Abstract
Bactrocera minax is a major citrus pest in China, Bhutan, and India. It is univoltine and exhibits pupal diapause during winter. To better understand pupal diapause in this pest, we investigated pupal survival and pupal developmental duration under field and laboratory conditions. Specifically, we tested if pupal chilling was required for diapause development and termination. Nearly all mature larvae collected at the end of the citrus season entered pupal diapause. For pupae exposed in the field, natural chilling for less than 3 months resulted in more than 70 % mortality. However, exposure to winter conditions for 3 months or more both decreased pupal mortality and developmental duration when pupae were returned to the laboratory and held under constant temperature (25 °C). When pupae were gathered from the field in November and exposed to different chilling regimes in the laboratory, the chilling duration (30 vs 60 days) had significantly more impact on pupal survival than the specific chilling temperature (6, 8, 10, or 12 °C constant). However, both chilling duration and chilling temperature impacted on the pupal developmental duration, with longer chilling duration and higher temperatures decreasing pupal developmental duration. In conclusion, we demonstrated that pupal diapause development and termination in B. minax is strongly influenced by chilling conditions. Increasing cold exposure led to significantly and consistently faster adult eclosion and improved synchronization of adult emergence. This knowledge will help with the laboratory rearing of B. minax, an essential step in the long-term management of this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Chinese citrus fruit fly, B. minax (Enderlein) (Diptera: Tephritidae), is one of the most economically important citrus pests in China, Bhutan, and north-west India (Dorji et al. 2006; Wang and Luo 1995). Like nearly all Bactrocera species (Daane and Johnson 2010; Goncalves and Torres 2011; Han et al. 2011; Canale and Benelli 2012), the fly lays its eggs into the fruit of host plants, where the subsequent larvae emerge and feed, before leaving the fruit to pupate in the soil (Wang and Luo 1995). Throughout its geographic range, B. minax is univoltine and oligophagous on species and varieties of the genus Citrus (White and Wang 1992), which has a centre of diversity in this region (Shan 2008; Han et al. 2011). Within the genus Bactrocera, B. minax is highly unusual in having a winter pupal diapause, presumably an evolved response to surviving the very cold winters which occur in its native range (Fan et al. 1994). The overwintering pupae last for 160–170 days, with emergence synchronized to the fruiting season of citrus (Wang and Luo 1995).
It is generally considered that diapause in insects is a widespread adaptation to avoiding seasonal environmental stress (Kostal 2006; Denlinger 2002; Danks 2007), with environmental temperature playing a key role in diapause development (Moribe et al. 2001; Stross 1966; Terao et al. 2012; Turnock et al. 1983). In many univoltine, temperate tephritid species, pupal diapause occurs commonly as an adaptive strategy to avoid the seasonal stresses of very cold winters (Moraiti et al. 2012; Teixeira and Polavarapu 2001; Yasuda et al. 1994). The initiation and duration of pupal winter diapause in tephritids is associated with temperatures experienced during the larval and pupal stages (Yasuda et al. 1994; Vankirk and Aliniazee 1982; Teixeira and Polavarapu 2005a, b). Across insects, chilling is considered one of the most common mechanisms by which winter diapause is terminated in the field (Hodek 1996, 2002; Tauber et al. 1986), and this also occurs in tephritids (Teixeira and Polavarapu 2002, 2005a; Vankirk and Aliniazee 1982). For example, chilling at 4–15 °C served as a terminating factor to complete the refractory phase of pupal diapause in blueberry maggot, Rhagoletis mendax Curran (Teixeira and Polavarapu 2005a). Chilling at 3–9 °C was adequate for completion of pupal diapause of the western cherry fruit fly, Rhagoletis indifferens Curran (Vankirk and Aliniazee 1982).
The pest status of B. minax justifies intensive research into its sustainable management (Zhou et al. 2012). However, a current inability to break pupal diapause means that research is severely hampered, while the long-term mass rearing of flies for use in the Sterile Insect Technique (SIT) (Krafsur 1998) is not possible. Given the extensive literature indicating that cold temperatures play a role in insect diapause termination, we evaluated the effects of temperature on B. minax pupal diapause. More explicitly, we carried out the following studies: (i) assessment of B. minax pupal diapause duration and adult eclosion success following differential exposure of pupae to cold conditions in the field; and (ii) the effects of different chilling regimes (varying both temperature and exposure periods) on B. minax pupal diapause duration and eclosion in the laboratory.
Materials and methods
Collection, maintenance, and assessment of pupae
Oranges infested with B. minax maggots were gathered from an abandoned orchard in Sandouping (30°81′N, 111°05′E), Hubei Province, China, and placed over sand in an outdoor field cage to allow larvae to complete development and pupate. These pupae were subsequently collected for use in two sets of experiments (see below). Before used in trials, collected pupae were surface-sterilized by emersion in 1 % bleach, rinsed in distilled water and then air dried on filter paper. During all trials, pupae were held by being buried in sterile sand (~3 cm) in plastic dishes to which distilled water was periodically added to maintain moderate moisture. Where pupae were held in constant temperature incubators (see below), a photo-regime of 16:8 (L:D) and 70 ± 5 % RH were maintained. Pupae were checked daily during trials to assess adult emergence (=pupal developmental duration) and pupal condition (dead pupae turned black and were easily told apart from healthy pupae). Following the definitions of Hodek and Hodková (1988), the pupal developmental duration was calculated as time from when chilling ceased [either following removal of pupae from the field (Trial 1) or following chilling treatments (Trial 2)] until the adult emerged.
Trial 1: field sampling survey
The objective of Trial 1 was to determine the effects of winter temperatures on pupal diapause duration and termination in B. minax. Pupae were collected approximately monthly from the field cage (20 November and 21 December 2009, and 27 January, 25 February, 27 March 2010) and then brought to the laboratory where they were maintained at 25 ± 2 °C in an incubator. In Hubei Province, mature larvae pupate from early to late November (YC Dong and CY Niu, pers. obs.), so a collection in November equates to the start of the pupal diapause period. In addition to the pupae recovered from the field cage, B. minax infested oranges were collected on 20 October 2009 and returned directly to the laboratory, where the fruit and subsequent pupae were held at a constant 25 ± 2 °C. These pupae, which never experienced natural field chilling, were used as a control. Morphogenesis was assessed through changes in the external appearance of the pupae during the period of non-diapausing individuals development (an obvious mark resulting from the mouthpart flaking off the puparium, and eye and wing formation seen under strong light).
The sample sizes of pupae collected from the field were as follows: 1,050 (n = 3 separate field collections, control), 1,013 (n = 3, November 2009), 1,135 (n = 4, December 2009), 443 (n = 3, January 2010), 331 (n = 3, February 2010), and 449 (n = 3, March 2010). Field air temperatures (daily maximum, minimum, and mean) for the period of pupal sampling were obtained from the Yichang Meteorological Station (30°70′N, 111°30′E), approximately 27 km from the field cages. Direct comparisons of air temperatures at the field site with those from the meteorological station showed them to be very similar (YC Dong and CY Niu, pers. obs.), but because of the altitudinal differences we would expect the field site to be marginally cooler than the weather station.
Trial 2: laboratory cold chilling treatment
The objective of this trial was to assess the effects of various cold temperatures and exposure periods on B. minax pupal diapause development. Batches of 25 pupae, collected from the field cage in late November 2011, were incubated in moist sand at either 6, 8, 10 or 12 °C, for durations 30 or 60 days, before being transferred into a 22 ± 1 °C incubator. A control group was held at a constant 22 ± 1 °C. Each combination of treatments (i.e., temperature × duration combination) was replicated five times.
Data analysis
Statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, Illinois, USA). Following tests for normality, pupal survival rates and developmental durations after transfer to the laboratory for different field collections in Trial 1 were compared using one-way analysis of variance (ANOVA), with Duncan’s test for multiple comparisons (post hoc test). Differences in treatment means for pupal survival and developmental duration after transfer to 22 °C in Trial 2 were analyzed using a general linear model (GLM) with “Temperature” and “Chilling duration” as the independent factors; the interaction between these factors was also assessed.
For both Trials 1 and 2 the adult emergence patterns over the time (i.e., days after transfer to the warmer environment) for each replicate for each treatment were fitted to sigmoid curves following the approach of Kostal and Havelka (2001). Two emergence parameters (slope and Et50) were then calculated from the equations for the curves. The slope (of the central, rapidly elevating part of the sigmoid curve) is an indicator of the level of synchrony of adult emergence, while the Et50 is the estimated time in days needed for emergence of 50 % of adults. Differences between treatments, within a trial, for values of Et50 and slope were tested to evaluate if different chilling treatments led to synchronization of adult emergence. Comparisons of the mean slope and Et50 between treatments was done using one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc tests of difference between all pairs of test variants (Prism 5.0, GraphPad Software, Inc.).
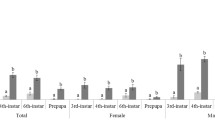
Across all trials, males consistently emerged from pupation a few days earlier than females (for example control in Trial 2: males 117.2 ± 28.6 days, n = 42; females 119.2 ± 20.2 days, n = 64). However, we did not separate male from female data for analyses because the general emergence pattern of both sexes in response to treatments was the same (YC Dong and CY Niu, unpublished data). Therefore, males and females were combined for all analyses.
Results
Trial 1: field sampling survey
The dates of field sampling and recorded temperatures are shown in Fig. 1. The temperature decreased through October and fluctuated between 0 and 10 °C from mid-November to mid-February (then the temperature began to rise). By the end of March, when the last pupal samples were collected, mean temperatures were around 15 °C.
Of the 1,050 “control” pupae, i.e., those returned to the laboratory as mature maggots in infested fruits with no chilling experience, 39 (3.71 %) directly initiated morphogenesis and emerged as adults within 130 days (median = 78 days, range 65–130 days).The remaining pupae entered diapause and died under constant temperature of 25 °C. The fact that 96 % of larvae in fruit, brought into warm conditions with a long-photoperiod regime, still entered pupal diapause implies that diapause entry is driven by factors impacting on the larvae at an earlier stage of their development.
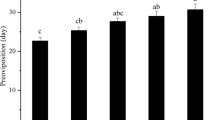
The data of pupal survival and developmental duration in Trials 1 and 2 followed normal distribution (P > 0.05). Significant differences were recorded in pupal survival (F 5,13 = 81.53, P < 0.01) and pupal developmental duration (F 5,13 = 90.92, P < 0.001) among the different sampling dates. Pupal survival increased significantly when the pupae were exposed to natural chilling before being brought into the laboratory (warmer environment). Indeed, pupal survival of the first three samples (Nov., Dec. and Jan. i.e. where pupae experienced mean temperatures inferior to 10 °C for ≤2 months) was not higher than 30 % (Fig. 2a). The pupal developmental duration for individuals collected from November onwards was significantly shorter than the control treatment, where pupae had experienced no chilling, but pupal developmental duration for samples collected in November and December were not significantly different from each other (Fig. 2b).
Mean (±SD) Bactrocera minax pupal survival (a) and pupal developmental duration (b) after field collection and transfer to laboratory conditions. Pupae of known age were collected in the field at five dates, returned to the laboratory and kept under constant temperature (25 °C). Control pupae were not exposed to any field conditions but were returned as mature larvae to the laboratory on 20 October 2009, where they were also kept at constant temperature (25 °C). Histograms bearing different letters are significantly different at the P < 0.05 level
Trial 2: laboratory cold chilling treatment
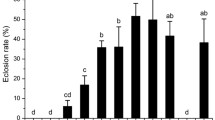
Chilling duration, but not chilling temperature, significantly influenced B. minax pupal survival, although there was also a significant interaction between temperature and duration (Table 1). Pupal survival combined across all temperature treatments was highest for 30 days of chilling, and significantly less for 60 days of chilling or no chilling (Fig. 3a). Mean pupal survival did not differ between the four chilling temperatures and varied between 60 and 80 % (Fig. 3b).
Mean (±SD) Bactrocera minax pupal survival at (a) two chilling durations (30 or 60 days) and (b) four chilling temperatures (6, 8, 10, or 12 °C) and pupal developmental duration at (c) two chilling durations (30 or 60 days) and (d) four chilling temperatures (6, 8, 10, or 12 °C). With the exception of the control treatment (=0 days chilling), the trial was run as a replicated, fully factorial design, thus the mean for each “chilling time” (except the control) is the combined result for the four chilling temperatures and vice versa. The control, with no chilling time, was run only at 22 °C. Pupal developmental duration was assessed only after removing from the chilling treatment. Each temperature/chilling time combination was replicated five times, and each replicate treatment consisting of 25 pupae. Asterisk indicates significant difference between chilling duration 30 and 60 days at P < 0.05 (a, c), histograms bearing different letters are statistically different at P < 0.05 (d)
Pupal developmental duration after chilling was significantly affected by both chilling duration and chilling temperature, but these two factors did not interact significantly (Table 1). Pupal developmental duration declined significantly with increasing chilling duration; from 126 days with no chilling to 63 days after 2 months (60 days) of chilling (Fig. 3c). In a similar manner, when pupae were exposed to increasing temperature, the pupal developmental duration decreased (from 81 days at 6 °C to 64 days at 12 °C) (Fig. 3d).
Cumulative emergence patterns
The adult emergence data from Trial 1 suggests that differential cold exposure leads to some synchronization of adult emergence. Increasing cold exposure in the field led to significantly and consistently faster adult eclosion (i.e., decreased pupal developmental duration) when pupae were returned to the laboratory (Fig. 4; Table 2). However, the shortened pupal developmental duration did not fully compensate for the extra chilling time. For example the Et50 of pupae collected in March (32 days) was only 6 days shorter than the Et50 of pupae collected in February (38 days) (Table 2), despite the extra month of chilling. Different laboratory chilling treatments (Trial 2) significantly affected both the Et50 and slope of the adult emergence curves (Table 3), but the slopes were not modified in any consistent fashion that could be related to synchronization of adults in the field (Fig. 5; Table 3). Rather, pupal developmental duration seemed to be related more directly to day degree exposure, with pupae exposed to higher temperatures for longer periods emerging more rapidly than pupae exposed to cooler temperatures for shorter periods (Fig. 5; Table 3).
Bactrocera minax cumulative adult emergence curves following transfer of pupae from winter field conditions to a constant temperature (25 °C). Pupae of known age were collected from the field on five occasions and then returned to the laboratory and kept under 25 °C temperature (i.e., Trial 1). Pupae were assessed for adult emergence every day, but for ease of presentation data is presented as cumulative 5-day counts
Bactrocera minax cumulative adult emergence curves following transfer of pupae from different laboratory chilling regimes to a constant 22 °C. The different chilling regimes were a control (no chilling), or combinations of four constant temperatures (6, 8, 10, 12 °C) for 30 days (a) and 60 days (b) (i.e. Trial 2). Pupae were assessed for adult emergence every day, but for ease of presentation data is presented as cumulative 5-day counts
Discussion
Cold chilling in both the laboratory and field shortened the pupal developmental duration in B. minax. The results from Trial 1 indicate that in the field B. minax’s pupal developmental duration declined, while pupal survival increased, as pupae underwent increasing cold exposure, inferring that cold temperature is a crucial factor in pupal diapause termination in this species. The inference from the field results is supported by the laboratory results (Trial 2) which confirm that cold chilling is a prerequisite for pupal diapause termination in B. minax, leading to a combination of a high percentage of successful adult eclosion. In addition, synchronized eclosions occur over a relatively short time. The post pupal developmental duration of approximately 35 days for pupae field collected in March matches well with the known spring adult emergence of this species. It also helps synchronizing the presence of newly sexually mature adults (approximately 1 month after eclosion) with the susceptible fruiting season of Citrus hosts (Dorji et al. 2006; Zhou et al. 2012). The different effects of low temperature on emergence synchronization between field and laboratory trials suggests that pupal diapause termination might be related to fluctuating temperature chilling rather than constant low temperature chilling or warm temperature condition after chilling (i.e., 22 vs 25 °C). Testing a wide range of chilling temperatures and constant versus fluctuating temperatures should be done in follow up trials to fully understand diapause termination in this species.
A finding that chilling is essential for diapause development and termination in B. minax is in agreement with findings for many other winter diapausing species (Smith and Jones 1991; Kostal et al. 2000; Nomura and Ishikawa 2000; Bosch and Kemp 2003; Terao et al. 2012; Collier et al. 1994), including other tephritids (Vankirk and Aliniazee 1982; Teixeira and Polavarapu 2005a, b, c, d; Dambroski and Feder 2007). That the pupal developmental duration in B. minax shortened with extended chilling duration suggests that flies exposed to longer chilling duration require fewer heat units for adult emergence: this result was also found for apple maggot fly, Rhagoletis pomonella (Smith and Jones 1991).
The actual temperatures involved in chilling of B. minax pupae played subtle effects on pupal survival and emergence in the laboratory, for example pupal mortality was significantly greater at 6 °C chilling than 10 °C chilling (though intermediate values were recorded at 8 °C and 12 °C). How this might affect pupae in the field (where temperatures fluctuate) is unclear, but there is evidence that exposure to lower temperatures increase stress in B. minax. In field studies, Tang et al. (2012) considered temperature and soil moisture the most important factors influencing the survival of diapausing B. minax individuals, with the relatively limited distribution of the species in China reflecting the impacts of winter low temperatures and high soil moisture. Pupal survival in our field cage trial reached a maximum of ~65 % when pupae were undisturbed for most of the winter, similar to but slightly higher than the 58 % B. minax pupal survival rate reported by Lv (2006) following 9 years of field studies. As wild B. minax pupae are subject to mortality agents other than cold (predators, pathogens, etc.), the similarity of Lv’s data with our own gives us confidence that the field cage exposure closely mimicked natural conditions.
Diapause, slowed development, or both?
One interpretation which could be made from some of the gathered data is that pupal development in B. minax is not a true diapause, but simply slowed development owing to cold winter temperatures. Pupal developmental duration and cumulative adult emergence patterns in the laboratory suggest that at constant 6, 8, 10, and 12 °C, pupal developmental duration decreased and survival increased as the temperature increased, suggesting that pupal development is occurring (albeit slowly) through simple day-degree accumulation. However, field collected pupae stored under 25 °C constant, without any chilling, almost entirely failed to emerge. It hints that pupal development is not simply temperature driven (we have two additional field seasons of data supporting this observation, YC Dong and CY Niu, unpublished data). In contrast to results at 25 °C, a slightly colder temperature (22 °C) enabled 50 % of pupae to emerge. Thus there is likely a true pupal diapause in B. minax because it failed to develop under “optimal” temperature of 25 °C.
Implications for management of B. minax
In our experiments approximately 3.7 % of B. minax pupae, without cold experience, were non-diapausing and directly initiated morphogenesis and emerged successfully under a warmer condition. A similar observation, involving a similar percentage of individuals, has been recorded in Rhagoletis (Vankirk and Aliniazee 1982). It is considered that variability in diapause entry within some univoltine flies is an evolutionary life-strategy which can promote survival under unpredictable environmental conditions (Ragland et al. 2009). Separating non-diapausing individuals from the larger population, and adopting them to laboratory conditions, has promise for the long-term development of a non-diapausing strain, which would be of great value for both basic research and SIT operations (Krafsur 1998; Kuriwada et al. 2011; Lauzon and Potter 2012). Such selection has been achieved in R. pomonella (Baerwald and Boush 1967).
Despite chilling being a commonly accepted method for hastening diapause termination, the injection or topical application of pharmacological agents, such as hormones or their analogues, tricarboxylic acid intermediates and upstream metabolites and hexane, also shows strong promise for the diapause termination in insects (Xu et al. 2012; Zhang et al. 2011; Fujiwara and Denlinger 2007; Kidokoro et al. 2006; Baird 1972; Zdarek and Denlinger 1975). In contrast to chilling treatments, pharmacological manipulation may be a much quicker way to break pupal diapause. The combined application of traditional chilling approaches (presented in this paper), and novel diapause termination procedures using chemical treatments (currently underway), may offer the possibility to rear B. minax as needed for routine laboratory experiments (without the constraints posed by the fly’s univoltine biology). Such progress is an essential step for developing the SIT against B. minax.
References
Baerwald RJ, Boush MG (1967) Selection of a nondiapausing race of apple maggot. J Econ Entomol 60:682–684
Baird CR (1972) Termination of pupal diapause in Cuterebra tenebrosa (Diptera: Cuterebridae) with injections of ecdysterone. J Med Entomol 9:77–80
Bosch J, Kemp WP (2003) Effect of wintering duration and temperature on survival and emergence time in males of the orchard pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environ Entomol 32:711–716
Canale A, Benelli G (2012) Impact of mass-rearing on the host seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szepligeti) (Hymenoptera: Braconidae). J Pest Sci 85:65–74
Collier RH, Elliott MS, Finch S (1994) Development of the overwintering stages of the carrot fly, Psila rosae (Diptera: Psilidae). Bull Entomol Res 84:469–476
Daane KM, Johnson MW (2010) Olive fruit fly: managing an ancient pest in modern times. Annu Rev Entomol 55:151–169
Dambroski HR, Feder JL (2007) Host plant and latitude-related diapause variation in Rhagoletis pomonella: a test for multifaceted life history adaptation on different stages of diapause development. J Evol Biol 20:2101–2112. doi:10.1111/j.1420-9101.2007.01435.x
Danks H (2007) The elements of seasonal adaptations in insects. Can Entomol 139:1–44
Denlinger DL (2002) Regulation of diapause. Ann Rev Entomol 47:93–122
Dorji C, Clarke AR, Drew RAI, Fletcher BS, Loday P, Mahat K, Raghu S, Romig MC (2006) Seasonal phenology of Bactrocera minax (Diptera: Tephritidae) in western Bhutan. Bull Entomol Res 96:531–538
Fan JA, Zhao XQ, Zhu J (1994) A study on the cold-resistance and diapause in Tetradacus citri Chen. J Southwest Agric Univ 16:532–534
Fujiwara Y, Denlinger DL (2007) High temperature and hexane break pupal diapause in the flesh fly, Sarcophaga crassipalpis, by activating ERK/MAPK. J Insect Physiol 53:1276–1282
Goncalves MF, Torres LM (2011) The use of the cumulative degree-days to predict olive fly, Bactrocera oleae (Rossi), activity in traditional olive groves from the northeast of Portugal. J Pest Sci 84:187–197
Han P, Wang X, Niu C, Dong Y, Zhu J, Desneux N (2011) Population dynamics, phenology, and overwintering of Bactrocera dorsalis (Diptera: Tephritidae) in Hubei Province, China. J Pest Sci 84:289–295
Hodek I (1996) Diapause development, diapause termination and the end of diapause. Eur J Entomol 93:475–488
Hodek I (2002) Controversial aspects of diapause development. Eur J Entomol 99:163–174
Kidokoro K, Wata K, Fujiwara Y, Takeda M (2006) Effects of juvenile hormone analogs and 20-hydroxyecdysone on diapause termination in eggs of Locusta migratoria and Oxya yezoensis. J Insect Physiol 52:473–479
Kostal V (2006) Eco-physiological phases of insect diapause. J Insect Physiol 52:113–127
Kostal V, Havelka J (2001) Low temperature storage of larvae and synchronization of adult emergence in the predatory midge Aphidoletes aphidimyza. Cryobiology 42:112–120. doi:10.1006/cryo.2001.2311
Kostal V, Shimada K, Hayakawa Y (2000) Induction and development of winter larval diapause in a drosophilid fly, Chymomyza costata. J Insect Physiol 46:417–428
Krafsur ES (1998) Sterile insect technique for suppressing and eradicating insect populations: 55 years and counting. J Agr Entomol 15:303–317
Kuriwada T, Kumano N, Shiromoto K, Haraguchi D (2011) Pre-exposure to sex pheromone did not affect mating behavior in the sweetpotato weevil Cylas formicarius. J Pest Sci 84:93–97
Lauzon CR, Potter SE (2012) Description of the irradiated and nonirradiated midgut of Ceratitis capitata Wiedemann (Diptera: Tephritidae) and Anastrepha ludens Loew (Diptera: Tephritidae) used for sterile insect technique. J Pest Sci 85:217–226
Lv ZZ (2006) Eclosion, mating and oviposition behavior of the Chinese citrus fly, Bactrocera minax (Enderlein), in Yichang region. Plant Quar 20:215–216
Moraiti CA, Nakas CT, Papadopoulos NT (2012) Prolonged pupal dormancy is associated with significant fitness cost for adults of Rhagoletis cerasi (Diptera: Tephritidae). J Insect Physiol 58:1128–1135
Moribe Y, Niimi T, Yamashita O, Yaginuma T (2001) Samui, a novel cold-inducible gene, encoding a protein with a BAG domain similar to silencer of death domains (SODD/BAG-4), isolated from Bombyx diapause eggs. Eur J of Biochem 268:3432–3442
Nomura M, Ishikawa Y (2000) Biphasic effect of low temperature on completion of winter diapause in the onion maggot, Delia antiqua. J Insect Physiol 46:373–377
Ragland GJ, Fuller J, Feder JL, Hahn DA (2009) Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J Insect Physiol 55:344–350
Shan Y (2008) Present situation, development trend and countermeasures of citrus industry in China. J Chin Inst Food Sci Technol 8:1–8
Smith SL, Jones VP (1991) Alteration of apple maggot (Diptera: Tephritidae) emergence by cold period duration and rain. Environ Entomol 20:44–47
Stross R (1966) Light and temperature requirements for diapause development and release in Daphnia. Ecology 47:368–374
Tang S, Gong QT, Dou W, Wang JJ, Zhao ZM (2012) Effects of temperature, soil humidity and depth of buried pupae on adult emergence of Bactrocera minax. Acta Phytophy Sinica 39:137–141
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, Oxford, p 411
Teixeira LAF, Polavarapu S (2001) Postdiapause development and prediction of emergence of female blueberry maggot (Diptera: Tephritidae). Environ Entomol 30:925–931
Teixeira LAF, Polavarapu S (2002) Phenological differences between populations of Rhagoletis mendax (Diptera: Tephritidae). Environ Entomol 31:1103–1109
Teixeira LAF, Polavarapu S (2005a) Diapause development in the blueberry maggot Rhagoletis mendax (Diptera: Tephritidae). Environ Entomol 34:47–53
Teixeira LAF, Polavarapu S (2005b) Heat stress inhibits the completion of pupal diapause in Rhagoletis mendax (Diptera: Tephritidae). Ann Entomol Soc Am 98:197–204
Teixeira LAF, Polavarapu S (2005c) Expression of heat shock protein 70 after heat stress during pupal diapause in Rhagoletis mendax (Diptera: Tephritidae). Ann Entomol Soc Am 98:966–972
Teixeira LAF, Polavarapu S (2005d) Evidence of a heat-induced quiescence during pupal development in Rhagoletis mendax (Diptera: Tephritidae). Environ Entomol 34:292–297
Terao M, Hirose Y, Shintani Y (2012) Effects of temperature and photoperiod on termination of pseudopupal diapause in the bean blister beetle Epicauta gorhami. J Insect Physiol. doi:10.1016/j.jinsphys.2012.02.009
Turnock W, Lamb R, Bodnaryk R (1983) Effects of cold stress during pupal diapause on the survival and development of Mamestra configurata (Lepidoptera: Noctuidae). Oecologia 56:185–192
Vankirk JR, Aliniazee MT (1982) Diapause developmnet in the western cherry fruit fly, Rhagoletis indifferens Curran (Diptera, Tephridae). J Appl Entomol 93:440–445
Wang X, Luo L (1995) Research progress in the Chinese citrus fruit fly. Entomol Knowl 32:310–315
White IM, Wang X (1992) Taxonomic notes on some dacine (Diptera: Tephritidae) fruit flies associated with citrus, olives and cucurbits. Bull Entomol Res 82:275–279
Xu WH, Lu YX, Denlinger DL (2012) Cross-talk between the fat body and brain regulates insect developmental arrest. Proc Natl Acad Sci USA. doi:10.1073/pnas.1212879109
Yasuda T, Narahara M, Tanaka S, Wakamura S (1994) Thermal responses in the citrus fruit fly, Dacus tsuneonis: evidence for a pupal diapause. Entomol Exp Appl 71:257–261
Zdarek J, Denlinger DL (1975) Action of ecdysoids, juvenoids, and non-hormonal agents on termination of pupal diapause in the flesh fly. J Insect Physiol 21:1193–1202
Zhang Q, Nachman RJ, Kaczmarek K, Zabrocki J, Denlinger DL (2011) Disruption of insect diapause using agonists and an antagonist of diapause hormone. Proc Natl Acad Sci USA 108(41):16922–16926
Zhou XW, Niu CY, Han P, Desneux N (2012) Field evaluation of attractive lures for the fruit fly Bactrocera minax (Diptera: Tephritidae) and their potential use in spot sprays in Hubei Province (China). J Econ Entomol 105(4):1277–1284
Acknowledgments
We thank David Denlinger and Vladimír Koštál for valuable suggestions on the manuscript. We also acknowledge three anonymous reviewers for their constructive comments, which help us to improve the quality of this manuscript. This study was financially supported by the Fundamental Research Funds for the Central Universities (2011PY055), National Science Foundation of China (No. 31071690) and the International Atomic Energy Agency (via Research Contract No. 16015 to C.N. and Expert Mission Contract CPR/5/020-01-01 to A.R.C.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Dong, YC., Wang, ZJ., Clarke, A.R. et al. Pupal diapause development and termination is driven by low temperature chilling in Bactrocera minax . J Pest Sci 86, 429–436 (2013). https://doi.org/10.1007/s10340-013-0493-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-013-0493-y