Abstract
Temperature is a critical environmental factor in the ecology of fish, significantly influencing their physiology and behavior. Previous studies on the effect of temperature have focused on the metabolic and growth rates of individual fish and on the social behavior of fish shoals in placid water. In this paper, we investigate the effect of changing temperature on shoals of giant danios as they swim in a water tunnel at 22, 25, and 28 °C. Fish activity is quantified in terms of rheotaxis and tail-beat frequency measured automatically by tracking fish shape. Fish social behavior is quantified in terms of average nearest neighbor distance (ANND) and polarization. Results show that both social behavior and individual activity are significantly affected by change in temperature. In particular, fish maximize their activity and ANND at 28 °C, while their polarization is maximized at 22 °C. These findings suggest that temperature influences both the social behavior and the energy expenditure of fish, whereby lower temperatures lead to more cohesive shoals with reduced fish activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Changes in temperature have critical effects on living organisms (Cossins and Bowler 1987; Deeming and Ferguson 1991; Pörtner et al. 2005), causing long-term variations in their reproduction rates (Torricelli et al. 1985; Visser et al. 2009; Schaper et al. 2012), growth rates (Gillooly et al. 2001), and geographical distribution (Beitinger and Fitzpatrick 1979; Mora and Ospina 2001). In particular, reproduction rates are affected due to the fact that temperature is perceived as an important seasonal cue, which modulates the duration of parental care of unhatched fish eggs (Torricelli et al. 1985) and possibly triggers an onset of egg laying in birds (Visser et al. 2009; Schaper et al. 2012). Growth rates are influenced by temperature due to physiological changes, such as increased metabolic rates in ectotherms (Zuo et al. 2012) and stomach evacuation rates in Atlantic Salmon (Austreng et al. 1987). Changes in geographical distributions as a function of temperature variations have been related to the species’ thermal tolerance (Beitinger and Fitzpatrick 1979; Mora and Ospina 2001), which defines their natural habitat.
Among poikilothermic fish, temperature is considered as the most important abiotic factor, with fish being capable of detecting changes as low as 0.03 °C (Beitinger and Fitzpatrick 1979; Krause et al. 1998). Like other organisms, fish also have a preferred temperature range (Beitinger and Fitzpatrick 1979; Coutant 1987; Mora and Ospina 2001), which determines their geographical distribution (Beitinger and Fitzpatrick 1979; Caissie 2006) and influences the biodiversity of their ecosystem (Caissie 2006). Temperature varies on the basis of several factors all of which occur naturally in freshwater fish habitats (Bunn and Arthington 2002; Liao 2007), such as altitude, time of day, season (Arscott et al. 2001; Gardner et al. 2003), water depth (Sinokrot and Gulliver 2000), anthropogenic activities (Caissie 2006), and changes in water flow (Sinokrot and Gulliver 2000; Caissie 2006; Olden and Naiman 2010), with higher temperatures occurring at low flow rates.
The majority of studies that investigate the effect of temperature on fish are performed either in the presence of shoal mates (Weetman et al. 1999; Pritchard et al. 2001) or water flow (Keenleyside 1955); little is known on how fish respond collectively to temperature variations as they swim in a water flow. The presence of flow is central to fish life as it elicits a propensity to swim against the current (rheotaxis) (Montgomery et al. 1997; Suli et al. 2012; Kalueff et al. 2013), and enables prey detection (Kanter and Coombs 2003) and stream-finding for reproduction (Johnson et al. 2012). For social fish, the presence of water flow has been shown to be a determinant of schooling, whereby the coordinated swimming of a fish school has been associated to a reduced energy expenditure by group members (Herskin and Steffensen 1998; Killen et al. 2012; Marras and Porfiri 2012; Polverino et al. 2013).
Here, the objective is to integrate the presence of social enrichment with water flow to study the effect of temperature on shoals of giant danio (Devario aequipinnatus), a freshwater fish that is extensively used in biological studies (Wong et al. 2005; Biga and Meyer 2009). Full-body orientation and shape tracking is developed to quantify social behavior and individual activity of multiple fish in the water tunnel. Social behavior is measured in terms of average nearest neighbor distance and group alignment (polarization), while individual activity is quantified in terms of tail-beat frequency and rheotaxis. Based on prior studies on the influence of temperature on cohesion in guppies and zebrafish (Weetman et al. 1998; Pritchard et al. 2001), we predict that an increase in temperature will lead to more cohesive groups in danios. Similarly, in agreement with prior studies on teleost fish that show a significant change in metabolic rate with variation in temperature (Clarke and Johnston 1999), we predict that giant danios will be more active at higher temperatures. We hypothesize that at higher temperatures, as oxygen levels drop, fish will rheotact more to increase their oxygen intake; this increase in individual rheotaxis, in turn, will likely produce an increase in group polarization. Ultimately, we expect to find a relationship between activity and social behavior, whereby fish swimming in cohesive schools will display lower tail-beat frequencies due to reduced energy costs (Svendsen et al. 2003; Killen et al. 2012).
Materials and methods
The experimental procedure described in this work was approved by the Animal Welfare Oversight Committee at New York University Polytechnic School of Engineering (previously called Polytechnic Institute of New York University), protocol number AWOC-2013-103. Both the housing and the experimental procedure were designed to minimize stress to the animals.
Animals
Giant danios were selected in this study for their relatively large size that ease behavioral observations and strong schooling tendency (Viscido et al. 2004; Biga and Meyer 2009). Giant danios, a member of the cyprinids family, are a social species whose striped color pattern aids in their schooling behavior (Rosenthal and Ryan 2005). Juvenile fish were acquired from an online aquarium source (LiveAquaria.com, Rhinelander, Wisconsin, USA).
At the time of the experiments, individuals were approximately 5 cm in body length and were housed in three separate holding tanks, approximately 100 L each, with a maximum stocking density of 0.3 fish per liter. Each holding tank had a re-circulating filtration system that maintained water quality (pH, ammonia, nitrate, and nitrite levels). Although the sex of the subjects was not determined, none of the fish displayed a swollen belly at the time of the experiments, indicating that the females were homogeneous with respect to reproductive state.
The temperatures of the holding tanks were maintained at three different test values (22, 25, and 28 °C), continuously monitored through a submersible thermometer in each tank. An IceProbe IPAC-50W chiller (Nova Tec, San Rafael, California, USA) was used to maintain the 22 °C temperature, while an Aqueon Submersible Aquarium heater (Aqueon, Franklin, Wisconsin, USA) was utilized for 25 and 28 °C. Lighting was maintained in a 12 h light and 12 h dark photoperiod. Animals were fed daily at 7 pm with commercial flake food (Aqueon, Franklin, Wisconsin, USA).
A total of 90 experimentally naïve fish were tested for the study. All fish were acclimatized for at least 15 days prior to testing.
Experimental setup
Experiments were conducted in a Blazka-type water tunnel (Visser et al. 2009) to reproduce a natural stream environment. The dimensions of the experimental section of the tunnel were 90 cm × 15 cm × 15 cm in length, width, and height, respectively, as shown in Fig. 1. The experimental region was sectioned between plastic honeycomb grids. A Logitech Webcam Pro 9000 webcam (Logitech, California, USA) was placed 75 cm above the experimental section filming at 15 frames s−1. To assist in tracking, the bottom of the tank was covered with a white contact paper that enhanced the contrast of the fish with the background. A black curtain was used to isolate the apparatus during the experimental session. The tunnel was instrumented with a heater or a chiller, analogous to those used in the holding tanks, for 2 days prior to the experiment to obtain the corresponding temperature.
Procedure
Three experimental conditions corresponding to water temperatures of 22, 25, and 28 °C were considered in this study. These values were selected on the basis of the naturally occurring temperature range of this species in their geographical distribution (McClure et al. 2006; Prasad et al. 2009; Arunachalam et al. 2013). The flow rate was maintained constant, independent of the temperature, at 0.025 m/s, corresponding to approximately half-body length per second. This value allowed the experiment to continue for 15 min without any visible sign of fatigue in the subjects. Ten trials were conducted per condition, with each trial consisting of a 10-min habituation period followed by a 5-min experimental time. At the beginning of a trial, experimentally naïve fish in groups of three were transferred from the holding tank with the analogous water temperature to the water tunnel. Tests were performed between 1 and 7 pm from August to September 2013.
Multi-shape tracking
Overhead videos were processed offline using a multi-target tracking algorithm developed in MATLAB R2011a (Mathworks, Natick, Massachusetts, USA). The tracking algorithm processed the video frames to compute the two-dimensional position, velocity, orientation, and shape of each fish. The position and velocity were estimated using a linear filtering method described in (Butail et al. 2013). Briefly, a fixed background was subtracted from an image to isolate individual blobs corresponding to each fish. Fish position was measured as the center of each blob and fish velocity was estimated as the value that minimized the error between two successive frames of the same fish (Butail et al. 2013). Fish identities between frames were maintained using a global nearest-neighbor optimization method (Cox 1993) that minimized the combined distance between blob-fish pairs on an image. The resolution of occlusions, the estimation of the orientation and shape of each fish were carried out as follows.
Post background-subtraction, image blobs were filtered based on pixel size. The average fish size was estimated from 500 such blob appearances. Occluded blobs were identified as those whose size was more than two standard deviations above the average value. Once detected, a large blob was split into individual fish by fitting Gaussian mixture model with an optimization routine (Dempster et al. 1977).
If un-occluded, an ellipse was fit on the blob whose center marked the fish position, selected as the origin of the fish body frame. Fish orientation was estimated based on the observation that if the blob within the ellipse were partitioned along the minor axis, the side with more foreground pixels would be the head. The major axis of the ellipse, aligned along the fish body and positively directed towards the head, was selected as the fish orientation h. The body frame was completed by an axis normal to the orientation n in the counter-clockwise direction.
Blob pixels were transformed to the body frame (Butail and Paley 2012) using a frame transformation. Specifically, the fish centroid c was initially subtracted from each two-dimensional pixel location p on the blob to give a new pixel location \( \widehat{\boldsymbol{p}}=\boldsymbol{p}-\boldsymbol{c} \). Then, the new pixel location was projected onto the fish body frame as follows  where (·)T denotes vector transposition. A quadratic curve of the form y = s
1
x + s
2
x
2 was fit to the pixel positions in the body frame using the least-squares method (Strang and Aarikka 1986), with x and y being pixel locations in the body frame. Fish shape was represented in the form of the two coefficients (s
1, s
2) of the quadratic curve in the body frame (Fig. 2). Although a quadratic curve representation sufficed for our purposes, higher dimensional representations of the fish shape may be used at the expense of computational effort (Butail and Paley 2012).
where (·)T denotes vector transposition. A quadratic curve of the form y = s
1
x + s
2
x
2 was fit to the pixel positions in the body frame using the least-squares method (Strang and Aarikka 1986), with x and y being pixel locations in the body frame. Fish shape was represented in the form of the two coefficients (s
1, s
2) of the quadratic curve in the body frame (Fig. 2). Although a quadratic curve representation sufficed for our purposes, higher dimensional representations of the fish shape may be used at the expense of computational effort (Butail and Paley 2012).
The estimates of fish position, orientation, and shape were propagated to the next frame and updated on the basis of new measurements. For an occluded blob, the same procedure of quadratic curve fitting was applied to each split portion.
Behavioral parameters and analysis
Fish behavior was measured using the trajectory data output from the tracking system. In particular, position, velocity, orientation, and shape were used to quantify social behavior and individual activity of each fish as a function of time.
Social behavior was quantified in terms of the average nearest neighbor distance (ANND) and polarization. ANND, a measure of group cohesion, was defined as the average of the planar distances between each fish and its closest neighbor (Parrish et al. 2002). Polarization, a measure of group coordination, was defined as the magnitude of the average orientation at each time step. This value ranges from 0 to 1, with 1 implying that all fish are oriented in the same direction (Pitcher 1986; Miller and Gerlai 2012). Polarization values close to zero indicate a low degree of coordination; for three fish, for example, zero polarization would imply orientations spaced 120° apart.
Fish tail-beat frequency was used to quantify individual activity. Successive frames where the fish was at least 2 cm away from the tank walls for more than 2 s were used to compute tail-beat frequency. The time series of the tail tip trajectory from the selected frame sequences was input to a Fast Fourier transform (FFT) algorithm to extract frequency information (Bracewell 1989; Duhamel and Vetterli 1990). The dominant frequency, defined as the frequency corresponding to the maximum magnitude was selected as the tail-beat frequency for that frame sequence (Supplementary information). The tail-beat frequency for a given trial was computed as the average tail-beat frequency of all fish in that trial. Finally, to measure positive rheotaxis, defined as the tendency of fish to swim against the flow, the average of the cosine of the angles between individual orientation and the flow direction was recorded. A value of 1 implied that all the fish were swimming against the flow where as a −1 implied that the fish were swimming downstream.
First, one-way multivariate ANOVA was performed to check if the combined group of four behavioral measures could be used to demonstrate a change in temperature. Then, all behavioral parameters were compared separately using a one-way analysis of variance (ANOVA) with the temperature as the independent variable and the behavioral parameter as the dependent variable. Post-hoc comparisons, wherever significance was found, were conducted using Tukey-HSD test. Linear correlations between tail-beat frequency and ANND and polarization were computed to investigate dependence between individual activity and social behaviors. The significance level was set at p < 0.05.
Results
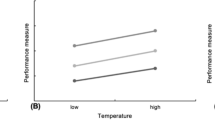
Multivariate analysis shows that, as a group, the behavioral measures failed to differ significantly from each other at different temperatures (p = 0.089). Group cohesion, measured by the ANND, varied significantly as a function of temperature (Table 1). Post-hoc comparisons indicate that fish were significantly less cohesive (ANND = 9.34 cm), at 28 °C than at 25 °C (ANND = 4.04 cm). Group coordination, measured by the polarization, also varied significantly as a function of the temperature, with post-hoc comparisons revealing that fish were significantly more polarized at the lowest temperature of 22 °C than at 25 °C, although the average polarization was always greater than 0.7.
ANOVA comparisons of individual fish activity show that fish tail-beat frequency varied significantly as a function of the temperature. Post-hoc comparisons reveal that the tail-beat frequency was significantly lower from 3.4 Hz at 28 °C to 2.4 Hz at 25 °C. Positive rheotaxis was also found to be significantly affected by the temperature with post-hoc comparisons indicating that fish are significantly more aligned with the flow at 22 °C than at 25 °C. Correlation between behavioral measures indicates that only ANND was positively correlated with tail-beat frequency (r 2 = 0.23, p < 0.01; Supplementary information).
Discussion
Our results show that variations in water temperature produced a significant change in social behavioral measures, namely ANND and polarization; however, the direction of change for ANND was opposite to our predictions. In particular, fish were less cohesive at the highest testing temperature and were more polarized at the lowest testing temperature. In agreement with prior studies, temperature also significantly affected individual activity, whereby fish tail-beat frequency was the highest at the maximum testing temperature. Finally, we found that fish orientation with the flow was a function of the water temperature.
Differently from previous studies on zebrafish and guppies (Weetman et al. 1998; Pritchard et al. 2001), where it was found that fish swam closer to each other as the temperature was raised by 4 °C from 22 to 26 °C, in our experiments we found that giant danios were almost twice as far from their closest neighbor at 28 °C than at 25 °C. A contrasting aspect in our experiment is the presence of the water flow, which compared to placid water, demands a high energy expenditure from schooling behavior (Johansen et al. 2010), and was likely a determinant of the observed social response. Specifically, this response may be related to variations in oxygen concentration, similar to experiments in (Domenici et al. 2002, 2013), where a decrease in cohesion was associated with hypoxia. Therein, it was suggested that a decrease in the available oxygen, particularly for fish swimming in trailing and middle positions, could force them to swim far from each other in order to increase their oxygen intake. In this context, it is possible that fish swimming in a flow at higher temperature experience a similar shortage of available oxygen due to (a) a high energy expenditure from an increased metabolic rate (Gillooly et al. 2001) and (b) an associated decrease in oxygen levels (Kramer 1987; Ficke et al. 2007), driving the shoal members far apart (Domenici et al. 2002, 2013). With respect to ecological considerations, fish cohesion is related to the efficiency of fish to forage and detect threats (Webster et al. 2007), that is, fish forage and are able to detect predators better when they swim close to each other. Consequently, a decrease in shoal cohesion enforced by environmental changes could adversely affect feeding rates and increase predation risk.
The remarkable change in fish polarization and rheotaxis from the nominal temperature of 25 °C to either direction is possibly related to different reasons. At 22 °C, social interaction is likely to contribute to the group coordination since the fish were relatively close to each other. High polarization offers anti-predatorial advantages (Weetman et al. 1998; Miller and Gerlai 2012), and, while there was no such stimulus present in our study, we cannot discount the association with increased oxygen levels, when the fish would likely avoid the water surface, with a threat perception in our experiments (Kramer 1987). Another possible hypothesis is that mutual alignment between fish pairs is a contributing factor in the reduction of energy expenditures through hydrodynamic cues (Herskin and Steffensen 1998; Svendsen et al. 2003; Killen et al. 2012; Marras and Domenici 2013). In particular, it is possible that at the lower temperature of 22 °C, an accompanied decrease in the metabolic rate (Gillooly et al. 2001) causes the fish to swim closer to each other and align their bodies to reduce their swimming cost. Conversely, at 28 °C, when the fish are not as close to each other, rheotaxis (Keenleyside and Hoar 1954; Montgomery et al. 1997; Suli et al. 2012) is probably the main reason for the observed high polarization. The low rheotaxis values at 25 °C are in agreement with another study where flow speeds of 3 cm/s were used to investigate the effect of sensory deprivation on rheotactic behavior (Bak-Coleman et al. 2013). At higher temperatures, fish may rheotact more so that they can increase their oxygen intake to spend less energy by aligning themselves against the water flow.
Fish activity measured in terms of tail-beat frequency varied with the temperature, with a significantly higher tail-beat frequency at the highest testing temperature of 28 °C. The observed change in activity as a function of temperature is in agreement with previous studies on groups of fish (Weetman et al. 1998; Clarke and Johnston 1999; Peck et al. 2006), although experiments with single fish in a water tunnel indicate that temperature plays a secondary role on the tail-beat frequency, which is instead modulated by the flow speed (Rome and Alexander 1990). The higher tail-beat frequency observed at 28 °C suggests that fish spend more energy (Herskin and Steffensen 1998; Steinhausen et al. 2005), possibly due to higher metabolic rates (Gillooly et al. 2001). These results have a direct implication on fish biology from environmental changes that cause an increase in temperature (Williamson et al. 2008). For example, in-situ observations have revealed that higher activity may cause imbalances in fish energy budgeting, in which lower rates of net energy are available for growth and reproduction (Rennie et al. 2005). Further experiments are required on an evolutionary time-scale to investigate whether changes in activity due to temperature can influence the species as a whole.
A positive correlation between individual activity and social behavior was also found in our results. In particular, fish cohesion (ANND) decreased (increased) with tail-beat frequency, indicating that as fish become more active they tend to stay apart. However, the causal relationship between these two behaviors may not be uniform through the entire range of temperatures. As discussed before, at high temperatures it is likely that activity and cohesion are independently regulated, due to an increase in metabolic rates and decrease in oxygen levels, respectively. Instead, at lower temperatures, social behavior may affect individual activity through hydrodynamic cues (Herskin and Steffensen 1998; Svendsen et al. 2003; Killen et al. 2012; Marras and Domenici 2013), whereby trailing fish that are able to utilize hydrodynamic cues at 22 and 25 °C display lower tail-beat frequencies.
Among the several parameters driving the global climate change, temperature is considered the most important (Cossins and Bowler 1987). We found that a moderate change of 3 °C in temperature is responsible for a remarkable variation of both social behavior and individual activity of giant danios. While the temperature variation considered here is compatible with diurnal and seasonal changes in the natural habitats of giant danios (McClure et al. 2006), our results indicate that it differentially influences fish cohesion, polarization, tail beat frequency, and rheotaxis. Analyses of these results indicate that a complex trade-off exists between individual activity and social behavior, which may depend on several factors, such as hydrodynamic cues and oxygen levels.
References
Arscott DB, Tockner K, Ward J (2001) Thermal heterogeneity along a braided floodplain river (Tagliamento River, northeastern Italy). Can J Fish Aquat Sci 58(12):2359–2373
Arunachalam M, Raja M, Vijayakumar C, Malaiammal P, Mayden RL (2013) Natural history of zebrafish (Danio rerio) in India. Zebrafish 10(1):1–14
Austreng E, Storebakken T, Åsgård T (1987) Growth rate estimates for cultured Atlantic salmon and rainbow trout. Aquaculture 60(2):157–160
Bak-Coleman J, Paley DA, Coombs S (2013) The spatiotemporal dynamics of rheotactic behavior depends on flow speed and available sensory information. J Exp Biol 216:4011–4024
Beitinger TL, Fitzpatrick LC (1979) Physiological and ecological correlates of preferred temperature in fish. Am Zool 19(1):319–329
Biga PR, Meyer J (2009) Growth hormone differentially regulates growth and growth-related gene expression in closely related fish species. Comp Biochem Physiol A 154(4):465–473
Bracewell RN (1989) The fourier transform. Sci Am 260(6):86–95
Bunn SE, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manag 30(4):492–507
Butail S, Paley DA (2012) Three-dimensional reconstruction of the fast-start swimming kinematics of densely schooling fish. J R Soc Interface 9(66):77–88
Butail S, Bartolini T, Porfiri M (2013) Collective response of zebrafish shoals to a free-swimming robotic fish. PLoS One 8(10):e76123
Caissie D (2006) The thermal regime of rivers: a review. Freshw Biol 51(8):1389–1406
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68(5):893–905
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, London
Coutant CC (1987) Thermal preference: when does an asset become a liability? Environ Biol Fish 18(3):161–172
Cox IJ (1993) A review of statistical data association techniques for motion correspondence. Int J Comput Vis 10(1):53–66
Deeming DC, Ferguson MWJ (1991) Physiological effects of incubation temperature on embryonic development in reptiles and birds. Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, pp 147–171
Dempster AP, Laird NM, Rubin DB (1977) Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Ser B 39(1):1–38
Domenici P, Ferrari RS, Steffensen JF, Batty RS (2002) The effect of progressive hypoxia on school structure and dynamics in Atlantic herring Clupea harengus. Proc R Soc Lond B Biol Sci 269(1505):2103–2111
Domenici P, Herbert N, Lefrançois C, Steffensen J, McKenzie D (2013) The effect of hypoxia on fish swimming performance and behaviour. In: Swimming physiology of fish. Springer Berlin Heidelberg, pp 129–159
Duhamel P, Vetterli M (1990) Fast Fourier transforms: a tutorial review and a state of the art. Signal Process 19(1):259–299
Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish 17(4):581–613
Gardner B, Sullivan PJ, Lembo J, Arthur J (2003) Predicting stream temperatures: geostatistical model comparison using alternative distance metrics. Can J Fish Aquat Sci 60(3):344–351
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293(5538):2248–2251
Herskin J, Steffensen J (1998) Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J Fish Biol 53(2):366–376
Johansen J, Vaknin R, Steffensen JF, Domenici P (2010) Kinematics and energetic benefits of schooling in the labriform fish, striped surfperch Embiotoca lateralis. Mar Ecol Prog Ser 420:221–229
Johnson NS, Muhammad A, Thompson H, Choi J, Li W (2012) Sea lamprey orient toward a source of a synthesized pheromone using odor-conditioned rheotaxis. Behav Ecol Sociobiol 66(12):1557–1567
Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SCF, Weng W, Bally-Cuif L, Schneider H (2013) Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10(1):70–86
Kanter MJ, Coombs S (2003) Rheotaxis and prey detection in uniform currents by Lake Michigan mottled sculpin (Cottus bairdi). J Exp Biol 206(1):59–70
Keenleyside MH (1955) Some aspects of the schooling behaviour of fish. Behaviour 8:183–248
Keenleyside MH, Hoar WS (1954) Effects of temperature on the responses of young salmon to water currents. Behaviour 7(2/3):77–87
Killen SS, Marras S, Steffensen JF, McKenzie DJ (2012) Aerobic capacity influences the spatial position of individuals within fish schools. Proc R Soc Biol Sci Ser B 279(1727):357–364
Kramer DL (1987) Dissolved oxygen and fish behavior. Environ Biol Fish 18(2):81–92
Krause J, Staaks G, Mehner T (1998) Habitat choice in shoals of roach as a function of water temperature and feeding rate. J Fish Biol 53(2):377–386
Liao JC (2007) A review of fish swimming mechanics and behaviour in altered flows. Philos Trans R Soc Lond B Biol Sci 362(1487):1973–1993
Marras S, Domenici P (2013) Schooling fish under attack are not all equal: some lead, others follow. PLoS One 8(6):e65784
Marras S, Porfiri M (2012) Fish and robots swimming together: attraction towards the robot demands biomimetic locomotion. J R Soc Interface 9(73):1856–1868
McClure M, McIntyre P, McCune A (2006) Notes on the natural diet and habitat of eight danionin fishes, including the zebrafish Danio rerio. J Fish Biol 69(2):553–570
Miller N, Gerlai R (2012) From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio). PLoS One 7(11):e48865
Montgomery JC, Baker CF, Carton AG (1997) The lateral line can mediate rheotaxis in fish. Nature 389(6654):960–963
Mora C, Ospina A (2001) Tolerance to high temperatures and potential impact of sea warming on reef fishes of Gorgona Island (tropical eastern Pacific). Mar Biol 139(4):765–769
Olden JD, Naiman RJ (2010) Incorporating thermal regimes into environmental flows assessments: modifying dam operations to restore freshwater ecosystem integrity. Freshw Biol 55(1):86–107
Parrish JK, Viscido SV, Grünbaum D (2002) Self-organized fish schools: an examination of emergent properties. Biol Bull 202(3):296–305
Peck MA, Buckley LJ, Bengtson DA (2006) Effects of temperature and body size on the swimming speed of larval and juvenile Atlantic cod (Gadus morhua): implications for individual-based modelling. Environ Biol Fish 75(4):419–429
Pitcher TJ (1986) Functions of shoaling behavior in teleost fishes. In: Pitcher TJ (ed) Springer US, Boston, pp 294–337
Polverino G, Phamduy P, Porfiri M (2013) Fish and robots swimming together in a water tunnel: robot color and tail-beat frequency influence fish behavior. PLoS One 8(10):e77589–e77589
Pörtner HO, Langenbuch M, Michaelidis B (2005) Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: from Earth history to global change. J Geophys Res (C Oceans) 110(C9):C09S10
Prasad A, Venkataramana G, Thomas M (2009) Fish diversity and its conservation in major wetlands of Mysore. J Environ Biol 30(5):713–718
Pritchard VL, Lawrence J, Butlin RK, Krause J (2001) Shoal choice in zebrafish, Danio rerio: the influence of shoal size and activity. Anim Behav 62(6):1085–1088
Rennie MD, Collins NC, Shuter BJ, Rajotte JW, Couture P (2005) A comparison of methods for estimating activity costs of wild fish populations: more active fish observed to grow slower. Can J Fish Aquat Sci 62(4):767–780
Rome LC, Alexander RM (1990) The influence of temperature on muscle velocity and sustained performance in swimming carp. J Exp Biol 154(1):163–178
Rosenthal GG, Ryan MJ (2005) Assortative preferences for stripes in danios. Anim Behav 70(5):1063–1066
Schaper SV, Dawson A, Sharp PJ, Gienapp P, Caro SP, Visser ME (2012) Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. Am Nat 179(2):E55–E69
Sinokrot BA, Gulliver JS (2000) In-stream flow impact on river water temperatures. J Hydraul Res 38(5):339–349
Steinhausen MF, Steffensen JF, Andersen NG (2005) Tail beat frequency as a predictor of swimming speed and oxygen consumption of saithe (Pollachius virens) and whiting (Merlangius merlangus) during forced swimming. Mar Biol 148(1):197–204
Strang G, Aarikka K (1986) Introduction to applied mathematics, vol 16. Wellesley-Cambridge, Wellesley
Suli A, Watson GM, Rubel EW, Raible DW (2012) Rheotaxis in larval zebrafish is mediated by lateral line mechanosensory hair cells. PLoS One 7(2):e29727
Svendsen JC, Skov J, Bildsoe M, Steffensen JF (2003) Intra-school positional preference and reduced tail beat frequency in trailing positions in schooling roach under experimental conditions. J Fish Biol 62(4):834–846
Torricelli P, Lugli M, Gandolfi G (1985) A quantitative analysis of the fanning activity in the male Padogobius martensi (Pisces: Gobiidae). Behaviour 92(3/4):288–301
Viscido SV, Parrish JK, Grünbaum D (2004) Individual behavior and emergent properties of fish schools: a comparison of observation and theory. Mar Ecol Prog Ser 273:239–249
Visser ME, Holleman LJ, Caro SP (2009) Temperature has a causal effect on avian timing of reproduction. Proc R Soc Biol Sci Ser B 276(1665):2323–2331
Webster MM, Goldsmith J, Ward AJW, Hart PJB (2007) Habitat-specific chemical cues influence association preferences and shoal cohesion in fish. Behav Ecol Sociobiol 62:273–280
Weetman D, Atkinson D, Chubb JC (1998) Effects of temperature on anti-predator behaviour in the guppy, Poecilia reticulata. Anim Behav 55(5):1361–1372
Weetman D, Atkinson D, Chubb JC (1999) Water temperature influences the shoaling decisions of guppies, Poecilia reticulata under predation threat. Anim Behav 58(4):735–741
Williamson CE, Dodds W, Kratz TK, Palmer MA (2008) Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Front Ecol Environ 6(5):247–254
Wong KY, Adolph AR, Dowling JE (2005) Retinal bipolar cell input mechanisms in giant danio. I. Electroretinographic analysis. J Neurophysiol 93(1):84–93
Zuo W, Moses ME, West GB, Hou C, Brown JH (2012) A general model for effects of temperature on ectotherm ontogenetic growth and development. Proc R Soc Biol Sci Ser B 279(1734):1840–1846
Acknowledgments
This research was supported by the National Science Foundation under Grant numbers CMMI-0745753, and CMMI-1129820, and the Mitsui USA Foundation through a fellowship to Tiziana Bartolini. The authors are also thankful to Giovanni Polverino for useful discussions and to Nicole Abaid and Simone Macrí for their careful review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 181 kb)
Rights and permissions
About this article
Cite this article
Bartolini, T., Butail, S. & Porfiri, M. Temperature influences sociality and activity of freshwater fish. Environ Biol Fish 98, 825–832 (2015). https://doi.org/10.1007/s10641-014-0318-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0318-8