Abstract
The objective of this study was to describe the epidemiological and molecular surveillance of rotaviruses in tap water, recycled water, and sewage sludge in Thailand from 2007 to 2018. Three hundred and seventy tap water, 202 recycled water, and 72 sewage sludge samples were collected and processed to detect the rotavirus VP7 gene using RT-nested PCR. Rotavirus G genotypes were identified by DNA sequencing and phylogenetic analysis. The frequency of rotavirus detection was 0.54% of the tap water samples, 30.2% of the recycled water samples, and 50.0% of the sewage sludge samples. During the 12-year surveillance, G1 was prevalent most years and constantly predominant in recycled water and sewage sludge. G2 was identified in a tap water sample and in recycled water samples. G3 and G9 were observed in both recycled water and sewage sludge samples. The uncommon G6 rotavirus strain was identified in one recycled water sample. The rotavirus VP4 gene was detected in rotavirus strains with an identified G genotype using RT-multiplex nested PCR. The unusual P[6] genotype was the most frequently detected, followed by mixed P[6]/[4] and P[4] genotypes. Phylogenetic analysis of both G and P genotypes showed a close genetic relationship with sequences of human rotavirus strains. The high nucleotide identity of the rotavirus strains found in this study to human rotavirus strains suggests that the rotaviruses are derived from human source. These results represent useful epidemiological and molecular information for evaluating rotavirus distribution in water for consumption and irrigation, and in biosolids for agricultural application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rotavirus is the most common cause of acute gastroenteritis in children in upper income countries as well as in low- and middle-income countries. Rotavirus is estimated to account for approximately 39% of diarrheal patients under 5 years of age admitted to hospital and an estimated 200,000 deaths each year (Global Burden of Diarrhoeal Diseases Collaborators 2017). The medical and societal burden due to rotavirus gastroenteritis has been dramatically reduced after the introduction of two licensed human rotavirus vaccines from 2006. However, their effectiveness appears to be significantly lower in low-income countries, and immunization programs have not yet reached many countries, particularly those in Africa and Asia with high childhood mortality (Mokomane et al. 2018). Rotaviruses belong to the genus Rotavirus in the family Reoviridae (Estes and Greenberg 2013). Based upon the antigenic response to antibodies targeting the middle layer protein VP6, rotaviruses are classified into eight species (A–H) and two tentative species (I and J) infecting human and animal hosts. Group A rotavirus is the most common species causing acute gastroenteritis in humans. The outer layer proteins VP7 and VP4 are the basis of a binary classification system for G types (glycoprotein) and P types (protease-sensitive protein), respectively. The group A rotavirus G and P genotypes are defined according to nucleotide sequence differences of genes 9 (VP7) and 4 (VP4). At least 36 G genotypes and 51 P genotypes have been identified (RCWG 2018) and six G/P genotype combinations: G1P[8], G2P[4], G3P[8], G4P[8], G9P[8] and G12P[8] are mainly associated with the global rotavirus disease burden (Dóró et al. 2014).
Infants and young children infected with human rotavirus always show acute diarrheal symptoms, whereas adults are frequently reinfected with rotavirus but with mild to moderate or no clinical manifestations. Rotaviruses are contagious, shed in large amounts in the feces of infected individuals, and quite stable in the environment. The virus is transmissible by the fecal–oral route either through direct person-to-person contact or through contaminated water and food (Estes and Greenberg 2013). Environmental contamination of surface water and discharge of wastewater into drinking water sources are risk factors for waterborne outbreaks caused by enteric pathogens (Moriera and Bondelind 2017). Waterborne outbreaks caused by rotavirus have increasingly become a public health concern, and they have been shown to be associated with contaminated tap water (Martinelli et al. 2007; Mellou et al. 2014), municipal water supply (Scarcella et al. 2009), large water depository from a water well which supplied drinking water (Koroglu et al. 2011), and water distribution system failure (Altzibar et al. 2015). Rotaviruses have been detected in sewage in Italy (Ruggeri et al. 2015), Germany (Leifels et al. 2016), Spain (Santiso-Bellón et al. 2020), Venezuela (Rodríguez-Díaz et al. 2009), Brazil (Staggemeier et al. 2017), and China (Zhou et al. 2016). Additionally, the virus is resistant to different wastewater treatment procedures as reported from France (Prevost et al. 2015), Brazil (Assis et al. 2018), and Iran (Kargar et al. 2013).
In Thailand, rotavirus is the leading cause of acute diarrhea in children under 5 years of age, and is responsible for one-third of gastroenteritis cases (Sakpaisal et al. 2019), as well as occasionally infecting adults who require hospital admission (Kittigul et al. 2014b; Tacharoenmuang et al. 2020). A variety of rotavirus strains have been detected in river and irrigation canal samples (Kittigul et al. 2014a), suggesting that evidence-based reports of rotaviruses in humans and the environment are essential for informed public health management. However, there have been few studies monitoring rotavirus continually in water for consumption and reuse purposes, and in sewage sludge for agricultural uses. The aim of this study was to assess the prevalence, genotypes, and molecular characteristics of group A rotavirus in tap water, recycled water, and sewage sludge samples, which are possible sources of rotavirus contamination, in Thailand during 2007–2018.
Materials and Methods
Water and Sewage Sludge Samples
From 2007 to 2018, 370 tap water samples (5 L per sample; 2007‒2011 and 20 L per sample; 2012‒2018) comprising 30‒32 samples collected during June–July each year were obtained from the Bangkok Metropolitan Region, Thailand. The tap water samples obtained from the water production, transmission and distribution system in Bangkok had been passed through water treatment processes including clarification (activated sludge), sand/anthracite coal filters and chlorine disinfection. Samples of 202 recycled water samples (5 L per sample) consisting of 7‒24 samples (1‒2 samples per month) each year between 2007 and 2018, and 72 sewage sludge samples (5 g per sample) consisting of 6‒12 samples (1‒2 samples per two months) each year between 2012 and 2018 were collected at a wastewater treatment plant (WWTP) located in Bangkok Metropolitan Region. This WWTP capacity is 16,000 m3 per day and the plant receives urban sewage produced by approximately 60,000 inhabitants. The recycled water samples were taken from the flow at the effluent after a secondary treatment system (an activated sludge process) and a tertiary treatment system with a dual media filter and a disinfection process. The sewage sludge samples were obtained from semi-solid waste that is a by-product of sewage treatment after a mechanical sludge dewatering process with lime stabilization. The tap water, recycled water, and sewage sludge samples collected were transported to the laboratory and processed to concentrate the virus.
Virus Processing
The tap water and recycled water samples were processed using an adsorption-elution method with membrane filtration as described previously (Kittigul et al. 2014a). Briefly, the water samples were adjusted to pH 3.5 and supplemented with aluminum chloride to a final concentration of 0.0015 N. A mixed cellulose ester membrane with a 0.45 µm pore size and a diameter of 47 mm (Pall Corporation, Ann Arbor, MI, USA) for small volumes or a 90 mm (Advantec®, Tokyo, Japan) for large volumes of water were used for the filtration and viral adsorption. The bound virus on the membrane was eluted by adding 2.9% tryptose phosphate broth (TPB) containing 6% glycine, pH 9.0 and the eluate was adjusted to pH 7.0–7.4. Volumes of the concentrates were reduced using a vacuum centrifuge (UNIEQUIP Laborgeratebau und-vertriebs GmbH, Munich, Germany) to 0.75–3.9 mL for the tap water and 1.3–2.2 mL for the recycled water. The sewage sludge samples were processed using an adsorption-elution method as described previously (Kittigul et al. 2014a). Briefly, the samples were mixed with deionized water (20 mL) and adjusted to pH 5.0. The virus was eluted twice by adding 2.9% TPB containing 6% glycine, pH 9.0 followed by 0.5 M arginine-0.15 M NaCl, pH 7.5. Volumes of the eluates were reduced using a vacuum centrifuge to 0.7–1.4 mL. All concentrates were stored at − 80 °C until used for nucleic acid extraction.
RNA Extraction
Viral RNAs in the concentrated water and sewage sludge samples (140 μL each) were extracted using the QIAamp® viral RNA extraction kit (QIAGEN Gmbh, Hilden, Germany) according to the manufacturer’s instruction. Extracted RNAs were eluted in 60 μL of the eluent buffer and stored at − 80 °C until rotavirus analysis.
RT-Nested PCR Amplification for Rotavirus G Genotypes
Group A rotavirus RNA was amplified by RT-nested PCR assay as described previously (Kittigul et al. 2014a) using VP7 specific primers: RV1 (GTCACATCATACAATTCTAATCTAAG) and RV2 (CTTTAAAAGAGAGAATTTCCGTCTG) for RT-PCR; and RV3 (TGTATGGTATTGAATATACCAC) and RV4 (ACTGATCCTGTTGGCCAWCC) for nested PCR. In the first round (RT-PCR), the cycling conditions consisted of reverse transcription at 41 °C for 60 min; 94 °C for 2 min, PCR of 25 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s, followed by a final extension at 72 °C for 3 min. In the second round (nested PCR), the RT-PCR product was further amplified under the same conditions of amplification as used for the first round RT-PCR for 40 cycles. The molecular size of PCR products was determined by agarose gel electrophoresis and ethidium bromide staining. The expected rotavirus amplicon was 346-bp.
Rotavirus P Genotyping
Rotavirus screening was performed using RT-nested PCR with VP7 specific primers and the positive samples were further genotyped for both VP7 (G-type) and VP4 genotypes (P type). G genotypes were identified using DNA sequencing and phylogenetic analysis. Rotavirus VP4 was detected using RT-multiplex nested PCR as described previously (Kittigul et al. 2014b). P genotypes were identified using DNA sequencing and phylogenetic analysis.
DNA Sequencing and Phylogenetic Analysis
The rotavirus PCR products from the VP7 and VP4 assays were purified using a QIAquick PCR Purification Kit or a QIAquick Gel Extraction Kit (QIAGEN Gmbh, Hilden, Germany) following the manufacturer’s protocol. The purified PCR products were subjected directly to DNA sequencing. The nucleotide sequences of the VP7 and VP4 genes were compared with those of reference strains available in the GenBank database using the BLAST (Basic Local Alignment Search Tool) server. Phylogenetic analysis was conducted using MEGA (Molecular Evolutionary Genetic Analysis), version 6.0 (Tamura et al. 2013).
GenBank Accession Numbers
The nucleotide sequences of rotavirus obtained from tap water, recycled water, and sewage sludge samples in this study were deposited in GenBank and were assigned accession numbers for VP7 genes: MT423524–MT423614 and for VP4 genes: MW075439–MW075464.
Results
Detection of Rotavirus in Water and Sewage Sludge Samples
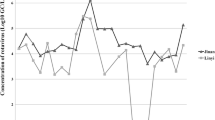
In laboratory experiments, the sensitivity of RT-nested PCR for detection as determined by seeding known rotavirus amounts into the concentrates from tap water, recycled water, and sewage sludge was 6.69 × 103 genome copies/mL or 2.23 copies/reaction. In analysis of the collected environmental samples, the group A rotavirus detection results showed that 0.54%, 30.2%, and 50.0% of the tap water, recycled water, and sewage sludge samples, respectively, were positive for rotavirus by RT-nested PCR (Table 1). In two separate years (2009 and 2013), a single tap water sample out of 30 (1/30, 3.33% for both years) was found to be positive for rotavirus RNA after which the collected tap water samples were consistently negative. The recycled water samples obtained from the wastewater treatment system showed the presence of rotavirus RNA with the highest frequency of rotavirus detection occurring in the year 2007 (5/7, 71.4%) and levels declined consistently until 2011, after which levels again increased. The collection of sewage sludge samples started in the year 2012 and rotavirus RNA was detected continuously in all years evaluated (2012‒2018) with the highest frequency occurring in 2018 (4/6, 67%) (Fig. 1). Rotavirus RNA could be detected in the recycled water and sewage sludge samples all year round, with the highest frequency in the winter months (November to February) through to the summer months (March to May) of Thailand (data not shown).
Genotype Distribution of Rotavirus
Among group A rotaviruses giving positive results by RT-nested PCR, most rotavirus strains (91/99, 91.9%) could be characterized and identified using DNA sequencing and phylogenetic analysis. The most frequent genotype of rotavirus detected in the present study was G1 (61/91, 67% of rotaviruses typed), followed by G3 (14/91, 15.4%), G9 (11/91, 12.1%), G2 (4/91, 4.4%), and G6 (1/91, 1.1%), although the distribution of the G genotypes varied according to the type of sample. Genetic diversity was observed in the recycled water samples and five (G1, G2, G3, G6, and G9) of the six rotavirus genotypes associated with the majority of human rotaviral disease were identified. Rotavirus genotypes G1, G3, and G9 were found in the sewage sludge samples. Of the two tap water samples positive for rotavirus, one sample was identified as belonging to the G2 genotype. According to the year of sample collection, one to four rotavirus genotypes were found in each year. The G1 genotype was found in the samples at the highest frequency in nine of the 12 years evaluated. The G2 genotype was found in the years 2009, 2010, and 2013; G3 in the years 2012, 2014‒2017; G6 in the year 2013; and G9 in the years 2008, 2013, 2015‒2017 (Fig. 2).
Of 91 group A rotavirus strains with identified G genotype, 59 (64.8%) could be genotyped for P type, and 9 (9.9%) were untypeable for the P type. P[6] (40/91, 44.0%) was the most frequently detected, followed by mixed P[6]/[4] (10/91, 11.0%) and P[4] (9/91, 9.8%). The recycled water samples gave positive results for rotavirus VP4 at a higher frequency (42/91, 46.1%) than the sewage sludge samples (26/91, 28.6%). Since different genotypes of rotaviruses might be present in an environmental sample, the combination of the G and P types in recycled water and sewage sludge samples could not be determined.
Molecular Characterization of Rotavirus G and P Genotypes
The 61 G1 rotavirus strains clustered with strains from the G1 lineage I (39 samples; 19 recycled water and 20 sewage sludge) and G1 lineage II (22 samples; 14 recycled water and 8 sewage sludge). The G1-I lineage strains exhibited 97.59–100% nucleotide identity with human rotavirus group A strains detected in Thailand, Taiwan, India, and Lebanon. The G1-II lineage strains exhibited 97.24–100% nucleotide identity with human rotavirus group A strains detected in Thailand, India, Russia, Italy, and Belgium (Fig. 3a). The 4 G2 rotavirus group A strains clustered with strains from the G2 lineage IV (3 recycled water samples and 1 tap water sample). These strains exhibited 99.66–100% nucleotide identity with human rotavirus group A strains detected in Taiwan and Japan, and with the rotavirus strain (Oys093) found in oyster from Thailand. The 14 G3 rotavirus strains grouped together with strains from the G3 lineage I (4 samples; 2 each for recycled water and sewage sludge) and with strains in the G3 lineage IX (10 samples; 8 recycled water and 2 sewage sludge). The G3-I lineage strains exhibited 98.98–99.66% nucleotide identity with human rotavirus group A strains detected in Pakistan and Iran. The G3-IX lineage strains exhibited 98.30–99.32% nucleotide identity with human rotavirus group A strains detected in Thailand and Pakistan. One rotavirus group A strain clustered with strains from the G6 lineage I. This G6-I lineage strain exhibited 95.92% nucleotide identity and was closely related to the human rotavirus group A strain identified in Belgium. The 11 G9 rotavirus strains grouped together with strains from the G9 lineage III (9 samples; 7 recycled water and 2 sewage sludge) and strains in the G9 lineage VI (2 samples; 1 each for recycled water and sewage sludge). The G9-III lineage strains separated into two branches with 97.19–100% nucleotide identity and were closely related to human rotavirus group A strains found in Thailand, Taiwan, Japan, India, and Lebanon. The G9-VI lineage strains exhibited 96.49–97.89% nucleotide identity with human rotavirus group A strains detected in China and Japan (Fig. 3b).
Phylogenetic trees obtained from partial nucleotide sequences of the VP7 gene of group A rotavirus together with known human and animal rotavirus strains from the GenBank database. The trees of rotavirus G genotypes are shown: G1 (a), and G2–G9 (b). The strains labeled with filled circles, filled squares, and filled star indicate rotaviruses detected in recycled water, sewage sludge, and tap water, respectively. The phylogenetic trees were constructed using the Maximum Likelihood Method with 1000 bootstrap replicates. Percent bootstrap support is indicated by the value at each node when the value was ≥ 70%. The scale bar at the bottom of the tree indicates genetic distance
The 26 rotavirus strains with an identified G genotype were used in a phylogenetic analysis of the VP4 gene. Most of them (76.9%) clustered with strains from the P[6] lineage Ia exhibiting 98.73–100% nucleotide identity with human rotavirus group A strains detected in Thailand, Pakistan, South Africa, Ethiopia, and Indonesia. Some rotavirus strains (23.1%) clustered with strains from the P[4] lineage IV exhibiting 99.58–100% nucleotide identity with human rotavirus group A strains detected in Bangladesh, Taiwan, Korea, and Vietnam (Fig. 4).
Phylogenetic trees obtained from partial nucleotide sequences of the VP4 gene of group A rotavirus together with known human and animal rotavirus strains from the GenBank database. The trees of rotavirus P genotypes are shown. The strains are labeled with filled circles and filled squares which indicate rotaviruses detected in recycled water, and sewage sludge, respectively. The phylogenetic trees were constructed using the Maximum Likelihood Method with 1000 bootstrap replicates. Percent bootstrap support is indicated by the value at each node when the value was ≥ 70%. The scale bar at the bottom of the tree indicates genetic distance
Discussion
Source water contamination with rotavirus is a public health concern of considerable significance. Wastewater often becomes an irrigation water source and insufficient treatment of the wastewater may cause outbreaks of viral diseases. Enteric viruses including rotavirus are required for virus risk management (Sano et al. 2016). This study undertook a longitudinal continuous epidemiological surveillance of group A rotavirus in tap water, recycled water, and sewage sludge for twelve consecutive years (2007–2018) in Thailand. Only two samples containing rotavirus were observed in tap water, which is a lower detection rate (0.54%) than that in previously reported in Brazil (16.4%) (Kluge et al. 2014). Although the prevalence of rotavirus in tap water was low, it is of significant concern, as this finding implies that the rotavirus had passed through the treatment process and entered the tap water distribution system. Rotavirus-contaminated tap water influences drinking water quality, which is considered to be a resource for safe drinking water. Adequate treatment and disinfection of drinking water has been documented to reduce the potential risk from human rotavirus (World Health Organization 2017).
Water derived from a WWTP is intended for recycling, agriculture reuse, and discharge into natural aquatic receiving environments. The presence of rotavirus in recycled water also supports the resistance of rotavirus to wastewater treatment, as shown in previous studies (Kargar et al. 2013; Prevost et al. 2015; Assis et al. 2018; Prado et al. 2019). Improvement of the treatment process could remove group A rotavirus in treated wastewater (Ibrahim et al. 2020), in the effluents from WWTPs (Randazzo et al. 2019) and in the drinking water from water treatment plants (Atabakhsh et al. 2019). Meanwhile, sewage sludge, which is obtained after the treatment process, had the highest rotavirus detection rate. Given that sewage sludge is often used as a source of nutrients in agricultural applications, the presence of rotavirus RNA is a cause for significant concern. To our knowledge, this is the first report on the presence of rotavirus in sewage sludge, and this finding suggests that rotavirus has the potential to enter the environment with concomitant adverse effects on human health, including acute gastroenteritis. Although the RT-nested PCR method used in this study cannot discriminate between the presence of infectious and non-infectious viral particles in water and sewage sludge, the high rates of detection suggest the dispersal of rotavirus into the environment.
In Thailand, severe flooding occurred in many areas throughout the country in 2011; however, rotavirus was not detected in either tap water or recycled water collected in that year. This is in contrast to a previous study that demonstrated enteric virus contamination of drinking water from wells due to a flood in Italy (Masciopinto et al. 2019). The difference may result from the source of water supplies and water treatment processes, especially in flood situations. After 2011, rotaviruses increased significantly in both recycled water and sewage sludge samples suggesting the persistent contamination of environmental sources from the agricultural applications of these resources. The seasonality of rotavirus infection in tropical regions may vary from place-to-place and from year to year. The peak of rotavirus present in the recycled water and sewage sludge was found in samples from the winter season of Thailand; however, the high prevalence was also observed in samples from the summer season, corresponding to previous reports of the rotavirus prevalence in patients with acute gastroenteritis (Sakpaisal et al. 2019; Tacharoenmuang et al. 2020).
During all years of the sampling period (2007–2018), the predominant G1 strain similar to human rotavirus strains was distributed in the recycled water and sewage sludge samples. Rotavirus G1 strains were prevalent in domestic sewage in Venezuela, 2007–2008 (Rodríguez-Díaz et al. 2009), urban and hospital sewage in Iran, 2010–2011 (Kargar et al. 2013), and sewage from WWTPs in Italy, 2010–2011 (Ruggeri et al. 2015). Other genotypes including G2, G3, and G9 similar to human rotavirus strains found in this study to a lesser extent correspond to a previous study in Italy, 2010–2011 (Ruggeri et al. 2015), whereas a study in Spain, 2015–2016, revealed the circulation of G2, G3, G9, but not G1 in raw sewage from WWTPs (Silva-Sales et al. 2020). Our previous study on samples from irrigation canals and rivers in Thailand during 2006–2007 revealed the highest prevalence of G3 followed by G1, G2, and G9 strains also demonstrating similarity to human rotavirus strains (Kittigul et al. 2014a). Interestingly, in the present study, rotavirus G2 strains were only identified in a tap water sample and in recycled water samples and were not present in sewage sludge. It is possible that this genotype might be resistant to water treatment and disinfection processes. Nevertheless, recycled water which had passed through the water treatment process also contained other rotavirus genotypes suggesting similar resistance of G1, G3, G6, and G9. The uncommon G6 genotype was detected in a recycled water sample and it is related phylogenetically to G6 strains in humans. This finding is consistent with the previous study in Italy (Ruggeri et al. 2015) suggesting that a rare G6 strain may have circulated in the city population, being shed with feces in large amounts into the wastewater and remains in the water for reuse purposes.
The G1 strain being the most prevalent rotavirus in environmental samples is in agreement with other studies in patients with acute gastroenteritis during 2007–2018 in Italy (de Waure et al. 2020), the same period as this study, and in a long 35-year observation (1984–2019) undertaken in Russia (Novikova et al. 2020). Similarly, the predominance of G1 and other genotypes (G2, G3, and G9) of rotavirus strains found in the current study are in accordance with earlier studies performed in samples from patients with acute gastroenteritis in Thailand during 2008–2010 (Sakpaisal et al. 2019), 2011–2014 (Chieochansin et al. 2016), and 2014–2016 (Tacharoenmuang et al. 2020). Of note, the decrease of G1 and the emergence of G3 as seen in recycled water and sewage sludge between 2014 and 2016 correspond to the rotavirus genotypes observed in samples from children and adults with acute gastroenteritis during the same period (Tacharoenmuang et al., 2020). Human rotavirus strains detected in environmental samples such as sewage sludge suggest the virus is circulating in the general population, being shed into wastewater and probably resisting wastewater treatment systems.
Since the authors used a more stringent methodology (RT-nested PCR) for the rotavirus group A detection and characterization of rotavirus G-type than for the VP4 genotyping (P type) which was carried out using RT-multiplex nested PCR, it is possible that this is reflected in the higher frequency of G-type rotavirus detected. Additional P genotyping in identified G rotavirus strains revealed an interesting finding of the predominance of P[6] followed by P[4] which are similar to human rotavirus strains, suggesting that these strains may have originated from human rotavirus. P[8] and P[4] are the most common VP4 types found in humans (Dóró et al. 2014) whereas P[6] is a rare genotype in various countries including Thailand (Chieochansin et al. 2016; Sakpaisal et al. 2019; Tacharoenmuang et al. 2020). The unusual rotavirus G1P[6] strain is reported to be mainly responsible for acute diarrhea in hospitalized children and adult patients in India (Jain et al. 2016). The common P type, P[8], was not detected in the recycled water and sewage sludge samples. The present study may imply a high resistance of P[6] and P[4] to the treatment process of wastewater.
This study has some limitations. Initially, virus recovery of the methodology was not determined using an internal control, although a high sensitivity of the RT-nested PCR was obtained for different kinds of environmental samples. In addition, determination of the viral loads in the rotavirus-positive samples was not undertaken. A highly sensitive RT-nested PCR methodology was used for the group A rotavirus detection and characterization of the G-type, but this method does not allow detection of mixed G genotypes in the same sample. Further investigations of the rotavirus present in the environment at the molecular level will provide crucial data on group A rotavirus epidemiology and strain diversity.
In conclusion, our study shows the epidemiological trends of rotavirus in tap water, recycled water, and sewage sludge, and common G and uncommon P rotavirus genotypes circulating in the environment in Thailand. Continuous and ongoing screening for rotavirus in potable water and environmental samples is necessary to improve public health and for proper assessment and management of the risks of acute gastroenteritis.
References
Altzibar, J. M., Zigorraga, C., Rodriguez, R., Leturia, N., Garmendia, A., Rodriguez, A., et al. (2015). Outbreak of acute gastroenteritis caused by contamination of drinking water in a factory, the Basque Country. Journal of Water and Health, 13(1), 168–173.
Assis, A. S. F., Fumian, T. M., Miagostovich, M. P., Drumond, B. P., da Rosa, E., & Silva, M. L. (2018). Adenovirus and rotavirus recovery from a treated effluent through an optimized skimmed-milk flocculation method. Environmental Science and Pollution Research International, 25(17), 17025–17032.
Atabakhsh, P., Kargar, M., & Doosti, A. (2019). Molecular surveillance of human rotaviruses in drinking water and investigation of the efficiency of their removal in Isfahan water treatment plant. Environmental Monitoring and Assessment, 191(12), 759.
Chieochansin, T., Vutithanachot, V., Phumpholsup, T., Posuwan, N., Theamboonlers, A., & Poovorawan, Y. (2016). The prevalence and genotype diversity of human rotavirus A circulating in Thailand, 2011–2014. Infection, Genetics and Evolution, 37, 129–136.
de Waure, C., Sarnari, L., Chiavarini, M., Ianiro, G., Monini, M., Alunno, A., et al. (2020). 10-year rotavirus infection surveillance: Epidemiological trends in the pediatric population of Perugia Province. International Journal of Environmental Research and Public Health, 17(3), 1008.
Dóró, R., László, B., Martella, V., Leshem, E., Gentsch, J., Parashar, U., et al. (2014). Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infection, Genetics and Evolution, 28, 446–461.
Estes, M. K., & Greenberg, H. B. (2013). Rotaviruses. In D. M. Knipe & P. M. Howley (Eds.), Fields virology (6th ed., Vol. II, pp. 1347–1401). Philadelphia: Lippincott Williams & Wilkins.
Global Burden of Diarrhoeal Diseases Collaborators. (2017). Estimates of global, regional, national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet Infectious Diseases, 17(9), 909–948.
Ibrahim, C., Hammami, S., Pothier, P., Khelifi, N., & Hassen, A. (2020). The performance of biological and tertiary wastewater treatment procedures for rotaviruses A removal. Environmental Science and Pollution Research International, 27(6), 5718–5729.
Jain, S., Thakur, N., Vashistt, J., Grover, N., Krishnan, T., & Changotra, H. (2016). Predominance of unusual rotavirus G1P[6] strain in North India: An evidence from hospitalized children and adult diarrheal patients. Infection, Genetics and Evolution, 46, 65–70.
Kargar, M., Javdani, N., Najafi, A., & Tahamtan, Y. (2013). First molecular detection of group A rotavirus in urban and hospital sewage systems by nested-RT PCR in Shiraz, Iran. Journal of Environmental Health Science & Engineering, 11(1), 4.
Kittigul, L., Panjangampatthana, A., Rupprom, K., & Pombubpa, K. (2014a). Genetic diversity of rotavirus strains circulating in environmental water and bivalve shellfish in Thailand. International Journal of Environmental Research and Public Health, 11(2), 1299–1311.
Kittigul, L., Swangsri, T., Pombubpa, K., Howteerakul, N., Diraphat, P., & Hirunpetcharat, C. (2014b). Rotavirus infection in children and adults with acute gastroenteritis in Thailand. The Southeast Asian Journal of Tropical Medicine and Public Health, 45(4), 816–824.
Kluge, M., Fleck, J. D., Soliman, M. C., Luz, R. B., Fabres, R. B., Comerlato, J., et al. (2014). Human adenovirus (HAdV), human enterovirus (hEV), and genogroup A rotavirus (GARV) in tap water in southern Brazil. Journal of Water and Health, 12(3), 526–532.
Koroglu, M., Yakupogullari, Y., Otlu, B., Ozturk, S., Ozden, M., Ozer, A., et al. (2011). A waterborne outbreak of epidemic diarrhea due to group A rotavirus in Malatya, Turkey. New Microbiologica, 34(1), 17–24.
Leifels, M., Hamza, I. A., Krieger, M., Wilhelm, M., Mackowiak, M., & Jurzik, L. (2016). From lab to lake—evaluation of current molecular methods for the detection of infectious enteric viruses in complex water matrices in an urban area. PLoS ONE, 11(11), e0167105.
Martinelli, D., Prato, R., Chironna, M., Sallustio, A., Caputi, G., Conversano, M., et al. (2007). Large outbreak of viral gastroenteritis caused by contaminated drinking water in Apulia, Italy, May-October 2006. Eurosurveillance, 12(4), E070419.
Masciopinto, C., De Giglio, O., Scrascia, M., Fortunato, F., La Rosa, G., Suffredini, E., et al. (2019). Human health risk assessment for the occurrence of enteric viruses in drinking water from wells: Role of flood runoff injections. The Science of The Total Environment, 666, 559–571.
Mellou, K., Katsioulis, A., Potamiti-Komi, M., Pournaras, S., Kyritsi, M., Katsiaflaka, A., et al. (2014). A large waterborne gastroenteritis outbreak in central Greece, March 2012: Challenges for the investigation and management. Epidemiology and Infection, 142(1), 40–50.
Mokomane, M., Kasvosve, I., de Melo, E., Pernica, J. M., & Goldfarb, D. M. (2018). The global problem of childhood diarrhoeal diseases: Emerging strategies in prevention and management. Therapeutic Advances in Infectious Disease, 5(1), 29–43.
Moriera, N. A., & Bondelind, M. (2017). Safe drinking water and waterborne outbreaks. Journal of Water and Health, 15(1), 83–96.
Novikova, N. A., Sashina, T. A., Epifanova, N. V., Kashnikov, A. U., & Morozova, O. V. (2020). Long-term monitoring of G1P[8] rotaviruses circulating without vaccine pressure in Nizhny Novgorod, Russia, 1984–2019. Archives of Virology, 165(4), 865–875.
Prado, T., de Castro Bruni, A., Barbosa, M. R. F., Garcia, S. C., de Jesus Melo, A. M., & Sato, M. I. Z. (2019). Performance of wastewater reclamation systems in enteric virus removal. The Science of The Total Environment, 678, 33–42.
Prevost, B., Lucas, F. S., Goncalves, A., Richard, F., Moulin, L., & Wurtzer, S. (2015). Large scale survey of enteric viruses in river and wastewater underlines the health status of the local population. Environment International, 79, 42–50.
Randazzo, W., Piqueras, J., Evtoski, Z., Sastre, G., Sancho, R., Gonzalez, C., et al. (2019). Interlaboratory comparative study to detect potentially infectious human enteric viruses in influent and effluent waters. Food and Environmental Virology, 11(4), 350–363.
RCWG, Rotavirus Classification Working Group (2018) Newly assigned genotypes—update May 29th. 2018. Retrieved October 15, 2019 from https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg.
Rodríguez-Díaz, J., Querales, L., Caraballo, L., Vizzi, E., Liprandi, F., Takiff, H., et al. (2009). Detection and characterization of waterborne gastroenteritis viruses in urban sewage and sewage-polluted river waters in Caracas, Venezuela. Applied and Environmental Microbiology, 75(2), 387–394.
Ruggeri, F. M., Bonomo, P., Ianiro, G., Battistone, A., Delogu, R., Germinario, C., et al. (2015). Rotavirus genotypes in sewage treatment plants and in children hospitalized with acute diarrhea in Italy in 2010 and 2011. Applied and Environmental Microbiology, 81(1), 241–249.
Sakpaisal, P., Silapong, S., Yowang, A., Boonyasakyothin, G., Yuttayong, B., Suksawad, U., et al. (2019). Prevalence and genotypic distribution of rotavirus in Thailand: A multicenter study. American Journal of Tropical Medicine and Hygiene, 100(5), 1258–1265.
Sano, D., Amarasiri, M., Hata, A., Watanabe, T., & Katayama, H. (2016). Risk management of viral infectious diseases in wastewater reclamation and reuse: Review. Environment International, 91, 220–229.
Santiso-Bellón, C., Randazzo, W., Pérez-Cataluña, A., Vila-Vicent, S., Gozalbo-Rovira, R., Muñoz, C., et al. (2020). Epidemiological surveillance of norovirus and rotavirus in sewage (2016–2017) in Valencia (Spain). Microorganisms, 8(3), 458.
Scarcella, C., Carasi, S., Cadoria, F., Macchi, L., Pavan, A., Salamana, M., et al. (2009). An outbreak of viral gastroenteritis linked to municipal water supply, Lombardy, Italy, June 2009. Eurosurveillance, 14(29), 19274.
Silva-Sales, M., Martínez-Puchol, S., Gonzales-Gustavson, E., Hundesa, A., & Gironès, R. (2020). High prevalence of rotavirus A in raw sewage samples from Northeast Spain. Viruses, 12(3), E318.
Staggemeier, R., Heck, T. M. S., Demoliner, M., Ritzel, R. G. F., Röhnelt, N. M. S., Girardi, V., et al. (2017). Enteric viruses and adenovirus diversity in waters from 2016 Olympic venues. The Science of The Total Environment, 586, 304–312.
Tacharoenmuang, R., Komoto, S., Guntapong, R., Upachai, S., Singchai, P., Ide, T., et al. (2020). High prevalence of equine-like G3P[8] rotavirus in children and adults with acute gastroenteritis in Thailand. Journal of Medical Virology, 92(2), 174–186.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729.
World Health Organization. (2017). Guidelines for drinking-water quality (4th ed.). Geneva: World Health Organization.
Zhou, N., Lv, D., Wang, S., Lin, X., Bi, Z., Wang, H., et al. (2016). Continuous detection and genetic diversity of human rotavirus A in sewage in eastern China, 2013–2014. Virology Journal, 13, 153.
Acknowledgement
The proof reading of this manuscript was supported by the Language Center, Faculty of Graduate Studies, Mahidol University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kittigul, L., Pombubpa, K. Rotavirus Surveillance in Tap Water, Recycled Water, and Sewage Sludge in Thailand: A Longitudinal Study, 2007–2018. Food Environ Virol 13, 53–63 (2021). https://doi.org/10.1007/s12560-020-09450-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-020-09450-0