Abstract
This study developed and evaluated chitosan-sodium alginate capsules containing the probiotic Lacticaseibacillus rhamnosus GG using extrusion and emulsification techniques. The encapsulated L. rhamnosus GG cells were also evaluated for technological and probiotic-related physiological functionalities, as well as when incorporated in UHT and powdered milk. Extrusion (86.01 ± 1.26%) and emulsification (74.43 ± 1.41%) encapsulation techniques showed high encapsulation efficiency and high survival rates of L. rhamnosus GG during 28 days of refrigeration and room temperature storage, especially emulsification capsules (> 81%). The encapsulated L. rhamnosus GG cells showed high survival rates during exposure to simulated gastrointestinal conditions (72.65 ± 1.09–114.15 ± 0.44%). L. rhamnosus GG encapsulated by extrusion and emulsification performed satisfactorily in probiotic-related physiological (pH and bile salts tolerance) and technological properties (positive proteolytic activity, diacetyl and exopolysaccharides production, high NaCl tolerance (> 91%), besides having high heat tolerance (> 76%)). L. rhamnosus GG in extrusion and emulsification capsules had high survival rates (> 89%) and did not significantly affect physicochemical parameters in Ultra-High Temperature (UHT) and powdered milk during storage. The results demonstrate that L. rhamnosus GG can be successfully encapsulated with alginate-chitosan as a protective material through extrusion and emulsification techniques. UHT and powdered milk could serve as appropriate delivery systems to increase the intake of this encapsulated probiotic by consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, the interest in functional foods has greatly increased worldwide, especially foods carrying probiotics [1]. Probiotics are living microorganisms that confer health benefits to consumers when administered in sufficient doses [2]. Protecting and preserving probiotic live cells in the delivery food matrix is crucial to reaching their claimed health-related benefits [3]. Furthermore, severe pH conditions in the stomach and the presence of bile salts in the small intestine are the main barriers limiting the arrival of probiotic cells to the ileum and colon, where these microorganisms interact with the host-residing intestinal microbiota [4]. Dairy products have been the most common food matrix to deliver probiotics, although studies assessing powdered milk as a probiotic vehicle are still scarce [5, 6].
The increase in the stability of probiotics has been a challenge in functionalizing foods with these microorganisms [7, 8]. Encapsulation could be an effective technology to overcome this limitation since it could protect probiotic cells from adverse conditions imposed by the intrinsic characteristics of the delivering foods and during passage through the gastrointestinal tract [9]. The encapsulation of probiotics using natural or synthetic polymers could have the advantages of being highly targeted, presenting low cytotoxicity, and adequate stability [10,11,12]. The selection of wall materials to produce the capsules and the technique used in the manufacture are of paramount importance and strictly affect the final morphology and functional properties of the produced capsules [13].
Probiotic encapsulation could help extend the typical short storability of some probiotic-supplemented products and maintain cell viability and functionality during passage through the gastrointestinal tract [14], besides avoiding contact with harsh conditions, like acidic environments, that could increase thermal stress to probiotic cells [15]. Chitosan has been typically ineffective as a coating material to increase probiotic cell survival when used alone [16]. However, other coating materials could be combined with chitosan to encapsulate probiotic bacteria, such as whey protein, poly-lysine, sodium caseinate, and sodium alginate [17].
The chitosan coating (as a multi-component compound) on negatively charged capsules of calcium alginate could increase the physical and chemical stability of the produced capsules, besides decreasing the destructive effects of calcium ion chelating agents on the capsule structure [18, 19]. D-mannuronic acid and L-glucuronic acid, linked by glycosidic bonds, comprise the alginate molecule, a polymer widely used to encapsulate probiotics [17]. Calcium alginate capsules can be produced using emulsion and extrusion techniques [15] and combining calcium alginate with polymers from different sources could produce a uniform and homogeneous mixture [18].
Lacticaseibacillus rhamnosus GG is among the lactic acid bacteria strains most tested for probiotic application, showing a high potential to adhere to and pass through the gastrointestinal tract, besides having antimicrobial activity, resistance to lysozyme, phenol, and antibiotics, and antioxidant activity [13, 20,21,22]. However, studies evaluating chitosan and sodium alginate mixture to encapsulate L. rhamnosus GG and incorporating encapsulated cells in delivery food are still scarce. This study aimed to develop chitosan-sodium alginate capsules containing L. rhamnosus GG using extrusion and emulsification techniques. The encapsulated L. rhamnosus GG cells were evaluated regarding technological and probiotic-related physiological functionalities, as well as when incorporated in whole Ultra-High Temperature (UHT) and powdered milk.
Materials and Methods
Probiotic Cultivation Conditions
Lacticaseibacillus rhamnosus GG (ATCC 53103) was obtained from Chr. Hansen A/S (Denmark). Before use in the assays, the strain was inoculated into 9 mL of de Man, Rogosa, and Sharpe (MRS) broth (Oxoid, Melbourne, Australia) and incubated at 37 ± 0.5 °C for 24 h under anaerobiosis (Anaerobic System Anaerogen, Oxoid, Hampshire, UK). The cells were collected with centrifugation (4000 × g, 10 min, 4 °C), washed twice with sterilized distilled water, and suspended in 4 mL of sterile distilled water for subsequent use as control-free cells and to produce the capsules [23]. The inoculum suspension had a cell concentration of approximately 9 log CFU/mL when plated on MRS agar (Oxoid).
Encapsulation of L. rhamnosus GG Cells
Extrusion Technique
The suspended L. rhamnosus GG cells were mixed with 40 mL of sterile sodium alginate solution (2% w/v) (D 3247 AJAX Chemicals Ltd., Sydney, Australia). The obtained cell suspension was placed in a sterile syringe and injected through a 0.11-mm needle (2 cm of distance from needle to solution) into sterile 0.05 mol/L calcium chloride (CaCl2) containing 0.1 g/100 g Span 80®. After 30 min of gelification in 0.05 mol/L CaCl2, the capsules were washed with sterile distilled water and filtered (Whatman Grade 4, GE Healthcare, Chicago, IL, USA). Low-molecular-weight chitosan (0.5 g) (Sigma-Aldrich, St. Louis, MA, USA) was dissolved in 100 mL of lactic acid solution (1% v/v), with pH adjusted to 5.7–6.0 using 1 mol/L NaOH, and sterilized with autoclavation (121 °C, 15 min, 1 atm) [23]. The capsules were placed in the chitosan solution for 40 min under magnetic stirring (56 × g). Afterward, the capsules were washed with sterile distilled water, filtered, and stored under refrigeration (4 ± 0.5 °C) in a sterile 0.05 mol/L CaCl2 solution [23]. The capsule size was approximately 2 mm, measured using a caliper.
Emulsification Technique
The suspended L. rhamnosus GG cells were mixed with 40 mL of sterile sodium alginate solution (2% w/v) (D 3247 AJAX Chemicals Ltd.) and added 2 mL of a sterile CaCO3 (500 mM) solution. After homogenization, the mixture was dispersed into soybean oil (200 mL) under magnetic stirring (56 × g), and after 15 min of emulsification, 40 mL of soybean oil containing glacial acetic acid (7 mL) was added to the emulsion using a sterile syringe, injected through a 0.11-mm needle into the mixture, and magnetic stirring (56 × g) continued to permit CaCO3 solubilization. Afterward, 300 mL of 0.05 mol/L CaCl2 solution was added to the flask wall and kept under magnetic stirring (56 × g) for 10 min. The capsules were settled down, the top layer of the oily phase was removed by aspiration, and the capsules were harvested with two subsequent washings to remove remnant oil, filtered (Whatman), and placed in the chitosan solution (as described in the “Extrusion Technique” section) for 40 min under magnetic stirring (56 × g). Afterward, the capsules were washed, filtered, and stored under refrigeration (4 ± 0.5 °C) in a sterile 0.05 mol/L CaCl2 solution [24]. The capsule size was approximately 0.5 mm, measured using a caliper.
Encapsulation Efficiency of L. rhamnosus GG Cells
Encapsulation efficiency (EE) was determined as the fraction of L. rhamnosus GG viable cells in each capsule compared to the viable cells in the feed bacterial cell dispersion.
The EE (%) was determined using Eq. 1:
N is the count of encapsulated viable cells, and N0 is the count of viable cells before the encapsulation.
One gram of the capsule was dispersed in 10 mL of sodium citrate solution (3% w/v), the suspension was shaken in a vortex until the complete capsule dissolved, and aliquots (0.1 mL) were dispersed in sterile peptone water (peptone 0.1 g/100 mL, 0.9 mL) for serial dilutions. Aliquots (0.1 mL) of each dilution were plated on MRS agar by the microdrop technique and incubated under anaerobiosis (Anaerogen) at 37 ± 0.5 °C for 48 h. The viable cell counts (log CFU/mL) were determined at the end of the incubation [25].
Survival of L. rhamnosus GG in the Capsules During Storage
The survival of L. rhamnosus GG in the extrusion and emulsification capsules was determined at 7, 14, 21, and 28 days of refrigeration (4 ± 0.5 °C) and room temperature (25 ± 0.5 °C) storage, following suspension preparation, plating, and incubation described in the “Encapsulation Efficiency of L. rhamnosus GG Cells” section. The viable cells of L. rhamnosus GG in the capsules were counted, and the results were expressed as survival rates (%) determined using Eq. 2:
where log N is the viable cell count (log CFU/mL) of L. rhamnosus GG in capsules at each monitored storage time interval, and log N0 is the viable cell count (log CFU/mL) of L. rhamnosus GG after the encapsulation.
The detection limit of the tests for enumerating L. rhamnosus GG viable cells was 1 log CFU/mL.
Evaluation of Probiotic-Related Physiological Properties of L. rhamnosus GG
pH and Bile Salts Tolerance
L. rhamnosus GG suspension (1% v/v, control-free cells as described in the “Probiotic Cultivation Conditions” section) and L. rhamnosus GG capsules (1% w/v) were inoculated into phosphate buffer solution (PBS, 10 mL) with pH adjusted to 7.2, 5, 3, and 2 using 1 M HCl or supplemented with bile salts (0.1%, 0.15%, 0.2%, 0.3%, or 1%, w/v) (Sigma‐Aldrich) under stirring (150 rpm) [26]. After 3 h of incubation under the tested conditions, an aliquot (0.1 mL) of the suspension was used to determine the survival rates of free and encapsulated cells of L. rhamnosus GG, as described in the “Survival of L. rhamnosus GG in the Capsules During Storage” section.
Exposure of L. rhamnosus GG to Simulated Gastrointestinal Conditions
L. rhamnosus GG suspension (1%, v/v, control-free cells as described in the “Probiotic Cultivation Conditions” section) and L. rhamnosus GG capsules (1% w/v) were exposed to simulated gastrointestinal conditions in UHT whole milk (10 mL; Camponesa, Lagoa da Prata, MG, Brazil). The simulation occurred continuously in sterile bottles to mimic esophageal-stomach, duodenum, and ileum conditions. Mechanical agitation simulated peristaltic movements, and experiments were done in an incubator at 37 ± 0.5 °C with rotation adjustment in each phase. The esophageal-stomach condition was simulated with 25 mg of pepsin diluted in 1 mL of 0.1 M HCl, added at a rate of 0.05 mL/mL, with a gradual decrease of pH with 1 M HCl (pH 5.5/10 min, pH 3.8/20 min, and pH 2.0/60 min) under stirring (130 rpm). Duodenal conditions were simulated with 2 g pancreatin/L of 0.1 M NaHCO3 and 12 g bovine bile salts/L of 0.1 M NaHCO3, pH adjusted for 5 with 0.1 M NaHCO3, and exposure time of 30 min under stirring (45 rpm). Ileal conditions were simulated with pH adjusted to 6.5 with 0.1 M NaHCO3, and the exposure time was 60 min under stirring (45 rpm) [26]. All enzymes and components were obtained from Sigma-Aldrich. At each digestion phase, an aliquot (0.1 mL) of the suspension was used to determine the viable cells of L. rhamnosus GG in the capsules. The results were expressed as survival rates of free and encapsulated cells of L. rhamnosus GG, as described in the “Survival of L. rhamnosus GG in the Capsules During Storage” section.
Technological Properties of L. rhamnosus GG
Proteolytic and Lipolytic Activity
Proteolytic activity was evaluated using an aliquot (10 μL) of L. rhamnosus GG culture (control, i.e., free cells) grown anaerobically in MRS broth (20–24 h, 37 ± 0.5 °C, Anaerogen), as well as of dissolved capsules previously released by homogenizing 1 g of each capsule with sodium citrate solution (3% w/v, 1 mL) using a vortex until visible complete dissolution. Aliquots (10 μL) were streaked onto plate count agar (HiMedia, Mumbai, India) supplemented with (10% w/v) skimmed milk (Camponesa) and incubated under anaerobiosis (37 ± 0.5 °C, 72 h). The appearance of a clear zone surrounding the colonies was indicative of proteolytic activity.
For evaluating the lipolytic activity, an aliquot (10 μL) of L. rhamnosus GG (culture control, i.e., free cells) and dissolved capsules were plated on tributyrin agar (Sigma-Aldrich) and incubated under anaerobiosis (37 ± 0.5 °C, 72 h). The appearance of a clear zone surrounding the colonies was indicative of lipolytic activity [26].
Exopolysaccharide and Diacetyl Production
L. rhamnosus GG culture (1%, v/v, control, i.e., free cells) and L. rhamnosus GG capsules (1% w/v of extrusion and emulsification capsules) were added in 10 mL of MRS broth containing 2% glucose (Sigma-Aldrich) and incubated under anaerobiosis (37 ± 0.5 °C, 72 h, Anaerogen). The cells were centrifuged (4000 rpm, 10 min, 4 °C), mixed at a rate of 1:2 with 95% (v/v) cold ethanol (Fmaia, Belo Horizonte, MG, Brazil), and maintained at 4 ± 0.5 °C for 24 h to induce the exopolysaccharide (EPS) precipitation. EPS precipitates were qualitatively evaluated as positive ( +) or negative ( −) for EPS production [27].
L. rhamnosus GG culture (1%, v/v, control, free cells) and L. rhamnosus GG capsules (1% w/v of extrusion and emulsification capsules) were added to 10 mL of UHT whole milk (Camponesa). After 24 h of incubation (37 ± 0.5 °C), 0.5 mL of 1% (w/v) α-naphthol (Sigma-Aldrich) and 16% (w/v) KOH (Sigma-Aldrich) were mixed with bacterial culture (1 mL) in UHT whole milk and incubated (37 ± 0.5 °C, 10 min). The formation of a red ring at the top of the mixture indicated diacetyl production [26, 27]. The diacetyl production was classified as weak ( +), medium (+ +), and strong (+ + +) based on the intensity of the red ring [26].
Heat Temperature and Salt Tolerance
The resistance of free (control cells as described in the “Probiotic Cultivation Conditions” section) and encapsulated L. rhamnosus GG cells to high temperatures (55, 65, 75, 85, and 95 °C for 30 s) was evaluated using sterile distilled water as a suspending medium. For the free cells, 1 mL of the suspension was collected, and for the extrusion and emulsification capsules, 1 g was collected and transferred to test tubes, each containing 9 mL of sterile distilled water. The samples were subjected to water bath thermal treatments (Dist DI950 M, Florianópolis SC, Brazil). Afterward, the samples were cooled (1 min in an ice-cold vessel) to room temperature (25 ± 0.5 °C) [28], and an aliquot (0.1 mL) was used to determine the survival rates of L. rhamnosus GG in capsules, as described in the “Survival of L. rhamnosus GG in the Capsules During Storage” section.
Salt (sodium chloride, NaCl) tolerance was evaluated using L. rhamnosus GG free cells (1%, v/v, control) and L. rhamnosus GG capsules (1% w/v), which were transferred to tubes containing 10 mL of MRS broth supplemented with different NaCl concentrations (0, 1, 3, and 5%, w/v), and incubated under anaerobiosis (37 ± 0.5 °C, 24 h, Anaerogen) [26]. At the end of the incubation period, the survival rates of L. rhamnosus GG in the capsules were determined, as described in the “Survival of L. rhamnosus GG in the Capsules During Storage” section.
Survival of Encapsulated L. rhamnosus GG in Whole UHT and Powdered Milk
L. rhamnosus GG capsules (1% w/v of extrusion and emulsification capsules) were inoculated in whole UHT milk (100 mL) and powdered milk (100 g) (Camponesa) and stored for 28 days under refrigeration (4 ± 0.5 °C). On days 0, 7, 14, 21, and 28 of storage, an aliquot (1 mL and 1 g, respectively) of the inoculated UHT and powdered milk was collected to determine survival rates of L. rhamnosus GG, as described in the “Survival of L. rhamnosus GG in the Capsules During Storage” section.
At the same storage intervals, some physicochemical parameters of UHT and powdered milk were determined. The pH values were determined using a potentiometer with a combined glass electrode for pH determination (Model Q400AS, Diadema, SP, Brazil). The titratable acidity (expressed as mmol H+/100 g) was determined by titration with 0.1 N NaOH. The soluble solids (°Brix) were determined using a digital refractometer (Hanna Instruments, model HI 96801, São Paulo, SP, Brazil) at 25 ± 0.5 °C and the moisture content was determined using standard procedures [29].
Statistical Analysis
The assays were performed in triplicate on three independent occasions, and the results were expressed as average ± standard deviation. Initially, the data were assessed via descriptive analysis (average and standard deviation) to obtain the description of the variables. Subsequently, the data were submitted to inferential analyses to determine significant differences (p ≤ 0.05) using Student’s t-test or ANOVA followed by post hoc Tukey’s test. The statistical analysis was performed using the software SPSS 22 (Statistical Package for Social Sciences).
Results and Discussion
Encapsulation Efficiency and Survival Rates of Encapsulated L. rhamnosus GG During Storage
Figure 1 shows the encapsulation efficiency of alginate-chitosan capsules prepared using extrusion and emulsification techniques was around 86.01 ± 1.26% and 74.43 ± 1.41%, respectively. Encapsulation efficiency refers to the concentration of probiotic viable cells within the capsules compared to the initial probiotic concentration in the suspension used to prepare them. Various factors could impact encapsulation efficiency, including the concentration of sodium alginate and CaCl2 used in the capsule formulation, the encapsulated microorganism, and the method and particle size used to formulate the capsules [30].
The primary advantage of using polymers to encapsulate bioactive components through the extrusion and emulsification techniques could be entrapping bacterial cells within the matrix during the capsule formation. It could prevent unintended bacterial cell release into the supplemented product, a common occurrence with other encapsulation techniques, including freeze-drying, spray-drying, and fluidized bed drying [18, 31]. Moreover, the extrusion and emulsification techniques could offer the benefit of employing mild conditions during capsule formation, aiding in maintaining high bacterial cell survival rates [32].
Similar encapsulation efficiency for L. rhamnosus GG cells (84–93%) was reported in early studies using spray-drying and gum Arabic blended with agave fructans, maltodextrin, inulin, and trehalose as wall materials [13]. Sodium alginate used as an encapsulation material in this study yielded satisfactory encapsulation efficiency, although the extrusion technique has shown greater efficiency in encapsulation than emulsification technique to encapsulate L. rhamnosus GG.
Table 1 shows the survival rates of L. rhamnosus GG in the extrusion and emulsification capsules during 28 days of room and refrigeration temperature storage. The survival rates of L. rhamnosus GG in the extrusion capsule (82.20 ± 0.98–85.03 ± 1.37%) were higher than in the emulsification capsule (69.80 ± 0.84–71.05 ± 0.24%) on day 7 of room and refrigeration temperature storage (p ≤ 0.05). The survival rate decreased on day 14 of room temperature storage (p ≤ 0.05), especially in the extrusion capsule (< 1.00 ± 0.00%). The survival rate of L. rhamnosus GG in the extrusion capsule was low until 28 days of room temperature storage (< 1.00 ± 0.00%). The survival rates of L. rhamnosus GG in the emulsification capsule were higher on days 21 and 28 of room temperature storage (75.48 ± 1.03% and 81.77 ± 1.09%, respectively) than in the extrusion capsule (p ≤ 0.05).
Bacterial metabolic activity is typically high above 22 °C, which can lead to reduced viability and cell death over time [32], agreeing with the results showing low survival rates of L. rhamnosus GG in the extrusion capsules under room temperature storage. However, it does not agree with the results showing high survival rates of L. rhamnosus GG in the emulsification capsules under room temperature storage. The sustained survival rates of L. rhamnosus GG in the emulsification capsules indicate the efficacy of the emulsification technique in protecting bacterial cells from adverse and stressful conditions over time [13].
The survival rates of L. rhamnosus GG in the extrusion capsule on day 7 of refrigerated storage (85.03 ± 1.37%) were higher than on days 14, 21, and 28 (62.20 ± 0.71–77.47 ± 0.66%) and higher than extrusion capsule under room temperature storage (p ≤ 0.05). The survival rate of L. rhamnosus GG in the emulsification capsule under refrigerated storage increased over time (69.80 ± 0.84% to 89.82 ± 0.45%), with a higher survival rate on day 28 day of storage. The higher survival rates of L. rhamnosus GG in the emulsification capsules indicate superior performance of the emulsification technique in protecting probiotic cells during storage rather than the extrusion technique [33]. The prolonged survival of L. rhamnosus GG cells in the emulsification capsule under refrigeration storage could be linked to the reduced bacterial metabolic activity induced by low temperatures, as well as the increased capsule stability over time [32].
Probiotic-Related Physiological Functionality Properties of Encapsulated L. rhamnosus GG
pH and Bile Salts Tolerance
Table 2 shows the survival rates of L. rhamnosus GG in the extrusion and emulsification capsules when exposed to different pH values (7.2, 5, 3, and 2) and bile salt concentrations (0.1, 0.15, 0.2, 0.3, 1%). After 3 h of exposure to pH 7.2 and 5, L. rhamnosus GG had a higher survival rate in the extrusion capsule (99.86 ± 1.11–99.72 ± 2.15%) (p ≤ 0.05) than in the emulsification capsule (52.23 ± 1.28–77.69 ± 1.11%) and control (61.01 ± 1.03–71.26 ± 1.18%). After 3 h of exposure to pH 3, the survival rate of L. rhamnosus GG sharply decreased in the extrusion and emulsification capsules (< 1.00 ± 0.00%) (p ≤ 0.05). However, after 3 h of exposure to pH 2, the highest survival rates of L. rhamnosus GG were detected in the emulsification capsule (99.90 ± 0.24%), followed by extrusion capsule (94.28 ± 1.23%) and control (40.42 ± 1.31%). Notably, the encapsulated L. rhamnosus GG cells had increased tolerance to low pH, indicating that encapsulation protected these cells from pH variations. Previous studies reported the potential use of sodium alginate and chitosan to encapsulate lactobacilli, with efficacy in protecting bacterial cells from acidic environments [34,35,36].
After exposure to 0.1, 0.15, and 0.2% bile salts, L. rhamnosus GG had the highest survival rates in the extrusion capsule (96.05 ± 1.18–99.81 ± 2.33%) (p ≤ 0.05), followed by emulsification capsule (79.79 ± 2.17–91.60 ± 1.50%) and control (57.47 ± 1.44–65.51 ± 1.07%). After exposure to 0.3 and 1% bile salts, L. rhamnosus GG had the highest survival rate in the emulsification capsule (76.38 ± 1.25% and 40.03 ± 1.25%, respectively) (p ≤ 0.05), followed by extrusion capsule (64.49 ± 2.06% and 16.32 ± 1.74%, respectively) and control (20.11 ± 2.04% and 9.57 ± 1.54%, respectively).
Bile and acid tolerance are important indicators of the survival capacity of probiotic strains during the gastrointestinal tract passage to reach the human colon [37]. Overall, the higher the bile salt concentration, the lower the L. rhamnosus GG survival rates [28, 37]. However, L. rhamnosus GG showed higher survival rates in the extrusion and emulsification capsules when exposed to all tested bile salt concentrations compared to the control. These results agree with early studies showing that calcium alginate and chitosan protect encapsulated bacterial cells from damage induced by bile salts [28, 38].
Exposure to Simulated Gastrointestinal Conditions
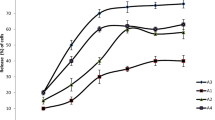
Figure 2 shows the survival rates of L. rhamnosus GG encapsulated with extrusion and emulsification techniques when exposed to different phases of the simulated gastrointestinal digestion. During the initial phases of the simulated gastrointestinal digestion, where the pH was 5.5 and 3.8 (esophageal-stomach), the survival rates of L. rhamnosus GG in the extrusion and emulsification capsules (83.01 ± 2.5–108.46 ± 1.1%) did not differ (p > 0.05) or were higher (p ≤ 0.05) than in control (91.87 ± 0.88–95.62 ± 0.87%).
At the most acidic pH of the esophageal-stomach phase (pH 2.0), L. rhamnosus GG had the greatest survival rate in the emulsification capsule (106.04 ± 0.35%), followed by the extrusion capsule (99.35 ± 0.49%) and control (86.87 ± 0.88%). In the duodenal phase (pH 5.0), the survival rate of L. rhamnosus GG decreased (p ≤ 0.05) in the emulsification capsule (81.91 ± 1.5%) and increased (p ≤ 0.05) in the extrusion capsule and control (114.15 ± 0.44% and 91.87 ± 1.02%, respectively). At the end of the simulated gastrointestinal digestion (ileal phase, pH 6.5), L. rhamnosus GG had a survival rate similar of around 73.38 ± 0.71% and 72.65 ± 1.09% in the extrusion and emulsification capsules, respectively.
L. rhamnosus free cells typically have a sharp viability loss when exposed to simulated gastrointestinal conditions [13, 19]. Encapsulating probiotic microorganisms could increase the protection against adverse conditions due to pH variations and the action of bile salts and enzymes when exposed to the stomach and intestinal fluids [39]. Even with differences between the formulated capsules, L. rhamnosus GG cells had high survival rates (above 70%) in the extrusion and emulsification capsules, indicating that encapsulated cells could keep their probiotic functionality due to surviving passage through the gastrointestinal tract [21].
Alginate and chitosan as wall materials to encapsulate bacterial cells have been linked to high resistance to gastric fluids because of their typical cationic behavior in media [32, 40]. Furthermore, the mucoadhesive properties of natural polymers, such as alginate and chitosan, used to produce encapsulated L. rhamnosus GG cells could help high populations of live bacterial cells to reach the colon [18], where these cells could adhere to the intestinal epithelial cells and perform the desired claimed beneficial effects on the host [21, 41, 42].
Technological Properties of Encapsulated L. rhamnosus GG
Proteolytic and Lipolytic Activity
L. rhamnosus GG cells in the extrusion and emulsification capsules and control had positive results for proteolytic activity and negative results for lipolytic activity (Table 3). Proteolysis causes enzymatic protein degradation to produce medium- or low-molecular-weight peptides and free amino acids, affecting flavor and food texture [43]. L. rhamnosus GG cells commonly show high proteolytic activity when tested as encapsulated or free cells [44].
Lactic acid bacteria commonly have weak or no lipolytic activity, although this property varies among bacterial species [45]. Enterococci commonly have higher lipolytic activity than other lactic acid bacteria species, while Lactococcus and Lactobacillus commonly have weak lipolytic activity [46]. The weak lipolytic activity prevents or reduces the possibility of developing rancidity or off-flavors [47], and this feature may make L. rhamnosus GG cells encapsulated using extrusion and emulsification capsules techniques candidates for use in dairy products [48].
Exopolysaccharide and Diacetyl Production
L. rhamnosus GG in the extrusion and emulsification capsules and control produced EPS but had a low capacity for diacetyl production (Table 3). Microbial EPS, a secondary metabolite produced by some probiotic strains, could exert several health-related benefits, such as antitumor [49], antibacterial, antioxidant [50, 51], immunomodulatory [52], and intestinal microbiota modulatory effects [53]. EPS produced by L. rhamnosus GG effectively mitigates oxidative damage and apoptosis in intestinal epithelial cells [54] and ameliorates intestinal inflammation [55].
L. rhamnosus GG cells can use the available substrate to produce diacetyl and acetoin as by-products. Moreover, L. rhamnosus GG increases diacetyl in different food substrates, such as oats and coconut [56]. Diacetyl, a volatile compound from citrate metabolism, can impart distinct characteristics to fermented products, particularly dairy products [57]. The natural diacetyl aroma is associated with creamy and buttery flavor in dairy products (e.g., butter, cheese, and fermented milk), affecting consumer acceptance, besides having antimicrobial effects that could be exploited by the food industry [58].
Heat and NaCl Tolerance
Table 3 shows the survival rates of L. rhamnosus GG in the extrusion and emulsion capsules and control when exposed to different temperatures (28, 55, 65, 75, 85, and 95 °C) and NaCl concentrations (1, 3, and 5%). L. rhamnosus GG in the extrusion and emulsification capsules had high survival rates (≥ 100%) when exposed to 28, 55, and 65 °C, which did not differ (p > 0.05) or were higher (p ≤ 0.05) than control (100 ± 0.01, 74.97 ± 1.44%, and 57.27 ± 1.17%, respectively). L. rhamnosus GG in the emulsification capsule had a survival rate 103.13 ± 1.20% when exposed to 75 °C, while the survival rates decreased to 97.11 ± 1.18% and 54.86 ± 1.11% in the extrusion capsule and control, respectively. Similarly, L. rhamnosus GG had a higher survival rate (p ≤ 0.05) in the emulsification capsule when exposed to 85 and 95 °C (95.15 ± 1.17% and 89.67 ± 1.49%, respectively), followed by the extrusion capsule (87.89 ± 1.13% and 76.89 ± 1.26%, respectively) and control (44.85 ± 1.40% and 38.74 ± 1.17%, respectively).
Heating is one of the main stresses to bacterial cells during food processing. Various bacterial cell functions are greatly disturbed when exposed to high temperatures, which cannot be countered by the cellular response system, leading to viability loss and cell death [59]. The higher stability of encapsulated L. rhamnosus GG cells should be a favorable technological attribute to their incorporation in foods exposed to heating during processing [60]. An early study reported a higher tolerance of L. rhamnosus GG cells encapsulated with whey protein and gum Arabic [61]. The increased tolerance of some lactic acid bacteria to high temperatures has been linked to increased EPS production, helping bacterial cells survive harsh environmental conditions [62].
The survival rates of L. rhamnosus GG in the extrusion and emulsification capsules exposed to 1% NaCl (97.26 ± 0.85% and 99.52 ± 1.75%, respectively) were higher than the control (94.26 ± 1.62%) (p ≤ 0.05). Only L. rhamnosus GG in the extrusion capsule decreased the survival rate (94.17 ± 1.65%) when exposed to 3% NaCl compared to 1% NaCl (p ≤ 0.05). However, L. rhamnosus GG in the extrusion and emulsification capsules and control had high survival rates (approximately 91%) when exposed to 5% NaCl, representing a high NaCl tolerance, which is a positive feature for using probiotics in salted processed foods [63].
Performance of Encapsulated L. rhamnosus GG in UHT and Powdered Milk
Table 4 shows the survival rates of L. rhamnosus GG in the extrusion and emulsification capsules incorporated in whole UHT and powdered milk during 28 days of refrigeration storage. L. rhamnosus GG in the extrusion capsule had an overall higher survival rate in UHT milk than in powdered milk during storage (p ≤ 0.05), while emulsification capsules had a higher (p ≤ 0.05) or similar survival rate (p > 0.05) in powdered milk compared to UHT milk.
On days 7 to 14 of storage, the survival rate of L. rhamnosus GG in the extrusion (114.88 ± 1.07% to 116.18 ± 1.06%) and emulsification (105.22 ± 1.29% to 109.20 ± 2.10%) capsules increased in UHT milk (p ≤ 0.05). Powdered milk had highest survival rate (121.86 ± 2.03–114.98 ± 2.07%) of L. rhamnosus GG in the emulsification capsule (p ≤ 0.05), while the survival rate of L. rhamnosus GG in the extrusion capsule was approximately 91% (p ≤ 0.05).
On day 21 of storage, L. rhamnosus GG in the extrusion capsule had the highest survival rate (113.29 ± 1.06%) in UHT milk (p ≤ 0.05), while L. rhamnosus GG in the emulsification capsule had the lowest survival rate in powdered milk (105.81 ± 2.16%) (p ≤ 0.05). On day 28 of storage, L. rhamnosus GG in the extrusion and emulsification capsules decreased the survival rate in powdered milk (89.35 ± 0.32% and 101.37 ± 1.0%, respectively) (p ≤ 0.05). The highest survival rate on day 28 of storage was detected in UHT milk for the extrusion capsule (112.28 ± 2.18%), followed by the emulsification capsule (105.90 ± 1.16%).
An important difficulty in incorporating probiotics in foods is the bacterial viability loss during storage [22]. The results showed overall high survival rates of L. rhamnosus GG in the extrusion and emulsification capsules in UHT and powdered milk during 28 days of refrigeration storage, which could be linked to the capsule size, tested probiotic, and/or post-acidification processes during storage [39]. These factors could affect the diffusion capacity of components within and outside the capsule due to the alginate porosity, suggesting that the nutrient passage could be used by the encapsulated bacterial cells to keep their viability during storage [22].
The survival rates of L. rhamnosus GG in the extrusion and emulsification capsules on day 28 of refrigeration storage were higher than previously reported for encapsulated L. rhamnosus cells in apple juice and yogurt during 22 and 30 days of refrigeration storage, respectively [22]. The extrusion and emulsification capsules could provide a favorable environment for L. rhamnosus GG cells and a physical barrier against environmental conditions affecting their viability and survival [13, 64].
The physicochemical characteristics of milk can be changed due to probiotic supplementation [65]. Table 4 shows the results of the measured physicochemical parameters of UHT and powdered milk supplemented with L. rhamnosus GG in the extrusion and emulsification capsules during 28 days of refrigeration storage. The titratable acidity increased during storage in UHT milk supplemented with L. rhamnosus GG in the extrusion and emulsification capsules (1.9 ± 0.06 to 2.6 ± 0.13 and 1.7 ± 0.10 to 2.6 ± 0.06°Brix, respectively) (p ≤ 0.05), while decreased in powdered milk (2.6 ± 0.13 for 1.6 ± 0.01 and 2.5 ± 0.13 to 1.6 ± 0.06°Brix, respectively) (p ≤ 0.05).
The pH values increased on day 7 of storage in UHT and powdered milk supplemented with L. rhamnosus GG in the extrusion and emulsification capsules but decreased on day 14 of storage (p ≤ 0.05). Overall, the pH values did not differ between UHT and powdered milk supplemented with L. rhamnosus GG in extrusion or emulsification capsule at the same storage period but differed during storage. The increase in titratable acidity and reduction in pH values during storage in UHT and powdered milk supplemented with L. rhamnosus GG in the extrusion and emulsification capsules agree with results of previous studies with milk supplemented with encapsulated lactic acid bacteria [32, 65], which could be linked to increased lactic acid concentrations due to lactose degradation [66].
The total soluble solids decreased (p ≤ 0.05) and did not change (p > 0.05) during storage in UHT and powdered milk supplemented with L. rhamnosus GG in the extrusion and emulsification capsules, respectively. As soluble solids decrease, the moisture content commonly increases in foods [65, 66]. The moisture did not change during storage in UHT milk supplemented with L. rhamnosus GG in the extrusion and emulsification capsules (88.1 ± 0.01–89.3 ± 1.41%) (p > 0.05) but increased in powdered milk regardless of the supplemented capsules (1.0 ± 0.36 to 4.4 ± 0.41%) (p ≤ 0.05). Powdered milk commonly has moisture content varying from approximately 3 to 5% [67]. Moisture can affect bacterial cell viability and survival in powdered foods even when in encapsulated cells [17]; however, low moisture is recommended for maintaining stability during prolonged storage of probiotic powder foods [68].
Conclusion
Chitosan-sodium alginate capsules containing L. rhamnosus GG were successfully developed with extrusion and emulsification techniques. Extrusion and emulsification encapsulation techniques showed high encapsulation efficiency and high survival rates of L. rhamnosus GG during refrigeration and room temperature storage and during exposure to simulated gastrointestinal conditions. Still, L. rhamnosus GG in the extrusion and emulsification capsules performed satisfactorily in probiotic-related physiological and technological properties. L. rhamnosus GG in the extrusion and emulsification capsules had high survival rates and did not significantly affect physicochemical parameters in UHT and powdered milk during storage. These results indicated that L. rhamnosus GG can be effectively encapsulated with an alginate-chitosan mixture as wall materials using extrusion and emulsification techniques, as well as UHT and powdered milk could be suitable delivery matrices to enhance the consumption of this encapsulated probiotic among consumers.
Data Availability
No datasets were generated or analysed during the current study.
References
Moreno I, Marasca ETG, de Sá PBZR et al (2018) Evaluation of probiotic potential of bacteriocinogenic lactic acid bacteria strains isolated from meat products. Probiotics Antimicro Prot 10:762–774. https://doi.org/10.1007/s12602-018-9388-9
Hill C, Guarner F, Reid G et al (2014) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Mandracchia B, Palpacuer J, Nazzaro F, Bianco V, Rega R, Ferraro P, Grilli S (2018) Biospeckle decorrelation quantifies the performance of alginate-encapsulated probiotic bacteria. IEEE J Sel Top Quantum Electron 25:1–6. https://doi.org/10.1109/JSTQE.2018.2836941
Vandenplas Y, Huys G, Daube G (2015) Probiotics: An update. J Pediatr 91:6–21. https://doi.org/10.1016/j.jped.2014.08.005
Bradford R, Reyes V, Bonilla F et al (2019) Development of milk powder containing Lactobacillus plantarum NCIMB 8826 immobilized with prebiotic hi-maize starch and survival under simulated gastric and intestinal conditions. Food Prod Process Nutr 1:10. https://doi.org/10.1186/s43014-019-0011-6
Shen Y, Zhang J, Ma M et al (2024) Lacticaseibacillus paracasei JY025 fortified milk powder: In vitro digestion characteristics and accelerated storage stability. Food Sci Technol 198:115937. https://doi.org/10.1016/j.lwt.2024.115937
De Prisco A, Mauriello G (2016) Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci Technol 48:27–39. https://doi.org/10.1016/j.tifs.2015.11.009
Pereira EPR, da Graça JS, Ferreira BM et al (2023) What are the main obstacles to turning foods healthier through probiotics incorporation? A review of functionalization of foods by probiotics and bioactive metabolites. Food Res Int. https://doi.org/10.1016/j.foodres.2023.113785
Ragavan ML, Das N (2018) Process optimization for microencapsulation of probiotic yeasts. Front Biol 13:197–207. https://doi.org/10.1007/s11515-018-1495-1
Bekhit M, Sánchez-González L, Messaoud GB, Desobry S (2016) Encapsulation of Lactococcus lactis subsp. lactis on alginate/pectin composite microbeads: Effect of matrix composition on bacterial survival and nisin release. J Food Eng 180:1–9. https://doi.org/10.1016/j.jfoodeng.2016.01.031
Rutz JK, Borges CD, Zambiazi RC, da Rosa CG, da Silva MM (2016) Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem 202:324–333. https://doi.org/10.1016/j.foodchem.2016.01.140
Koupantsis T, Pavlidou E, Paraskevopoulou A (2016) Glycerol and tannic acid as applied in the preparation of milk proteins–CMC complex coavervates for flavour encapsulation. Food Hydrocol 57:62–71. https://doi.org/10.1016/j.foodhyd.2016.01.007
Barajas-Álvarez P, González-Ávila M, Espinosa-Andrews H (2022) Microencapsulation of Lactobacillus rhamnosus HN001 by spray drying and its evaluation under gastrointestinal and storage conditions. Food Sci Technol 153:112485. https://doi.org/10.1016/j.lwt.2021.112485
Melchior S, Marino M, D’Este F, Innocente N, Nicoli MC, Calligaris S (2021) Effect of the formulation and structure of monoglyceride-based gels on the viability of probiotic Lactobacillus rhamnosus upon in vitro digestion. Food Funct 12:351–361. https://doi.org/10.1039/D0FO01788D
Zanjani MAK, Tarzi BG, Sharifan A, Mohammadi N, Bakhoda H, Madanipour MM (2012) Microencapsulation of Lactobacillus casei with calcium alginate-resistant starch and evaluation of survival and sensory properties in cream-filled cake. Afr J Microbiol Res 6:5511–5517. https://doi.org/10.5897/AJMR12.972
Suvarna S, Dsouza J, Ragavan ML et al (2018) Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci Biotechnol 27:745–753. https://doi.org/10.1007/s10068-018-0310-8
Luca L, Oroian M (2021) Influence of different prebiotics on viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 10:710. https://doi.org/10.3390/foods10040710
Qi X, Simsek S, Ohm JB, Chen B, Rao J (2020) Viability of Lactobacillus rhamnosus GG microencapsulated in alginate/chitosan hydrogel particles during storage and simulated gastrointestinal digestion: role of chitosan molecular weight. Soft Matter 16:1877–1887. https://doi.org/10.1039/C9SM02387A
Santos MA, Machado MT (2021) Coated alginate–chitosan particles to improve the stability of probiotic yeast. Int J Food Sci Tech 56:2122–2131. https://doi.org/10.1111/ijfs.14829
Rajoka MSR, Mehwish HM, Siddiq M et al (2017) Identification, characterization, and probiotic potential of Lactobacillus rhamnosus isolated from human milk. Food Sci Technol 84:271–280. https://doi.org/10.1016/j.lwt.2017.05.055
Capurso L (2019) Thirty years of Lactobacillus rhamnosus GG: A review. J Clin Gastroent 53:S1–S41. https://doi.org/10.1097/MCG.0000000000001170
Romero-Chapol OO, Varela-Pérez A, Castillo-Olmos AG, García HS, Singh J, García-Ramírez PJ, Viveros-Contreras R, Figueroa-Hernández CY, Cano-Sarmiento C (2022) Encapsulation of Lacticaseibacillus rhamnosus GG: Probiotic survival, in vitro digestion and viability in apple juice and yogurt. Appl Sci 12:2141. https://doi.org/10.3390/app12042141
Krasaekoopt W, Bhandari B, Deeth H (2003) Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J 13:3–13. https://doi.org/10.1016/S0958-6946(02)00155-3
Chen M, Mustapha A (2012) Survival of freeze-dried microcapsules of α-galactosidase producing probiotics in a soy bar matrix. Food Microbiol 30:68–73. https://doi.org/10.1016/j.fm.2011.10.017
Sánchez MT, Ruiz MA, Lasserrot A, Hormigo M, Morales ME (2017) An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: protection, survival and stability study. Food Hydrocoll 69:67–75. https://doi.org/10.1016/j.foodhyd.2017.01.019
de Albuquerque TMR, Garcia EF, de Oliveira AA et al (2018) In vitro characterization of Lactobacillus strains isolated from fruit processing by-products as potential probiotics. Probiotics Antimicro Prot 10:704–716. https://doi.org/10.1007/s12602-017-9318-2
de Almeida Júnior WLG, da Silva FÍ, de Souza JV, da Silva CDA, da Costa MM, Dias FS (2015) Characterization and evaluation of lactic acid bacteria isolated from goat milk. Food Control 53:96–103. https://doi.org/10.1016/j.foodcont.2015.01.013
Verruck S, de Carvalho MW, de Liz GR, Amante ER, Vieira CRW, Amboni RDDMC, Prudencio ES (2017) Survival of Bifidobacterium BB-12 microencapsulated with full-fat goat’s milk and prebiotics when exposed to simulated gastrointestinal conditions and thermal treatments. Small Rumin Res 153:48–56. https://doi.org/10.1016/j.smallrumres.2017.05.008
AOAC - ASSOCIATION OF OFFICIAL AGRICULTURAL CHEMISTS (2016) Official methods of analysis of Association of Official Analytical Chemists International, 20th edn. AOAC International, Salt Lake City
Hugues-Ayala AM, Sarabia-Sainz JAI, González-Rios H, Vázquez-Moreno L, Montfort GRC (2020) Airbrush encapsulation of Lactobacillus rhamnosus GG in dry microbeads of alginate coated with regular buttermilk proteins. Food Sci Technol 117:108639. https://doi.org/10.1016/j.lwt.2019.108639
Nedović V, Kalušević A, Manojlović V, Petrović T, Bugarski B (2013) Encapsulation systems in the food industry. In: Taoukis P, Stoforos N, Karathanos V (eds) Advances in food process engineering research and applications. Food Eng Series. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-7906-2_13
Dimitrellou D, Kandylis P, Lević S, Petrović T, Ivanović S, Nedović V, Kourkoutas Y (2019) Encapsulation of Lactobacillus casei ATCC 393 in alginate capsules for probiotic fermented milk production. Food Sci Technol 116:108501. https://doi.org/10.1016/j.lwt.2019.108501
Xu M, Gagné-Bourque F, Dumont MJ, Jabaji S (2016) Encapsulation of Lactobacillus casei ATCC 393 cells and evaluation of their survival after freeze-drying, storage and under gastrointestinal conditions. J Food Eng 168:52–59. https://doi.org/10.1016/j.jfoodeng.2015.07.021
Varela-Pérez A, Romero-Chapol OO, Castillo-Olmos AG, García HS, Suárez-Quiroz ML, Singh J, Figueroa-Hernández CY, Viveros-Contreras R, Cano-Sarmiento C (2022) Encapsulation of Lactobacillus gasseri: characterization, probiotic survival, in vitro evaluation and viability in apple juice. Foods 11:740. https://doi.org/10.3390/foods11050740
Oberoi K, Tolun A, Altintas Z, Sharma S (2021) Effect of alginate-microencapsulated hydrogels on the survival of Lactobacillus rhamnosus under simulated gastrointestinal conditions. Foods 10:1999. https://doi.org/10.3390/foods10091999
Abbaszadeh S, Gandomi H, Misaghi A, Bokaei S, Noori N (2014) The effect of alginate and chitosan concentrations on some properties of chitosan-coated alginate beads and survivability of encapsulated Lactobacillus rhamnosus in simulated gastrointestinal conditions and during heat processing. J Sci Food Agric 94:2210–2216. https://doi.org/10.1002/jsfa.6541
Tripathi MK, Giri SK (2014) Probiotic functional foods: Survival of probiotics during processing and storage. J Funct Foods 9:225–241. https://doi.org/10.1016/j.jff.2014.04.030
Chen HY, Li XY, Liu BJ, Meng XH (2017) Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J Funct Foods 29:248–255. https://doi.org/10.1016/j.jff.2016.12.015
Frakolaki G, Giannou V, Kekos D, Tzia C (2021) A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit Rev Food Sci Nutr 61:1515–1536. https://doi.org/10.1080/10408398.2020.1761773
Ramos PE, Cerqueira MA, Teixeira JA, Vicente AA (2018) Physiological protection of probiotic microcapsules by coatings. Crit Rev Food Sci Nutr 58:1864–1877. https://doi.org/10.1080/10408398.2017.1289148
Patel M, Siddiqui AJ, Hamadou WS et al (2021) Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from Lactobacillus rhamnosus with its physicochemical and functional properties. Antibiotics 10:1546. https://doi.org/10.3390/antibiotics10121546
Tong L, Zhang X, Hao H et al (2021) Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in DSS-induced colitis mice. Nutrients 13:3319. https://doi.org/10.3390/nu13103319
Chávez de la Vega MI, Alatorre-Santamaría S, Gómez-Ruiz L, García-Garibay M, Guzmán-Rodríguez F, González-Olivares LG, Cruz-Guerrero AE, Rodríguez-Serrano GM (2021) Influence of oat β-glucan on the survival and proteolytic activity of Lactobacillus rhamnosus GG in milk fermentation: optimization by response surface. Fermentation 7:210. https://doi.org/10.3390/fermentation7040210
Fortuin J, Hellebois T, Iken M, Shaplov AS, Fogliano V, Soukoulis C (2024) Stabilising and functional effects of Spirulina (Arthrospira platensis) protein isolate on encapsulated Lacticaseibacillus rhamnosus GG during processing, storage and gastrointestinal digestion. Food Hydrocoll 149:109519. https://doi.org/10.1016/j.foodhyd.2023.109519
Xu Y, Tian Y, Cao Y et al (2019) Probiotic properties of Lactobacillus paracasei subsp. paracasei L1 and its growth performance-promotion in chicken by improving the intestinal microflora. Front Physiol 10:937. https://doi.org/10.3389/fphys.2019.00937
García-Cano I, Rocha-Mendoza D, Ortega-Anaya J et al (2019) Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl Microbiol Biotechnol 103:5243–5257. https://doi.org/10.1007/s00253-019-09844-6
Sonei A, Dovom MRE, Yavarmanesh M (2024) Evaluation of probiotic, safety, and technological properties of bacteriocinogenic Enterococcus faecium and Enterococcus faecalis strains isolated from lighvan and koozeh cheeses. Int Dairy J 148:105807. https://doi.org/10.1016/j.idairyj.2023.105807
Barzegar H, Alizadeh Behbahani B, Falah F (2021) Safety, probiotic properties, antimicrobial activity, and technological performance of Lactobacillus strains isolated from Iranian raw milk cheeses. Food Sci Nutr 9:4094–4107. https://doi.org/10.1002/fsn3.2365
El-Deeb NM, Yassin AM, Al-Madboly LA et al (2018) A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer. Microb Cell Fact 17:29. https://doi.org/10.1186/s12934-018-0877-z
Min WH, Fang X-B, Wu T, Fang L, Liu CL, Wang J (2019) Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J Biosci Bioeng 127:758–766. https://doi.org/10.1016/j.jbiosc.2018.12.004
Tang W, Zhou J, Xu Q, Dong M, Fan X, Rui X et al (2020) In vitro digestion and fermentation of released exopolysaccharides (r-EPS) from Lactobacillus delbrueckii ssp. bulgaricus SRFM-1. Carbohydr Polym 230:115593. https://doi.org/10.1016/j.carbpol.2019.115593
Chaisuwan W, Jantanasakulwong K, Wangtueai S, Phimolsiripol Y, Chaiyaso T, Techapun C et al (2020) Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Biosci 35:100564. https://doi.org/10.1016/j.fbio.2020.100564
Allonsius CN, van den Broek MFL, De Boeck I, Kiekens S, Oerlemans EFM, Kiekens F et al (2017) Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb Biotechnol 10:1753–1763. https://doi.org/10.1111/1751-7915.12799
Li J, Li Q, Gao N, Wang Z, Li F, Li J et al (2021) Exopolysaccharides produced by Lactobacillus rhamnosus GG alleviate hydrogen peroxide-induced intestinal oxidative damage and apoptosis through the Keap1/Nrf2 and Bax/Bcl-2 pathways in vitro. Food Funct 12:9632–9641. https://doi.org/10.1039/d1fo00277e
Li J, Li Q, Wu Q et al (2023) Exopolysaccharides of Lactobacillus rhamnosus GG ameliorate Salmonella typhimurium-induced intestinal inflammation via the TLR4/NF-κB/MAPK pathway. J Animal Sci Biotechnol 14:23. https://doi.org/10.1186/s40104-023-00830-7
Ningtyas DW, Bhandari B, Bansal N, Prakash S (2019) Flavour profiles of functional reduced-fat cream cheese: Effects of β-glucan, phytosterols, and probiotic L. rhamnosus. Food Sci Technol 105:16–22. https://doi.org/10.1016/j.lwt.2019.01.063
Joshi SR, Koijam K (2014) Exopolysaccharide production by a lactic acid bacterium, Leuconostoc lactis isolated from ethnically fermented beverage. Natl Acad Sci Lett 37:59–64. https://doi.org/10.1007/s40009-013-0203-6
Clark S, Winter CK (2015) Diacetyl in foods: A review of safety and sensory characteristics. Compr Rev Food Sci Food Saf 14:634–643. https://doi.org/10.1111/1541-4337.12150
Wang Y, Hao F, Lu W et al (2020) Enhanced thermal stability of lactic acid bacteria during spray drying by intracellular accumulation of calcium. J Food Eng 279:109975. https://doi.org/10.1016/j.jfoodeng.2020.109975
Hao F, Fu N, Ndiaye H et al (2021) Thermotolerance, survival, and stability of lactic acid bacteria after spray drying as affected by the increase of growth temperature. Food Bioprocess Technol 14:120–132. https://doi.org/10.1007/s11947-020-02571-1
Yin M, Chen M, Yuan Y, Liu F, Zhong F (2024) Encapsulation of Lactobacillus rhamnosus GG in whey protein isolate-shortening oil and gum Arabic by complex coacervation: Enhanced the viability of probiotics during spray drying and storage. Food Hydrocoll 146:109252. https://doi.org/10.1016/j.foodhyd.2023.109252
Zhang K, Liu S, Liang S (2023) Exopolysaccharides of lactic acid bacteria: structure, biological activity, structure-activity relationship, and application in the food industry: A review. Int J Biol Macromol 1:128733. https://doi.org/10.1016/j.ijbiomac.2023.128733
Nguyen PT, Nguyen TT, Vo TNT et al (2021) Response of Lactobacillus plantarum VAL6 to challenges of pH and sodium chloride stresses. Sci Rep 11:1301. https://doi.org/10.1038/s41598-020-80634-1
Ding WK, Shah NP (2008) Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int Food Res 15:219–232
Richards M, Buys EM, De Kock HL (2016) Survival analysis, consumer perception and physico-chemical analysis of low fat UHT milk stored for different time periods. Int Dairy J 57:56–61. https://doi.org/10.1016/j.idairyj.2016.02.037
Siddique F, Riffatb S, Arshadc M et al (2016) Effect of storage temperature on the physiochemical properties of Ultra High Temperature (UHT) milk. Int J Food Allied Sci 2:52–57
Nese C, Palugan L, Cerea M, Pinto JF (2020) Preparation and characterization of a powder manufactured by spray drying milk based formulations for the delivery of theophylline for pediatric use. Int J Pharm 580:119227. https://doi.org/10.1016/j.ijpharm.2020.119227
Bhagwat A, Bhushette P, Annapure US (2020) Spray drying studies of probiotic Enterococcus strains encapsulated with whey protein and maltodextrin. Beni-Suef Univ J Basic Appl Sci 9:33. https://doi.org/10.1186/s43088-020-00061-z
Funding
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Brazil (process no 300885/2021–0), and Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco—FACEPE (APQ-0263–2.12/19).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.J.A.A.A., L.R.R.B, T.C.M.S. Formal analysis: A.J.A.A.A., L.R.R.B, T.C.M.S. Funding acquisition: E.L.S, T.C.M.S. Investigation: A.J.A.A.A., K.F.D.F., H.M.A.N., S.P.A.O., L.A.A.L. Methodology: A.J.A.A.A., L.R.R.B, T.M.R.A., C.E.V.O., M.L.C., E.L.S., T.C.M.S. Project administration: T.C.M.S. Supervision: L.R.R.B, T.C.M.S. Validation: A.J.A.A.A., L.R.R.B, T.C.M.S. Writing – original draft: A.J.A.A.A., L.R.R.B, T.M.R.A., K.B.S., E.L.S., T.C.M.S. Writing – review & editing: A.J.A.A.A., T.M.R.A., K.B.S., E.L.S.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Athayde, A.J.A.A., Berger, L.R.R., de Albuquerque, T.M.R. et al. Physiological and Technological Properties of Probiotic Lacticaseibacillus rhamnosus GG Encapsulated with Alginate-Chitosan Mixture and Its Incorporation in Whole Milk. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10345-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10345-w