Abstract
Corynebacterium glutamicum has been utilized for industrial amino acid production, especially for monosodium glutamate (MSG), the food-additive for the “UMAMI” category of taste sensation, which is one of the five human basic tastes. Glutamate export from these cells is facilitated by the opening of mechanosensitive channels in the cell membrane within the bacterial cell envelope following specific treatments, such as biotin limitation, addition of Tween 40 or penicillin. A long-unsolved puzzle still remains how and why C. glutamicum mechanosensitive channels are activated by these treatments to export glutamate. Unlike mechanosensitive channels in other bacteria, these channels are not simply osmotic safety valves that prevent these bacteria from bursting upon a hypo-osmotic shock. They also function as metabolic valves to continuously release glutamate as components of a pump-and-leak mechanism regulating the cellular turgor pressure. Recent studies have demonstrated that the opening of the mechanosensitive channel, MscCG, mainly facilitates the efflux of glutamate and not of other amino acids and that the “force-from-lipids” gating mechanism of channels also applies to the MscCG channel. The bacterial types of mechanosensitive channels are found in cell-walled organisms from bacteria to land plants, where their physiological functions have been specialized beyond their basic function in bacterial osmoregulation. In the case of the C. glutamicum MscCG channels, they have evolved to function as specialized glutamate exporters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gram-positive soil bacterium Corynebacterium glutamicum is a model microorganism employed in industrial production of amino acids, especially monosodium glutamate, which is used as a food-additive to achieve the “UMAMI” taste. The history of C. glutamicum as a glutamate overproducer began in the 1950s when Japanese scientists Kinoshita and Udaka isolated this bacterium (Kinoshita et al. 1957) and discovered their ability to secrete massive amounts of glutamate. Later, bacterial fermentation led to the glutamate production, thereby replacing completely a chemical synthesis with a biological process. Subsequently, this bacterium has been extensively studied to examine its unique physiology as well as to improve the glutamate production.
The unique characteristic of the C. glutamicum bacteria is their ability to overproduce glutamate only when the cell envelope (the cell membrane and the cell wall) is influenced by specific treatments such as biotin limitation, addition of fatty acid ester surfactants or antibiotics that inhibit the cell wall synthesis (Shiio et al. 1962; Nara et al. 1964; Takinami et al. 1965). Glutamate production requires two main processes: (i) a change of the metabolic flow by inhibiting the 2-oxoglutarate dehydrogenase complex (ODHC); and (ii) the opening of mechanosensitive channels. The change in glutamate synthesis by the inhibition of the 2-oxoglutarate dehydrogenase complex (ODHC) was studied exclusively in the past, and it has been clearly demonstrated that ODHC is inhibited under glutamate-producing conditions to change the metabolic flow towards synthesis (Kawahara et al. 1997). On the other hand, the mechanism of glutamate secretion remained elusive until the major glutamate exporter in C. glutamicum was identified. This is because all treatments that trigger glutamate secretion damage the structure of the bacterial cell envelope. It was thus initially thought that glutamate simply leaked from the cells as a consequence of damaged membranes. Nevertheless, a specific glutamate carrier was contemplated, which finally found support through the discovery of MscCG (originally named NCgl1221). This MscS-like mechanosensitive channel functions as a major glutamate exporter (Nakamura et al. 2007). Recent studies have provided further evidence for the glutamate efflux occurring exclusively due to the opening of MscCG (Becker et al. 2013; Hashimoto et al. 2012).
Mechanosensory transduction is an essential physiological process in all living cells and involves the conversion of mechanical stimuli into electro-chemical intracellular signals (Murthy et al. 2017). Although bacteria do not have a complex nervous system, they can sense and adapt to various changes in their environments that are frequently characterized by changes in osmotic pressure (Cox et al. 2018). Consequently, bacterial mechanosensory transduction is largely an osmoregulatory process modified by evolution to maintain and regulate the cellular turgor. To meet the environmental challenges of osmotic fluctuations the strong cell wall is also equipped with mechanosensitive channels, which are activated by an increase in membrane tension caused by hypo-osmotic shock. Two types of mechanosensitive channels, MscS and MscL, were identified electrophysiologically in the E. coli plasma membrane (Martinac et al. 1987; Sukharev et al. 1993). They differ largely in their conductance (1 and 3 nS, respectively), half activation (6 and 12 mN/m, respectively), and gating kinetics (Sukharev et al. 1993; Nomura et al. 2012). In contrast, while MscL-like channels function exclusively as osmo-protective valves, MscS-like channels are characterized by large structural differences that during the evolution have diversified their physiological functions. In fact, MscS homologs are not only in bacteria, but also in almost all cell-walled organisms, including fungi, algae, and land plants (Wilson et al. 2013; Pivetti et al. 2003; Malcolm and Maurer 2012), They function in osmoregulation as well as in calcium signaling, chloroplast organization, wound healing, and pollen germination (Nakayama et al. 2012a; Nakayama et al. 2007; Nakayama et al. 2014; Haswell and Meyerowitz 2006; Hamilton and Haswell 2017; Haswell et al. 2008; Zou et al. 2016).
In this review, we briefly summarize recent progress in understanding the structure and function of mechanosensitive channels in C. glutamicum. We will emphasize the MscCG channel that, as an MscS-like channel, has evolved to acquire its specialized function as a glutamate exporter. Specific treatments are required to increase the membrane tension for glutamate efflux. When Kinoshita and Udaka isolated C. glutamicum as an efficient glutamate-producing bacterium they used an elaborated screening method combined with a bioautographic bio-assay with the Leuconostoc mesenteroides strain (Udaka 1960). Later, three specific treatments (biotin limitation, fatty acid ester surfactants, and β-lactam antibiotics) were adopted to trigger the glutamate efflux in C. glutamicum (Fig. 1).
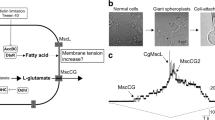
Three major specific treatments triggering glutamate efflux in C. glutamicum. Biotin limitation, fatty acid ester surfactants (Tween 40), and β-lactam antibiotics (Penicillin) induce the glutamate secretion. Biotin limitation decreases total lipid amount and causes a lipid component change and lipid saturation. Fatty acid ester surfactants, such as Tween 40, are also known to affect the fatty acid synthesis in C. glutamicum and emulsifying the membrane lipids may change the surface tension in the membrane. Penicillin treatment inhibits the cell wall synthesis. These treatments cause an increase in membrane tension, which activates specific carriers for glutamate efflux in the C. glutamicum membrane
Biotin limitation, reducing total membrane lipids amount
C. glutamicum requires biotin for its growth since this bacterium lacks the enzymes for biotin biosynthesis (Shiio et al. 1962). In the metabolic pathway for glutamate synthesis, the activity of 2-oxoglutarate dehydrogenase complex (ODHC) may be inhibited by biotin limitation conditions (Kawahara et al. 1997). Moreover, biotin limitation decreases the activity of acetyl-CoA carboxylase, a complex of DtsR and AccBC, a component of biotin-containing enzyme complex needed for the synthesis of fatty acids for the plasma membrane (Kimura et al. 1999). Reduced lipids may alter the membrane mechanical properties and cause the permeable membrane to secrete glutamate in C. glutamicum. To support this hypothesis, Hoischen and Krämer (Hoischen and Kramer 1990) demonstrated that under biotin limitation condition, the total amount of the membrane lipids was almost halved, and the ratio of saturated and unsaturated lipids (mostly palmitic acid (C16:0) and oleic acid (C18:1)) in the membrane was increased. However, initiation of glutamate efflux is only associated with the decrease of total membrane lipids (Hoischen and Kramer 1990) (Fig. 2). Therefore, it was concluded that changes in membrane composition is a necessary but not sufficient condition for glutamate efflux. Moreover, they implied existence of a carrier-mediated glutamate secretion system by changing lipid environments while limiting biotin, and suggested that the carrier should be sensitive to the total membrane lipid rather than the lipid components.
The mechanism of glutamate production in C. glutamicum during biotin limitation. a Metabolic flow under the normal culture and biotin limitation conditions. The activity of the 2-oxoglutarate dehydrogenase complex (ODHC) inhibits the synthesis of glutamate and the lipid synthesis is also inhibited by the reduced activity of acetyl-CoA carboxylase, a complex of DtsR and AccBC, under biotin limitation. Under glutamate-producing conditions, oxaloacetate is supplied from phosphoenolpyruvate (Hasegawa et al. 2008). b The ratio of the saturation and unsaturated membrane lipids. c The ratio of membrane lipid components under the normal culture (blue) and biotin limitation conditions (orange) are shown. Note that the plasma membrane of C. glutamicum is exceptional, being exclusively composed of negatively charged phospholipids, phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylinositol mannoside (PIM), phosphatidic acid (PA), and cardiolipin (DPG), respectively
Fatty acid ester surfactants emulsifying membrane lipids
The addition of non-ionic surfactants (Tween 40 and Tween 60) can change metabolic flow and trigger the glutamate efflux in C. glutamicum (Takinami et al. 1965). When they are added, C. glutamicum stops growth and starts to synthesize and secrete glutamate similarly to biotin limitation condition. Since Tweens are used as emulsifier in pharmaceuticals, cosmetics, cleaning compounds, etc., they change the surface tension of the lipid bilayer by reducing interfacial tension between water and membrane lipids. In addition, they are used as detergent for cell lysis, nuclei isolation, and cell fractionation. An interesting fact is that only Tween 40 and 60, which have monopalmitate and monostearate as the type of fatty acid, respectively, trigger the glutamate secretion in C. glutamicum, whereas Tween 20 and 80 do not. Although the mechanism of the glutamate efflux by adding Tween 40 remains unclear, it is known that the ratio of palmitic acid (C16:0) and oleic acid (C18:1) in the membrane is increased and the total lipid amount is also decreased after this treatment. It has also been suggested that by acting on the DtsR, a subunit of acetyl-CoA carboxylase, Tween 40 and 60 affect the fatty acid synthesis (Kimura et al. 1999; Kimura et al. 1997; Kimura et al. 1996). These facts suggest that membrane tension change causes glutamate secretion in C. glutamicum and membrane lipids may influence the glutamate efflux. In support of this notion, a recent study reported that MscCG behaves very differently in cell membranes of C. glutamicum compared to E. coli, which greatly differ in their lipid composition (Nakayama et al. 2018).
Cell wall synthesis inhibition, removing the cell wall
Unlike other Gram-positive bacteria, C. glutamicum possess a mycobacteria-type cell wall (Laneelle et al. 2013). Its structure mainly consists of three components: a peptidoglycan layer, an arabinogalactan layer, and a mycolic acids layer. The mycolic acid layer is considered to be the outer-most permeability barrier to solutes and antibacterial drugs. Interestingly, inhibition of the cell wall synthesis using a sublethal concentration of antibiotics (e.g., penicillin which inhibits the peptidoglycan synthesis (Nara et al. 1964; Hirasawa et al. 2018), and ethambutol which inhibits the arabinogalactan synthesis (Radmacher et al. 2005)), has also been demonstrated to trigger the glutamate secretion in C. glutamicum. After weakening the cell wall of the bacterium, osmotic pressure becomes increasingly uncompensated, and membrane tension increases because of the turgor pressure, eventually resulting in cytolysis. Therefore, the glutamate efflux under the sublethal concentration of antibiotics is also related to the increased membrane tension.
Mechanosensitive channels are molecular entities of the long-elusive glutamate exporter
The “puzzle of membrane permeability for glutamate” in C. glutamicum remained unresolved for a long time. Since all treatments used to trigger the glutamate efflux damage the bacterial cell envelope structure, a generally accepted explanation for glutamate efflux was the “leak model” of the glutamate export through the damaged membrane. However, the existence of a specific carrier for the glutamate efflux has also been suspected for a long time. This model controversy ended by the discovery of the major glutamate exporter in the form of the MscS-like mechanosensitive channel MscCG (NCgl1221). Several C. glutamicum mutants, which secrete glutamate constitutively without any treatment, were isolated to study the mechanism of glutamate overproduction. Using these strains, Nakamura et al. discovered in 2007 that all mutant strains had mutations in the ncgl1221 gene, which is homologous to the gene encoding the bacterial mechanosensitive channel of small conductance MscS in E. coli (Nakamura et al. 2007). This finding ended the model controversy by firmly establishing the MscCG mechanosensitive channel as the glutamate exporter in C. glutamicum.
Mechanotransduction in bacteria
All living cells continuously experience mechanical force, such as gravity, osmotic pressure, touch or sound. In order to convert mechanical stimuli into electrical signals in nervous system, for example, mechanosensitive channels play a central role in this signaling process. Bacterial cells also sense and adapt to their surroundings susceptible to frequent changes in osmolarity by expressing mechanosensitive channels. For over 30 years, Martinac et al. developed a bacterial patch-clamp technique for recording ion channel activities from E. coli giant spheroplasts. They discovered two types of mechanosensitive channel activity in the cytoplasmic membrane of this Gram-negative bacterium (Fig. 3a) (Martinac et al. 1987; Sukharev et al. 1993). This first report on the existence of mechanosensitive channels in bacteria provided clear evidence for mechanosensory transduction as part of bacterial osmoregulatory processes. Later, these two types of mechanosensitive channels were cloned (Sukharev et al. 1994; Levina et al. 1999), and their 3D structures were elucidated by X-ray crystallography (Fig. 3b) (Chang et al. 1998; Bass et al. 2002). Since these channels have a distinctly different ion conductance and activation threshold, the small conductance channel was named MscS (Mechanosensitive channel of Small conductance) and large one MscL (Mechanosensitive channel of Large conductance) (Sukharev et al. 1993; Levina et al. 1999). The physiological function of MscS and MscL in E. coli has been established as osmotic safety valves protecting bacterial cells from hypo-osmotic shock (Levina et al. 1999). When shocked, water influx inflates the cells and MscS and MscL become activated by increased membrane tension. Opening of their large pores enables the release of ions and small organic osmolytes, which helps to keep cellular turgor pressure within its physiological limits and thus prevent bacterial cells from bursting (Bialecka-Fornal et al. 2015).
Two types of mechanosensitive channel activities MscS and MscL in the inner membrane of E. coli. a The schematic images depicting patch-clamp recording from the E. coli giant spheroplasts. E. coli cells are enlarged, and the inside-out excised patch configuration is generated from the cell-attached configuration. Mechanosensitive channels are activated by applying suction to the patch membrane to increase membrane tension. b The current traces of the mechanosensitive channels MscS and MscL in the E. coli membrane. Upper and lower traces show the channel currents and the negative pressure applied to the membrane, respectively. MscS is always activated first at pressure level PS mmHg followed by MscL activation at the pressure level of PL mmHg
Osmoregulation in C. glutamicum
An interesting question is whether the physiological role of mechanosensitive channels is the same in C. glutamicum as in E. coli. As a soil bacterium, the former must have developed osmoregulation to adapt to sudden changes in osmolarity of its environment. Efficient efflux of compatible solutes is one system, and it has been demonstrated that C. glutamicum continuously and preferentially releases glycine, betaine and proline to adjust osmolarity after a sudden reduction in external osmolarity (Krämer 2009). Interestingly, a release of similar size molecules, such as glutamate or lysine, is restricted, and ATP is completely retained even after severe shock (Ruffert et al. 1997). The channels involved in osmoregulation in C. glutamicum were investigated to establish whether they were similar to the mechanosensitive channels in E. coli. As a result, the double-deletion mutant of MscS and MscL homologs showed almost the same survival rate as the WT cells upon hypo-osmotic shock (Nottebrock et al. 2003). Although under severe hypo-osmotic conditions, the survival rate of the C. glutamicum double-deletion mutant was significantly decreased, it is not comparable to the E. coli MscS and MscL double-deletion mutant where the mortality rate was almost 100%. Consequently, the channels involved in osmoregulation in C. glutamicum differ from E. coli mechanosensitive channels. Mechanosensitive channels involved in the glutamate efflux under glutamate-producing conditions are specialized for this purpose.
Structural and functional features of the C. glutamicum mechanosensitive channels
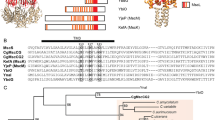
In the C. glutamicum ATCC13032 genome, only one MscS (MscCG) and one MscL homolog can be found. Recently, another mechanosensitive channel, called MscCG2, was identified as a “minor” glutamate exporter in a different C. glutamicum strain Z-188 (Wang et al. 2018). Interestingly, only MscCG functions as the main glutamate exporter under glutamate-producing conditions. Even though MscCG is an MscS homolog, its predicted secondary structure contains four transmembrane helices, which thus differs from the E. coli canonical MscS structure containing three transmembrane helices (Fig. 4). In comparison, the minor exporter MscCG2 does not have the fourth C-terminal helix like MscCG and its molecular size is almost the same as the size of the canonical MscS. Moreover, it has been reported that a truncation of the C-terminal extension causes the spontaneous glutamate efflux in this MscCG truncation mutant (Becker and Krämer 2015). Thus, it is likely that the additional C-terminal helix in MscCG is functionally important for MscCG specialization as a glutamate exporter. After its molecular identification as the glutamate exporter, MscCG was examined by the patch-clamp technique. It was not clear whether it can function as a mechanosensitive channel given that it is not involved in the osmo-protection in C. glutamicum like the canonical MscS in E. coli. Börngen et al. (Börngen et al. 2010) demonstrated that MscCG expressed in the E. coli membrane can be activated by stretching the giant spheroplast patches, clearly indicating that MscCG is a mechanosensitive channel. The channel is selective for cations over anions (PK/PCl~3) and has smaller conductance than MscS (~ 300 pS) (Börngen et al. 2010). In addition, Hashimoto et al. expressed MscCG in Bacillus subtilis giant provacuoles and confirmed passive glutamate efflux through the opening of MscCG channel (Hashimoto et al. 2012; Hashimoto et al. 2010). Furthermore, Nakayama et al. investigated whether the spontaneous glutamate efflux in C. glutamicum was caused by a lower activation threshold of the mutant MscCG and found significant changes in the mechanosensitivity and gating hysteresis of the MscCG mutant (Nakayama et al. 2012b). These findings provided direct evidence that the mechanosensitive activation of MscCG causes glutamate efflux in C. glutamicum.
Gain-of-function mutations of C. glutamicum MscCG cause spontaneous glutamate efflux. W15CSLW is an insertion mutation at the extracellular N-terminal loop. Δ110 is a truncation mutation at the extracellular C-terminal loop, and P424L is a point mutation at the C-terminal loop (top), respectively. The secondary structure of E. coli canonical MscS and the crystal structure of the monomer and homoheptamer MscS protein (bottom)
If the spontaneous glutamate efflux is caused by the lower activation threshold of MscCG, the gain-of-function mutations must hold information about mechanosensitivity of MscCG. Interestingly, the mutations can be classified into two types, third transmembrane helix (TM3) and extracellular loops. In the canonical MscS, TM3 is known as a pore-lining helix and mutations in this region change the pore constriction. On the other hand, the extracellular loop-type mutations are not seen in MscS. They include a point mutation (P424L), an insertion at the N-terminal loop (W15CSLW) and a truncation at the C-terminal loop (Δ110) in MscCG (Fig. 4). These extracellular loop mutations thus suggest a possible regulation of the MscCG mechanosensitivity from the extracellular side.
Gating mechanism of bacterial mechanosensitive channels is consistent with the “Force-From-Lipids” (FFL) principle (Martinac et al. 1990; Teng et al. 2015). If MscCG is a “genuine” mechanosensitive channel, and its mechanosensitive gating causes glutamate efflux, how do specific treatments trigger glutamate efflux by activating only MscCG, but not other mechanosensitive channels in C. glutamicum? The gating mechanism of the canonical bacterial mechanosensitive channels MscS and MscL are known to gate according to the “Force-From-Lipids (FFL)” principle. This principle states that a membrane protein, such as a mechanosensitive channel embedded in the membrane lipid bilayer, should always sense the transbilayer pressure profile, which is a strong anisotropic internal bilayer stress along the bilayer thickness (Cantor 1999) (see also Martinac et al., article in this issue of Biophys Rev). Mechanical stimuli applied to the membrane bilayer change the pressure profile, which leads to conformational changes of mechanosensitive channels MscS and MscL because they have specific mechanisms to directly sense the membrane tension. MscS and MscL channels have been functionally reconstituted into artificial lipid bilayer systems (Martinac et al. 2010; Rosholm et al. 2017), and a difference in FFL gating mechanisms resulting from their different structures has been reported (Nomura et al. 2012). MscL is sensitive to the change of membrane thickness because of a hydrophobic mismatch between the lipid bilayer and the channel, and tilting of its transmembrane helices initiates the channel opening. An amphipathic N-terminal short helix of MscL on the cytoplasmic side functions as an anchor. Pulling on this helix by stretching and thinning the bilayer opens the channel by tilting its transmembrane TM1 helix (Bavi et al. 2016; Martinac et al. 2017). Consequently, the interaction with the inner leaflet of the bilayer is important for MscL gating. On the other hand, MscS is sensitive to membrane stiffness rather than membrane thickness, and the gating model of MscS has been suggested to follow the “Jack-in-the-box” mechanism (Malcolm et al. 2015). The resting stress from the transbilayer pressure in addition to the lipids, which protrude into the MscS structural voids between the TM2 and TM3 helices created by a kink of the G113 residue, stabilizes the channel in its closed state. Upon application of mechanical stimuli, MscS is envisaged to open due to reduction in pressure in certain regions of the transbilayer pressure profile and removal of lipids from the structural voids (Pliotas et al. 2015) (Fig. 5).
The predicted gating model of C. glutamicum MscCG. The closed state of E. coli MscS is stabilized in the membrane by lipid molecules penetrating into the cavity between TM2 and TM3 created by the G113 kink. The “Jack-in-the-box” model has been suggested for the MscS gating in which lipids move out from the cavity when membrane tension increases (left). Dotted arrows show removal of the lipid molecules from the cavity. C. glutamicum MscCG does not have the G113 kink that bends and separates TM3 into two sub-helices TM3a and TM3b as in E. coli MscS (bottom). The extracellular N- and C-terminal loops are potential stabilizers of the MscCG closed state since the mutations in these parts cause the spontaneous glutamate efflux. The fourth transmembrane helix of MscCG is therefore, the most peripheral helix to sense the force-from-lipids resulting from membrane tension (right)
Mechanosensitive gating mechanism of MscCG
In order to understand the mechanosensitive gating of C. glutamicum MscCG, how does the FFL principle apply to the MscCG gating mechanism given what we learned from MscS? The “Jack-in-the-box” model does not seem to fit for the MscCG channel because MscCG does not have a kink similar to the G113 kink in MscS. Therefore, there is no interdigitation of lipid molecules in this channel. In addition, C. glutamicum membrane lipid components are mostly negatively charged lipids, which include phosphatidylglycerol and cardiolipin (Hoischen and Krämer 1990), and thus one would expect the lateral pressure profile to somewhat differ from that of the E. coli membrane. Moreover, MscCG has the fourth transmembrane helix that is not seen in MscS. Consequently, changes in the pressure profile should affect the MscCG structure differently from the MscS structure. Both N- and C-terminal extracellular loops of MscCG may stabilize the closed state of the channel differently from MscS given that deletion or insertion of amino acid residues in these loops causes constitutive glutamate efflux in C. glutamicum (Fig. 5). These possibilities need to be studied in the future.
Experimental methods for the study of C. glutamicum mechanosensitive channels
In order to study the gating mechanism of C. glutamicum mechanosensitive channels, several experimental approaches have been developed as described next.
Bacterial patch-clamp techniques
Bacterial cells are too small to directly apply the patch-clamp technique. Also, their thick cell wall does not allow formation of a tight giga-ohm seal between the cell membrane and the pipette glass wall, which is a prerequisite for single-channel recording. Bacterial enlargement methods have thus been developed to overcome these problems (Martinac et al. 2013). For the initial characterization of its mechanosensitive properties the C. glutamicum MscCG was expressed and characterized in E. coli membrane (Börngen et al. 2010). Compared to MscS of E. coli, gating of MscCG exhibits much slower kinetics (Nakayama et al. 2013). Furthermore, MscS is characterized by strong channel inactivation upon a mechanical stimulus. In contrast, MscCG does not exhibit any inactivation or desensitization but remains open and gating upon a sustained mechanical stimulus (Nakayama et al. 2013). MscCG has also been studied in giant provacuoles of Bacillus subtilis (Hashimoto et al. 2010), which is a Gram-positive bacterium like C. glutamicum. Like in the E. coli giant spheroplasts, MscCG was activated by suction applied to the B. subtilis membrane as well. The MscCG channel was also shown to pass not only glutamate but also aspartate and phenylalanine (Hashimoto et al. 2012), which indicates that MscCG has a potential to serve as a versatile exporter of amino acids.
Molecular dynamics simulation
The gating of canonical bacterial mechanosensitive channels MscS and MscL has been predicted by the molecular dynamics simulation based on the experimental data (Sawada and Sokabe 2015; Sawada et al. 2012; Sotomayor et al. 2007; Anishkin et al. 2008). The advantage of the simulation is that one can determine the most stable structure to be equilibrated from energetic point of view in a different membrane environment. C. glutamicum produces glutamate under biotin limitation conditions. In addition, the reduced total lipids amount and the increased ratio of saturated and unsaturated lipids, palmitic acid (C16:0) and oleic acid (C18:1), may be the factors required to open MscCG. However, it is difficult to study how reduced amount of total lipids and the ratio of palmitic acid and oleic acid affect the gating of mechanosensitive channels experimentally. Consequently, similar to MscL and MscS, molecular dynamics simulations can also be employed to study the gating behavior of MscCG channels in bilayers consisting of different total lipid amounts, lipid components, and saturation levels of the membrane lipids. The modeled lipid bilayers with different ratios of palmitic and oleic acids can be generated and tested whether the MscCG channel gating is affected when the total lipids are reduced by 25% and/or the ratio of saturated and unsaturated lipids is changed like the C. glutamicum membrane under the biotin limitation condition.
Micropipette aspiration and the patch-fluorometry technique
Mechanosensitive channels sense FFL, and therefore membrane lipid components have a strong influence on the gating of mechanosensitive channels. In fact, recent studies have shown that the gating of MscS is affected by cardiolipin and lipid saturation in liposomal membranes (Ridone et al. 2015; Ridone et al. 2018). The cell membrane of C. glutamicum is composed of very unique lipid components and its major lipids are negatively charged, including phosphatidylglycerol (PG) and cardiolipin (DPG), whereas the E. coli cell membrane contains phosphatidylethanolamine (PE) as a major lipid component. Therefore, C. glutamicum MscCG gates in a completely different membrane environment in the native membrane of this bacterium to which it must have adapted and specialized as the main glutamate exporter. As previously shown, the FFL mechano-sensing mechanism of MscS differs from that of MscL. This is because MscS is more sensitive to membrane stiffness than MscL, whereas MscL is more sensitive to membrane thickness (Nomura et al. 2012). Under glutamate-producing conditions, C. glutamicum changes its membrane lipid composition and saturation while simultaneously reducing the total lipid content. Consequently, the membrane mechanical properties are expected to change dramatically during this process. To evaluate the membrane mechanical properties, both micropipette aspiration technique and patch-fluorometry technique were used to calculate Young’s modulus of the membrane as an indication of its stiffness (Bavi et al. 2014; Sun et al. 2014). Both membranes were found to greatly differ in their stiffness. The area elasticity module KA of the C. glutamicum membrane was determined to be ~ 15 mN/m, which is characteristic of a very soft membrane, whereas KA of the E. coli membrane was with ~ 44 mN/m three times larger, indicating a stiffer, more elastic membrane (Nakayama et al. 2018). Since the C. glutamicum membrane contains mostly negatively charged lipids, the membrane is extremely soft and expandable when stretched. These findings suggest that mechanosensitive channels of C. glutamicum gate in a very different membrane environment compared to the E. coli membrane.
Glutamate productivity assay
Since MscCG was discovered during the screening of the glutamate-overproducing strains without any specific treatments, the spontaneous glutamate export can be used as an indicator of the functionality of MscCG. One of the mutations of MscCG, A111V is a point mutation in the pore-lining transmembrane helix, which causes spontaneous glutamate export in C. glutamicum. This mutant channel has significantly lower activation threshold and stronger gating hysteresis than the WT channel in E. coli spheroplasts. This suggests that the MscCG gain-of-function (GOF) mutation causes spontaneous glutamate export. By using this spontaneous glutamate export phenotype, mutations of MscCG can be screened to identify the functional region of this channel. Although the functionality of bacterial mechanosensitive channels has been evaluated traditionally by measuring survival rate upon hypo-osmotic shock in E. coli, spontaneous glutamate efflux in C. glutamicum would present a new screening method for identifying the physiological function of mechanosensitive channels.
Future prospects
The “puzzle of the glutamate efflux” in C. glutamicum has been solved after the discovery of the MscCG channel, which functions as the major glutamate exporter in this bacterium. Given that the gating of bacterial mechanosensitive channels is based on the “Force-From-Lipids” principle it is plausible to assume that the specific treatments (biotin limitation, Tween 40, and penicillin) that have been used to trigger the glutamate secretion in C. glutamicum, increase membrane tension sufficiently to activate the MscCG channel. However, what remains unclear is how these treatments increase membrane tension to activate the MscCG channel only and not the other mechanosensitive channels present in the C. glutamicum cell membrane. What has been learned thus far from the studies of MscS-like channels is that their structural diversity also causes their mechanosensitive gating to differ significantly from their basic function as osmotic nanovalves. They have specialized functions in diverse processes, such as amino acid efflux, Ca2+ regulation, chloroplast organization, and cell division. Based on the current knowledge, MscCG seems not to have evolved to function as an osmotic nanovalve protecting bacterial cells from lysing upon a hypo-osmotic shock. Consequently, although as one of MscS-like channels MscCG shares a number of characteristics with MscS, its gating apparently presents a new paradigm of the mechanosensitive channel gating given the differences between its structure and that of MscS. Understanding this new mechano-sensation paradigm promises to lead to a solution to the long-elusive puzzle of the C. glutamicum glutamate efflux.
References
Anishkin A, Kamaraju K, Sukharev S (2008) Mechanosensitive channel MscS in the open state: modeling of the transition, explicit simulations, and experimental measurements of conductance. J Gen Physiol 132(1):67–83
Bass RB, Strop P, Barclay M, Rees DC (2002) Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298(5598):1582–1587
Bavi N et al (2014) Biophysical implications of lipid bilayer rheometry for mechanosensitive channels. Proc Natl Acad Sci U S A 111(38):13864–13869
Bavi N et al (2016) The role of MscL amphipathic N terminus indicates a blueprint for bilayer mediated gating of mechanosensitive channels. Nat Commun 7:11984
Becker M, Krämer R (2015) MscCG from Corynebacterium glutamicum: functional significance of the C-terminal domain. Eur Biophys J 44(7):577–588
Becker M et al (2013) Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta 1828(4):1230–1240
Bialecka-Fornal M, Lee HJ, Phillips R (2015) The rate of osmotic downshock determines the survival probability of bacterial mechanosensitive channel mutants. J Bacteriol 197(1):231–237
Börngen K et al (2010) The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta 1798(11):2141–2149
Cantor RS (1999) Lipid composition and the lateral pressure profile in bilayers. Biophys J 76(5):2625–2639
Chang G, Spencer RH, Lee AT, Barclay MT, Rees DC (1998) Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282(5397):2220–2226
Cox CD, Bavi N, Martinac B (2018) Bacterial Mechanosensors. Annu Rev Physiol 80:71–93
Hamilton ES, Haswell ES (2017) The tension-sensitive ion transport activity of MSL8 is critical for its function in pollen hydration and germination. Plant Cell Physiol 58(7):1222–1237
Hasegawa T, Hashimoto K-I, Kawasaki H, Nakamatsu T (2008) Changes in enzyme activities at the pyruvate node in glutamate-overproducing Corynebacterium glutamicum. J Biosci Bioeng 105(1):12–19
Hashimoto K et al (2010) The protein encoded by NCgl1221 in Corynebacterium glutamicum functions as a mechanosensitive channel. Biosci Biotechnol Biochem 74(12):2546–2549
Hashimoto K et al (2012) Glutamate is excreted across the cytoplasmic membrane through the NCgl1221 channel of Corynebacterium glutamicum by passive diffusion. Biosci Biotechnol Biochem 31 76(7):1422–1424
Haswell ES, Meyerowitz EM (2006) MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol 16(1):1–11
Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM (2008) Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol 18(10):730–734
Hirasawa T, Saito M, Yoshikawa K, Furusawa C, Shmizu H (2018) Integrated analysis of the transcriptome and metabolome of Corynebacterium glutamicum during penicillin-induced glutamic acid production. Biotechnol J 13(5):e1700612
Hoischen C, Krämer R (1990) Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol 172(6):3409–3416
Kawahara Y, Takahashi-Fuke K, Shimizu E, Nakamatsu T, Nakamori S (1997) Relationship between the glutamate production and the activity of 2-oxoglutarate dehydrogenase in Brevibacterium lactofermentum. Biosci Biotechnol Biochem 61(7):1109–1112
Kimura E, Abe C, Kawahara Y, Nakamatsu T (1996) Molecular cloning of a novel gene, dtsR, which rescues the detergent sensitivity of a mutant derived from Brevibacterium lactofermentum. Biosci Biotechnol Biochem 60(10):1565–1570
Kimura E, Abe C, Kawahara Y, Nakamatsu T, Tokuda H (1997) A dtsR gene-disrupted mutant of Brevibacterium lactofermentum requires fatty acids for growth and efficiently produces L-glutamate in the presence of an excess of biotin. Biochem Biophys Res Commun 234(1):157–161
Kimura E et al (1999) Glutamate overproduction in Corynebacterium glutamicum triggered by a decrease in the level of a complex comprising DtsR and a biotin-containing subunit. Biosci Biotechnol Biochem 63(7):1274–1278
Kinoshita S, Udaka S, Shimono M (1957) Studies on the amino acid fermentation on the various microorganism. J Gen Appl Microbiol 3(3):193–205
Krämer R (2009) Osmosensing and osmosignaling in Corynebacterium glutamicum. Amino Acids 37(3):487–497
Laneelle MA, Tropis M, Daffe M (2013) Current knowledge on mycolic acids in Corynebacterium glutamicum and their relevance for biotechnological processes. Appl Microbiol Biotechnol 97(23):9923–9930
Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18(7):1730–1737
Malcolm HR, Maurer JA (2012) The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem 13(14):2037–2043
Malcolm HR, Blount P, Maurer JA (2015) The mechanosensitive channel of small conductance (MscS) functions as a Jack-in-the box. Biochim Biophys Acta 1848:159–166
Martinac B, Buechner M, Delcour AH, Adler J, Kung C (1987) Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A 84(8):2297–2301
Martinac B, Adler J, & Kung C (1990) Mechanosensitive ion channels of E. coli activated by amphipaths Nature 348(6298):261–263
Martinac B et al (2010) Studying mechanosensitive ion channels using liposomes. Methods Mol Biol 606:31–53
Martinac B, Rohde PR, Cranfield CG, Nomura T (2013) Patch clamp electrophysiology for the study of bacterial ion channels in giant spheroplasts of E. coli. Methods Mol Biol 966:367–380
Martinac AD, Bavi N, Bavi O, Martinac B (2017) Pulling MscL open via N-terminal and TM1 helices: a computational study towards engineering an MscL nanovalve. PLoS One 12(8):e0183822
Murthy SE, Dubin AE, Patapoutian A (2017) Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18(12):771–783
Nakamura J, Hirano S, Ito H, Wachi M (2007) Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce L-glutamic acid production. Appl Environ Microbiol 73(14):4491–4498
Nakayama Y, Fujiu K, Sokabe M, Yoshimura K (2007) Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci U S A 104(14):5883–5888
Nakayama Y, Yoshimura K, Iida H (2012a) Organellar mechanosensitive 1 channels in fission yeast regulate the hypo-osmotic shock response. Nat Commun 3:1020
Nakayama Y, Yoshimura K, Iida H (2012b) A gain-of-function mutation in gating of Corynebacterium glutamicum NCgl1221 causes constitutive glutamate secretion. Appl Environ Microbiol 78(15):5432–5434
Nakayama Y, Yoshimura K, Iida H (2013) Electrophysiological characterization of the mechanosensitive channel MscCG in Corynebacterium glutamicum. Biophys J 105(6):1366–1375
Nakayama Y, Hirata A, Iida H (2014) Mechanosensitive channels Msy1 and Msy2 are required for maintaining organelle integrity upon hypoosmotic shock in Schizosaccharomyces pombe. FEMS Yeast Res 14(6):992–994
Nakayama Y, Komazawa K, Bavi N, Hashimoto K, Kawasaki H, Martinac B (2018) Evolutionary specialization of MscCG, an MscS-like mechanosensitive channel, in amino acid transport in Corynebacterium glutamicum. Sci Rep 27;8(1):12893. https://doi.org/10.1038/s41598-018-31219-6
Nara T, Samejima H, Kinoshita S (1964) Effect of penicillin on amino acid fermentation. Agric Biol Chem 28(2):120–124
Nomura T et al (2012) Differential effects of lipids and lyso-lipids on the mechanosensitivity of the mechanosensitive channels MscL and MscS. Proc Natl Acad Sci U S A 109(22):8770–8775
Nottebrock D, Meyer U, Kramer R, Morbach S (2003) Molecular and biochemical characterization of mechanosensitive channels in Corynebacterium glutamicum. FEMS Microbiol Lett 218(2):305–309
Pivetti CD et al (2003) Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev 44 67(1):66–85
Pliotas C et al (2015) The role of lipids in mechanosensation. Nat Struct Mol Biol 22(12):991–998
Radmacher E et al (2005) Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits L glutamate efflux of Corynebacterium glutamicum. Microbiology 151(Pt 5):1359–1368
Ridone P, Nakayama Y, Martinac B, Battle AR (2015) Patch clamp 1 characterization of the effect of cardiolipin on MscS of E. coli. Eur Biophys J 44(7):567–576
Ridone P et al (2018) “Force-from-lipids” gating of mechanosensitive channels modulated by PUFAs. J Mech Behav Biomed Mater 79:158–167
Rosholm KR et al (2017) Activation of the mechanosensitive ion channel MscL by mechanical stimulation of supported droplet-hydrogel bilayers. Sci Rep 7:45180
Ruffert S, Lambert C, Peter H, Wendisch VF, Krämer R (1997) Efflux of compatible solutes in Corynebacterium glutamicum mediated by osmoregulated channel activity. Eur J Biochem 247(2):572–580
Sawada Y, Sokabe M (2015) Molecular dynamics study on protein-water interplay in the mechanogating of the bacterial mechanosensitive channel MscL. Eur Biophys J 44(7):531–543
Sawada Y, Murase M, Sokabe M (2012) The gating mechanism of the bacterial mechanosensitive channel MscL revealed by molecular dynamics simulations: from tension sensing to channel opening. Channels (Austin) 6(4):317–331
Shiio I, Otsuka SI, Takahashi M (1962) Effect of biotin on the bacterial formation of glutamic acid. I. Glutamate formation and cellular premeability of amino acids. J Biochem 51:56–62
Sotomayor M, Vasquez V, Perozo E, Schulten K (2007) Ion conduction through MscS as determined by electrophysiology and simulation. Biophys J 92(3):886–902
Sukharev SI, Martinac B, Arshavsky VY, Kung C (1993) Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J 65(1):177–183
Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C (1994) A large-conductance mechanosensitive channel in E. coli encoded by mscL alone Nature 368(6468):265–268
Sun Y, Sun TL, Huang HW (2014) Physical properties of Escherichia coli spheroplast membranes. Biophys J 107(9):2082–2090
Takinami K, Yoshii H, Tsuri H, Okada H (1965) Biochemical effects of fatty acid and its derivatives on L-glutamin acid fermentation. Agric Biol Chem 29(4):351–359
Teng J, Loukin S, Anishkin A, Kung C (2015) The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch 467(1):27–37
Udaka S (1960) Screening method for microorganisms accumulating metabolites and its use in the isolation of Micrococcus glutamicus. J Bacteriol 79:754–755
Wang Y et al (2018) A novel Corynebacterium glutamicum l-glutamate exporter. Appl Environ Microbiol 84(6)
Wilson ME, Maksaev G, Haswell ES (2013) MscS-like mechanosensitive channels in plants and microbes. Biochemistry 52(34):5708–5722
Zou Y et al (2016) A gain-of-function mutation in Msl10 triggers cell death and wound-induced hyperaccumulation of jasmonic acid in Arabidopsis. J Integr Plant Biol 58(6):600–609
Acknowledgements
We acknowledge the Japanese Society for Promotion of Science (JSPS) for a fellowship to YN, and the National Health and Medical Research Council of Australia for a Principal Research Fellowship to BM.
Author information
Authors and Affiliations
Contributions
Y. N., Y. S., H. K., and B. M. wrote the manuscript.
Corresponding author
Ethics declarations
Funding
This work was supported by the Discovery Project DP180102813 grant from the Australian Research Council.
Conflict of interest
Yoshitaka Nakayama declares that he has no conflicts of interest. Ken-ichi Hashimoto declares that he has no conflicts of interest. Yasuyuki Sawada declares that he has no conflicts of interest. Masahiro Sokabe declares that he has no conflicts of interest. Hisashi Kawasaki declares that he has no conflicts of interest. Boris Martinac declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Nakayama, Y., Hashimoto, Ki., Sawada, Y. et al. Corynebacterium glutamicum mechanosensitive channels: towards unpuzzling “glutamate efflux” for amino acid production. Biophys Rev 10, 1359–1369 (2018). https://doi.org/10.1007/s12551-018-0452-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-018-0452-1