Abstract
Corynebacterium glutamicum is used in microbial biotechnology for the production of amino acids, e.g., glutamate and lysine. Excretion of glutamate into the surrounding medium under production conditions is mediated by MscCG, an MscS-type mechanosensitive channel. In difference to most other MscS-type channel proteins, MscCG carries, in addition to the N-terminal pore domain, a long C-terminal domain that amounts to about half of the size of the protein and harbors an additional transmembrane segment. Here we study the impact of the C-terminal domain on both functions of MscCG as mechanosensitive channel and as glutamate exporter. Sequential truncations of the C-terminal domain were applied, as well as deletion of particular subdomains, replacement of these segments by other amino acid sequences, and sequence randomization. Several parameters of cell physiology and bioenergetics of the obtained mutants related to both glutamate excretion and response to osmotic stress were quantified. All three subdomains of the C-terminal domain, i.e., the periplasmic loop, the fourth transmembrane segment, and the cytoplasmic loop, proved to be of core significance for MscCG function, in particular for glutamate excretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corynebacterium glutamicum, a member of the Corynebacteriales order of actinomycetes, is a Gram-positive soil bacterium and one of the model organisms in modern microbial biotechnology relevant for the production of a number of small metabolites (Becker and Wittmann 2012). C. glutamicum is well known for its ability to excrete amino acids, in particular l-glutamate and l-lysine. Both in its normal habitat, the soil, and in the context of biotechnological production processes, it faces strong changes in external osmolality. For this reason it is equipped with effective defense mechanisms against both hypo- and hyperosmotic stress.
One of the specific properties of C. glutamicum is the peculiar structure of both its cell wall and its plasma membrane. The cell wall of C. glutamicum is composed of a peptidoglycan layer covalently linked to arabinogalactan, which carries covalently bound mycolic acids, i.e., branched and hydroxylated long-chain fatty acids. They form a semipermeable outer bilayer, an exceptional feature of actinomycetes different from other Gram-positive bacteria (Jarlier and Nikaido 1994). Also, the plasma membrane of C. glutamicum is exceptional, being exclusively composed of negatively charged phospholipids, i.e., phosphatidylglycerol (PG), phosphatidylinositol (PI), and cardiolipin (DPG), respectively (Ozcan et al. 2007).
The first line of defense of bacterial cells against hypo-osmotic stress is provided by mechanosensitive (MS) channels as emergency valves (Martinac 2001). Different types of MS channels have been characterized in detail by several groups, the major components being MscL, MscS, and MscM (Berrier et al. 1996). Besides their physiological function and biochemical and mechanistic properties, the three-dimensional structure of some of them has also been studied in great depth in recent years (Bass et al. 2002; Naismith and Booth 2012). For C. glutamicum, one member each of MscL and MscS-type channels has been identified, both on the genomic, the electrophysiological, and the functional level (Borngen et al. 2010; Nottebrock et al. 2003). The observation that C. glutamicum is not significantly more sensitive to severe hypo-osmotic stress in the absence of both channels argues for the fact that the plasma membrane of this organism may harbor other MS channels beyond the two identified, which are not recognized in the genome because of a lack of sequence similarity to MS channels of known structure from other bacteria.
The exceptional biotechnological significance of C. glutamicum is closely related to its ability for efficient amino acid excretion. Since 1988, a number of active and regulated amino acid excretion systems (lysine, threonine, methionine, and branched-chain amino acids) have first been functionally described (Krämer 1994) and then genetically defined (Eggeling and Sahm 2003). Export of these amino acids follows a common physiologic pattern, based on the fact that they are terminal products of anabolic pathways (Krämer 1994). Glutamate, on the other hand, is a metabolite of the central metabolism and its excretion is correlated to a particular physiologic state of ‘metabolic overflow’ (Teixeira de Mattos and Neijssel 1997). Moreover, glutamate excretion can be triggered by a number of manipulations all related to alterations of the cell wall and/or the plasma membrane (Krämer 1994; Laneelle et al. 2013; Sano 2009). Among these are additions of various antibiotics, e.g., penicillin, which interfere with the synthesis of cell wall components, as well as agents and conditions, which alter the composition of the plasma membrane, e.g., local anesthetics and fatty acid auxotrophy (Krämer 1994). The mechanistic correlation of these different manipulations with the common consequence of glutamate excretion is still not well understood.
The export system responsible for glutamate excretion has long withstood identification. In a seminal publication, Nakamura et al. (2007) correlated the ability to effectively excrete glutamate with the presence of the MscS type MS channel in C. glutamicum. Working with a different strain (ATCC 13869), this protein was called NCgl1221 in the original publication (Nakamura et al. 2007); in C. glutamicum ATCC 13032 it was named MscCG (Borngen et al. 2010). The presence of the NCgl1221 channel of C. glutamicum was correlated to glutamate excretion (Nakamura et al. 2007), however, not excluding a situation where this membrane protein may have only a regulatory impact on glutamate excretion. This complication is known, for example, for sugar phosphate transport in E. coli (Island and Kadner 1993). Also the observation that the rate of penicillin-induced glutamate excretion is relatively constant and not significantly influenced by a widely varying expression level of MscCG and its mutant forms (Becker et al. 2013; Borngen et al. 2010) argued for MscCG being a triggering factor of excretion rather than the excretion pore itself. In a later publication, by introducing loss-of-function (LOF) mutations in the channel part of MscCG of C. glutamicum, however, we were able to prove that the exported glutamate in fact moves through the pore domain of MscCG (Becker et al. 2013).
Thorough electrophysiological studies proved that MscCG from C. glutamicum is in fact a classic MscS-type channel, however, with some peculiarities (Becker et al. 2013; Borngen et al. 2010; Nakayama et al. 2013). Besides responding to hypo-osmotic stress like a typical MS channel, it was shown to be also essential in fine-tuning the cell’s response to hyperosmotic stress, which may be a property of MS channels in other bacteria, too (Borngen et al. 2010). The conductance of MscCG is rectifying and somewhat smaller than that of MscS from E. coli. The MscCG channel has a slight preference for cations, unlike MscS, which exhibits a slight preference for anions (Borngen et al. 2010; Sukharev 2002; Sukharev et al. 1993). On the other hand, it lacks the typical inactivation and desensitization phenomenon as observed for MscS from E. coli (Borngen et al. 2010; Nakayama et al. 2013). The N-terminal part of MscCG from C. glutamicum representing its pore domain has a significant sequence similarity to E. coli MscS (Borngen et al. 2010). In addition, MscCG harbors a long C-terminal extension, which was found to lack sequence similarity to any other MS channel and is specific for MscS-type channels from bacteria of the genus Corynebacteriaceae (Becker et al. 2013). The functional significance of the C-terminal domain of MscCG is the main focus of this work.
Consequently, MscCG seems to provide two different functions for the cell, i.e., being an MS channel, on the one hand, and representing the exit pathway for glutamate under conditions of overflow metabolism, on the other. The simplest way to combine these two aspects, in particular in view of a constantly high cytoplasmic glutamate concentration observed in C. glutamicum (Gutmann et al. 1992), is to assume MscCG being an MS channel strongly preferring glutamate as a substrate. The analysis of metabolites exported from C. glutamicum upon a hypo-osmotic shift, however, revealed that the major solutes excreted under these conditions are the compatible solutes betaine, ectoine, and proline (Nottebrock et al. 2003; Ruffert et al. 1997). Moreover, electrophysiology did not indicate any specificity or preference of MscCG for glutamate as substrate (Becker et al. 2013; Nakayama et al. 2013), and consequently, this explanation can be ruled out.
In the present analysis, the impact of a variety of genetic manipulations of the C-terminal domain of MscCG is correlated with a broad range of putative consequences of these manipulations for functional properties of this channel as well as for energetic parameters of C. glutamicum cells in which MscCG is embedded with the aim to obtain information about the significance of the C-terminal domain for the function of MscCG.
Materials and methods
Strains, media, and growth conditions
Escherichia coli strains DH5αmcr (Grant et al. 1990) and C. glutamicum wild-type strain ATCC13032 and its derivatives (this work) were used (Tab. S1 in the supporting material). E. coli was grown at 37 °C in Luria–Bertani medium, C. glutamicum at 30 °C in brain heart infusion (BHI, Difco, Detroit, USA) or in CgXII medium (pH 7.0) containing per l: 20 g (NH4)2SO4, 5 g urea, 1 g KH2PO4, 1.6 g K2HPO4, 42 g MOPS, 2.9 g NaCl, 4 % glucose, 0.25 g MgSO4, 0.01 g CaCl2, 0.2 mg biotin, 30 mg protocatechuate, 1 ml of a solution of trace elements (Rübenhagen et al. 2000). For growth under different osmolalities, MM1 medium (pH 7.0) was used containing per l: 5 g (NH4)2SO4, 5 g urea, 2 g KH2PO4, 2 g K2HPO4, 3 g NaCl, 4 % glucose, 0.25 g MgSO4, 0.01 g CaCl2, 0.2 mg biotin, 30 mg protocatechuate, 1 ml of a solution of trace elements and 0, 200, or 400 mM NaCl. Antibiotics were added in concentrations of 100 µg/ml for E. coli and 25 µg/ml for C. glutamicum.
Strain construction
Deletion of the mscCG gene (NCgl1221) was performed by crossover PCR (Jakoby et al. 1999) and double homologous recombination using the suicide vector pK19mobsacB_ΔmscCG (Borngen et al. 2010), and was verified by PCR analysis. For complementation the mscCG gene was amplified by PCR using C. glutamicum ATCC 13032 chromosomal DNA as template. The amplified fragment was cleaved by BamHI and SalI and ligated into BamHI/SalI-cleaved pEKex2, resulting in pEKex2_mscCG or pEKex2_mscCG-his, respectively. Truncations of mscCG (mscCGΔ110_his, mscCGΔ132_his mscCGΔ247_his) were subcloned from pET29b into the E. coli-C. glutamicum shuttle vector pEKex2 (Becker et al. 2013). For this purpose, fragments were cleaved by B1pI and XbaI. Upon refill of 3′-end overhangs, fragments were ligated into pEKex2 cleaved by Ecl136II.
The other truncations of mscCG as listed in Tab S1 were cloned by amplification of the truncated gene with customized primer pairs, containing a NotI restriction site for ligating the cleaved fragments (BamHI/NotI) into the pre-cleaved plasmid pEKEx2_mscCG_his. Site-directed mutagenesis for generating GOF and LOF mutations in pEKex2_mscCG_his was performed using the Stratagene Quickchange Site-Directed Mutagenesis protocol to introduce amino acid replacements by using mutagenic primer pairs. Subsequent to the PCR, the methylated initial template DNA was digested by the addition of 1 μl DpnI and the residual DNA was transformed into E. coli DH5αmcr-competent cells.
Deletion of the fourth transmembrane segment (pEKex2_Δ406-423_his) or conserved sequences (pEKex2_mscCGΔ392-405_his) were performed by three consecutive PCRs. At first, two fragments with overlapping ends by omitting the DNA sequence of the fourth transmembrane segment or the desired sequence were generated. In a third reaction, both DNA fragments were fused and finally ligated into the pre-cleaved vector pEKEx2_mscCG_his, after digestion with BamHI/NotI. The replacement by a poly-alanine (polyA) sequence (pEKex2_mscCG_polyA406-432_his, pEKex2_mscCG_polyA394-405_his) was performed in a similar way applying three consecutive PCRs.
Changing or randomizing the sequence of the cytoplasmic loop was performed by subsequent PCR, fusing the generated DNA template with primers of a length of about 100 nucleotides with overlapping ends. The original amino acid sequence was shuffled and translated into a DNA sequence, taking the codon usage of C. glutamicum ATCC 13032 into account. Amplified fragments were subcloned into the pJET1.2 vector and were used as template for the next, overlapping PCR. The final fused fragment was cleaved by BamHI/NotI and ligated into the BamHI/NotI-cleaved pEKex2. The artificial transmembrane helix (pEKEx2_Δ406-423_tmX_his) was constructed by replacement and insertion of a sequence coding for the transmembrane segment of a nondimerizing mutant of human glycophorin A (ITLILFGVMALVIGTILLY) (Nasie et al. 2013), using customized primers. Amplified and fused DNA fragments were cleaved by BamHI and NotI and ligated into the BamHI/NotI-cleaved pEKex2. Constructs with combinations of LOF/GOF mutations, truncations, replaced or randomized sequences were generated by processing intermediate DNA fragments using PCR, cloning in the pEKEx2-vector and using the generated intermediate plasmids as template for further PCRs. The plasmids were introduced into ATCC 13032 ΔmscCG by electroporation. All strains and plasmids are listed in Tab. S1.
Uptake and efflux of glycine betaine and glutamate
Synthesis of [14C]-labeled glycine betaine was performed as described previously (Landfald and Strom 1986). For measurement of betaine efflux in response to hypo-osmotic stress, C. glutamicum strains were grown aerobically at 30 °C at 125 rpm overnight in BHI medium containing 500 mM NaCl. Cells were harvested by centrifugation and washed twice under hypo-osmotic conditions in 100 mM MES/Tris, pH 8.0, 4 °C, containing 5 mM Na2HPO4 and 5 mM K2HPO4. This hypo-osmotic washing step results in efflux of the majority of compatible solutes, which were taken up or synthesized during the growth period. Uptake of the labeled betaine was performed by incubating the cells at an OD600 of 4 for 80 min at 30 °C in a hyper-osmotic buffer (100 mM MES/Tris pH 8.0) containing 0.9 M NaCl, 30 mM glucose, 30 mM urea, and 30 mM KCl. Shortly after transfer into the uptake buffer, [14C]-glycine betaine at a final concentration of 1 mM (25,000 cpm/ml) was added. After loading of the cells with labeled betaine, they were stored on ice until being used. To measure the efflux, cells were centrifuged and resuspended in 100 mM MES/Tris pH 8.0 containing various concentrations of NaCl to obtain appropriate osmolalities. After 15 s, 200-µl samples were withdrawn and cells were rapidly separated from the surrounding medium by silicone oil centrifugation (oil density of 1.09 kg/l for osmolalities >1.2 osmol/kg and 1.03 kg/l for osmolalities <1.2 omol/kg) and counted.
Glutamate excretion was induced during the exponential growth phase by the addition of 0.15 % Tween 60 or 6 U/ml Penicillin G. For the biotin-limited conditions, a 24-h pre-culture in CgXII minimal medium containing 0.5 µg/l biotin was necessary (Gutmann et al. 1992). Concentrations of external glutamate were determined by HPLC analysis.
Determination of membrane potential
The membrane potential was determined as described earlier (Krämer and Lambert 1990). The membrane potential was calculated from the distribution of the permeant cation [14C]-tetraphenylphosphonium bromide (5 µM final concentration, specific radioactivity 0.995 Ci/mol). Rapid separation of extra- and intracellular fluids was performed by using silicone oil centrifugation with perchloric acid in the bottom layer (Rottenberg 1979). The obtained values of membrane potential were corrected for unspecific probe binding by the addition of a mixture of 20 µM valinomycin and 5 µM nigericin.
Protein synthesis and Western-blot analysis
To control the expression of mscCG mutants, cells were disrupted using a Ribolyser (Thermo Co.) three times at maximum speed of 6.5 for 45 s. After separation of cell debris, membranes were isolated by centrifugation and resuspended in PBS buffer, pH 7.5. Protein concentration was measured by the Bradford technique (Bradford 1976). Western-blot analysis was performed as described (Rübenhagen et al. 2000) using antibodies raised against 6xHis-tag (Qiagen, Hilden, Germany) and against MscCG (Eurogentec, Cologne, Germany). As secondary antibodies, anti-mouse-(AP) and anti-rabbit-(AP) (Sigma) antibodies were used.
Results
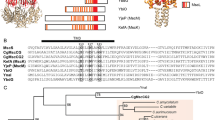
The pore domain of MscCG (aa 1–286) harbors three transmembrane segments, identical to other MscS-type channels (Becker et al. 2013; Wilson et al. 2013). The additional and specific C-terminal domain of MscCG carries a fourth transmembrane segment (Borngen et al. 2010), and can thus, based on topologic considerations, be separated into three subdomains (Fig. 1a), namely the terminal periplasmic subdomain (aa 423–533, 111 amino acid residues), the transmembrane segment (aa 406–422, 17 residues), and the cytoplasmic loop (aa 287–405, 119 residues). The significance of these three subdomains for MscCG function was investigated by several types of manipulations, such as (1) sequential truncations, (2) deletion of parts of these subdomains, (3) replacement of parts of the subdomains by segments with similar or different properties, (4) sequence randomization of individual subdomains, and (5) combination of some of these mutations with loss of function (LOF) mutations of the pore domain of MscCG. The mutational strategy also took into account that some regions showed significant sequence similarity amongst homologous MscS-type proteins with the Corynebacteriaceae family (Figs. 1b, 2, 3, 4, 5).
Topology of MscCG and location of three representative truncation mutants. a Individual domains of MscCG are defined by numbers of amino acid residues (underlined), whereas selected truncation constructs are indicated by arrows. The N-terminal domain (amino acid residues 1–286) resembles the pore domain, aa 287–405 the cytoplasmic loop, aa 406–422 the fourth transmembrane domain, and aa 423–533 the periplasmic C-terminal loop. The segment from N to Δ247 equals MscS from E. coli, whereas the MscCG-specific C-terminal domain covers the segment from Δ247 to C. The transmembrane segments are indicated. Both N- and C-termini face the periplasm. b Schematic drawing of the amino acid sequence of the C-terminal domain of MscCG. The three subdomains, cytoplasmic loop, transmembrane segment (TMS), and periplasmic terminal loop are indicated on the top of the sequence bar, which is tagged with the corresponding amino acid residue numbers of MscCG. The region showing a significant sequence similarity to the C-terminal domains of MscS-type proteins in Corynebacteriaceae is indicated below the bar
Graphic representation of constructs used in this chapter. Numbers at the left-hand side of the construct symbols refer to the same numbers in Table 1 where the characteristic data are provided, as well as to the numbers in the text in square brackets
Graphic representation of constructs used in this chapter. Numbers at the left-hand side of the construct symbols refer to the same numbers in Table 1 where the characteristic data are provided, as well as to the numbers in the text in square brackets
Graphic representation of constructs used in this chapter. Numbers at the left-hand side of the construct symbols refer to the same numbers in Table 1 where the characteristic data are provided, as well as to the numbers in the text in square brackets
Graphic representation of constructs used in this chapter. Numbers at the left-hand side of the construct symbols refer to the same numbers in Table 1 where the characteristic data are provided, as well as to the numbers in the text in square brackets
The functional properties of the obtained mutant forms of MscCG that were studied here included aspects of general physiology (cell growth, expression strength), parameters directly related to MS channel function (electrophysiological properties, membrane potential, and betaine accumulation), as well as aspects of the ability of MscCG to function as glutamate excretion system (both spontaneous and penicillin-induced glutamate excretion). Most of these parameters are indirectly related to MscCG function, since they were quantified in intact cells. A significant change in steady-state membrane potential upon (over)expression of recombinant forms of MscCG, for example, indicates a change in ion permeability as a consequence of channel activity. Similarly, a change in the ability of the cell to accumulate the compatible solute betaine under conditions of hyper-osmotic stress is an indirect measure of the contribution of MscCG to the steady state of cytoplasmic betaine concentration as a consequence of betaine influx via the BetP active uptake system and the passive efflux via MscCG (Borngen et al. 2010).
In all these studies and analyses, a particular caveat has to be taken into account. It has been shown that massive overexpression of MscCG may directly lead to an increase in glutamate excretion, without any further manipulations of the protein itself (Becker et al. 2013; Nakamura et al. 2007). For this reason, expression strength had to be quantified and was modulated by varying the concentration of the inductor IPTG. The former helps to recognize possible overexpression effects (Fig. S1); the latter provides proper conditions for a meaningful comparison of various recombinant forms of MscCG. Table 1 summarizes all parameters measured for the various mutant forms of MscCG under study.
Spontaneous glutamate efflux is an indicator for MscCG functionality
In the first report on this channel being a glutamate efflux protein, a mutant was obtained using an unbiased approach, which caused spontaneous glutamate excretion already without the addition of the inducing agent penicillin (Nakamura et al. 2007). This mutant was found to be truncated in the C-terminal domain due to the presence of an insertion element in the original gene. We have studied effects of stepwise truncation of the C-terminal domain, which led to significant functional changes of MscCG (Becker et al. 2013). In the present work, this analysis was broadly extended both with respect to the constructs studied as well as concerning the type of functional consequences (Fig. 2; Table 1). Truncation by 21 (construct No. 6 in Table 1) and 53 amino acid residues [7], respectively, did not cause a significant phenotype. The loss of 97 [8] amino acid residues reduced the membrane potential of the cell and the capability for betaine accumulation, and the truncation of 110 [9] amino acid residues creates, as described before (Becker et al. 2013), a form of MscCG with drastically changed properties. The presence of the ΔC110 mutant of MscCG [9], although hardly expressed, strongly inhibits growth, significantly lowers the membrane potential, and abolishes betaine accumulation. Moreover, it leads to spontaneous glutamate excretion, similar to that reported previously for the comparable mutant NCgl1221(V419::IS1207) (Nakamura et al. 2007). The broad comparison of results in Table 1 in fact demonstrates that MscCG ΔC110 [9] is the only mutant form that leads to spontaneous glutamate excretion at a low expression level, except the introduction of a GOF mutation [5]. Table 1 furthermore shows that introduction of a LOF mutation by double amino acid replacement (Q112L and V115S [4]), which is effective in the wild-type background, is not able to block spontaneous glutamate excretion by the MscCG ΔC110 mutant [21] (Becker et al. 2013).
The reason for the exceptional properties of MscCG Δ110 [9] are not fully understood at the moment. It may be suggested, on the one hand, that this is a consequence of the absence of the periplasmic domain. There are a number of arguments, however, which rule out this simple explanation (Table 1). C-terminal truncation by 128 [10] or 141 amino acid residues [12], respectively, does not lead to such a phenotype, although the periplasmic subdomain is also missing. Moreover, mutant MscCG ΔC110_rd287-401 [24], which carries the same truncation and, in addition, a cytoplasmic loop with randomized sequence, does not lead to spontaneous glutamate excretion, although being significantly higher expressed than the MscCG ΔC110 form of the protein [9]. It has to be pointed out, on the other hand, that MscCG ΔC110 [9] ends at its C-terminal side right at the end of the transmembrane subdomain lacking any periplasmic extension. It may be envisaged that this creates a structural problem for proper insertion of the C-terminal domain of the protein. The properties of MscCG ΔC110_rd287-401 [24] mentioned above, however, which carries the same C-terminal end and lacks spontaneous glutamate excretion, argues against this hypothesis. Finally, it should be noted that mutations being located directly at either end of the fourth transmembrane segment of MscCG seem to be critical. Truncation at the C-terminal end leads to the MscCG_ΔC110 mutant [9] with its deleterious properties for the cell, and results in a situation where survival of C. glutamicum is only possible at an extremely low expression level of this mutant form. A mutant where the C-terminal domain is truncated directly at the N-terminal end (MscCG_ΔC132 [11]) could, in spite of intense efforts, never be expressed.
Spontaneous glutamate excretion as a result of mscCG overexpression
It has been shown before that strong overexpression of MscCG and its mutant forms may lead to spontaneous glutamate excretion (Becker et al. 2013). This observation is fully in line with the fact that introducing gain-of-function (GOF) mutations into the pore domain of MscCG [5] similarly leads to enhanced glutamate excretion (Becker et al. 2013; Nakamura et al. 2007; Nakayama et al. 2012) (Fig. 3; Table 1). As a matter of fact, this consequence was also observed for many of the mutant forms of MscCG studied here (Table 1). In this context, it is interesting to consider which of the mutant proteins do not give rise to this effect (Table 1). Besides a trivial case, i.e., overexpression of the LOF mutant [5], the three truncated forms MscCG ΔC128 [10], ΔC141 [12], and ΔC247 [13], all lacking the fourth transmembrane segment located within the C-terminal domain, belong to this class. Besides those forms, selective deletion of the transmembrane segment (MscCG Δ406-423 [14]) or replacement of it by a poly-alanine stretch of amino acids (MscCG polyA406-423 [15]) has the same consequence. Spontaneous efflux, however, is observed when the original transmembrane segment is replaced by an artificial transmembrane domain taken from glycophorin, an eukaryotic membrane protein (MscCG Δ406-423_tmX [16]). Of similar interest in this context is the fact that randomization of the cytoplasmic loop completely blocks spontaneous glutamate excretion even upon strong overexpression of the mutant MscCG rd287-401 [19].
It is interesting to note that the presence or lack of the fourth transmembrane domain leads to similar consequences even in the case of the strongly compromised mutant form MscCG Δ110 [9]. Replacement of this domain with a stretch of alanine residues (MscCG Δ110_polyA406-423 [22]) abolishes spontaneous glutamate excretion in the MscCG Δ110 background, whereas replacement by the glycophorin transmembrane domain does not [23].
We also studied the possible significance of the conserved stretch of amino acid residues 392-405. We found that deletion of this segment (MscCG Δ392-405 [17]) also blocks spontaneous glutamate excretion upon overexpression of the construct. We believe, however, that this result is due to an effect on the adjacent transmembrane segment, because of several reasons. First, by comparing the wild-type protein, a mutant protein in which this particular stretch of amino acid residues has been replaced by alanine (MscCG polyA392-405 [18]), and the mutant MscCG rd287-401 [19], we observed spontaneous glutamate excretion in the case of the first two proteins but not with the latter, although all three are lacking this particular motif. Second, when comparing MscCG ΔC128 [10], MscCG ΔC141 [12], and MscCG Δ392-405 [13], only the latter two mutant forms lack this particular motif, whereas glutamate excretion is blocked in all three of them.
The significance of the cytoplasmic loop for MscCG function
The integrity of the cytoplasmic loop was found to be of core significance for the functional properties of MscCG (Fig. 4; Table 1). In the previous sections, it was already demonstrated that randomizing the amino acid sequence of the cytoplasmic loop resulted in loss of spontaneous glutamate efflux both in the MscCG ΔC110 mutant [24] and as a consequence of MscCG overexpression [19]. The properties of the MscCG rd287-401 mutant [19] show that randomization in fact leads to another interesting consequence, namely the inhibition of penicillin-induced glutamate efflux mediated by MscCG. The only other mutation having this effect was found to be the LOF mutation [4]. Furthermore, the inhibitory strength of randomizing the cytoplasmic loop is weakened in the MscCG ΔC110 mutant [9, 24], which per se is strongly biased towards efflux. In this mutant, cytoplasmic loop randomization is still able to block the spontaneous efflux but fails to inhibit the penicillin-induced glutamate efflux.
The gating behavior of MscCG mutant forms is altered
We already previously suggested that the gating behavior of some of the studied MscCG mutants may be altered, although their ion conductance as determined in patch-clamp experiments was found to be essentially unchanged (Becker et al. 2013). We observed that the membrane potential in C. glutamicum cells expressing various mutant forms of MscCG differed in dependence whether the determination was carried out before or after application of a hypo-osmotic downshift (Table 1). It should be noted that the established procedure to measure betaine accumulation includes a washing step under hypo-osmotic conditions before the stimulatory hyperosmotic shock is applied, in order to remove previously accumulated compatible solutes from the cytoplasm (Becker et al. 2014).
An instructive set of data is provided by the sequence of C-terminally truncated mutant forms of MscCG also when combined with an LOF mutation [4, 6–10, 12] (Fig. 5; Table 1). Interestingly, the capability of betaine accumulation turns out to be the most sensitive parameter, followed by membrane potential after application of a hypo-osmotic downshift, whereas the measured membrane potential in cells which did not experience hypo-osmotic challenge was unchanged with the single exception of the MscCG ΔC110 mutant [9]. In the wild-type form as well as the first truncation mutant MscCG ΔC21 [3, 6], no difference depending on application of a hypo-osmotic shock was observed. All other mutants were found to be compromised in betaine accumulation and membrane potential after shock application to various extents. As in the case of other properties, the MscCG ΔC110 mutant [9] showed the most extreme phenotype. Not surprisingly, growth without application of hypo-osmotic stress follows the values measured for membrane potential under the same conditions. The differentiation in dependence of the history of previously experienced hypo-osmotic challenge can be fully abolished again by introducing an LOF mutation in the case of the MscCG ΔC141 [20]. A partial reversion is observed in case of the MscCG ΔC110 mutant [21]. In spite of the observed differences of these mutant forms described above, essentially the same conductance was measured for all of them (Becker et al. 2013).
Discussion
The analysis described here should answer the following questions on the role of the C-terminal domain of MscCG in glutamate excretion and/or mechanosensitive solute efflux. (1) Can we identify specific alterations in the C-terminal domain of MscCG that lead to a higher or a lower activity of glutamate efflux, respectively? This would help to assign specific functions to particular subdomains. (2) Is the effect of these manipulations additive and/or subtractive? This question addresses cooperative effects of subdomains. (3) Do these alterations only affect glutamate excretion or do they also have an impact on solute efflux? This refers to the question of whether and how close the two different functions of MscCG are mechanistically related.
Elaborate studies using X-ray crystallography and molecular modeling have fundamentally contributed to our understanding of the mechanics and the functional significance of MS channels (Bass et al. 2002; Naismith and Booth 2012; Sukharev and Sachs 2012). Because a 3D structure of MscCG is not available, we used biochemical and genetic approaches to unravel specific features of this membrane protein. Since MscCG from C. glutamicum differs from ‘normal’ MS channels in certain aspects, the question arose as to whether it also has structural properties different from those of other known bacterial MS proteins. The obvious structural peculiarity of MscCG is its specific C-terminal domain (Becker et al. 2013). Previous studies indicated that this C-terminal domain critically influences in particular its function as a specific glutamate exporter in C. glutamicum. Specific mutations in this domain have been shown to lead to a significantly enhanced capacity of glutamate excretion (Becker et al. 2013; Borngen et al. 2010; Nakamura et al. 2007). Even more surprising was the finding that the C-terminal domain of MscCG from C. glutamicum, when fused to the C-terminal end of E. coli MscS, is able to significantly increase the basically very low capacity of MscS for glutamate excretion, too (Becker et al. 2013).
An equally interesting argument in favor of this dual function of MscCG is based on the fact that glutamate excretion is a regulated process and can be triggered by a number of specific stimuli. There is an enormous amount of work in the biotechnological literature dealing with methods to trigger glutamate efflux from C. glutamicum (Krämer 1994; Sano 2009), which are all linked to compromising the integrity of the cell wall and/or plasma membrane. It should be noted that unspecific membrane leakage being the explanation for glutamate efflux has been ruled out long ago (Gutmann et al. 1992; Hoischen and Krämer 1990). In view of the complex and heterogeneous nature of manipulations triggering glutamate efflux, it is difficult to suggest a common mechanistic explanation. An appropriate biochemical approach for solving this question would be functional reconstitution in proteoliposomes. Although MscCG can be isolated and purified in sufficient quantities, we did not succeed in reconstituting it in functionally active form even when using a phospholipid composition similar to that of C. glutamicum (unpublished results).
In the present work, we demonstrated that in fact all three subdomains of the C-terminal domain of MscCG, namely the terminal periplasmic loop, the transmembrane segment, and the cytoplasmic loop proved to be important for various functional aspects of MscCG. As a possible explanation for the functional contribution of the periplasmic loop comprising about 110 amino acid residues, interaction of this stretch of amino acids with the interior side of the cell wall could be imagined. This, however, is not very likely since it needs more than 50 amino acid residues to be removed before a significant phenotype is observed. On the other hand, stepwise truncation of the periplasmic end further down to its complete removal revealed a gradual decrease of gating efficiency of the pore domain of MscCG, thus indicating a physical interaction of this part of the periplasmic loop with the N-terminal pore domain of MscCG.
The presence of the fourth transmembrane segment of MscCG within the C-terminal domain also proved to be of core functional significance. Without this subdomain, spontaneous glutamate excretion was never observed, even upon strong overexpression of MscCG constructs and even in the background of the strongly compromised mutant MscCG Δ110. It is interesting to note that this requirement is not sequence specific, since the replacement of the original transmembrane domain (amino acid residues 406–424) by the α-helical transmembrane segment of the eukaryotic membrane protein glycophorin could functionally fully replace this subdomain of MscCG. Replacement with a stretch of alanine residues of the same size, on the other hand, closely resembles the properties of the mutant in which the original transmembrane segment was deleted. It may be envisaged that this membrane stretch of amino acids is required for a correct placement of the hydrophilic parts of the C-terminal subdomain, i.e. both the cytoplasmic and the periplasmic loop, with respect to the N-terminal pore domain of MscCG.
The functional significance of the cytoplasmic loop qualitatively differs from that of the other two subdomains. It is in fact the correct sequence of this stretch of about 120 amino acid residues which matters, since a loop with identical length and amino acid composition whose amino acid sequence was randomized changed the functional properties of MscCG. By introducing this modification, the tendency for glutamate excretion of MscCG was found to be strongly decreased, leading to a complete loss of excretion by the full-length MscCG upon penicillin induction, and to a significant reduction of activity of the particular MscCG ΔC110 mutant.
Besides the general pattern, a number of specific situations have to be considered, too. Functional interaction between the N-terminal pore domain and the C-terminal domain of MscCG is further indicated by the impact of LOF mutations. Inhibition of MscCG function was found to be complete by introducing a LOF mutation into both the wild-type and the MscCG ΔC141 mutant, both being strongly expressed, whereas it was only partially effective in blocking efflux in the MscCG ΔC110 mutant, which is particularly prone to solute excretion. It should be noted that introduction of the LOF mutation significantly improves the viability of the C. glutamicum cells harboring these mutants forms, as indicated by both membrane potential and betaine accumulation. As a matter of fact, the MscCG ΔC110 mutant form of MscCG causes a very strong phenotype and results in significant alterations of biochemical properties of MscCG. It obviously abolishes proper gating, compromises cellular energetics and is consequently very toxic for the cell even at an extremely low expression level. Besides a partial reversion of its strong phenotype by introducing a LOF mutation, its properties can be fully amended by changing the sequence of the cytoplasmic loop (randomization). The latter construct, as well as other combinations of mutations, also indicates a cooperative behavior of some of these alterations, or, in other words, a cooperative behavior or the three subdomains in their action on the N-terminal pore domain of MscCG.
The functional properties of the MscCG ΔC247 mutant remains difficult to explain in the framework of the other results. This mutant, in which MscCG from C. glutamicum is truncated down to the size of E. coli MscS thus lacking the complete C-terminal domain is still capable of penicillin-induced glutamate excretion with high activity. Its properties are definitely different from the wild-type MscCG, as shown by a reduced ability of gating and by the lack of spontaneous glutamate excretion after strong expression; however, a significant extent of functionality is retained without the presence of the original C-terminal domain. We would like to point out that this is in line with our previous findings studying fusion constructs of E. coli MscS with the C-terminal domain of MscCG (Becker et al. 2013). The corresponding MscS channel from E. coli was shown to be capable of penicillin-induced glutamate efflux at a very low level, but this activity could be significantly enhanced upon fusion of the C-terminal domain of MscCG. Again, the pore domain of the MscS channel harboring the solute channel is in principle able to function as inducible glutamate efflux system, the effectivity of which can be enhanced by the presence of and by the interaction with the particular C-terminal domain.
It has to be pointed out that the basic electrophysiological properties of a number of mutant forms of MscCG, which have been determined in detail in previous publications (Becker et al. 2013; Borngen et al. 2010; Nakayama et al. 2013), were not found to be significantly altered by the introduced structural changes. In other words, the basic structural coordinates of the pore domain should be retained in all the mutant forms studied. This obviously does not hold for other functional aspects, e.g., gating probability. As a consequence of the experiments reported here, we conclude that structural alterations in the C-terminal domain of MscCG have a significant and direct impact on the function of this channel as a glutamate exporter. Since the pore conductance of various mutants in the C-terminal domain was not found to be changed, its impact on the pore domain of MscCG has to be on the regulatory level. Structural alterations of the C-terminal domain of MscCG thus do not directly change the geometry and thus the conductance of the channel, but may have a strong impact on its gating probability. It should be noted at this point, however, that for electrophysiological measurements, MscCG and mutant forms of it are expressed in E. coli spheroplasts (Becker et al. 2013; Borngen et al. 2010), whereas in the experiments reported here, MscCG is embedded in the C. glutamicum plasma membrane. As mentioned above, these two membranes fundamentally differ in their lipid composition, which may give rise to differences in function, too.
How can these results be combined into a mechanistic explanation? Undoubtedly, the C-terminal domain physically interacts with the N-terminal pore domain of MscCG being able to regulate its function to some extent. The specificity, and possibly the strength of this interaction, critically depend on the structural integrity of the C-terminal domain. A hot spot for interaction seems to be the additional transmembrane segment of the C-terminal domain, although the specificity of this interaction has to be provided not by the hydrophobic stretch of amino acids but both by regions of the periplasmic domain adjacent to the transmembrane segment, and, in particular by the cytoplasmic domain. One of the most interesting results obtained in this work indicates that this putative interaction of the cytoplasmic domain is sequence specific. This could in principle be explained either by the presence of specific binding motifs or by the requirement of covalent modifications, e.g., phosphorylation, at sequence specific sites within the loop. Both suggestions need further experimentation for elucidation. Undoubtedly, the availability of a three-dimensional structure of MscCG would strongly facilitate the mechanistic understanding of this interesting channel protein.
References
Bass RB, Strop P, Barclay M, Rees DC (2002) Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298:1582–1587
Becker J, Wittmann C (2012) Systems and synthetic metabolic engineering for amino acid production—the heartbeat of industrial strain development. Curr Opin Biotechnol 23:718–726
Becker M, Borngen K, Nomura T, Battle AR, Marin K, Martinac B, Kramer R (2013) Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta 1828:1230–1240
Becker M, Maximov S, Becker M, Meyer U, Wittmann A, Kramer R (2014) Analysis of putative protomer crosstalk in the trimeric transporter BetP: the heterotrimer approach. Biochim Biophys Acta 1837:888–898
Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A (1996) Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol 151:175–187
Borngen K, Battle AR, Moker N, Morbach S, Marin K, Martinac B, Kramer R (2010) The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta 1798:2141–2149
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Eggeling L, Sahm H (2003) New ubiquitous translocators: amino acid export by Corynebacterium glutamicum and Escherichia coli. Arch Microbiol 180:155–160
Grant SG, Jessee J, Bloom FR, Hanahan D (1990) Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci USA 87:4645–4649
Gutmann M, Hoischen C, Krämer R (1992) Carrier-mediated glutamate secretion by Corynebacterium glutamicum under biotin limitation. Biochim Biophys Acta 1112:115–123
Hoischen C, Krämer R (1990) Membrane alteration is necessary but not sufficient for effective glutamate secretion in Corynebacterium glutamicum. J Bacteriol 172:3409–3416
Island MD, Kadner RJ (1993) Interplay between the membrane-associated UhpB and UhpC regulatory proteins. J Bacteriol 175:5028–5034
Jakoby M, Krämer R, Burkovski A (1999) Nitrogen regulation in Corynebacterium glutamicum: isolation of genes involved and biochemical characterization of corresponding proteins. FEMS Microbiol Lett 173:303–310
Jarlier V, Nikaido H (1994) Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol Lett 123:11–18
Krämer R (1994) Secretion of amino acids by bacteria: physiology and mechanism. FEMS Microbiol Rev 13:75–93
Krämer R, Lambert C (1990) Uptake of glutamate in Corynebacterium glutamicum. 2. Evidence for a primary active transport system. Eur J Biochem 194:937–944
Landfald B, Strom AR (1986) Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol 165:849–855
Laneelle MA, Tropis M, Daffe M (2013) Current knowledge on mycolic acids in Corynebacterium glutamicum and their relevance for biotechnological processes. Appl Microbiol Biotechnol 97:9923–9930
Martinac B (2001) Mechanosensitive channels in prokaryotes. Cell Physiol Biochem 11:61–76
Naismith JH, Booth IR (2012) Bacterial mechanosensitive channels–MscS: evolution’s solution to creating sensitivity in function. Annu Rev Biophys 41:157–177
Nakamura J, Hirano S, Ito H, Wachi M (2007) Mutations of the Corynebacterium glutamicum NCgl1221 gene, encoding a mechanosensitive channel homolog, induce l-glutamic acid production. Appl Environ Microbiol 73:4491–4498
Nakayama Y, Yoshimura K, Iida H (2012) A gain-of-function mutation in gating of Corynebacterium glutamicum NCgl1221 causes constitutive glutamate secretion. Appl Environ Microbiol 78:5432–5434
Nakayama Y, Yoshimura K, Iida H (2013) Electrophysiological characterization of the mechanosensitive channel MscCG in Corynebacterium glutamicum. Biophys J 105:1366–1375
Nasie I, Steiner-Mordoch S, Schuldiner S (2013) Topology determination of untagged membrane proteins. Methods Mol Biol 1033:121–130
Nottebrock D, Meyer U, Krämer R, Morbach S (2003) Molecular and biochemical characterization of mechanosensitive channels in Corynebacterium glutamicum. FEMS Microbiol Lett 218:305–309
Ozcan N, Ejsing CS, Shevchenko A, Lipski A, Morbach S, Krämer R (2007) Osmolality, temperature, and membrane lipid composition modulate the activity of betaine transporter BetP in Corynebacterium glutamicum. J Bacteriol 189:7485–7496
Rottenberg H (1979) Non-equilibrium thermodynamics of energy conversion in bioenergetics. Biochim Biophys Acta 549:225–253
Rübenhagen R, Rönsch H, Jung H, Krämer R, Morbach S (2000) Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J Biol Chem 275:735–741
Ruffert S, Lambert C, Peter H, Wendisch VF, Krämer R (1997) Efflux of compatible solutes in Corynebacterium glutamicum mediated by osmoregulated channel activity. Eur J Biochem 247:572–580
Sano C (2009) History of glutamate production. Am J Clin Nutr 90:728S–732S
Sukharev S (2002) Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J 83:290–298
Sukharev S, Sachs F (2012) Molecular force transduction by ion channels: diversity and unifying principles. J Cell Sci 125:3075–3083
Sukharev SI, Martinac B, Arshavsky VY, Kung C (1993) Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J 65:177–183
Teixeira de Mattos MJ, Neijssel OM (1997) Bioenergetic consequences of microbial adaptation to low-nutrient environments. J Biotechnol 59:117–126
Wilson ME, Maksaev G, Haswell ES (2013) MscS-like mechanosensitive channels in plants and microbes. Biochemistry 52:5708–5722
Acknowledgments
We gratefully acknowledge valuable discussions with Boris Martinac, Takeshi Nomura, Andrew Battle, and Yoshitaka Nakayama (Victor Chang Cardiac Research Institute, Sydney, Australia) within the joint project on MscCG. We would like to thank Anja Wittmann for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue: Biophysics of Mechanotransduction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Becker, M., Krämer, R. MscCG from Corynebacterium glutamicum: functional significance of the C-terminal domain. Eur Biophys J 44, 577–588 (2015). https://doi.org/10.1007/s00249-015-1041-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-015-1041-x