Abstract
In recent decade, polycystic ovarian syndrome (PCOS) has become one of the main fertility disorders in females. Other than genetic factors, the etiology of this disease includes environmental factors, especially endocrine disrupting compounds (EDC). Bisphenol A (BPA) is a prominent EDC enormously used in manufacturing of various substances. Increased exposure to these substances on a daily basis throughout life, from prenatal to adult stages, has resulted in deleterious changes in female reproductive system. These changes include PCOS-like phenotypes such as hyperandrogenism, cystic ovaries and anovulation. Although studies in human are limited, several reports are available in animal models wherein BPA has been shown to directly affect ovarian development, folliculogenesis and steroidogenesis, thereby causing PCOS-like symptoms. Hypothalamus and pituitary are considered to be the most significant endocrine tissues involved in maintaining the structure and functions of ovary. BPA being an endocrine disruptor severely affects these tissues by modulating the synthesis and release of gonadotropin releasing hormone and gonadotropins from hypothalamus and pituitary, respectively. However, in light of reports available, effect of BPA on hypothalamus and pituitary do not corroborate with those on ovary. The current review suggests that BPA-induced PCOS-like phenotypes might be due to its direct action on ovary while alteration in hypothalamo-pituitary-ovarian axis seems to play a minor role. The authors through this review also intend to direct the attention of readers and policy makers towards the fact that despite the well-known negative effects of BPA exposure, manufacturing and use of BPA-containing substances is continuing, especially in developing countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovarian syndrome (PCOS), a disorder resulting from the alteration of reproductive, endocrine and metabolic functions, is worldwide considered as the most common reproductive disorder in women of fertile age (Fenichel et al. 2017; Liu et al. 2021). In India, the occurrence of this disorder ranges from 3.7 to 22.5% and it has been observed that urban women have 0.1 times higher odds of developing the disorder than their rural counterparts (Ganie et al. 2019; Joshi et al. 2014; Gill et al. 2012; Bharathi et al. 2017). The prevalence of PCOS has been shown to be associated with obesity, type 2 diabetes, glucose tolerance, abdominal adiposity, cardiovascular diseases and lifestyle (Escobar-Morreale 2018). Since the clinical manifestations of this syndrome are diverse, there was a need to set a basis for its diagnosis. While in 1990, the National Institute of Health considered hyperandrogenism and ovulatory dysfunctions (including altered menstrual cycles) as the diagnostic parameters for PCOS, the Rotterdam criteria of 2003 added a third feature i.e., presence of polycystic ovarian morphology (Zawadski and Dunaif 1992; Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004). Currently, the clinical diagnosis is based on whether an individual meets at least two of the three above mentioned phenotypes. In recent years, in addition to genetic and metabolic determinants, the endocrine disrupting chemicals (EDCs) have emerged as major environmental factor in inducing PCOS. Various synthetic chemicals have been categorized under EDCs as these molecules have the capacity of mimicking the action of natural hormones and in turn causing endocrinopathy (Crisp et al. 1998). These range from commercial plasticisers (bisphenol A and pthalates), paints (tributyltin), drug ingredients (diethylstilbesterol) to polyhalogenated aromatic hydrocarbons used in pesticides and herbicides (biphenyls and dioxins) (Palioura and Diamanti-Kandarakis 2013; Rutkowska and Diamanti-Kandarakis 2016).
Among EDCs, bisphenol A or 2,2-Bis(4-hydroxyphenyl)propane (BPA) is an omnipresent molecule that mimics estrogenic action. It has been shown to adversely affect a wide range of female reproductive functions such as development of ovary and other reproductive tissues, menstrual/estrous cycle, folliculogenesis, ovarian steroidogenesis, ovulation, fertilization, implantation, and survival as well as development of zygote (Palioura and Diamanti-Kandarakis 2015; Pivonello et al. 2020). The link between BPA and PCOS has been largely drawn based on population studies wherein women with PCOS showed high serum and urinary BPA concentrations (Takeuchi et al. 2004; Hossein et al. 2017; Akin et al. 2015; Tarantino et al. 2013; Vahedi et al. 2016). To elucidate the specific role of BPA in causing PCOS, several in vivo, ex vivo and in vitro experiments have been conducted in animal models. These experiments have explicitly shown that BPA exposure causes structural and functional changes in the ovary similar to those observed in PCOS. Also, BPA alters secretion of gonadotropin releasing hormone (GnRH) and gonadotropins from hypothalamus and pituitary, respectively. However, BPA-induced changes at the level of ovary in most of the studies do not correspond with changes in hypothalamus and pituitary. The present review is aimed to answer whether BPA-induced PCOS-like changes in ovary is due to direct action or by altering hypothalamic-hypophyseal functions or both.
Bisphenol A: Routes of Exposure, Accumulation and Action
The endocrine disruptor BPA is a major constituent in food packaging materials, bottles, flame retardants, water supply tanks and pipes. BPA is capable of leaching into consumables such as food and water on exposure to heat (Vandenberg et al. 2007). Therefore, humans are exposed to it largely through food and water that accounts for almost 90% of the overall route of exposure (Geens et al. 2012). Intake of BPA can also occur through air or mere surface contact via exposure to BPA-containing non-dietary products such as aerosol, medical equipment, thermal paper, etc. (Abraham and Chakraborty 2020; Vahedi et al. 2016).
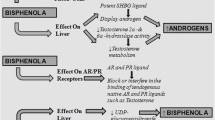
The metabolism and bioaccumulation of BPA has been schematically represented in Fig. 1. Liver is the main site for metabolism of BPA wherein enzymes uridine diphosphate glucuronosyltransferase and phenol sulfotransferase are reported to cause glucuronidation and sulfonation of the BPA molecule, respectively (Yokota et al. 1999; Pritchett et al. 2002). This conjugation process makes BPA hydrophilic and inactive, thus allowing its excretion via urine. The half-life of the conjugated BPA is ~ 5.3 h (Völkel et al. 2002) which is sufficient enough to cause its deconjugation in tissues such as lung, liver, kidney and placenta by a critical enzyme beta-glucuronidase, thereby making the molecule active again. The active form of BPA gets released into the circulation leading to its bioaccumulation in certain tissues (Ginsberg and Rice 2009). For instance, it has been reported that fat accumulates approximately triple the amount of BPA than other tissues due to BPA's lipophilic nature (Csanády et al. 2002). Hence, tissues such as ovary that are surrounded by large amount of fat become more susceptible to being exposed to BPA (Fernandez et al. 2007) and is probably one of the main reason for pronounced deleterious effect of BPA on female reproduction than male reproduction.
Metabolism and bioaccumulation of bisphenol A (BPA) in human (schematic representation in a female body). BPA is converted into inactive form following conjugation in liver. This is catalysed by enzymes uridine diphosphate glucuronosyltransferase and phenol sulfotransferase. The conjugated BPA is either excreted out via urine or deconjugated by an enzyme beta-glucuronidase in various tissues, importantly lung and kidney. The deconjugated BPA which is active gets accumulated in fat
Dodds and Lawson (1936) for the first time described the estrogenic property of BPA while investigating its role in maintenance of the vaginal estrus phase in ovariectomised rats. Competitive binding assays have shown that BPA binds to human estrogen receptors (ER) with lesser affinity as compared to 17β-estradiol (Chapin et al. 2008). This decrease in affinity is due to structural differences causing steric hindrance in attachment of BPA to the ligand binding domain of the ERs. BPA acts as estrogen agonist via ER alpha (ERα) (Ascenzi et al. 2006) and is capable to translate its effect through genomic as well as non-genomic pathways (Nadal et al. 2000). During non-genomic actions, BPA generally involves ERK/MAPK (extracellular regulated kinase/mitogen-activated protein kinase), PI3K-AKT (phosphatidylinositol 3-kinases - serine/threonine protein kinase) and cytoplasmic Ca2+-dependent signalling pathways (Bolli et al. 2008; Marino et al. 2012). In addition to ERα, agonistic action of BPA is mediated through a non-classical estrogen receptor G protein-coupled receptor 30 following intracellular Ca2+ signalling mechanism (Alonso-Magdalena et al. 2005). Interestingly, BPA also acts as an antagonist to sex steroids when it binds to ER beta (ERβ) (Ascenzi et al. 2006) and androgen receptor (AR) (Xu et al. 2005; Wang et al. 2017). Other receptors employed by bisphenols are aryl hydrocarbon receptor (AHR), pregnane X receptor and peroxisome proliferator-activated receptor (PPARγ) that are reported to inhibit follicle growth, induce hypercholesterolemia and cause proliferation of pre-adipocytes, respectively (Riu et al. 2011; Sui et al. 2012; Ziv-Gal et al. 2013; Boucher et al. 2014).
Based on a three generation study in rats, World Health Organization, Food and Drug Administration (2009) labelled the BPA dose of 5 mg/kg bw/day as ‘No-observed-adverse-effect-level’ (NOAEL), the highest concentration that has no adverse morphological effect. Thereafter, up to the dose of 50 mg BPA /kg bw/day has been considered as ‘lowest-observed-adverse-effect-level’ (LOAEL) by the United States Environmental Protection Agency (US EPA 2010). It is noteworthy to mention that several studies in animal models are now available that have shown the detrimental effects of BPA even at doses many times lower than NOAEL. The tolerable daily intake value for BPA has been deduced to be 0.05 mg/kg bw/day which is greater than the highest daily intake of BPA as seen in adolescents (European Food Safety Authority, EFSA 2015). However, such permissible levels do not take into account the cumulative effect that BPA has on the health of an individual exposed to it during its lifetime. Eventually, due to toxic nature of BPA, restriction has been imposed by developed nations on its use in various products with special emphasis on baby items (Ministry of Environment and Energy, MOEE 2012). In spite of toxic effect of BPA, it is still being used in India (Shrinithivihahshini et al. 2014) though policy has been formulated to prevent BPA usage (Mahamuni and Shrinithivihahshini 2017). In lieu of BPA, some alternative analogues such as BPS [bis-(4-hydroxyphenyl)sulfone] and BPF [4,4’-dihydroxydiphenyl methane] have been introduced worldwide. However, these molecules are also reported to have antiandrogenic, estrogenic and thyroidogenic actions (Rochester and Bolden 2015) and hence, their use is debatable.
Effect of BPA on Hypothalamo-Pituitary-Ovarian Axis
GnRH and Gonadotropins
The hypothalamo-pituitary axis plays pivotal role in regulating the female reproductive system. Gonadotropin releasing hormone (GnRH) released from hypothalamus in pulsatile manner is under the control of various endogenous factors, importantly kisspeptin (Oakley et al. 2009) which is secreted from neurons of anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) of brain (Fig. 2). Thereafter, GnRH stimulates the production and release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from gonadotrophs of anterior pituitary. Low frequency pulses of GnRH stimulate the synthesis and release of FSH while high frequency leads to production and release of LH (Ferris and Shupnik 2006). The gonadotropins in turn regulate the ovarian functions including folliculogenesis, steroidogenesis and ovulation. Estrogen produced from ovary regulates GnRH and gonadotropin secretion via feedback mechanisms, either directly at the level of hypothalamus and pituitary, or indirectly by regulating KISS production (Fig. 2).
Bisphenol A (BPA) effect on hypothalamo-pituitary-ovarian axis. BPA induces an increase in number of kisspeptin (KISS)-secreting neurons located in anteroventral periventricular nucleus (AVPV) and secretion of KISS peptide. These neurons exhibit an upregulation in expression of estrogen receptor alpha (ERα) after BPA exposure. BPA enhances KISS-induced pulse frequency of gonadotropin releasing hormone (GnRH) secretion from hypothalamic neurons and thereby production of luteinising hormone (LH) from anterior pituitary. At the level of ovary, BPA inhibits folliculogenesis, causes cyst formation and promotes anovulation. On steroidogenesis, it had differential effects on estrogen, stimulatory on testosterone and inhibitory on progesterone. The effect of BPA on estrogen feedback pathways is largely unexplored. [arcuate nucleus, ARC; follicle stimulating hormone, FSH]
The effect of BPA on secretion of KISS, GnRH and gonadotropins is summarized in Fig. 2. It has been shown that BPA increases the number of KISS1-secreting neurons of AVPV region (Naulé et al. 2014), and upregulates the expression of KISS1 mRNA and ERα protein in these neurons (Monje et al. 2010; Xi et al. 2011; Wang et al. 2014b, a). Since BPA binds with ERα, it is possible that by increasing the expression of this receptor it is enhancing its own effect on KISS-secreting neurons. In addition to effect on KISS, exposure to BPA at perinatal, postnatal, pubertal or adult stages in different animal models is reported to cause an increase in GnRH pulse frequency (Fernández et al. 2009; 2010; Gámez et al. 2015), upregulation in expression of GnRH mRNA (Xi et al. 2011; Wang et al. 2014b, a) and enhancement in post-transcriptional processing of GnRH mRNA (Monje et al. 2010). Parallel to GnRH, many of these studies report an increase in level of LH (Monje et al. 2010; Lee et al. 2013; Wang et al. 2014b, a; Zhou et al. 2014; Gámez et al. 2015). In case of PCOS patients, a positive association is observed between serum level of BPA and LH (Vahedi et al. 2016; Rutkowska et al. 2020). On the contrary, a few studies report the decrease in serum level of LH after BPA exposure (Savabieasfahani et al. 2006; Fernández et al. 2009; Zaid et al. 2018). Since GnRH regulates the release of LH by upregulating inositol trisphosphate pathway (IP3) and BPA causes decrease in IP3 production, Fernández et al. (2009) have speculated that decrease in LH production after BPA exposure could be due to inhibition of release mechanism and not the synthesis. Besides, BPA is shown to delay and reduce the amplitude the preovulatory LH surge in adult rat (López-Rodríguez et al. 2019). In the same study, GnRH regulator phoenixin and clock genes such as period circadian regulator 1 (Per1) and brain and muscle ARNT-like 1 (Bmal1) are also shown to be downregulated by BPA, thereby leading to disruption of LH surge (Loganathan et al. 2019; López-Rodríguez et al. 2019).
With regard to the effect of BPA on production and release of FSH, the reports are contradictory. BPA has been reported to have stimulatory (Xi et al. 2011; Wang et al. 2014b, a; Zhou et al. 2014), inhibitory (Zaid et al. 2018) and no effect (Fernández et al. 2009; Lee et al. 2013; Gámez et al. 2015) on FSH mRNA and serum level. The inhibitory or no effect of BPA could be seen in light of the fact that GnRH pulse frequency increases under the effect of BPA, and FSH synthesis and release depends on slow pulsatile release of GnRH. However, the reason behind the stimulatory effect of BPA on FSH level is not clear and needs further investigation. Nevertheless, it is evident from these studies that BPA has the potential to disrupt the KISS1-GnRH-gonadotropin production.
Ovarian Functions
The effect of BPA on ovarian functions, folliculogenesis and steroidogenesis, either directly or via modulating hypothalamo-hypophyseal axis is depicted in Figs. 2 and 3.
Bisphenol A (BPA)-induced changes in ovarian folliculogenesis. Exposure to BPA disrupts the natural process of folliculogenesis. BPA inhibits germ cell nest breakdown by upregulating anti-apoptotic factors and downregulating apoptotic factors leading to formation of multiovular follicles. The premature transition of primordial follicles to primary follicles is enhanced by BPA via PI3K-AKT signalling pathway that decreases the expression of transcription factor Pten involved in maintaining the pool of primordial follicles. Further, BPA induces the formation of large abnormal antral follicles by increasing its antral cavity and inhibiting granulosa cell (GC) proliferation and meiotic maturation. The abnormal antral follicles get transformed into cysts. Besides BPA increases number of atretic follicles by upregulating expression of p27, and it alters LH surge thereby causing anovulation. [FAS cell surface death receptor, Fas; caspase 8, Casp8; B-cell lymphoma 2, Bcl2; BCL2-like protein 4, Bax; BCL2 antagonist/killer 1, Bak1; B-cell lymphoma extra-large, Bclxl; phosphatidylinositol-3-kinase and serine/threonine protein kinase pathway, PI3K-AKT; phosphatase and tensin homologue, Pten; cyclin D2, Ccnd2; transformation-related protein 53, Trp53; cyclin dependent kinase inhibitor, p27; luteinising hormone, LH]
Folliculogenesis
Primordial germ cells originating from epiblast migrate to genital ridge during embryonic stage and give rise to oogonia. These germ cells enter into meiosis which gets arrested at diplotene stage of prophase I to form primary oocytes. They are required to break off from the germ cell nest and get surrounded by a single layer of follicular cells to form primordial follicles (Pepling 2006; Tingen et al. 2009). A few primordial follicles from its pool are selected for growth and transformation into antral follicles. It is noteworthy to mention that transformation from primordial to secondary/preantral follicle is independent of gonadotropins. Under the influence of gonadotropins, preantral follicles are transformed into antral/Graffian follicles and eventually into preovulatory follicles. Prior to ovulation, meiosis which was arrested at diplotene stage of prophase I is resumed and gets arrested again at metaphase II. These secondary oocytes/ova are released out from preovulatory follicles at the time of ovulation.
Numerous studies have shown the association between BPA, oocyte formation, follicular development and ovulation (Pivonello et al. 2020). These associations have been schematically represented in Fig. 3. BPA is shown to adversely affect the transformation of oogonia into primary oocytes by inhibiting germ cell nest breakdown (Zhang et al. 2012; Zhao et al. 2014; Miao et al. 2015; Berger et al. 2016). Germ cell nest is maintained by estrogen and its breakdown occurs due to upregulation of anti-apoptotic factors and downregulation of pro-apoptotic factors (Sarraj and Drummond 2012). The balance between apoptotic and anti-apoptotic factors gets disrupted due to exposure of BPA. In vitro treatment of postnatal mice ovary with BPA has resulted in a significant increase in expression level of two prominent anti-apoptotic factors, B-cell lymphoma 2 (Bcl2) and B-cell lymphoma extra-large (Bclxl) (Zhou et al. 2015). The same study also reports decrease in expression of extrinsic apoptotic pathway factors, FAS cell surface death receptor (Fas) and Caspase 8 (Casp8). A similar observation has been made in another study wherein level of Bcl2 is shown to increase concomitantly with decrease in pro-apoptotic factors of intrinsic apoptotic pathway, BCL2-like protein 4 (Bax) and BCL2 Antagonist/Killer 1 (Bak1) (Wang et al. 2014b, a). Often incomplete germ cell nest breakdown leads to the appearance of multiovular follicles (MOFs) in adult ovary (Pepling 2006; Tingen et al. 2009) and number of such malformed follicles is shown to increase post BPA exposure in mice and lamb (Suzuki et al. 2002; Rivera et al. 2011). In BPA-exposed postnatal lamb ovary, an increase in ERs with an increase in MOFs tempted them to speculate that extended action of estrogen via its receptor would have caused inhibition in germ cell nest breakdown leading to formation of the malformed follicles. In addition to impairment of oogenesis and induction of abnormal follicles formation, BPA reduces the pool of primordial follicles by enhancing its premature transformation into primary follicles (Rodríguez et al. 2010; Rivera et al. 2011; Zhao et al. 2014). In vitro treatment of rat postnatal ovary with BPA is reported to upregulate PI3K-AKT pathway that is known for its involvement in follicular development (Liu et al. 2006; Cecconi et al. 2012). Another study corroborates the BPA-induced acceleration in number of primordial follicle entering into growth (Hu et al. 2018). In this study in mice, treatment with BPA inhibited phosphatase and tensin homologue (Pten), the transcription factor known to maintain the pool of primordial follicles (Reddy et al. 2008).
In addition to affecting the pool of primordial follicles and follicular selection, BPA adversely affects the development of antral follicles (Fig. 3). After exposure to BPA, antral follicles become abnormally large-sized due to enlarged antrum (Adewale et al. 2009; Zaid et al. 2018) thereby contributing in formation of cysts (Zaid et al. 2018), the characteristic feature of ovary in case of PCOS. BPA has been reported to reduce granulosa cell proliferation of preantral and antral follicles (Xu et al. 2002; Lenie et al. 2008; Peretz et al. 2012). Since ER antagonists could not block this effect on antral follicles even though BPA enhances ovarian ER expression, it has been suggested that BPA would have carried out its effect on development of antral follicles following nongenomic/non-estrogenic pathway (Peretz et al. 2012). Probably, BPA might have caused abnormal antral follicle development via AHR as expression of this receptor is reported to increase in gonads after in utero exposure to BPA (Nishizawa et al. 2005). Further, in Ahr knockout mice, BPA failed to induce abnormal follicular growth (Ziv-Gal et al. 2013), corroborating the involvement of AHR by BPA in translating its effect at the level of antral follicles. In farm animals in which androgen is shown to play important role in later stages of follicular development (Sen and Hammes 2010; Prizant et al. 2014), BPA-induced decrease in expression of ovarian ARs suggests that BPA affects antral follicles by inhibiting the action of endogenous androgen (Rivera et al. 2015; Santamaría et al. 2016). BPA-induced abnormal growth of follicles could be due to alteration in cell cycle regulators that leads to reduction in the proliferation of granulose cells. Peretz et al. (2012) identified two such factors, cyclin D2 (CCND2) and transformation-related protein 53 (TRP53), in antral follicles of mice. It has been reported that BPA upregulates TRP53 that in turn downregulates CCND2 and results in inhibition of granulosa cell proliferation in antral follicles (Peretz et al. 2012). For comprehensive understanding, influence of BPA on genetic regulation of folliculogenesis is summarized in Fig. 3.
BPA not only affects the granulosa cells, it also inhibits meiotic resumption and maturation of oocytes (Hunt et al. 2003; Can et al. 2005; Susiarjo et al. 2007; Lawson et al. 2011; Chao et al. 2012). BPA exposure is reported to cause aneuploidy due to improper centrosome and spindle microtubular organization (Hunt et al. 2003; Can et al. 2005; Chao et al. 2012), and inhibition of germinal vesicle breakdown (Lenie et al. 2008; Chao et al. 2012). BPA-induced acceleration in number of growing follicles eventually leads to their atresia (Rivera et al. 2011; Peretz et al. 2012; Lee et al. 2013; Gámez et al. 2015; Zaid et al. 2018). The increased expression of p27 in oocytes and granulosa cells of antral follicles has been highlighted as one of the main reasons for their atresia in lamb exposed postnatally to BPA (Rivera et al. 2011). It is noteworthy that p27 can activate caspases in oocyte and granulosa cells thereby causing cell death (Rajareddy et al. 2007). Taken together, it can be speculated that the above mentioned effects of BPA on ovary at the level of germ cell nest breakdown, formation of follicles, development and maturation of oocytes, and atresia of follicles ultimately lead to reduction in number of antral follicles or formation of abnormal antral follicles not capable of ovulation (Fig. 3). As a result, the histological observation of these ovaries often showed reduced number of corpora lutea (Takeuchi et al. 2004; Adewale et al. 2009; Zaid et al. 2018; López-Rodríguez et al. 2019) due to reduced ovulation or anovulation, another characteristic feature of PCOS patients. In conclusion, BPA-induced PCOS-like features in ovary could be due to lack of factors favouring folliculogenesis and excess production of factors inducing cell death.
Steroidogenesis
It is a matter of debate whether hyperandrogenism is the cause or effect of PCOS. Nonetheless, elevated serum testosterone and high BPA level in urine and serum have been observed in women with PCOS (Takeuchi and Tsutsumi 2002; Konieczna et al. 2018; Akin et al. 2015). A similar observation has been made in girls showing precocious puberty (Lee et al. 2014). In addition, BPA has been reported to increase serum level of free testosterone in PCOS patients (Kandaraki et al. 2011; Tarantino et al. 2013) which might be due to its property of displacing testosterone from sex hormone binding globulin (Déchaud et al. 1999). The increased level of testosterone is shown to decrease clearance of BPA from circulation; thereby hyperandrogenism is suggested to have an additive effect on BPA titre in PCOS patients (Takeuchi et al. 2006). The correlation between androgen and BPA has been examined using animal models. In postnatal rats, treatment with BPA has resulted in an increase in serum level of testosterone (Fernández et al. 2010). This was further validated by in vitro experiment where BPA had positive effect on steroid acute regulatory proteins (StAR), steroidogenic enzymes such as cholesterol side chain cleavage enzyme (Cyp11a) and 17α-hydroxylase, and testosterone production by thecal cells isolated from immature rat ovary (Zhou et al. 2008). On the contrary, in mice, high concentration of BPA is shown to downregulate the expression of StAR and Cyp11a and consequently, testosterone production by antral follicles (Peretz et al. 2011; Peretz and Flaws 2013).
Efforts have also been made to examine correlation between BPA and estrogen, and the results are contradictory. A positive correlation has been suggested in BPA-exposed women factory workers in which high level of serum estrogen has been recorded as compared to unexposed individuals (Miao et al. 2015). Similarly, high urinary BPA and serum estrogen levels have been reported in girls with precocious puberty (Lee et al. 2014). In contrast, the analysis of estrogen serum level along with urinary BPA level in women facing infertility issues and undergoing in vitro fertilization treatment showed a negative correlation (Mok-Lin et al. 2010; Bloom et al. 2011; Ehrlich et al. 2013). This gets support from in vitro studies where human granulosa cells treated with BPA exhibited downregulation of aromatase expression (CYP19A) and estradiol production (Kwintkiewicz et al. 2010; Wang et al. 2017). The relationship between BPA and estrogen levels remains controversial even in non-human animal models. In vivo studies in rat and mice have shown that exposure to BPA, whether in prenatal, postnatal or in adult stages, have upregulated the expression of Cyp19a and serum level of estrogen (Fernández et al. 2010; Xi et al. 2011; Naulé et al. 2014; Wang et al. 2014b, a; Gámez et al. 2015; Zaid et al. 2018). On the other hand, in vitro studies report decrease in Cyp19a expression and estradiol production under the effect of BPA (Zhou et al. 2008; Peretz et al. 2011). In another in vitro study in which human granulosa cell line (KGN) was used, BPA is shown to upregulate the expression of PPARγ that in turn inhibited the FSH-induced CYP19A expression (Kwintkiewicz and Giudice 2008). The difference in results of in vitro and in vivo studies might be because the latter involves interplay of endogenous factors and hypothalamo-hypophyseal-ovarian (HPO) axis with BPA.
With regard to effect of BPA on another female sex steroid progesterone, in vivo as well as in vitro studies in human and several animals revealed its inhibitory effect on production of progesterone (Zhou et al. 2008; Fernández et al. 2010; Grasselli et al. 2010; Peretz et al. 2011; Peretz and Flaws 2013; Mansur et al. 2016; Samardzija et al. 2018; Zaid et al. 2018; Qi et al. 2020). Surprisingly, expression of StAR and several steroidogenic enzymes such as Cyp11a, Cyp17a and 3β- hydroxysteroid dehydrogenase (3β-Hsd) are reported to increase in response to BPA (Zhou et al. 2008; Samardzija et al. 2018; Qi et al. 2020). The decrease in production of progesterone despite increased expression of steroidogenic enzymes could be seen in light of a study where exposure to BPA is demonstrated to enhance the expression of ATP binding cassette subfamily A member 1 (ABCA1) which is known to cause efflux of cholesterol, thereby decreasing the availability of substrate for steroid biosynthesis (Qi et al. 2020). This gets support from a study in PCOS patients in whom an increase in ABCA1 gene polymorphism is seen as compared to normal individuals (Karadeniz et al. 2011). In response to BPA exposure, sequestering of cholesterol in the perinuclear areas of steroidogenic cells also needs to be considered as a probable reason for decrease in progesterone production (Samardzija et al. 2018). In addition, we propose that BPA-induced decline in number of corpora lutea resulting from disrupted follicular growth and ovulation needs to be taken into account for decreased progesterone production since luteinised follicular cells are the main source of progesterone in an adult female. However, it is not yet clear how production of testosterone and estrogen increases when level of cholesterol decreases due to its efflux or sequestering.
Conclusion
The endocrine disruptor BPA enhances the synthesis of GnRH by directly regulating the GnRH-secreting neurons and also indirectly by altering the production of KISS from its neurons of AVPV region. An increased level of GnRH in turn stimulates LH secretion though contradictory results are reported with regard to its effect on FSH secretion. In addition, both in vivo and in vitro exposure to BPA causes structural and functional abnormalities of ovary that are often similar to PCOS-like phenotypes such as hyperandrogenism, formation of cysts and anovulation. It appears that BPA causes PCOS-like symptoms by directly affecting the ovary and also indirectly through HPO axis. However, many of the BPA-induced changes at the level of hypothalamus and pituitary have not been seen in accordance with the functional changes in ovary in response to BPA. The increased production of GnRH and gonadotropins after exposure to BPA instead of stimulating folliculogenesis, production of female sex steroids and ovulation in ovary, caused reduction in number of primordial follicles, formation of abnormal and atretic follicles, decrease in testosterone to estrogen ratio, inhibition in production of progesterone, and restriction on ovulation.
It is to be noted that majority of studies dealing with the effect of BPA on reproductive axis are focused on non-primates. In human, reports are limited to correlating plasma and urinary BPA levels with PCOS symptoms. BPA seems to have species-specific effects and hence, conclusions derived from non-human studies should not be extrapolated to humans. Well-designed in vivo and in vitro studies in human are required for investigating the involvement of HPG axis in BPA-induced PCOS-related changes in ovary. Also, the dose of BPA and route of administration has significant impact on its effect and therefore should be carefully considered while designing these experiments. In addition, studies need to be directed towards understanding molecular mechanisms of BPA action leading to PCOS-like symptoms. Nonetheless, enough evidence is available to mark this EDC as a highly dangerous chemical affecting female fertility. Despite this, developing and under-developed countries are still far away from putting a cap on the use of BPA-containing substances. In the interest of human and animal welfare, research needs to be focussed on developing mechanisms to antagonizing the effect of BPA and enhancing its clearance from the body.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Abraham, Anna, and Paromita Chakraborty. 2020. A review on sources and health impacts of bisphenol A. Reviews on Environmental Health 35: 201–210. https://doi.org/10.1515/reveh-2019-0034.
Adewale, Heather B., Wendy N. Jefferson, Retha R. Newbold, and Heather B. Patisaul. 2009. Neonatal bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biology of Reproduction 81: 690–699. https://doi.org/10.1095/biolreprod.109.078261.
Akın, L., M. Kendirci, F. Narin, S. Kurtoglu, R. Saraymen, M. Kondolot, S. Koçak, and F. Elmali. 2015. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatrica 104: e171–e177. https://doi.org/10.1111/apa.12885.
Alonso-Magdalena, Paloma, Ouahiba Laribi, Ana B. Ropero, Esther Fuentes, Cristina Ripoll, Bernat Soria, and Angel Nadal. 2005. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environmental Health Perspectives 113: 969–977. https://doi.org/10.1289/ehp.8002.
Ascenzi, Paolo, Alessio Bocedi, and Maria Marino. 2006. Structure-function relationship of estrogen receptor alpha and beta: Impact on human health. Molecular Aspects of Medicine 27: 299–402. https://doi.org/10.1016/j.mam.2006.07.001.
Berger, Amelia, Ziv-gal Ayelet, Cudiamat Jonathan, Wei Wang, Zhou Changqing, and Jodi A. Flaws. 2016. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reproductive Toxicology 60: 39–52. https://doi.org/10.1016/j.reprotox.2015.12.004.The.
Bloom, Michael S., Dongsul Kim, Frederick S. Vom Saal, Julia A. Taylor, Gloria Cheng, Julie D. Lamb, and Victor Y. Fujimoto. 2011. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertility and Sterility 96: 672–677. https://doi.org/10.1016/j.fertnstert.2011.06.063.
Bolli, Alessandro, Paola Galluzzo, Paolo Ascenzi, Giovanna Del Pozzo, Immacolata Manco, Maria Teresa Vietri, Luigi Mita, Lucia Altucci, Damiano Gustavo Mita, and Maria Marino. 2008. Laccase treatment impairs bisphenol A-induced cancer cell proliferation affecting estrogen receptor alpha-dependent rapid signals. IUBMB Life 60: 843–852. https://doi.org/10.1002/iub.130.
Boucher, J.G., A. Boudreau, and E. Atlas. 2014. Bisphenol a induces differentiation of human preadipocytes in the absence of glucocorticoid and is inhibited by an estrogen-receptor antagonist. Nutrition and Diabetes 4: e102–e108. https://doi.org/10.1038/nutd.2013.43.
Can, Alpean, O. Semiz, and O. Cinar. 2005. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Molecular Human Reproduction 11: 389–396. https://doi.org/10.1093/molehr/gah179.
Cecconi, Sandra, Annunziata Mauro, Valerio Cellini, and Felice Patacchiola. 2012. The role of Akt signalling in the mammalian ovary. International Journal of Developmental Biology 56: 809–817. https://doi.org/10.1387/ijdb.120146sc.
Chao, HuHe., Xi Feng Zhang, Bo. Chen, Bo. Pan, Lian Jun Zhang, Lan Li, Xiao Feng Sun, Qing Hua Shi, and Wei Shen. 2012. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochemistry and Cell Biology 137: 249–259. https://doi.org/10.1007/s00418-011-0894-z.
Chapin, Robert E., Jane Adams, L. Kim Boekelheide, Earl Gray, Simon W. Hayward, Peter S.J.. Lees, Barry S. McIntyre, et al. 2008. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Research Part B - Developmental and Reproductive Toxicology 83: 157–395. https://doi.org/10.1002/bdrb.20147.
Crisp, Thomas M., Eric D. Clegg, Ralph L. Cooper, William P. Wood, David G. Andersen, Karl P. Baetcke, Jennifer L. Hoffmann, et al. 1998. Environmental endocrine disruption: An effects assessment and analysis. Environmental Health Perspectives 106: 11–56. https://doi.org/10.1289/ehp.98106s111.
Csanády, G., H. Oberste-Frielinghaus, B. Semder, C. Baur, K. Schneider, and J. Filser. 2002. Distribution and unspecific protein binding of the xenoestrogens bisphenol A and daidzein. Archives of Toxicology 76: 299–305. https://doi.org/10.1007/s00204-002-0339-5.
Déchaud, Henri, Claire Ravard, Francine Claustrat, Aude Brac De La. Perrière, and Michel Pugeat. 1999. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG). Steroids 64: 328–334. https://doi.org/10.1016/S0039-128X(98)00114-7.
Dodds, E.C., and Wilfrid Lawson. 1936. Synthetic strogenic agents without the phenanthrene nucleus. Nature 137: 996. https://doi.org/10.1038/137996a0.
EFSA. No consumer health risk from bisphenol A exposure. Press release on 21 January 2015; accessed from http://www.efsa.europa.eu/en/press/news/150121.htm.
Ehrlich, Shelley, Paige L. Williams, Russ Hauser, Stacey A. Missmer, Jackye Peretz, Antonia M. Calafat, and Jodi A. Flaws. 2013. Urinary bisphenol A concentrations and cytochrome P450 19 A1 (Cyp19) gene expression in ovarian granulosa cells: An in vivo human study. Reproductive Toxicology 42: 18–23. https://doi.org/10.1016/j.reprotox.2013.06.071.
Escobar-Morreale, Héctor. F. 2018. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nature Reviews Endocrinology 14: 270–284. https://doi.org/10.1038/nrendo.2018.24.
Fenichel, Patrick, Charlotte Rougier, Sylvie Hieronimus, and Nicolas Chevalier. 2017. Which origin for polycystic ovaries syndrome: Genetic, environmental or both? Annales D’endocrinologie (paris) 78: 176–185. https://doi.org/10.1016/j.ando.2017.04.024.
Fernandez, M.F., J.P. Arrebola, J. Taoufiki, A. Navalón, O. Ballesteros, R. Pulgar, J.L. Vilchez, and N. Olea. 2007. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reproductive Toxicology 24: 259–264. https://doi.org/10.1016/j.reprotox.2007.06.007.
FernándezMaria, MarinaBianchi, Lux-Santos. Victoria, and Libertun Carlos. 2009. Neonatal exposure to bisphenol A alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environmental Health Perspectives 117: 757–762. https://doi.org/10.1289/ehp.0800267.
FernándezNadia, MarinaBourguignon, Lux-Lantos. Victoria, and Libertun Carlos. 2010. Neonatal exposure to bisphenol A and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environmental Health Perspectives 118: 1217–1222. https://doi.org/10.1289/ehp.0901257.
Ferris Heather, A., and Margaret A. Shupnik. 2006. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GnRH1. Biology of Reproduction 74: 993–998. https://doi.org/10.1095/biolreprod.105.049049.
Gámez, J.M., R. Penalba, N. Cardoso, P. Scacchi Bernasconi, S. Carbone, O. Ponzo, M. Pandolfi, P. Scacchi, and R. Reynoso. 2015. Exposure to a low dose of bisphenol A impairs pituitary-ovarian axis in prepubertal rats. Effects on early folliculogenesis. Environmental Toxicology and Pharmacology 39: 9–15. https://doi.org/10.1016/j.etap.2014.10.015.
Ganie, M.A., V. Vasudevan, I.A. Wani, M.S. Baba, T. Arif, and A. Rashid. 2019. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. The Indian Journal of Medical Research 150: 333–344. https://doi.org/10.4103/ijmr.IJMR_1937_17.
Geens, Tinne, Dominique Aerts, Carl Berthot, Jean Pierre Bourguignon, Leo Goeyens, Philippe Lecomte, Guy Maghuin-Rogister, et al. 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food and Chemical Toxicology 50: 3725–3740. https://doi.org/10.1016/j.fct.2012.07.059.
Gill, H., P. Tiwari, and P. Dabadghao. 2012. Prevalence of polycystic ovary syndrome in young women from North India: A Community-based study. Indian Journal of Endocrinology and Metabolism 16 (Suppl 2): S389–S392. https://doi.org/10.4103/2230-8210.104104.
Ginsberg, Gary, and Deborah C. Rice. 2009. Does rapid metabolism ensure negligible risk from bisphenol A? Environmental Health Perspectives 117: 1639–1643. https://doi.org/10.1289/ehp.0901010.
Grasselli, F., L. Baratta, L. Baioni, S. Bussolati, R. Ramoni, S. Grolli, and G. Basini. 2010. Bisphenol A disrupts granulosa cell function. Domestic Animal Endocrinology 39: 34–39. https://doi.org/10.1016/j.domaniend.2010.01.004.
Hossein Rashidi, B., M. Amanlou, T. Behrouzi Lak, M. Ghazizadeh, F. Haghollahi, M. Bagheri, and B. Eslami. 2017. The association between Bisphenol A and polycystic ovarian syndrome: A case-control study. Acta Medica Iranica 55: 759–764.
Hu, Ying, Dong Zhi Yuan, Wu. Yi, Yu. Lin Lin, Xu. Liang Zhi, Li Min Yue, Lin Liu, et al. 2018. Bisphenol A initiates excessive premature activation of primordial follicles in mouse ovaries via the PTEN signaling pathway. Reproductive Sciences 25: 609–620. https://doi.org/10.1177/1933719117734700.
Hunt, Patricia A., Kara E. Koehler, and Martha Susiarjo. 2003. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Current Biology 13: 546–553. https://doi.org/10.1016/S0960-9822(03)00189-1.
Joshi, B., S. Mukherjee, A. Patil, A. Purandare, S. Chauhan, and R. Vaidya. 2014. A cross-sectional study of polycystic ovarian syndrome among adolescent and young girls in Mumbai, India. Indian Journal of Endocrinology and Metabolism 18: 317–324. https://doi.org/10.4103/2230-8210.131162.
Kandaraki, Eleni, Antonis Chatzigeorgiou, Sarantis Livadas, Eleni Palioura, Frangiscos Economou, Michael Koutsilieris, Sotiria Palimeri, Dimitrios Panidis, and Evanthia Diamanti-Kandarakis. 2011. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. Journal of Clinical Endocrinology and Metabolism 96: 480–484. https://doi.org/10.1210/jc.2010-1658.
Karadeniz, Muammer, Mehmet Erdoan, Zengi Ayhan, Murat Yalcn, Murat Olukman, Sevki Cetinkalp, Gulinnaz E. Alper, et al. 2011. Effect of G2706A and G1051A polymorphisms of the ABCA1 gene on the lipid, oxidative stress and homocystein levels in Turkish patients with polycystc ovary syndrome. Lipids in Health and Disease 10: 1–8. https://doi.org/10.1186/1476-511X-10-193.
Konieczna, Aleksandra, Dominik Rachoń, Katarzyna Owczarek, Paweł Kubica, Agnieszka Kowalewska, B.łażej Kudłak, Andrzej Wasik, and Jacek Namieśnik. 2018. Serum bisphenol A concentrations correlate with serum testosterone levels in women with polycystic ovary syndrome. Reproductive Toxicology 82: 32–37. https://doi.org/10.1016/j.reprotox.2018.09.006.
KwintkiewiczGiudice, JakubLinda C. 2008. Endocrine disruptor bisphenol A induces expression of peroxisome proliferator-activated receptor γ which contributes to down-regulation of FSH-stimulated aromatase expression and estradiol production in human granulosa KGN Cells. Biology of Reproduction 78: 199. https://doi.org/10.1093/biolreprod/78.s1.199.
Kwintkiewicz, Jakub, Yoshihiro Nishi, Toshihiko Yanase, and Linda C. Giudice. 2010. Peroxisome proliferator-activated receptor-γ mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environmental Health Perspectives 118: 400–406. https://doi.org/10.1289/ehp.0901161.
Lawson, Crystal, Mary Gieske, Brenda Murdoch, Ping Ye, Yunfei Li, Terry Hassold, and Patricia A. Hunt. 2011. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biology of Reproduction 84: 79–86. https://doi.org/10.1095/biolreprod.110.084814.
Lee, Seung Gee, Ji Young Kim, Jin Yong Chung, Yoon Jae Kim, Ji Eun Park, Oh. Seunghoon, Yong Dal Yoon, Ki Soo Yoo, Young Hyun Yoo, and Jong Min Kim. 2013. Bisphenol a exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environmental Health Perspectives 121: 663–669. https://doi.org/10.1289/ehp.1205823.
Lee, SuHyeon, Se Mi. Kang, Man Ho Choi, Jeongae Lee, Mi Jung Park, Shin Hye Kim, Won Yong Lee, Jongki Hong, and Bong Chul Chung. 2014. Changes in steroid metabolism among girls with precocious puberty may not be associated with urinary levels of bisphenol A. Reproductive Toxicology 44: 1–6. https://doi.org/10.1016/j.reprotox.2013.03.008.
Lenie, Sandy, Rita Cortvrindt, Ursula Eichenlaub-Ritter, and Johan Smitz. 2008. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Genetic Toxicology and Environmental Mutagenesis 651: 71–81. https://doi.org/10.1016/j.mrgentox.2007.10.017.
Liu, Jingjing, Wu. Qunhong, Yanhua Hao, Mingli Jiao, Xing Wang, Shengchao Jiang, and Liyuan Han. 2021. Measuring the global disease burden of polycystic ovary syndrome in 194 countries: Global burden of disease study 2017. Human Reproduction 36: 1108–1119. https://doi.org/10.1093/humrep/deaa371.
Liu, Kui, Singareddy Rajareddy, Lian Liu, Krishna Jagarlamudi, Karin Boman, Gunnar Selstam, and Pradeep Reddy. 2006. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: New roles for an old timer. Developmental Biology 299: 1–11. https://doi.org/10.1016/j.ydbio.2006.07.038.
Loganathan, Neruja, Ashkan Salehi, Jennifer A. Chalmers, and Denise D. Belsham. 2019. Bisphenol A Alters Bmal1, Per2, and Rev-Erba mRNA and Requires Bmal1 to increase neuropeptide Y expression in hypothalamic neurons. Endocrinology 160: 181–192. https://doi.org/10.1210/en.2018-00881.
López-Rodríguez, David, Delphine Franssen, Elena Sevrin, Arlette Gérard, Cédric. Balsat, Silvia Blacher, Agnès Noël, and Anne Simone Parent. 2019. Persistent vs transient alteration of folliculogenesis and estrous cycle after neonatal vs adult exposure to bisphenol A. Endocrinology 160: 2558–2572. https://doi.org/10.1210/en.2019-00505.
Mahamuni, Duraisamy, and Nirmaladevi Dhandayudapani Shrinithivihahshini. 2017. Need for regulatory policies in India, on the use of bisphenol A in food contact plastic containers. Current Science 113: 861–868. https://doi.org/10.18520/cs/v113/i05/861-868.
Mansur, Abdallah, Michal Adir, Gil Yerushalmi, Ariel Hourvitz, Hila Gitman, Yuval Yung, Raoul Orvieto, and Ronit Machtinger. 2016. Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Human Reproduction 31: 1562–1569. https://doi.org/10.1093/humrep/dew088.
Marino, Maria, Marco Pellegrini, Piergiorgio La Rosa, and Filippo Acconcia. 2012. Susceptibility of estrogen receptor rapid responses to xenoestrogens: Physiological outcomes. Steroids 77: 910–917. https://doi.org/10.1016/j.steroids.2012.02.019.
Miao, Maohua, Wei Yuan, Fen Yang, Hong Liang, Zhijun Zhou, Runsheng Li, Ersheng Gao, and De Kun Li. 2015. Associations between bisphenol a exposure and reproductive hormones among female workers. International Journal of Environmental Research and Public Health 12: 13240–13250. https://doi.org/10.3390/ijerph121013240.
MOEE, Ministry of Environment and energy, Government of Sweden, Government prohibits Bisphenol A in baby food bottles. Press Release by Ministry of Environment and energy, Government Offices of Sweden, 2012; accessed from http://www.government.se/contentassets/df02c1bf39d842a8a61809ec0748ecdd/press-releases-2010-2014.
Mok-Lin, E., S. Ehrlich, P.L. Williams, J. Petrozza, D.L. Wright, A.M. Calafat, X. Ye, and R. Hauser. 2010. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International Journal of Andrology 33: 385–393. https://doi.org/10.1111/j.1365-2605.2009.01014.x.
Monje, L., J. Varayoud, M. Muñoz-de-Toro, E.H. Luque, and J.G. Ramos. 2010. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor alpha expression in nuclei controlling estrous cyclicity. Reproductive Toxicology 30: 625–634. https://doi.org/10.1016/j.reprotox.2010.08.004.
Nadal, Angel, Ana B. Ropero, Ouahiba Laribi, Marjorie Maillet, Esther Fuentes, and Bernat Soria. 2000. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proceedings of the National Academy of Sciences of the United States of America 97: 11603–11608. https://doi.org/10.1073/pnas.97.21.11603.
Naulé, Lydie, Marie Picot, Mariangela Martini, Caroline Parmentier, Hélène. Hardin-Pouzet, Matthieu Keller, Isabelle Franceschini, and Sakina Mhaouty-Kodja. 2014. Neuroendocrine and behavioral effects of maternal exposure to oral bisphenol A in female mice. Journal of Endocrinology 220: 375–388. https://doi.org/10.1530/JOE-13-0607.
Nishizawa, Hanako, Maki Morita, Miki Sugimoto, Satoshi Imanishi, and Noboru Manabe. 2005. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. Journal of Reproduction and Development 51: 315–324. https://doi.org/10.1262/jrd.16008.
Oakley, Amy E., Donald K. Clifton, and Robert A. Steiner. 2009. Kisspeptin signaling in the brain. Endocrine Reviews 30: 713–743. https://doi.org/10.1210/er.2009-0005.
Palioura, E., and E. Diamanti-Kandarakis. 2013. Industrial endocrine disruptors and polycystic ovary syndrome. Journal of Endocrinological Investigation 36: 1105–1111. https://doi.org/10.1007/BF03346762.
Palioura, Eleni, and Evanthia Diamanti-Kandarakis. 2015. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Reviews in Endocrine and Metabolic Disorders 16: 365–371. https://doi.org/10.1007/s11154-016-9326-7.
Pepling, M.E. 2006. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis 44: 622–632. https://doi.org/10.1002/dvg.
Peretz, Jackye, Zelieann R. Craig, and Jodi A. Flaws. 2012. Bisphenol a inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biology of Reproduction 87: 1–11. https://doi.org/10.1095/biolreprod.112.101899.
Peretz, Jackye, and Jodi A. Flaws. 2013. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicology and Applied Pharmacology 271: 249–256. https://doi.org/10.1016/j.taap.2013.04.028.
Peretz, Jackye, Rupesh K. Gupta, Jeffrey Singh, Isabel Hernández-Ochoa, and Jodi A. Flaws. 2011. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicological Sciences 119: 209–217. https://doi.org/10.1093/toxsci/kfq319.
Pivonello, Claudia, Giovanna Muscogiuri, Antonio Nardone, Francesco Garifalos, Donatella Paola Provvisiero, Nunzia Verde, Cristina De Angelis, et al. 2020. Bisphenol A: An emerging threat to female fertility. Reproductive Biology and Endocrinology 18: 1–33. https://doi.org/10.1186/s12958-019-0558-8.
Pritchett, J.J., R.K. Kuester, and I.G. Sipes. 2002. Metabolism of bisphenol A in primary cultured hepatocytes from mice, rats, and humans. Drug Metabolism and Disposition 30: 1180–1185. https://doi.org/10.1124/dmd.30.11.1180.
Prizant, Hen, Norbert Gleicher, and Aritro Sen. 2014. Androgen actions in the ovary: Balance is key. Journal of Endocrinology 222: 141–151. https://doi.org/10.1530/JOE-14-0296.
Qi, Junfeng, Lida Liu, Jie Yang, Xueying Gao, and Wei Zhang. 2020. Bisphenol A decreases progesterone synthesis in human ovarian granulosa cells. Birth Defects Research 112: 1843–1849. https://doi.org/10.1002/bdr2.1817.
Rajareddy, Singareddy, Pradeep Reddy, Du. Chun, Lian Liu, Krishna Jagarlamudi, Wenli Tang, Yan Shen, et al. 2007. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Molecular Endocrinology 21: 2189–2202. https://doi.org/10.1210/me.2007-0172.
Reddy, Pradeep, Lian Liu, Deepak Adhikari, Krishna Jagarlamudi, Singareddy Rajareddy, Yan Shen, Du. Chun, et al. 2008. Oocyte-specific deletion of PTEN causes premature activation of the primordial follicle pool. Science 319: 611–613. https://doi.org/10.1126/science.1152257.
Riu, Anne, Marina Grimaldi, Albane le Maire, Gilbert Bey, Kevin Phillips, Abdelhay Boulahtouf, Elisabeth Perdu, Daniel Zalko, William Bourguet, and Patrick Balaguer. 2011. Peroxisome proliferator-activated receptor γ is a target for halogenated analogs of bisphenol A. Environmental Health Perspectives 119: 1227–1232. https://doi.org/10.1289/ehp.1003328.
Rivera, Oscar E., Jorgelina Varayoud, Horacio A. Rodríguez, Mónica. Muñoz-de-Toro, and Enrique H. Luque. 2011. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reproductive Toxicology 32: 304–312. https://doi.org/10.1016/j.reprotox.2011.06.118.
Rivera, Oscar E., Jorgelina Varayoud, Horacio A. Rodríguez, Clarisa G. Santamaría, Verónica L. Bosquiazzo, Mario Osti, Norberto M. Belmonte, Mónica. Muñoz-de-Toro, and Enrique H. Luque. 2015. Neonatal exposure to xenoestrogens impairs the ovarian response to gonadotropin treatment in lambs. Reproduction 149: 645–655. https://doi.org/10.1530/REP-14-0567.
Rochester, Johanna R., and Ashley L. Bolden. 2015. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environmental Health Perspectives 123: 643–650. https://doi.org/10.1289/ehp.1408989.
Rodríguez, Horacio A., Noelia Santambrosio, Clarisa G. Santamaría, Mónica. Muñoz-de-Toro, and Enrique H. Luque. 2010. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reproductive Toxicology 30: 550–557. https://doi.org/10.1016/j.reprotox.2010.07.008.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop. 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility 81: 19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004.
Rutkowska, A.Z., and E. Diamanti-Kandarakis. 2016. Polycystic ovary syndrome and environmental toxins. Fertility and Sterility 106: 948–958. https://doi.org/10.1016/j.fertnstert.2016.08.031.
Rutkowska, Aleksandra, Aleksandra Olsson, Kamila Wilczewska, Łukasz Łaczman, J. Justyna Kuliczkowska-płaksej, Andrzej Wasik Diana, et al. 2020. Bisphenol A impacts hormonal profile in patients with polycystic ovary syndrome but not in healthy women. Gynecological and Reproductive Endocrinology & Metabolism 1: 43–47.
Samardzija, Dragana, Kristina Pogrmic-Majkic, Svetlana Fa, Bojana Stanic, Jovana Jasnic, and Nebojsa Andric. 2018. Bisphenol A decreases progesterone synthesis by disrupting cholesterol homeostasis in rat granulosa cells. Molecular and Cellular Endocrinology 461: 55–63. https://doi.org/10.1016/j.mce.2017.08.013.
Santamaría, Clarisa, Milena Durando, Mónica Muñoz. De. Toro, Enrique H. Luque, and Horacio A. Rodriguez. 2016. Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. Journal of Steroid Biochemistry and Molecular Biology 158: 220–230. https://doi.org/10.1016/j.jsbmb.2015.11.016.
Sarraj, Mai A., and Ann E. Drummond. 2012. Mammalian foetal ovarian development: Consequences for health and disease. Reproduction 143: 151–163. https://doi.org/10.1530/REP-11-0247.
Savabieasfahani, Mozhgan, Kurunthachalam Kannan, Olga Astapova, Neil P. Evans, and Vasantha Padmanabhan. 2006. Developmental programming: Differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology 147: 5956–5966. https://doi.org/10.1210/en.2006-0805.
Sen, Aritro, and Stephen R. Hammes. 2010. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Molecular Endocrinology 24: 1393–1403. https://doi.org/10.1210/me.2010-0006.
Shrinithivihahshini, N.D., D. Mahamuni, and N. Praveen. 2014. Bisphenol A migration study in baby feeding bottles of selected brands available in the Indian market. Current Science 106: 1081–1084.
Sui, Yipeng, Ni. Ai, Se Hyung Park, Jennifer Rios-Pilier, Jordan T. Perkins, William J. Welsh, and Changcheng Zhou. 2012. Bisphenol A and its analogues activate human pregnane X receptor. Environmental Health Perspectives 120: 399–405. https://doi.org/10.1289/ehp.1104426.
Susiarjo, Martha, Terry J. Hassold, Edward Freeman, and Patricia A. Hunt. 2007. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genetics 3: 0063–0070. https://doi.org/10.1371/journal.pgen.0030005.
Suzuki, Atsuko, Akika Sugihara, Kaoru Uchida, Tomomi Sato, Yasuhiko Ohta, Yoshinao Katsu, Hajime Watanabe, and Taisen Iguchi. 2002. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reproductive Toxicology 16: 107–116. https://doi.org/10.1016/S0890-6238(02)00005-9.
Takeuchi, Toru, and Osamu Tsutsumi. 2002. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochemical and Biophysical Research Communications 291: 76–78. https://doi.org/10.1006/bbrc.2002.6407.
Takeuchi, Toru, Osamu Tsutsumi, Yumiko Ikezuki, Yasushi Takai, and Yuji Taketani. 2004. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocrine Journal 51: 165–169. https://doi.org/10.1507/endocrj.51.165.
Takeuchi, Toru, Osamu Tsutsumi, Yumiko Ikezuki, Y. Kamei, Y. Osuga, T. Fujiwara, Y. Takai, M. Momoeda, and T. Yano. 2006. Elevated serum bisphenol A levels under hyperandrogenic conditions may be caused by decreased UDP-glucuronosyltransferase activity. Endocrine Journal 53: 485–491.
Tarantino, G., R. Valentino, C. Di Somma, V. D’Esposito, F. Passaretti, G. Pizza, V. Brancato, F. Orio, P. Formisano, A. Colao, and S. Savastano. 2013. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clinical Endocrinology 78: 447–453. https://doi.org/10.1111/j.1365-2265.2012.04500.x.
Tingen, Candace, Alison Kim, and Teresa K. Woodruff. 2009. The primordial pool of follicles and nest breakdown in mammalian ovaries. Molecular Human Reproduction 15: 795–803. https://doi.org/10.1093/molehr/gap073.
USEPA, Bisphenol A (BPA) action plan summary. Environmental Protection Agency, USA, 2015; retrieved from http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/bpa.html.
Vahedi, M., A. Saeedi, S.L. Poorbaghi, M. Sepehrimanesh, and M. Fattahi. 2016. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environmental Science and Pollution Research International 23: 23546–23550. https://doi.org/10.1007/s11356-016-7573-5.
Vandenberg, Laura N., Russ Hauser, Michele Marcus, Nicolas Olea, and Wade V. Welshons. 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24: 139–177. https://doi.org/10.1016/j.reprotox.2007.07.010.
Völkel, Wolfgang, Thomas Colnot, György. A. Csanády, Johannes G. Filser, and Wolfgang Dekant. 2002. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chemical Research in Toxicology 15: 1281–1287.
Vidya Bharathi, R., S. Swetha, J. Neerajaa, J. Varsha Madhavica, Dakshina Moorthy Janani, S.N. Rekha, S. Ramya, and B. Usha. 2017. An epidemiological survey: Effect of predisposing factors for PCOS in Indian urban and rural population. Middle East Fertility Society Journal 22: 313–316. https://doi.org/10.1016/j.mefs.2017.05.007.
Wang, Wei, Katlyn S. Hafner, and Jodi A. Flaws. 2014a. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicology and Applied Pharmacology 276: 157–164. https://doi.org/10.1016/j.taap.2014.02.009.In.
Wang, Xiaoli, Fei Chang, Yinyang Bai, Fang Chen, Jun Zhang, and Ling Chen. 2014b. Bisphenol A enhances kisspeptin neurons in anteroventral periventricular nucleus of female mice. Journal of Endocrinology 221: 201–213. https://doi.org/10.1530/JOE-13-0475.
Wang, Yuan, Qinling Zhu, Xuan Dang, Yaqiong He, Xiaoxue Li, and Yun Sun. 2017. Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecological Endocrinology 33: 21–25. https://doi.org/10.1080/09513590.2016.1184641.
World Health Organization. 2009. BISPHENOL A (BPA)—Current state of knowledge and future actions by WHO and FAO. Food and Agriculture Organization of the United Nations, International Food Safety Authorities Network: 1–6.
Xi, Wei, C.K.F. Lee, W.S.B. Yeung, John P. Giesy, M.H. Wong, Xiaowei Zhang, Markus Hecker, and Chris K.C.. Wong. 2011. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reproductive Toxicology 31: 409–417. https://doi.org/10.1016/j.reprotox.2010.12.002.
Xu, Jiping, Yutaka Osuga, Tetsu Yano, Yutaka Morita, Xiaohui Tang, Toshihiro Fujiwara, Yasushi Takai, et al. 2002. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochemical and Biophysical Research Communications 292: 456–462. https://doi.org/10.1006/bbrc.2002.6644.
Xu, Li Chun, Hong Sun, Jian Feng Chen, Qian Bian, Jie Qian, Ling Song, and Xin Ru Wang. 2005. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology 216: 197–203. https://doi.org/10.1016/j.tox.2005.08.006.
Yokota, Hiroshi, Hidetomo Iwano, Mari Endo, Tsutomu Kobayashi, Hiroki Inoue, Shin Ichi Ikushiro, and Akira Yuasa. 1999. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochemical Journal 340: 405–409. https://doi.org/10.1042/0264-6021:3400405.
Zaid, Siti Sarah, Shatrah Othman Mohamad, and Normadiah M. Kassim. 2018. Protective role of Ficus deltoidea against BPA-induced impairments of the follicular development, estrous cycle, gonadotropin and sex steroid hormones level of prepubertal rats. Journal of Ovarian Research 11: 1–9. https://doi.org/10.1186/s13048-018-0466-0.
Zawadski, J.K., and A. Dunaif. 1992. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Polycystic Ovary Syndrome, ed. A. Dunaif, JR Givens, and F. Haseltine, 377–384. Blackwell Scientific.
Zhang, Han Qiong, Xi Feng Zhang, Lian Jun Zhang, Hu He. Chao, Bo. Pan, Yan Min Feng, Lan Li, Xiao Feng Sun, and Wei Shen. 2012. Fetal exposure to bisphenol a affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Molecular Biology Reports 39: 5651–5657. https://doi.org/10.1007/s11033-011-1372-3.
Zhao, Qian, Yan Ma, Ning Xia Sun, Chen Ye, Qing Zhang, Shu Han Sun, Xu. Chen, Fang Wang, and Wen Li. 2014. Exposure to bisphenol A at physiological concentrations observed in Chinese children promotes primordial follicle growth through the PI3K/Akt pathway in an ovarian culture system. Toxicology in Vitro 28: 1424–1429. https://doi.org/10.1016/j.tiv.2014.07.009.
Zhou, Changqing, W. Wang, J. Peretz, and J. Flaws. 2015. Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Physiology & Behavior 57: 87–99. https://doi.org/10.1016/j.reprotox.2015.05.012.Bisphenol.
Zhou, Jue, Qu. Fan, Yue Jin, and Dong Xia Yang. 2014. The extracts of pacific oyster (Crassostrea gigas) alleviate ovarian functional disorders of female ratswith exposure to bisphenol A through decreasing FSHR expression in ovarian tissues. African Journal of Traditional, Complementary and Alternative Medicines 11: 1–7. https://doi.org/10.4314/ajtcam.v11i5.1.
Zhou, Wei, Jiayin Liu, Lianming Liao, Suping Han, and Jinyong Liu. 2008. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Molecular and Cellular Endocrinology 283: 12–18. https://doi.org/10.1016/j.mce.2007.10.010.
Ziv-Gal, Ayelet, Zelieann R. Craig, Wei Wang, and Jodi A. Flaws. 2013. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reproductive Toxicology 23: 1–7.
Acknowledgements
The second author Ananya Banerjee is indebted to Council of Scientific and Industrial Research, New Delhi, India for financial assistance [Nov/06/2020(i)EU-V].
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: Reetuparna Basak; Literature search and data analysis: Reetuparna Basak and Ananya Banerjee; Writing original draft: Reetuparna Basak and Ananya Banerjee; Review and editing: Reetuparna Basak, Ananya Banerjee and Umesh Rai.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Consent to Participate
Consent hereby provided by the authors.
Consent for Publication
Consent hereby provided by the authors.
Ethics Approval
Not applicable.
Humans and Animals Rights Statement
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basak, R., Banerjee, A. & Rai, U. Demystifying Bisphenol A-Induced Alterations in Hypothalamic-Pituitary-Ovarian Functions Leading to Polycystic Ovarian Syndrome. Proc Zool Soc 74, 466–478 (2021). https://doi.org/10.1007/s12595-021-00407-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-021-00407-0