500 mg) was dissolved in 10 ml PBS. That was parallel to 500,000 µg /10000 µl PBS which was equivalent to 50 µg /1 µl, then a concentration outcome of 150 µg /3 µl PBS was calculated. The concentration was completed to 100 µl by adding 97 µl PBS to reach a final concentration of 150 µg /100 µl PBS/mouse. NPs were administered SC in a dose of 150 µg /100 µl PBS /mouse from the previously prepared vials. PBS was the suitable solvent used for mucus and NPs in vivo (Nebendahl 2000).
Evaluation of antischistosomal activity
-
Parasitological Parameters by assessing TWB (Al-Kazzaz et al. 2016) were evaluated, the percentage of reduction of worm burden in all infected groups (Fallon et al. 1995), tissue egg count/gm intestine and liver was done (Cheever 1968) and the percentage of reduction of ova count in intestine and liver in all infected groups was calculated (El-Ansary et al. 2007). Oogram pattern was studied (Pellegrino and Faria 1965) and ova were classified according to their stages of development (mature, immature, and dead) using (× 40 objective).
-
Histopathological parameters (Bancroft and Steven 1975) were done to assess the number, diameter, type of granulomas (cellular, fibrous, fibro-cellular) and state of egg (intact or degenerated). Mean granuloma number was calculated (Mahmoud and Warren 1974). Liver sections were graded according to the criteria of hepatocytes affected and for inflammation and vacuolation as mild (0–25%), moderate (26–50%) or severe (> 51%).
-
Biochemical oxidative stress evaluation
-
Estimation of NO levels was done in liver tissues by using a NO assay kit (ENZO Life Sciences, Catalogue no. ADI-917–020). According to the kit instructions, Quantification is performed by measuring absorption at 540–570 nm.
-
Estimation of GSH levels which were determined using a glutathione Colorimetric Detection Kit (Detect X®, Arbor-Assays, Catalog No. K006-H1) in the liver homogenates following the method of Ellman (1959), based on the reduction of Ellman’s reagent with GSH to produce a yellow compound.
Statistical analysis
Recorded data were analyzed using, SPSS Inc., Chicago, Illinois, USA version 23.0. The quantitative data were presented using; ANOVA, Post-Hoc test: LSD, Kruskal Wallis test., Mann Whitney U test and Chi-square (x2) test of significance compare proportions between qualitative parameters. The confidence interval was set to 95% and the margin of error accepted was set to 5%. P-value < 0.05 was considered significant, P-value < 0.001 was considered highly significant and P-value > 0.05 was considered insignificant. LC50 and LC90 were calculated by linear regression to test and estimate the mortality percentage of a quantitative variable based on its relationship to concentration.
Results
Results of the in vitro study
Concerning the effect of mucus on cercaria and miracidia, there was a highly significant relation (P < 0.001) among the different concentrations along successive intervals. There was a positive relationship between concentration of mucus and death of cercariae and miracidia. However, there was an inverse relation between the concentrations of mucus along the increased time intervals. It was noted that mucus had a better lethal effect on cercariae than miracidia (Table 1).
Concerning the lethal effect of NPs on cercaria and miracidia there was a highly significant relation (P < 0.001) among the different concentrations along the time intervals. It was noted that increasing concentration of NPs was accompanied by an increase of cercaricidal effect and lethal effect on miracidia in shorter time intervals. There was a positive relationship between the concentration of NPs and death of cercariae and miracidia. However, there was an inverse relationship between concentrations of NPs along the time intervals. It was noted that NPs had better lethal effect on miracidia than on cercariae in most of the concentrations along the different time intervals (Table 2).
As regard the effect of mucus on S. mansoni adult males and females, there was a highly significant difference (P < 0.001) among the different concentration of mucus (100, 80 and 60 µg/ml) along the 3 day-interval. The results of lethal effect of mucus on females and males were almost equivocal except for the effect after 72 h where the effect of mucus on females was better than on adult males. As regard the effect of mucus on males, the LC50 was 69.91 µg/ml and the LC90 was 110.93 µg/ml. However, as regard the effect of mucus on females it showed a lower scale value of effective concentrations than males (Tables 3 and 4).
Regarding the effect of NPs on S. mansoni adult males and females, there was a highly significant difference (P < 0.001) from NPs concentration of (100 µl/ml) along the duration of the 3 days. As regard the effect of NPs on males, the LC50 was 240 µl/ml and the LC90 was 400 µl/ml. However, as regard the effect of NPs on females LC50 was 123.33 µl/ml and LC90 was 190 µl/ml, denoting again a better overall impact on females than males with a lower scale value of effective concentrations (Tables 3 and 4). Collectively, mucus showed a better outcome as regard lethal effect on males and females compared to NPs.
Results of the in vivo study
Comparing TWB reduction percentages among the studied groups including PZQ (I, III, V) they revealed an outcome of 100% followed by mucus (group II) which showed a percentage of 43.39% and NPs (group IV) which showed only 26.14%. These results reflected that the effect of PZQ either alone or combined with mucus or NPs were equivocal revealing best outcomes. There was a highly significant relation (P < 0.001) among the studied groups concerning the numbers of copula and the total count. There was a significant difference (P < 0.05) among the studied groups concerning the number of males (Table 5).
There was a highly statistical relation (P < 0.001) among all the studied groups according to the percentage of immature, mature and dead egg counts. As regard the immature stages, the groups including PZQ (I, III, V) were the most efficient in decreasing the mean outcome of immature stages (Fig. 1c, d, g, h; k, l). However, a little decrease was recorded from the mucus and NPs groups (II, IV) (Fig. 1e, f; i, j) compared to the control (Fig. 1a, b; m, n, o). As regard the dead stages, the groups including PZQ (I, III, V) (Fig. 1c, d; g, h; k, l) were the most efficient in increasing the mean outcome of dead stages. However, a little increase was recorded from the mucus and NPs groups (II, IV) (Fig. 1e, f; i, j) compared to the control (Fig. 1m, n, o). The effect of PZQ and NPs combination (group V) (Fig. 1k, l) showed the best outcome with a higher increase in dead stages followed by combined PZQ and mucus (group III) (Fig. 1g, h) then PZQ (group I) (Fig. 1c, d). No outcome was shown from mucus (group II) or NPs (group IV) (Fig. 1i, j) (Table 6).
Histopathological examination of liver sections in different study groups. a, b: Liver section from infected non-treated mice (group C2) showing hepatocytes with centrally located nuclei (black arrow), central vein (red arrow), and large cellular granuloma (white arrow), composed of collection of lymphocytes intra-sinusoidal (green arrows). Adjacent hepatocytes showed hydropic change and focal atypical hyperplasia. c, d: Liver section from mice infected and treated with PZQ (group I) showing hepatocytes with centrally located nuclei (black arrow), central vein (red arrow) and a small fibrocellular granuloma with markedly degenerated miracidium (white arrow). The granuloma contour is regular and well demarcated from the surrounding tissue. e, f: Liver section from mice infected and treated with mucus (group II) showing hepatocytes with centrally located nuclei (black arrow), central vein (red arrows), dilated and congested blood sinusoids (yellow arrows), moderate collection of intrasinusoidal lymphocytes (green arrows) and a fibro cellular granuloma (white arrow) with densely packed inflammatory cells and intact cellular miracidium inside the ovum. g, h: Liver section from mouse infected and treated with combined PZQ and mucus (group III) showing almost normal hepatocytes with centrally located nuclei (black arrow), central vein (red arrows) and a small hepatic fibro cellular granuloma (white arrow) with markedly degenerated miracidium. The granuloma contour is regular and well demarcated from the surrounding tissue. i, j: Liver section from mice infected and treated by NPs (group IV) showing hepatocytes with centrally located nuclei (black arrow), central vein (red arrows), with kupffer cell hyperplasia (green arrow). A fibro cellular granuloma (white arrow) with densely packed inflammatory cells and intact cellular miracidium inside the ovum is seen. Scattered lymphocytes are shown all over the section. k, l: Liver section from mice infected and treated with combined PZQ and NPs (group V) showing hepatocytes with centrally located nuclei (black arrow), central vein (red arrow), dilated and congested blood sinusoids (yellow arrows) with kupffer cell hyperplasia (green arrow), collection of lymphocytes (blue arrow) and a small hepatic fibro cellular granuloma (white arrow) with markedly degenerated miracidium. The granuloma contour is regular and well demarcated from the surrounding. m: Liver section from non-infected non-treated mice (C1) showing normal hepatic architecture, with normal blood sinusoids (yellow arrow) and normal hepatocytes with centrally located nuclei (black arrow) and central vein (red arrows). n: Liver section from mice non-infected and mucus treated (C3) showing almost normal hepatic architecture with normal blood sinusoids (yellow arrow), normal hepatocytes with centrally located nuclei (black arrow) and central vein (red arrow). o: Liver section from non-infected mice NPs treated (C4) showing almost normal hepatic architecture with normal blood sinusoids (yellow arrow), normal hepatocytes with centrally located nuclei (black arrow) and central vein (red arrows)
There was a highly significant relation (P < 0.001) among the studied groups as regards ova count/gm liver and intestine compared to the control (C2). Comparing ova count among groups, combined PZQ and NPs (group V) showed the best outcome followed by combined PZQ and mucus (group III), then PZQ (group I) then NPs (group IV) and last was mucus (group II). Though the groups including PZQ showed the best results, still all the groups showed a quietly apart result from the control (C2). The highest reduction was seen from the combined PZQ and NPs (group V) (Table 7).
As regard the percentage of reduction in granuloma diameter, there was a highly statistical relation (P < 0.001) among the studied groups (I, III, V) (Fig. 1c, d; g, h; k, l). There was a reduction in granuloma diameter in all groups in comparison to the control group. The best outcome was revealed from the mice group treated with PZQ and NPs followed by PZQ and mucus (group III) (Fig. 1g, h), PZQ (group I) (Fig. 1c, d), mucus (group II) (Fig. 1e, f) and the least outcome was from NPs (group IV) (Fig. 1i, j) (Table 8).
As regard the number of granulomas in successive power fields, there was a highly statistical relation (P < 0.001) among the studied groups PZQ (group I), PZQ and mucus (group III) and a significant relation (P < 0.05) among groups NPs (group IV), PZQ and NPs (group V) in comparison to C2. The best outcome was revealed from the mice group treated with PZQ and NPs (group V), followed by PZQ and mucus (group III), PZQ (group I), NPs (group IV) and the least outcome was from the mucus (group II) (Table 9).
As regard fibro cellular granuloma there was a highly significant relation (P < 0.001) among all the studied groups in comparison to control (C2) (Fig. 1a, b). Combined PZQ and NP (group V) (Fig. 1k, l) showed a maximal increase in number of fibro cellular granulomas exceeding the control (C2) followed by PZQ and mucus (group III) (Fig. 1g, h) then PZQ (group I) (Fig. 1c, d), NPs (group IV) (Fig. 1i, j) and mucus (group II) (Fig. 1e, f), respectively. As regard cellular granuloma there was a significant relation (P < 0.05) among all the studied groups. Combined PZQ and NPs (group V) (Fig. 1k, l) showed a maximal decrease in number of cellular granulomas followed by PZQ and mucus (group III) (Fig. 1g, h) then PZQ (group I) (Fig. 1c, d), then NPs (group IV) (Fig. 1i, j) and finally mucus (group II) (Fig. 1e, f). No fibrous granulomas were detected in all groups. The best outcome was revealed from the mice group treated with PZQ and NPs (group V) (Fig. 1k, l) followed by PZQ and mucus (group III) (Fig. 1g, h), PZQ (group I) (Fig. 1c, d), NPs (group IV) (Fig. 1i, j) and the least outcome was from the mucus (group II) (Fig. 1e, f) (Table 10).
As regard number of degenerated S. mansoni eggs, there was a highly significant relation (P < 0.001) among all the studied groups. Combined PZQ and NP (group V) showed a maximal increase in number of degenerated eggs followed by PZQ and mucus (group III) then PZQ (group I), mucus (group II) and NPs (group IV) in comparison to the control group (C2) (Table 11). Consequently, it was shown that the combination of mucus or NPs to PZQ potentiated its outcome.
There was a highly significant difference (P < 0.001) among the studied groups as regards level of NO in liver tissue. The best outcome was recorded from combined PZQ and NPs (group V) followed by individual PZQ (group I), individual mucus (group II), combined PZQ and mucus (group III) and then from individual NPs (group IV) while the least level of NO resulted from non-infected non-treated group C1 reflecting the best response (Table 12).
There was a highly significant difference (P < 0.001) among the studied groups as regards level of GSH in liver tissue. Infected non-treated (group C2) showed the least level of GSH reflecting a maximal increase in lipid peroxidation. The highest level was reported in PZQ treated group (group I) followed by combined PZQ and NPs (group V), combined PZQ and mucus (group III), individual NPs (group IV) and then individual mucus (group II). All treated groups showed a response in increasing GSH in approaching levels to group C1 best outcome (Table 13).
Discussion
The results of GC/MS showed that mucus of B. alexandrina contains indole, lactic acid, diphenyl (trimethylsilyl) phosphine, silanol, trimethyl, carbonate (2:1), oxalic acid, urea, glycerol, myo-Inositol and hydroxybutanoic acid. It is interesting to note that myo-inositol also enhanced bacterial eradication and therapy efficacy against infections (Zhao et al. 2018). Many studies also reported that myo-inositol improves the host’s ability to eliminate antibiotic-resistant Escherichia coli (E. coli) (Chen et al. 2015; Jiang et al. 2014, 2015). The diphenylphosphine was observed to have good antibacterial activity against E. coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas syringae pv. Salmonella enterica serotype Typhmurium SL 1344 and Streptococcus mutans ATCC 25175 (Sarıöz et al. 2018). A prior in vivo study showed that Glycerol Monolaurate (GML) was used to kill Trichomonas spp. and Candida spp. (Strandberg et al. 2010). Also, Schlievert and Peterson (2012) showed that GML has a broad range of activity against pathogenic bacteria and Mycoplasma.
The observed higher activity of the therapeutic extracts against miracidia is advantageous since killing one miracidium prevents the formation of thousands of cercariae which would contaminate the water and infect the people who get in contact with such water bodies (Obare et al. 2016). Expectantly, in our results, NPs had a better lethal effect on miracidia than on cercariae in most of the used concentrations along the different time intervals. Still cercaria eradication is a crucial way to terminate infection. Our results showed that mucus had a better lethal effect on cercariae than miracidia using the different concentrations along the successive time intervals. That runs in parallel with those recorded by Abu Zeid et al. (2016) who revealed that cercariae were more sensitive to stem alkaloids and ellagitannins and less sensitive to root alkaloids than miracidia.
Obare et al. (2016) formerly revealed that there was a significant difference in susceptibility of miracidia and cercariae to plants extracts as miracidia were more sensitive than cercariae. Abu Zeid et al. (2016) revealed that miracidia were more sensitive to ellagitannins and root alkaloids than cercariae and that was reversed with stem alkaloids reflecting that each drug has its own impact according to its selective mechanism of its action.
In our study, the effect of mucus on S. mansoni adult females and males was almost equivocal except for the effect after 72 h where the effect of mucus on adult females was better. The effect of NPs on S. mansoni adult females was better than adult males at the highest used concentration of 100 µl/ml after 72 h. However, at 48 h the same concentration had a better lethal impact on males.
Previous studies have shown more susceptibility of female schistosomes to artesunate (Mitsui et al. 2009), N-alkylamino-thiosulfuric acids (Penido et al. 1994; Guimarãesma et al. 2015). Also, Mitsui et al. (2009) revealed that female worms were more susceptible to artesunate and artemether than male worms. Also, Barth et al. (1997) and Manneck et al. (2010) revealed that female worms were significantly more susceptible than male worms to their tested drugs. Targeting an impact on females is a hope of drugs’ potency since females eradication will stop disease progression and infection continuation. Yet, there will be no mating without males so, their eradication is still that important.
From our demonstrated results of LC50 and LC90 values, it was concluded that a better overall impact on females than males was documented using lower concentrations. On the contrary, Khalil et al. (2016) revealed that Calotropis procera ethanolic stem extract has more anti-schistosomal activities on adult male schistosomes than female ones. The same was repeatedly described in some in vitro studies (Sanderson et al. 2002; De Melo. Et al. 2011). Also, Moraes et al. (2011, 2013) demonstrated that carvacryl acetate exhibited an optimal in vitro activity against the adult stage with no differential sensitivity between males and females worms.
All those data confirmed that, responses of drugs vary on males or females, also the scale of efficacy may vary at whether low or high concentrations are potent. In our study, the higher concentration scale was proportional to an increased lethal effect while in other studies, the lower scale of concentrations was more potent as Queiroz et al. (2021) who revealed that adult female worms were more susceptible than males after in vitro incubation with cnicin especially at low concentrations whereas, no mortality in male worms was observed. Probably that is depending on the pharmacological properties of the drugs used.
Regarding our in vivo results, the effect of PZQ either alone or combined with mucus or NPs was equivocal showing the best outcomes, with 100% TWB reduction. On the contrary, El Lakany et al. (2019) reported that the percentage of worm reduction was significantly increased in S. mansoni infected mice in combined treated groups with PZQ and omeperazole at 4 weeks post infection as compared to mice treated with PZQ or omeprazole alone.
In our study, mucus and NPs showed a TWB reduction of 43.39% and 26.14%, respectively. That was like Seif el-Din et al. (2013) who revealed that administration of Quinine or Halofantrine alone produced significant reductions in males, females and total number of worms compared to infected group. That is still giving a promise for developing synergy with repeating the study and alternating the concentration scales.
The effect of PZQ and NPs combination showed a higher increase in dead stages of eggs followed by combined PZQ and mucus then PZQ. That was previously shown by Abou-Shady et al. (2016) who revealed that treatment of S. mansoni infected mice with a single oral dose of 200 mg/kg of mefloquine combined to 500 mg/kg of praziquantel resulted in a 100% increase in percentage of dead eggs. That emphasizes that anti-schistosomal chemotherapy is considered effective against S. mansoni infection when the oogram pattern shows increased percentage of dead ova (Pellegrino et al. 1962).
The mature stages decreased predominately from combined PZQ and NPs, then combined PZQ and mucus followed by PZQ. Thus, the combination effect exceeded the individual PZQ effect. The same groups caused disappearance of the immature stages affirming that anti-schistosomal treatment is considered effective against S. mansoni infection when the oogram pattern shows disappearance of about 50% or more of the mature ova or if there was complete absence of one or more of the immature stages (Pellegrino et al. 1962).
Similarly, there was complete loss of immature eggs and increase in the percentages of dead eggs in the combined groups treated with PZQ and mefloquine in low and high doses respectively (El-Lakkany et al. 2011). Also, Ibrahim et al. (2019) revealed that PZQ combined with Pentoxifylline caused the disappearance of immature egg stages; decrease in the number of mature eggs and increase in the number of dead eggs and this agreed with the findings of Botros et al. (1996). Moreover, Hegazy et al. (2018) reported that the highest mean dead egg count and lowest mean immature & mature egg count were observed in PZQ and Artesunate combined group compared to infected control group.
The highest reduction rate of ova count in intestine and liver tissue was recorded from combined PZQ and NPs followed by combined PZQ and mucus, then individual PZQ then NPs and last from mucus. Elkersh et al. (2016) revealed that the highest reduction rate was obtained by combined mefloquine and Echinacea purpurea for ova count in liver and intestine, and by combined PZQ and Echinacea purpurea which showed significant reduction in tissue and intestinal egg load more than individual PZQ. This confirms that effective combinations may add to the potential of PZQ in decreasing ova count in tissue and intestine.
There was a relevant potential for NPs on histopathological findings in liver when compared to the rest of the groups and the control after PZQ. That runs in parallel to Hamed et al. (2010) who in a trial for finding a new vaccine stated that NPs of Biomphlaria snails protected against S. mansoni infection in mice through reduction in worm count, egg lodge, liver enzymes, liver energetic parameters and liver histopathology.
In our study, the effect of B. alexandrina mucus and NPs on hepatic granuloma number, size, increase of fibro cellular type, decrease of cellular type and the degenerated egg state, highlighted that PZQ and NPs combination showed the best outcome followed by PZQ and mucus, individual PZQ, individual mucus then individual NPs.
Many studies were previously documented on the assessment of prognosis through evaluating hepatic granulomas. Farid et al. (2013) reported that the microscopic examination of liver sections of infected mice showed a large cellular granuloma with living central ova. While liver sections from infected mice treated with combined PZQ with fluro-methyl-ketone or Vinyl sulfone showed a great reduction in granuloma size as small cellular granuloma with central degenerated ova, indicating that increase in fibro cellular and decrease in cellular granulomas is a reflection for good prognosis of the disease, that was confirmed by our results.
Mantawy et al. (2011) revealed that liver sections of infected mice treated with PZQ together with onion-garlic mixture showed fluctuated and noticeable degrees of improvement in comparison to infected group represented by: small sized, late fibro cellular granuloma, in addition, decrease in cellular constituents and degenerative changes in eggs.
Likewise, El Lakany et al. (2011) stated that there was a significant reduction in the granuloma diameters in groups treated with combined PZQ and mefloquine in comparison with groups treated with the different doses of PZQ alone. All treated groups showed size-variable, well-demarcated or circumscribed fibro cellular granulomas with central ova encircling living or dead miracidia, surrounded by lymphocytes, epithelioid cells, neutrophils, eosinophils and collagen bundles.
Stressing on the impact of combination, Abdel Hafeez et al. (2012) documented diminished granuloma size and number following combined PZQ to alpha lipoic acid. Also, Seif el-Din et al. (2013) reported that the combined treatment regimen of ketoconazole and quinine after S. mansoni infection had an added merit of maximal amelioration of histopathological changes where the liver sections showed remarkably better cellular architecture upon comparing with the S. mansoni infected untreated and quinine treated groups. Granulomas showed either partially or almost completely degenerated miracidia beside the minimal surrounding inflammatory reactions.
Yang et al. (2021) documented that the combination of PZQ with DW-3-15 not only significantly attenuated the egg burden in the liver but also reduced the area of egg induced granuloma, indicating that the combination therapy not only possesses antiparasitic activity but also inhibits the formation of egg-induced granulomas. Thus, compound treatment alleviated liver lesions and protected the liver in cases of schistosomiasis. Again, similar findings were published by Elkersh et al. (2016) who documented that combined regimen of Echinacea purpurea with PZQ had a highly significant reduction of hepatic granulomas diameter and number.
Free radicals are formed next to the eggs of S. mansoni in a process of granulomatous inflammation in which eggs of the parasite are destroyed and some cells of defense, such as macrophages and eosinophils, are attracted to the inflammation site. Some antioxidant enzymes and molecules are also attracted to destroy the free radicals present in the inflammatory region (Hogan et al. 2002; Stavitsky 2004; Neves et al. 2006).
The expression of antioxidant enzymes can be affected by many factors, such as inflammatory processes, some diseases and immunodeficiency, resulting in an imbalance between free radicals present in high quantities and few antioxidants sufficiently formed to destroy them (Alger et al. 2002; Bolukbas et al. 2005; Barbosa et al. 2007).
A major constituent of mucus, myo-inositol is classified as a vitamin-like nutrient which plays an important role in preventing oxidative damage and increasing antioxidant enzyme activities (Jiang et al. 2015; Shiau et al. 2005).
Overproduction of hepatic NO in response to parasitic infection can be considered one of the factors that is responsible for inducing oxidative stress and inflicting tissue injury (Dkhil et al. 2014). Excess NO is involved in the production of peroxynitrite, which is a reactive nitrogen species that oxidizes cellular structures, causing lipid peroxidation impairing the function of certain enzymes. NO production is also positively associated with tissue fibrosis due to its induction of fibrogenic cytokines and increased collagen synthesis (Parola and Robino 2001).
GSH plays a role in many biological processes, including the synthesis of protein, maintenance of cellular activity, xenobiotics, and reactive aldehydes detoxification such as Malondialdehyde (MDA), metabolism and cell acting protection against free radicals (Meister 1991; Jordao 1998). GSH is an intracellular reductant that plays a major role in catalysis, metabolism and transport. It protects cells against free radicals, peroxides, and other toxic compounds (Hiraishi 1994).
As regards level of NO in liver tissue, non-infected non-treated group showed the least level of NO. Other than individual NPs and infected non-treated group, all responses after treatment were considerable decreasing NO levels. The best outcome was recorded from the combined PZQ and NPs followed by individual PZQ, then individual mucus, combined PZQ and mucus and lastly individual NPs.
The infected non-treated group showed the least level of GSH reflecting a maximal increase in lipid peroxidation. The highest level of GSH was reported from PZQ treated group followed by combined PZQ and NPs, combined PZQ and mucus, individual NPs, and lastly individual mucus. All treated groups showed a response in decreasing GSH in approaching levels compared to non-infected non-treated group.
Formerly, Al-Olayan et al. (2016) proved that Schistosomiasis caused a significant increase in the liver levels of NO compared with the control group. Ceratonia siliqua pod extract (CPE) treatments significantly reversed this elevation in NO levels. Also, treatment of infected mice with CPE increased hepatic GSH contents (Al-Olayan 2016).
Similar findings were published by Fahmy et al. (2014) who documented that the content of GSH was significantly decreased in hepatic tissue of infected non-treated mice group compared to control group. These results agree with the reports of Mahmoud and Elbessoumy (2013) and Kadry et al. (2013). Accordingly, Hamed (2006) found that glutathione level decreased after parasitic infection and Gharib et al. (1999) attributed the decreased level of glutathione to the increased cytotoxicity with H2O2 which is produced as a result of inhibition of GSH that keeps glutathione in its reduced form. Fahmy et al. (2014) also reported that administration of Mefloquine caused a significant increase in the content of GSH compared to infected non-treated groups.
Schistosomiasis imbalanced the hepatocellular antioxidant system and liberated the free radicals, as evidenced by the decrease in GSH level and the increased levels of NO in hepatic tissue. It has been reported that schistosomiasis disturbs the levels of enzymatic and non-enzymatic antioxidants which impairs the liver GSH content of mice and decreases the hepatic antioxidant capacity, inducing the generation of lipid peroxides which may play a main role in the pathology associated with schistosomiasis (Cunha et al. 2012; Amer et al. 2013).
Conclusions
The study concluded that mucus and NPs had a prophylactic effect as antischistosomal therapeutics regarding their cercaricidal, miracidicidal and anti-schistosomal effect in vitro. This can be applied by using formulations containing treatments of B. alexandrina mucus or NPs in water canals to benefit from its cicaricidal and miracidicidal effects as a biological control measure. Moreover, their combination could be considered as a sharing therapeutic adding to PZQ potentiality in vivo for further investigations. The study highlighted that the combination of mucus or NPs with PZQ could add to PZQ potentiality.
References
Abd-ElAzeem HH, Osman GY, El-Sabbagh SM, Sheir SK (2020) Antibacterial activity of some terrestrial gastropods from Egypt against Staphylococcus aureus and Escherichia coli. Egypt J Zool EJZ Publisher Zool Soc AR Egypt 1110–6344:2682–3160
Abdel-Hafeez EH, Ahmad AK, Abdulla AM, Aabdel-Wahab S, Mosalem FA (2012) Therapeutic effect of alpha lipoic acid combined with praziquantel on liver fibrosis induced by Schistosoma mansoni challenged mice. Parasitol Res 111(2):577–586
Abou Rayia DM, Ashour DS, Abo Safia HS, Abdel Ghafar MT, Amer RS, Saad AE (2023) Human umbilical cord blood mesenchymal stem cells as a potential therapy for schistosomal hepatic fibrosis: an experimental study. Pathogens Global Health 117(2):190–202
Abou-Shady OM, Mohammed SS, Attia SS, Yusuf HS, Helmy DO (2016) Therapeutic effect of mefloquine on Schistosoma mansoni in experimental infection in mice. J Parasit Dis 40:259–267
Abu Zeid KH, El-Badawy MF, Gumaa SA, Ismael A, Shohayeb MM (2016) In vitro lethal effect of ellagitannins of the fruit rind of Punica Granatum and stem and root alkaloids on the miracidia and cercariae of Schistosoma mansoni. IOSR J Pharm Biol Sci 11(6):80–88
Akers MJ, Fites AL, Robinson RL (1987) Types of parenteral administration. J Parenter Sci Technol 41:88–95
Alger HM, Sayed AA, Stadecker MJ, Williams DL (2002) Molecular and enzymatic characterisation of Schistosoma mansoni thioredoxin. Int J Parasitol 32:1285–1292
Al-Kazzaz MA, El-Sayad MH, Abu-Helw SA (2016) Antischistosomal activity of Mirazid in experimental schistosomiasis mansoni: exploring the controversy. Parasitologists United J 9:31–36
Al-Olayan EM, El-Khadragy MF, Alajmi RA, Othman MS, Bauomy AA, Ibrahim SR, Abdel Moneim AE (2016) Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complement Altern Med 16:1–1
Aly I, Gouida MS, Sayed HEL, Attiyah SMN, Shaker S, Elleboudy NA, Ghoname SI (2020) Efficiency of three extracts of Carica papaya as molluscicidal and anti-schistosomal agents against Biomphalaria alexandrina and Schistosoma mansoni by flow cytometry. J Pharm Res Int 32(11):31–41
Amer OS, Dkhil MA, Al-Quraishy S (2013) Antischistosomal and hepatoprotective activity of Morus alba leaves extract. Pak J Zool 45(2):387–393
Bancroft JD, Steven A (1975) Histopathological stains and their diagnostic uses. Churchill Living Stone Edinburgh. https://doi.org/10.1155/2013/710647
Baranovskii DS, Klabukov ID, Arguchinskaya NV, Yakimova AO, Kisel AA, Yatsenko EM, Ivanov SA, Shegay PV, Kaprin AD (2022) Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig 9:7. https://doi.org/10.21037/sci-2022-025
Barbosa E, Moreira EAM, Faintuch J, Pereima MJL (2007) Suplementação de antioxidantes: enfoque em queimados. Rev Nutr 20:693–702
Barth LR, Fernandes APM, Ribeiro-Paes JT, Rodrigues V (1997) Effects of Goyazensolide during in Vitro Cultivation of Schistosoma mansoni. Mem Inst Oswaldo Cruz 92(3):427–429
Lee HJ, Lee JK, Lee H, Shin JW, Carter JE, Sakamoto T, Jin HK, Bae JS (2010) The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer’s disease. Neurosci Lett 481(1):30–35
Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O (2005) Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis 5:1–7
Bortolotti D, Trapella C, Bernardi T, Rizzo R (2016) Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br J Biomed Sci 73:49–50
Botros SS, Doughty BL, Shaker ZA, Akl MM, Sharmy R, Diab TM, Hassanein HI (1996) Efficacy of an antipathology vaccine in murine schistosomiasis administered with and without chemotherapy. Int J Immunopharmacol 18:707–718
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cardoso LS, Barreto ADSR, Fernandes JS, Oliveira RR, De Souza RDP, Carvalho EM, Araujo MI (2013) Impaired lymphocyte profile in schistosomiasis patients with periportal fibrosis. Clin Dev Immunol 710647:8. https://doi.org/10.1136/bmj.d2651
Cheever AW (1968) Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull WHO 39:328–331
Chen XH, Zhang BW, Li H, P, X. (2015) Myo-inositol improves the host’s ability to eliminate balofloxacin-resistant Escherichia coli. Sci Rep 5:10720–10731
Coyne K, Laursen JR, Yoshino TP (2015) In Vitro Effects of Mucus from the Mantle of Compatible (Lymnaea elodes) and Incompatible (Helisoma trivolvis) Snail Hosts on Fascioloides magna Miracidia. J Parasitol 101(3):351–357
Cunha GMM, Silva VMA, Bessa KDG, Bitencourt MAO, Macêdo UBO, Freire-Neto FP, Martins RR, Assis CF, Lemos TMAM, Almeida MG, Freire ACG (2012) Levels of oxidative stress markers: correlation with hepatic function and worm burden patients with schistosomiasis. Acta Parasitol 57:160–166
Da Silva DM, Martins JLR, De Oliveira DR, Florentino IF, Da Silva DPB, Dos Santos FCA, Costa EA (2018) Effect of allantoin on experimentally induced gastric ulcers: Pathways of gastroprotection. Eur J Pharmacol 821:68–78
Del Villar LP, Burguillo FJ, López-Abán J, Muro A (2012) Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of schistosomiasis. PLoS ONE 7:e45867–e45882
Dkhil MA, Adel Moneim AE, Al-Quraishy S (2014) Berberine protects against Schistosoma mansoni induced oxidative damage in renal and testicular tissues of mice. Pak J Zool 46:763–771
Eissa M, Bardicy S, Tadros T (2011) Bioactivity of miltefosine against aquatic stages of Schistosoma mansoni and Schistosoma haematobium and their snail hosts supported by scanning electron microscopy. Parasit Vect 73:4–11
El Lakany AR, El Gendy DI, Alshenawy HA, Abdel Ghaffar AE (2019) Omeprazole as an Adjuvant to Praziquantel in Treatment of Experimental Schistosomiasis mansoni. Med J Cairo Univ 87(5):3127–3135
El Ridi R, Tallima H, Dalton JP, Donnelly S (2014) Induction of protective immune responses against schistosomiasis using functionally active cysteine peptidases. Front Genet 5:119–126
El-Ansary AK, Ahmed SA, Aly SA (2007) Antischistosomal and liver-protective effects of Curcuma longa extract in Schistosoma mansoni infected mice. Indian J Exp Biol 45:791–801
Elkersh WM, Mohamed AH, Rady AA, Moharm IM, Mahdy AS (2016) Assessment of Echinacea purpurea and mefloquine in treatment of Schistosoma mansoni. Menoufia Med J 31:354–364
El-Lakkany NM, Seif el-Din SH, Sabra AA, Hammam OA (2011) Pharmacodynamics of mefloquine and praziquantel combination therapy in mice harboring juvenile and adult Schistosoma mansoni. Mem Inst Oswaldo Cruz Rio J 106(7):814–822
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
El-Rigal NS, Metwally NM, Mohamed AM, Mohamed NZ, Rizk MZ (2011) Protection against oxidative damage induced by Schistosoma mansoni using susceptible/resistant nucleoproteins from Biomphalaria alexandrina Snails. Asian J Biol Sci 4:445–456
El-Shennawy SF, Abdel Aaty HE, Radwan NA, Abdel-Hameed DM, Alam-Eldin YH, El-Ashkar AM, Abu-Zahra FA (2015) Therapeutic potential of mesenchymal stem cells on early and late experimental hepatic schistosomiasis model. J Parasitol 101(5):587–597. https://doi.org/10.1645/15-754.1
Fahmy SR, Rabia I, Mansour EM (2014) The potential role of mefloquine against Schistosoma mansoni infection by prohibition of hepatic oxidative stress in mice. J Basic Appl Zool 67:40–47
Fallon PG, Sturrock RF, Niang AC, Doenhoff MJ (1995) Short report: diminished susceptibility to praziquantel in a Senegal isolate of Schistosoma mansoni. Am J Trop Med Hyg 53:61–62
Farid A, Abdel Malek A, Rabie I, Helmy A, El Amir AM (2013) Overview on cysteine protease inhibitors as chemotherapy for Schistosomiasis mansoni in mice and also its effect on the parasitological and immunological profile. Pak J Biol Sci 16:1849–1861
Gharib B, Abdallahi OM, Dessein H, De Reggi M (1999) Development of eosinophil peroxidase activity and concomitantalteration of antioxidant defenses in the liver of mice infected withSchistosoma mansoni. J Hepatol 30:594–602
Gryseels B, Polman K, Clerinx J, Kestens L (2006) Human schistosomiasis. Lancet 368:1106–1118
Guimaraes MA, de Oliveira RN, Véras LM, Lima DF, Campelo YD, Campos SA, Kuckelhaus SA, Pinto PL, Eaton P, Mafud AC, Mascarenhas YP (2015) Anthelmintic activity in vivo of epiisopiloturine against juvenile and adult worms of Schistosoma mansoni. PLoS Negl Trop Dis 9(3):e0003656
Hamed MA (2006) Excretory–secretory product of Fasciola hepatica worm protects against Schistosoma mansoni infection in mice.Indian. J Exp Biol 44:554–561
Hamed MA, Ali SA, Aly HF, El-Rigal NS, Rizk MZ (2010) Biomphalaria alexandrina snails as immunogens against Schistosoma mansoni infection in mice. Mem Inst Oswaldo Cruz 105(7):879–888
Hegazy LA, Al Motiam MH, Abd El-Aal NF, Ibrahim SM, Mohamed HK (2018) Evaluation of artesunate and praziquantel combination therapy in murine Schistosomiasis mansoni. Iran J Parasitol 13(2):193–203
Helmy M, Mahmoud S, Fahmy Z (2009) Schistosoma mansoni: effect of dietary zinc supplement on egg granuloma in Swiss mice treated with praziquantel. Exp Parasitol 122:310–317
Hiraishi H, Terano A, Ota S, Mutoh H, Sugimoto T, Harada T, Razandi M, Ivey KJ (1994) Protection of cultured rat gastric cells against oxidant-induced damage by exogenous glutathione. Gastroenterology 106:1199–1207
Hogan LH, Wang M, Suresh M, Co DO, Weinstock JV, Sandor M (2002) CD4+ TCR repertoire heterogeneity in Schistosoma mansoni-induced granulomas. J Immunol 169:6386–6393
Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO (2008) Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol 6(10):2297–2313
Holtfreter MC, Loebermann M, Klammt S, Sombetzki M, Bodammer P, Riebold D, Kinzelbach R, Reisinger EC (2011) Schistosoma mansoni:schistosomicidal effect of mefloquine and primaquine in vitro. Exp Parasitol 127(1):270–276
Ibrahim A, Abdel-Tawab H, Hussein T (2019) Pentoxifylline and/or praziquantel reduce murine schistosomiasis histopathology via amelioration of liver functions. Egypt J Aquat Biol Fish 23(5):121–133
Jiang WD, Liu Y, Hu K, Li S, Feng L, Zhou X (2014) Copper exposure induces oxidative injury, disturbs the antioxidant system, and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: protective effects of myo-inositol. Aquat Toxicol 155:301–313
Jiang WD, Liu Y, Jiang J, Wu P, Feng L, Zhou X (2015) Copper exposure induces toxicity to the antioxidant system via th destruction of Nrf2/ARE signalling and caspase-3-regulated DNA damage in fish muscle: amelioration by myo-inositol. Aquat Toxicol 159:245–255
Jones M (2019) Gas chromatography-mass spectrometry from national historic chemical landmarks. Am Chem Soc. www.acs.org/landmarks
Jordao JA, Chiarello PG, Bernardes MM, Vannucchi HA (1998) Peroxidacao lipidica etanol: papel da glutationa reduzida e da vitamina E. Rev Inst Med Trop Sao Paulo 31:434–449
Kadry SM, Mohamed AM, Farrag EM, Dalia B, Fayed DB (2013) Influence of some micronutrients and Citharexylum quadrangular extract against liver fibrosis in Schistosoma mansoni infectedmice. Afr J Pharm Pharmacol 7(38):2628–2638
Keiser J, Chollet J, Xiao S, Mei J, Jiao P, Utzinger J, Tanner M (2009) Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. Trop Dis 21:15–22
Keystone JS (1987) Seizures and electroencephalograph changes associated with oxamniquine therapy. Am J Trop Med Hyg 27:360–362
Khalil LMM, Azzam AM, Mohamed HAM, Aboueldahab MM, Taha HA, Soliman MI (2016) in vitro effects of the stem extracts of the plant calotropis procera on schistosoma mansoni adult worms. Egypt J Zool 66:205–216
King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG (2011) Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis 5:e1321–e1336
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:220–227
Kotb MA (2012) Molecular mechanisms of ursodeoxycholic acid toxicity & side effects: ursodeoxycholic acid freezes regeneration & induces hibernation mode. Int J Mol Sci 13:8882–8914. https://doi.org/10.3390/ijms13078882
Lee KA, Bindereif A, Green MR (1988) A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech 5(2):22–31
Liang YS, Bruce JI, Boyd DA (1987) Laboratory cultivation of Schistosome vector snails and maintenance of Schistosome life cycles. Proc. 1st Sino. Am Symp 1:34–48
Lukong KE, Chang KW, Khandjian EW, Richard S (2008) RNA-binding proteins in human genetic disease. Trends Genet 24(8):416–425
Mahmoud EA, Elbessoumy AA (2013) Effect of curcumin on hematological, biochemical and antioxidants parameters in Schis-tosoma mansoni infected mice. Int J Sci 2:1–14
Mahmoud AEF, Warren KS (1974) Anti-inflammatory effects of tartar emetic and niridazole: suppression of Schistosome egg granuloma. J Immunol 112:222–228
Manneck T, Haggenmuller Y, Keiser J (2010) Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 137(1):85–98
Mantawy MM, Ali HF, Rizk MZ (2011) Therapeutic effects of Allium sativum and Allium cepa in Schistosoma mansoni experimental infection. Rev Inst Med Trop 53(3):155–163
Mathaes R, Koulov A, Joerg S, Mahler HC (2016) Subcutaneous injection volume of biopharmaceuticals-pushing the boundaries. J Pharm Sci 105:2255–2259
Meister A (1991) Glutathione deficiency produced by inhibition of its synthesis, and its reversal applications in research and therapy. Pharmacol Ther 51:155–194
De Melo NI, Magalhaes LG, De Carvalho CE, Wakabayashi KA, Ramos RC, Mantovani ALL, Turatti ICC, Rodrigues V, Groppo M, Cunha WR, Veneziani RCS, Crotti AEM (2011) Schistosomicidal activity of the essential oil of Ageratum conyzoides L. (Asteraceae) against adult Schistosoma mansoni worms. Molecules 16(1):762–773
Mitsui Y, Miura M, Aoki Y (2009) In vitro effects of artesunate on the survival of worm pairs and egg production of Schistosoma mansoni. J Helminthol 83:7–11
Mohamed SS, Abdelmksoud HF, Sabry HY, Mahmoud S, El Komi W, Abu Shousha T, El-Ashkar AM (2023) Cholagogue additive effect of ursodeoxycholic acid to Praziquantel on murine schistosomiasis mansoni: Parasitological and histopathological studies. PUJ 16(1):32–40. https://doi.org/10.2168/puj.2023.174365.1195
Moraes J, Nascimento C, Lopes PO, Nakano E, Yamaguchi LF, Kato MJ, Kawano T (2011) Schistosoma mansoni: In vitro schistosomicidal activity of piplartine. Exp Parasitol 127(2):357–364
Moraes JD, Carvalho AAL, Nakano E, De Almeida AAC, Marques DHD, Andrade LN, De Freitas RM, De Sousa DP (2013) Antihelmenthic activity of carvacryl acetate against schistosoma mansoni. Parasitol Res 112:603–610
Musiał-Wysocka A, Kot M, Majka M (2019) The pros and cons of mesenchymal stem cell-based therapies. Cell Transp 28(7):801–812
Nebendahl K (2000) Routes of administration. Chapter: 24 in the laboratory rat. Academic Press, San Diego, CA, pp 463–483
Neves RH, Alencar ACMB, Aguila MB, Mandarim-de-Lacerda CA, Machado-Silva JR, Gomes DC (2006) Hepatic stereology of schistosomiasis mansoni infected-mice fed a high-fat diet. Mem Inst Oswaldo Cruz 101:253–260
Obare B, Yole D, Nonoh J, Lwande W (2016) Evaluation of cercaricidal and miracicidal activity of selected plant extracts against larval stages of Schistosoma Mansoni. J Nat Sci Res 6:2224–3186
Onyema AM (2019) Extraction, phytochemical, physicochemical and toxicological study of ginger lily, leaf and snail (Archachatina marginata) slime for potential use as anti-diabetic drug delivery. IOSR J Appl Chem 12:08–19
Parola M, Robino G (2001) Oxidative stress-related molecules and liver fibrosis. J Hepatol 35(2):297–306
Pellegrino J, Faria J (1965) The oogram method for the screening of drugs in schistosomiasis mansoni. Am J Trop Med Hyg 14:363–369
Pellegrino J, Oliveira CA, Faria J, Cunha AS (1962) New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg 11:201–215
Penido MLO, Nelson DL, Vieira LQ, Coelho PMZ (1994) Schistosomicidal activity of alkylaminoctanethiosulfuric acids. Mem Inst Oswaldo Cruz 89:595–602
Queiroz LS, Ferreira EA, Mengarda AC, Almeida AD, Pinto PD, Coimbra ES, de Moraes J, Denadai AM, Da Silva Filho AA (2021) In vitro and in vivo evaluation of cnicin from blessed thistle (Centaurea benedicta) and its inclusion complexes with cyclodextrins against Schistosoma mansoni. Parasitol Res 120:1321–1333
Sabry HY, Hassan SI, Mahmd SH, Ezzat AR, Fahmy ZH (2004) Modification of resistance to reinfection with Schistosoma mansoni in mice by combined administration of praziquantel and non-steroidal anti-inflammatory drugs. Egypt J Med Sci 25:839–859
Sanderson L, Bartlett A, Whitfield PJ (2002) In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J Helminthol 76(3):241–247
Sarıöz Ö, Malgaç B, Sürme Y, İlk S, Karaarslan M (2018) studies on the heavy metal removal efficiency and antibacterial activity of 2-(diphenylphosphino) aminopyridine. Maced J Chem Chem Eng 37:53–60
Schlievert PM, Peterson ML (2012) Glycerol Monolaurate Antibacterial Activity in Broth and Biofilm Cultures. PLoS ONE 7(7):e40350
Seif el-Din SH, Sabra AA, Hammam OA, El-Lakkany NM (2013) Effect of Ketoconazole, a Cytochrome P450 Inhibitor, on the Efficacy of Quinine and Halofantrine against Schistosoma mansoni in Mice. Korean J Parasitol 51(2):165–175
Shiau SY, Su SL (2005) Juvenile tilapia (Oreochromis niloticus x Oreochromis aureus) requires dietary myo-inositol for maximal growth. Aquaculture 243:273–277
Smithers SR, Terry RJ (1965) Infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of adult worms. Parasitol 55:695–700
Stavitsky AB (2004) Regulation of granulomatous inflammation in experimental models of schistosomiasis. Infect Immun 72:1–12
Strandberg KL, Peterson ML, Lin YC, Pack MC, Chase DJ, Schlievert PM (2010) Glycerol monolaurate inhibits Candida and Gardnerella vaginalis in vitro and in vivo but not Lactobacillus. Antimicrob Agents Chemother 54:597–601
Tempone AG (2007) Cutaneous secretion from the giant African snail, Achatina fulica, as a source of Antileishmanial compounds. Rev Inst 66(1):73–77
Tsoutsos D, Kakagia D, Tamparopoulos K (2009) The efficacy of Helix aspersa Muller extract in the healing of partial thickness burns: a novel treatment for open burn management protocols. J Dermatol Treat 20:219–222
Turner PV, Brabb T, Pekow C, Vasbinder MA (2011) Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50(5):600–613
Utzinger J, N’Goran EK, N’Dri A, Lengeler C, Xiao S, Tanner M (2000) Oral artemether for prevention of Schistosoma mansoni infection: randomized controlled trial. Lancet 355:1320–1325
WHO (2010): Scistosomiasis, fact sheet No.115. World Health Organization.Available at: < http:// www.who.int/ mediacentre/ facesheets/ fs115/ en/ index.html>.
Yang ZY, Liu ZH, Zhang YN, Li C, Liu L, Pu WJ, Xie SQ, Xu J, Xia CM (2021) Synergistic effect of combination chemotherapy with praziquantel and DW-3-15 for Schistosoma japonicum in vitro and in vivo. Parasit Vect 14:550–564
Yoles TK, Moore DV, Guisti D, Ripsam CL, Meleney HE (1947) A technique for perfusion of laboratory animals for the recovery of schistosomes. J Parasitol 33:491–526
Yousif F, Hifnawy MS, Soliman G, Boulos L, Labib T, Mahmoud S, Ramzy F, Yousif M, Hassan I, Mahmoud K, El Hallouty S, El Gendy M, Gohar L, Manawaty M, Fayyad W, El Menshawi B (2007) Large-scale in vitro screening of Egyptian native and cultivated plants for schistosomicidal activity. Pharm Biol 45:501–510
Zerivitz K, Akusjärvi G (1989) An improved nuclear extract preparation method. Gene Anal Tech 6(5):101–109
Zhang S, Coultas KA (2013) Identification of plumbagin and sanguinarine as effective chemotherapeutic agents for treatment of schistosomiasis. Int J Parasitol Drugs Drug Resist 3:28–34
Zhao XL, Chen H, Zhong KK, Li L, Kong HX (2018) Myo-inositol as an adjuvant to florfenicol against Aeromonas hydrophila infection in common carp Cyprinus carpio. FEMS Microbiol Lett 365:212–219
Funding
There has been no financial support for this work that could have influenced its outcome.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors have not disclosed any competing interests.
Ethical approval
The study was applied according to the regulation of the Ethics Committee of Faculty of Medicine Ain Shams University (FMASU MS 575/2020) and the Schistosome Biology Supply Center at TBRI in accordance with those of the Higher Ministry of Education.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nafie, E.H., Abou-Gamra, M.M., Mossalem, H.S. et al. Evaluation of the prophylactic and therapeutic efficacies of mucus and tissue nucleoproteins extracted from Biomphalaria alexandrina snails on schistosomiasis mansoni. J Parasit Dis 48, 551–569 (2024). https://doi.org/10.1007/s12639-024-01692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-024-01692-0