Abstract
Parkinson’s disease is a progressive neurodegenerative movement disorder. We aimed to investigate the effects of regular swimming exercise and melatonin applied in the 6-Hydroxydopamine-induced Parkinson’s disease rats by analysing dendritic spine of striatal neurons. Twenty-four male Wistar albino rats were used. 6-Hydroxydopamine unilaterally injected four (control, exercise, melatonin and exercise + melatonin) groups were included in the study. Tyrosine hydroxylase expression was detected by immunohistochemistry. Neurons and structures were identified from three-dimensional images by Neurolucida software. There was not any apparent difference for tyrosine hydroxylase positive neurons in the substantia nigra pars compacta and fibres in the striatum between the lesion sides of hemiparkinsonian groups. The treatment groups blocked the apomorphine-induced increase in rotations compared to the control group. In stepping test, the treatment groups prevented the loss of stepping in the contralateral side of hemiparkinsonian groups. The melatonin mostly had a positive effect on motor activity tests. In morphological analyses, the 6-Hydroxydopamine-induced lesion led to the reduction of the total dendritic length and number of branches. In the treatment groups, the reduction of the dendritic parameters was not observed. 6-Hydroxydopamine lesion led to a decrease in the total spine density, spine densities of thin and mushroom types. The exercise and melatonin treatments prevented the loss of spine density. The exercise treatment prevented the loss of spine density of mushroom type spines. The melatonin treatment blocked the loss of spine density of stubby type. In conclusion, these results provide evidence for effective additional protective therapeutic strategies for Parkinson’s disease. In conclusion, results from the current study provide evidence for swimming exercise and melatonin as a promising candidate for effective additional protective strategies for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative movement disorder characterized by motor (asymmetric onset of bradykinesia, rigidity, resting tremor, postural instability) and non-motor (depression, sleep disturbances, memory deficits) symptoms (Samii et al. 2004). The main pathology underlying PD is the selective loss of the dopaminergic neurons in the Substantia nigra pars compacta (SNc) and their axons and terminals in the striatum which have been suggested to arise as a consequence of a variety of factors including ageing, oxidative stress, inflammation, mitochondrial dysfunction and environmental toxins. The other major mechanism involved in in the pathogenesis of PD is the accumulation of Lewy bodies, composed of α-synuclein, in multiple systems. When the reduction in dopaminergic neuron terminals reaches about 50–70% the clinical manifestation of the motor systems of the disease become evident which are consequence of changes in basal ganglia circuitry (Kordower et al. 2013; Morais et al. 2012).
The striatum is the major component of the basal ganglia motor system that is composed of mostly medium spiny neurons (MSNs) and a small proportion of interneurons. These MSNs are the principal target of SNc dopamine neurons and play a vital role in not only movement but also motivation and learning (Shohamy 2011). These neurons possess a high density of dendritic spines that are specialized small protrusions that receive afferent synaptic inputs and sites of synaptic regulation and integration (Arellano et al. 2007). In PD and its models, presumably as a consequence of the loss of dopamine input, there is a reduction in dendritic length and spine density of MSNs and also MSNs a loss of axospinous synapses (Gagnon et al. 2017; Graves and Surmeier 2019; Ingham et al. 1993; Solis et al. 2007).
Dopamine replacement therapy is an effective treatment for PD, especially for relieving motor symptoms and indeed, in animal models PD-like motor impairments can be attenuated by acute levodopa (L-DOPA) treatment (Hossain and Weiner 1993; Paillé et al. 2007). However, L-DOPA treatment does not prevent dopaminergic neuron death and is associated with long-term motor complications (Filali and Lalonde 2016). In view of this, it is critical that neuroprotective therapies are developed and indeed, pharmacological and non-pharmacological treatments have been recently been studied including treatment with antioxidants (Rammo et al. 2019; Sarkar et al. 2016; Spivey 2011).
Melatonin (N-acetyl-5-methoxytryptamine) is an antioxidant endocrine hormone, secreted mainly by the pineal gland that regulates the circadian rhythm, modulation of immune and neuroendocrine functions and brain homeostasis. Melatonin also possess anti-apoptotic, anti-inflammatory and anti-oxidative properties. It prevents lipid peroxidation and also decreases the harmful effects of free radicals by stimulating the secretion of antioxidant enzymes (Lin et al. 2013; Ozsoy et al. 2015; Reiter et al. 2016). Melatonin is highly lipophilic and easily passes the blood–brain barrier and cell membranes, features that potentially useful in therapeutics. Furthermore, serum melatonin levels have been reported to be lower in patients with PD and correlate with the severity of the disease. However, the data relating the effects of melatonin substitution therapy in PD treatment is limited and contradictory (Lin et al. 2014; Paul et al. 2018).
Physical exercise is a promising non-pharmacological approach to ameliorate neurodegeneration in PD and has a favourable impact on clinical symptoms of PD possibly through the control of reactive oxygen species levels and neurotrophic factors. Neurotrophic factors are secretory proteins that have been shown to reduce oxidative stress and dopamine depletion (Bouzid et al. 2018; Feter et al. 2019) acting like endogenous antioxidants and having beneficial effects on brain plasticity (Shirvani et al. 2019). Although there is evidence of the beneficial effects of physical exercise on PD, the data is limited; there is still not a general consensus about the type or duration of physical activity required in PD. Water-based exercise programmes demonstrated better results compared to land-based exercises in PD (Ayán et al. 2014; Cugusi et al. 2019). Water-based programmes modulated antioxidant enzyme activity, increased superoxide dismutase (SOD) activity, reduced catalase (CAT) activity, and reduced SOD/CAT activity ratio in PD individuals (Dani et al. 2020). Aquatic exercise enhances physical mobility and oxidative capacity of major muscle group which leads to improve balance and gait of people with PD. Aquatic-based physical exercise programme, once a week for 5 months, has been reported to have some positive effects on gait kinematic parameters of the typical gait pattern in PD (Ayan and Cancela 2012; Lauze et al. 2016). Moderate swimming exercise decreased rotations which is corresponding to neuroprotective effect in Parkinsonian rats (Boracı et al. 2020).
In the current study, we aimed to investigate the effects of regular swimming exercise and melatonin applied in the 6-OHDA induced PD rats. In view of the changes in striatal MSNs that have been reported in PD and in animal models (Gagnon et al. 2017; Graves and Surmeier 2019; Ingham et al. 1993; Solis et al. 2007), that presumably underlie the motor symptoms, we chose to by analysing dendrite and spine morphology of striatal neurons.
Materials and methods
Animals and groups
A total of 24 male Wistar albino rats (250 ± 10 g; 9 weeks of age) were used in this study. The rats were obtained from Marmara University Experimental Animal Research Centre. All experimental protocols were approved by Marmara University Animal Care and Use Committee (approval code: 075.2016.mar). The experiments were performed in compliance with the Turkish law on the use of animals in experiments.
The rats were kept in a room with controlled temperature (20 °C ± 2 °C) under a 12-h light/12-h dark cycle. The animals were fed with standard rat feed and allowed free access to food and water. The animals were randomly divided into 4 groups (6 in each group). The experimental groups in detailed were as follows:
-
1.
6 OH-DA injected + control (P-Control)
-
2.
6 OH-DA injected + melatonin (P-Mel)
-
3.
6 OH-DA injected + exercise (P-Exc)
-
4.
6 OH-DA injected + exercise + melatonin (P-Exc + Mel)
Animal model
The 6-OHDA was administered by stereotaxic injection. The rats were anaesthetized with a mixture of ketamine (100 mg/kg) and xylazine (50 mg/kg) were administered intraperitoneally. The 6-OHDA was administered to the rostral (in mm: AP: – 2.1, L: 2.0, V: – 7.8) and caudal (AP: – 4.3, L: 1.5 V: – 7.8) right medial forebrain bundle (MFB) (Paxinos and Watson 2007). A total of 8 µl 6-OHDA (Sigma H116, St Louis, MO, USA) (5 mg/10 ml, 4 µl at each site) dissolved in 0.9% saline solution were injected at a rate of 1 µl/min (Unzai et al. 2017) using a Hamilton syringe (Stoelting/10 µl) and an infusion pump (kdScientific). After the injection the syringe was left in place for 5 min and slowly retracted. At the end of the procedure, 5 ml 0.9% saline was injected subcutaneously. The animals were fed with various soft and small particulate foods in the separate cages for the first 24 h postoperatively and then the animals were fed ad libitum for 12 h in light–dark environment.
Melatonin preparation
Melatonin (Sigma, St Louis, MO, USA, M5250) was dissolved in ethanol, and diluted in saline solution at a final proportion of 5:95. The final concentration of the melatonin 10 mg/ml which was injected intraperitoneally at a dose of 10 mg/kg at the same time of 30 days. The drug was prepared freshly every day, before the injection. The melatonin injection was started 3 days after 6-OHDA infusion.
Exercise protocol
Three days after the lesion was created, the exercise regime was started for the two groups for PD model. Regular swimming training, consisting of 5 times a week for 30 min, was performed for six weeks. The exercise protocol is considered moderately intense according to World Health Organization guidelines (Chen and Chang 2010). A water tank with a height and diameter of 80 × 80 cm was used for swimming exercise. After the tank was filled with water to a depth of 40 cm and at a temperature of 31 ± 1 °C, the rats were placed in the water. During the first two weeks, the rats were acclimatized to the water for increasing minutes on each day. After the first two weeks, rats in the exercise group were accustomed to the swimming activity. Then 30 min swimming protocol was applied for four weeks. The rats were dried with towels after swimming and were kept warm with the help of the heater. Swimming was performed at the same time every day and the rats with better adaptation were noted. The swimming exercise was restarted 3 days after 6-OHDA infusion.
Motor activity tests
On the 30th day following the injection of the 6-OHDA, apomorphine-induced rotation and the stepping tests were performed. The aim of these tests was to assess the degree of degeneration of SNc dopamine neurons and any neuroprotective effects of the exercise or melatonin.
Apomorphine-induced rotation test
Apomorphine hydrochloride (Sigma, St Louis, MO, USA, A4393) was administered subcutaneously at a dose of 0.05 mg/kg/ml to examine the rotation of rats. The rats placed in a plastic circular arena and observed for 40 min. The basal movement status of the rats and the number of turns to the side contralateral to the lesion were noted.
Stepping test
To monitor akinesia, the stepping test was performed (Olsson et al. 1995). The 6-OHDA induced lesion resulted in motor deficit on the contralateral side paw of the lesion, while the impairment on the ipsilateral side to the lesion was ambiguous.
In the test, the animals brought to the test medium and allowed to settle for at least 5 min. Each rat was forced to step on a flat surface with one of the anterior limbs touching the ground. The test was repeated twice a day for 3 consecutive days for both palmar and dorsal surfaces of the anterior limbs. Mean values of dorsal and palmar direction of steps for each animal were calculated.
Tissue collection
The rats were deeply anaesthetized with ketamine (100 mg/kg, intraperitoneally) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde (PFA) (Merck, Germany 1.04005.1000), (0.1 M phosphate buffer, pH 7.4). The brains were removed to divide into three parts using a 1-mm slicer. The first 4 mm part of the brain was placed into 4% PFA fixative solution for tyrosine hydroxylase (TH) staining. The next 6 mm part was used for into the Golgi staining. The last part which includes the SN was taken into 4% PFA fixative solution for TH staining. After 24 h of fixation, the brains were cryoprotected with 30% sucrose solution until saturated.
Immunohistochemistry
For analysis of TH, serial coronal brain Sects. (40 µm) were obtained in the anteroposterior direction using a frozen sliding microtome (Thermo Scientific, HM 430, Germany). One in three of the sections was taken for TH immunohistochemistry. The sections were rinsed in PBS (phosphate buffer saline) three times for 5 min and then incubated in a PBS solution containing 80% methanol and 3% H2O2 for 20 min at room temperature to block endogenous peroxidase activity. After rinsing in PBS three times, the sections were incubated in blocking serum solution (10% goat serum) (Merck, Darmstadt, Germany) for 1 h, followed by incubation in primary antibody (polyclonal rabbit anti-rat TH, 150 µl aliquot in 1:130 dilution in 20 ml carrier solution) (Pel-Freez, Rogers, AR, USA, P40101) overnight at room temperature. After rinsing in PBS three times, the sections were incubated in carrier solution including 150 µl biotinylated anti-rabbit IgG (Vector labs, BA, USA, 1000) secondary antibody for 2 h. They were then rinsed in PBS and subsequently incubated in avidin–biotin- horseradish peroxidase complex (10 ml/Vectastain ABC kit Elite, Vector Laboratories, PK-6100) for 1 h at room temperature. Immunoreactivity was revealed by incubating the sections in a 0.05% solution of 3.3’-diaminobenzidine (DAB) (Sigma, St Louis, MO, USA, D5905) for 2 min. After washing in PBS, the sections were mounted on gelatin-coated slides and examined under a light microscope.
Golgi impregnation
Golgi impregnation was used to observe the morphological features of neural microstructures, especially dendritic spines. The brain tissues were cut in 100 µm sections on a cryostat (Leica biosystems, CM 1950, USA) at – 20 °C. Then the sections were impregnated using a FD Rapid GolgiStain Kit, (FD Neurotechnologies) according to the manufacturers’ instructions.
Light microscopic analysis
Golgi-impregnated sections were examined using an Olympus BX51 microscope and Q Imaging Retiga-2000R camera. Neurons and structures were identified from three-dimensional images using Neurolucida (MBF Bioscience) software. The neurons with somatodendritic morphology of MSNs and with high density of dendritic spines without any sign of incomplete staining were reconstructed (Olsson et al. 1995; Yuste 2010). MSN selection and neuronal structures classification were made carefully by an observer blinded to the experimental groups. Neuron selection criteria were applied in accordance with previous literature (Jacobs et al. 1997): First, soma should be centrally located in a 100 µm section to reduce the possibility that distal dendritic branches were cut. Second, the neuron was clearly separated from adjacent structures. Third, the neuronal structures were adequately impregnated. Fourth, distal dendritic branches should be observed to form natural endings (Fig. 1). For each group, 4 neurons on the lesioned side were analysed giving a total of 192 dendrites. The same analysis was also done at the contralateral sides. For quantitative measures, neuron body then the axon, dendrite and their structures were drawn using a 60 X objective. Then, the dendritic spines of the first 10 µm length from the branching point on the secondary dendrites (nearest branch point) were marked and categorised according to dendritic spine head and neck ratio using a 100 X objective. The software preserves three-dimensional details of the neuronal structures so that the proper analysis of the spine is provided (Fig. 2). Spines were classified based on the ratio of spine head and neck diameter. Thin (ratio < 2), mushroom (ratio > 2) stubby (ratio < 1) (Peters and Kaiserman-Abramof 1970; Arellano et al. 2007), and branched (bifurcated head) spines were defined (Spires et al. 2004) (Fig. 3). In the first step of spine tracing, the size of the head was determined, then the neck of spine was extended to the dendritic shaft. Sholl analysis was performed for each neuron to obtain dendrite parameters and intersection numbers per concentric circles which were 10 µm in diameter, starting from the point at the centroid of the cell body (Braak and Braak 1985; Sholl 1953). The dendritic parameters were analysed by Neurolucida Explorer; total dendritic length (total length of primary and secondary dendritic branches), number of dendritic branches (total number of primary and secondary dendritic segments), total spine density (spines/10 µm dendrite), morphologic classification of spines (thin, mushroom, stubby, branched) and density of each type.
3D re-constructions of individual dendrites of MSNs in each of the PD groups. The secondary dendritic branches are shown in orange (P-Control, P-Mel, P-Exc) and white (P-Exc + Mel colours. The spine types were represented with different colours (blue-mushroom type, red-thin type, green-stubby type, pink-branched type). a P-Control group b P-Mel group c P-Exc group d P-Exc + Mel group
Statistical analysis
Data were analysed using GraphPad prism 6. The suitability of the data for normal distribution was evaluated by Kolmogorov–Smirnov test. Descriptive statistics were shown as mean ± standard error of mean (SEM), median, minimum and maximum value for continuous variables. The variables which fit the normal distribution, Sample t-test and One-way analysis of variance (ANOVA), post hoc Dunnett’s and Tukey’s multiple comparison tests were used. The variables which did not fit the normal distribution, Mann–Whitney U test and Kruskal–Wallis test, post hoc Dunn’s multiple comparisons test were used. The data were represented as “F value, p value” for one‐way ANOVA and “p value” for Kruskal–Wallis test. If p < 0.05, the difference was considered significant.
Results
The degree of akinesia and the effect of treatment groups on motor activity were measured by stepping and rotation tests. Dendritic features and spine morphology were observed using Neurolucida software on MSN. The differences at motor activity and the changes in dendritic and spine structure for each group were analysed in detail. Data from 24 rats were analysed.
Motor activity analyses
Apomorphine-induced rotation test
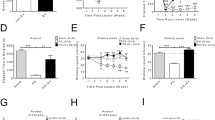
Consistent with many other studies, the injection of 6-OHDA into the MFB led to a motor asymmetry such that challenge with apomorphine caused the animals in each of the groups to rotate in a contralateral direction over 40 min on 30th day of lesion (Fig. 4). The injection of apomorphine provoked a strong contralateral turning response in the P-Control group (269.7 ± 20.1 turns/40 min). At 30 days post-lesion the groups treated with melatonin and/or subjected to the exercise regime (P-Mel, P-Exc + Mel and P-Exc) exhibited significantly less rotation in response to the apomorphine injection compared to the control (F = 5.90; p = 0.0047) Interestingly, the treatment with melatonin alone (P-Mel group 146.7 ± 28.6; p = 0.002) led to a greater reduction in rotations compared to all other PD treatment groups (P-Exc 170.3 ± 19.3; p = 0.01, P-Exc + Mel 176.7 ± 19.8; p = 0.02; One-way ANOVA, post hoc Dunnett's multiple comparison test, n = 6) Fig. 5.
Apomorphine-induced rotation test on the 30th day post-lesion. There was a statistically significant decrease in the number of contralateral rotations in P-Mel, P-Exc + Mel and P-Exc groups compared to P-Control group (F = 5.97; p = 0.0047, One-way ANOVA, post hoc Dunnett’s multiple comparison test). The reduction in the number of rotations of P-Mel group (p < 0.01) was more prominent than that of P-Exc and P-Exc + Mel groups (p < 0.05). The data are shown as the mean ± SEM of n = 6 rats in per group (*p < 0.05, **p < 0.01)
Stepping test data on the 30th day post-lesion. The stepping test of ipsilateral and contralateral sides of palmar and dorsal direction data were performed and monitored. Bonferroni adjusted multiple comparison test were performed. There were significant differences between ipsilateral palmar and contralateral palmar sides and between ipsilateral dorsal and contralateral dorsal sides (p < 0.001; F: 134.8; I: 2.34 df:3)
Stepping test
The statistical analyses were performed to compare the ipsilateral (lesion) sides to the contralateral sides of either palmar or dorsal directions in each group. We also analysed the stepping perfomance at both directions comparing treatment groups to the P-Control. The data obtained from the stepping test were consistent with the loss of dopamine neurons in the SNc in that the number of steps in the palmar direction on the side contralateral to the 6-OHDA injection were consistently reduced in PD groups compared to the ipsilateral side. The number of steps in the palmar direction decreased on the contralateral side compared to the ipsilateral side in each PD group side (p < 0.001; F: 134.8; I: 2.34 df: 3).
Two-way ANOVA, post hoc Bonferroni test, n = 6. The number of steps in the dorsal direction also decreased on the contralateral side compared to the lesion side in each PD group (p < 0.001; F: 134.8; I: 2.34 df: 3).
TH immunohistochemistry
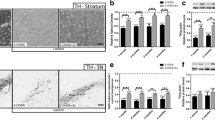
TH immunoreactivity was observed under light microscopy to confirmed the dopaminergic neurons loss due to the 6-OHDA injection. Similar degrees of TH staining were observed in the striatum (Fig. 6a) and SNc (Fig. 6b) between the lesion sides of PD groups.
Photomicrograph of (a) striatum (b) SNc and ventral tegmental area (VTA) after 6-OHDA lesion. TH immunoreactivity in the P-Control group comparing the lesion (right) and contralateral (left) sides under light microscope. TH immunoreactivity showed loss of labelling on the lesion side in P-Control group. The contralateral side of the P-Control group showed brown colour TH staining in the striatum. The lesion side showed no labelling due to dopaminergic cell loss
Morphological analyses of MSNs
Dendrites
The 6-OHDA injected animals showed morphological changes of the dendritic arbours and spine features of MSNs in the striatum ipsilateral to the lesion. The 6-OHDA injection led to reduction in the total dendritic length of MSNs on the lesion side (127.4 ± 39.52 μm) of the P-Control group compared to the contralateral side (366.3 ± 58.73 μm) (t = 3.37, df = 10; p = 0.007; Sample t-test, n = 6; Fig. 7a). Additionally, 6-0HDA induced Parkinsonian rats showed significant decrease in the number of dedritic branches (t = 3.98, df = 10; p = 0.002; Sample t-test, n = 6) on the lesion side of the P-Control group compared to the contralateral side (p < 0.001; Fig. 7b). The treatment with melatonin (378.2 ± 39.5 μm; p = 0.018; n = 6), the exercise regime (341.1 ± 57.9 μm; p = 0.047; n = 6) and a combination of both (380.7 ± 38 μm; p = 0.017; n = 6) ameliorated this reduction in total dendritic length compared to the P-Control group (F = 4.22, p = 0.018; One-way ANOVA Dunnett’s multiple comparison test, n = 6; Fig. 7c). The treatment with melatonin, the exercise regime and a combination of both increased the number of dendritic branches compared to the P-Control group (F = 6.45, p = 0.003; One-way ANOVA Dunnett’s multiple comparison test, n = 6; Fig. 7d).
a Comparison of the dendritic features of MSNs between either PD groups or lesion and contralateral sides of P-Control group. There was a statistically significant reduction in the total dendritic length (µm) on the lesion side of P-Control group compared to the contralateral side (p < 0.05, Sample t-test, n = 6). Data are shown as mean ± SEM, **p<0.001. b Comparison of the dendritic features of MSNs between either PD groups or lesion and contralateral sides of P-Control group. There was a statistically significant decrease in the number of dendritic branches on the lesion side of P-Control group compared to the contralateral side (p < 0.05, Sample t-test, n = 6). Data are shown as mean ± SEM, *p < 0.05. c Comparison of the dendritic features of MSNs between either PD groups or lesion and contralateral sides of P-Control group. The reduction in the total dendritic length (µm) was blocked by the melatonin, exercise or combination of the both (p < 0.05, One-way ANOVA Dunnett’s multiple comparison test, n = 6). Data are shown as mean ± SEM, *p < 0.05. d Comparison of the dendritic features of MSNs between either PD groups or lesion and contralateral sides of P-Control group. The effects of melatonin (p < 0.0001), exercise (p < 0.05) and combination of the two (p < 0.05) showed a statistically significant increase in the number of dendritic branches compared to the P-Control group (p < 0.05, One-way ANOVA Dunnett’s multiple comparison test, n = 6). Data are shown as mean ± SEM, *p < 0.05, ***p< 0.0001
Spines
The 6-OHDA injection led to significant reduction in the total spine density of MSNs on the lesion side of P-Control group compared to the contralateral side (p = 0.026, Mann–Whitney U test, n = 6; Fig. 8a). The densities of thin (Fig. 8b) and mushroom (Fig. 8c) types of P-Control group decreased in the lesion side compared to the contralateral side (p = 0.021, Mann–Whitney U test, n = 6). However, we did not observe any significant influence of the lesion sides of the P-Control on the spine densities of stubby (Fig. 8d) and branched (Fig. 8e) types compared with the contralateral sides (p > 0.05). Treatment with melatonin, subjecting the animals to the exercise regime combination significantly ameliorated the reduction in total spine density (p = 0.038; Fig. 9a), spine densities of thin (p = 0.018; Fig. 9b) and mushroom (p = 0.019; Fig. 9c) types compared to the P-Control group (p < 0.05; Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6). We also found that the exercise regime showed a significant increase in the spine density of mushroom type compared to the P-Control group (p = 0.014; Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6; Fig. 9c). The treatment with melatonin (0.5 ± 0.1 µm, n = 6) led to a significant increase in the spine density of stubby type compared to the P-Control (0.05 ± 0.03 µm, n = 6) group (p = 0.004; Fig. 9d). Branched type of spine had rarely observed. There was no significant density difference between P-Control and PD treatment groups (p > 0.05; Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6; Fig. 9e).
a Comparison of spine morphology of MSNs between the lesion and contralateral sides of P-Control group. The effects of 6-OHDA on the total spine density (spines/10 µm) of MSNs. There was a significant reduction in the total spine density on the lesion side of the P-Control group compared to the contralateral side (p < 0.05, Mann–Whitney U test, n = 6). Data are shown as mean ± SEM, *p < 0.05. b Comparison of spine morphology of MSNs between the lesion and contralateral sides of P-Control group. The effects of 6-OHDA on the total spine density (spines/10 µm) of MSNs. The spine density of thin type (thin spines/10 µm) decreased on the lesion side compared to the contralateral side in the P-Control group (p < 0.05, Mann–Whitney U test, n = 6). Data are shown as mean ± SEM, *p < 0.05. c Comparison of spine morphology of MSNs between the lesion and contralateral sides of P-Control group. The spine density of mushroom type (mushroom spines/10 µm) decreased on the lesion side compared to the contralateral side in the P-Control group (p < 0.05, Mann–Whitney U test, n = 6). Data are shown as mean ± SEM, *p < 0.05. d Comparison of spine morphology of MSNs between the lesion and contralateral sides of P-Control group. There was no significant difference between the spine density of stubby type (stubby spines/10 µm) on the lesion and contralateral sides of the P-Control group (p > 0.05, Mann–Whitney U test, n = 6). Data are shown as mean ± SEM, *p < 0.05. e Comparison of spine morphology of MSNs between the lesion and contralateral sides of P-Control group. There was no significant difference between the spine density of branched type (branched spines/10 µm) on the lesion and contralateral sides of the P-Control group (p > 0.05, Mann–Whitney U test, n = 6). Data are shown as mean ± SEM, *p < 0.05
a Comparison of spine morphology of MSNs between PD treatment groups and P-Control groups. The reduction in the total spine density (spines/10 µm) ameliorated by the P-Exc + Mel group compared to the P-Control (p < 0.05, Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6). Data are shown as mean ± SEM, *p < 0.05. b Comparison of spine morphology of MSNs between PD treatment groups and P-Control groups. The reduction in the spine density of thin type (thin spines/10 µm) was prevented by the P-Exc + Mel group (p < 0.05, Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6). Data are shown as mean ± SEM, *p< 0.05. c Comparison of spine morphology of MSNs between PD treatment groups and P-Control groups. The treatment with exercise and also combination of both increased the spine density of mushroom type (mushroom spines/10 µm) compared to the P-Control (p < 0.05, Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6). Data are shown as mean ± SEM, *p < 0.05. d Comparison of spine morphology of MSNs between PD treatment groups and P-Control groups. The treatment with melatonin led to increase in the spine density of stubby type (stubby spines/10 µm) compared to the P-Control group (p < 0.05; Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6). Data are shown as mean ± SEM, *p < 0.05. e Comparison of spine morphology of MSNs between PD treatment groups and P-Control groups. PD treatment groups had no effect on the spine density of branched type (branched spines/10 µm), p < 0.05; Kruskal–Wallis test, Dunn’s multiple comparisons test, n = 6
Discussion
The main objective of the present study was to investigate the potential protective effects of melatonin and swimming exercise on the functional and morphological deficits caused by 6-OHDA induced Parkinsonism in rats, i.e. following 6-OHDA lesions of the midbrain. The main findings are that melatonin, swimming exercise and a combination of the two ameliorated at least some of the motor deficits and the alterations in the morphological characteristics of dendrites and spines of the principal target of midbrain dopamine neurons, the MSNs, in the striatum.
In this study, we demonstrated a reduction in motor performance after the 6-OHDA lesion monitored by stepping and rotation tests and that treatment with melatonin prevented the motor performance impairment. The morphological analysis of the striatum showed that 6-OHDA lesion led to a reduction in the total dendritic length and number of dendritic branches in MSNs. The treatment with melatonin, exercise and combination of the two ameliorated these dendritic changes. Furthermore, the 6-OHDA lesion reduced the total spine density and the spine densities of thin and mushroom types in the control group. However, the combination of the melatonin and exercise blocked this. Additionally, the treatment with exercise prevented the reduction of the mushroom type spine density while melatonin treatment blocked the reduction of the stubby type spine density.
Effects of melatonin and exercise on 6-OHDA-induced motor deficits
Unilateral 6-OHDA injection into the MFB affects nigrostriatal system that develops motor disturbances caused by high neuronal loss. The dopamine depletion is evidenced by contralateral rotational behaviour in response to dopamine agonists (Sholl 1953). The 6-OHDA mechanism causes oxidative stress, which is implicated in the pathogenesis of PD, and that melatonin and exercise have positive impacts on this mechanism (Chagniel et al. 2012). The behavioural testing in PD models is used to characterize the extent of neurological damage and therapeutic efficacy (Blesa et al. 2015). The behavioural parameters of this study showed that forelimb akinesia was distinct in P-Control group compared to PD treatment groups. The results of apomorphine-induced rotation test showed decrease in rotations in the treatment groups. Melatonin, exercise and combination of both blocked the apomorphine-induced increase in rotations. The effect of melatonin was more prominent compared to the other treatment modalities. The stepping test demonstrated that 6-OHDA induced lesion led to a significant loss of motor performance in PD groups. The comparison of ipsilateral and contralateral stepping tests of each group showed that the number of contralateral side steps decreased significantly.
There is conflicting data on the effect of melatonin on locomotor activity (Burton et al. 1991; Kim et al. 1998). In a study of 6-OHDA lesioned rats administrated doses higher and lower than physiological dose of melatonin apomorphine-induced rotational behaviour diminished at the higher dose (Sharma et al. 2007). In contrast, it has been reported that melatonin (10 mg/kg) administration had no effect on locomotor activity in MPTP-induced Parkinsonism in mice (Khaldy et al. 2003). Similarly, short-term melatonin (10 mg/kg) treatment has been reported to not increase the locomotor activity following 6-OHDA treatment in rats (Yildirim et al. 2014). In PD patients, melatonin supplementation ameliorated sleep quality but without improvement of motor disability (Medeiros et al. 2007). Our findings were consistent with the positive effect of melatonin on locomotor performance in animal model of PD (Patki and Lau 2011; Tancheva et al. 2021).
Effects of melatonin and exercise on 6-OHDA-induced morphological changes
We quantitatively analysed the dendritic features and spine morphology of MSNs in the striatum and showed that 6-OHDA induced lesion led to reduction in the total dendritic length and also the number of dendritic branches. Treatment with melatonin, the exercise regime and a combination of both blocked this reduction in the total dendritic length and the number of dendritic branches. The treatment with melatonin was more effective than other treatments in the number of dendritic branches.
The melatonin and regular swimming exercise influenced spine morphology in Parkinsonian rats including the total spine density and various types of spine densities. We examined the four main types of spines that were classified according to their head and neck proportions i.e. thin, mushroom, stubby and branched types. It has been suggested that various spine types reflect the different functions and also critical for synaptic function (Gipson and Olive 2017). Therefore, morphological alterations of spines provide important information about neuronal plasticity.
The morphological analysis of MSNs in the 6-OHDA lesioned rats demonstrated significant protection in the total spine density and of the thin and mushroom type of spines which were consistent with the previous findings (Smith et al. 2009; Soderstrom et al. 2010; Suarez et al. 2016). The treatment with a combination of the melatonin and exercise prevented this reduction in the spine densities. The treatment with swimming exercise inhibited the loss of the density of mushroom type. The treatment with melatonin blocked the loss of the density of stubby type. These results revealed that the morphological change of spines of the MSNs were consistent with the results of the motor behavioural tests.
In the literature, the length of dendrites and the density of dendritic spines on the MSNs were reported to be decreased in experimental models of PD which was correlated with our results (Gerfen 2006; Ingham et al. 1993; Soderstrom et al. 2010; Walker et al. 2012). Dopamine regulates the formation and the density of dendritic spines and dopamine depletion results in shorter dendritic spines with a decreased density. Many studies have shown different experimental models of the PD in various of species resulted in decrease of spine density (Villalba et al. 2009; Villalba and Smith 2010, 2011a). Stephens et al. (2005) showed decrease of spine density of human with PD. Zaja-Milatovic et al. (2005) showed decrease the dendritic length and spine density as our study. Spine density gradually decreased with age in α-synuclein transgenic mouse model of PD (Parajuli et al. 2020a).
The treatments were applied immediately after the injection of 6-OHDA showed that melatonin and exercise have protective effects on the morphological changes that occur in MSNs following the 6-OHDA-induced Parkinsonism. Thus, the decrease in the total dendritic length and number of dendritic branches further to that the densities of total, thin and mushroom spines were prevented by the combined exercise and melatonin treatments and exercise alone had an effect of the blocked the reduction in spine density, at least for those of the mushroom type. Mushroom types are stable, mature, large and least dynamic spines which form strong synaptic connections and may represent memory storage spines (Grutzendler et al. 2002). Thin types are more dynamic spines which represent small synapse and act as learning spines. We also observed that the treatment with melatonin increased spine density of stubby type. Stubby types are dynamic and immature spines and consider as a general precursor of mushroom and thin spine types (Bourne and Harris 2007; Hayashi and Majeweska 2005). Branched types very rarely observed spine. The branched type did not show any significant difference by treatments in this study. Various spine morphologies were detected in the striatum of C57BL/6 male mice by focussed ion beam/scanning electron microscope. Contraversly, the branched type spines were frequently seen among them in the dorsolateral striatum (Parajuli et al. 2020b).
The mainstay in PD treatment are the dopaminergic medications, especially for relieving motor symptoms. However medical treatments do not have any effects on the dopamine neuron death nor on the onset of non-motor symptoms (Amara et al. 2019; Connolly and Lang 2014) i.e. current treatments do not alter the course of the disease. It is thus of importance that new treatment modalities are identified that have the ability to modify the course of the disease improve the life quality and the life expectancy of patients with PD.
The physical activity has been suggested as a novel treatment method and the data are accumulating about the beneficial effects of physical activity in PD. Shin et al. (2017) showed that treadmill exercise suppressed immobility time and enhanced climbing time in the rotenone-injected rats in an experimental model. Askar et al. (2019) reported that, swimming exercise improved the motor performance in PD rodent model which might be associated with the increasing density of dopaminergic neurons in corpus striatum. Goes et al. (2014) investigated the potential neuroprotective effects of swimming training for 4 weeks in a mouse model of PD induced by 6-OHDA and reported that swimming training was effective in attenuating depressive-like behaviour in the tail suspension test, increase in the number of falls in the rotarod test, impairment on long-term memory in the object recognition test, increasing antioxidants and decreasing free oxidant radicals. The authors also suggested swimming exercise as a non-pharmacological tool to reduce the symptoms of PD. Zhao et al. (2017) also investigated the effects of a new exercise training regimen including dry-land swimming and paraspinal muscle stretching for 30 min/workday for one year on PD patients and reported that this exercise method was effective in improving mobility disorder, balance, and cardiac function over a 1-year period. Toy et al. (2014) demonstrated that treadmill exercise increases MSN spine densities and dendritic arborization in MPTP-lesioned mice. The author also suggested that exercise has beneficial effect on striatum of healthy brain. In this study, the exercise resulted in protection of motor function and also alteration in dendritic structure. The exercise regime blocked the reduction in the number of dendritic length and branches. The complex dendritic arbours have the potential to host more synapses. Besides, the effect of exercise increased the density of mushroom type in Parkinsonian rats that reflects the synaptic form of spine types.
Melatonin, other promising treatment modality, is an endocrine hormone and with its antioxidant and neuroprotective effects, it has been suggested as a potential agent in the treatment of some neurological diseases such as PD (Alghamdi 2018; Sharma et al. 2007). Saravanan et al. (2007) revealed the effects of melatonin against rotenone‐induced oxidative stress in a hemiparkinsonian rat model. They reported that rotenone‐induced hydroxyl radicals in the isolated mitochondria were significantly scavenged by melatonin; and rotenone‐induced decrease in glutathione level and changes in antioxidant enzyme activities in the SN were restored with melatonin. Ozsoy et al. (2015) reported that, melatonin supplementation reduced dopaminergic neuron death and increased antioxidant levels in SN samples in a 6-OHDA induced rat Parkinsonism model. They also reported that, starting melatonin treatment before creating experimental PD was more effective on observed changes; defining the preventive role of melatonin in PD. Singhal et al. (2011) also reported that melatonin offers nigrostriatal dopaminergic neuroprotection against maneb and paraquat induced PD by the modulation of oxidative stress and apoptotic mechanism. Li et al. (2017) reported that melatonin replacement therapy significantly alleviated the neurotoxicity in a rat model of PD. In another rat model, Rasheed et al. (2018) reported that melatonin improves motor functions by upregulation of TH in striatum of the brain and melatonin inhibits the striatal degeneration. Toxic α‐synuclein accumulation causes Lewy body production, which is a mediator of neurodegeneration cell death in PD. Melatonin has also been shown to interfere with α‐synuclein and to reduce the neurotoxicity associated with α‐synuclein (Lin et al. 2007; Ono et al. 2012). Furthermore, Naskar et al. (2013) reported that melatonin ameliorated the striatal MSNs spine loss especially with L-DOPA in MPTP mice. In present study, melatonin mostly protected the motor activities of parkinsonian rats and also dendritic morphological alterations. The mushroom and thin types spine densities and total dendrite length and the number of branches were positively affected by the combined treatment of melatonin and exercise. The stubby spine is more unstable form of spine and may reflect the synaptic plasticity. Treatment with melatonin increased the density of stubby type (Tønnesen et al. 2014).
Besides, motor activity tests and Neurolucida analysis results were not compatible with TH-cell loss. According to TH immunohistochemistry, there was not an apparent difference between TH-positive neurons on the lesion side of all PD groups. TH, the rate limiting enzyme for dopamine synthesis, is widely used marker of dopaminergic depletion in the 6-OHDA rat model. Kordower et al. (2013) showed that loss of TH was seen in SNc neuronal cell bodies proved the whole neuronal depredation early after PD diagnosis. Furthermore, the authors also reported that the density of TH-positive fibres in the dorsal striatum was affected in a moderate-to-mild manner at 1 year following diagnosis and the density gradually diminished until years 4–5. Similarly, Ma et al. (2009) also reported that in a MPTP mice model of PD, melatonin pre-treatment potentially was neuroprotective for all the SNc cells in toxicity and death and prevented the loss of TH-positive cells.
There are some limitations of this study that should be mentioned. First, this is an experimental study carrying all bias associated with species differences. Second, long-term follow-up results were not obtained. Third, swimming that has been suggested as a treatment modality in this study in PD may not always be possible for PD patients, especially in advanced stages of the disease.
Our study showed the alterations on dendrites and spines in response to 6-OHDA injection and the effects of melatonin and swimming exercise in the striatum of Parkinsonian rats. There is not a consensus on the function of different types of dendritic spines in the literature. The information provided from this study will also contribute to the understanding of the changes of dendritic spines in response to various conditions.
Conclusion
In conclusion, results from the current study provide evidence for swimming exercise and melatonin as a promising candidate for effective additional protective strategies for PD. Further, prospective, clinical studies are warranted to define the exact role of these treatment modalities in this disease.
Data accessibility statement
Data is available if required.
References
Alghamdi BS (2018) The neuroprotective role of melatonin in neurological disorders. J Neurosci Res 96:1136–1149. https://doi.org/10.1002/jnr.24220
Amara AW, Chahine L, Seedorff N, Caspell-Garcia CJ, Coffey C, Simuni T (2019) Self-reported physical activity levels and clinical progression in early Parkinson’s disease. Parkinsonism Relat Disord 61:118–125. https://doi.org/10.1016/j.parkreldis.2018.11.006
Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R (2007) Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci 1:131–143. https://doi.org/10.3389/neuro.01.1.1.010.2007
Askar MH, Hussein AM, Al-Basiony SF, Meseha RK, Metias EF, Salama MM, Antar A, El-Sayed A (2019) Effects of exercise and ferulic acid on alpha synuclein and neuroprotective heat shock protein 70 in an experimental model of Parkinsonism disease. CNS Neurol Disord Drug Targets 18:156–169. https://doi.org/10.2174/1871527317666180816095707
Ayán C, Cancela J (2012) Feasibility of 2 different water-based exercise training programs in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil 93:1709–1714. https://doi.org/10.1016/j.apmr.2012.03.029
Ayán C, Cancela JM, Gutiérrez-Santiago A, Prieto I (2014) Effects of two different exercise programs on gait parameters in individuals with Parkinson’s disease: a pilot study. Gait Posture 39:648–651. https://doi.org/10.1016/j.gaitpost.2013.08.019
Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson’s disease. Front Neuroanat 9:91. https://doi.org/10.3389/fnana.2015.00091
Boracı H, Kirazlı Ö, Gülhan R, Yıldız Sercan D, Şehirli ÜS (2020) Neuroprotective effect of regular swimming exercise on calretinin-positive striatal neurons of Parkinsonian rats. Anat Sci Int 95:429–439. https://doi.org/10.1007/s12565-020-00538-y
Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17(3):381-386. https://doi.org/10.1016/j.conb.2007.04.009
Bouzid MA, Filaire E, Matran R, Robin S, Fabre C (2018) Lifelong voluntary exercise modulates age-related changes in oxidative stress. Int J Sports Med 39:21–28. https://doi.org/10.1055/s-0043-119882
Braak H, Braak E (1985) Golgi preparations as a tool in neuropathology with particular reference to investigations of the human telencephalic cortex. Prog Neurobiol 25:93–139. https://doi.org/10.1016/0301-0082(85)90001-2
Burton S, Daya S, Potgieter B (1991) Melatonin modulates apomorphine-induced rotational behaviour. Experientia 47:466–469. https://doi.org/10.1007/BF01959946
Chagniel L, Robitaille C, Lebel M, Cyr M (2012) Striatal inhibition of calpains prevents levodopa-induced neurochemical changes and abnormal involuntary movements in the hemiparkinsonian rat model. Neurobiol Dis 45:645–655. https://doi.org/10.1016/j.nbd.2011.10.011
Chen W, Chang MH (2010) New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr Neonatol 51:69–79. https://doi.org/10.1016/S1875-9572(10)60014-9
Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. JAMA 311:1670–1683. https://doi.org/10.1001/jama.2014.3654
Cugusi L, Manca A, Bergamin M, Di Blasio A, Monticone M, Deriu F, Mercuro G (2019) Aquatic exercise improves motor impairments in people with Parkinson’s disease, with similar or greater benefits than land-based exercise: a systematic review. J Physiother 65:65–74. https://doi.org/10.1016/j.jphys.2019.02.003
Dani C, Proença IT, Marinho J, Peccin P, da Silva IRV, Nique S, Striebel V, Pochmann D, Elsner VR (2020) Aquatic exercise program-modulated oxidative stress markers in patients with Parkinson’s disease. Neural Regen Res 15:2067–2072. https://doi.org/10.4103/1673-5374.276337
Feter N, Spanevello RM, Soares MSP, Spohr L, Pedra NS, Bona NP, Freitas MP, Gonzales NG, Ito LGMS, Stefanello FM, Rombaldi AJ (2019) How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol Behav 201:42–52. https://doi.org/10.1016/j.physbeh.2018.12.002
Filali M, Lalonde R (2016) Neurobehavioral anomalies in the Pitx3/ak murine model of Parkinson’s disease and MPTP. Behav Genet 46:228–241. https://doi.org/10.1007/s10519-015-9753-3
Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, Parent M (2017) Striatal neurons expressing D1 and D2 receptors are morphologically distinct and differently affected by dopamine denervation in mice. Sci Rep 7:41432. https://doi.org/10.1038/srep41432
Gerfen CR (2006) Indirect-pathway neurons lose their spines in Parkinson disease. Nat Neurosci 9:157–158. https://doi.org/10.1038/nn0206-157
Gipson CD, Olive MF (2017) Structural and functional plasticity of dendritic spines–root or result of behavior? Genes Brain Behav 16:101–117. https://doi.org/10.1111/gbb.12324
Goes AT, Souza LC, Filho CB, Del Fabbro L, De Gomes MG, Boeira SP, Jesse CR (2014) Neuroprotective effects of swimming training in a mouse model of Parkinson’s disease induced by 6-hydroxydopamine. Neuroscience 256:61–71. https://doi.org/10.1016/j.neuroscience.2013.09.042
Graves SM, Surmeier DJ (2019) Delayed spine pruning of direct pathway spiny projection neurons in a mouse model of Parkinson’s disease. Front Cell Neurosci 13:32–37. https://doi.org/10.3389/fncel.2019.00032
Grutzendler J, Kasthuri N, Gan WB (2002) Long-term dendritic spine stability in the adult cortex. Nature 420:812–816. https://doi.org/10.1038/nature01276
Hayashi Y, Majewska AK (2005) Dendritic spine geometry: functional implication and regulation. Neuron 46(4):529–532. https://doi.org/10.1016/j.neuron.2005.05.006
Hossain MA, Weiner N (1993) Dopaminergic functional supersensitivity: effects of chronic L-dopa and carbidopa treatment in an animal model of Parkinson’s disease. J Pharmacol Exp Ther 267:1105–1111
Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW (1993) Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res 93:17–27. https://doi.org/10.1007/BF00227776
Jacobs B, Driscoll L, Schall M (1997) Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol 386:661–680
Khaldy H, Escames G, León J, Bikjdaouene L, Acuña-Castroviejo D (2003) Synergistic effects of melatonin and deprenyl against MPTP-induced mitochondrial damage and DA depletion. Neurobiol Aging 24:491–500. https://doi.org/10.1016/s0197-4580(02)00133-1
Kim YS, Joo WS, Jin BK, Cho YH, Baik HH, Park CW (1998) Melatonin protects 6-OHDA-induced neuronal death of nigrostriatal dopaminergic system. NeuroReport 9:2387–2390. https://doi.org/10.1097/00001756-199807130-00043
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136:2419–2431. https://doi.org/10.1093/brain/awt192
Lauzé M, Daneault JF, Duval C (2016) The effects of physical activity in Parkinson’s disease: a review. J Parkinsons Dis 6:685–698. https://doi.org/10.3233/JPD-160790
Li Y, Wang SM, Guo L, Zhu J, Wang Y, Li L, Zhao YX (2017) Effects of melatonin levels on neurotoxicity of the medial prefrontal cortex in a rat model of Parkinson’s disease. Chin Med J (engl) 130:2726–2731. https://doi.org/10.4103/0366-6999.218025
Lin AM, Fang SF, Chao PL, Yang CH (2007) Melatonin attenuates arsenite-induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res 43:163–171. https://doi.org/10.1111/j.1600-079X.2007.00456.x
Lin L, Meng T, Liu T, Zheng Z (2013) Increased melatonin may play dual roles in the striata of a 6-hydroxydopamine model of Parkinson’s disease. Life Sci 92:311–316. https://doi.org/10.1016/j.lfs.2013.01.007
Lin L, Du Y, Yuan S, Shen J, Lin X, Zheng Z (2014) Serum melatonin is an alternative index of Parkinson’s disease severity. Brain Res 1547:43–48. https://doi.org/10.1016/j.brainres.2013.12.021
Ma J, Shaw VE, Mitrofanis J (2009) Does melatonin help save dopaminergic cells in MPTP-treated mice? Parkinsonism Relat Disord 15:307–314. https://doi.org/10.1016/j.parkreldis.2008.07.008
Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lourdes SM, de Bruin VM (2007) Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease. J Neurol 254:459–464. https://doi.org/10.1007/s00415-006-0390-x
Morais LH, Lima MM, Martynhak BJ, Santiago R, Takahashi TT, Ariza D, Barbiero JK, Andreatini R, Vital MA (2012) Characterization of motor, depressive-like and neurochemical alterations induced by a short-term rotenone administration. Pharmacol Rep 64:1081–1090. https://doi.org/10.1016/s1734-1140(12)70905-2
Naskar A, Manivasagam T, Chakraborty J, Singh R, Thomas B, Dhanasekaran M, Mohanakumar KP (2013) Melatonin synergizes with low doses of L-DOPA to improve dendritic spine density in the mouse striatum in experimental Parkinsonism. J Pineal Res 55:304–312. https://doi.org/10.1111/jpi.12076
Olsson M, Nikkhah G, Bentlage C, Björklund A (1995) Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15:3863–3875. https://doi.org/10.1523/JNEUROSCI.15-05-03863.1995
Ono K, Mochizuki H, Ikeda T, Nihira T, Takasaki J, Teplow DB, Yamada M (2012) Effect of melatonin on alpha-synuclein self-assembly and cytotoxicity. Neurobiol Aging 33:2172–2185. https://doi.org/10.1016/j.neurobiolaging.2011.10.015
Ozsoy O, Yildirim FB, Ogut E, Kaya Y, Tanriover G, Parlak H, Agar A, Aslan M (2015) Melatonin is protective against 6-hydroxydopamine-induced oxidative stress in a hemiparkinsonian rat model. Free Radic Res 49:1004–1014. https://doi.org/10.3109/10715762.2015.1027198
Paillé V, Henry V, Lescaudron L, Brachet P, Damier P (2007) Rat model of Parkinson’s disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord 22:533–539. https://doi.org/10.1002/mds.21308
Parajuli LK, Wako K, Maruo S, Kakuta S, Taguchi T, Ikuno M, Yamakado H, Takahashi R, Koike M (2020a) Developmental changes in dendritic spine morphology in the striatum and their alteration in an A53T α-synuclein transgenic mouse model of Parkinson’s disease. eNeuro. https://doi.org/10.1523/ENEURO.0072-20.2020a
Parajuli LK, Urakubo H, Takahashi-Nakazato A, Ogelman R, Iwasaki H, Koike M, Kwon HB, Ishii S, Oh WC, Fukazawa Y, Okabe S (2020b) Geometry and the organizational principle of spine synapses along a dendrite. eNeuro. https://doi.org/10.1523/ENEURO.0248-20.2020
Patki G, Lau YS (2011) Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson’s disease. Pharmacol Biochem Behav 99:704–711. https://doi.org/10.1016/j.pbb.2011.06.026
Paul R, Phukan BC, Justin Thenmozhi A, Manivasagam T, Bhattacharya P, Borah A (2018) Melatonin protects against behavioral deficits, dopamine loss and oxidative stress in homocysteine model of Parkinson’s disease. Life Sci 192:238–245. https://doi.org/10.1016/j.lfs.2017.11.016
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Peters A, Kaiserman-Abramof IR (1970) The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat 127:321–355. https://doi.org/10.1002/aja.1001270402
Rammo R, Schwalb JM, Air EL (2019) MRI-guided DBS for Parkinson’s disease. In: Goodman RR Surgery for Parkinson’s Disease. Springer Nature Switzerland AG, 67–79: (1st ed.)
Rasheed MZ, Andrabi SS, Salman M, Tabassum H, Shaquiquzzaman M, Parveen S, Parvez S (2018) Melatonin improves behavioral and biochemical outcomes in a rotenone-induced rat model of Parkinson’s disease. J Environ Pathol Toxicol Oncol 37:139–150. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2018025666
Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J Pineal Res 61:253–278. https://doi.org/10.1111/jpi.12360
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363:1783–1793. https://doi.org/10.1016/S0140-6736(04)16305-8
Saravanan KS, Sindhu KM, Mohanakumar KP (2007) Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res 42:247–253. https://doi.org/10.1111/j.1600-079X.2006.00412.x
Sarkar S, Raymick J, Imam S (2016) Neuroprotective and therapeutic strategies against Parkinson’s disease: Recent perspectives. Int J Mol Sci 17:904–935. https://doi.org/10.3390/ijms17060904
Sharma R, McMillan CR, Niles LP (2007) Neural stem cell transplantation and melatonin treatment in a 6-hydroxydopamine model of Parkinson’s disease. J Pineal Res 43:245–254. https://doi.org/10.1111/j.1600-079X.2007.00469.x
Shin MS, Kim TW, Lee JM, Sung YH, Lim BV (2017) Treadmill exercise alleviates depressive symptoms in rotenone-induced Parkinson disease rats. J Exerc Rehabil. 13:124–129. https://doi.org/10.12965/jer.1734966.483.
Shirvani H, Aslani J, Mohammadi ZF, Arabzadeh E (2019) Short-term effect of low-, moderate-, and high-intensity exercise training on cerebral dopamine neurotrophic factor (CDNF) and oxidative stress biomarkers in brain male Wistar rats. Comp Clin Pathol 28:369–376. https://doi.org/10.1007/s00580-018-2885-0
Shohamy D (2011) Learning and motivation in the human striatum. Curr Opin Neurobiol 21:408–414. https://doi.org/10.1016/j.conb.2011.05.009
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387–406
Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP (2011) Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J Pineal Res 50:97–109. https://doi.org/10.1111/j.1600-079X.2010.00819.x
Smith Y, Villalba RM, Raju DV (2009) Striatal spine plasticity in Parkinson’s disease: pathological or not? Parkinsonism Relat Disord 15:156–161. https://doi.org/10.1016/S1353-8020(09)70805-3
Soderstrom KE, O’Malley JA, Levine ND, Sortwell CE, Collier TJ, Steece-Collier K (2010) Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur J Neurosci 31:478–490. https://doi.org/10.1111/j.1460-9568.2010.07077.x
Solis O, Limón DI, Flores-Hernández J, Flores G (2007) Alterations in dendritic morphology of the prefrontal cortical and striatum neurons in the unilateral 6-OHDA-rat model of Parkinson’s disease. Synapse 61:450–458. https://doi.org/10.1002/syn.20381
Spires TL, Grote HE, Garry S, Cordery PM, Van Dellen A, Blakemore C, Hannan AJ (2004) Dendritic spine pathology and deficits in experience-dependent dendritic plasticity in R6/1 Huntington’s disease transgenic mice. Eur J Neurosci 19:2799–2807. https://doi.org/10.1111/j.0953-816X.2004.03374.x
Spivey A (2011) Rotenone and paraquat linked to Parkinson’s disease: human exposure study supports years of animal studies. Environ Health Perspect 119:A259. https://doi.org/10.1289/ehp.119-a259a
Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, Ingham CA (2005) Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience 132:741–754. https://doi.org/10.1016/j.neuroscience.2005.01.007
Suarez LM, Solis O, Aguado C, Lujan R, Moratalla R (2016) L-DOPA oppositely regulates synaptic strength and spine morphology in D1 and D2 striatal projection neurons in dyskinesia. Cereb Cortex 26:4253–4264. https://doi.org/10.1093/cercor/bhw263
Tancheva L, Lazarova M, Saso L, Kalfin R, Stefanova M, Uzunova D, Atanasov AG (2021) Beneficial Effect of Melatonin on Motor and Memory Disturbances in 6-OHDA-Lesioned Rats. J Mol Neurosci 41:1–11. https://doi.org/10.1007/s12031-020-01760-z
Tønnesen J, Katona G, Rózsa B, Nägerl UV (2014) Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci 17:678–685. https://doi.org/10.1038/nn.3682
Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vučković MG, Jakowec MW (2014) Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol Dis 63:201–209. https://doi.org/10.1016/j.nbd.2013.11.017
Unzai T, Kuramoto E, Kaneko T, Fujiyama F (2017) Quantitative analyses of the projection of individual neurons from the midline thalamic nuclei to the striosome and matrix compartments of the rat striatum. Cereb Cortex 27:1164–1181. https://doi.org/10.1093/cercor/bhv295
Villalba RM, Lee H, Smith Y (2009) Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol 215:220–227. https://doi.org/10.1016/j.expneurol.2008.09.025
Villalba RM, Smith Y (2010) Striatal spine plasticity in Parkinson’s disease. Front Neuroanat 4:133–139. https://doi.org/10.3389/fnana.2010.00133
Villalba RM, Smith Y (2011a) Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys. J Comp Neurol 519:989–1005. https://doi.org/10.1002/cne.22563
Walker RH, Moore C, Davies G, Dirling LB, Koch RJ, Meshul CK (2012) Effects of subthalamic nucleus lesions and stimulation upon corticostriatal afferents in the 6-hydroxydopamine-lesioned rat. PLoS ONE 7:e32919. https://doi.org/10.1371/journal.pone.0032919
Yildirim FB, Ozsoy O, Tanriover G, Kaya Y, Ogut E, Gemici B, Dilmac S, Ozkan A, Agar A, Aslan M (2014) Mechanism of the beneficial effect of melatonin in experimental Parkinson’s disease. Neurochem Int 79:1–11. https://doi.org/10.1016/j.neuint.2014.09.005
Yuste R (2010) Dendritic spines. MIT Press, Cambridge
Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ (2005) Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64:545–547. https://doi.org/10.1212/01.WNL.0000150591.33787.A4
Zhao M, Hu C, Wu Z, Chen Y, Li Z, Zhang M (2017) Effects of coordination and manipulation therapy for patients with Parkinson disease. Int J Neurosci 127:762–769. https://doi.org/10.1080/00207454.2016.1248839
Acknowledgements
We are grateful to Prof. J. Paul Bolam for his insightful comments on this manuscript and for the language revision.
Funding
This study was supported by the Marmara University Scientific Research Projects Coordination Unit (project number: SAG-C-DRP-131016–0440).
Author information
Authors and Affiliations
Contributions
SG and ÜSŞ found the topic, performed the experiment and analysed, wrote the manuscript. ÖK performed the immunohistochemistry and motor activity tests, HB performed the swimming exercise, SDY contributed the analysis at Neurolucida software. HRY contributed to the statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
This project is based upon the PhD thesis of Sinem Gergin. The authors declare that they have no conflict of interest.
Ethical approval
All experimental protocols were approved by Marmara University Animal Care and Use Committee (approval code: 075.2016.mar). The experiments were performed in compliance with the Turkish law on the use of animals in experiments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MOV 12991 KB)
Supplementary file2 (MOV 53941 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gergin, S., Kirazlı, Ö., Boracı, H. et al. The effects of regular swimming exercise and melatonin on the neurons localized in the striatum of hemiparkinsonian rats. Anat Sci Int 98, 204–219 (2023). https://doi.org/10.1007/s12565-022-00688-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-022-00688-1