Abstract
To investigate the effects of starvation and acclimation temperature on the escape ability of juvenile rose bitterling (Rhodeus ocellatus), we measured the fast-start escape and constant acceleration swimming performance of fish fasted for 0 (control), 1 and 2 weeks and half-lethal periods (6 or 4 weeks) at two temperatures (15 and 25 °C). Fish acclimated at a high temperature exhibited shorter response latency (R), higher maximum linear velocity (V max) and longer escape distance during escape movement (D 120ms) than those at the low temperature. Starvation resulted in a significant decrease in V max and D 120ms at either low or high temperature and a significant increase in R at only the high temperature in the half-lethal period groups (P < 0.05). The relationship between V max (Y, m s−1) and starvation time (X, week) was Y 15 = −0.062X + 1.568 (r = −0.665, n = 36, P < 0.001) at low temperature and Y 25 = −0.091X + 1.755 (r = −0.391, n = 40, P = 0.013) at high temperature. The relationship between U cat (Y, cm s−1) and starvation time (X, week) was Y 15 = −1.649X + 55.418 (r = −0.398, n = 34, P = 0.020) at low temperature and Y 25 = −4.917X + 62.916 (r = −0.793, n = 33, P < 0.001) at high temperature. The slopes of equations showed a significant difference between low and high temperature (F 1,63 = 9.688, P = 0.003), which may be due to the different energy substrate utilization when faced with food deprivation at different temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Locomotor ability is a key organismal performance trait in the chain of interactions that link morphological, physiological and biochemical traits to fitness (Arnold 1983). The essential ecological functions, such as predator–prey interactions, reproductive activities or foraging, are dependent upon an animal’s capacity for movement in many fish species (Marras et al. 2010). The fast-start swimming is initiated by the Mauthner cells and characterized by a rapid acceleration that is anaerobically fueled (Eaton et al. 1977; Domenici and Blake 1997), which is commonly used by fish species as a component of anti-predator behavior used to evade an attack by a potential predator (Webb 1986; Law and Blake 1996; Walker et al. 2005). The constant acceleration test (CAT) evaluates the swimming performance that fish may employ when being pursued by strong swimming predators, trying to escape a fishing trawl or maneuvering through strong currents (Marras et al. 2010). The fast-start escape movement, which is completed in seconds, is powered by intracellular stores of adenosine triphosphate (ATP) and creatine phosphate (PCr), and it is most likely limited by neuromuscular morphology and physiology (Reidy et al. 2000). Constant acceleration swimming in fish generally involves the use of three endogenous fuels stored within the white muscle: glycogen, ATP and PCr. In the early stages of acceleration swimming, energy is largely derived from the breakdown of PCr and ATP (Dobson and Hochachka 1987; Marras et al. 2010), whereas glycogenolysis provides the majority of the ATP required to sustain muscular exertion at later stages (Dobson and Hochachka 1987; Wood 1991).
Because of environmental and seasonal changes, such as temporal and spatial patchiness of food availability, long and/or short periods of starvation are common for aquatic species (Méndez and Wieser 1993). The ecophysiological effects of starvation on fish species have been studied for several decades (e.g., Jobling 1980; Sheridan and Mommsen 1991; Kieffer and Tufts 1998; Martínez et al. 2003; Fu et al. 2011; Pang et al. 2014). Such research demonstrated that starvation resulted in the reduction in energy reserves and the impairment of physiological functions (Méndez and Wieser 1993; Martínez et al. 2003; McCue 2010; Tang et al. 2010; Fu et al. 2011; Zhao et al. 2012; Luo et al. 2013). Previous research showed that starvation had a profound negative effect on both fast-start and constant acceleration swimming performance in some fish species (Wang et al. 2012; Pang et al. 2014). Temperature is an important abiotic factor in the habitats of ectothermic animals and has been called the ‘ecological master factor’ for animals (Brett 1971). Fish are subjected to large diurnal and seasonal changes in temperature as ectotherms in natural water bodies (Lee et al. 2003; Claireaux et al. 2006). Temperature may have direct, negative effects on the swimming performance in fish species through physical and biochemical manners (Kieffer and Tufts 1998; Lee et al. 2003; Claireaux et al. 2006; Lyon et al. 2008; Burt et al. 2012; Penghan et al. 2014a). Temperature also indirectly affects the swimming performance, as the food supply of fish displayed strong seasonal oscillations (Shulman 1974). Under natural conditions, fish must face two exogenous stresses, low temperature and insufficient food resource, at the same time (e.g., in winter). Consequently, fish possibly developed different strategies of swimming activity to face starvation at different temperatures (Pang et al. 2014).
The rose bitterling (Rhodeus ocellatus) is a small, freshwater fish species belonging to the family Cyprinidae. It is one of the most abundant fish species in southern China and typically lives in still or slow-flowing water with dense aquatic vegetation and sand-silt bottom, such as lowland ponds, canals, slow-flowing rivers, backwaters and oxbows (Ding 1994). Thus, maintaining the fast-start escape and constant acceleration swimming performance is vital for its predator avoidance. The water temperature in southern China showed large seasonal changes that varied from approximately 15 °C in winter to 25 °C in summer in recent years. The aims of this study were to: (1) assess the effects of starvation (0, 1 and 2 weeks and half-lethal period) and acclimation temperature (15 and 25 °C) on both fast-start and constant swimming performances and (2) test whether the responses of measured swimming performance to starvation varied with the acclimation temperature in this species.
Materials and methods
Experimental fish and holding conditions
Juvenile rose bitterling (0.5–0.9 g, n = 300) was caught from local pond in Chongqing, China, in autumn. The fish were maintained in a water recirculating system (1.5 m × 0.6 m × 0.5 m) at Chongqing Normal University for 2 weeks before the experiments. During this period, the temperature of the fresh dechlorinated water was maintained at 20 ± 0.5 °C, the water oxygen content was maintained above 7.0 mg L−1, the pH ranged from 6.5 to 7.3, and the ammonia-N was maintained below 0.025 ppm. The photoperiod was maintained at 12-h light/12-h dark light cycle. The fish were fed daily to satiation at 9:00 a.m. with a commercial diet (Tongwei Group, Chengdu, China; composition: 41.2 % protein; 8.5 % lipid; 25.7 % carbohydrate and 12.3 % ash).
Experimental protocol
Temperature acclimation
After 2 weeks in the recirculating tank, 300 fish were randomly selected and divided into two groups (15 and 25 °C) of 150 fish, and individuals from each temperature group were then transferred to two similar water recirculating systems (allowing for 15 % mortality during starvation, 300 fish were acclimated in the present experiment). The water temperature was 20 °C when the fish were transferred, and the temperature was then increased or decreased by 1 °C day−1 until it reached the prescribed temperature (Lee et al. 2003). The fish were maintained at the experimental temperature for another 2 weeks. During the acclimation period, the fish were fed once daily to satiation.
Starvation treatment
Once the temperature acclimation was complete, 20 fish from each temperature group were transferred to two similar water recirculating systems as a pilot experiment to determine their half-lethal (50 % death) period (the half-lethal periods were 6 and 4 weeks at low and high temperature, respectively). The remaining fish from each temperature group were fasted for different periods (0, 1 and 2 weeks and the half-lethal period). Fish individuals were randomly selected along with starvation. The fish in the control group (0 weeks) were fasted for 24 h before any swimming performance measurements for the reduction of digestion effects. The rearing conditions during the experimental period were consistent with those used during the temperature acclimation period.
Measurement of swimming performance
Measurement of fast-start swimming performance
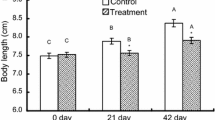
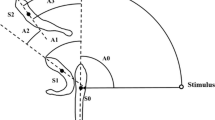
The fast-start instrument was developed by the Laboratory of Evolutionary Physiology and Behaviour, Chongqing Normal University. The device included a Basler A504K high-speed camera (500 frames s−1; www.baslerweb.com), a light emitting diode (LED) matrix light source and a tank (40 cm × 40 cm × 15 cm) engraved with 1 cm × 1 cm grid lines on the bottom. Electrodes at two inside walls of the tank were connected to a 20 V direct current, and both electrodes and the LED light shared a control switch (Yan et al. 2012). The sides of the experimental tank were lined with black paper. The water in the tank was 10 cm deep. The fish were anesthetized with neutralized tricaine methane sulfonate (MS-222, 50 mg L−1), and the body mass and length of experimental fish were measured to the nearest 0.1 g and cm, respectively (Table 1). Then, the experimental fish was marked dorsally at the center of mass position with titanium oxide. After 2 h of recovery, the fish were individually transferred to the experimental glass tank and kept for another 1 h. Escape responses were elicited by an electrical impulse (0.5 V cm−1; 30 ms) administered when the fish maintained a position at the center of the filming zone. The recording was initiated as soon as the LED (synchronized with the electrical stimulus) was illuminated (0 ms delay). The high-speed camera was used to record the entire escape process (time span 3 s). The resulting images were analyzed using image processing software (ACDsee 10) and digitized using TpsUnil and TpsDig software (http://life.bio.sunysb.edu/morph/) to define the track of the centroid of the locomotion performed by the fish during its escape response. Based on the centroid locomotion track and the time interval of each adjacent track, the following variables were calculated. Response latency (R) was defined as the time elapsed between the initiation of the stimulus (LED light) and the time when the escape behavior was observed. The maximum linear velocity (V max) was calculated from the maximum distance moved by the center of mass in 2 ms (two consecutive video frames). The maximum linear acceleration (A max) was further calculated by the change of V max. The escape distance during the first 120 ms (approximate time course of fast-start movement in rose bitterling) (D 120ms) was the total distance moved by the center of mass during the first 120 ms immediately after the stimulus.
Measurements of U cat
A Brett-type swimming tunnel respirometer with a swim chamber that had a 19.9-cm2 cross-sectional area was used to measure the fish’s U cat [total volume 3.1 L; similar to Pang et al. (2010)]. The fish were anesthetized, and the body mass and length of experimental fish were measured to the nearest 0.1 g and cm, respectively (Table 1). Then, fish were individually transferred into the swim tunnel and were allowed to recover for 1 h at a water velocity of 6 cm s−1. The flow of aerated water through the respirometer was maintained continuously during this recovery period. The water temperature in the swimming chamber was controlled within ±0.2 °C using a water bath connected to a stainless steel heat exchanger. The water velocity in the swim tunnel was then steadily increased at a rate of 0.1667 cm s−2 (10 cm s−1 min−1). A computer outputted various electric pulse to the stepping motor of swimming tunnel respirometer and thus changed the water velocity in the tunnel. The water was accelerated at this rate until the fish were exhausted. The water velocity at which the fish were exhausted was used as the value of the U cat (Reidy et al. 2000; Marras et al. 2010). Fatigue was defined as the failure of the fish to move away from the rear honeycomb screen of the swimming chamber for 20 s (Lee et al. 2003).
Date analysis and statistics
STATISTICA 6.0 (StatSoft, Inc, Tulsa, OK, USA) was used for the data analysis. All of the values were presented as the mean ± SE, and P < 0.05 was used as the level of statistical significance. The effects of temperature (20 and 25 °C) and starvation periods (1, 2 weeks and half-lethal) on R, V max, A max, D 120ms and U cat were determined using a two-way covariance analysis of variance (ANCOVA) with body length as the covariate. The ANOVA was followed by a Duncan’s multiple range test (among different starvation groups) or t test (between two temperature groups) if a statistical evaluation comparing different starvation or temperature group values was necessary. The relationships between V max, U cat and starvation time (T) at each temperature were also determined using a one-way covariance analysis of variance (ANCOVA); i.e., we performed a regression for each treatment group and then did a comparison of their coefficients.
Results
R
The temperature and starvation both had significant effects on R (P < 0.05; Table 2). The R was generally shorter at the high temperature when compared to those at the low temperature (P = 0.026). Starvation resulted in an increase in R at the high temperature, but not at the low temperature (P < 0.05).
V max
The temperature and starvation both had significant effects on V max (P < 0.05; Table 2). Fish acclimated at the high temperature generally showed higher V max than those acclimated at the low temperature (P = 0.006). V max gradually decreased with the starvation period, and there was a significant difference between the control and half-lethal period groups at both temperatures (P = 0.001). The relationship between V max (Y, m s−1) and starvation time (X, week) was Y 15 = −0.062X + 1.568 (r = −0.665, n = 36, P < 0.001) at the low temperature and Y 25 = −0.091X + 1.755 (r = −0.391, n = 40, P = 0.013) at the high temperature (Fig. 1). The ANCOVA indicated that the slopes of equations showed no significant difference between the low and high temperatures (F 1,72 = 0.796, P = 0.375).
A max
The acclimation temperature resulted in a significantly higher A max (P = 0.025), while starvation showed no significant effect on A max (Table 2).
D 120ms
The temperature and starvation both had significant effects on D 120ms (P < 0.001). D 120ms was generally longer at the high temperature than at the low temperature (P < 0.001). Fish in the half-lethal period showed significantly shorter D 120ms than those of the control group at the high temperature and all of the other three groups at the low temperature (P < 0.05).
U cat
Starvation resulted in a significant decrease in U cat in both temperature groups (P < 0.001, Table 2). In the control group, fish acclimated at the high temperature showed a significantly higher U cat than fish acclimated at the low temperature; however, this difference vanished as fish in the high temperature showed a more profound decrease with starvation (31 vs. 18 % after starvation for the half-lethal period). The relationship between U cat (Y, cm s−1) and starvation time (X, week) was Y 15 = −1.649X + 55.418 (r = −0.398, n = 34, P = 0.020) at the low temperature and Y 25 = −4.917X + 62.916 (r = −0.793, n = 33, P < 0.001) at the high temperature (Fig. 2). The ANCOVA indicated that the slopes of equations showed significant difference between the low and high temperatures (F 1,63 = 9.688, P = 0.003).
Discussion
Effect of starvation on fast-start and constant acceleration swimming performance
Many ectotherms can withstand weeks, months or even years of food deprivation without mortality (Biro et al. 2004; Wang et al. 2012). However, the half-lethal periods of rose bitterling were only 6 and 4 weeks at the low temperature and high temperature, respectively, which may be related to the small body mass and low energy reserve in rose bitterling (Biro et al. 2004).
There were many factors that could affect the ‘successful escape’ of fish, such as body size, habitation, environmental factors and body functionalities (Webb 1976; Domenici and Blake 1991; Domenici 2003; Gerry et al. 2012; Penghan et al. 2014a). Among them, the fast-start escape ability and constant acceleration swimming performance are two of the main determining factors of a ‘successful escape’ in fish species (Penghan et al. 2014a). In the present study, rose bitterling showed a typical C-start movement (Domenici 2003). The maximum swimming speed in both fast-start and acceleration swimming (i.e., V max and U cat), which were the two most reliable parameters of these two swimming capacities, showed no decrease until fish were fasted to the half-lethal period at both temperatures (except that U cat at the high temperature showed a significant decrease after 2 weeks of starvation). Thus, the results suggested that short-term starvation had no effect on both swimming capacities, hence the escape ability of this fish species. This result was consistent with the results of previous studies in crucian carp (Carassius carassius) and southern catfish (Silurus meridionalis), which showed 28 and 30 days, respectively, of starvation had no effect on V max (Penghan et al. 2014b; Yan et al. 2015). The conservativeness of fast-start and acceleration swimming performances to starvation was possibly due to their important roles in prey–predator interactions which became utmost important under starvation (Domenici and Blake 1997; Reidy et al. 2000; Penghan et al. 2014b; Yan et al. 2015).
In the present study, the starvation treatments resulted in a 25 and 21 % decrease in V max and a 17 and 30 % decrease in U cat after starvation for the half-lethal period. These results meant that both swimming capacities and the escape ability were eventually impaired after long-term starvation. These results were consistent with the results of previous studies in some fish species, which showed a 81 % decrease in V max after 6 weeks of starvation in qingbo (Spinibarbus sinensis), a 20 % decrease in V max in southern catfish after 4 weeks of starvation and a 32 % decrease in U cat in crucian carp after approximately 4 weeks of starvation (Wang et al. 2012; Penghan et al. 2014b; Yan et al. 2015). It is because that the energy stores in fish, such as ATP and PCr in the muscles, which play an important role during both swimming performances would profoundly decrease after long-term starvation (Fournier and Weber 1994; Weber 2011; Zhao et al. 2012). Furthermore, long periods of starvation can lead to the degradation of skeletal muscle, as structural proteins are catabolized for fuel (Killen et al. 2014), while the atrophic muscle fibers might impair the contraction function of muscles and, hence, the fast-start and constant acceleration swimming performances (Méndez and Wieser 1993; Maddock and Burton 1994; Martínez et al. 2003). In the present study, the long-term starvation treatments produced significant decreases in the condition factor at both temperatures. These results suggested that the muscle glycogen level decreased to some extent, which may have had a significant effect on U cat (Kieffer and Tufts 1998; Tang et al. 2010; Zhao et al. 2012). This might be the reason that fish at the high temperature showed a significantly lower U cat after 2 weeks of starvation than the control group. The V max and U cat at the low temperature showed significant decreases until the half-lethal period, as muscle glycogen decreased more rapidly at the high temperature and only affected U cat. Nevertheless, long-term starvation eventually resulted in the impairment in the fast-start and acceleration swimming performances, and the effect manifested more profoundly in U cat when fish were acclimated at the high temperature.
Effect of acclimation temperature on fast-start and constant acceleration swimming performance
In this study, the V max and U cat of non-starvation rose bitterling were higher at the high temperature than at the low temperature. This result was similar to previously published data in some fish species (e.g., Lyon et al. 2008; Yan et al. 2012; Penghan et al. 2014a). The reduction in swimming performance with a decreasing temperature could stem from changes in both the external and internal environments of the fish, e.g., increased water viscosity, and hence, increased drag force when swimming at low temperatures (Temple and Johnston 1997), reduced metabolic power and skeletal muscle contractility generated by the muscle (Randall and Brauner 1991; Day and Butler 2005), decreased biochemical reaction rates (Franklin 1998) and lower contents of some energy substrates (PCr and ATP) in the bodies of fish living in cold water (Kieffer 2000; Kieffer et al. 1994).
Interestingly, the slopes of equations which described the relationship between U cat and the starvation period showed a significant difference between the low and high temperatures, while this was not the case between V max and the starvation period according to the analysis results of ANCOVA. These results suggested that V max decreased similarly to the starvation period between the two temperatures, while U cat decreased more profoundly at the high temperature. The measured value of U cat at the high temperature was even lower than that at the low temperature at 2 weeks and the half-lethal period (not significantly different). The cytosolic ATP and PCr are the most likely fuels to be utilized during the short-term bursts that comprise fast-start swimming (Reidy et al. 2000), whereas glycogenolysis within the white muscle provides the majority of the ATP required to sustain muscular exertion during U cat (Dobson and Hochachka 1987; Wood 1991). Therefore, the different responses in swimming patterns may be because of the different energy substrate utilization when faced with food deprivation at different temperatures, as the muscle glycogen storage was more susceptible to change than ATP and PCr during starvation. It was worthy to note that although the V max showed a similar tendency to starvation between the high and low temperatures, R increased significantly with starvation at the high temperature, while it showed no change at the low temperature. It has been suggested that the individual with a short response time (R) toward a predator may possess a better chance to survive predator–prey encounters (Domenici and Kapoor 2010). Thus, the change of R might also cause a significant effect on the escape ability of rose bitterling under different conditions.
In conclusion, both the starvation treatment and temperature acclimation had profound effects on the fast-start escape and constant acceleration swimming performances in rose bitterling. Both swimming performances were quite conservative after short-term starvation, while they eventually decreased after long-term starvation (half-lethal period) and the possible depletion of muscle fuel storage, such as ATP, PCr and glycogen. Fish acclimated at the high temperature showed superior fast-start and constant acceleration swimming performances, as anticipated. However, U cat decreased more profoundly at the high temperature, and there was no difference between the two temperatures after starvation, although this was not the case in V max. The different responses in the swimming patterns may be because of the different energy substrate utilized when the fish faced food deprivation at different temperatures.
References
Arnold SJ (1983) Morphology, performance and fitness. Am Zool 23:347–361
Biro PA, Morton AE, Post JR, Parkinson EA (2004) Over-winter lipid depletion and mortality of age-0 rainbow trout (Oncorhynchus mykiss). Can J Fish Aquat Sci 61:1513–1519
Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relation in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus mykiss). Am Zool 11:99–113
Burt JM, Hinch SG, Patterson DA (2012) Developmental temperature stress and parental identity shape offspring burst swimming performance in sockeye salmon (Oncorhynchus nerka). Ecol Freshw Fish 21:176–188
Claireaux G, Couturier C, Groison AL (2006) Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass (Dicentrarchus labrax). J Exp Biol 200:3420–3428
Day N, Butler PJ (2005) The effects of acclimation to reversed seasonal temperatures on the swimming performance of adult brown trout Salmo trutta. J Exp Biol 208:2683–2692
Ding RH (1994) The fishes of Sichuan. Sichuan Publishing House of Science and Technology, Chengdu, pp 174–176
Dobson GP, Hochachka PW (1987) Role of glycolysis in adenylate depletion and repletion during work and recovery in teleost white muscle. J Exp Biol 129:125–140
Domenici P (2003) Habitat, body design and the swimming performance of fish. In: Bels VL, Gasc JP, Casinos A (eds) Vertebrate biomechanics and evolution. BIOS Scientific, Milton Park, pp 137–160
Domenici P, Blake RW (1991) The kinematics and performance of the escape response in the angelfish (Pterophyllum eimekei). J Exp Biol 156:187–205
Domenici P, Blake R (1997) The kinematics and performance of fish fast-start swimming. J Exp Biol 200:1165–1178
Domenici P, Kapoor BG (2010) Fish locomotion. Science, Enfield, pp 123–170
Eaton RC, Bombardieri RA, Meyer DH (1977) The Mauthner initiated startle response in teleost fish. J Exp Biol 66:65–81
Fournier RA, Weber JM (1994) Locomotory energetics and metabolic fuel reserves of the Virginia opossum. J Exp Biol 197:1–16
Franklin CE (1998) Studies of evolutionary temperature adaptation: muscle function and locomotor performance in Antarctic fish. Clin Exp Pharmacol Physiol 25:753–756
Fu SJ, Pang X, Cao ZD, Peng JL, Yang GJ (2011) The effects of fasting on the metabolic interaction between digestion and locomotion in juvenile southern catfish (Silurus meridionalis. Comp Biochem Physiol A 158:498–505
Gerry SP, Robbins A, Ellerby DJ (2012) Variation in fast-start performance within a population of polyphenic bluegill (Lepomis macrochirus). Physiol Biochem Zool 85:697–703
Jobling M (1980) Effects of starvation on proximate chemical composition and energy utilization of plaice Pleuronectes platessa L. J Fish Biol 17:325–334
Kieffer JD (2000) Limits to exhaustive exercise in fish. Comp Biochem Physiol A 126:161–179
Kieffer JD, Tufts BL (1998) Effects of food deprivation on white muscle energy reserves in rainbow trout (Oncorhynchus mykiss): the relationships with body size and temperature. Fish Physiol Biochem 19:239–245
Kieffer J, Currie S, Tufts B (1994) Effects of environmental temperature on the metabolic and acid-base responses of rainbow trout to exhaustive exercise. J Exp Biol 194:299–317
Killen SS, Marras S, McKenzie DJ (2014) Fast growers sprint slower: effects of food deprivation and refeeding on sprint swimming performance in individual juvenile European sea bass. J Exp Biol 217:859–865
Law T, Blake R (1996) Comparison of the fast-start performances of closely related, morphologically distinct threespine sticklebacks (Gasterosteus spp.). J Exp Biol 199:2595–2604
Lee CG, Farrell AP, Lotto A, MacNctt MJ, Hinch SG, Healey MC (2003) The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J Exp Biol 206:3239–3251
Luo YP, Wang W, Zhang YR, Huang QD, Lim D (2013) Effects of starvation on the excess post-exercise oxygen consumption of juvenile Nile tilapia (Oreochromis niloticus). Mar Freshw Behav Physiol 45:333–342
Lyon JP, Ryan TJ, Scroggie MP (2008) Effects of temperature on the fast-start swimming performance of an Australian freshwater fish. Ecol Freshw Fish 17:184–188
Maddock DM, Burton MPM (1994) Some effects of starvation on the lipid and skeletal muscle layers of the winter flounder, Pleuronectes americanus. Can J Zool 72:1672–1679
Marras S, Claireaux G, McKenzie DJ, Nelson JA (2010) Individual variation and repeatability in aerobic and anaerobic swimming performance of European sea bass, Dicentrarchus labrax. J Exp Biol 213:26–32
Martínez M, Guderley H, Dutil JD, Winger PD, He P, Walsh SJ (2003) Condition, prolonged swimming performance and muscle metabolic capacities of cod Gadus morhua. J Exp Biol 206:503–511
McCue MD (2010) Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A 156:1–18
Méndez G, Wieser W (1993) Metabolic responses to food deprivation and refeeding in juveniles of Rutilus rutilus (Teleosteei: Cyprinidae). Environ Biol Fish 36:73–81
Pang X, Cao ZD, Peng JL, Fu SJ (2010) The effects of feeding on the swimming performance and metabolic response of juvenile southern catfish, Silurus meridionalis, acclimated at different temperatures. Comp Biochem Physiol A 155:253–258
Pang X, Yuan XZ, Cao ZD, Fu SJ (2014) The effects of fasting on swimming performance in juvenile qingbo (Spinibarbus sinensis) at two temperatures. J Therm Biol 42:25–32
Penghan LY, Cao ZD, Fu SJ (2014a) Effect of temperature and dissolved oxygen on swimming performance in crucian carp. Aquat Biol 21:57–65
Penghan LY, Cao ZD, Fu SJ (2014b) Effect of starvation on swimming performance of juvenile carp. Chin J Ecol 33:2756–2760
Randall D, Brauner C (1991) Effects of environmental factors on exercise in fish. J Exp Biol 160:113–126
Reidy SP, Kerr SR, Nelson JA (2000) Aerobic and anaerobic swimming performance of individual Atlantic cod. J Exp Biol 203:347–357
Sheridan MA, Mommsen TP (1991) Effects of nutritional state on in vivo lipid and carbohydrate metabolism of coho salmon Oncorhynchus kisutch. Gen Comp Endocr 81:473–483
Shulman GE (1974) Life cycles of fish: physiology and biochemistry. Wiley, New York, pp 257–258
Tang HF, Cao ZD, Fu SJ (2010) The relationship among resting metabolic rate, body composition and excess post-excess oxygen consumption during fasting in silurus asotus. Acta Hydrobiol Sin 34:190–195
Temple GK, Johnston IA (1997) The thermal dependence of fast-start performance in fish. J Therm Biol 22:391–401
Walker JA, Ghalambor CK, Griset OL, McKenney D, Reznick DN (2005) Do faster starts increase the probability of evading predators? Funct Ecol 19:808–815
Wang F, Chen BJ, Cao ZD, Wang YX, Fu SJ (2012) The influence of starvation on fast-start performance of Spinibar bussinensis. Acta Ecol Sin 32:291–296
Webb PW (1976) The effect of size on fast-start performance of rainbow trout (Salmo gaidneri) and a consideration of piscivorous predator–prey interactions. J Exp Biol 65:157–177
Webb PW (1986) Effect of body form and response threshold on the vulnerability of four species of teleost prey attacked by largemouth bass (Micropterus salmoides). Can J Fish Aquat Sci 43:763–771
Weber JM (2011) Metabolic fuels: regulating fluxes to select mix. J Exp Biol 214:286–294
Wood CM (1991) Acid–base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish. J Exp Biol 160:285–308
Yan GJ, He XK, Cao ZD, Fu SJ (2012) The trade-off between steady and unsteady swimming performance. J Therm Biol 37:424–431
Yan GJ, He XK, Cao ZD, Fu SJ (2015) Effects of fasting and feeding on the fast-start swimming performance of southern catfish Silurus meridionalis. J Fish Biol 86:605–614
Zhao WW, Pang X, Peng JL, Cao ZD, Fu SJ (2012) The effects of hypoxia acclimation, exercise training and fasting on swimming performance in juvenile qingbo (Spinibarbus sinensis). Fish Physiol Biochem 38:1367–1377
Acknowledgments
This study was funded by a grant from the National Science Foundation of China (NSFC 31300340; 31300341). All experiments were conducted according to the Guidelines on the Humane Treatment of Laboratory Animals established by the Ministry of Science and Technology of the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Liu-Yi Penghan and Xu Pang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Penghan, LY., Pang, X. & Fu, SJ. The effects of starvation on fast-start escape and constant acceleration swimming performance in rose bitterling (Rhodeus ocellatus) at two acclimation temperatures. Fish Physiol Biochem 42, 909–918 (2016). https://doi.org/10.1007/s10695-015-0184-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0184-0