Abstract
Human bocavirus (HBoV) is predominantly found in the respiratory tract infections and in the stool of patients with gastroenteritis symptoms. However, data on the prevalence of HBoV genotypes in environmental samples are limited. Here we addressed the prevalence of HBoV in sewage collected from three different wastewater treatment plants in Egypt. HBoV-1, HBoV-2, and HBoV-3 were detected, whereas none of the samples were positive for HBoV-4. The median concentration of HBoV in influent samples was 8.5 × 103 GC/l for HBoV-1, 3.0 × 104 GC/l for HBoV-2, and 2.5 × 104 GC/l for HBoV-3. The concentration was reduced but not completely removed in the effluent samples. The median concentration in the outlet samples was 2.9 × 103 GC/l for HBoV-1, 4.1 × 103 GC/l for HBoV-2, and 2.1 × 103 GC/l for HBoV-3. Moreover, no seasonality pattern of HBoVs was found. The high incidence of HBoV in sewage samples provided an evidence of its circulation in the local population. Although the role of HBoV in respiratory or gastro-intestinal infections still remains to be fully elucidated, the risk of infection via contaminated water should be taken into consideration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Waterborne viruses represent a major health risk to population. Direct or indirect discharge of treated and untreated wastewater is considered to be a meaningful cause of water contamination. Enteric viruses are associated with respiratory infection, hepatitis, conjunctivitis, myocarditis, and infectious diarrhea disease that is considered to be a common cause of morbidity and mortality in younger ages (Fong and Lipp 2005; Smits et al. 2016). Despite the progress in molecular detection methods, 40% of infectious diarrhea cases remain with unknown etiology (Ong et al. 2016).
Human bocavirus (HBoV) was first described in 2005 (Allander et al. 2005) and classified as a member of Parvoviridae (subfamily Parvovirinae, genus Bocavirus). HBoVs are non-enveloped viruses with single-stranded DNA, 5 Kb in length. The genome consists of three ORFs: the first and third ORFs encode two non-structural proteins NS-1 and NP-1, respectively. The second ORF encodes two major structural proteins (VP1 and VP2) (Zhao et al. 2012). Four genotypes, HBoV-1(Allander et al. 2005), HBoV-2 (Kapoor et al. 2009), HBoV-3 (Arthur et al. 2009), and HBoV-4 (Kapoor et al. 2010), have recently been identified. It was reported that HBoV subtypes were evolved as a result of a high frequency degree of recombination at NP1 and VP1 genes between and within bocavirus species (Kapoor et al., 2010).
There is a limitation concerning the pathogenesis of human bocavirus due to the lack of a simplified cell culture system or animal model. Human bocavirus could be detected in nasopharyngeal aspirates (Allander et al. 2005; Neske et al. 2007; Song et al. 2010; Koseki et al. 2012), stool (Kapoor et al. 2009; Cashman and O’Shea 2012; Zhao et al. 2012; Zhang et al. 2015), and intestinal biopsies (Kapoor et al. 2011). Furthermore, it could also be found in river water (Hamza et al. 2009) and wastewater samples (Blinkova et al. 2009; Myrmel et al. 2015; Iaconelli et al. 2016). Notably, HBoV-1 is mainly associated with respiratory infection; however, it was also detected in patients suffering from gastroenteritis (Vicente et al., 2007; Schildgen 2013), whereas, HBoV-2, HBoV-3, and HBoV-4 are mainly found in the stool (Jartti et al. 2012; Schildgen 2013).
HBoV was successfully cultured in cell culture system that mimics the human airway environment, but HBoV mRNA could only be detected by RT-PCR (Dijkman et al. 2009; Huang et al. 2012), Due to the lack of a versatile cell culture system, detection of human bocavirus is extensively rely on the basis of molecular techniques. Currently, several real-time PCR, conventional single round PCR, and nested PCR techniques that targeting NS1, NP1-, and Vp1 gene have been devised for HBoV detection in clinical and environmental samples (Allander et al. 2005; Hamza et al. 2009; Kapoor et al. 2009; Kantola et al. 2010; Santos et al. 2010; Zhao et al. 2012). Despite the high performance of qPCR, especially for the multiplex assay developed by Kantola et al. (2010), the aforementioned assays were unable to distinguish between the genetically similar subtypes HBoV-2 and HBoV-4.
The clinical data of HBoV prevalence in Egypt are limited and no environmental data are available. To the best of our knowledge, in Egypt, only HBoV-1 has been recently reported in children showing a manifestation of lower respiratory tract infection (Abdel-Moneim et al. 2016). Thus, in the present study, we aimed to estimate the prevalence of HBoV genotypes (1–4) and their removal during the municipal wastewater treatment for a period of one year.
Materials and Methods
Sampling Sites and Processing
Sixty-six raw and treated sewage samples were collected (2014–2015) from three wastewater treatment plants Zenin (WWTP-1), Balaks (WWTP-2), and El Berka (WWTP-3) located in Greater Cairo. The designed capacity of WWTP-2 and WWTP-3 is 600,000 m3/day, while for WWTP-1 is 330 000 m3/day. The three WWTP used activated sludge as a secondary treatment step. The treated effluents from WWTP-1 are discharged into the Nahia drainage system then to the River Nile at the Rosette branch following into the Mediterranean Sea. The effluents of WWTP-2 are discharged to Shibin al Kanater drain then to the river Nile at the Damietta branch following into the Mediterranean Sea. The final effluents from WWTP-3 are discharged into the Bahr El-Bakar drainage system and finally into Al Manzalah lake. The populations served by the wastewater treatment plants are approximately 1,320,000 for WWTP-1, 1,200,000 for WWTP-2, and 2,200,000 for WWTP-3.
Samples were collected from both influent and effluent in a total volume 5 l. Virus concentration was accomplished using the virus adsorption elution method previously described in (USEPA 2001). Briefly, samples were prepared by adding 1 M MgCl2 to reach final concentration 0.05 M for each 1 l of samples. For the filtration process, a negatively charged HA nitrocellulose membrane, 0.45 µm pore size, 142 mm diameter was used. The membrane was rinsed with 0.5 mM H2SO4, pH 4 prior to the virus recovery by 70 ml an organic elution buffer (3% beef extract, 0.05 M glycine pH 9.4). The eluate was subjected to further re-concentration step by the organic flocculation procedure.
Viral Nucleic Acid Extraction
Viral nucleic acids were extracted from 200 µl of concentrated suspension using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Standard precautions were followed to avoid contamination. Murine norovirus was used as an internal control to identify the occurrence of PCR inhibition. Comparing the Ct value of MNV spiked to environmental samples to the negative control, no inhibitory effect was observed (data not shown).
Detection and Quantification by qPCR
All primers used in the current study are listed in Table 1. For HBoV-1, the quantification protocol targets NP1 gene was used according to Hamza et al. (2009). A single sense primer was shared in HBoV -2, 3 and -4 quantification and the qPCR of HBoV-2 and -4 used the same antisense primer Kantola et al. (2010). Since the quantification system devised by Kantola et al. (2010) was unable to differentiate between HBoV-2 and 4, a subsequent sequencing step was performed for the positive samples.
DNA standards were prepared for the quantification of viral nucleic acids. For HBoV-1, the standard was prepared by cloning PCR fragment into the pSC-Aamp/kan vector using a StrataClone PCR cloning kit according to the manufacturer’s instructions (Stratagene, CA, USA), as previously described by Hamza et al. (2009). In case of HBoV genotypes 2/4 and 3, two HBoV positive sewage samples (sample ID: BI-2 and ZI-12 for both HBoV-2, 4 and HBoV-3, respectively) were used for the amplification of PCR products using the primers listed in Table 1. The obtained PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) to be used afterward as a standard curve during quantification. Nucleic acid concentrations of the purified products were determined by Quant-iT dsDNA HS Assay (Invitrogen, Carlsbad, CA, USA) using Qubit Fluorometer (Invitrogen, Carlsbad, CA, USA). The number of DNA copies was determined by multiplying the DNA concentration by Avogadro’s constant and dividing by the product size and average weight of a base pair.
The standard curves of HBoV-1, HBoV-2, 4, and HBoV-3 were prepared by tenfold serial dilution of the DNA standard ranging from 5 × 101 to 5 × 106 copies/reaction. For HBoV-1, the slop was −3.6, the coefficient of determination (R2) was 0.99, and the reaction efficiency was 90%. In case of HBoV-2, 4 and HBoV-3, the mean value of the slope was −3.46, the mean of R2 was 0.99, and the reaction efficiency was ≥96%.
TaqMan probe assay was used for HBoV-1 quantification. In case of HBoV-2, 3, and 4, SYBR green qPCR assay was conducted. Real-time qPCR reactions were performed in a total volume of 20 µl containing 1x (10 µl) Quantitect probe PCR kit (Qiagen, Hilden, Germany) with uracil-N-glycosylase (UNG), 0.6 µM for both forward and reverse primers and 0.2 µM Taqman probe/SYBR green. To avoid possible carryover PCR contamination, the cycling program was preceded by 2 min at 50 °C for UNG activation followed by 95 °C for 15 min as initial activation step for HotStart Taq DNA Polymerase and 45 cycle of 2-step cycling for 15 s at 94 °C and 1 min at 60 °C. Negative controls were included in each run as nuclease free water. The amplification and data analysis were performed using Rotorgene 3000 cycler system (Corbett Research). Virus concentration per liter GCL was calculated according to the following equation:
where GC is genome copy number per reaction, DF is the dilution factor for the volume reductions that occur during the concentration, DNA extraction and qPCR steps, and V is volume of original water sample assayed in liters.
Genotyping and Sequences Analysis
Positive samples from influent were subjected to subsequent PCR targeting NS1 gene to confirm the circulating genotypes using the primers listed in Table 1 for the respective genotype. The amplification condition was started with initial denaturation step 15 min at 95 °C followed by 40 cycles of 94 °C for 15 s, 53 °C for 30 s, and 72 °C for 1 min. All amplicons were purified using QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and used for direct sequencing. Sequences data were aligned by BioEdit software version 7.1.11. Moreover, MEGA 6 software was used for phylogenetic analysis and neighbor-joining (NJ) method with a matrix of Kimura’s 2-parameter model to infer the tree topology. Sequences were analyzed in terms of sequence homology using BLAST software. All sequences were deposited in GenBank under accession numbers KX962105 – KX962158.
Results
Detection and Quantification of Human Bocaviruses
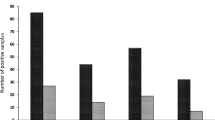
In the present study, three out of four human bocavirus genotypes were detected in both influent and effluent using qPCR that targets NP-1 and NS-1 genes. The data were confirmed by genotyping of positive samples. The detection rates of different HBoV genotypes are presented in Table 2.
HBoV-1
HBoV-1 was detected in 38 out of 66 samples. The positive samples were 27 samples from influents and 11 effluents with 79.4% and 34.3%, respectively, in accordance to the number of samples collected from each (Table 2). HBoV-1 was not detected in WWTP-2 outlet. For WWTP-1, the viral concentration ranged from 6.0 × 103 to 4.9 × 104 GC/l in influent and 1.64 × 103–4.35 × 103 GC/l in effluent (Fig. 1). In WWTP-2, the viral concentration ranged from 5.50 × 102 to 1.76 × 104 GC/l in influent with complete removal in effluent. The presence of HBoV-1 in WWTP-3 was 1.09 × 103 to 2.44 × 104 GC/l and 1.65 × 103–5.54 × 103 GC/l in both inlet and outlet, respectively. The highest median concentrations were determined in WWTP-1 for influent (2.78 × 104 GC/l) and effluent (3.42 × 103 GC/l) as well.
The treatment process reduced but did not completely remove the virus as roughly estimated from the difference between viral concentrations in the influent and effluent samples. There was 0–1 log10 viral reduction observed in these WWTPs samples (Fig. 2).
HBoV-2/4
Since there is a possible cross-reaction between the genetically similar HBoV-2 and 4 viruses, the data were further confirmed by genotyping of positive samples. A high prevalence of HBoV-2 was observed in influent and effluent of the three WWTPs (Table 2). HBoV-2 virus was detected in all samples collected from WWTP-1 with viral concentrations ranging from 2.29 × 103 to 1.2 × 105 GC/l and 5.43 × 102–7.54 × 103 GC/l in the influent and effluent, respectively (Fig. 1). Similarly, it was detected in most of WWTP-3 samples (20/22) with viral titers ranging from 9.4 × 103 to 1.23 × 105 GC/l in influent and 2.86 × 103–4.44 × 104 GC/l in effluent. Data showed that in WWTP-1 and WWTP-3, the reduction of HBoV-2 was ~0–3 log10, whereas, in WWTP-2 ~0–1 log10, viral reduction could be obtained (Fig. 1).
HBoV-3
Likewise, HBoV-3 showed a high abundance in the influent and effluent samples (94%) (Table 2). The virus was detected in almost all samples collected from WWTP-1 as well as WWTP-3. The collective virus concentration in all positive influent samples ranged between 1 × 103 and 1.98 × 105 GC/l which was reduced to 6.9 × 103 and 3.3 × 104 GC/l in the effluent samples. The efficiency of the treatment process varied between the treatment plants as shown in Fig. 1.
Phylogenetic and Sequences Analysis
Further sequence analysis step was performed targeting partial NS-1 gene. Since the qPCR assays for HBoV-1 and -3 were specific for each genotype, randomly selected positive samples from influent were selected and subjected to sequencing. Phylogenetic analysis of HBoV-1 sequence data showed that all isolates were clustered with HBoV-1 reference strains retrieved from GenBank (Fig. 3). Also, a high degree of nucleotide identity was observed within the studied isolate (100%) and 98.9–99.6% between the studied isolates and reference strains except isolate (ID: HBoV-1/Egy/ZI-6) showed 97.6% homology with both the studied isolate and reference strains.
Phylogenetic analysis of partial sequences of HBoV NS-1 gene. (dark circle) represent HBoV-1 isolates and (dark triangle) for HBoV-3 isolates. The tree was constructed using Neighbor-Joining method with Kimura 2-parameter model and the reliability of the tree was evaluated by bootstrapping of 1000 replicates. Bootstrap values above 70% are shown. The variation rate among sites was modeled with gamma distribution
Overall, for HBoV-3, all isolates were clustered with HBoV-3 reference strains. Phylogenetic analysis of HBoV-3 sequences showed that seven isolates were clustered with the Australian strain (FJ948861) and with 99.4–99.7% nucleotide homology (Fig. 3). These seven isolates also showed 99.7% of genetic similarity between each other and 98.8% with the rest of studied isolates. Furthermore, four isolates were clustered with the Brazilian isolate (GQ867666) and shared 99.1–99.4% of homology.
Concerning the HBoV-2 and -4, due to the inability of qPCR assay to distinguish between these two genotypes, almost all influent samples were subjected to sequencing. It became evident that all isolates belonged to HBoV-2 based on the BLAST search and phylogenetic analysis (Fig. 4). HBoV-2 isolates shared 99.3% nucleotide similarity with Chinese isolates (KM624025, JX257046). Only one isolate (HBoV-2/Egy/BI8) showed 99.7% similarity with (FJ170279- isolate—PK-2255- Pakistan).
Phylogenetic analysis of partial sequences of HBoV-2 NS-1 gene. (dark circle) represent HBoV-2 isolates. The tree was constructed using Neighbor-Joining method with Kimura 2-parameter model and the reliability of the tree was evaluated by bootstrapping of 1000 replicates. Bootstrap values above 70% are shown. The variation rate among sites was modeled with gamma distribution
Discussion
Different studies showed the prevalence of human enteric viruses in Nile River and sewage in Egypt (Villena et al. 2003; Ali et al. 2004; Pintó et al. 2007; Kamel et al. 2010). However, there are no data available concerning the newly identified viral candidates of a possible waterborne infection. The present study represents a quantitative surveillance and genetic characterization of HBoVs in three WWTPs located in Greater Cairo.
Real-time PCR was used to examine sixty-six sewage samples that were collected from influent and effluent of three different WWTPs. The surveillance of human bocaviruses in water and sewage was addressed in few studies worldwide. The first detection of HBoV in river water was in the river Ruhr, Germany, and HBoV-1 was predominant in ~41% of collected samples. The viral concentration ranged between 3 × 101 and 2 × 103 GC/l (Hamza et al. 2009). The present work showed that HBoV-1 could be detected in 57.6% of sewage samples compared to 92.4% for HBoV-2 and 94% for HBoV-3; however, none of the examined sequences were related to HBoV-4. Interestingly, HBoV-2 and 3 were found in all influent samples of the WWTPs. The relative abundance of HBoV-2 and 3 is in agreement with Blinkova et al. (2009), who indicated a high dissemination of HBoV in environmental samples from USA and the circulating strains were HBoV-1, -2, and -3 but genotype 4 was also not detected. Similarly, raw and treated sewage in Norway reported a high prevalence of genotypes 2 and 3 compared to genotype 1 but genotype 4 was not found (Myrmel et al. 2015). Recently, HBoV-4 was detected only in 1 out of 134 Italian sewage samples, despite the high distribution of genotypes 2 and 3 in the population (Iaconelli et al. 2016).
Quantitatively, the median levels of HBoV in influents were 8.46 × 103 GC/l for HBoV-1, 2.95 × 104 GC/l for HBoV-2, and 2.45 × 104 GC/l for HBoV-3, whereas in effluents were 2.87 × 103, 4.06 × 103, and 2.07 × 103 GC/l for the three genotypes, respectively (Fig. 2). The virus concentrations obtained here are quite similar to the values obtained by (Iaconelli et al. 2016). It should be noted that the efficiency of treatment process expressed as log reduction is a rough estimation because the transit time between the influent and effluent was not considered. Nevertheless, examining the influent and effluent samples gave us some insight into viral contamination in treated wastewater.
Indeed, the presence of viral pathogens in the environment depends on rates of infection and shedding within the host population. Accordingly, the high detection rate of HBoVs in sewage samples is an evidence of the high incidence of HBoV in human population in Egypt.
The virus might be associated with undiagnosed gastroenteritis or respiratory disease in Egypt. Particularly, it was found that HBoV could be excreted in asymptomatic patients (Kapoor et al. 2011; Proenca-Modena et al. 2013) or frequently detected as a co-infection with other respiratory or enteric viruses, as described elsewhere (Rasanen et al. 2010; Wang et al. 2010). Although HBoV is associated with a high prevalence of other viral pathogens, especially norovirus and rotavirus, evidence supporting the causative role of HBoV in gastroenteritis is currently lacking (Ong et al. 2016). Excretion of HBoV in a symptomatic patient raises further questions regarding the possible etiologic role of HBoVs in either respiratory or acute gastroenteritis infections and whether it is a true pathogen or an innocent bystander (Broccolo et al. 2015; Ong et al. 2016).
In this study, the widespread of HBoV-2 and HBoV-3 in comparison to HBoV-1 could presumably be due to differences in tissue tropism or pathogenesis that may influence its transmission route and ability to establish persistence. HBoV-2 and HBoV-3 were primarily detected in stool from patients with acute diarrhea (Arthur et al. 2009; Kapoor et al. 2009; Santos et al. 2010; Zhao et al. 2012), while, HBoV-1 is mainly associated with respiratory tract infection.
Additionally, no clear seasonal pattern of HBoV in environmental samples was observed. This finding is consistence with other reports from Germany (Hamza et al. 2009) and Italy (Iaconelli et al. 2016) where the virus was found throughout the year. However, there is less concordance between published clinical studies regarding the seasonality of HBoV. Some suggested that the virus might have seasonal peak in the spring/early summer months (Choi et al. 2006; Nawaz et al. 2012), whereas others observed a seasonal peak during winter (Chow et al. 2008; Ong et al. 2016).
Recently, a high rate of nosocomial infection with HBoV-1 was detected among children suffering from lower respiratory tract infections in Egypt (Abdel-Moneim et al. 2016). Abdel-Moneim and co-workers proposed also that HBoV-1 might play a potential role in the induction of Colorectal cancer (Abdel-Moneim et al. 2016).
Although the qPCR data might lack the correlation with viral infectivity (Hamza et al. 2011), the existence of HBoV in the outlet could be infectious and poses a threat of contamination to the population. Due to the lack of a commercially available cell culture system or animal model, it was not possible to evaluate the viral infectivity that could help in health risk assessment. However, the cell culture systems devised by Dijkman et al. (2009) and Huang et al. (2012) may represent a breakthrough in understanding HBoV replication and transmission as well.
Conclusion
As far as we know, there are no previous studies conducted in Egypt to explore the circulation of human bocaviruses in environmental samples. The data obtained in this study revealed a high level of prevalence of human bocaviruses, particularly HBoV-2 and HBoV-3 in both treated and untreated wastewater samples, Therefore, a potential role of water in HBoV transmission and the threats to population should be taken into consideration. Further studies are still required to answer the questions concerning viral infectivity, disinfectants, persistence, and the risk of infection via contaminated water.
References
Abdel-Moneim, A. S., El-Fol, H. A., Kamel, M. M., Soliman, A. S. A., Mahdi, E. A., El-Gammal, A. S., et al. (2016a). Screening of human bocavirus in surgically excised cancer specimens. Archives of Virology, 161(8), 2095–2102.
Abdel-Moneim, A. S., Kamel, M. M., Hamed, D. H., Hassan, S. S., Soliman, M. S., Al-Quraishy, S. A., et al. (2016b). A novel primer set for improved direct gene sequencing of human bocavirus genotype-1 from clinical samples. Journal of Virological Methods, 228, 108–113.
Ali, M. A., Al-Herrawy, A. Z., & El-Hawaary, S. E. (2004). Detection of enteric viruses, Giardia and Cryptosporidium in two different types of drinking water treatment facilities. Water Research, 38(18), 3931–3939.
Allander, T., Tammi, M. T., Eriksson, M., Bjerkner, A., Tiveljung-Lindell, A., & Andersson, B. (2005). Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proceedings of the National Academy of Sciences USA, 102(36), 12891–12896.
Arthur, J. L., Higgins, G. D., Davidson, G. P., Givney, R. C., & Ratcliff, R. M. (2009). A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathogens, 5(4), e1000391.
Blinkova, O., Rosario, K., Li, L., Kapoor, A., Slikas, B., Bernardin, F., et al. (2009). Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. Journal of Clinical Microbiology, 47(11), 3507–3513.
Broccolo, F., Falcone, V., Esposito, S., & Toniolo, A. (2015). Human bocaviruses: possible etiologic role in respiratory infection. Journal of Clinical Virology, 72, 75–81.
Cashman, O., & O’Shea, H. (2012). Detection of human bocaviruses 1, 2 and 3 in Irish children presenting with gastroenteritis. Archives of Virology, 157(9), 1767–1773.
Choi, E. H., Lee, H. J., Kim, S. J., Eun, B. W., Kim, N. H., Lee, J. A., et al. (2006). The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clinical Infectious Diseases, 43(5), 585–592.
Chow, B. D., Huang, Y. T., & Esper, F. P. (2008). Evidence of human bocavirus circulating in children and adults, Cleveland, Ohio. Journal of Clinical Virology, 43(3), 302–306.
Dijkman, R., Koekkoek, S. M., Molenkamp, R., Schildgen, O., & van der Hoek, L. (2009). Human Bocavirus Can Be Cultured in Differentiated Human Airway Epithelial Cells. Journal of Virology, 83(15), 7739–7748.
Fong, T. T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews, 69(2), 357–371.
Hamza, I. A., Jurzik, L., Uberla, K., & Wilhelm, M. (2011). Methods to detect infectious human enteric viruses in environmental water samples. International Journal of Hygiene and Environmental Health, 214(6), 424–436.
Hamza, I. A., Jurzik, L., Wilhelm, M., & Uberla, K. (2009). Detection and quantification of human bocavirus in river water. Journal of General Virology, 90(11), 2634–2637.
Huang, Q., Deng, X., Yan, Z., Cheng, F., Luo, Y., Shen, W., et al. (2012). Establishment of a Reverse Genetics System for Studying Human Bocavirus in Human Airway Epithelia. PLoS Pathogens, 8(8), e1002899.
Iaconelli, M., Divizia, M., Della Libera, S., Di Bonito, P., & La Rosa, G. (2016). Frequent detection and genetic diversity of human bocavirus in urban sewage samples. Food and Environmental Virology, 8, 289–295.
Jartti, T., Hedman, K., Jartti, L., Ruuskanen, O., Allander, T., & Soderlund-Venermo, M. (2012). Human bocavirus-the first 5 years. Reviews in Medical Virology, 22(1), 46–64.
Kamel, A. H., Ali, M. A., El-Nady, H. G., Aho, S., Pothier, P., & Belliot, G. (2010). Evidence of the co-circulation of enteric viruses in sewage and in the population of Greater Cairo. Journal of Applied Microbiology, 108(5), 1620–1629.
Kantola, K., Sadeghi, M., Antikainen, J., Kirveskari, J., Delwart, E., Hedman, K., et al. (2010). Real-time quantitative PCR detection of four human bocaviruses. Journal of Clinical Microbiology, 48(11), 4044–4050.
Kapoor, A., Hornig, M., Asokan, A., Williams, B., Henriquez, J. A., & Lipkin, W. I. (2011). Bocavirus episome in infected human tissue contains non-identical termini. PLoS ONE, 6(6), e21362.
Kapoor, A., Simmonds, P., Slikas, E., Li, L., Bodhidatta, L., Sethabutr, O., et al. (2010). Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. Journal of Infectious Diseases, 201(11), 1633–1643.
Kapoor, A., Slikas, E., Simmonds, P., Chieochansin, T., Naeem, A., Shaukat, S., et al. (2009). A newly identified bocavirus species in human stool. Journal of Infectious Diseases, 199(2), 196–200.
Koseki, N., Teramoto, S., Kaiho, M., Gomi-Endo, R., Yoshioka, M., Takahashi, Y., et al. (2012). Detection of human bocaviruses 1 to 4 from nasopharyngeal swab samples collected from patients with respiratory tract infections. Journal of Clinical Microbiology, 50(6), 2118–2121.
Myrmel, M., Lange, H., & Rimstad, E. (2015). A 1-year quantitative survey of noro-, adeno-, human boca-, and Hepatitis E Viruses in raw and secondarily treated sewage from two plants in Norway. Food and Environmental Virology, 7(3), 213–223.
Nawaz, S., Allen, D. J., Aladin, F., Gallimore, C., & Iturriza-Gomara, M. (2012). Human bocaviruses are not significantly associated with gastroenteritis: results of retesting archive DNA from a case control study in the UK. PLoS ONE, 7(7), e41346.
Neske, F., Blessing, K., Tollmann, F., Schubert, J., Rethwilm, A., Kreth, H. W., et al. (2007). Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. Journal of Clinical Microbiology, 45(7), 2116–2122.
Ong, D. S., Schuurman, R., & Heikens, E. (2016). Human bocavirus in stool: a true pathogen or an innocent bystander? Journal of Clinical Virology, 74, 45–49.
Pintó, R. M., Alegre, D., Dominguez, A., El-Senousy, W. M., Sanchez, G., Villena, C., et al. (2007). Hepatitis A virus in urban sewage from two Mediterranean countries. Epidemiology and Infection, 135(2), 270–273.
Proenca-Modena, J. L., Martinez, M., Amarilla, A. A., EspÍNola, E. E., Galeano, M. E., FariÑA, N., et al. (2013). Viral load of human bocavirus-1 in stools from children with viral diarrhoea in Paraguay. Epidemiology and Infection, 141(12), 2576–2580.
Rasanen, S., Lappalainen, S., Kaikkonen, S., Hamalainen, M., Salminen, M., & Vesikari, T. (2010). Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiology and Infection, 138(9), 1227–1234.
Santos, N., Peret, T. C., Humphrey, C. D., Albuquerque, M. C., Silva, R. C., Benati, F. J., et al. (2010). Human bocavirus species 2 and 3 in Brazil. Journal of Clinical Virology, 48(2), 127–130.
Schildgen, O. (2013). Human bocavirus: lessons learned to date. Pathogens, 2(1), 1–12.
Smits, S. L., Osterhaus, A. D., & Koopmans, M. P. (2016). Newly identified viruses in human gastroenteritis: pathogens or not? Pediatric Infectious Disease Journal, 35(1), 104–107.
Song, J. R., Jin, Y., Xie, Z. P., Gao, H. C., Xiao, N. G., Chen, W. X., et al. (2010). Novel human bocavirus in children with acute respiratory tract infection. Emerging Infectious Diseases, 16(2), 324–327.
USEPA (2001). Manual of methods for virology. EPA/600/4-84/013. USEPA, Cincinnati, USA: 6–62.
Vicente, D., Cilla, G., Montes, M., Perez-Yarza, E. G., & Perez-Trallero, E. (2007). Human bocavirus, a respiratory and enteric virus. Emerging Infectious Diseases, 13(4), 636–637.
Villena, C., El-Senousy, W. M., Abad, F. X., Pinto, R. M., & Bosch, A. (2003). Group A rotavirus in sewage samples from Barcelona and Cairo: emergence of unusual genotypes. Applied and Environment Microbiology, 69(7), 3919–3923.
Wang, K., Wang, W., Yan, H., Ren, P., Zhang, J., Shen, J., et al. (2010). Correlation between bocavirus infection and humoral response, and co-infection with other respiratory viruses in children with acute respiratory infection. Journal of Clinical Virology, 47(2), 148–155.
Zhang, D. M., Ma, M. M., Wen, W. T., Zhu, X., Xu, L., He, Z. J., et al. (2015). Clinical epidemiology and molecular profiling of human bocavirus in faecal samples from children with diarrhoea in Guangzhou, China. Epidemiology and Infection, 143(11), 2315–2329.
Zhao, H., Zhao, L., Sun, Y., Qian, Y., Liu, L., Jia, L., et al. (2012). Detection of a bocavirus circular genome in fecal specimens from children with acute diarrhea in Beijing, China. PLoS One, 7(11), e48980.
Acknowledgement
This work was conducted within a short-term fellowship funded by the Egyptian Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamza, H., Leifels, M., Wilhelm, M. et al. Relative Abundance of Human Bocaviruses in Urban Sewage in Greater Cairo, Egypt. Food Environ Virol 9, 304–313 (2017). https://doi.org/10.1007/s12560-017-9287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9287-3