Abstract
In Parkinson’s disease (PD), physical therapy is one of the mainstays of supportive treatment modalities. This study focused on the neuroprotective effect of regular exercise on striatal calretinin positive interneurons in a rat model of PD. 6-hydroxydopamine (6-OHDA) was injected unilaterally into the medial forebrain bundle of Wistar rats. 6-OHDA lesioned (Parkinsonian) and unlesioned (control) rats were divided into sedentary and exercise groups. Exercise groups had daily swimming sessions for 30 min for 6 weeks. After 6-OHDA injections, an apomorphine-induced rotation test was performed (0.05 mg/kg, subcutaneous) at the 3rd and 6th weeks. At the end of the 6th week, brains were removed following transcardiac perfusion. The brain sections were stained immunohistochemically for tyrosine hydroxylase and calretinin reactivity. The number of rotations was significantly lower in Parkinsonian exercise group compared to Parkinsonian sedentary group at the 6th week (p = 0.024) and there was significant difference between Parkisonian sedentary groups at the 3rd and 6th weeks (p < 0.002). The calretinin positive interneurons significantly increased in the Parkinsonian exercise group compared to Parkinsonian sedentary group (p = 0.0003) and control exercise group (p < 0.0001). To conclude, the swimming exercise led to a striking increment of calretinin positive interneurons in the striatum of Parkinsonian rat. These findings indicated that the neuroprotective mechanism of exercise increased the number of striatal calretinin positive interneurons that might generate new approaches for the mechanism of neuroprotection. We concluded that striatal calretinin positive interneurons have an important role in the neuroprotective mechanisms of exercise in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative movement disorder. The approaches for the treatment focused on the symptoms due to the fact that there is no cure for the disease (Balestrino and Schapira 2020). A variety of treatment modalities are being in use, including drug therapy, deep brain stimulation and supportive treatments. The physical exercise is the clinically approved component of these supportive treatments. The efficacy of physical exercise, especially on motor functions, is variable and based upon the frequency, intensity, time and type of exercise (Ammann et al. 2014).
By demonstrating an improved physical capacity and increased oxidative capacity of the major muscle groups with no sign of stress, swimming exercise is accepted as a well-liked treatment of PD among experiments in recent years (Haobam et al. 2005; Contarteze et al. 2008). The swimming is a natural ability of rats, and therefore, it is also preferred in experimental rat models (Souza et al. 2009).
Several studies in PD animal models claim neuroprotective effects of exercise on dopaminergic neurons and increase in neurotrophic factors (Garza et al. 2004; Neeper et al. 1995; Gomez-Pinilla et al. 1998). It is mentioned that treadmill exercise increases cell proliferation as well as receptors for neurotrophic factors BDNF (brain derived neurotrophic factor) and GDNF (glial cell line-derived neurotrophic factor) in the striatum after brain damage (Yi et al. 2009). Both vigorous and voluntary exercises lead to an increase in neurotrophic factors. These neurotrophic factors can pass through the blood–brain barrier, activating the cAMP response element binding protein (CREB) and synapsin 1 (Tajiri et al. 2010). The neurotrophic factors bind to tyrosine kinase to regulate CREB, a cellular transcription factor that activates the transcription of tyrosine hydroxylase (TH), leading to increased synthesis activity of dopaminergic neurons.
The activity of striatal neurons is affected by the degeneration of the dopaminergic neurons. The dopaminergic nerve fibers are essential for the regulation of axon terminals and neurons in the striatum (Nicola et al. 2000). Recently, the rise in intracellular calcium concentration was highlighted to be the reason for the cell death and degeneration of the dopaminergic neurons in PD. The abnormalities in calcium levels may trigger injurious effects, proteases and endonucleases. The regulation of the calcium concentration may be achieved by intracellular calcium binding proteins like calbindin, calretinin and parvalbumin (Heizmann et al. 1990).
Numerous studies focus on the dopaminergic neurons expressing calcium-binding proteins in the nigrostriatal pathway. The calcium binding proteins have essential roles in intracellular calcium buffering and in shaping the synaptic responses of the neurons (Schmidt 2012). The literature indicates that the modification of these calcium-binding proteins may be achieved by the action of BDNF. In view of the neurotrophic effects of BDNF, the variable changes in the calcium binding protein expressions may be related with the neuroprotection mechanism of the neurons. Furthermore, calbindin and calretinin are more closely associated with the neuroprotective mechanism and correlated positively with resistance to neurodegeneration. Following the exposure to acute injuries and neurotoxins, the expression of calcium binding proteins may increase or the capacity of the calcium binding protein expressions of the neurons may change as a protective stance (Fairless et al. 2019).
The rat striatum comprises 90–95% spiny projection neurons and 5–10% aspiny interneurons (Graveland and DiFiglia 1985). The striatum consists of two compartments called the striosome (patch) and matrix, due to the various associations of afferent pathways, neuropeptides and receptors (Crittenden and Graybiel 2011; Drago et al. 1996; Gerfen 1985). Projections from the striatal patches (dorsal striatum) arise to the area of the cell bodies and proximal dendrites of the neurons located in the substantia nigra pars compacta (Gerfen 1985). The interneurons in rat striatum are classified into four subclasses according to their marker protein expression patterns: choline acetyltransferase (ChAT) + , calretinin + , neuropeptide Y + , and PV + . Although, there are fewer interneurons than neurons in the striatum, they are essential regarding motor functions and cognition (Ma et al. 2014a). The striatal interneurons have crucial functions in the circuit of basal ganglia. The fast spiking GABAergic parvalbumin positive interneurons maintain the cortical influences to medium spiny neurons. Moreover, calretinin positive interneurons exert monosynaptic inhibition on medium spiny neurons which can delay or block spiking. Striatal interneurons regulate the response of medium spiny neurons to cortical input. The powerful GABAergic modulation of spike timing in the medium spiny neurons is mostly affected by the GABAergic interneurons. (Tepper 2004).
In the human striatum, three subgroups of calretinin positive aspiny interneurons were classified according to their soma size (large, medium, and small). It was shown that the small aspiny interneurons were denser in the sensorimotor and limbic regions. Medium and large aspiny interneurons were dispersed homogeneously throughout the striatum. Calretinin positive interneurons were found in high numbers in the associative territories (caudate nucleus and precommissural putamen).
Injection of 6-OHDA into the medial forebrain bundle leads to degeneration of dopaminergic neurons and affects motor and cognitive functions of the animals. Interneuron lesions are also responsible for detoriation of motor and cognitive functions. There are controversial results in the literature. A study put forward that the number of calretinin positive interneurons decreased (Ma et al. 2014a, b) and another study presented that the dopaminergic neurons expressing calretinin were mostly intact after the 6-OHDA lesion (Tsuboi et al. 2000). An experimental study investigating the effects of exercise on calretinin positive interneurons in the dentate gyrus of diabetic rat models showed an increase in the number of the calretinin positive interneurons (Nam et al. 2011).
In the current study, we would like to enlighten the effect of regular swimming exercise (30 min/5 days/6 weeks) on number of striatal calretinin positive interneurons in 6-OHDA rat model, since the striatal interneurons may be responsible for the motor recovery.
Materials and methods
Male Wistar albino rats (300–350 g, 10 weeks, n = 21) were used in the experiments. The animals were supplied by the Marmara University Animal Center and caged individually at 21 °C on a 12-h light/dark cycle (lights out from 08:00 to 20:00) with unlimited food and water access. All experiments were carried out with the approval of Marmara University Ethical Committee for Experimental Animals (No: 013.2016.mar).
6-OHDA (Sigma H116, St Louis, MO, USA; dissolved in saline % 0.9 NaCl) was used to PD rat model. To achieve extensive degeneration in dopaminergic neurons, animals had two unilateral injections of 6-OHDA (8 μg/4 μl per site) into the medial forebrain bundle along the nigrostriatal pathway using stereotaxic method.
The bregma was used as the reference point and the coordinates of the lesions were anteroposterior: − 2.1 mm, lateral: 2.0 mm, ventral: − 7.8 mm for the first injection and anteroposterior: − 4.3, lateral: 1.5, ventral: − 7.8 for the second injection (Paxinos and Watson 2007).
Following the stereotaxic surgery, control group and 6-OHDA lesioned (Parkinsonian) rats were further divided into sedentary and exercise groups:
-
(1)
Sedentary group without lesion: Control sedentary group (C-Sed, n = 4),
-
(2)
Exercise group without lesion: Control exercise group (C-Exc, n = 5),
-
(3)
Sedentary group with lesion-Parkinsonian sedentary group (P-Sed, n = 6),
-
(4)
Exercise group with lesion-Parkinsonian exercise group (P-Exc, n = 6),
One week after the stereotaxic surgery, the P-Exc and C-Exc groups were subjected to daily regular swimming exercise of 30 min for 5 consecutive days a week, for 6 weeks. Firstly, the rats were adapted to exercise by 10 min swimming session daily, for 2 weeks. Then, the rats were allowed to swim for 30 min for 6 weeks. All animals were able to swim and they did not stop swimming during the session but they were slower towards to the end of the exercise. The swimming sessions were performed in a pool (100 cm × 50 cm × 50 cm) filled with lukewarm water (32 °C ± 2 °C) to a depth of 35 cm. The intensity of the exercise protocol was considered as moderate exercise according to World Health Organization (WHO) guidelines (Chen and Chang 2010). Sedentary groups (P-Sed and C-Sed) were kept in their cage during 6 weeks.
To measure the extent of motor impairment induced by the lesion rotation test was held on the 21st day (3rd week) and at the 6th week following 6-OHDA injections (Fig. 1) The rotational behavior was induced by subcutaneous apomorphine injection (0. 05 mg/kg) and evaluated visually by counting the number of contralateral and ipsilateral rotations to the injection side for 30 min. The contralateral rotations were noted and statistically analyzed.
Following termination of the exercise program, the animals were deeply anesthetized with ketamine (100 mg/kg, intraperitoneally), transcardially perfused with 50 mM phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) in 100 mM phosphate buffer at pH 7.4. The brain was removed, post fixed in the fixative overnight, and transferred into a 30% sucrose solution for cryoprotection.
The anterior and posterior coordinates of the striatum were marked on each brain according to Paxinos and Watson Rat Brain Atlas (bregma, 2.52 mm and − 3.24 mm).
For immunohistochemistry investigations, serial coronal brain sections (40 µm) were taken in the anteroposterior direction using a sliding microtome. One of three sections was taken for TH and calretinin immunohistochemistry procedure, respectively. Thirty-six sections were obtained from each rat brain.
For TH immunohistochemistry, the sections were rinsed in PBS three times for 5 min and incubated in 80 ml methanol and 3% H2O2 for 20 min (at room temperature) to block endogenous peroxidase activity. After rinsing in PBS, the sections were incubated in blocking serum solution (10 ml PBS, 1 ml normal goat serum, 0.1 g bovin serum albumin (BSA) and 30 µl Triton™ X-100 (Merck, Darmstadt, Germany) for 1 h, followed by incubation in primary antibody (anti-tyrosine hydroxylase antibody (Pel Freez/cat no: P40101) in 1:500 10 ml carrier solution) for overnight at room temperature. After rinsing period, sections were incubated in 150 µl biotinylated anti-rabbit IgG secondary antibody for 2 h (Vector labs), then rinsed in PBS and subsequently incubated in avidin-biotinylated complex for 1 h at room temperature (Vector lab, ABC kit). Immunoreactivity was visualized by incubating the sections in a solution of 3,3′-diaminobenzidine (DAB) (10 ml PBS, 5 mg DAB, 100 µl 3% H2O2). The sections were mounted on gelatin-coated slides and examined under light microscope.
Calretinin immunohistochemistry was performed by rinsing the mounted sections three times in PBS and incubating overnight at room temperature in PBS containing 1:500 anti-calretinin antibody (Merck), 0.3 Triton™ X-100 (Merck), and 1% serum (Sigma-Aldrich). After PBS rinsing, the sections were incubated for 90 min in a secondary antibody solution of Alexa Fluor 488 donkey anti-mouse IgG (Invitrogen, Carlsbad, CA, USA). The sections were examined at × 40 magnification, under flourescence microscope. The interneurons were counted in each section (Paxinos and Watson 2007). The calretinin positive interneurons of all groups were homogenously distributed throughout the whole striatum. Four areas at × 40 magnification in the striatum were photographed in each section. A total number of calretinin positive interneurons in 36 sections were calculated for each animal to analyze statistically.
Calretinin positive interneuron diameter was calculated by randomly selecting twenty interneurons from each rat. The diameter measurements were realized at a final magnification of × 400 using Image J Software (NIH, Bethesda, MD, USA, https://imagej.net). Maximum and minimum diameters for each interneuron were measured and divided by two to obtain average diameter. The measurements were taken by an observer who was blinded to the groups. The number of rotations following apomorphin challenge test in Parkinsonian groups, the quantity and diameter of calretinin positive interneurons in both control and Parkinsonian groups were presented as mean ± standard error of mean (SEM). Rotation data of the 3rd week vs 6th week in P-Sed vs P-Exc groups and data of calretinin positive interneurons from Sedentary vs Exercise groups in Control vs Parkinsonian groups were analyzed by two-way ANOVA followed by post-hoc Tukey test in Graph-Pad PRISM 6.0 (GraphPad Software Inc., San Diego, CA, USA) program.
Results
The number of rotations of the P-Exc and P-Sed groups, TH immunoreactivity in the striatum and substantia nigra, interneuron diameters and the number of calretinin positive interneurons in the striatum of all groups were evaluated. Following 6-OHDA lesioning, rotational behavior in apomorphin challenge was significantly affected by time (F (1, 11) = 31.07; p = 0.002) and exercise (F (1, 11) = 20.23; p = 0.0009) in Parkinsonian groups. There was no interaction between time and exercise on rotational behavior (F (1, 11) = 1.96, p = 0.19). Briefly, the number of rotations showed a significant increase in the 6th week compared to the 3rd week in sedentary groups (p = 0.002), whereas there was not any significant increase in the P-Exc group (p = 0.058) (Fig. 2). Furthermore, swimming exercise significantly decreased the number of rotations in the P-Exc group when compared to P-Sed groups at both the 3rd (126.6 ± 16.2 vs 187 ± 6.5; p = 0.052) and 6th weeks (208 ± 5 vs 323 ± 7; p = 0.02) time points.
Rotation numbers of P-Exc and P-Sed groups. At the 3rd week, there was no significant difference (p = 0.05) between P-Exc and P-Sed groups. P-Sed showed a significant increase (p < 0.001) in the number of rotations between the 3rd week and 6th week. P-Exc group showed a significant difference (p = 0.01) compared to P-Sed group at the 6th week. The rotations of P-Exc group did not show any significant difference (p = 0.04) between the 3rd and 6th weeks
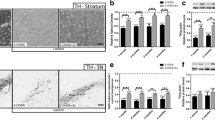
The calretinin positive interneurons in the striatum of all sedentary and exercise groups (Figs. 3, 4, 5, 6) were counted under fluorescent microscopy. The number of calretinin positive neurons (Fig. 7) was significantly affected by both 6-OHDA lesioning [F (1, 16) = 43.39, p < 0.0001] and exercise (F (1, 16) = 7.943, p = 0.01). There was a significant interaction between 6-OHDA lesioning and exercise (F (1, 16) = 26.03; p = 0.0001). The number of calretinin positive neurons in the striatum was 272.5 ± 26.2 in C-Sed group and 375.25 ± 27.3 in P-Sed group. Although, there were higher neurons in P-Sed group, this difference was not statistically significant (p > 0.05). The number of calretinin positive interneurons in exercise groups was 181 ± 8.7 in C-Exc group and 616.4 ± 43.1 in the P-Exc group. The increase in the number of calretinin interneurons in P-Exc group compared to C-Exc was strongly significant (p < 0.0001). Swimming exercise did not show any significant effect in the number of calretinin positive interneurons of the control groups (C-Sed vs C-Exc, p > 0.05) but significantly increased the number of calretinin positive interneurons in P-Exc group compared to P-Sed group (p = 0.0003).
Number of calretinin positive interneurons in P-Sed, P-Exc, C-Sed and C-Exc. There were higher number of interneurons in P-Sed group compared to C-Sed group, but this was not statistically significant (p > 0.05). The increase in the number of calretinin positive interneurons in P-Exc group compared to C-Exc was strongly significant (p < 0.0001). The number of calretinin positive interneurons in P-Exc group compared to P-Sed group was significantly increased (p = 0.0003). There was no significant difference in between C-Sed and C-Exc (p > 0.05)
The mean diameter of the calretinin positive interneurons did not show any significant difference between the groups. The mean diameter of the interneurons in the C-Sed and C-Exc groups was 11.3 ± 0.20 and 11 ± 0.10 µm. The mean diameter of the calretinin positive interneurons located in the striatum of the P-Sed and P-Exc groups was 11.70 ± 0.69 µm and 10.76 ± 0.35 µm, respectively.
TH immunoreactivity was examined under light microscopy to verify the effect of 6-OHDA injections and 6 weeks of exercise. 6-OHDA injections decreased the TH immunostaining of striatum and substantia nigra in the lesioned sides of both P-Sed and P-Exc groups compared to unlesioned sides. However, P-Sed and P-Exc groups showed similar labelling of TH in lesioned sides (Figs. 8, 9).
Discussion
Exercise is an indispensable component of the neurologic rehabilitation for PD in physiotherapy. In this study, the neuroprotective role of swimming exercise on calretinin positive interneurons in the striatum of 6-OHDA lesioned Parkinsonian rats was investigated. A moderate swimming exercise program was held for 6 weeks for both control and 6-OHDA lesioned animals. Parkinsonian groups, sedentary and exercise, were challenged with apomorphine at the 3rd and 6th weeks of exercise program. The results of the apomorphine-induced rotation test (on the 3rd and 6th weeks after 6-OHDA injection) revealed a decrease in rotations in the exercise group, supporting the hypothesis for a positive neuroprotective effect of exercise on PD (Aguiar et al. 2006).
Furthermore, P-Exc group showed a significant increase in the number of calretinin positive interneurons in the striatum compared to the P-Sed and C-Exc groups. The P-Sed group had a slight increase in the number of calretinin positive interneurons, whereas C-Exc group had a slight decrease. These findings suggested that 6-OHDA injection caused an increase in the number of calretinin positive interneurons and more prominently the swimming exercise had a positive effect on the number of striatal calretinin positive interneurons in Parkinsonian rats.
Acute injuries such as axotomy, head trauma or exposure to neurotoxins such as MPTP caused an increase in calcium binding protein expression (Lowenstein et al. 1994; Ng et al. 1996). The argument was based on that if neuronal death did not occur as a result of a disease or an insult, neuron had capacity to express more calcium binding proteins as a protective mechanism (Drago et al. 1996; Fairless et al. 2019). The increase in calretinin positive interneurons in P-Exc group may be related with a compensatory process in pathological circumstances. As it is considered a protective mechanism, we did not expect an increase of calretinin positive interneurons in both of the control groups. These findings made us to consider that the presence of calretinin can be attributed to the neuroprotective effect.
Katarzyna Billing-Marczak et al. (1999) mentioned that the calcium binding proteins may act in different ways. For example, some studies suggested that calretinin acts as a calcium modulator due to its limited calcium binding capacity. Moreover, calretinin may show cooperativity in calcium binding between the different EF-hand domains, with an increasing calcium binding affinity as the calcium concentration increases. The calcium binding proteins are classified due to the resistance/susceptibility to the degeneration (Billing-Marczak and Kuznicki 1999). The calretinin and calbindin are more closely associated with resistance (Fairless et al. 2019). Accordingly, this study investigated the change in striatal calretinin positive interneurons as a neuroprotective mechanism following the 6-OHDA lesion.
The literature debates that the expression of calcium binding proteins like calbindin and calretinin may have essential roles on neuroprotective mechanism for the striatal neurons. Our study showed that 6-OHDA lesion lead to increment of calretinin positive interneurons in the striatum, at post-lesion 6th week, similar to previous findings (Mura et al. 2000). Authors suggested that dopamine depletion may lead to an increase in the expression of calretinin in striatal cells which was mostly apparent at post-lesion 3rd week and then decreased toward normal levels at the 6th, 10th, and 18th weeks following the 6-OHDA lesion. Mura et al.’s study emphasized that the increase in the 3rd week may be related to dopamine/glutamate balance, to a shift in the striatal excitatory/inhibitory balance toward excitation. This compensatory mechanism was proposed as an explanation for the increase in the number of calretinin positive interneurons. Our study also suggested a striking increase in the calretinin positive interneurons in the P-Exc group compared to C-Exc group. That significant result is another finding of our study which may indicate that the apparent difference in the number of the calretinin positive interneurons may be related to the 6-OHDA lesion. This increase in calretinin expression was most likely due to pathologic processes mentioned before. In the study of Mura et al (2000) expression of calretinin in striatal cells returned to normal levels at the 6th, 10th, and 18th weeks following the 6-OHDA lesion. However, in our study the increase in the calretinin positive interneurons continued through the 6th week following the lesion in the P-Exc group. This sustained increase in the number of calretinin positive interneurons in the P-Exc group can be attributed to the effect of exercise, although the mechanism of it needs further investigation.
On the other hand, a reduction in striatal calretinin positive interneurons was observed after the 6-OHDA lesion (Ma et al. 2014b). The calcium-binding protein calretinin influences the maintenance of intracellular calcium homeostasis. Calretinin probably provides protection against the massive calcium entry which may result from over-stimulation of glutamate receptors. Thus, Ma et al. (2014a, b) proposed that a reduction of calretinin positive interneurons in striatum may cause a decreased protection against to excitotoxicity following dopaminergic depletion (Ma et al. 2014b).
Several studies showed that 6-OHDA injection into the striatum led to a severe decline in TH + neurons and a decrease in calretinin positive neurons (Tsuboi et al. 2000) (Xenias et al. 2015). The dopaminergic neurons expressing calretinin are mostly found in the ventral tegmental area and substantia nigra pars compacta (Mattson 2007; Tsuboi et al. 2000; Bezprozvanny 2009). The dopaminergic and noradrenergic neurons in the midbrain are essential for motor systems and defensive behaviors against stress factors and show some differences in the expression of calcium-binding proteins. The results addressed the neuroprotective influence of calretinin, based upon the finding that the remaining axons of dopaminergic neurons were expressing calretinin (Tsuboi et al. 2000).
In accordance with these studies, the increase in the number of calretinin positive interneurons in our study was probably due to a neuroprotective mechanism provided by exercise. Several studies have focused on the relationship between the frequency and duration of exercise (Jang et al. 2017; Chuang et al. 2017). In rotenone-induced Parkinsonian rats, treadmill-running exercise for fourteen consecutive days induced an amelioration in nigrostriatal dopaminergic loss of neurons, fibers, and motor functions (Shi et al. 2017). Another study discovered that treadmill exercise exhibited a neuroprotective effect on the degeneration of the nigrostriatal dopaminergic neurons (Shin et al. 2017). Vigorous exercises, particularly for the lower extremities, are more effective than voluntary exercise in enhancing the secretion of neurotrophic factors. Neurotrophic factors are regulated by exercise and can act as growth factors for the continuation of surviving neuronal populations. Neurotrophic factors, such as BDNF, GDNF, IGF (Insulin-like growth factor) and VEGF(Vascular Endothelial Growth Factor), play an important role in neuronal viability, key for the treatment of PD (Alberts et al. 2016, Tuszynski 2002). TH gene transcription can increase the activation of BDNF and GDNF enzymes, which further activate TH. The modifications of the calretinin and calbindin may be regulated by the action of BDNF. In our study, effect of regular swimming exercise on the number of calretinin positive interneurons may be due to the regulation of the neurotrophic factors like BDNF (Fairless et al. 2019).
In another research, it was determined that 4 weeks exercise prior to a lipopolysaccharide injection was effective in preventing a decrease in BDNF in the nigrostriatal pathway, but 2- or 3-week exercise after the injection was insufficient to prevent the loss of dopaminergic neurons (Wu et al. 2011). There is limited information regarding the efficiency of neurotrophic factors on TH regulation in the nigrostriatal pathway after exercise. Nevertheless, it could be claimed that exercise is effective on the motor symptoms and leads to the regulation of neurotrophic factors that regulate the viability of dopaminergic neurons (da Silva et al. 2016).
Moreover, exercise training increases the concentration of insulin and IGF-1. However, the exercise decreases the concentrations of tau and amyloid precursor protein (APP) in diabetic rats (Diegues et al. 2014).
In this way, a study was focused on the effect of the exercise on the expression of the proteins involved in the insulin/IGF-1 pathway. They found out that the exercise training protocol was not able to reduce the phosphorylation of tau protein and APP (Borges et al. 2017). Recently, a study focused on the effect of mild and progressive intensity treadmill running protocols on Parkinsonian rats. The procedure was applied for 4 weeks and the results revealed that progressive exercise intensity (initiating by 20 min, then 50 min and 60 min) had greater neuroprotective effects against 6-OHDA lesion. Progressive intensity treadmill notably reduced rotational behavior of rats. This type of exercise enhanced the levels of cerebral dopamine neurotrophic factor (CDNF) and mesencephalic astrocyte-derived neurotrophic factor (MANF) in the striatum (Fallah Mohammadi et al. 2019). Our results also suggest that the swimming exercise has a neuroprotective effect on PD by inducing a decrease in the number of rotations and an increase in calretinin positive interneurons in the striatum.
Acute L-dopa treatment prevented the increase of striatal calretinin positive interneurons in the ipsilateral side with unilateral lesion (Mura et al. 2000). In contrast with this result, our study evokes that the swimming exercise treatment induces an increase in the striatal calretinin positive interneurons.
In a study, it was emphasized that calretinin was expressed by medium-sized (7–20 lm), aspiny, interneurons with a diameter of 12–20 µm but further studies showed that calretinin positive interneurons were aspiny, and these interneurons were relatively sparse in the striatum (Bennett and Bolam 1993; Wu and Parent 2000). The ratio of calretinin positive interneurons in the ventromedial region of striatum is greater than in the dorsolateral region of striatum (Ma et al. 2014b). The calretinin positive interneurons were found mostly in dorsomedial striatum and had medium-sized soma (10–15 µm in diameter) in rats (Reiner et al. 1995). Moreover, Rymar et al. (2004) examined the calretinin positive interneurons in the striatum of Sprague–Dawley rat brains (Rymar et al. 2004). They found out that cell bodies of calretinin positive interneurons were mainly medium sized and round, oval, or fusiform. Calretinin positive interneurons in the rat striatum were categorized as small (≤ 7 µm), medium-sized (7.1–20 µm), and large (20 µm) (Kawaguchi et al. 1995). Medium-sized cells (7.1–20 µm) constitute 91% of neurons of these three groups. In our study, there was no difference in the diameter of calretinin positive interneurons localized in the lesioned side of the rat striatum of P-Sed and P-Exc groups. The diameter of the calretinin positive interneurons in C-Sed and C-Ex groups were about 11.3 ± 0.20 µm and 11 ± 0.10 µm and the mean diameter of P-Sed and P-Exc groups were 11.70 and 10.76 µm, respectively. These results support the literature which highlights that calretinin positive interneurons are mostly medium sized in the rat striatum. The swimming exercise for 6 weeks did not display any difference in TH + neurons in the lesioned side of the rat brains. Further studies are needed to elucidate the mechanism of action of vigorous and chronic exercise on the dopaminergic neurons and calretinin positive interneurons located in the striatum.
Due the neuroprotective effect of the calretinin, we searched for the role of the striatal calretinin positive interneurons in the 6-OHDA lesioned rats. The striatal interneurons are essential for coordinating and regulating network function. Electrophysiological studies showed that most striatal GABAergic interneurons are classified as FSIs and persistent and low-threshold spike interneurons (PLTSs). Neurons are classified physiologically as PLTSs may also include several subtypes of GABAergic interneurons. The calretinin-expressing interneurons are less in number in rodents compared to primates and their electrophysiological properties are not well described. Under normal conditions, PLTS interneurons make sparse, weak inhibitory projections onto spiny projection neurons but in Parkinson; large, rhythmic inhibitory inputs project onto spiny projection neurons (Gittis et al. 2010).
In conclusion, this study suggests that regular exercise increases calretinin positive interneurons in the striatum of Parkinsonian rats and decreases rotation severity. We conclude that calretinin is closely involved in the neuroprotective effect of exercise on the striatal interneurons of Parkinsonian rats.
References
Aguiar LM, Nobre HV Jr, Macedo DS, Oliveira AA, Freitas RM, Vasconcelos SM, Cunha GM, Sousa FC, Viana GS (2006) Neuroprotective effects of caffeine in the model of 6-hydroxydopamine lesion in rats. Pharmacol Biochem Behav 84:415–419
Alberts JL, Phillips M, Lowe MJ, Frankemolle A, Thota A, Beall EB, Feldman M, Ahmed A, Ridgel AL (2016) Cortical and motor responses to acute forced exercise in Parkinson's disease. Parkinsonism Relat Disord 24:56–62
Ammann BC, Knols RH, Baschung P, de Bie RA, de Bruin ED (2014) Application of principles of exercise training in sub-acute and chronic stroke survivors: a systematic review. BMC Neurol 14:167
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27:27–42
Bennett BD, Bolam JP (1993) Characterization of calretinin-immunoreactive structures in the striatum of the rat. Brain Res 609:137–148
Bezprozvanny I (2009) Calcium signaling and neurodegenerative diseases. Trends Mol Med 15:89–100
Billing-Marczak K, Kuznicki J (1999) Calretinin–sensor or buffer–function still unclear. Pol J Pharmacol 51:173–178
Borges ME, Ribeiro AM, Pauli JR, Arantes LM, Luciano E, de Moura LP, de Almeida Leme JA, Medeiros A, Bertolini NO, Sibuya CY, Gomes RJ (2017) Cerebellar Insulin/IGF-1 signaling in diabetic rats: effects of exercise training. Neurosci Lett 639:157–161
Chen W, Chang MH (2010) New growth charts for Taiwanese children and adolescents based on World Health Organization standards and health-related physical fitness. Pediatr Neonatol 51:69–79
Chuang CS, Chang JC, Cheng FC, Liu KH, Su HL, Liu CS (2017) Modulation of mitochondrial dynamics by treadmill training to improve gait and mitochondrial deficiency in a rat model of Parkinson's disease. Life Sci 191:236–244
Contarteze RV, Manchado Fde B, Gobatto CA, de Mello MA (2008) Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol 151:415–422
Crittenden JR, Graybiel AM (2011) Basal Ganglia disorders associated with imbalances in the striatal striosome and matrix compartments. Front Neuroanat 5:59
Diegues JC, Pauli JR, Luciano E, de Almeida Leme JA, de Moura LP, Dalia RA, de Araujo MB, Sibuya CY, de Mello MA, Gomes RJ (2014) Spatial memory in sedentary and trained diabetic rats: molecular mechanisms. Hippocampus 24:703–711
Drago J, Gerfen CR, Westphal H, Steiner H (1996) D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience 74:813–823
Fairless R, Williams SK, Diem R (2019) Calcium-binding proteins as determinants of central nervous system neuronal vulnerability to disease. Int J Mol Sci 20:3–7
Fallah Mohammadi Z, Falah Mohammadi H, Patel DI (2019) Comparing the effects of progressive and mild intensity treadmill running protocols on neuroprotection of parkinsonian rats. Life Sci 229:219–224
Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA (2004) Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav 77:209–220
Gerfen CR (1985) The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol 236:454–476
Gittis AH et al (2010) Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci 30:2223–2234 (PubMed: 20147549)
Gomez-Pinilla F, So V, Kesslak JP (1998) Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience 85:53–61
Graveland GA, Difiglia M (1985) The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res 327:307–311
Haobam R, Sindhu KM, Chandra G, Mohanakumar KP (2005) Swim-test as a function of motor impairment in MPTP model of Parkinson's disease: a comparative study in two mouse strains. Behav Brain Res 163:159–167
Heizmann CW, Rohrenbeck J, Kamphuis W (1990) Parvalbumin, molecular and functional aspects. Adv Exp Med Biol 269:57–66
Jang Y, Koo JH, Kwon I, Kang EB, Um HS, Soya H, Lee Y, Cho JY (2017) Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson's disease mice. Brain Res 1655:186–193
Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC (1995) Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18:527–535
Lowenstein DH, Gwinn RP, Seren MS, Simon RP, McIntosh TK (1994) Increased expression of mRNA encoding calbindin-D28K, the glucose-regulated proteins, or the 72 kDa heat-shock protein in three models of acute CNS injury. Brain Res Mol Brain Res 22:299–308
Ma Y, Feng Q, Ouyang L, Mu S, Liu B, Li Y, Chen S, Lei W (2014a) Morphological diversity of GABAergic and cholinergic interneurons in the striatal dorsolateral and ventromedial regions of rats. Cell Mol Neurobiol 34:351–359
Ma Y, Zhan M, Ouyang L, Li Y, Chen S, Wu J, Chen J, Luo C, Lei W (2014b) The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav Brain Res 266:37–45
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6:337–350
Mura A, Feldon J, Mintz M (2000) The expression of the calcium binding protein calretinin in the rat striatum: effects of dopamine depletion and L-DOPA treatment. Exp Neurol 164:322–332
Nam SM, Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK (2011) Differential effects of treadmill exercise on calretinin immunoreactivity in type 2 diabetic rats in early and chronic diabetic stages. J Vet Med Sci 73:1037–1042
Neeper SA, Gomez-Pinilla F, Choi J, Cotman C (1995) Exercise and brain neurotrophins. Nature 373:109
Ng MC, Iacopino AM, Quintero EM, Marches F, Sonsalla PK, Liang CL, Speciale SG, German DC (1996) The neurotoxin MPTP increases calbindin-D28k levels in mouse midbrain dopaminergic neurons. Brain Res Mol Brain Res 36:329–336
Nicola SM, Surmeier J, Malenka RC (2000) Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23:185–215
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn
Reiner A, Medina L, Figueredo-Cardenas G, Anfinson S (1995) Brainstem motoneuron pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS. Exp Neurol 131:239–250
Rymar VV, Sasseville R, Luk KC, Sadikot AF (2004) Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol 469:325–339
Schmidt H (2012) Three functional facets of calbindin D-28k. Front Mol Neurosci 5:25
Shi K, Liu X, Qiao D, Hou L (2017) Effects of treadmill exercise on spontaneous firing activities of striatal neurons in a rat model of Parkinson's disease. Mot Control 21:58–71
Shin MS, Kim TW, Lee JM, Ji ES, Lim BV (2017) Treadmill exercise alleviates nigrostriatal dopaminergic loss of neurons and fibers in rotenone-induced Parkinson rats. J Exerc Rehabil 13:30–35
da Silva PG, Domingues DD, de Carvalho LA, Allodi S, Correa CL (2016) Neurotrophic factors in Parkinson's disease are regulated by exercise: evidence-based practice. J Neurol Sci 363:5–15
Souza MA, Oliveira MS, Furian AF, Rambo LM, Ribeiro LR, Lima FD, Dalla Corte LC, Silva LF, Retamoso LT, Dalla Corte CL, Puntel GO, de Avila DS, Soares FA, Fighera MR, de Mello CF, Royes LF (2009) Swimming training prevents pentylenetetrazol-induced inhibition of Na+, K+-ATPase activity, seizures, and oxidative stress. Epilepsia 50:811–823
Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I (2010) Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res 1310:200–207
Tepper JM, Bolam JP (2004) Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 14:685–692
Tsuboi K, Kimber TA, Shults CW (2000) Calretinin-containing axons and neurons are resistant to an intrastriatal 6-hydroxydopamine lesion. Brain Res 866:55–64
Tuszynski MH (2002) Growth-factor gene therapy for neurodegenerative disorders. Lancet Neurol 1:51–57
Wu Y, Parent A (2000) Striatal interneurons expressing calretinin, parvalbumin or NADPH-diaphorase: a comparative study in the rat, monkey and human. Brain Res 863:182–191
Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, Wu CW, Kuo YM (2011) Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun 25:135–146
Xenias HS, Ibanez-Sandoval O, Koos T, Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci 35:6584–6599
Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, Lee IS, Won MH, Seong JK, Yoon YS (2009) Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res 34:1039–1046
Acknowledgements
This manuscript is produced from master’s thesis of Hatice Boracı entitled with “The neuroprotective effect of regular swimming exercise on the dopaminergic neurons localized in the striatum of Parkinsonian rats”. This work was funded by Marmara University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boracı, H., Kirazlı, Ö., Gülhan, R. et al. Neuroprotective effect of regular swimming exercise on calretinin-positive striatal neurons of Parkinsonian rats. Anat Sci Int 95, 429–439 (2020). https://doi.org/10.1007/s12565-020-00538-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-020-00538-y