Abstract
The embryonic developmental response of two abalone species (disk abalone Haliotis discus discus, giant abalone Haliotis gigantea) to a drop in salinity with different exposure times was investigated to gain a better understanding of the reasons for the decrease in natural stock populations. Two experimental designs—first, combinations of two salinities (34 and 17 psu) and four exposure times (0, 1, 2 and 4 h), and second, combinations of three salinities (34, 24 and 14 psu) and four exposure times (0, 1, 3 and 6 h)—were tested on disk and giant abalone eggs to determine the effects on hatching onset time, hatching success, percentage of abnormality and survival rate. Hatching onset time increased significantly for both species as salinity dropped and exposure time increased. Both species followed a decreasing trend in terms of hatching success as exposure time increased at low salinity levels. As for abnormality, both species showed a significant negative effect of low salinity and long exposure times. Giant abalone showed better adaptability to long exposure time at low salinity levels, and hence a higher survival rate, than disk abalone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Torrential rainfall is one of the main external factors affecting coastal marine species during spawning seasons. Heavy rain over a certain period may influence embryonic development of coastal marine organisms. The earliest life stages are the most sensitive in the life cycle of a bivalve, and as the larva develops into a benthic juvenile, its tolerance towards various environmental conditions increases (Bayne et al. 1976). This is true not only for bivalves, but for any species in their early stages, when they need the proper care and environmental conditions in which to develop. In a review article by Przeslawski (2004), it was noted that very few studies have considered more than one environmental factor at a time, thus leaving a fundamental gap in our understanding of how independent environmental factors influence embryonic development in gastropod egg masses.
Disk abalone H. discus discus (kuro awabi), giant abalone H. gigantea (megai awabi) and Japanese abalone H. discus hannai (ezo awabi) are the three most commonly consumed abalones in Japan. There has been a dramatic decline in abalone fishing all over the world. Globally, legal landings from abalone fisheries dropped from 20,000 mt in the 1970s to 6500 mt in 2015 (Cook 2016). In Japan, Oba (2000) reported that nationwide catches of abalone plunged from 6466 tons in 1970 to roughly 2000 tons three decades later. Abalone wild stock populations in Japan have been declining over recent decades, and there are many theories as to why this is happening. Overharvesting is commonly cited as the major reason for the wild stock population decline. To help tackle this problem, the Japanese government introduced a marine stock enhancement program in 1963 (Masuda and Tsukamoto 1998; Kitada 1999; Kitada and Kishino 2006), and abalone was one of the first targets to help restore the wild stock population (Inoue 1976). Although the Japanese large-scale stock enhancement program was expected to sustain the fishery production of abalone, it was reported that stocking cultured juveniles did not contribute to the enhancement of wild abalone stocks (Seki and Sano 1998; Seki and Taniguchi 2000). Scientists have thus hypothesized that there may be other factors that play a larger role in the reduced abalone wild stocks, such as temperature, salinity, turbidity or pH.
In addition, the main spawning season for H. discus hannai in Japan is from August to October, and for H. discus discus and H. gigantea, spawning generally extends from October to January, when seawater temperatures increase or decrease to 20 °C (Sasaki 2005). Among these three species, several studies on H. discus hannai have been carried out, whereas H. discus discus and H. gigantea are poorly studied. Previous field surveys of H. discus hannai reported that 0-year-old juveniles disappeared when seawater temperatures from February to March decreased to less than 5–8 °C in coastal waters of Iwate Prefecture (Saido 2002; Musashi 2006). Since H. discus discus and H. gigantea are found in the southern parts of Honshu, and H. discus hannai is distributed along the Pacific coast in northern Honshu, studies on H. discus discus and H. gigantea are needed to properly understand them. Because H. discus discus and H. gigantea do not spawn during the rainy seasons in Japan, recent unpredictable weather patterns caused by climate change have made investigations difficult, as these months can experience heavy rainfall as a result of delayed typhoons at the end of October to the middle of November (Japan Meteorological Agency 2018), which could be a reason for the high mortality rates, hence the purpose of the current study.
A few studies have reported on salinity tolerance in marine invertebrate species including Palaemon affinis (Kirkpatrick and Jones 1985), kuruma prawn Marsupenaeus japonicus (Charmantier-Daures et al. 1988), blue crab Callinectes similis (Guerin and Stickle 1997), green scallop Chlamys opercularis (Paul 1980), bay scallop Pecten maximus (Strand et al. 1993), mussel Mytilopsis leucophaeata (Verween et al. 2007), pearl oyster Pinctada imbricata (O'Connor and Lawler 2004) and Akoya pearl oyster Pinctada fucata (Arisman et al. 2018). Only three studies on salinity tolerance have been carried out in abalone species. The first, by Singhagraiwan et al. (1992) on donkey’s ear abalone Haliotis asinina, reported that H. asinina juveniles could tolerate salinity levels as low as 20.5 psu without acclimation and as low as 12.5 psu with acclimation (gradual decrease of 2.5 psu per day). Chen and Chen (2000) and Cheng et al. (2004) then studied salinity tolerance and immune response, respectively, in Haliotis diversicolor supertexta. Chen and Chen (2000) suggested that H. diversicolor supertexta juveniles maintained at 25 psu at 20 °C were able to survive salinity levels in the range of 17–37 psu, and those maintained in 35 psu at 20 °C survived salinities in the range of 20–45 psu, when exposed to gradually increasing and decreasing salinity. However, less is known of the effect of salinity drop and exposure time on the early-stage development of abalones.
Therefore, the objective of this study is to determine the combined effects of salinity change and exposure time on disk abalone and giant abalone eggs with regard to hatching onset time, hatching success rate, percentage of abnormality and survival rate.
Materials and methods
Egg collection and preparation
During the spawning season in November 2018, both disk and giant abalone eggs were obtained from the Mie Prefectural Sea Farming Center in Hamajima, Shima City, Mie Prefecture, Japan. Eggs were collected and transferred to Mie University in Styrofoam boxes with an ambient salinity of 34 psu. About 3 h after artificial insemination, eggs were provided for the following two experiments. Seawater at different salinities was prepared in the laboratory by diluting commercial sea-salt powder (LIVESea Salt, Delphis Co. Ltd., Japan) with distilled water, and then sterilized using an autoclave at 110 °C for 20 min.
Experiment 1
For each species, 12 replicates per treatment were prepared, and each replicate was stocked with 36 eggs that were transferred into six-well plastic microplates and incubated at a temperature of 20 ± 1 °C under white-light conditions and a 12:12 h light/dark cycle. The experiment was carried out over a period of 5 days. The effects of salinity change and exposure time on both species were tested by reducing the salinity from ambient (34 psu; control) to 17 psu at four different exposure times of 0 (control), 1, 2 and 4 h. Eggs were carefully counted and transferred to each well containing 5 ml of sterilized seawater. A gradual change and recovery of salinity of the solution was achieved using an automatic pipette at a rate of 1 ml each time until the desired salinity was achieved. It has been argued that results obtained from experimental methods with rapid salinity changes are not representative of the changes that occur in the natural environment, as they provide little opportunity for animals to acclimate (Davenport et al. 1975); hence this experiment focused on a gradual decrease and recovery of salinity. Salinity levels in the control group were kept constant at 34 psu, as they followed identical water exchange procedures using the same 34 psu medium. Hatching onset times were recorded at the time of hatching for each of the treatments. Hatching was determined when free-swimming viable larvae were visible in the plastic microplate wells. Hatching success rate and abnormality rate were recorded 24 h after hatching, and the survival rate was observed and recorded under a dissecting microscope (SZ61, Olympus, Japan) at day 5 post-hatch. Abnormal individuals were determined using a stereoscope (CKX53, Olympus, Co. Ltd, Japan), based on the external malformations that appeared mainly around the edge of larval shells in experimental groups with salinity change.
Experiment 2
Experiment 2 was conducted with six replicates per treatment, and each replicate was stocked with 90 eggs in six-well microplates under the same temperature and light conditions as in experiment 1. The experiment was carried out over a period of 5 days. The effects of salinity change and exposure time on both species were tested by reducing the salinity from 34, 24 and 14 psu with exposure times of 0, 1, 3 and 6 h. Egg transfer and gradual change and recovery of salinity were performed as described in experiment 1. Hatching onset time was recorded at the time of hatching for each of the treatments. Hatching success rate and abnormality rate were recorded 24 h after hatching, and the survival rate was recorded at day 5 post-hatch using the same criteria as in experiment 1.
Statistical analysis
Statistical analysis (SPSS version 16, SPSS Inc., Chicago, IL, USA) of data was carried out and results presented as mean ± standard error (SE). Data were subjected to one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (HSD) post hoc multiple range test to estimate the differences between treatments. An independent-samples t test was used to determine any differences between species within treatments. In experiment 2, two-way ANOVA was performed to determine whether salinity or exposure time, or a combination of both, had any effects on either species. The level of significant difference was set at p < 0.05.
Results
Hatching onset time
In experiment 1, there were significant differences in hatching onset time among treatments in both species (Table 1a). The control group and disk abalone group with 1 h treatment demonstrated a significantly longer time to onset of hatching than that of giant abalone.
For experiment 2, the treatments were more precisely conducted in terms of salinity drop and exposure time in order to determine whether salinity drop or exposure time made any difference in hatching onset time. As salinity dropped and exposure time increased, the time to onset of hatching also increased significantly within treatments (Table 1b). Significant differences in hatching onset time between the two species were observed with the control, 24 psu 1 h, 14 psu 1 h, and 24 psu 3 h treatments. With treatment of 14 psu 6 h, both species demonstrated a low hatching rate, so no data were recorded for hatching onset time.
Hatching success rate
With regard to hatching success in experiment 1, both species followed a decreasing trend as exposure time increased (Fig. 1a). Among treatments, the giant abalone control group demonstrated significantly higher hatching success than those treated for 2 and 4 h, whereas for disk abalone, the hatching success rates for the control 0, 1 and 2 h treatments were significantly different from the rate with 4 h treatment. With the 4 h treatment, giant abalone demonstrated a significantly higher hatching success rate than disk abalone, 56% and 24%, respectively (p < 0.05).
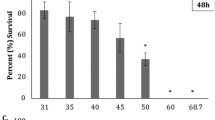
Hatching success rate 24 h post-hatch (a), percentage of abnormality 24 h post-hatch (b) and survival rate 5 days post-hatch (c) of disk abalone H. discus discus and giant abalone H. gigantea at a salinity level of 17 psu in experiment 1. Different letters indicate a significant difference between treatments, and asterisks (*) denote a significant difference between species in the same treatment (p < 0.05)
In experiment 2, the hatching success rate within the same exposure time with different salinities, e.g., 24 psu 1 h and 14 psu 1 h treatments, showed a decreasing trend which was almost significant in all groups (Fig. 2a). There were no significant differences between abalone species in hatching success rates among all treatments except the 14 psu 1 h treatment. In the 14 psu 6 h treatment, the hatching success rate was considered to be low, as no disk abalone eggs hatched and only 3% of giant abalone eggs hatched. Two-way ANOVA indicated that salinity and exposure time had a significant interaction effect on the hatching success rate of disk abalone [F (2, 35) = 21.74, p < 0.05] and giant abalone [F (2, 35) = 26.90, p < 0.05].
Hatching success rate 24 h post-hatch (a), percentage of abnormality 24 h post-hatch (b) and survival rate 5 days post-hatch (c) of disk abalone H. discus discus and giant abalone H. gigantea in experiment 2. Different letters indicate a significant difference between treatments, and asterisks (*) denote a significant difference between species in the same treatment (p < 0.05)
Abnormality
With regard to percentage of abnormality in experiment 1, both species showed an increasing trend as exposure time increased at 17 psu salinity (Fig. 1b). The percentage of abnormality in experiment 2 was at its highest for most of the treatments (Fig. 2b). There were significant differences between the control group, 24 psu 1 h treatments and most of the other treatments. According to Table 2, the results show that both salinity and exposure time had interaction effects on the percentage of abnormality of disk abalone [F (2, 35) = 60.78, p < 0.05] and giant abalone [F (2, 35) = 12.64, p < 0.05].
Survival rate
The survival rates in experiment 1 decreased for both species as exposure time increased. The survival rates of disk abalone were significantly lower than those of giant abalone for all treatments except at 2 h exposure (Fig. 1c). Furthermore, disk abalone recorded a 100% mortality at 4 h exposure time.
The percentage of survival in experiment 2 decreased as salinity levels were reduced and exposure time increased (Fig. 2c). Disk abalone recorded 100% mortality at 14 psu 3 h and 14 psu 6 h treatments. Results of two-way ANOVA in Table 2 indicate that exposure time did not have a significant effect on the survival rate of disk abalone [F (2, 35) = 3.11, p > 0.05], but salinity did have a significant effect on the survival rate [F (2, 35) = 5.05, p < 0.05]. No significant interaction effect was found between salinity and exposure time on the survival rate for disk abalone [F (2, 35) = 0.50, p > 005], but a significant interaction effect on survival was observed in giant abalone [F (2, 35) = 4.80, p < 0.05]. Thus, according to the results, in terms of survival, giant abalone proved to have significantly greater resistance to stressful salinity conditions than disk abalone.
Discussion
In marine coastal waters characterized by unstable hydrological regimes, invertebrate embryos and larvae are strongly affected by salinity decreases during summer (Kashenko 2000). This factor is crucial in the survival and development of the larvae of stenohaline species, including abalone. Results from this study help explain how rainfall (amount and period of downfall) during the spawning season can affect the early-life-stage development of disk and giant abalone embryos. In the current study, satisfactory development of disk abalone and giant abalone embryos and larvae occurred in conditions very close to those in which spawning is conducted (temperature of 20 ± 1 °C and salinity of 34 psu).
In this experiment, temperature was kept constant at 20 ± 1 °C to determine whether a drop in salinity at different exposure times had an effect on hatching. The results in experiment 1 shown in Table 1a indicate that the increase in exposure times (1, 2 and 4 h) at a low salinity level of 17 psu had a significant effect on the hatching onset time of both abalone species. With experiment 2 (Table 1b) being more detailed, it also confirmed that a drop in salinity from 34 to 24 psu and then to 14 psu, along with an increase in exposure time, significantly prolonged the time to onset of hatching and embryo development in both abalone species. O'Connor and Lawler (2004) noted that temperature and salinity affected the speed and success of early development of marine coastal species and reported that Pinctada imbricata embryos showed little tolerance to reduced salinity (< 29 psu) and did not develop at temperatures (~14 °C) experienced in Port Stephens, Australia. Similar findings by Madrones-Ladja (2002) revealed that windowpane oyster Placuna placenta embryos did not develop beyond the gastrula stage at 16 psu, and no development was observed at salinity lower than 16 psu. While it appears that salinity does play a vital role in the early-stage development of abalone species, it must be noted that a few studies have reported that as embryos develop, they seem to become more tolerant to a wider range of salinities (Struhsaker and Costlow 1969; Pechenik 1983; Richmond and Woodin 1996).
In experiment 1 (Fig. 1a), the hatching success rate gradually decreased as salinity and exposure time increased, but in experiment 2 (Fig. 2a), it is notable that a salinity of 14 psu with 3 h and 6 h exposure had a significantly greater effect on the hatching success rate of both species relative to the other treatments, suggesting that exposure time had a greater influence than salinity; however, as mentioned in the results section, two-way ANOVA indicated that salinity and exposure time had a significant interaction effect on the hatching success rate of disk abalone. Similar results were reported by O'Connor and Lawler (2004), who found that reductions in salinity caused an incremental reduction in the rate of both embryo development and embryo yield.
In a recent study by Legat et al. (2017) on the effects of salinity on fertilization and larviculture in the mangrove oyster Crassostrea gasar, the results showed that the amount of abnormal larvae was significantly higher in salinity of 14 psu than 21, 28 and 35 psu. Because both experiments showed an increasing trend in percentage of abnormality as salinity dropped and exposure time increased, it could be noted that both salinity and exposure time have an effect on the normal development of abalone larvae.
Finally, at day 5 after hatching, the survival of giant abalone indicated better adaptability to low-salinity conditions and longer exposure time compared with disk abalone (Fig. 1c). In particular, disk abalone treatment at 4 h exhibited 100% mortality. Chen and Chen (2000) found that the survival of abalone H. diversicolor supertexta was highly dependent on salinity. Madrones-Ladja (2002) reported that extended exposure to low salinity resulted in slow larval growth in P. placenta, extending the time required for development to settlement size and reducing survival. Legat et al. (2017) showed that mangrove oyster larvae cultivated in salinity between 28 and 35 psu were able to reach the pediveliger phase and begin the settlement process, whereas in this study, it was observed that both abalones cultured in salinity of 14 and 24 psu and extended exposure times were unable to form D-shaped veliger shells. Legat et al. (2017) further noted that this salinity restriction in the pediveliger phase could be related to larval energy requirements in executing morphological and anatomical changes for settlement, a stage when the species presents higher energy demand and requires optimal conditions for development.
In conclusion, hatching onset time increased significantly for both species as salinity decreased and exposure time increased. Both abalone species demonstrated a decreasing trend in terms of hatching success rate as exposure time increased at low salinity levels. With regard to abnormality, both abalones were greatly affected by the drop in salinity and increased exposure time. Both species were influenced by stressful low-salinity conditions, but giant abalone showed greater adaptability to long exposure time, and hence a higher survival rate, than disk abalone.
References
Arisman N, Istiqomah N, Yoshimatsu T (2018) Impact of short-term hyposalinity stress on Akoya pearl oyster (Pinctada fucata). Asian Fish Sci 31:265–275
Bayne BL, Thompson RJ, Widdows J (1976) Marine mussels: their ecology and physiology. In: Bayne BL (ed) Physiology I. Cambridge Scientific Press, Cambridge, pp 121–206
Charmantier-Daures M, Charmantier G, Trilles JP (1988) Tolerance a la salinite et osmoregulation chez les post-larves de (Penaeus japonicus) et P. chinensis Effet de la temperature. Aqua Liv Res 1:267–276
Chen J, Chen W (2000) Salinity tolerance of (Haliotis diversicolor supertexta) at different salinity and temperature levels. Aquaculture 181:191–203
Cheng W, Juang F, Chen J (2004) The immune response of taiwan abalone (Haliotis diversicolor supertexta) and its susceptibility to Vibrio parahaemolyticus at different salinity levels. Fish Shellfish Immunol 16:295–306
Cook PA (2016) Recent trends in worldwide abalone production. J Shellfish Res 35:581–583
Davenport J, Gruffydd LID, Beaumont AR (1975) An apparatus to supply water of fluctuating salinity, and its use in the study of the salinity tolerance of the larvae of scallop (Pectin maximus L.). J Mar Biol Assoc UK 55:391–409
Guerin JL, Stickle WB (1997) Effect of salinity on survival and biogenetics of juvenile lesser blue crabs (Callinectes similis). Mar Biol 129:63–69
Inoue M (1976) Stocking effectiveness of abalone. In: The Japanese Society for Fisheries Science (ed) Stocking effectiveness of Fish and Shellfish. Koseisha-Koseikaku, Tokyo, pp 9–25 (in Japanese)
Japan Meteorological Agency (2018) 1.2 Climate in Japan. In: Japan Meteorological Agency (ed) Climate Change Monitoring Report 2017. Japan Meteorological Agency, Tokyo, pp 16–21
Kashenko SD (2000) Acclimation of sea cucumber (Apostichopus japonicus) to decreased salinity at the blastula and gastrula stages: its effect on the desalination resistance of larvae at subsequent stages of development. Rus J Mar Biol 26:422–426
Kirkpatrick K, Jones MB (1985) Salinity tolerance and osmoregulatory of a prawn (Palaemon affinis) Milne Edwards (Carida: Palaemonidae). J Exp Mar Biol Ecol 93:61–70
Kitada S (1999) Effectiveness of Japan's stock enhancement programmes: current perspectives. In: Howell BR, Moksness E, Svåsand T (eds) Stock enhancement and sea ranching. Blackwell Publishing, Oxford, pp 103–131
Kitada S, Kishino H (2006) Lessons learned from Japanese marine finfish stock enhancement programmes. Fish Res 80:101–112
Legat JFA, Puchnick-Legat A, Gomes CHAM, Suhnel S, de Melo CMR (2017) Effects of salinity on fertilzation and larviculture of the mangrove oyster (Crassostrea gasar) in the laboratory. Aquaculture 468:545–548
Madrones-Ladja JA (2002) Salinity effect on the embryonic development, larval growth and survival at metamophosis of (Placuna placenta) Linnaeus (1758). Aquaculture 214:411–418
Masuda R, Tsukamoto K (1998) Stock enhancement in Japan: review and perspective. Bull Mar Sci 62:337–358
Musashi T (2006) Stocking effectiveness of Ezo abalone Haliotis discus hannai in Iwate Prefecture Japan. Nippon Suisan Gakkaisha 72:467–470 (in Japanese)
Oba T (2000) Awabi bunka to nihonjin. Seizando Shoten, Tokyo (in Japanese)
O'Connor WA, Lawler NF (2004) Salinity and temperature tolerance of embryos and juveniles of pearl oyster (Pinctada imbricata) Röding. Aquaculture 229:493–506
Paul JD (1980) Salinity-temperature relationships in the green scallop (Chlamys opercularis). Mar Biol 56:295–300
Pechenik JA (1983) Egg capsules of (Nucella lapillus) protect against low low-salinity stress. J Exp Mar Biol Ecol 71:165–179
Przeslawski R (2004) A review of the effects of environmental stress on embryonic development within intertidal gastropod egg masses. Molls Res 24:43–63
Richmond CE, Woodin SA (1996) Short-term fluctuations in salinity: effects on planktonic invertebrate larvae. Mar Ecol Prog Ser 133:167–177
Saido T (2002) Recovery of the abalone Haliotis discus hannai and its causes in Iwate Prefecture Japan. Kaiyo Mon 34:477–481 (in Japanese)
Sasaki R (2005) Abalone. In: Mori K (ed) Aquaculture system Vol. 3: Molluscs, Crustaceans, Sea Urchins and Algae. Koseisha Koseikaku, Tokyo, pp 85–120 (in Japanese)
Seki T, Sano M (1998) An ecological basis for the restoration of Japanese abalone populations. Bull Tohoku Natl Fish Res Inst 60:23–40 (in Japanese with English abstract)
Seki T, Taniguchi K (2000) Rehabilitation of northern Japanese abalone, Haliotis discus hannai populations by transplanting juveniles. Can Spec Publ Fish Aquat Sci 130:72–83
Singhagraiwan T, Doi M, Sasaki M (1992) Salinity tolerance of juvenile donkey's ear abalone, (Haliotis asinina L.). Thai Mar Fish Res Bull 3:71–77
Strand O, Solberg PT, Andersen KK, Magnesen T (1993) Salinity tolerance of juvenile scallops (Pecten maximus L.) at low temperature. Aquaculture 115:169–179
Struhsaker J, Costlow JJ (1969) Some environmental effects on the larval development of (Littorina picta) Mesogastropoda, reared in the laboratory. Malacologia 9:403–419
Verween A, Vincx M, Degraer S (2007) The effect of temperature and salinity on the survival of (Mytilopsis leucophaeata) larvae (Mollusca, Bivalvia): The search for environmental limits. J Exp Mar Biol Ecol 348:111–120
Acknowledgements
The authors would like to acknowledge the Mie Prefectural Sea Farming Center and its staff for providing fertilized eggs of both species of abalone to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manuel, A.V., Tu, P.T.C., Tsutsui, N. et al. Effect of salinity change and exposure time on the egg stages of two abalone species Haliotis discus discus and H. gigantea. Fish Sci 86, 27–33 (2020). https://doi.org/10.1007/s12562-019-01366-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-019-01366-4