Abstract
Dacus frontalis (Diptera: Tephritidae) is an emerging species affecting fruit production in Africa and may pose a serious risk to the Cucurbitaceae fruit producing industry in Europe in response to climate change. To understand how temperature affects the fitness and population dynamics of this species and consequently its invasive potential, we investigated for the first time the survival and development time of immature stages, longevity and fecundity of D. frontalis adults in the laboratory at four constant temperatures of 15, 20, 25 and 30 °C. In addition, the lower developmental threshold and thermal constant were calculated using a temperature summation model. Results showed that the rearing temperature has a significant effect on the survival, development, reproduction, and longevity of the pumpkin fruit fly. The highest survival rates of eggs, larvae, pupae, adult females and males were observed at 20 °C. The development time of immature stages and from egg to adult, decreased significantly with increasing temperature from 15 to 30 °C. Females produced a significantly higher number of eggs at 20 °C, and no oviposition was observed at 15 °C. Pupae were able to survive at 15 °C with the longest development time, suggesting that this tephritid species can overwinter as pupae in the field in North Africa. The thermal constant of egg, larval, and pupal stages were 33, 95, and 210 DD, respectively. The minimum temperature threshold of egg, larval, and pupal stages were 4.6, 13.5, and 9.5 °C, respectively. These thermal requirements may explain the seasonality of D. frontalis observed in North Africa. Implications for pest management and potential geographical distribution are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pumpkin fruit fly, Dacus frontalis Becker (Diptera:Tephritidae), is one of the economically damaging fruit fly species that negatively impacts food security in areas where it is established and in newly invaded areas (Elghadi & Port, 2019; Foottit & Adler, 2009; Hafsi et al., 2015a). This fruit fly species originated from South Africa and is widely distributed throughout Africa and the Middle East (GBIF Secretariat, 2023). In recent years, in response to global warming, that relaxes climatic barriers to establishment, D. frontalis has gradually spread and expanded its geographical distribution to new areas, becaming a serious pest threatening the cucurbit fruit industry in these areas. Since the first detection of D. frontalis in Libya in 1992, it has spread rapidly to most areas of North Africa, invading Tunisia, Algeria, and most recently Morocco in 2017 (Elghadi & Port, 2019; Hafsi et al., 2015a). This tephritid species was considered locally as a serious pest of fruit species belonging to Cucurbitaceae family (Ekesi et al., 2007; El-Harym & Belqat, 2017; Hafsi et al., 2015a) and could be established as a very serious agricultural quarantine pest.
In Africa, D. frontalis is a major pest of commercial and wild cucurbit fruits (Hafsi et al., 2015a), and can infest some solanaceous fruits such as Solanum melongena (L.). As a tephritid species, direct damage is caused by the larval stages when feeding in the pulp of fruits, and indirect damage is caused by secondary microorganisms, which are often introduced by adult females when ovipositing, thus causing them to fall to the ground (Badii et al., 2015). Infestation by tephritid species makes the fruit unmarketable, resulting in severe economic losses (Grechi et al., 2022; White & Elson-Harris, 1992). Heavy infestations of cucurbit fruits by D. frontalis have been reported in Egypt, Libya, Iraq, and Tunisia, causing devastating losses that can reach to 60–100% in some areas (Al-Jorany et al., 2019; Elghadi & Port, 2019; Shawkit et al., 2011). For example, in Iraq 68% of the total fruit production was damaged (Al-Jorany et al., 2019) and in Libya 100% losses of cucurbit fruit production were observed (Elghadi & Port, 2019).
Efforts to suppress D. frontalis populations has always relied on the application of bait sprays containing toxic organophosphate insecticides such as diazinon or fenthion during the fruiting season. Often, the last insecticide treatment is applied when the fruits is ripening, making it difficult which to respect the safety period before harvest (Ba-Angood, 1977). This raises concerns among growers, as chemical treatments do not always prevent fruit damage and fail to suppress the fly population (Hafsi et al., 2015b). Recently, emphasis has been placed on implementing safer environmental measures to control D. frontalis, such as the use of entomopathogenic fungi for the biological control (Elghadi & Port, 2019). Therefore, there is a need to explore other control methods to effectively manage this pest and develop an eradication strategy before it spreads to other regions of the world.
Understanding the thermal requirements of insect species is essential for the development of phytosanitary measures and Integrated Pest Management (IPM) programmes (Bale, 2002; Kalaitzaki et al., 2023; Schlesener et al., 2020), especially for invasive pests and in the case of global warming. Studying the thermal requirements of exotic insects can provide also information about insect phenology, population density thresholds and timing of insecticide applications (Wiman et al., 2016). Likewise, it will help to the improve rearing techniques for the implementation of IPM strategies including sterile insect technique (SIT), autocidal and biological control approaches (Pfab et al., 2018; Winkler et al., 2020). In addition, the rapid invasion of D. frontalis in North Africa and the Middle East raises questions about its environmental niche breadth and its responses to climate change.
Tephritidae, as ectotherms insect species, are particularly vulnerable to thermal perturbations. The distribution and physiological processes of tephritid species can be affected by extreme temperatures (Pieterse et al., 2017; Terblanche et al., 2011; Weldon et al., 2018), which vary considerably in space and time (Bonebrake & Deutsch, 2012; Gutierrez & Ponti, 2014). Extreme high temperatures can have multiple negative effects on insects, either directly by inducing mortality or sterility associated with cellular and tissue damage (Duyck et al., 2004; Hill et al., 2020; Sinclair et al., 2012; Terblanche et al., 2010), or indirectly by negatively affecting key features of physiological activity (Régnière et al., 2012). Furthermore, low temperature extremes can also have direct consequences (Burikam et al., 1992; Jessup & Baheer, 1990), thus affecting the overwintering survival (Merkel et al., 2019) and insect population dynamics either by reducing growth rate, developmental rate, and reproductive activity (Huang et al., 2020). The response to extreme temperatures may differ between insect families and species. A temperature of 35ºC can induce larval mortality and female sterility in three Ceratitis species (Duyck & Quilici, 2002). Moreover, this temperature of 35 °C reduces the population growth of other tephritid species such as Ceratitis capitata (Wiedemann), Bactrocera dorsalis (Hendel) and B. cucurbitae (Coquillett), suggesting that a temperature range of 35 ºC is more limiting for their establishment in a new area than lower temperatures (Vargas et al., 2000). Given that insect populations can cope with thermal extremes using a variety of mechanisms and strategies (Terblanche et al., 2015), there is need to study and to understand these tolerances and adaptations.
Despite the economic importance of D. frontalis in cucurbit crops, little is known about the effects of temperature variation on the fitness of this tephritid species. This information can support the success of control strategies against this pest in different regions and ecosystems. It can also help to understand the presence or absence of this insect in a given region and it’s invasion success in a such area. Thus, this finding is the first that study the immature life history parameters and estimate the adult performance of D. frontalis reared under four constant temperatures.
Materials and methods
Insect rearing
The study was conducted with a laboratory strain of D. frontalis that is established in the Entomology laboratory at Higher Agronomic Institute of Chott-Meriem. The strain of D. frontalis was initially started from samples of field infested cucumber fruits (Cucumis sativus L.) that are collected between september and october 2014 in Kairouan area (Center of Tunisia) (Hafsi et al., 2015a). Larvae were reared on cucumber fruits for three to five generations. However, adults were reared on an optimal diet which contained a mixture of yeast hydrolysate and sugar at 1:3 ratio. Laboratory rearing was conducted under constant conditions in an environmental chamber set at 25 ± 1 °C; 12:12 h (light: dark) photoperiod, and 70 ± 5% RH. Thermal tolerance experiments were done under four constant temperatures (15, 20, 25, and 30 °C) in a separate environmental chamber (MEMMERT, France) under constant conditions of relative humidity (70 ± 5%), and photoperiod (L12:D12).
Egg, larval, and pupal development
Eggs were collected from an artificial egg-laying device placed for 2-hours into laboratory D. frontalis strain. The device consisted of a half of yellow ball (3 cm diameter) with 100 small holes through which the females laid their eggs. A piece of 5 g of cucumber fruit was placed inside the device so that its odor stimulated egg-laying. Eggs were carefully collected with a camel brush and a sample of 100 eggs were placed on black humid blotting paper inside a petri dish. At each tested temperature, ten replications were maintained with 100 eggs each. Egg developmental duration and survivorship for each temperature were determined by observation of egg hatching every 12 h for 4 days.
Newly emerged larvae (< 4 h) were placed on slit made on the surface of premature cucumber fruit with camel brush for a final density of 1 larva per 10 g of cucumber. This density prevents intraspecific resource competition. After that, each cucumber fruit was placed in a plastic container aerated by meshed openings and layered with dry sand for pupation. In total, ten replications were done at each tested temperature. Every 48 h during 45 days, all containers were examined and pupae were collected and placed individually into a small plastic container containing sand. Towards the end of the pupal stage, the number and the sex of newly emerged adults was recorded daily (Duyck & Quilici, 2002) to determine the time and the percentage of adult emergence. The sex ratio was calculated for each tested temperature as the total number of emerged females/total number of emerged males.
Adult survival, longevity and female fecundity
At each tested temperature, thirty pairs of newly emerged males and females (< 8 h) were held individually in transparent plastic cups of 1 L volume aerated by meshed openings. Adult flies had free access to food (sugar and protein with 1:3 ratio) and water. Half of the yellow ball (3 cm diameter) with 100 small holes containing a small portion of cucumber fruit (5 g) was placed daily in each transparent plastic cup as oviposition substrate (Duyck et al., 2002). The number of eggs oviposited per female per day and the adult mortality were recorded daily, until the death of all adults, to determine the pre-oviposition and oviposition duration, fecundity, and longevity of D. frontalis females and males at each tested temperature.
Temperature summation model
The temperature summation model was used to predict the development rate of individual life stages (Duyck & Quilici, 2002), and was calculated using regression analysis between temperature and development rate. To establish this relationship, the development time of individual life stages (i.e. the time required for 50% of individuals to complete a given biological stage) was determined at a series of constant temperatures. The lower development threshold t (i.e. the temperature at which the development rate is zero) was then determined by extrapolation of the regression line back to the x-axis. The thermal constant K (i.e. the number of day degrees above the lower threshold required to complete development) was calculated from the regression equation using the relationship y = K / (x – t) (Duyck & Quilici, 2002).
Statistical analyses
All statistical analysis was carried out using R version 3.6.1 (R Development Core Team, 2008) via the interface R studio (version 1.2.5). Survival rate of each life stage was analyzed using a general linear model (GLM) with a binomial error (logit link) as a function of temperature. Development time of each life stages (eggs, larvae, pupae), female and male longevity, and female fecundity were analyzed by analysis of variance (ANOVA) as a function of temperature. The data were checked for normality by using the Shapiro-Wilk test and student’s t-test was used to compare development and survival for each life stage of D. frontalis between temperature treatments. A GLM with binomial error (logit link) was used to determine how the sex-ratio of D. frontalis were affected by temperature.
Results
Survival rate of immature stage of D. frontalis under constant temperatures
Dacus frontalis successfully survived from egg to adult stage at all studied temperatures (Table 1). The survival rate of each immature stage was signifcantly affected by temperature (egg: F3, 241 = 10.04, P < 0.001; larvae: F3, 372 = 6.77, P < 0.001; pupae: F3, 372 = 8.59, P < 0.001; Table 1). In the low temperature treatment (15 °C), the egg, larval, and pupal stage had significantly the lowest survival rate. Eggs were the most tolerant to low and high-temperatures with the highest survival rate of 56.80 and 85.20% at 15 and 25 °C, respectively. Among the different tested temperature, the highest survival rate was observed at 20 °C for the larval and pupal stages, but not for the egg.
Developmental time and rate of immature stages of D. frontalis and sex-ratio under constant temperatures
Dacus frontalis was able to complete its development from egg to adult at all tested temperatures. The developmental time of each life stage and from egg to adult emergence was found to significantly affected by temperature (eggs: ΔDev3, 244 = 11,297; P < 0.001; larvae: ΔDev3, 375 = 491; P < 0.001; pupae: ΔDev3, 372 = 459; P < 0.001) (Table 2). In response to an increase in temperature from 15 to 30 °C, there was a reduction in the time required to complete the development of each immature stage and the total development form egg to adult, with the shortest being at 30 °C. The development time from egg to adult ranged from 100.26 days at 15 °C to 23.90 days at 30 °C.
For D. frontalis, a strong and positive linear relationship was observed between temperature and development rate of each life stage (Fig. 1, R² = 0.95, 0.94, 0.93 for egg, larval and pupal stages, respectively; P < 0.0001). Lower temperature thresholds for egg, larvae and pupae development were estimated as 4.6, 13.5, and 9.5 °C, respectively. The day degree (DD) requirements to complete the egg, larvae, and pupae development were 32, 95, and 210 DD, respectively.
The proportion of females in emerged D. frontalis pupae -were not significantly affected by temperature treatments (ΔDev3, 140 = 198; P = 0.734).
Adult survival, longevity, and fecundity of D. frontalis under constant temperatures
With the exception of a temperature of 15 °C, at which females did not lay eggs, D. frontalis successfully survived and laid eggs at all other tested temperatures. The survival rates of females and males were found to be significantly affected by temperature (ΔDev3, 4546 = 4965, P < 0.001; ΔDev3, 4546 = 5434, P < 0.001, respectively) (Table 1). Females and males of D. frontalis had significantly higher survival at 20 °C with 84.86 and 75.58%, respectively.
Overall, temperature treatments had a significant effect on the longevity of male and female D. frontalis. Adult longevity was significantly affected by temperature for both sexes (female: F3, 84 = 125.59, P < 0.001; male: F3, 84 = 70.49, P < 0.001) (Fig. 2). At 15 °C, the longevity was significantly shorter compared to all other tested temperatures, not exceeding 11 and 10 days for females and males, respectively. Except for 15 °C, and when the temperature was increased from 20 to 30 °C, the longevity of either female or male adults gradually decreased, suggesting that temperature stress shortens the lifespan of both sexes.
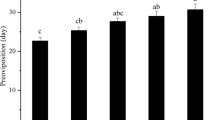
The preoviposition period of D. frontalis females was significantly affected by temperature treatments (F2, 67 = 46.70, P < 0.001). Females of D. frontalis were not able to produce eggs at low temperatures (15 °C). The duration of preoviposition was shortest at 25 °C (6.23 days) (Fig. 3). Apart from this temperature, preoviposition was significantly prolonged reaching a maximum of 10.35 days at 20 °C. With the increase in temperature, the preoviposition period of D. frontalis was first shortened and then prolonged.
The oviposition duration of adults (Fig. 3) and female fecundity (Fig. 4) were significantly affected by temperature treatments (F2, 67 = 27.43, P < 0.001; F2, 3371 = 5.84, P = 0.015, respectively). Females of D. frontalis were not able to produce eggs at low temperatures ≤ 15 °C. With increasing temperature, the oviposition period of D. frontalis was significantly shortened. Regarding female fecundity, during this longest oviposition period, D. frontalis females produced significantly the highest number of eggs (14.58 eggs/female/day) at 20 °C. Overall, with the exception of 15 °C, the average number of eggs produced per female decreased significantly with increasing temperatures.
Discussion
In this study, we investigated, for the first time, the effect of temperature on the development, survival, reproduction, and sex ratio of the invasive tephritid species, D. frontalis. We showed a strong and positive linear relationship between temperature and development rate for egg, larval and pupal stages. The temperature infuences significantly the development time of each life stage of D. frontalis. This tephritid species is able to develop from egg to adult over a temperature range of 15 to 30ºC, and the development time of each life stage decreases with increasing temperature. Our results are consistent with that of previous studies on other tephritid species (Choudhary et al., 2020; Duyck & Quilici, 2002; Duyck et al., 2004; Tanga et al., 2015).
Considering the survival and development rate of the different developmental stages, our results suggest that 20 and 30 °C represent an optimal temperature for this pest, as D. frontalis had the highest survival rate and shorter development time at these temperatures. These parameters are considered as important criteria for mass rearing of tephritid species (Duyck & Quilici, 2002; Duyck et al., 2004; Krainacker et al., 1989). In addition, our resultswere consistent with those observed in the field where larvae of D. frontalis were found in cucumber and zucchini crops only when field temperatures ranged from 17 to 30 °C in Iraq and in Tunisia (Al-Soltany et al., 2020; Hafsi et al., 2015a). The ability of eggs of D. frontalis to tolerate low and high experimental temperatures (15 and 30 °C) compared to larvae and pupae is expected because eggs are often laid close to the peel of the host fruit and exposed to more extreme temperatures compared to the larvae, which are able to move inside the pulp of the fruit, protecting them from extreme low or high temperatures (Grout & Stoltz, 2014; Pieterse et al., 2017).
The positive response of immature stages of D. frontalis to different thermal environments may explain its current distribution, but also suggests that it can adapt, and invade new areas. In addition to tropical climates, D. frontalis is present in colder climates such as in South Africa and the Mediterranean area. The lower developmental threshold for egg, larval and pupal stages of D. frontalis was estimated in our study as 4.6, 13.5, and 9.5 °C, respectively, compared to 11.6, 10.2, 11.2 °C for C. capitata (Duyck & Quilici, 2002) that is able to establish under different climatic zones (White & Elson-Harris, 1992) including Mediterranean areas. The invasion of D. frontalis in new areas of Mediterranea and Europe can be expected as the minimum temperature threshold of this species are lower than those of C. capitata, particularly during egg stages.
Our results on adult longevity, female oviposition, and pre-oviposition period of D. frontalis revealed that these parameters varied with temperature, suggesting that 20 °C is an optimal temperature for best adult performance. However, the determinal effect of prolonged exposure to low temperatures was observed in adult flies, which were unable to reproduce and live for more than 11 days at 15 °C. This suggests that even mild winter temperatures are detrimental to the adult survival and reproductive potential of this tephritid species and the low temperatures (15 °C) may affect ovarianmaturity in females. During winter, sexually mature female flies will resorb developing oocytes and become sexually and ovipositionally inactive (Fletcher, 1975). Thermal tolerance studies on Ceratitis and Bactrocera tephritid species, showed no ovarian maturation of some adults when reared at 15 °C (Duyck & Quilici, 2002; Duyck et al., 2004). This finding showed that thermal requirements are higher for oviposition than for survival and development of immature and adult life stages. Such results are often observed in Bactrocera species, which were considered to be reproductively “diapausing” (Fletcher, 1987). Therefore, thermal requirements can explain the seasonality of D. frontalis observed in the field in North Africa (Al-Soltany et al., 2020; Hafsi et al., 2015a), where no adult flies and no fruit damage were observed during the winter season. It is possible that lower temperatures experienced during winter may limit the reproduction success during this period. However, survival rates and lower temperature thresholds may allow a slow development of egg and larvae during winter and may have probably contributed to favouring its invasion in Mediterranean areas.
Pupae are also able to survive at low temperature with long period of development time, suggesting that this species can overwinter as pupae in the field. In insects, diapause is recognized as the most common mechanism of overwintering (Clarke et al., 2019), which can occur most commonly in the egg or pupal stage (Denlinger, 2002; Tauber & Tauber, 1976). In Dacini fruit flies, the winter diapause is often unknown (Clarke et al., 2019). Dacini species are known as tropical and subtropical species and are generally not associated with temperate regions, but, some species have successfully extended their geographic range into seasonally cold climates where winter temperatures may be limiting (Clarke et al., 2019). This is the case for Bactrocera olea and Bactrocera minax, which are able to overwinter as diapausing pupae (Dong et al., 2013; Tzanakakis, 2003). Given the importance of this phenomenon in the successful tolerance of insects to low temperatures and in population dynamics, it should be taken into account as one of the important parameters in the biosecurity risk assessment and in the implementation of the pest management strategy of this tephritid species.
For practical implications, the results of this study provide useful information on the effect of temperature on the development and survival of D. frontalis under laboratory conditions, which is a first step before analysing more complex ecological relationships. For example, our data can be integrated among other parameters to improve the modelling system of fruit fly population dynamics in order to implement better monitoring and pest management, and to determine the quarantine risk associated with these flies (Ahn et al., 2022; Rossini et al., 2020). Also, our data provide information that may be useful to improve rearing methods of D. frontalis, as temperature plays a key role in the rearing process of tephritid species (Brévault & Quilici, 2000). A compromise between a short development time and a high survival rate would be to rear eggs, larvae, and pupae at 25 °C. However, adults should be reared at 20 °C to produce more eggs. In addition, knowledge of optimal temperatures is important to maintain natural enemies population which might be reared (Brévault & Quilici, 2000) on D. frontalis as part of an IPM strategy or for mass rearing of this tephritid species for sterile insect technique (SIT) programs that could be implemented for their control and eradication.
References
Ahn, J. J., Choi, K. S., & Huang, Y. B. (2022). Thermal effects on the development of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) and model validation. Phytoparasitica, 50, 601–616.
Al-Jorany, R., Al-Zubaidy, H., & Flair, S. (2019). Economic losses and economic threshold of Cucurbit fruit fly Dacus ciliatus (Loew) and Greater Melon fruit fly Dacus frontalis (Becker) on Cucumber Cucumis Staivus L. in middle of Iraq. Journal of Kerbala for Agricultural Sciences, 2, 106–117.
Al-Soltany, A. H., Al-Dahwy, S. S., & Ali, A. A. (2020). Evaluation of the bifenthrin 10% EC in both normal and nanoparticules against Dacus frontalis in cucumbers field. Plant Archives, 20, 1131–1135.
Ba-Angood, S. A. S. (1977). Control of the melon fruit fly, Dacus frontalis Becker (Diptera: Trypetidae), on cucurbits. Journal of Horticultural Sciences, 52, 545–547.
Badii, K., Billah, M., Afreh-Nuamah, K., Obeng-Ofori, D., & Nyarko, G. (2015). Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa. African Journal of Agricultural Research, 10, 1488–1498.
Bale, J. (2002). Insects and low temperatures: From molecular biology to distributions and abundance. Philosophical Transactions of the Royal Society of London Series B Biological Sciences, 357, 849–862.
Bonebrake, T. C., & Deutsch, C. A. (2012). Climate heterogeneity modulates impact of warming on tropical insects. Ecology, 93, 449–455.
Brévault, T., & Quilici, S. (2000). Relationships between temperature, development and survival of different life stages of the tomato fruit fly, Neoceratitis cyanescens. Entomologia Experimentalis et Applicata, 94, 25–30.
Burikam, I., Sarnthoy, O., Charernsom, K., Kanno, T., & Homma, H. (1992). Cold temperature treatment for mangosteens infested with the oriental fruit fly (Diptera: Tephritidae). Journal of Economic Entomolgy, 85, 2298–2301.
Choudhary, J. S., Mali, S. S., Naaz, N., Mukherjee, D., Moanaro, L., Das, B., Singh, A., Rao, M. S., & Bhatt, B. (2020). Predicting the population growth potential of Bactrocera zonata (Saunders)(Diptera: Tephritidae) using temperature development growth models and their validation in fluctuating temperature condition. Phytoparasitica, 48, 1–13.
Clarke, A. R., Merkel, K., Hulthen, A. D., & Schwarzmueller, F. (2019). Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) overwintering: An overview. Austral Entomology, 58, 3–8.
Denlinger, D. L. (2002). Regulation of diapause. Annual Review of Entomology, 47, 93–122.
Dong, Y. C., Wang, Z. J., Clarke, A. R., Pereira, R., Desneux, N., & Niu, C. Y. (2013). Pupal diapause development and termination is driven by low temperature chilling in Bactrocera minax. Journal of Pest Science, 86, 429–436.
Duyck, P. F., & Quilici, S. (2002). Survival and development of different life stages of three Ceratitis spp (Diptera: Tephritidae) reared at five constant temperatures. Bulletin of Entomological Research, 92, 461–469.
Duyck, P. F., Sterlin, J. F., & Quilici, S. (2004). Survival and development of different life stages of Bactrocera zonata (Diptera: Tephritidae) reared at five constant temperatures compared to other fruit fly species. Bulletin of Entomological Research, 94, 89–93.
Duyck, P. F., Quilici, S., & Glenac, S. (2002). Comparative study of the developmental biology of three species of fruit flies (Ceratitis spp.) (Diptera: Tephritidae), pests of fruit crops on Réunion Island. In: B. N. Barnes (Ed.), Proceedings of the 6th International Fruit Fly Symposium 6–10 May 2002 (pp. 67–69). Stellenbosch, South Africa.
Ekesi, S., Lux, S., & Billah, M. (2007). Field comparison of food-based synthetic attractants and traps for African tephritid fruit flies: Development of improved attractants and their integration into fruit fly SIT management programmes. IAEA Vienna. https://inis.iaea.org/collection/NCLCollectionStore/_Public/38/115/38115160.pdf#page=212. Accessed 2021
Elghadi, E., & Port, G. (2019). Use of Entomopathogenic Fungi for the Biological Control of the Greater Melon Fly Dacus frontalis in Libya. In: D. Perez-Staples, F. Diaz-Fleischer, P. Montoya, & M. Vera (Eds.), Area-Wide Management of Fruit Fly Pests (1st ed., pp. 251–265).
El-Harym, Y., & Belqat, B. (2017). First checklist of the fruit flies of Morocco, including new records (Diptera, Tephritidae). Zookeys, 702, 137–171.
Fletcher, B. (1975). Temperature-regulated changes in the ovaries of overwintering females of the Queensland fruit fly, Dacus Tryoni. Australian Journal of Zoology, 23, 91–102.
Fletcher, B. (1987). The biology of dacine fruit flies. Annual Review of Entomology, 32, 115–144.
Foottit, R. G., & Adler, P. H. (2009). Insect biodiversity: Science and society. Wiley.
Grechi, I., Preterre, A. L., Lardenois, M., & Ratnadass, A. (2022). Bactrocera dorsalis invasion increased fruit fly incidence on mango production in Reunion Island. Crop Protection, 161, 1–9.
Grout, T. G., & Stoltz, K. C. (2014). Developmental rates at constant temperatures of three economically important Ceratitis spp. (Diptera: Tephritidae) from southern Africa. Environmental Entomolgy, 36, 1310–1317.
Gutierrez, A. P., & Ponti, L. (2014). Analysis of invasive insects: Links to climate change. In L. H. Ziska, & J. S. Dukes (Eds.), Invasive species and global climate change (4th ed., pp. 45–61). CABI.
Hafsi, A., Abbes, K., Harbi, A., Ben Othmen, S., Limem, E., Elimem, M., Ksantini, M., & Chermiti, B. (2015a). The pumpkin fly Dacus frontalis (Diptera: Tephritidae): A new pest of curcubits in Tunisia. EPPO Bulletin, 45, 209–213.
Hafsi, A., Abbes, K., Harbi, A., Rahmouni, R., & Chermiti, B. (2015b). Comparative efficacy of Malathion and spinosad bait sprays against Ceratitis capitata Wiedmann (Diptera: Tephritidae) in Tunisian citrus orchards. Journal of Entomology and Zoology Studies, 3, 246–249.
Hill, S. J., Silcocks, S. C., & Andrew, N. R. (2020). Impacts of temperature on metabolic rates of adult Extatosoma tiaratum reared on different host plant species. Physiological Entomology, 45, 7–15.
Huang, Y., Gu, X., Peng, X., Tao, M., Peng, L., Chen, G., & Zhang, X. (2020). Effect of short-term low temperature on the growth, development, and reproduction of Bactrocera tau (Diptera: Tephritidae) and Bactrocera cucurbitae. Journal of Economic Entomology, 113, 2141–2149.
Jessup, A. J., & Baheer, A. (1990). Low-temperature storage as a quarantine treatment for kiwifruit infested with Dacus tryoni (Diptera: Tephritidae). Journal of Economic Entomology, 83, 2317–2319.
Kalaitzaki, A., Amara, A., Dervisoglou, S., Perdikis, D., Τzοbanoglou, D., Koufakis, I., & Tsagkarakis, Α. (2023). Effect of host plant species and temperature on the development and survival of the plant bug Closterotomus Trivialis (Costa) (Hemiptera: Miridae). Phytoparasitica, 51, 19–28.
Krainacker, D., Carey, J., & Vargas, R. (1989). Size-specific survival and fecundity for laboratory strains of two tephritid (Diptera: Tephritidae) species: Implications for mass rearing. Journal of Economic Entomology, 82, 104–108.
Merkel, K., Schwarzmueller, F., Hulthen, A. D., Schellhorn, N., Williams, D., & Clarke, A. R. (2019). Temperature effects on overwintering phenology of a polyphagous, tropical fruit fly (Tephritidae) at the subtropical/temperate interface. Journal of Applied Entomology, 143, 754–765.
Pfab, F., Stacconi, M. V. R., Anfora, G., Grassi, A., Walton, V., & Pugliese, A. (2018). Optimized timing of parasitoid release: A mathematical model for biological control of Drosophila suzukii. Theoretical Ecology, 11, 489–501.
Pieterse, W., Terblanche, J. S., & Addison, P. (2017). Do thermal tolerances and rapid thermal responses contribute to the invasion potential of Bactrocera dorsalis (Diptera: Tephritidae)? Journal of Insect Physiology, 98, 1–6.
R Development Core Team. (2008). R: A language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Régnière, J., Powell, J., Bentz, B., & Nealis, V. (2012). Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. Journal of Insect Physiology, 58, 634–647.
Rossini, L., Contarini, M., Giarruzzo, F., Assennato, M., & Speranza, S. (2020). Modelling Drosophila suzukii adult male populations: A physiologically based approach with validation. Insects, 11, 1–15.
Schlesener, D. C., Wollmann, J., Krüger, A. P., Martins, L. N., Teixeira, C. M., Bernardi, D., & Garcia, F. R. (2020). Effect of temperature on reproduction, development, and phenotypic plasticity of Drosophila suzukii in Brazil. Entomologia Experimentalis et Applicata, 168, 817–826.
Secretariat. (2023). GBIF backbone taxonomy. Checklist dataset. Retrieved November 22, 2023, from https://doi.org/10.15468/39omei
Shawkit, M. A., Basam, A. N., Hussian, F., Edan, L. H., & Mahmood, A. R. (2011). Population density and biological studies of two cucucrbit flies species: Dacus ciliates Loew and Dacus frontalis Beecker (Diptera: Tephritidae). Journal of Madenat Alelem University College, 3, 78–84.
Sinclair, B. J., Williams, C. M., & Terblanche, J. S. (2012). Variation in thermal performance among insect populations. Physiological and Biochemical Zoology, 85, 594–606.
Tanga, C. M., Manrakhan, A., Daneel, J. H., Mohamed, S. A., Fathiya, K., & Ekesi, S. (2015). Comparative analysis of development and survival of two Natal fruit fly Ceratitis rosa Karsch (Diptera, Tephritidae) populations from Kenya and South Africa. ZooKeys, 540, 467–487.
Tauber, M. J., & Tauber, C. A. (1976). Diapause maintenance, terminantion, and postdiapose. Annual Review of Entomology, 21, 81–107.
Terblanche, J. S., Hoffmann, A. A., Mitchell, K. A., Rako, L., Le Roux, P. C., & Chown, S. L. (2011). Ecologically relevant measures of tolerance to potentially lethal temperatures. Journal of Experimental Biology, 214, 3713–3725.
Terblanche, J. S., Karsten, M., Mitchell, K. A., Barton, M. G., & Gibert, P. (2015). Physiological variation of insects in agricultural landscapes: potential impacts of climate change. In: C. Björkman, & P. Niemelä (Eds.), Climate Change and Insect Pests. (8th ed., pp 92–118).
Terblanche, J. S., Nyamukondiwa, C., & Kleynhans, E. (2010). Thermal variability alters climatic stress resistance and plastic responses in a globally invasive pest, the Mediterranean fruit fly (Ceratitis capitata). Entomologia Experimentalis et Applicata, 137, 304–315.
Tzanakakis, M. E. (2003). Seasonal development and dormancy of insects and mites feeding on olive: A review. Netherlands Journal of Zoology, 52, 87–224.
Vargas, R. I., Walsh, W. A., Kanehisa, D., Stark, J. D., & Nishida, T. (2000). Comparative demography of three hawaiian fruit flies (Diptera: Tephritidae) at alternating temperatures. Annals of the Entomological Society of America, 93, 75–81.
Weldon, C. W., Nyamukondiwa, C., Karsten, M., Chown, S. L., & Terblanche, J. S. (2018). Geographic variation and plasticity in climate stress resistance among southern African populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Scientific Reports, 8, 1–13.
White, I. M., & Elson-Harris, M. M. (1992). Fruit flies of economic significance: Their identification and bionomics. CAB International.
Wiman, N. G., Dalton, D. T., Anfora, G., Biondi, A., Chiu, J. C., Daane, K. M., Gerdeman, B., Gottardello, A., Hamby, K. A., & Isaacs, R. (2016). Drosophila suzukii population response to environment and management strategies. Journal of Pest Science, 89, 653–665.
Winkler, A., Jung, J., Kleinhenz, B., & Racca, P. (2020). A review on temperature and humidity effects on Drosophila suzukii population dynamics. Agricultural and Forest Entomology, 22, 179–192.
Acknowledgements
This publication was produced with the financial support of the European Union within the framework of the ENI Cross-Border Cooperation Programme Italy-Tunisia 2014–2020 through the INTEMAR project -IS_2.1_073 Innovations in the integrated control of insect pests and pathogens recently introduced on vegetable crops. Its content is the sole responsibility of the project beneficiary and does not necessarily reflect the opinions of the European Union and those of the Managing Authority. This study used the facilities provided by the High Agronomic Institute of Chott-Mariem, University of Sousse, Tunisia. We think the student H. Mannai that contributed to measuring D. frontalis life history traits during here internship.
Funding
This study was produced with the financial support of the European Union within the framework of the ENI Cross-Border Cooperation Programme Italy-Tunisia 2014–2020 through the INTEMAR project -IS_2.1_073 Innovations in the integrated control of insect pests and pathogens recently introduced on vegetable crops.
Author information
Authors and Affiliations
Contributions
A.H., K.A., and B.C. contributed to the study conception, design and/or writing of the manuscript. Material preparation, data collection and analysis were performed by A.H. and K.A. Statistical analysis was approved by P-F.D. The first draft of the manuscript was written by A.H. All authors reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hafsi, A., Abbes, K., Duyck, PF. et al. Life-history traits of Dacus frontalis Becker (Diptera: Tephritidae) reared at four constant temperatures. Phytoparasitica 52, 16 (2024). https://doi.org/10.1007/s12600-024-01132-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01132-y