Abstract
An increasing number of papers have been published on the effects of ocean acidification and warming on fishes over the last several years. However, there is little information on how these environmental changes affect the swimming behavior of fish. This study examined the escape response under elevated CO2 concentration and temperature of the Japanese anchovy Engraulis japonicus. Following acclimation to four conditions (CO2 400/1000 ppm, temperature 15/19 °C) for 1 month, the fish were tested for escape response through kinematic analysis of startle reactions to a mechanical stimulus. The response was recorded with a high speed video camera of 500 frames per second. The result showed turning rate was significantly higher at 19 °C than at 15 °C. Neither CO2 nor temperature affected the kinematic parameters analyzed (the escape trajectory, swimming velocity, acceleration, escape direction, or frequency of single and double bends), with the exception of the turning rate that was significantly higher at 19 °C than at 15 °C. However, we must clarify how future oceanic environmental changes affect escape responses of schooling fish and prey-predator interactions under more rigorous experimental conditions, to elaborate our prediction capacity for the trajectory of anchovy populations and thereby assess possible implications for anchovy fisheries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The atmospheric CO2 concentration has increased from 280 ppm in the late 1700s to 390 ppm in 2011, mainly due to the burning of fossil fuels and land use change [1]. The CO2 concentration is projected to reach 940 ppm in 2100 in accordance with the representative concentration pathway (RCP) 8.5, the most carbon-intensive future projection scenario among the RCPs [1, 2]. The ocean has absorbed about 30 % of the anthropogenic CO2 emitted to the air, which has resulted in a decrease of average surface ocean pH by 0.1 units since the beginning of the industrial revolution [1]. The reduction in seawater pH, together with associated changes in seawater carbonate chemistry, is termed ocean acidification. Under the RCP 8.5 projection, average surface ocean pH will further decrease by 0.30–0.32 units by the end of the twenty-first century [1]. The rapidly changing oceanic environmental conditions will likely alter the structure and function of marine ecosystems [3, 4], which will undermine productivity of fisheries and other ecosystem services of the ocean [1].
Reflecting mounting concern about the fate of marine ecosystems in the coming decades, there has been a rapid growth of scientific literature attempting to predict how marine species and ecosystems will respond to these environmental changes [4–7]. Although earlier studies of ocean acidification focused mainly on invertebrates, more attention has been paid to fishes during the last few years. Recent papers on the ocean acidification effect on fish have demonstrated that ocean acidification disrupts olfactory discrimination of chemical cues [8], visual perception of a predator fish [9], which may be related to impaired retinal function [10], and auditory ability to discriminate sounds appropriate for settling [11]. In addition to these findings on sensory functions, several studies have also shown that CO2 could have detrimental impacts on the brain function of fish, which may have direct implications for fish survival. For example, Ferrari et al. [12] demonstrated clear evidence that acclimation to CO2 (850 ppm) would suppress the learning ability of juvenile fish. Elevated CO2 also disrupts the behavioral lateralization of fish, which may influence efficacy of an escape response [13]. More directly, coral reef fish larvae pretreated with CO2 (700 and 850 ppm) and released to a natural reef showed significantly higher mortality, though the cause-and-effect relationship of this finding is somewhat obscure [14]. In contrast, the data by Allan et al. [15] demonstrated that when predators acclimated to control CO2 conditions are interacted with prey fish acclimated to 880 ppm CO2, capture success rate decreased with no change in predation rate (capture success/number of attack). There is some evidence for the involvement of a neurotransmitter, gamma-aminobutyric acid (GABA), in alterations of sensory and behavioral functions under elevated CO2 [16–18], but the mechanism(s) for the cognitive and behavioral disturbance by CO2 needs more scrutiny. In comparison, much less is known about swimming responses to elevated ambient CO2 [19–22].
One crucial behavior that determines survivorship of fish is the escape response [23]. In response to a predation risk, fish generally show a behavior called C-start, which determines whether or not fish can avoid a predator. The escape response (C-start) is the highest swimming speed attainable by a fish and occurs during the “flight or fight” response to predators. C-start begins with unilateral contraction of trunk musculature, bending the fish’s body into a C shape (stage 1) and may be followed by contraction of the contralateral trunk musculature (stage 2) [24]. An escape response consists of non-locomotion variables, which relate to sensory perception of a stimulus and processing of the sensory input to trigger a response (responsiveness, i.e., whether a fish responds to a stimulus or not, escape latency, and directionality) and locomotion variables, which directly govern kinematics of the escape response (turning rate, distance, swimming speed, and acceleration) [25]. Because escape responses are usually triggered by a specific type of cells in the central nervous system (the Mauthner cells) [26], one might expect that CO2 would somehow modify or disrupt escape responses of fish.
Since ocean acidification will proceed concurrently with temperature rise [1, 2], it is crucial to understand how these two environmental changes will affect marine species and ecosystems in concert. Under the RCP 8.5 pathway, surface ocean temperature is projected to rise 2 °C by the year 2100 [2]. Numerous papers have already been published on the effect of temperature on various aspects of fish biology [see [27], [28] for review] because temperature is one of the most powerful environmental factors to modulate biological activities [29]. Similar to all other animal groups, temperature almost invariably exerts strong influences on development [30–32], behavior [33–35], metabolism [36, 37], growth [38–40], and swimming [41–43] of fishes. It has also been reported that temperature affects tail-beat frequency, muscle twitch contraction, and aversive behavior of fish [44–46] and that increasing CO2 levels can reduce the swimming ability of both predators and prey [15, 47]. Domenici et al. [48] showed that elevated temperature attenuated the magnitude of lateralization in a marine damselfish without affecting the directionality of turning behavior, whereas elevated CO2 significantly reversed the turning bias. To our knowledge, no study has been conducted on the interactions of temperature and CO2 on the escape response of fish.
The aim of this study was to examine both separate and combined effects of temperature and CO2 on the escape responses of fish. The Japanese anchovy Engraulis japonicus was selected in our study for the following two reasons. First, it is commercially very important, being ranked ninth in the list of major marine fish species in production, amounting to the annual global catch of 1.3 million tonnes in 2012 [49]. In fact, both the top and 21st species in the list are from the genus Engraulis (Peruvian anchovy E. ringens and European anchovy E. encrasicolus, respectively), underpinning the importance of the genus in fisheries production. Second, the Japanese anchovy plays an important role in the marine ecosystem as prey of higher tropic predators such as skipjack tuna [50] and, as such, experiences a high mortality rate due to predation. The Japanese anchovy is found in the western north and central Pacific including the Yellow Sea, East China Sea, and Sea of Japan, showing changes in distribution pattern with resource abundance [51, 52]. These ocean regions are the main distribution areas of the Japanese anchovy and are among the most rapidly warming large marine ecosystems around the world [53]. Thus, studying the escape response of Japanese anchovy to elevated CO2 and temperature should provide useful information to foresee the trajectory of this important fishery species into the future ocean.
Materials and methods
Ethics statement
Animal care and experimental procedures for the experiment were approved by the Animal Care and Use Committee of the Institute for East China Sea Research, Nagasaki University (Permit no. ECSER13-01) in accordance with the Guidelines for Animal Experimentation of Nagasaki University.
Experimental animals
In January 2013, ~200 adult Japanese anchovy [total length = 99.3 ± 9.9 mm (mean ± SD, N = 25)] were purchased from a fisherman in Saikai-shi, Nagasaki, Japan (33°05′ N, 129°41′ E), who maintained the anchovies in fish cages as bait for skipjack tuna fishing. The fish were transferred to the Institute for East China Sea Research of Nagasaki University, Japan and stocked in two 500 L tanks supplied with a continuous flow of fresh seawater at a flow rate of 4 L/min. Fish were fed artificial pellet (diameter 2.3 mm, Otohime EP2, Marubeni Nisshin Co. Ltd., Tokyo) by using a custom-made automatic feeding machines every 30 min from 9 am to 5 pm with a daily ration of 5 % body weight. The Japanese anchovy turned out to be highly sensitive to handling stress, and a high mortality occurred within 3 days of transportation. The fish were maintained for 2 weeks before being acclimated to the experimental conditions described below.
Acclimation
After 2-weeks transport, 80 fish were randomly chosen, divided into four groups of 20 each and acclimated to four conditions: control condition (seawater equilibrated with ambient air containing 400 ppm CO2 and temperature at 15 °C), high CO2 condition (seawater equilibrated with air containing CO2 at a concentration of 1000 ppm and temperature at 15 °C), high temperature condition (CO2 same as control and temperature at 19 °C), and combined condition (CO2 1000 ppm and temperature at 19 °C. There were two acclimation tanks for each treatment (a total of eight tanks), and 10 fish were stocked in each tank (20 fish per treatment). The acclimation tanks were 100 L in capacity and supplied with filtered (1 µm) seawater at a flow rate 0.4 L/min. The overflow from the tanks was drained without recirculating. Seawater in the acclimation tanks was bubbled with atmospheric air for control or CO2-enriched air for high CO2 conditions at a flow rate of 20 L/min. Water temperatures of 15 and 19 °C were controlled by a submersible heater and a thermostat. The fish were fed artificial pellets at a daily ration of 5 % of body weight in three portions. Excess food was removed daily by siphoning. Water quality in each tank was monitored at 10:00 am daily for dissolved oxygen (DO) concentration, pH (NBS scale), and salinity. DO concentration was measured with a digital DO meter (YSI proODO dissolved oxygen meter, US) and never fell below 85 % saturation. Salinity was measured with a digital salinometer (Mettler-Toledo GmbH SG3, Switzerland). Water pH was measured with a digital pH meter (Mettler-Toledo GmbH SG8, Switzerland), calibrated with standard buffer solutions of pH 4.01, 6.86, and 9.18 (Nacalai Tesque Inc. Kyoto Japan). Alkalinity was measured with a total alkalinity titrator (Kimoto, ATT-05, Japan) at weekly intervals. Partial pressure of CO2 (pCO2) was calculated from measured seawater pH, temperature and total alkalinity by using the program CO2SYS [54]. The concentration of ammonia in seawater was measured twice weekly with an ammonia electrode (Orion 9512, Thermo Scientific, USA), calibrated with a standard solution of 1000 ppm NH3. The fish were acclimated to the four experimental conditions for 1 month before testing, between 4 February and 7 March 2013. The data of seawater temperature, oxygen saturation, and carbonate chemistry during acclimation are shown in Table 1.
We used every precaution to avoid any stressful treatment to the anchovies during acclimation, but daily cleaning of the tanks was apparently stressful to them. Many fish jumped out from the tanks or into the net covering the tanks. Thus, the number of fish decreased from 80 to 48 (Control 15, high CO2 12, high temperature 10, and combined 10) during the 1-month acclimation period.
Procedure
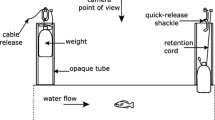
Seven dead fish (total length = 98.4 ± 4.5 mm and wet body mass = 8.80 ± 0.71 g; mean ± SD) were used to estimate the position of the center of mass (CM). The CM was estimated by hanging dorsally each dead fish with a needle. The balancing point of the body indicated the position of the CM at 43.64 ± 1.70 mm, i.e., 44.3 ± 0.2 % of the total length, from the snout. A black polyethylene tank (capacity 500 L, diameter 150 cm, height 80 cm) with a light yellow sheeting on the bottom was used for recording escape responses. The tank was filled with seawater to a depth of 20 cm. Escape responses were studied under the same CO2 and temperature conditions as during acclimation. The stimulus (a rubber parcel) was released through a 75 cm long PVC tube with a diameter of 6 cm by triggering an electromagnetic device. To avoid visual stimulation, the PVC tube was positioned at the center of the experimental tank, with the lower end 5 mm above the water surface. A high speed video camera (HAS-L1, Ditect Co., Japan) was installed 2 m above the experimental tank. A 180 W spotlight was mounted on the upper edge of the experimental tank and two 32 W fluorescent lamps were installed above the tank. The light intensity at the water surface of the testing tank was about 1800 lx. Each fish was tested individually and only once. Before triggering the electromagnetic stimulus, the anchovies were allowed to habituate themselves in the test tank for about 10 min until they started to swim in a normal manner. When the fish swam to the center of the tank, the stimulus was activated. A high speed video recorded the escape response at 500 frames/s. The video recording was triggered two seconds before the stimulus was dropped and lasted for 4 s.
Measured variables
The fish movement was sequentially tracked frame by frame by using ImageJ 1.46r (National Institutes of Health, USA). The x–y coordinates of the CM and the tip of the head were digitized in each frame. The following variables were calculated according to Lefrançois et al. [55]: (1) response latency, i.e., the time interval between the stimulus hitting the surface and the first detectable movement of the escape behavior; (2) response type, termed single bend (SB), when the tail did not recoil completely after the formation of the C or double bend (DB), when a full return flip of the tail occurred after initial contraction; (3) directionality, the “away” and “toward” responses, which were defined on the basis of the first detectable movement of fish being either oriented away or toward the stimulus; (4) stage 1 duration, the time between the first detectable fish movement and the onset of the return tail flip; (5) stage 2 duration, the time between the end of stage 1 and the end of the return tail flip; (6) total duration, the sum of the stage 1 and stage 2 durations; (7) initial orientation (A0), the angle between the line passing through the stimulus and the CM, and the line passing through the CM and the tip of the head at the onset of stage 1 (as shown in Fig. 1); (8) stage 1 angle (A1), the angle between the lines passing through the CM and the tip of the head at the onset and the end of stage 1; (9) stage 2 angle (A2), the angle between the lines passing through the CM and the tip of the head at the end of stage 1 and at the end of stage 2; (10) escape angle (A3), the sum of A1 and A2; (11) escape trajectory angle (A4), the sum of A0 and A3; (12) turning rate, calculated by dividing A1 by stage 1 duration; and (13) the cumulative distance (D), maximum speed (V max), and maximum acceleration (A max) were determined within a fixed 48 ms duration which was the time needed to complete stages 1 and 2 (see Results). Speed and acceleration were calculated by differentiation and double differentiation, respectively, by the cumulative distance for the time-series. A five point smoothing with polynomial regression was applied to calculate the speed and acceleration using QuickSAND software [56].
Schematic drawing of angular variable. The solid circles indicate the positions of the center of mass. The open circle indicate the position of stimulus hitting the water surface. S0, position of fish at the onset of stage 1; S1, position at the end of stage 1; S2, position at the end of stage 2; A0, initial orientation; A1, stage 1 angle; A2, stage 2 angle; A3, escape angle; A4, escape trajectory angle
Statistical analysis
Because of the unexpected mortality during the acclimation period, a total of 48 fish were used for testing C-start response. One fish in the high temperature group failed to respond to the stimulus. Therefore, data from 47 individuals were used for analysis (15 in the control treatment, 12 in the high CO2 treatment, 10 each in high temperature and combined treatments). The SPSS 16 was used to analyze all the measurement parameters. Two-way ANOVA with temperature and CO2 as fixed factors was used to compare response latency. A binary logistic regression was applied to analyze response type and directionality (away and toward the stimulus), in which the response type or directionality was regarded as the objective variable, and temperature, CO2 and their interaction were regarded as explanatory variables. The significance of these explanatory variables were assessed by using the Wald t test. The initial orientation was compared by a Kruskal–Wallis test, followed by Mann–Whitney U test for pairwise comparison. Mardia-Watson-Wheeler test was applied to detect differences in angular variance of escape direction between treatments. Because of multiple pairwise testing, Bonferroni correction was applied to adjust the alpha for each comparison. There were six hypotheses being tested at P value 0.05, and therefore, the new critical P value is either 0.05/6 or 0.008. Two-way ANCOVA was applied to assess the effect of temperature and CO2 on the s1 and s2 durations, turning rate, cumulative distance, maximum velocity, and maximum acceleration. Webb [57] observed that fast-start increased with size while Domenici and Blake [58] interpreted that the initial orientation of the fish had affected the directionality of escape. Therefore, the effect of both fish size and initial orientation were also included as explanatory variables to estimate all the parameters.
Results
Initial orientation and timing variable
The median of initial orientation angle was found to be 150.8, 98.8, 146.7, and 113.8 degrees in each of the control, high CO2, high temperature and combined treatments, respectively. The initial orientation angle was significantly smaller in high CO2 treatment, compared to the control and high temperature treatments (Table 2). The data on response latency, s1 and s2 durations are shown in Table 3. Two-way ANOVA detected no significant effect of temperature, CO2 or their interaction on the response latency (Table 4). Similarly, two-way ANCOVA failed to detect significant effects of temperature, CO2, or their interaction on the s1 duration or s2 duration (Table 4).
Directionality and escape trajectory
The proportions of fish whose first detectable movement was away from the stimulus in control, high CO2, high temperature and combined treatments were 40, 50, 60, and 60 %, respectively. Binary logistic regression indicated that temperature or CO2 did not have statistically significant effect on directionality (Table 4). Multiple Mardia-Watson-Wheeler test with adjusted P value showed no significant difference in escape trajectory angle between treatments (Table 2; Fig. 2).
Circular frequency distribution of escape trajectory angle of the Japanese anchovy defined as swimming direction at the end of stage 2 with respect to the stimulus orientation at 0°. The number beside a sector represents the number of fish that had an escape trajectory angle within each central angle division of 36°. a control, b high CO2, c high temperature, and d combined
Turning rate and response type
The turning rate was calculated for both single bend (SB) and double bend (DB) responses. The turning rate at 19 °C was significantly higher than those at 15 °C (Table 4; Fig. 3a). Binary logistic regression showed neither temperature nor CO2 affected response type (Table 4; Fig. 3b).
Kinematics
The mean (±SD) values of cumulative distance (D), maximum velocity (V max), and maximum acceleration (A max) within the average s2 duration (48 ms) of control, high CO2, high temperature and combined treatments are shown in Fig. 4. ANCOVA showed that there were no significant effects of temperature, CO2 or their interaction on D, V max, or A max (Table 4).
Discussion
This is the first study that has examined separate and combined effects of elevated temperature and CO2 on the escape response of fish. The results demonstrated that neither elevated temperature nor CO2 concentration or their combination affected any of the measured variables of solitary anchovies except turning rate, which was significantly higher at 19 °C than at 15 °C irrespectively of CO2 levels.
The maximum velocity (1.4–1.6 m/s) found in this study is in good agreement with the relationship between body length and V max obtained from the data from five species of fish (1.3 m/s for body length 0.1 m) [59]. The observed ranges of A max (63–117 m/s2) and turning rate (1.7–3.2 degrees/ms) are both well within the range reported for other fishes. Both the sum of stage 1 and stage 2 durations (44–48 ms) and response latency (5–7 ms) are relatively short, but are still within the range reported for other fishes from other studies [24, 25]. The relatively low proportion of away response (40–60 %) may have resulted from the initial orientation angles being larger than 90°. It has been shown that the proportion of away response can be as high as 90 % when a fish is stimulated sideways, but it decreases when the initial orientation angles deviate from 90° [25]. Our video recording showed that 50–75 % of fish was stimulated sideways.
Response latency is a behavioral measure directly correlated to the activity in the brainstem escape network that includes the two Mauthner cells [26], and thus the measurement of response latency yields important information with respect to the temperature dependence of the system. It has been reported that response latency decreases with temperature [60, 61]. The response latency in our study tended to be shorter at higher temperature (Table 3), but the difference was not statistically significant. This may have been due to the relatively small temperature difference used (4 °C) as compared with the much wider habitat temperature range of the Japanese anchovy (15–29 °C) [62].
The escape responses observed for the Japanese anchovy are somewhat different from those reported by Allan et al. [47], who studied the effects of ocean acidification on escape response in juvenile coral reef fish, Amphiprion melanopus. These authors tested escape responses in control (400 µatm) and high (1087 µatm) CO2 conditions, using juveniles produced by the parents that had been kept in the respective conditions for 11 days. The comparison between control and CO2-treated juveniles from control parents detected significant reductions in: (1) response distance (the total distance covered by the fish during the entire escape response until the fish stopped movement); (2) mean swimming speed; (3) V max; (4) the proportion of fish that showed away response in directionality; and (5) response duration (the duration of entire escape response). In addition, a significant increase was detected for the proportion of non-reactive fish, while no difference was found in response latency. CO2-treated juveniles from the parents acclimatized to high CO2 conditions largely showed intermediate responses. Data from this study showed no change in V max or the proportion of non-reacting fish (with only one fish in high temperature group failing to respond). It is not clear whether these differences in escape response between the species are due to the differences in life stages of test fish (i.e., juveniles vs. adults), test temperature (15 and 19 °C vs. 28.5 °C) or acclimation period (11 days vs. 1 month) or the neuromechanical nature of the kinematic behavior inherent to each species.
The turning rate was the only parameter that showed a significant difference between treatment groups in this study (Fig. 3a). Turning rate determines how fast a fish turns its body into C shape following a stimulus, and therefore, a higher turning rate would potentially lead to a higher rate of escape from predators. Walker et al. [63] demonstrated that the following abilities of the prey affected evasion outcome; the ability of the prey to generate rapid tangential acceleration (V max and A max), and the ability of the prey to rapidly rotate during the initial stage of the fast start (i.e., a higher turning rate), together with the evasion path of the prey relative to the strike path. An elevation of 4 °C increased turning rate from 0.65 degrees/ms (400 ppm CO2) to 1.44 degrees/ms (1000 ppm CO2). Temperature is also known to increase reaction distance, latency, responsiveness, distance, speed, and acceleration in fish [25]. These effects may be substantial when a test fish is subjected to acute temperature change, but will usually subside after temperature acclimation [44]. Considering the range of temperature rise projected (4–5 °C), which will gradually occur over the next decades, temperature will probably exert a minor, if any, direct influence on fish escape behavior. It is likely that increasing daily temperature fluctuations during extreme weather events would have much stronger effects on the behavior.
Although our data demonstrated the relative robustness of escape response by the Japanese anchovy under simulated future conditions, this does not imply that the fish will be subjected to a lower risk when attacked by a predator in the high CO2, warmer oceans. For instance, there is now ample evidence that the predicted CO2 level in 2100 will affect the sensory perception of external stimuli in fish, which is a crucial step in initiating an escape response (see Introduction). Thus, there is a possibility that prey fish would incur a higher risk of predation through impaired perception of approaching predators. On the other hand, how future oceanic environment may affect attack behaviour of predators needs to be examined for the mechanistic understanding of prey-predator interaction in changing marine conditions. Another important consideration is each species’ tendency to form schools or aggregations. The Japanese anchovy, like many other small pelagic fishes, forms a large school in nature [51]. Although schooling individuals can become solitary when they are sequentially attacked by predators [64], Domenici and Batty [65] reported that escape responses shown by fish in school may differ quantitatively in kinematics from those of solitary individuals, and they speculated that a solitary herring (a schooling species) may employ more toward responses, as a result of an alternative strategy aiming at increasing the unpredictability of the response. Namely, schooling fish can take advantage of the confusion effect while solitary fish cannot. However, these authors also pointed out that individual herrings in a school can be in effect solitary for a brief period. Therefore, to understand how these small pelagic fish will be affected by the future oceanic environmental changes and how the effect will propagate to higher trophic levels, it is crucial to analyze escape responses of the fish in both school and solitary conditions, and to investigate how prey-predator interaction of solitary and schooling fish will be influenced under simulated future oceanic conditions.
Recently, Cornwall, and Hurd (2015) reported that 94 % of papers published since 1993 in the field of ocean acidification research had employed experimental designs that fall short of the rigorous statistical requirements; treatments within manipulation experiments must all contain adequate numbers of randomly interspersed and independent treatment replicates [66]. To satisfy the level of independence of each experimental unit these authors recommend, each anchovy must have been acclimated to an experimental condition in a separate tank, or multiple anchovies must have been acclimated to multiple tanks with three or more header tanks per treatment. Though ideal, these designs would have been logistically highly demanding and practically quite difficult to achieve because the Japanese anchovy is a species that does not easily acclimate to laboratory conditions and needs extreme care to handle, which was evident from the unfortunate high mortality we experienced in this study. Repeated investigations from independent research institutions on different species of commercially important, small plegic fishes would improve our prediction capacity for the trajectory of these fishes and fishery resources in the future oceanic environment, as long as they meet statistical requirements to the maximum feasible extent to prevent pseudoreplications in the experimental design. With these limitations in mind, we still hope that the present results form a basis for further research to predict how commercially important, small pelagic fish will be affected by warmer and more acidified future oceans.
References
IPCC (2013) Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on climate change. Cambridge University Press, Cambridge
Meinshausen M, Smith SJ, Calvin K, Daniel JS, Kainuma MLT, Lamarque JF, Matsumoto K, Montzka SA, Raper SCB, Riahi K, Thomson A, Velders GJM, Vuuren DPP (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change 109:213–241
Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Hoegh-Guldberg O, Bruno JF (2010) The impact of climate change on the world’s marine ecosystems. Science 328:1523–1528
Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Ann Rev Mar Sci. doi:10.1146/annurev-marine-041911-111611.1118
Branch TA, DeJoseph BM, Ray LJ, Wagner CA (2013) Impacts of ocean acidification on marine seafood. Trends Ecol Evol 28:178–186
Harvey BP, Gwynn-Jones D, Moore PJ (2013) Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol Evol 3:1016–1030
Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106:1848–1852
Ferrari MCO, McCormick MI, Munday PL, Meekan MG, Dixson DL, Lönnstedt O, Chivers DP (2012) Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct Ecol 26:553–558
Chung W-S, Marshall NJ, Watson S-A, Munday PL, Nilsson GE (2014) Ocean acidification slows retinal function in a damselfish through interference with GABAA receptors. J Exp Biol 217:323–326
Simpson SD, Munday PL, Wittenrich ML, Manassa R, Dixson DL, Gagliano M, Yan HY (2011) Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol Lett 7:917–920
Ferrari MCO, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Andrew H, Chivers DP (2012) Effects of ocean acidification on learning in coral reef fishes. PLoS One. doi:10.1371/journal.pone.0031478
Domenici P, Allan B, McCormick MI, Munday PL (2012) Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol Lett 8:78–81
Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP (2010) Replenishment of fish populations is threatened by ocean acidification. Proc Natl Acad Sci USA 107:12930–12934
Allan BJM, Domenici P, McCormick MI, Watson S-A, Munday PL (2013) Elevated CO2 affects predator-prey interactions through altered performance. PLoS One. doi:10.1371/journal.pone.0058520
Nilsson GE, Dixson DL, Domenici P, McCormick MI, Sørensen C, Watson S-A, Munday PL (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nature Clim Change 2:201–204
Chivers DP, McCormick MI, Nilsson GE, Munday PL, Watson S-A, Meekan MG, Mitchell MD, Corkill KC, Ferrari MC (2014) Impaired learning of predators and lower prey survival under elevated CO2: a consequence of neurotransmitter interference. Glob Change Biol 20:515–522
Hamilton TJ, Holcombe A, Tresguerres M (2014) CO2-induced ocean acidification increases anxiety in Rockfish via alteration of GABAA receptor functioning. Proc R Soc B. doi:10.1098/rspb.2013.2509
Bignami S, Sponaugle S, Cowen RK (2013) Response to ocean acidification in larvae of a large tropical marine fish, Rachycentron canadum. Glob Change Biol 19:996–1006
Bignami S, Sponaugle S, Cowen RK (2014) Effects of ocean acidification on the larvae of a high-value pelagic fisheries species, mahi-mahi Coryphaena hippurus. Aquat Biol 21:249–260
Maneja RH, Frommel AY, Browman HI, Clemmesen C, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB (2013) The swimming kinematics of larval Atlantic cod, Gadus morhua L., are resilient to elevated seawater pCO2. Mar Biol 160:1963–1972
Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner HO, Lucassen M (2009) Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater Pco2. Aquat Toxicol 92:30–37
Domenici P (2010) Escape responses in fish: kinematics, performance and behavior. In: Domenici P, Kapoor BG (eds) Fish locomotion: an eco-ethological perspective. Science Publisher, New Hampshire, pp 123–170
Domenici P, Blake RS (1997) The kinematics and performance of fish fast-start swimming. J Exp Biol 200:1165–1178
Domenici P (2010) Context-dependent variability in the components of fish escape response: integrating locomotor performance and behavior. J Exp Zool 313A:59–79
Eaton RC, Lee RKK, Foreman MB (2001) The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol 63:467–485
Wood CM, McDonald DG (1997) Global warming: implications for freshwater and marine fish. Cambridge University Press, Cambridge
Currie S, Schulte P (2014) Thermal stress. In: Evans DH et al (eds) The physiology of fishes. CRC Press, Florida, pp 257–287
Willmer P, Stone G, Johnston I (2005) Environmental physiology of animals. Blackwell Science, Oxford
Boucher MA, McAdam SO, Shrimpton JM (2014) The effect of temperature and substrate on the growth, development and survival of larval white sturgeon. Aquaculture 430:139–148
Ma Z (2014) Food ingestion, prey selectivity, feeding incidence, and performance of yellowtail kingfish Seriola lalandi larvae under constant and varying temperatures. Aquac Int 22:1317–1330
Politis SN, Dahlke FT, Butts IAE, Peck MA, Trippel EA (2014) Temperature, paternity and asynchronous hatching influence early developmental characteristics of larval Atlantic cod, Gadus morhua. J Exp Mar Biol Ecol 459:70–79
Bartolini T, Butail S, Porfiri M (2014) Temperature influences sociality and activity of freshwater fish. Environ Biol Fishes 98:825–832
Johansen JL, Messmer V, Coker DJ, Hoey AS, Pratchett MS (2014) Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob Chang Biol 20:1067–1074
Malavasi S, Cipolato G, Cioni C, Torricelli P, Alleva E, Manciocco A, Tony M (2013) Effects of temperature on the antipredator behaviour and on the cholinergic expression in the European sea bass (Dicentrarchus labrax L.) juveniles. Ethology 119:592–604
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Tirsgaard B, Behrens JW, Steffensen JF (2015) The effect of temperature and body size on metabolic scope of activity in juvenile Atlantic cod Gadus morhua L. Comp Biochem Physiol 179A:89–94
Neuheimer AB, Thresher RE, Lyle JM, Semmens JM (2011) Tolerance limit for fish growth exceeded by warming waters. Nature Clim Change 1:110–113
Khan JR, Pether S, Bruce M, Walker SP, Herbert NA (2014) Optimum temperatures for growth and feed conversion in cultured hapuku (Polyprion oxygeneios)—is there a link to aerobic metabolic scope and final temperature preference? Aquaculture 430:107–113
Sun L, Chen H (2014) Effects of water temperature and fish size on growth and bioenergetics of cobia (Rachycentron canadum). Aquaculture 426–427:172–180
Almeida JR, Gravato C, Guilhermino L (2014) Effects of temperature in juvenile seabass (Dicentrarchus labrax L.) biomarker responses and behaviour: implications for environmental monitoring. Estuar Coasts 38:45–55
Cai L, Liu G, Taupier R, Fang M, Johnson D, Tu Z, Huang Y (2014) Effect of temperature on swimming performance of juvenile Schizothorax prenanti. Fish Physiol Biochem 40:491–498
Pang X, Yuan XZ, Cao ZD, Fu SJ (2013) The effects of temperature and exercise training on swimming performance in juvenile qingbo (Spinibarbus sinensis). J Comp Physiol 183B:99–108
Batty RS, Blaxter J, Fretwell K (1993) Effect of temperature on the escape responses of larval herring, Clupea harengus. Mar Biol 115:523–528
Johnson TP, Bennett AF (1995) The thermal acclimation of burst escape performance in fish: an integrated study of molecular and cellular physiology and organismal performance. J Exp Biol 198:2165–2175
Manciocco A, Toni M, Tedesco A, Malavasi S, Alleva E, Cioni C (2015) The acclimation of European sea bass (Dicentrarchus labrax) to temperature: behavioural and neurochemical responses. Ethology 121:68–83
Allan BJM, Miller GB, McCormick MI, Domenici P, Munday PL (2014) Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc R Soc B. doi:10.1098/rspb.2013.2179
Domenici PL, Allan BJM, Watson SA, McCormick MI, Munday PL (2014) Shifting from right to left: the combined effect of elevated CO2 and temperature on behavioural lateralization in a coral reef fish. PLoS One. doi:10.1371/journal.pone.0087969
FAO (2014) The state of world fisheries and aquaculture 2014. FAO, Rome
Takasuka A, Oozeki Y, Kimura R, Kubota H, Aoki I (2004) Growth-selective predation hypothesis revisited for larval anchovy in offshore waters: cannibalism by juveniles versus predation by skipjack tunas. Mar Ecol Prog Ser 278:297–302
Whitehead PJP, Nelson GJ, Wongratana T (1988) FAO species catalogue Vol. 7. Clupeoid fishes of the world (suborder Clupeoidei): an annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings, Part 2 Engraulididae. FAO Fisheries Synopsis, Rome
Funamoto T, Aoki I (2002) Reproductive ecology of Japanese anchovy off the Pacific coast of eastern Honshu, Japan. J Fish Biol 60:154–169
Belkin IM (2009) Rapid warming of large marine ecosystems. Prog Oceanogr 81:207–213
Pierrot D, Lewis E, Wallace D (2006) CO2SYS Dos program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory. US Department of Energy, Oak Ridge, Tennessee
Lefrançois C, Shingles A, Domenici P (2005) The effect of hypoxia on locomotor performance and behaviour during escape in Liza aurata. J Fish Biol 67:1711–1729
Walker JA (1998) Estimating velocities and accelerations of animal locomotion: a simulation experiment comparing numerical differentiation algorithms. J Exp Biol 201:981–995
Webb PW (1976) The effect of size on the fast-start performance of rainbow trout Salmo gairdneri, and a consideration of piscivorous predator-prey interactions. J Exp Biol 65:157–177
Domenici P, Blake RW (1993) Escape trajectories in angelfish (Pterophyllum eimekei). J Exp Biol 177:253–272
Domenici P (2001) The scaling of locomotor performance in predator–prey encounters: from fish to killer whales. Comp Biochem Physiol 131A:169–182
Preuss T, Faber DS (2003) Central cellular mechanisms underlying temperature-dependent changes in the goldfish startle-escape behaviour. J Neurosci 23:5617–5626
Webb PW (1978) Temperature effects on acceleration of rainbow trout, Salmo gairneri. J Fish Res Board Can 35:1417–1422
Hayasi S (1967) A note on the biology and fishery of the Japanese anchovy Engraulis japonica (Houttuyn). Califonia coorperative oceanic fisheries investigations, California
Walker JA, Ghalambor CK, Griset OL, McKenney D, Reznick DN (2005) Do faster starts increase the probability of evading predators? Funct Ecol 19:808–815
Major PF (1978) Predator-pray interactions to two schooling fishes Caranx ignobilis and Stolepholus purpureus. Anim Behav 26:760–777
Domenici P, Batty RS (1997) Escape behaviour of solitary herring (Clupea harengus) and comparisons with schooling individuals. Mar Biol 128:29–38
Cornwall CE, Hurd CL (2015) Experimental design in ocean acidification: problems and solutions. ICES J Mar Sci. doi:10.1093/icesjms/fsv118
Acknowledgments
This study was partly supported by Strategic Japanese-Chinese Cooperative Program on “Climate Change” (2012–2014). We thank Dr. Kazuki Yokouchi for his support with the statistical analysis of this study. Thanks are also due to Ms. Mizuri Murata and Ms. Peeraporn Punchai for their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasuchon, N., Yagi, M., Kawabata, Y. et al. Escape responses of the Japanese anchovy Engraulis japonicus under elevated temperature and CO2 conditions. Fish Sci 82, 435–444 (2016). https://doi.org/10.1007/s12562-016-0974-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-016-0974-z