Abstract
Cancer remains a global problem, with millions of new cases diagnosed yearly and countless lives lost. The financial burden of cancer therapy, along with worries about the long-term safety of existing medicines, necessitates the investigation of alternative approaches to cancer prevention. Probiotics generate chemopreventive compounds such as bacteriocins, short-chain fatty acids (SCFA), and extracellular polymeric substances (EPS), which have demonstrated the ability to impede cancer cell proliferation, induce apoptosis, and bolster the expression of pro-apoptotic genes. On the other hand, prebiotics, classified as non-digestible food ingredients, promote the proliferation of probiotics within the colon, thereby ensuring sustained functionality of the gut microbiota. Consequently, the synergistic effect of combining prebiotics with probiotics, known as the synbiotic effect, in dietary interventions holds promise for potentially mitigating cancer risk and augmenting preventive measures. The utilization of gut microbiota in cancer treatment has shown promise in alleviating adverse health effects. This review explored the potential and the role of probiotics and synbiotics in enhancing health and contributing to cancer prevention efforts. In this review, the applications of functional probiotics and synbiotics, the mechanisms of action of probiotics in cancer, and the relationship of probiotics with various drugs were discussed, shedding light on the potential of probiotics and synbiotics to alleviate the burdens of cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer caused by unregulated cell proliferation that leads to benign or malignant tumor growth is currently one of the top causes of death globally. About 19.3 million new cancer cases were recorded globally in 2020, leading to 10 million fatalities due to cancer’s lethal malignancy trait [1]. Nowadays, oncology medications are used alongside other treatment modalities, including chemotherapy, radiation therapy, and surgery [2]. In 2017, patients paid an average of $229,295.33 for breast cancer treatment [3]. Costs for breast cancer treatment ranged from $60,637 to $134,682, almost triple the cost from stage I to IV [4]. Moreover, a study has shown that the average distance for a patient to enrol for the facility in Malaysia was 41.4 km with a minimal cost of $14.08 [5]. Aside from the exorbitant cost of cancer treatments, the long-term safety of synthetic medications in contemporary cancer treatments is debatable. According to [6], the accumulation of toxins in the body from continuously administering chemotherapy medications might cause catastrophic side effects such as dermatologic, ventricular dysfunction, and ophthalmologic. In light of this, developing low-cost chemopreventive treatments for minimal toxicity is critical.
The dietary intake and contemporary lifestyle have also increased the occurrence of methemoglobin and other disorders [7]. In fact, more than half of cancer occurrences, diabetes and cardiovascular diseases, and deaths are preventable, in which 30% of cancer is related to nutrition intake and diet [8]. Recent research has highlighted the significant impact of probiotics and synbiotics on health, suggesting their potential as chemopreventive agents. Functional foods such as yogurt, kefir, miso, tempeh, sauerkraut, and sourdough are known to enhance health outcomes due to their probiotic properties [9]. Extensive studies have demonstrated the role of microbes in cancer biology, including their involvement in metabolic regulation, the gut-brain axis, immune system development, and gastrointestinal carcinogenesis. Importantly, probiotics in the gut microbiota have been shown to mitigate the adverse effects of chemotherapy [10,11,12]. Predominant intestinal microbes such as Lactobacillus and Bifidobacterium have been documented to bind with and deactivate carcinogens at the initial stages of colon carcinogenesis [13]. Lactobacillus plantarum KU15149 and Bifidobacterium pseudocatenulatum G7 have been reported to adhere to gut immune cells, influence toll-like receptor signaling, increase pro-inflammatory cytokine levels, and modulate T cells, thereby inhibiting cancer development [14, 15].
The global market for probiotics was valued at approximately $57.8 billion in 2022, with forecasts projecting growth to reach $85.4 billion by 2027 [16]. This growth is driven by their recognized benefits in improving gut health, enhancing livestock and pet performance, reducing mortality rates, and even in applications such as edible films and coatings that enhance food safety and preservation [17]. The probiotics industry has evolved into a multi-billion-dollar sector within the human consumption market alone. Probiotics have been employed to reduce mortality rates in fish, enhance growth in piglets, improve egg production quality in poultry, fortify immune defenses in fish, and mitigate Salmonella contamination in chickens [18,19,20,21,22]. Moreover, incorporating probiotics into edible films and coatings, such as a gelatin-based coating with inulin and Lacticaseibacillus rhamnosus used on fresh strawberries, has notably enhanced product stability and functional properties, maintaining probiotic viability while reducing pathogen counts and preserving strawberry quality, phenolic content, and antioxidant activity [23, 24].
This review aims to explore the potential of probiotics and synbiotics as chemopreventive agents, focusing on their mechanisms of action, the evidence supporting their role in cancer prevention, and the future directions for research in this field. By delving into the complex interactions between diet, gut microbiota, and cancer, this review seeks to provide a comprehensive overview of how probiotics and synbiotics could contribute to innovative and effective cancer prevention strategies.
The Health Benefits of Probiotics and Their Role in Cancer Prevention
Cancer development is multifactorial, influenced by genetics, environmental carcinogens, lifestyle choices, and dietary habits [25]. Diets high in fats and sugars and low in fiber can lead to an imbalance of gut microbiota (dysbiosis), which is linked to numerous diseases, including cancer [11, 26]. For instance, a high intake of polyunsaturated omega-6 fatty acids may alter gut microbiota, leading to metabolic disturbances and obesity, thereby increasing cancer risks [27]. Dysbiosis can also trigger inflammatory and immune responses that facilitate cancer development [28, 29]. The native gut microbe, Fusobacterium nucleatum, has been shown to invade tumor cells and initiate tumorigenesis, highlighting the critical role of microbiota regulation in cancer prevention [30].
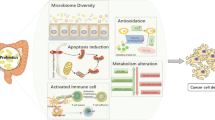
Probiotics, defined as live microorganisms that confer health benefits when administered in adequate amounts, have been reported to modulate gut microbiota in terms of enhancing barrier functions, reducing permeability, reducing systemic inflammation, preventing the translocation of potential carcinogens, and promoting an environment that is less conducive to cancerous growths (Table 1). Probiotics such as Bifidobacterium, Lactobacillus, Lactococcus, Streptococcus, and Enterococcus have been claimed to enhance gut health by maintaining intestinal pH, producing hydrogen peroxide to inhibit pathogen growth, and synthesizing antimicrobial peptides, thus creating an environment detrimental to pathogenic bacteria and beneficial for the host [31]. For example, Lactobacillus plantarum UBLP40 produces hydrogen peroxide against pathogens like Micrococcus luteus MTCC 106 and methicillin-resistant Staphylococcus aureus subsp. aureus ATCC® BAA-1720 to prevent the proliferation of harmful microbial colonies that can contribute to cancer progression [32].
Some probiotics produce short-chain fatty acids (SCFAs), including butyrate, propionate, and acetate, which serve as energy sources for colonocytes and regulate cell proliferation and apoptosis in cancer cells [58]. SCFAs such as those produced by Bifidobacterium animalis subsp. lactis GCL2505 and Lacticaseibacillus paracasei SD1 have demonstrated anti-cancer properties and help maintain a healthy intestinal microflora [59, 60]. Probiotics also enhance the body’s immune response against cancer cells by regulating cytokine production, which is critical for directing immune responses and improving surveillance and destruction of cancer cells. Enterococcus faecium S29 (EU 158,188) secretes antimicrobial compounds that compete with pathogenic bacteria in the gut mucosa, altering microbiota composition and reducing cancer development [61]. Similarly, the intake of Lactobacillus gasseri OLL2716: LG21 has been associated with a decrease in Clostridium perfringens levels (a bacterium linked to colorectal cancer) and an increase in beneficial SCFAs in the gut of colorectal cancer patients [62].
Moreover, probiotics play a role in enhancing mucin production and maintaining the integrity of the gut barrier. It is crucial for preventing the translocation of bacteria and harmful metabolites into the systemic circulation, which could lead to systemic inflammation and cancer [63]. Escherichia coli Nissle 1917 had been reported to inhibit the leaky gut condition by upregulating the zonula occludens-1 (ZO-1) in murine intestinal epithelial cells [64]. Lactobacillus casei GG has been shown to inhibit the translocation of specific pathogenic bacteria by upregulating the MUC2 gene expression, contributing to maintaining the gut barrier integrity [64]. Probiotics, such as Lactobacillus rhamnosus GG, have been shown to suppress procarcinogenic fecal enzymes like azoreductase, nitroreductase, and β-glucuronidase, which can convert procarcinogens into carcinogens [65]. Lactobacillus rhamnosus GG suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis [51, 66]. Lactulose and OF-IN significantly decreased beta-glucuronidase activity, whereas a tendency to a decreased beta-glucuronidase activity was observed after L. casei Shirota intake [51, 67, 68].
Probiotics have also been reported to inhibit cancer cell proliferation and induce apoptosis directly. Probiotics interact with and decrease toll-like receptors (TLR) on epithelial cells, macrophages, and lymphocytes, as well as regulate the synthesis of interleukin-10 (IL-10) and immunoglobulin A antibodies (IgA) to eradicate pathogenic bacteria in the intestine [69, 70]. For instance, Lactobacillus casei KCCM11072P (LC11072) exhibits antiproliferative activity against gastric cell lines by inhibiting NF-κB and mTOR-mediated signaling [71, 72]. The study demonstrates that the cell-free supernatant of Enterococcus faecalis KUMS-T48 displayed cytotoxic effects on gastric cancer cell lines [73]. Beyond these cellular effects, probiotics can also bind to and degrade carcinogenic substances in the intestinal lumen, reducing their potential harm [74]. Saccharomyces have demonstrated clinical and experimental effectiveness in gut diseases such as antibiotic-associated diarrhoea, Crohn’s disease, and irritable bowel syndrome [75]. Saccharomyces cerevisiae var. boulardii has been shown to protect the normal microbiota of the human gut, inhibit pathogenic infections, and exhibit immune-modulatory properties, making it beneficial for managing irritable bowel syndrome [76].
Synbiotics: Combining Probiotics and Prebiotics with Chemopreventive
Synbiotics, which synergistically combine probiotics and prebiotics, enhance the survival and efficacy of beneficial gut bacteria, offering significant chemopreventive benefits (Table 2). By pairing live beneficial microbes (probiotics) with non-digestible fibers (prebiotics), synbiotics ensure better microbial survival through the harsh gastric environment and improve colonization in the colon [77, 78]. It is critical in cancer prevention, as synbiotics effectively maintain a balanced gut microbiota, crucial for reducing systemic inflammation (a known factor in cancer progression). Synbiotics can be either complementary or synergistic. A complementary synbiotic comprises probiotics and prebiotics that interact separately to accomplish one or more health advantages. In contrast, in a synergistic synbiotic, the prebiotics are selected to increase the activities of the co-administered probiotics. For instance, lactulose (prebiotic) that does not promote the growth of Lactobacillus plantarum has been combined as a complementary synbiotic with L. plantarum to reduce colibacillosis in pigs [79]. L-arginine boosted the growth and survival of Lactobacillus rhamnosus and was combined as a synergistic synbiotics [80].
Some believe synbiotics are more beneficial than either probiotics or prebiotics alone because synbiotics display the qualities of both components [102, 103]. Studies suggest synbiotics may prevent and improve the health of diabetic individuals by enhancing the beneficial gut microbiota composition [104]. Diabetes occurs when insufficient insulin is produced, reducing nitric oxide bioavailability and developing endothelial dysfunction. Synbiotic fermented milk of Lactobacillus acidophilus ATCC® 4357™ with fructooligosaccharide and isomaltooligosaccharide efficiently reduces blood glucose levels [92]. A positive effect on reducing liver injury and insulin resistance was also reported in high-fat diet-induced rats treated with synbiotics of Lactobacillus paracasei B21060, arabinogalactan, and fructooligosaccharides [105]. Moreover, synbiotic supplements remarkably decreased insulin and fasting blood sugar levels [106]. Synbiotic food resulted in a notable increase in the production of plasma nitric oxide [107, 108]. Nitric oxide is a ubiquitous signalling molecule that contributes to the synthesis and secretion of insulin [109]. It was suggested that synbiotics improved endothelial nitric oxide synthase activity in umbilical vein endothelial cells. The synbiotic treatment also improved hormonal homeostasis and glycemic control and decreased the synthesis of inflammatory cytokines and phosphorylation in the insulin receptor in high-fat diet-induced rats [105, 110].
Patients with diabetes are likely to suffer from heart-related diseases [111], which can be alleviated by consuming synbiotic supplements to lower systolic and diastolic blood pressure [110]. The improvement in blood pressure in diabetics may be due to an improvement in nitric oxide production via probiotic actions. The increment of nitric oxide may aid the blood vessels in relaxing and maintaining low systolic and diastolic blood pressure [112]. The synbiotic effect of L. sporogenes and inulin reduces very low-density lipoprotein cholesterol and triacylglycerol [113]. A study using Enterococcus faecium CRL 183 and Lactobacillus helveticus ssp. jugurti 416 in combination with soybean and yacon extract revealed a reduction in triglyceride and total cholesterol levels, as well as a substantial increase in high-density lipoprotein (HDL) cholesterol in the treatment group [114]. High production of HDL cholesterol prevents the build-up of low-density lipoprotein (LDL) cholesterol, in which high LDL cholesterol content may deposit in the walls of the blood vessels and arteries, leading to atherosclerosis and heart attack. Reducing cholesterol levels is suggested due to the digestion and absorption of cholesterol in the intestine by the probiotics in the synbiotics [114]. Incorporating prebiotics in synbiotics further uplifts the probiotic’s survival and mechanism of action, enhancing cholesterol absorption in the small intestines.
The chemopreventive actions of synbiotics include enhancing gut barrier function to prevent the translocation of pathogenic bacteria and toxins, modulating immune responses to detect better and eliminate cancer cells, reducing exposure to carcinogens by altering gut pH and microbial environment, and promoting anti-inflammatory effects through the production of short-chain fatty acids like butyrate, propionate, and acetate [77]. These fatty acids are particularly effective in inhibiting tumor cell proliferation and inducing apoptosis. Clinical studies underscore the potential of synbiotics in reducing biomarkers of cancer progression, particularly in colorectal cancer, where they have helped decrease the incidence of polyps and adenomas. Furthermore, synbiotics are being explored for their role in supporting cancer treatment regimens, such as chemotherapy, by alleviating adverse effects like diarrhea and mucositis and maintaining gut integrity. Synbiotics of Lactobacillus acidophilus LA5 and B. animalis subsp. lactis BB-12 with germinated brown rice induced cancer cell apoptosis, thus suppressing colon tumor growth [115]. The chemopreventive effects of synbiotics (Lactobacillus gasseri 505 and Cudrania tricuspidata leaf extract) were also proven in in vivo studies on colitis-associated colorectal cancer mice [116]. Synbiotic-treated mice produce higher levels of SCFAs, suppressing the Staphylococcus linked to intestinal inflammation and colorectal cancer [117]. As a result, the inflammation and carcinogenesis in colorectal cancer tissues were decreased [117]. A notable increase of tumor necrosis factor-α production and apoptosis-regulating genes were also observed in the synbiotic-treated mice; the production of anti-apoptosis genes and tumor cell proliferation significantly decreased in the synbiotic-treated mice simultaneously [117]. Consequently, synbiotic-treated mice exhibited enhanced tumor repression and reduced cancer cell proliferation [117].
Recent Technologies and Mechanisms Involved in the Gut Microbiota Function
Growing evidence demonstrates gut microbiota’s significance in regulating anti-cancer treatments’ efficacy and toxicity [118]. Besides the commonly known food supplements for gut microbiota improvement, emerging techniques such as fecal microbiota transplantation (FMT) and bacteriophage exhibited promising strategies in modulating the involvement of gut microbe involvement in chemoprevention and cancer treatments.
Faecal Microbiota Transplantation (FMT)
FMT, or fecal microbiota transplantation, involves transferring fecal material from a healthy donor to a recipient who lacks a healthy gut microbiota composition. It has been found to significant advantages in immunotherapy as it reduces the side effects of treatment [119, 120]. FMT contributes to cancer prevention by restoring microbial diversity, reintroducing beneficial microbes, enhancing immunological function and tolerance, and detoxifying dietary carcinogens.
As previously mentioned, the gut microbiome plays a crucial role in modulating immune response and balance. An imbalance in gut microbiota is strongly associated with chronic inflammation and ultimately contributes to the development of cancer [121]. FMT enables patients to restore normal gut function by receiving a well-balanced microbiome from a donor [122]. The reintroduction of a selective beneficial gut microbiome through FMT alleviates disease symptoms, significantly minimizing the risk of cancer development. The beneficial microbes can produce various metabolites involved in the gastrointestinal tract’s homeostasis balance [95, 123]. In addition, the production of anti-carcinogenic metabolites such as SCFAs, EPS, and bacteriocin can eliminate pathogenic bacteria by induction of apoptosis and inhibiting cancer growth. The enriched microbiome from donors possesses hydrolytic enzymes that could rapidly break down and eliminate dietary carcinogens before they can destroy the gut intestinal lining and hence inhibit cancer development [124]. FMT from a donor with a microbiome adept at this detoxification process can equip the recipient’s gut with these beneficial bacteria. Thus, this improves the recipient’s ability to neutralize dietary carcinogens and reduces cancer risk [125].
FMT has been successfully utilized to treat Clostridioides difficile infections (CDI) [126], inflammatory bowel disorders [127], autism [128], chronic kidney disease, metabolic syndrome [129], and alteration of hepatitis B virus infection [130]. Foremost, an in-depth systematic review of the global incidence of FMT to treat CDI demonstrated that FMT appears safe in major indication conditions with mild or moderate adverse events [129, 131]. It has been shown that FMT improves the effectiveness of anti-programmed death-1 (PD-1) therapy in melanoma patients [132, 133]. Anti-PD-1 therapy is an immunotherapy for metastatic melanoma that utilizes antibodies PD-1 to prevent the interaction of PD1 and release CD8+ T cells from inhibition, thereby increasing anti-cancer activity. A combination of responder-derived FMT and anti-PD-1 therapy has shown clinical success in 30–40% of previously immunotherapy-refractory patients [133]. FMT and anti-PD-1 improved CD8+ T cell activation and changed gene expression inside the tumour microenvironment, indicating that gut microbiota modification with FMT could be a way to enhance anti-PD-1 treatment [133]. Furthermore, FMT minimises the severity of side effects from cancer treatment by restoring balance and reversing the loss of diversity in gut microbiota after cancer treatment [127]. A positive result was reported in the study of Bifidobacterium strains in improving the immunopathology associated with cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade. Bifidobacterium was reported to reduce autoimmunity after checkpoint blockade therapies without reducing the anti-cancer responses [134].
Despite FMT showing some promising features, it has some limitations that need refinement. For example, there are no standardization approaches to FMT. Various aspects, such as delivery methods, stool preparation, and donor selection, need to be considered. Limited data is available to pinpoint which gut bacteria are responsible for the treatment’s success or failure. The strongest evidence currently supports FMT’s effectiveness is limited to treating recurring CDI. However, the application of FMT for other disorders like metabolic syndrome, inflammatory bowel disease, digestive problems, and obesity is still in the pioneering research stage. Clinical trial results are inconsistent, limiting our understanding of the gut microbiome’s makeup and function. Therefore, this highlighted the need for a more precise and personalized approach to isolating and transferring beneficial bacteria. In short, FMT, as a potent medical procedure for the manipulation of gut health balance, holds great promise to reduce the risk of cancer and side effects. However, fortifying more understanding of the gut bacteria involved is important, and personalizing the treatment for better results across different diseases is important.
Bacteriophages
Bacteriophage therapy, or phage therapy, is a therapeutic approach that utilizes bacteriophages to combat bacterial infections. The mode of action of bacteriophage therapy involves attack, transfer, replication, and lysis. Initially, the bacteriophages target a susceptible host, followed by the transfer of genetic materials within the host, rapid replication, and, ultimately, the bursting of bacterial cells through lysis. Releasing new phages from the burst cells restores the phage attack cycle [135].
Recent advancements in understanding the role of bacteriophages in the gut microbiota have provided insights into their significance in human health. The interaction between bacteriophages and gut bacteria can contribute to the prevention of intestinal dysbiosis and associated diseases [136]. Changes in the gut microbiota have been associated with conditions such as ulcerative colitis, highlighting the impact of bacteriophages on gut health [137]. Extensive research has been conducted on the potential clinical applications of bacteriophages, including treating antibiotic-resistant bacteria in lung infections, and bone and joint problems [138, 139]. Studies have demonstrated that bacteriophages play a crucial role in preventing the formation of biofilms on medical devices [140]. Research has also indicated that bacteriophages have a dynamic influence on the gut microbiome through predation and gene transfers, affecting the ecological structure of gut bacteriophages and their role in diseases like inflammatory bowel disease [141, 142].
On the other hand, bacteriophage therapy is essential in maintaining gut homeostasis, detoxifying carcinogens, and modulating the immune system [143]. Bacteriophage therapy has proven effective in controlling the carcinogenic microbiome [144]. By assembling nanoparticles on their surface, bacteriophages can kill Fusobacterium nucleatum and activate immune cells, leading to a stronger anti-tumor immune response [145]. However, chemotherapy may still be more evident and compelling than bacteriophage treatment. Moreover, dietary modulation of bacteriophages can potentially impact the gut microbiome, reducing the risk of gastroenteritis, inflammatory bowel disease, and cancer [146]. The versatility of phages in medical applications is demonstrated by their investigation for use in vaccine development and immunotherapy [147]. Each phage has a specific target bacterial host, allowing it to infect and replicate within a particular type or strain of bacteria [148]. This targeted approach selectively eliminates the target bacterial host with minimal disruption to the gut microbiome [149, 150]. The gut phageome of healthy individuals is believed to play a critical role in maintaining a healthy gut ecosystem [123]. The escalating threat of antibiotic resistance, caused by the emergence of antibiotic-resistant bacteria (ARB) and antimicrobial resistance genes (AMR), necessitates alternative treatment options [151]. Phages can be effective against bacteria resistant to conventional antibiotics, providing a potential solution to the growing problem of antibiotic resistance. In conclusion, phage therapy is gaining increasing attention as a potential alternative or complementary treatment for bacterial infections and antimicrobial resistance. However, further research and development are required to establish its broader use and effectiveness.
Probiotics and Drug Interactions
Understanding interactions between probiotics and drugs is essential, especially for cancer patients undergoing chemotherapy. Probiotics may influence the pharmacokinetics of chemotherapy drugs by modulating drug metabolism and altering drug absorption, distribution, and excretion [152]. Furthermore, probiotics could affect the efficacy and toxicity of chemotherapy by mechanisms such as enzyme modulation and competition for binding sites [153].
In the context of irinotecan (a cancer drug), there are positive and negative interactions with gut microbiota. Gut bacteria such as Bacteroides vulgatus, E. coli, and Clostridium ramosum may produce β-glucuronidase, which transforms SN-38 G (an active metabolite) into SN-38 (an inactive metabolite that may cause neutropenia and diarrhea), thereby increasing the risk of side effects and reducing the effectiveness of cancer treatments. Contrarily, probiotics such as Lactobacillus reuteri and Bifidobacterium infantis do not affect the therapy with irinotecan as they lack the gene to produce β-glucuronidase. Administrating a bacterial β-glucuronidase inhibitor shielded the mice from gastrointestinal toxicity, indicating that microbial β-glucuronidase inhibitor could be clinically used to improve drug efficacy and minimize the side effects (154). VSL3 (the combination of Lactobacilli spp., Bifidobacteria spp., and Streptococcus thermophilus) has also been found to reduce irinotecan-induced diarrhea in animal studies by promoting the growth of intestinal cells and preventing cell death [155]. Bifidobacterium longum enriched with selenium has shown promise in reducing the risk of irinotecan-induced diarrhea in mice by enhancing enzyme activity and increasing gene expression [156, 157].
Beyond the interactions with cancer drugs, probiotics may enhance the effectiveness of antibiotics by maintaining a balanced gut microbiota, which potentially lowers the risk of infections during cancer treatment. For instance, the use of synbiotics (inulin, Lactobacillus rhamnosus, and Bifidobacterium lactis) has been found to reduce colorectal proliferation significantly, induce necrosis in colonic cells and increase the production of interferon γ in the cancer patients [74]. Clinical studies have also revealed positive outcomes when cancer patients took synbiotics after colorectal cancer surgery, including fewer infections, less diarrhea, quicker recovery of normal gut function, reduced use of antibiotics, lower risk of severe infections, and shorter hospital stays [158, 159].
Furthermore, probiotics have the potential to alter the immune system and increase the body’s response to vaccines, which could have implications for cancer prevention. Probiotics have been shown in studies to improve vaccine-induced immune responses. Bifidobacteria and Lactobacillus have been shown to boost seroconversion and seroprotection rates in adults receiving influenza vaccines, as well as increased interferon- (IFN-) production and better antibody responses in infants following Hepatitis B immunization [160,161,162]. Other probiotic strains like E. coli, Lactococcus lactis, and Bacillus species have also demonstrated the ability to bolster humoral and cellular immune responses [163,164,165].
Probiotics are also reported to assist in detoxifying the gut, potentially reducing the risk of cancer associated with pesticide exposure and heavy metals. Lactobacillus was found to sequester organophosphate pesticides, parathion, and chlorpyrifos. Remarkably, this pesticide-sequestering capability was observed to be independent of the metabolic activity of the bacteria, with both live and heat-killed Lactobacillus strains demonstrating similar pesticide-capturing abilities [166]. Numerous in vitro studies have also been conducted to investigate the potential of probiotics to exhibit detoxification properties, such as Lactobacillus plantarum on chromium, cadmium, and lead toxicity [167,168,169], Bacillus cereus on cadmium toxicity [170], and Lactobacillus on mercury toxicity [171]. These probiotics enhance the elimination of heavy metals from the body, reversing their adverse effects on gut microbiota.
Potential Challenges and Future Directions
Despite the growing body of research supporting probiotics in cancer prevention and treatment, several challenges and questions remain. The effects of probiotics can be highly strain-specific, necessitating further research to identify the most effective probiotic strains for cancer prevention and treatment. Determining optimal dosages and treatment durations and standardizing probiotic products in terms of quality, quantity, and labelling is essential to ensure consistent and reliable results across studies [77]. While probiotics are generally considered safe, certain patient populations, such as those with compromised immune systems, may be at risk of adverse effects, emphasizing the need to establish safety profiles for specific groups. Genetic stability, deleterious metabolic activity, and potential pathogenicity of probiotics over time must also be assessed according to the probiotics involved, particularly immunological effects in infants. Probiotics have been reported to confer infections by translocation throughout the digestive tract and intestines [172]. Individuals with weak immune responses, specifically long-term hospitalized patients, have an increased risk for infection. Lactose intolerant individuals might also experience bloating and gas issues upon consuming synbiotic food consisting of lactose due to D-lactic acidosis and the overgrowth of bacteria in the small intestines [173]. Biogenic amines, often released from fermented probiotic-based food products, have been reported to fluctuate and rise or reduce blood flow, which might trigger headaches in individuals [174].
As personalized medicine advances, tailoring probiotic usage based on an individual’s gut microbiota and genetic makeup may become an exciting avenue. One of the most significant innovations is the application of CRISPR-Cas systems. The CRISPR-Cas systems are being used in probiotics for cancer treatment by leveraging the ability of certain probiotic bacteria to colonize tumor regions and deliver the CRISPR-Cas9 system to the tumor site. It has been demonstrated Lactobacillus rhamnosus GG (LGG) can penetrate the hypoxia tumor center, allowing efficient delivery of the CRISPR-Cas9 system to the tumor region [175]. The process of genetically modifying probiotics involves several key steps: designing a specific guide RNA (gRNA) to target a desired DNA sequence within the probiotic, using the Cas9 enzyme to create a precise cut at the DNA target site, and then allowing the bacterial cell to repair the cut using its natural mechanisms, which can be harnessed to introduce or disrupt genes [176]. In the study by [175], the CRISPR-Cas9 system is used to knockdown tumor immunosuppression-related genes, which helps to amplify immunogenic cell death and reverse tumor immunosuppression. Consequently, Lactobacillus rhamnosus GG (LGG) efficiently colonizes the tumor area and activates the immune system, thus, enhancing the effectiveness of the CRISPR-Cas9 system in inducing ICD and lifting immunosuppression. It has been shown that the CRISPR-Cas9 system generates immune responses that effectively attack tumor cells in mice, contributing to the inhibition of tumor re-challenge in vivo, providing an immunological memory effect, and offering protection against lung metastasis [175].
The emerging of single-cell RNA sequencing (scRNA-seq) technologies also improves our understanding of gut microbiome and cancer relationship [176]. The study of gene profiles at the individual microbial cell provides the vast heterogeneity within microbial communities and sheds light on their potential roles and interactions in cancer pathology, particularly in how they influence the tumor microenvironment [177]. For example, scRNA-seq has been reported to profile the transcriptomes of cells, which helps understand the cellular landscapes of tumors and the development of personalized cancer treatments. It is suggested that these detailed profiles can be used to tailor therapies based on the specific cellular makeup of each patient’s tumor, potentially increasing the efficacy of treatments and reducing side effects [178]. The scRNA-seq has been claimed in advancing the cellular mechanisms and genetic mutations that facilitate resistance, leading to a better understanding to design therapies that improve patient therapeutic outcomes [179]. Additionally, scRNA-seq has been applied to study circulating tumor cells (CTCs), which are essential for understanding cancer metastasis and progression. Analyzing the gene expression profiles of CTCs at the single-cell level could enriche the comprehension of tumor biology and metastatic processes, offering for new therapeutic interventions [176]. To date, scRNA-seq has identified various cell populations within lung tumors, some of which are associated with poor prognosis; uncovered cellular heterogeneity and mechanisms of drug resistance in breast cancer; and identified cell populations linked to poor prognosis and drug resistance in ovarian cancer [179].
Conclusion
The genetic makeup in the gut microbiota is 100 times greater than that of humans. Gut microbes compensate for the weakness of humans’ metabolism by providing valuable metabolites through catabolism and anabolism reactions. Therefore, synbiotics have emerged as new functional foods for healthy immune balance. However, the minimal intake of probiotics and prebiotics to exert their maximum function is still debated. The technology to maintain, process, and mass produce synbiotic products for market demand are the critical aspects that need to be considered for overall benefits. Various technologies to formulate synbiotics in food products should be studied in depth. Omics technology allows the pathophysiology study of synbiotics to explain the health diseases and prevention mechanisms. Further, in vitro and in vivo clinical studies are also required to analyze the effect of synbiotic consumption in individuals with various health problems, which could provide sound evidence for maximum utilizing probiotics in treatments.
Data Availability
The data supporting the findings of this paper are available upon request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ (2020) Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother 129:110409. https://doi.org/10.1016/j.biopha.2020.110409
Yusoff J, Ismail A (2021) Out of pockets spending among breast cancer women receiving out-patient treatment in a tertiary teaching hospital in Kuala Lumpur Malaysia. Malaysian J Public Health Med 21(3):240–249. https://doi.org/10.37268/mjphm/21/3/art.1107
Blumen H, Fitch K, Polkus V (2016) Comparison of treatment costs for breast cancer, by tumor stage and type of service. Am Health Drug Benefits 9(1):23–32
Fitzpatrick OM, Murphy C, Duignan E, Egan K, Hennessy BT, Grogan L, ... Morris PG (2022) The cost of cancer care: how far would you go for a trial? Ir J Med Sci (1971). https://doi.org/10.1007/s11845-021-02915-6
Dy GK, Adjei AA (2013) Understanding, recognizing, and managing toxicities of targeted anticancer therapies: toxicities of targeted anticancer therapies. Cancer J Clin 63(4):249–279. https://doi.org/10.3322/caac.21184
Mandal D (2019) Food preservative chemistry: effects and side effects. J Indian Chem Soc 96:10
Bharat Helkar P, Sahoo A (2016) Review: food industry by-products used as a functional food ingredients. Int J Waste Resour 6(3). https://doi.org/10.4172/2252-5211.1000248
Banerjee P, Ray D (2019) Functional food: a brief overview. Int J Bioresource Sci 6(2):57–60. https://doi.org/10.30954/2347-9655.02.2019.2
Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A, Arcaro G (2019) Gut microbiota and cancer: how gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol/Hematol 143:139–147. https://doi.org/10.1016/j.critrevonc.2019.09.003
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, ... Chen ZS (2022) Microbiota in health and diseases. Sig Transduct Target Ther 7(1):1–28. https://doi.org/10.1038/s41392-022-00974-4
Wong SH, Kwong TNY, Wu C-Y, Yu J (2019) Clinical applications of gut microbiota in cancer biology. Sem Cancer Biol 55:28–36. https://doi.org/10.1016/j.semcancer.2018.05.003
Masood MI, Qadir MI, Shirazi JH, Khan IU (2011) Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol 37(1):91–98. https://doi.org/10.3109/1040841X.2010.536522
Han KJ, Lee J-E, Lee N-K, Paik H-D (2020) Antioxidant and anti-inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish kimchi. J Microbiol Biotechnol 30(4):591–598. https://doi.org/10.4014/jmb.2002.02052
Sun Y, Zhou J, Du H, Zhou Z, Han Y, Luo M, ... Xiao H (2024) The anti-inflammatory potential of a strain of probiotic Bifidobacterium pseudocatenulatum G7: in vitro and in vivo evidence. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.3c07935
MarketsandMarkets Research Pvt Ltd (2023, June 5) Global Probiotics Market: Projected Worth of $85.4 Billion by 2027 with an 8.1% CAGR. GlobeNewswire News Room. From https://www.globenewswire.com/en/news-release/2023/06/05/2682003/0/en/Global-Probiotics-Market-Projected-Worth-of-85-4-Billion-by-2027-with-an-8-1-CAGR.html. Accessed 5 Sept 2023
Arsène MMJ, Davares AKL, Andreevna SL, Vladimirovich EA, Carime BZ, Marouf R, Khelifi I (2021) The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet World 14(2):319–328. https://doi.org/10.14202/vetworld.2021.319-328
Faseleh Jahromi M, Wesam Altaher Y, Shokryazdan P, Ebrahimi R, Ebrahimi M, Idrus Z, ... Liang JB (2016) Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int J Biometeorol 60(7):1099–1110. https://doi.org/10.1007/s00484-015-1103-x
Huang C, Qiao S, Li D, Piao X, Ren J (2004) Effects of Lactobacilli on the performance, diarrhea Incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian-Australasian J Anim Sci 17(3):401–409. https://doi.org/10.5713/ajas.2004.401
Kim D, Beck BR, Heo SB, Kim J, Kim HD, Lee SM, ... Song SK (2013) Lactococcus lactis BFE920 activates the innate immune system of olive flounder (Paralichthys olivaceus), resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large-scale field studies. Fish Shellfish Immunol 35(5):1585–1590. https://doi.org/10.1016/j.fsi.2013.09.008
Pirarat N, Kobayashi T, Katagiri T, Maita M, Endo M (2006) Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunopathol 113(3):339–347. https://doi.org/10.1016/j.vetimm.2006.06.003
van Immerseel F, Russell JB, Flythe MD, Gantois I, Timbermont L, Pasmans F, ... Ducatelle R (2006) The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol 35(3):182–188. https://doi.org/10.1080/03079450600711045
Seyedzade Hashemi S, Khorshidian N, Mohammadi M (2022) An insight to potential application of synbiotic edible films and coatings in food products. Frontiers in Nutrition 9. https://doi.org/10.3389/fnut.2022.875368
Temiz NN, Özdemir KS (2021) Microbiological and physicochemical quality of strawberries (Fragaria × ananassa) coated with Lactobacillus rhamnosus and inulin enriched gelatin films. Postharvest Biol Technol 173:111433. https://doi.org/10.1016/j.postharvbio.2020.111433
National Cancer Institute (2021) What is cancer? - NCI. cgvArticle. From https://www.cancer.gov/about-cancer/understanding/what-is-cancer. Accessed 7 Oct 2023
Losso J (2021) Food processing, dysbiosis, gastrointestinal inflammatory diseases, and antiangiogenic functional foods or beverages. Ann Rev Food Sci Technol 12. https://doi.org/10.1146/annurev-food-062520-090235
Huang EY, Leone VA, Devkota S, Wang Y, Brady MJ, Chang EB (2013) Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. J Parenter Enter Nutr 37(6):746–754. https://doi.org/10.1177/0148607113486931
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535(7610):75–84. https://doi.org/10.1038/nature18848
Jain T, Sharma P, Are AC, Vickers SM, Dudeja V (2021) New insights into the cancer–microbiome–immune axis: decrypting a decade of discoveries. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.622064
Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, ... Meyerson M (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22(2):292–298. https://doi.org/10.1101/gr.126573.111
Markowiak P, Śliżewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9(9):1021. https://doi.org/10.3390/nu9091021
Ahire JJ, Jakkamsetty C, Kashikar MS, Lakshmi SG, Madempudi RS (2021) In vitro evaluation of probiotic properties of Lactobacillus plantarum UBLP40 isolated from traditional indigenous fermented food. Probiotics Antimicrob Proteins 13(5):1413–1424. https://doi.org/10.1007/s12602-021-09775-7
Ma EL, Choi YJ, Choi J, Pothoulakis C, Rhee SH, Im E (2010) The anti-cancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int J Cancer 127(4):780–790. https://doi.org/10.1002/ijc.25011
Lee N-K, Son S-H, Jeon EB, Jung GH, Lee J-Y, Paik H-D (2015) The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J Funct Foods 14:513–518. https://doi.org/10.1016/j.jff.2015.02.019
Kim Y, Oh S, Yun HS, Oh S, Kim SH (2010) Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells: antitumour activity of cb-EPS via autophagy. Lett Appl Microbiol no-no. https://doi.org/10.1111/j.1472-765X.2010.02859.x
Chen Z-F, Ai L-Y, Wang J-L, Ren L-L, Yu Y-N, Xu J, ... Fang J-Y (2015) Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Fut Microbiol 10(9):1433–1445. https://doi.org/10.2217/fmb.15.66
Castro MS, Molina MA, Di Sciullo P, Azpiroz MB, Leocata Nieto F, Sterín-Speziale NB, ... Manghi MA (2010) Beneficial activity of Enterococcus faecalis CECT7121 in the anti-lymphoma protective response: Anti-tumour effects exerted by Ent. faecalis CECT7121. J Appl Microbiol 109(4):1234–1243. https://doi.org/10.1111/j.1365-2672.2010.04747.x
Nakkarach A, Foo HL, Song AA-L, Mutalib NEA, Nitisinprasert S, Withayagiat U (2021) Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb Cell Fact 20(1):36. https://doi.org/10.1186/s12934-020-01477-z
Baldwin∗ C, Millette∗ M, † O, Ruiz D, Luquet MT, Lacroix M (2010) Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutr Cancer 62(3):371–378. https://doi.org/10.1080/01635580903407197
Chang J-H, Shim YY, Cha S-K, Reaney MJT, Chee KM (2012) Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J Med Microbiol 61(3):361–368. https://doi.org/10.1099/jmm.0.035154-0
Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, Aindelis G, Tompoulidou E, Lamprianidou EE, ... Chlichlia K (2016) Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 11(2):e0147960. https://doi.org/10.1371/journal.pone.0147960
Ghoneum M, Gimzewski J (2014) Apoptotic effect of a novel kefir product, PFT, on multidrug-resistant myeloid leukemia cells via a hole-piercing mechanism. Int J Oncol 44(3):830–837. https://doi.org/10.3892/ijo.2014.2258
Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V, Russo F (2012) Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer 64(7):1103–1111. https://doi.org/10.1080/01635581.2012.717676
Saxami G, Karapetsas A, Lamprianidou E, Kotsianidis I, Chlichlia A, Tassou C, ... Galanis A (2016) Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J Functi Foods 24:461–471. https://doi.org/10.1016/j.jff.2016.04.036
Walia S, Kamal R, Dhawan DK, Kanwar SS (2018) Chemoprevention by probiotics during 1,2-Dimethylhydrazine-induced colon carcinogenesis in rats. Dig Dis Sci 63(4):900–909. https://doi.org/10.1007/s10620-018-4949-z
Sadeghi-Aliabadi H, Mohammadi F, Fazeli H, Mirlohi M (2014) Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran J Basic Med Sci 17(10):815–819
Zhou X, Hong T, Yu Q, Nie S, Gong D, Xiong T, Xie M (2017) Exopolysaccharides from Lactobacillus plantarum NCU116 induce c-Jun dependent Fas/Fasl-mediated apoptosis via TLR2 in mouse intestinal epithelial cancer cells. Sci Rep 7:14247. https://doi.org/10.1038/s41598-017-14178-2
Borowicki A, Michelmann A, Stein K, Scharlau D, Scheu K, Obst U, Glei M (2010) Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr Cancer 1–1. https://doi.org/10.1080/01635581.2010.516874
Altonsy MO, Andrews SC, Tuohy KM (2010) Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: mediation by the mitochondrial pathway. Int J Food Microbiol 137(2–3):190–203. https://doi.org/10.1016/j.ijfoodmicro.2009.11.015
Gamallat Y, Meyiah A, Kuugbee ED, Hago AM, Chiwala G, Awadasseid A, ... Xin Y (2016) Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed Pharmacother 83:536–541. https://doi.org/10.1016/j.biopha.2016.07.001
Verma A, Shukla G (2013) Probiotics Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Sprague Dawley rats. Nutr Cancer 65(1):84–91. https://doi.org/10.1080/01635581.2013.741746
Zhang C, Du J, Peng Z (2015) Correlation between Enterococcus faecalis and persistent intraradicular infection compared with primary intraradicular infection: a systematic review. J Endod 41(8):1207–1213. https://doi.org/10.1016/j.joen.2015.04.008
Han KJ, Lee N-K, Park H, Paik H-D (2015) Anticancer and anti-inflammatory activity of probiotic Lactococcus lactis NK34. J Microbiol Biotechnol 25(10):1697–1701. https://doi.org/10.4014/jmb.1503.03033
Thirabunyanon M, Hongwittayakorn P (2013) Potential probiotic lactic acid bacteria of human origin induce antiproliferation of colon cancer cells via synergic actions in adhesion to cancer cells and short-chain fatty acid bioproduction. Appl Biochem Biotechnol 169(2):511–525. https://doi.org/10.1007/s12010-012-9995-y
Dubey V, Ghosh AR, Bishayee K, Khuda-Bukhsh AR (2016) Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: in vitro and in vivo approaches. J Funct Foods 23:66–79. https://doi.org/10.1016/j.jff.2016.02.032
Ayyash M, Abu-Jdayil B, Olaimat A, Esposito G, Itsaranuwat P, Osaili T, ... Liu S-Q (2020) Physicochemical, bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41. Carbohydr Polym 229:115462. https://doi.org/10.1016/j.carbpol.2019.115462
Cousin FJ, Jouan-Lanhouet S, Dimanche-Boitrel M-T, Corcos L, Jan G (2012) Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PLoS ONE 7(3):e31892. https://doi.org/10.1371/journal.pone.0031892
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54(9):2325–2340. https://doi.org/10.1194/jlr.R036012
Horiuchi H, Kamikado K, Aoki R, Suganuma N, Nishijima T, Nakatani A, Kimura I (2020) Bifidobacterium animalis subsp. lactis GCL2505 modulates host energy metabolism via the short-chain fatty acid receptor GPR43. Sci Rep 10(1):4158. https://doi.org/10.1038/s41598-020-60984-6
Thananimit S, Pahumunto N, Teanpaisan R (2022) Characterization of short chain fatty acids produced by selected potential probiotic Lactobacillus strains. Biomolecules 12(12):1829. https://doi.org/10.3390/biom12121829
El-Bestawy EA, Gohar YM, Wefky SM (2017) Antimicrobial phenolic compounds from Enterococcus faecium S29 (EU 158188): characterization and production optimization. Int J Environ Sci Technol 14(3):497–508. https://doi.org/10.1007/s13762-016-1161-6
Ohara T, Yoshino K, Kitajima M (2010) Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology 57(104):1411–1415
Liévin-Le Moal V, Servin AL (2014) Anti-infective activities of lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev 27(2):167–199. https://doi.org/10.1128/CMR.00080-13
La Fata G, Weber P, Mohajeri MH (2018) Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob Proteins 10(1):11–21. https://doi.org/10.1007/s12602-017-9322-6
Kaeid Sharaf L, Shukla G (2018) Probiotics (Lactobacillus acidophilus and Lactobacillus rhamnosus GG) in conjunction with Celecoxib (selective COX-2 inhibitor) modulated DMH-Induced early experimental colon carcinogenesis. Nutr Cancer 70(6):946–955. https://doi.org/10.1080/01635581.2018.1490783
Zhu Q, Gao R, Wu W, Qin H (2013) The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biology: J Int Soc Oncodevelopmental Biology Med 34(3):1285–1300. https://doi.org/10.1007/s13277-013-0684-4
Bouhnik Y, Flourie B, Andrieux C, Bisetti N, Briet F, Rambaud JC (1996) Effects of Bifidobacterium Sp fermented milk ingested with or without inulin on colonic bifidobacteria and enzymatic activities in healthy humans. Eur J Clin Nutr 50(4):269–273
Lee DK, Jang S, Baek EH, Kim MJ, Lee KS, Shin HS, ... Ha NJ (2009) Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids in Health Dis 8:21. https://doi.org/10.1186/1476-511X-8-21
Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Annals Nutr Metabolism 61(2):160–174. https://doi.org/10.1159/000342079
Ruiz L, Delgado S, Ruas-Madiedo P, Sánchez B, Margolles A (2017) Bifidobacteria and their molecular communication with the immune system. Front Microbiol 8:2345. https://doi.org/10.3389/fmicb.2017.02345
Hwang JW, Baek YM, Yang KE, Yoo HS, Cho CK, Lee YW, ... Jang IS (2013) Lactobacillus casei extract induces apoptosis in gastric cancer by inhibiting NF-κB and mTOR-mediated signaling. Integr Cancer Ther 12(2):165–173. https://doi.org/10.1177/1534735412442380
Salek F, Mirzaei H, Khandaghi J, Javadi A, Nami Y (2023) Apoptosis induction in cancer cell lines and anti-inflammatory and anti-pathogenic properties of proteinaceous metabolites secreted from potential probiotic Enterococcus faecalis KUMS-T48. Sci Rep 13(1):7813. https://doi.org/10.1038/s41598-023-34894-2
Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, ... Collins JK (2007) Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr 85(2):488–496. https://doi.org/10.1093/ajcn/85.2.488
Kelesidis T, Pothoulakis C (2012) Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Th Adv Gastroenterol 5(2):111–125. https://doi.org/10.1177/2F1756283X11428502
Abid R, Waseem H, Ali J, Ghazanfar S, Ali M, Elasbali G, Alharethi SH (2022) Probiotic yeast Saccharomyces: back to nature to improve human health. J Fungi 8(5):444. https://doi.org/10.3390/jof8050444
Pandey KR, Naik SR, Vakil BV (2015) Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol 52(12):7577–7587. https://doi.org/10.1007/s13197-015-1921-1
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, ... Sanders ME (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17(11):687–701. https://doi.org/10.1038/s41575-020-0344-2
Guerra-Ordaz AA, González-Ortiz G, La Ragione RM, Woodward MJ, Collins JW, Pérez JF, Martín-Orúe SM (2014) Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl Environ Microbiol 80(16):4879–4886. https://doi.org/10.1128/AEM.00770-14
Bijle MN, Neelakantan P, Ekambaram M, Lo ECM, Yiu CKY (2020) Effect of a novel synbiotic on Streptococcus mutans. Sci Rep 10(1):7951. https://doi.org/10.1038/s41598-020-64956-8
Lee C-W, Chen H-J, Chien Y-H, Hsia S-M, Chen J-H, Shih C-K (2019) Synbiotic combination of Djulis (Chenopodium Formosanum) and Lactobacillus acidophilus inhibits colon carcinogenesis in rats. Nutrients 12(1):103. https://doi.org/10.3390/nu12010103
Saito Y, Hinoi T, Adachi T, Miguchi M, Niitsu H, Kochi M, ... Ohdan H (2019) Synbiotics suppress colitis-induced tumorigenesis in a colon-specific cancer mouse model. PLoS ONE 14(6):e0216393. https://doi.org/10.1371/journal.pone.0216393
Kuo H-W, Chang C-C, Cheng W (2021) Synbiotic combination of prebiotic, cacao pod husk pectin and probiotic, Lactobacillus plantarum, improve the immunocompetence and growth of Litopenaeus vannamei. Fish Shellfish Immunol 118:333–342. https://doi.org/10.1016/j.fsi.2021.09.023
Bindels LB, Neyrinck AM, Claus SP, Le Roy C, Grangette C, Pot B, ... Delzenne NM (2016) Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J 10(6):1456–1470. https://doi.org/10.1038/ismej.2015.209
Mantzourani I, Terpou A, Bekatorou A, Mallouchos A, Alexopoulos A, Kimbaris A, ... Plessas S (2020) Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem 308:125658. https://doi.org/10.1016/j.foodchem.2019.125658
Celebioglu HU (2021) Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells. Arch Microbiol 203(3):1221–1229. https://doi.org/10.1007/s00203-021-02200-1
Hasan MT, Jang WJ, Kim H, Lee B-J, Kim KW, Hur SW, ... Kong I-S (2018) Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 82:544–553. https://doi.org/10.1016/j.fsi.2018.09.002
Shinde T, Perera AP, Vemuri R, Gondalia SV, Karpe AV, Beale DJ, ... Stanley R (2019) Synbiotic supplementation containing whole plant sugar cane fibre and probiotic spores potentiates protective synergistic effects in mouse model of IBD. Nutrients 11(4):818. https://doi.org/10.3390/nu11040818
Dias MC, Vieiralves NFL, Gomes MIFV, Salvadori DMF, Rodrigues MAM, Barbisan LF (2010) Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis. Food Chem Toxicol 48(3):772–780. https://doi.org/10.1016/j.fct.2009.12.003
Polakowski CB, Kato M, Preti VB, Schieferdecker MEM, Ligocki Campos AC (2019) Impact of the preoperative use of synbiotics in colorectal cancer patients: a prospective, randomized, double-blind, placebo-controlled study. Nutrition 58:40–46. https://doi.org/10.1016/j.nut.2018.06.004
Wong VW-S, Wong GL-H, Chim AM-L, Chu WC-W, Yeung DK-W, Li K, C.-T., Chan HL-Y (2013) Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol 12(2):256–262. https://doi.org/10.1016/S1665-2681(19)31364-X
Shafi A, Naeem Raja H, Farooq U, Akram K, Hayat Z, Naz A, Nadeem HR (2019) Antimicrobial and antidiabetic potential of synbiotic fermented milk: a functional dairy product. Int J Dairy Technol 72(1):15–22. https://doi.org/10.1111/1471-0307.12555
Liong M-T, Dunshea FR, Shah NP (2007) Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. Br J Nutr 98(04). https://doi.org/10.1017/S0007114507747803
Kariyawasam KMGMM, Lee N-K, Paik H-D (2021) Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Biosci 39:100835. https://doi.org/10.1016/j.fbio.2020.100835
Jerez-Morales A, Merino JS, Díaz-Castillo ST, Smith CT, Fuentealba J, Bernasconi H, ... García-Cancino A (2021) The administration of the synbiotic Lactobacillus bulgaricus 6c3 strain, inulin and fructooligosaccharide decreases the concentrations of indoxyl sulfate and kidney damage in a rat model. Toxins 13(3):192. https://doi.org/10.3390/toxins13030192
Westfall S, Lomis N, Prakash S (2018) A novel polyphenolic prebiotic and probiotic formulation have synergistic effects on the gut microbiota influencing Drosophila melanogaster physiology. Artif Cells Nanomed Biotechnol 46(sup2):441–455. https://doi.org/10.1080/21691401.2018.1458731
Tang T, Song J, Li J, Wang H, Zhang Y, Suo H (2020) A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley β-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohydr Polym 243:116398. https://doi.org/10.1016/j.carbpol.2020.116398
Simeoli R, Mattace Raso G, Lama A, Pirozzi C, Santoro A, Di Guida F, ... Meli R (2015) Preventive and therapeutic effects of Lactobacillus paracasei B21060–based synbiotic treatment on gut inflammation and barrier integrity in colitic mice. J Nutr 145(6):1202–1210. https://doi.org/10.3945/jn.114.205989
Morshedi M, Saghafi-Asl M, Hosseinifard E-S (2020) The potential therapeutic effects of the gut microbiome manipulation by synbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. J Translational Med 18(1):18. https://doi.org/10.1186/s12967-019-02169-y
Sefidgari-Abrasi S, Roshangar L, Karimi P, Morshedi M, Rahimiyan-Heravan M, Saghafi-Asl M (2021) From the gut to the heart: L. plantarum and inulin administration as a novel approach to control cardiac apoptosis via 5-HT2B and TrkB receptors in diabetes. Clin Nutr 40(1):190–201. https://doi.org/10.1016/j.clnu.2020.05.004
Le B, Ngoc APT, Yang SH (2020) Synbiotic fermented soymilk with Weissella cibaria FB069 and xylooligosaccharides prevents proliferation in human colon cancer cells. J Appl Microbiol 128(5):1486–1496. https://doi.org/10.1111/jam.14551
Nazhand A, Souto EB, Lucarini M, Souto SB, Durazzo A, Santini A (2020) Ready to use therapeutical beverages: focus on functional beverages containing probiotics, prebiotics and synbiotics. Beverages 6(2):26. https://doi.org/10.3390/beverages6020026
Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, ... Vyas BRM (2013) Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes 4(3):181–192. https://doi.org/10.4161/gmic.23919
Bock PM, Telo GH, Ramalho R, Sbaraini M, Leivas G, Martins AF, Schaan BD (2021) The effect of probiotics, prebiotics or synbiotics on metabolic outcomes in individuals with diabetes: a systematic review and meta-analysis. Diabetologia 64(1):26–41. https://doi.org/10.1007/s00125-020-05295-1
Mattace Raso G, Simeoli R, Iacono A, Santoro A, Amero P, Paciello O, ... Meli R (2014) Effects of a Lactobacillus paracasei B21060 based synbiotic on steatosis, insulin signaling and toll-like receptor expression in rats fed a high-fat diet. J Nutr Biochem 25(1):81–90. https://doi.org/10.1016/j.jnutbio.2013.09.006
Eslamparast T, Zamani F, Hekmatdoost A, Sharafkhah M, Eghtesad S, Malekzadeh R, Poustchi H (2014) Effects of synbiotic supplementation on insulin resistance in subjects with the metabolic syndrome: a randomised, double-blind, placebo-controlled pilot study. Br J Nutr 112(3):438–445. https://doi.org/10.1017/S0007114514000919
Bahmani F, Tajadadi-Ebrahimi M, Kolahdooz F, Mazouchi M, Hadaegh H, Jamal A-S, ... Asemi Z (2016) The consumption of synbiotic bread containing Lactobacillus sporogenes and inulin affects nitric oxide and malondialdehyde in patients with type 2 diabetes mellitus: randomized, double-blind, placebo-controlled trial. J Am College Nutr 35(6):506–513. https://doi.org/10.1080/07315724.2015.1032443
Cheng C-P, Tsai S-W, Chiu CP, Pan T-M, Tsai T-Y (2013) The effect of probiotic-fermented soy milk on enhancing the NO-mediated vascular relaxation factors: effect of probiotic-fermented soy milk on vascular relaxation factors. J Sci Food Agric 93(5):1219–1225. https://doi.org/10.1002/jsfa.5880
Gheibi S, Ghasemi A (2020) Insulin secretion: The nitric oxide controversy. EXCLI J 19:Doc1227. ISSN 1611–2156. https://doi.org/10.17179/EXCLI2020-2711
Tajabadi-Ebrahimi M, Sharifi N, Farrokhian A, Raygan F, Karamali F, Razzaghi R, ... Asemi Z (2017) A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp Clin Endocrinol Diabetes 125(01):21–27. https://doi.org/10.1055/s-0042-105441
Farrag A, Bakhoum S, Salem MA, El-Faramawy A, Gergis E (2013) The association between extracoronary calcification and coronary artery disease in patients with type 2 diabetes mellitus. Heart Vessels 28(1):12–18. https://doi.org/10.1007/s00380-011-0205-6
Velayati A, Kareem I, Sedaghat M, Sohrab G, Nikpayam O, Hedayati M, ... Hejazi E (2020) The effects of synbiotic supplementation on metabolic factors and systemic inflammation in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Res Square. https://doi.org/10.21203/rs.3.rs-16649/v1
Taghizadeh M, Hashemi T, Shakeri H, Abedi F, Sabihi S-S, Alizadeh S-A, Asemi Z (2014) Synbiotic food consumption reduces levels of triacylglycerols and VLDL, but not cholesterol, LDL, or HDL in plasma from pregnant women. Lipids 49(2):155–161. https://doi.org/10.1007/s11745-013-3867-2
Roselino MN, Pauly-Silveira ND, Cavallini DC, Celiberto LS, Pinto RA, Vendramini RC, Rossi EA (2012) A potential synbiotic product improves the lipid profile of diabetic rats. Lipids Health Dis 11(1):114. https://doi.org/10.1186/1476-511X-11-114
McDermott A, Weatherspoon D (2017) What’s the difference between HDL and LDL cholesterol? Healthline. From https://www.healthline.com/health/hdl-vs-ldl-cholesterol. Accessed 7 Jan 2022
Lin P-Y, Li S-C, Lin H-P, Shih C-K (2019) Germinated brown rice combined with Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis inhibits colorectal carcinogenesis in rats. Food Sci Nutr 7(1):216–224. https://doi.org/10.1002/fsn3.864
Oh NS, Lee JY, Kim Y-T, Kim SH, Lee J-H (2020) Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 12(1):1785803. https://doi.org/10.1080/19490976.2020.1785803
Huang J, Liu W, Kang W, He Y, Yang R, Mou X, Zhao W (2022) Effects of microbiota on anticancer drugs: current knowledge and potential applications. eBioMedicine 83. https://doi.org/10.1016/j.ebiom.2022.104197
Park S-Y, Seo GS (2021) Fecal microbiota transplantation: is it safe? Clin Endoscopy 54(2):157–160. https://doi.org/10.5946/ce.2021.072
Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, ... Ohashi K (2016) Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 128(16):2083–2088. https://doi.org/10.1182/blood-2016-05-717652
Du H, Liu Y, Zhang L, Zhan C, Chen J, Zhang M, ... Liang C (2020) Abnormal gut microbiota composition is associated with experimental autoimmune prostatitis-induced depressive-like behaviors in mice. Prostate 80(9):663–673. https://doi.org/10.1002/pros.23978
Li J, Cui H, Cai Y, Lin J, Song X, Zhou Z, Wang L (2018) Tong-Xie-Yao-Fang regulates 5-HT level in Diarrhea predominant irritable bowel syndrome through gut microbiota modulation. Front Pharmacol 9:1110. https://doi.org/10.3389/fphar.2018.01110
Das B, Nair GB (2019) Homeostasis and dysbiosis of the gut microbiome in health and disease. J Biosci 44(5):117. https://doi.org/10.1007/s12038-019-9926-y
Cao Y, Zhang L, Xiong F, Guo X, Kan X, Song S, ... Zheng C (2024) Effect of probiotics and fecal microbiota transplantation in dirty rats with established primary liver cancer. Fut Microbiol 19(2):117–129. https://doi.org/10.2217/fmb-2022-0234
Tanaka Y, Shimizu S, Shirotani M, Yorozu K, Kitamura K, Oehorumu M, ... Fukuzawa Y (2021) Nutrition and cancer risk from the viewpoint of the intestinal microbiome. Nutrients 13(10):3326. https://doi.org/10.3390/nu13103326
Stoff R, Wolf Y, Boursi B (2023) Fecal microbiota transplantation as a cancer therapeutic. Cancer J 29(2):102–108. https://doi.org/10.1097/PPO.0000000000000651
Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, ... Jenq RR (2018) Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 24(12):1804–1808. https://doi.org/10.1038/s41591-018-0238-9
Kang D-W, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, ... Krajmalnik-Brown R (2019) Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep 9(1):5821. https://doi.org/10.1038/s41598-019-42183-0
Baunwall SMD, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, Hvas CL (2020) Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 29–30:100642. https://doi.org/10.1016/j.eclinm.2020.100642
Wang J, Zhou X, Li X, Guo W, Zhu Q, Zhu B, ... Wang B (2022) Fecal microbiota transplantation alters the outcome of hepatitis B virus infection in mice. Front Cell Infect Microbiol 12:844132. https://doi.org/10.3389/fcimb.2022.844132
Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F (2021) Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther 53(1):33–42. https://doi.org/10.1111/apt.16148
Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, ... Boursi B (2021) Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371(6529):602–609. https://doi.org/10.1126/science.abb5920
Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin J-M, Morrison RM, ... Zarour HM (2021) Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science 371(6529):595–602. https://doi.org/10.1126/science.abf3363
Wang F, Yin Q, Chen L, Davis MM (2018) Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci 115(1):157–161
Azam AH, Tanji Y (2019) Bacteriophage-host arm race: an update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl Microbiol Biotechnol 103(5):2121–2131. https://doi.org/10.1007/s00253-019-09629-x
Vitetta L, Vitetta G, Hall S (2018) Immunological tolerance and function: associations between intestinal bacteria, probiotics, prebiotics, and phages. Front Immunol 9:2240. https://doi.org/10.3389/fimmu.2018.02240
Zuo T, Lu X-J, Zhang Y, Cheung CP, Lam S, Zhang F, ... Ng SC (2019) Gut mucosal virome alterations in ulcerative colitis. Gut 68(7):1169–1179. https://doi.org/10.1136/gutjnl-2018-318131
Genevière J, McCallin S, Huttner A, Pham T-T, Suva D (2021) A systematic review of phage therapy applied to bone and joint infections: an analysis of success rates, treatment modalities and safety. EFORT Open Rev 6(12):1148–1156. https://doi.org/10.1302/2058-5241.6.210073
Satta G, O’Callagharn C, Clokie MRJ, Di Luca M (2022) Advancing bacteriophages as a treatment of antibiotic-resistant bacterial pulmonary infections. Curr Opin Pulm Med 28(3):225–231. https://doi.org/10.1097/MCP.0000000000000864
Ryan EM, Gorman SP, Donnelly RF, Gilmore BF (2011) Recent advances in bacteriophage therapy: how delivery routes, formulation, concentration and timing influence the success of phage therapy. J Pharm Pharmacol 63(10):1253–1264. https://doi.org/10.1111/j.2042-7158.2011.01324.x
Dong S, Xin Z, He W, Zhang Y, Xiong J, Wang J, ... Fang X (2022) Correlation between the regulation of intestinal bacteriophages by green tea polyphenols and the flora diversity in SPF mice. Food Funct 13(5):2952–2965. https://doi.org/10.1039/D1FO03694G
Nishiyama H, Endo H, Blanc-Mathieu R, Ogata H (2020) Ecological structuring of Temperate bacteriophages in the inflammatory bowel disease-affected gut. Microorganisms 8(11):1663. https://doi.org/10.3390/microorganisms8111663
Lim ES, Zhou Y, Zhao G, Bauer IK, Droit L, Ndao IM, ... Holtz LR (2015) Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med 21(10):1228–1234. https://doi.org/10.1038/nm.3950
Kabwe M, Dashper S, Bachrach G, Tucci J (2021) Bacteriophage manipulation of the microbiome associated with tumour microenvironments-can this improve cancer therapeutic response? FEMS Microbiol Rev 45(5):fuab017. https://doi.org/10.1093/femsre/fuab017
Abed J, Maalouf N, Manson AL, Earl AM, Parhi L, Emgård JEM, Bachrach G (2020) Colon cancer-associated fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front Cell Infect Microbiol 10:400. https://doi.org/10.3389/fcimb.2020.00400
Marongiu L, Burkard M, Venturelli S, Allgayer H (2021) Dietary modulation of bacteriophages as an additional player in inflammation and cancer. Cancers 13(9):2036. https://doi.org/10.3390/cancers13092036
Hess KL, Jewell CM (2020) Phage display as a tool for vaccine and immunotherapy development. Bioeng Translational Med 5(1):e10142. https://doi.org/10.1002/btm2.10142
Batinovic S, Wassef F, Knowler SA, Rice DTF, Stanton CR, Rose J, ... Franks AE (2019) Bacteriophages in natural and artificial environments. Pathogens 8(3):100. https://doi.org/10.3390/pathogens8030100
Chegini Z, Khoshbayan A, Vesal S, Moradabadi A, Hashemi A, Shariati A (2021) Bacteriophage therapy for inhibition of multi drug-resistant uropathogenic bacteria: a narrative review. Ann Clin Microbiol Antimicrob 20(1):30. https://doi.org/10.1186/s12941-021-00433-y
Taati Moghadam M, Khoshbayan A, Chegini Z, Farahani I, Shariati A (2020) Bacteriophages, a new therapeutic solution for inhibiting multidrug-resistant bacteria causing wound infection: lesson from animal models and clinical trials. Drug Des Devel Ther 14:1867–1883. https://doi.org/10.2147/DDDT.S251171
Khoo SC, Goh MS, Alias A, Luang-In V, Chin KW, Ling Michelle TH, ... Ma NL (2022) Application of antimicrobial, potential hazard and mitigation plans. Environ Res 215:114218. https://doi.org/10.1016/j.envres.2022.114218
Hu P, Song W, Shan Y, Du M, Huang M, Song C, Zhang L (2015) Lactobacillus paracasei subsp. paracasei M5L induces cell cycle arrest and calreticulin translocation via the generation of reactive oxygen species in HT-29 cell apoptosis. Food Funct 6(7):2257–2265. https://doi.org/10.1039/c5fo00248f
Mego M, Holec V, Drgona L, Hainova K, Ciernikova S, Zajac V (2013) Probiotic bacteria in cancer patients undergoing chemotherapy and radiation therapy. Complement Ther Med 21(6):712–723. https://doi.org/10.1016/j.ctim.2013.08.018
Kong R, Liu T, Zhu X, Ahmad S, Williams AL, Phan AT, ... Wong STC (2014) Old drug new use—amoxapine and its metabolites as potent bacterial β-glucuronidase inhibitors for alleviating cancer drug toxicity. Clin Cancer Res 20(13):3521–3530. https://doi.org/10.1158/1078-0432.CCR-14-0395
Bowen JM, Stringer AM, Gibson RJ, Yeoh AS, Hannam S, Keefe DM (2007) VSL#3 probiotic treatment reduces chemotherapy-induced diarrhoea and weight loss. Cancer Biol Ther 6(9):1445–1450. https://doi.org/10.4161/cbt.6.9.4622
Cao S, Durrani FA, Rustum YM, Yu YE (2012) Ugt1a is required for the protective effect of selenium against irinotecan-induced toxicity. Cancer Chemother Pharmacol 69(4):1107–1111. https://doi.org/10.1007/s00280-011-1820-8
Qiu Y, Zhang J, Ji R, Zhou Y, Shao L, Chen D, Tan J (2019) Preventative effects of selenium-enriched Bifidobacterium longum on irinotecan-induced small intestinal mucositis in mice. Beneficial Microbes 10(5):569–577. https://doi.org/10.3920/BM2018.0096
Amitay EL, Carr PR, Gies A, Laetsch DC, Brenner H (2020) Probiotic/synbiotic treatment and postoperative complications in colorectal cancer patients: systematic review and meta-analysis of randomized controlled trials. Clin Translational Gastroenterol 11(12):e00268. https://doi.org/10.14309/ctg.0000000000000268
Chen Y, Qi A, Teng D, Li S, Yan Y, Hu S, Du X (2022) Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol 26(6):425–436. https://doi.org/10.1007/s10151-022-02585-1
Lei W-T, Shih P-C, Liu S-J, Lin C-Y, Yeh T-L (2017) Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients 9(11):1175. https://doi.org/10.3390/nu9111175
Soh SE, Ong DQR, Gerez I, Zhang X, Chollate P, Shek LP-C, ... Aw M (2010) Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant Hepatitis B vaccination. Vaccine 28(14):2577–2579. https://doi.org/10.1016/j.vaccine.2010.01.020
Wu B-B, Yang Y, Xu X, Wang W-P (2016) Effects of Bifidobacterium supplementation on intestinal microbiota composition and the immune response in healthy infants. World J Pediatr 12(2):177–182. https://doi.org/10.1007/s12519-015-0025-3
Khor C-S, Tsuji R, Lee H-Y, Nor’e S-S, Sahimin N, Azman A-S, … AbuBakar S, (2021) Lactococcus lactis strain plasma intake suppresses the incidence of dengue fever-like symptoms in healthy Malaysians: a randomized, double-blind placebo-controlled trial. Nutrients 13(12):4507. https://doi.org/10.3390/nu13124507
Michael H, Paim FC, Langel SN, Miyazaki A, Fischer DD, Chepngeno J, … Vlasova AN (2021) Escherichia coli Nissle 1917 Enhances innate and adaptive immune responses in a ciprofloxacin-treated defined-microbiota piglet model of human rotavirus infection. mSphere 6(2). https://doi.org/10.1128/msphere.00074-21
Santos FDS, Ferreira MRA, Maubrigades LR, Gonçalves VS, de Lara APS, Moreira C, Leivas Leite FP (2021) Bacillus toyonensis BCT-7112T transient supplementation improves vaccine efficacy in ewes vaccinated against Clostridium perfringens epsilon toxin. Journal of Applied Microbiology 130(3):699–706. https://doi.org/10.1111/jam.14814
Trinder M, McDowell TW, Daisley BA, Ali SN, Leong HS, Sumarah MW, Reid G (2016) Probiotic Lactobacillus rhamnosus reduces organophosphate pesticide absorption and toxicity to Drosophila melanogaster. Appl Environ Microbiol 82(20):6204. https://doi.org/10.1128/AEM.01510-16
Singh A, Saini P, Srivastava U, Kushwaha R (2021) Biosorption of cadmium and lead through Lactobacillus reuteri and Lactobacillus plantarum and its exopolysaccharides: a comparative study. Int J Curr Res Rev 13:30–35. https://doi.org/10.31782/IJCRR.2021.131507
Wu G, Xiao X, Feng P, Xie F, Yu Z, Yuan W, ... Li X (2017) Gut remediation: a potential approach to reducing chromium accumulation using Lactobacillus plantarum TW1-1. Sci Rep 7(1):15000. https://doi.org/10.1038/s41598-017-15216-9
Zhai Q, Wang G, Zhao J, Liu X, Tian F, Zhang H, Chen W (2013) Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol 79(5):1508–1515. https://doi.org/10.1128/AEM.03417-12
Jabeen Z, Irshad F, Habib A, Hussain N, Sajjad M, Mumtaz S, ... Hassan MN (2022) Alleviation of cadmium stress in rice by inoculation of Bacillus cereus. PeerJ 10:e13131. https://doi.org/10.7717/peerj.13131
Jiang X, Gu S, Liu D, Zhao L, Xia S, He X, Ge J (2018) Lactobacillus brevis 23017 relieves mercury toxicity in the colon by modulation of oxidative stress and inflammation through the interplay of MAPK and NF-κB signaling cascades. Front Microbiol 9:2425. https://doi.org/10.3389/fmicb.2018.02425
Butel M-J (2014) Probiotics, gut microbiota and health. Méd Mal Infect 44(1):1–8. https://doi.org/10.1016/j.medmal.2013.10.002
Rao SSC, Rehman A, Yu S, de Andino NM (2018) Brain fogginess, gas and bloating: a link between SIBO, probiotics and metabolic acidosis. Clin Translational Gastroenterol 9(6):162. https://doi.org/10.1038/s41424-018-0030-7
Kothari D, Patel S, Kim S-K (2019) Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother 111:537–547. https://doi.org/10.1016/j.biopha.2018.12.104
Yu J, Zhou B, Zhang S, Yin H, Sun L, Pu Y, ... Xu H (2022) Design of a self-driven probiotic-CRISPR/Cas9 nanosystem for sono-immunometabolic cancer therapy. Nat Commun 13(1):7903. https://doi.org/10.1038/s41467-022-35580-z
Sun G, Li Z, Rong D, Zhang H, Shi X, Yang W, ... Sun Y (2021) Single-cell RNA sequencing in cancer: applications, advances, and emerging challenges. Mol Ther Oncolytics 21:183–206. https://doi.org/10.1016/j.omto.2021.04.001
Saliba A-E, Westermann AJ, Gorski SA, Vogel J (2014) Single-cell RNA-seq: advances and future challenges. Nucleic Acids Res 42(14):8845–8860. https://doi.org/10.1093/nar/gku555
Lei Y, Tang R, Xu J, Wang W, Zhang B, Liu J, ... Shi S (2021) Applications of single-cell sequencing in cancer research: progress and perspectives. J Hematol Oncol 14(1):91. https://doi.org/10.1186/s13045-021-01105-2
Van de Sande B, Lee JS, Mutasa-Gottgens E, Naughton B, Bacon W, Manning J, ... Ferran E (2023) Applications of single-cell RNA sequencing in drug discovery and development. Nat Rev Drug Discov 22(6):496–520. https://doi.org/10.1038/s41573-023-00688-4
Chang X, Zheng Y, Xu K (2023) Single-cell RNA sequencing: technological progress and biomedical application in cancer research. Mol Biotechnol. https://doi.org/10.1007/s12033-023-00777-0
Acknowledgements
The authors express their gratitude to the Ministry of Higher Education Malaysia for providing financial assistance through the Fundamental Research Grant Scheme (FRGS) (FRGS/1/2022/WAB13/UMT/02/2)(Vot 59701).
Funding
This manuscript was supported by Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS) (FRGS/1/2022/WAB13/UMT/02/2)(Vot 59701).
Author information
Authors and Affiliations
Contributions
Conceptualization: N.L Ma; writing—original draft preparation: K.W Chin, S.C Khoo, and Richard Paul M.P; writing—review and editing: K.W Chin, N.L Ma, Vijitra Luang-In, and S.D Lam; funding acquisition: N.L Ma; supervision: N.L Ma. All authors commented on and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chin, K.W., Khoo, S.C., Paul, R.P.M. et al. Potential of Synbiotics and Probiotics as Chemopreventive Agent. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10299-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10299-z