Abstract
The Gram-positive soil bacterium Corynebacterium glutamicum is used in microbial biotechnology for the large-scale production of amino acids, e.g., l-glutamate and l-lysine. We have studied the response of this organism to hyperosmotic challenge at the level of both transcription and protein activity. Two systems responding to hyperosmotic stress in C. glutamicum are reviewed here, the two component system MtrAB and the glycine-betaine uptake system BetP. The osmosensory two-component system consists of the membrane-bound histidine kinase MtrB and the soluble response regulator MtrA. MtrB was shown to perceive a so far unknown physical stimulus related to hyperosmotic stress via the cytoplasmically oriented phosphorylation domain, and to transduce the signal to the DNA via MtrA. The secondary active transporter BetP takes up betaine in cotransport with two Na+ ions. BetP responds to hyperosmotic stress by increased transcription mediated via MtrAB signaling, and by instant activation of transport. In the mechanism of BetP activation, the C-terminal, regulatory domain of BetP, the cytoplasmic concentration of K+, and negative membrane surface charges are involved. The molecular mechanism of the activation process is discussed in relation to the recently published X-ray structure of BetP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are a number of bacterial membrane proteins capable of sensing external physical stimuli and transducing them into signals within the bacterial cell. Osmotic stress is a major cause generating this kind of external stimuli relevant to cells. Bacteria are frequently exposed to both hyperosmotic (high external osmolality) and hypoosmotic stress (low external osmolality). Typical conditions for soil bacteria, as an example, are dryness and sudden rainfall, respectively. Osmosensing, on the one hand, refers to mechanisms responsible for perceiving appropriate physical stimuli representative for osmotic stress and osmosignaling, on the other hand, describes transduction of these stimuli into productive cellular signals which may become efficient on the level of both protein activity and gene expression.
Hypoosmotic stress leads to immediate and uncontrolled influx of water, and the cell counteracts this life-threatening situation by instant opening of mechanosensitive channels, i.e., emergency valves leading to fast efflux of small solutes out of the cell thereby relieving the physical stress situation. Hyperosmotic stress, on the other hand, leads to water efflux out of the cell. This situation is counterbalanced by an increase of small, osmotically active solutes in the cytoplasm, the so-called compatible solutes. Compatible solutes cover a large variety of substances, e.g., amino acids (proline), amino acid derivatives (betaines, ectoine), oligosaccharides (trehalose), and heterosides (glucosylglycerol).
A number of osmoregulated receptor and transport systems have been investigated in detail. This refers to both aspects, namely hypoosmotic and hyperosmotic stress. Representative examples for responding to the former type of stress are mechanosensitive channels in bacteria, of the MscL, MscS, and MscK type (Booth et al. 2007). In the case of hyperosmotic stress, both receptor systems like KdpDE and EnvZ/OmpR from Escherichia coli (Hamann et al. 2008; Jung et al. 2001), or MtrAB from Corynebacterium glutamicum (Möker et al. 2004), and transport systems have been studied. There are three major model systems of bacterial osmoregulated uptake of the so-called compatible solutes, namely ProP from E. coli, OpuA from Lactococcus lactis, and BetP from C. glutamicum (for reviews see Poolman et al. 2004; Morbach and Krämer 2004, 2005b; Wood 2006, 2007). The membrane-bound sensor MtrAB and the glycine betaine transporter BetP from C. glutamicum will be the focus of this article.
Corynebacterium glutamicum is an apathogenic, rod-shaped soil bacterium which is of utmost significance in the biotechnology of large-scale amino acid production, mainly for l-glutamate and l-lysine. As a soil bacterium, it frequently faces hypo- and hyperosmotic stress in its natural habitat. It is equipped with at least two mechanosensitive channels for the response against hypoosmotic stress (Ruffert et al. 1999; Nottebrock et al. 2003). Under hyperosmotic conditions it accumulates glycine betaine, ectoine, and proline, the former two only by uptake due to the lack of an appropriate biosynthetic pathway, and the latter both by synthesis and uptake (Bremer and Krämer 2000; Morbach and Krämer 2005a, 2008). C. glutamicum harbors altogether five uptake systems for compatible solutes (Peter et al. 1998b; Morbach and Krämer 2005a), and four of them are osmoregulated, BetP, EctP, ProP, and LcoP. These transporters vary with respect to their substrate specificity, their mode of energetic coupling, their maximum activity, and their regulation in response to osmotic stress. BetP is the most active and the best studied of these uptake carriers. Whereas the regulatory action of BetP in response to osmotic stress solely refers to the level of protein activity, C. glutamicum also harbors stress perception systems which transduce their signal to the level of gene expression. Out of the 13 two-component signal transduction systems found in C. glutamicum (Kočan et al. 2006), the MtrAB system has been identified as a sensor system relevant to the response of C. glutamicum to hyperosmotic stress (Möker et al. 2004). An overview about the types of signals and systems involved in this scenario is presented in Fig. 1.
Stimulus perception and signal transduction in response to hypo- and hyperosmotic stress in C. glutamicum. Osmotic stress leads to regulation both on the level of transcription (red arrows) and protein activity (blue arrows) of uptake systems of compatible solutes (BetP, EctP, ProP, LcoP), mechanosenstive solute efflux systems (MscL, MscS), as well as biosynthesis pathways for compatible solutes
Both MtrAB and, in particular, BetP are model systems for the understanding of osmostress-related stimulus perception and signal transduction in bacteria. There is an obvious gap between the common occurrence of this kind of external stress situation for bacteria and our knowledge about the correct type of stimulus which is actually perceived by cells exposed to this kind of stress. A large variety of physical stimuli may in principle be relevant for perception by osmoregulated sensor and transport proteins, e.g., osmolality and/or ionic strength of the external medium or the internal space, a change in the concentration of particular solutes, e.g., ions, the water activity of the cytoplasm or the availability of free water, the hydration of particularly sensitive proteins, a change in physical properties of the membrane, e.g., curvature stress, etc. The three-model transport systems, BetP of C. glutamicum, ProP of E. coli, and OpuA of L. lactis, are examples where this question has been pursued to the molecular level. In the following, first sensing and signaling by the MtrAB receptor will be reviewed, and subsequently details of osmosensing and osmoregulation of the glycine betaine carrier BetP will be discussed.
Osmosensing and osmosignaling by the two-component system MtrAB from C. glutamicum
The most common type of bacterial sensory and signal transduction systems are two-component systems. Two-component systems are frequently used in bacterial cells to detect environmental changes. C. glutamicum harbors the relatively small number of 13 different two-component systems only (Kočan et al. 2006). Based on single knock-out strains, a number of them have been successfully assigned to particular functions and one of these is the MtrAB system, which was found to be related to osmotic stress response and cell wall biosynthesis (Möker et al. 2004).
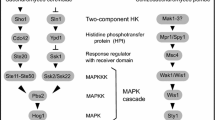
Two-component systems typically consist of a homodimeric membrane-bound histidine protein kinase, e.g., MtrB, and a soluble response regulator in the cytoplasm (MtrA), commonly acting as a transcription factor. The histidine protein kinase, upon being activated by an external stimulus, autophosphorylates at a histidine residue, and transfers the phosphoryl group to an aspartate of the response regulator, thereby affecting its DNA-binding properties, which leads to activation or repression of the transcription of particular genes (Fig. 2).
Schematic drawing of MtrB–MtrA signal transductionin response to osmotic stress. The membrane bound histidine kinase MtrB is composed of four domains, 1 the two transmembrane segments, 2 the external loop, 3 the HAMP domain, and 4 the catalytic domain responsible for autophosphorylation and phosphate transfer to MtrA. The soluble response regulator MtrA becomes phosphorylated be MtrB and binds to the DNA regulating the transcription of selective genes
MtrAB from C. glutamicum was found to regulate expression of both genes involved in cell wall biosynthesis and genes of three of the four carriers responsible for the accumulation of compatible solutes, betP, proP, and lcoP, as well as one of the mechanosensitive channels, mscL. Due to the fact that MtrAB responds to hyperosmotic stress and based on the function of the different targets, MtrA acts as an activator of the expression of uptake carriers genes, whereas it represses the expression of the gene of the MscL channel (Möker et al. 2004). We were basically interested in two questions: (1) which kind of physical stimulus may be recognized by MtrB as a measure of hyperosmotic stress, and (2) which part of MtrB may act as sensor domain perceiving this kind of stimulus. In order to answer these questions, experiments both in intact cells and in proteoliposomes harboring a functionally reconstituted MtrB–MtrA system were carried out (Möker et al. 2007a, b).
For investigating the kind of physical stimulus, proteoliposomes were the system of choice, since in intact cells composition and state of the cytoplasm cannot be controlled or manipulated in a quantitative way. For this purpose, MtrB was reconstituted in functionally active form into liposomes made from different phospholipids. We quantified autophosphorylation of MtrB as a measure for the extent of activation in response to the addition of a particular stimulating compound. In this system, we systematically varied a number of parameters, including the concentration of different chemical compounds like ions, amino acids, compatible solutes, sugars, and polyethylene glycol. Under all circumstances, the most efficient stimulation was caused by high K+ concentrations (Möker et al. 2007a). We could demonstrate, however, that this K+-dependent activation seems to be an unspecific feature of two-component systems, since the same activation was observed when DcuS from E. coli was analyzed instead of MtrB. The DcuS-DcuR system is a two-component system sensing C4-dicarboxylates and has no connection to osmoregulation whatsoever (Janausch et al. 2002). Stimulation by K+ has furthermore been reported also for other two-component systems (Voelkner et al. 1993; Jung et al. 2001). Significant MtrB-specific stimulation was observed upon addition of various solutes, sugars, amino acids, and polyethylene glycol to various extents. It seems unlikely that these compounds with very different chemical nature all bind to the same binding site; consequently, we proposed that they may act via a change of the hydration state of the MtrB protein (Möker et al. 2007b). As a simple functional model we assume that MtrB may be present in two different conformations, an active and an inactive one. Decreasing the hydration state of MtrB now is suggested to shift the conformational equilibrium towards the active state of the protein thus triggering its own autophosphorylation and subsequently phosphorylation of MtrA thereby starting signal transduction to the target genes.
Surprisingly, we observed that the added solutes lead to activation of MtrB only when present at the external side, but not in the internal space of the proteoliposomes, which indicates a side-specific action of the relevant stimulus. A critical limitation of reconstituted systems is the a priori unknown orientation of the membrane-embedded proteins. Upon insertion into the membrane, the reconstituted protein may be integrated either in a ‘right-side-out’, an ‘inside-out’, or a random orientation. We used a proteolytic approach and investigated the accessibility of the large, catalytic C-terminal domain of MtrB to carboxypeptidase (Möker et al. 2007b). We found that the C-terminally located Strep Tag II of MtrB was completely degraded in both intact liposomes and solubilized protein; consequently, MtrB is unidirectionally inserted in proteoliposomes in an ‘inside-out’ orientation. In view of the side-specific action of activating solutes, it can thus be concluded that the stimulus-sensing domain of MtrB should be located at the cytoplasmic side of the protein.
A general motif of two-component systems sensitive to external stimuli is a sensory input mechanism via the external loop of the membrane-bound histidine kinase (e.g., MtrB), as has been elegantly shown at the molecular level also for the DcuS protein which was used here as a control (Kneuper et al. 2005). This general scenario, however, does not seem to be valid for the sensory action of MtrB, since the signal input site was concluded to be located at the cytoplasmic side of MtrB. In order to study this in more detail, we constructed a series of mutants of MtrB, which either lack the periplasmic domain or the HAMP domain at the cytoplasmic side of MtrB which is inserted between the transmembrane domain and the activation domain (Fig. 2) (Möker et al. 2007b). In further constructs extending this analysis we expressed only the soluble part of MtrB, i.e., the activation domain and the HAMP domain, thus lacking the transmembrane parts. Moreover, a construct was generated in which the membrane part of E. coli DcuS (see above) was fused to the cytoplasmic soluble part of MtrB. Constructs lacking one of the two transmembrane domains of BetP could not be expressed. We found that all recombinant forms of MtrB were regulated similar to the wild type, both in intact cells and when isolated and reconstituted into proteoliposomes, provided they contain the cytoplasmically located activation domain. The soluble activation domain alone, not anchored to the membrane, however, was not active (Reihlen et al. unpublished results). On the other hand, the external loop seems to be dispensable for stimulus perception, which is in line with the observation of a side-specific stimulation from the cytoplasmic side of MtrB (see above). Furthermore, there seems to be no specific requirement for the transmembrane domains of MtrB, provided the activation domain of MtrB is somehow fixed to the membrane.
Consequently, MtrB belongs to a particular class of histidine protein kinases which sense environmental stimuli not via the externally located, exposed periplasmic loop. On the other hand, cytoplasmically located domains of MtrB seem to be relevant to stimulus perception, but not the HAMP domain located between the second transmembrane segment and the activation domain of MtrB. As a further interesting consequence, we conclude that the physiologically relevant stimulus generated by the external increase of osmolality does act from the cytoplasmic space. It has to be taken into account that an external hyperosmotic shift will lead to an instant increase in internal solute concentration because of the immediate efflux of water, and subsequently, this increase will be sustained by the accumulation of compatible solutes provided by biosynthesis or uptake. Interestingly, this resembles the situation which will be described in detail for the osmosensory glycine betaine carrier BetP (see below), which also is triggered by a concentration change of an internal solute. A schematic view of the stimulus perception and signal transduction of the MtrA–MtrB system is shown in Fig. 3.
The histidine kinase MtrB and various recombinant forms of it investigated to elucidate stimulus perception (for assignment of different domains of MtrB see legend to Fig. 2 and text)
Osmosensing, osmosignaling and osmoregulation by the glycine betaine transporter BetP from C. glutamicum
The first physical consequence of hyperosmotic stress for all cells is an immediate efflux of water due to the osmotic gradient. The first physiological answer of C. glutamicum immediately following water efflux is fast influx of K+, similar to that observed for E. coli (Dinnbier et al. 1988). It should be pointed out that the nature of the ion, which is electrically compensating this K+ flux, is not yet known for any bacterium. After the fast K+ response, a number of subsequent physiological responses of the bacterial cell are observed (Wood 1999), the most significant being accumulation of compatible solutes at high concentrations. These solutes fulfill at least two purposes: they increase the internal osmolality to sustain an appropriate cell turgor which is essential for survival of the cell, and they function as direct protectants for cellular proteins against denaturation under high osmotic conditions (Bolen and Rose 2008). Accumulation of compatible solutes in the cytoplasm can be achieved in two ways: by biosynthesis, provided an appropriate metabolic pathway is available, and by uptake, provided they are present in the surrounding medium. In terms of energy economics, uptake is always cheaper compared to biosynthesis; consequently, bacterial cells usually prefer taking up these solutes. Bacteria in general are equipped with a diversity of compatible solute uptake systems, divided into inducible and constitutive systems, on the one hand, and into substrate specific systems and those with a broad substrate spectrum, on the other. Bacteria in which these patterns have been studied to detail are, for example, Bacillus subtilis (Kempf and Bremer 1998), E. coli (Wood 1999), or C. glutamicum (Morbach and Krämer 2005a). Diverse types of carrier mechanisms are found for this purpose, primary active transporters, as well as secondary carriers including also TRAP type transporters (Grammann et al. 2002).
The situation in C. glutamicum seems to be somewhat simpler than in other bacteria, since only secondary transporters energetically coupled to electrochemical ion potentials are involved, however, the multitude of altogether five different transport systems creates some complexity (Peter et al. 1998b; Morbach and Krämer 2005a). C. glutamicum uses glycine betaine, ectoine, proline, and under the particular condition of nitrogen limitation and absence of external betaine or ectoine, also trehalose, as compatible solute. PutP, a proline transporter, is not activated by osmotic stress but provides this amino acid for anabolic purposes. EctP, ProP, and LcoP accept ectoine, proline and betaine with different affinity as a substrate and are all directly activated by osmotic stress. Direct activation by osmotic stress also holds for BetP which is specific for glycine betaine as a substrate and shows by far the highest transport capacity of the five carriers mentioned. Expression of the genes encoding BetP, ProP, and LcoP is induced by the two-component system MtrAB in response to hyperosmotic conditions on the level of transcription (see above), the transcriptional control of EctP has not been elucidated so far.
BetP is a secondary carrier belonging to the BCCT-type family of transporters (Peter et al. 1996; Krämer and Morbach 2004; Morbach and Krämer 2005b), glycine betaine uptake being energetically coupled to the membrane potential dependent co-transport of two sodium ions (Farwick et al. 1995; Krämer and Morbach 2004). It consists of 594 amino acids and can be divided into a core part of 12 transmembrane segments as well as two hydrophilic C- and N-terminal domains of around 50–55 amino acids each exposed to the cytoplasm (Peter et al. 1996, 1998a). BetP has been structurally characterized by 2D electron crystallography (Ziegler et al. 2004), and recently by X-ray crystallography to 3.3 Å resolution (Ressl et al. 2009).
Transport proteins are membrane-bound enzymes which, different to ‘normal’ enzymes, change the location and not the chemical nature of their substrates. Like enzymes, they do their job in lowering the activation energy of a particular reaction by utilizing conformational energy, in the case of transporters only with respect to the protein and not the substrate. The conformational change of a transporter during its catalytic activity (catalytic cycle) can conceptually be divided into four steps: (1) binding of substrate to the transporter with the substrate binding site exposed to one side of the membrane, (2) substrate translocation including a conformational change of the transporter, (3) release of substrate at the opposite side of the membrane, and (4) return of the transporter conformation resulting in exposure of the binding site to the original side of the membrane. The important aspect now, when considering a transporter which is regulated by an external stimulus, is the involvement of a second functional cycle, which simply includes two states: the active and the inactive state (activation cycle). These two cycles are not necessarily connected; however, it is obvious that the catalytic cycle will only proceed when the transporter is in an active state. Function and velocity of the catalytic cycle of a transporter are governed by the available driving force(s), which, in the case of BetP, is the sum of the electrochemical sodium potential and the betaine gradient, as well as by the availability of the two substrates, betaine and sodium, or in other words, the occupancy of the their respective binding sites at the transporter. Function of the activation cycle, on the other hand, will depend on the presence or absence of physical stimuli or parameters to which BetP is susceptible. A conceptual drawing of the two cycles is shown in Fig. 4.
Catalytic cycle and activation cycle of BetP. In the activation cycle, BetP alternates between the inactive (C) and the active state (C*). The shift from the inactive to the active state may be triggered by the cytoplasmic K+ concentration and by the membrane surface charge (see text). The catalytic cycle of BetP in the active state involves several conformational states, with the substrate binding site either exposed to the outside (C e *) or the inside (C i *), as well as the transition, or occluded state (C*), respectively
The topic of this review is the activation cycle of BetP, and our goal is to understand the molecular mechanism of regulation of this activation cycle. This task is more complicated as it may seem at the beginning. We will have to understand how BetP changes its functional state when being activated by hyperosmotic stress. Consequently, the first task would be to identify the kind of physical stimuli which are relevant for activation, which, immediately is followed by the second question, concerning the identification of the elements of BetP which are involved in this response (structural aspect) as well as concerning the understanding how they function during activation (dynamic aspect). Furthermore, the opposite side of this process is rather interesting as well. BetP will return to the inactive state when the external stress ceases; however, this is obviously not the most interesting situation in terms of physiology. It must be guaranteed that active and efficient betaine uptake into the cell ceases when the cell has reached osmotic compensation, or in other words, when enough betaine has been accumulated in the cytoplasm. Moreover, the latter response has to work in a fine-tuned way, i.e., BetP activity has to cease at different values of betaine accumulation upon exposure of the cell to different extent of hyperosmotic stress. In the following, results will be presented which suggest an elaborated concept for activation of BetP; however, our knowledge on the adaptive inactivation of BetP is still very limited.
Parameters influencing BetP activation by hyperosmotic stress
A number of parameters have been identified in the past years to contribute to the molecular mechanism of BetP activation. They are of both intrinsic (elements of BetP involved) and extrinsic nature (factors influencing BetP from the surrounding). The use of a functionally reconstituted system, where BetP is integrated into membranes of proteolipososmes, was a great help in this analysis (Rübenhagen et al. 2000). Only in proteoliposomes both hydrophilic sides of BetP are easily accessible to experimental variation and this holds true, in addition, to the variation of the lipid surrounding into which BetP is embedded. The advantage of such a reconstituted system is the possibility to separate the different parameters suggested to take part in the activation process and to test them individually. The influence of particular compounds (ions, neutral solutes), as an example, can be investigated in proteoliposomes in two different ways, (1) adding them only at the outside thereby changing their concentration and, in addition, initially generating an osmotic gradient, or (2) adding them on both sides of the membrane, thus generating only a symmetric concentration change. This system also has serious disadvantages, however, and they originate from the intended reduction of complexity from the intact cell to proteoliposomes. Liposomes will react as perfect osmometers; consequently, the influence of turgor or, in other words, a putative interaction of the plasma membrane, in which BetP is inserted, with the cell wall during osmotic stress, cannot be investigated.
Using the reconstituted system, a broad variety of solutes and conditions were tested for their contribution to BetP activation (Rübenhagen et al. 2000). Among them, solely K+ ions, as well as the chemically similar Cs+ and Rb+, were found to significantly activate BetP when present in concentrations higher than about 200 mM (Rübenhagen et al. 2001; Schiller et al. 2004b). Both Na+ and NH4 + had only a minor effect. The specificity of BetP with respect to the stimulating compound is an interesting aspect of the activation mechanism and clearly differs from the two other thoroughly studied osmoregulated transport systems, namely OpuA from L. lactis and ProP from E. coli. In the former case, the ionic strength was found to be the relevant stimulating parameter and the hydration state of the ProP protein was suggested for the latter (Biemans-Oldehinkel et al. 2006; Wood 2006). These results on BetP stimulation by K+ also raise a problem, since it seems rather difficult to imagine such a distinct specificity with respect to stimulation, and consequently, also concerning the binding to a so far unknown binding site at BetP, on the one hand, and an extremely low apparent affinity of this process with 200 mM or higher, on the other. If, however, K+ plays the pivotal role in BetP activation during osmotic stress, the range of 200 mM and higher is the correct range of effector concentration in terms of physiology, since the internal K+ concentration observed in C. glutamicum matches this concentration range.
Also the second external parameter relevant to BetP activation was identified using the reconstituted system. It has previously been observed that betP, when heterologously expressed in E. coli, although being catalytically active and responsive to osmotic activation, showed a shift in the optimum of activation to lower external osmolalities as compared to the situation in C. glutamicum, its homologous host. This observation was originally thought to reflect the significantly lower turgor pressure in the Gram-negative E. coli. Experiments using the reconstituted system, in which the headgroup composition of the phospholipids used was varied, however, resulted in a different explanation. It turned out the share of negatively charged phospholipids critically influences the activation cycle of BetP (Schiller et al. 2006). The larger this share, the higher the concentration of K+ necessary for maximum activation. The originally observed shift of the activation optimum of BetP in E. coli with respect to C. glutamicum is directly reflected by the fact that the E. coli plasma membrane contains only about 20% negatively charged phospholipids, whereas this type of lipids covers nearly 100% in C. glutamicum (Özcan et al. 2007). The two external parameters influencing the activity state of BetP can be integrated into the activation cycle concept of BetP (Fig. 4). Specific lipid requirement was also observed for the other two model systems in bacterial osmoregulation, ProP from E. coli, and OpuA from L. lactis (Tsatskis et al. 2005; van der Heide et al. 2001).
Already, in early attempts to mechanistically understand the response of BetP to hyperosmotic stress, it was observed that manipulations in the structure of the N- and C-terminal domain of the transporter protein directly influence its activation properties (Peter et al. 1998a). Truncations of the negatively charged N-terminal domain were found to reduce the sensitivity of BetP towards osmotic stress, whereas truncations of the C-terminal domain had even more drastic effects (Peter et al. 1998a; Schiller et al. 2004a, b). Deletion of the terminal 12 amino acids of the C-terminal domain did not significantly influence BetP function, whereas a truncation of 23 amino acids led to a reduced maximum activity and a concomitant loss of regulation (Peter et al. 1998a; Schiller et al. 2004a, b). Full deletion of this domain inactivated BetP. Consequently, the C-terminal domain was interpreted as a sensor domain or at least as being critically involved in osmosensing by BetP (Schiller et al. 2004a, b, 2006).
Besides this conclusion, another important information can be drawn from these results. Since wild-type BetP is inactive in the absence of osmotic stress, and particular mutants with amino acid replacements within the core part of the C-terminal domain in terms of function show significant activity under these conditions (Ott et al. 2008), the role of the C-terminal domain can be interpreted as being inhibitory for BetP function. When osmotic activation occurs in which the C-terminal domain plays a pivotal role, the terminal domain is supposed to change its conformation which then will lead to activation of BetP. Furthermore, when this concept is combined with the results concerning the dependence of activation on the surface charge of the surrounding membrane (see above), we conclude that the C-terminal domain of BetP has to somehow interact with the membrane surface. The membrane interacting state of BetP via its C-terminal domain is very likely to represent the inactive state of BetP. Consequently, we assume that upon the rise in the cytoplasmic K+ concentration which is the primary trigger of BetP activation, the C-terminal domain is released from the membrane bound state. This state is supposed to be the resting state keeping BetP catalytically inactive. The release from membrane interaction then triggers the onset of the catalytic cycle of transport.
In recent studies the function of the C-terminal domain was further elaborated by scanning mutagenesis and by a number of other techniques (Schiller et al. 2006; Ott et al. 2008). First, it was found that within the C-terminal domain of about 50 amino acids, a core part exists in which mutations leading to conformational effects, e.g., replacing amino acids by proline, abolishes the ability to become activated by osmotic stress although a significant level of basal activity was retained. Interestingly, this core part of the C-terminal domain has a different size when BetP was analyzed in C. glutamicum or in E. coli membranes, being larger in the less negatively charged membrane of E. coli. This observation may indicate that a highly negatively charged membrane surrounding stabilizes a particular conformation of the C-terminal domain required for proper sensing of osmotic stress and/or catalytic activity.
Further in vitro studies revealed that the C-terminal domain of BetP seems to interact during its function in the activation cycle of BetP also with other elements or parts of the transport protein. By surface plasmon resonance spectroscopy, we found that the C-terminal domain in fact shows significant binding to membrane surfaces, as already concluded above on the basis of functional studies (Ott et al. 2008). Experiments using peptide arrays, in which interaction of the C-terminal domain with a series of peptides representing the complete BetP sequence was tested, showed selective interaction of the C-terminal with the N-terminal domain as well as with at least one of the hydrophilic loops of the transporter. The latter result was corroborated by a competition experiment in proteoliposomes, in which synthetic peptides resembling the hydrophilic loops of BetP were added to the reconstituted BetP. Selected loops in fact suppressed the response of BetP to hyperosmotic stress, obviously by competition with the intrinsic loops of BetP for the C-terminal domain, whereas a loop peptide with randomized sequence had not effect (Ott et al. 2008).
A mechanistic model of BetP activation based on its 3D structure
The details obtained from biochemical studies as described above, have been integrated into mechanistic concepts of BetP function, in particular with respect to the activation cycle. Recently, a detailed X-ray structure of BetP was published (Ressl et al. 2009), which now provides a new and interesting basis for a revision und further development of this concept.
The basic mechanistic concept of BetP activation by hyperosmotic stress involves a change of the location of the C-terminal domain between a membrane-attached and a loop-attached state (Fig. 5a). The former conformational state, in which (parts of) the C-terminal domain are supposed to be attached to the surface of the surrounding membrane, is interpreted as the resting, or inactive state. It will be the preferred state at low cytoplasmic K+ concentration, i.e., at low osmotic stress, and will be favored by a high density of negative charges at the membrane surface. The latter conformational state, on the other hand, in with (parts of) the C-terminal domain are supposed to physically interact with other elements of BetP, namely the N-terminal domain and/or cytoplasmic loops, was suggested to represent the catalytically active state of BetP. This conformational state will be favored by the presence of a high cytoplasmic K+ concentration, i.e., at high osmotic stress, and will be also be favored by a decreased density of negative charges at the surrounding membrane surface. So far only a change in cytoplasmic K+ concentration has been identified as a trigger responsible for the functional switch between the two states. There are, however, indications for a second trigger, as will be discussed in the next chapter.
Structure-function correlation of BetP activation. a Elements of BetP activity control including the C-terminal, regulatory domain 1, the cytoplasmic concentration of K+ 2, and negative membrane surface charges 3. b Mechanistic concept of BetP activation involving the elements described in a. In this concept, in the resting state, the C-terminal domain is interacting with the adjacent membrane. Upon activation by an increase in cytoplasmic K+, the C-terminal domain switches its conformation, interacts with not yet defined regions of BetP, and as a result, BetP actively transports glycine betaine. c Structural elements of the functionally active BetP trimer derived from the X-ray structure (Ressl et al. 2009; Krämer and Ziegler 2009). The three individual monomers of BetP are differently color-coded. The C-terminal domains, which are recognized as long helices lying on the cytoplasmic surface of the adjacent BetP monomers, are not fully resolved in the X-ray structure (Ressl et al. 2009), their conformation was modeled in accordance with other biochemical data (Krämer and Ziegler 2009)
Due to the recent achievement of the X-ray structure of BetP (Ressl et al. 2009), we are now in the favorable position to critically evaluate this concept in the light of the structural data. The first and important aspect to be taken into account is the fact that BetP is a functional trimer, which was discovered already previously upon 2D electron crystallography of BetP (Ziegler et al. 2004). Consequently, for a mechanistic model, subunit interactions are possibly relevant. As a matter of fact, the X-ray structure clearly shows that the C-terminal domain of each BetP monomer is interacting primarily not with the same, but with the adjacent monomer (Fig. 5b). This situation necessarily leads to at least two important consequences. (1) There is a functional interaction between the three BetP subunits in the trimer. (2) Since the C-terminal domain is one of the interacting elements, this interaction will most probably be connected to the activation cycle of BetP. Unfortunately, only one of the three C-terminal domains of BetP is sufficiently resolved in the X-ray structure, and this particular domain is most probably not in a physiological conformation, since it was found to be responsible for crystal contacts between individual trimers (Ressl et al. 2009). Because of this fact, we are currently not able to decide exactly with which part of the cytoplasmic surface of the adjacent subunit the C-terminal domain does interact (Krämer and Ziegler 2009).
It is furthermore interesting to note that, in difference to most other secondary transporters crystallized to far, in the resolved X-ray structure BetP is in an occluded state, i.e., the intermediate state of the catalytic cycle of a carrier [a ‘frozen’ state (2) as described above for the catalytic cycle of a transporter] resembling the transition state of an enzyme. Since, however, we have currently no idea which state of the activation cycle the crystallized form of BetP does in fact represent, we are not able to draw conclusions between possible relations between the catalytic state (occluded) and the activity state of BetP. Furthermore, a decision on the suggested involvement of an interaction between the N- and the C-terminal domain during BetP activation is not yet possible, since the N-terminal domain is partially truncated in the crystallized form of BetP.
Further aspects of BetP activation
There are a large number of unsolved questions connected with the concept of BetP activation as presented here, and some of the questions which are either most interesting or most urgent to be solved will briefly be discussed in the following.
Adaptation to osmotic stress
As mentioned already at the beginning of this chapter, a conclusive model of BetP activation should not only include the mechanism of activation and deactivation, but should also explain adaptation to the state of osmotic compensation. We found that BetP activity is strongly decreased in cells of C. glutamicum after an appropriate amount of betaine has been taken up (Botzenhardt et al. 2004). This adaptive downregulation of activity was found not to depend on the increased substrate concentration (simple feedback control), nor on a changed internal K+ concentration, and most surprisingly, not even on the presence of the C-terminal domain (Botzenhardt et al. 2004). Consequently, this result is an indication for the presence of an alternative stimulus which may be relevant for regulation of BetP activation, at least in the case of adaptation to osmotic compensation. Based on several observations, we suggest that this additional type of stimulus may be related to the physical properties of the surrounding membrane and may be sensed by BetP via protein/membrane interaction.
Further stimuli related to hyperosmotic stress
As a matter of fact, this line of evidence for alternative types of stimuli relevant for BetP activation is strongly supported by investigations on chill sensitivity of BetP. We found that, besides being activated by hyperosmotic stress, BetP can be stimulated to a comparable extent by low temperatures (Özcan et al. 2005). Again, this type of activation was found to be independent of the presence of high K+ or an intact C-terminal domain. On the other hand, we found a correlation of the property to become chill activated with the lipid composition of the surrounding membrane, which was changed upon growth at low temperatures both with respect to headgroup and fatty acid distribution (Özcan et al. 2007). Recently, we found that in fact solely the fatty acid composition and not the headgroup distribution is modulating the activity cycle of BetP (Henrich and Krämer, unpublished results).
Activation and oligomeric state of BetP
Although the mechanistic concept of the BetP activation cycle is supported by a number of results obtained using different methodical approaches (see above), it is still not clear whether the trimeric nature of BetP is important for the functioning of both the catalytic and the activation cycle. With respect to the former aspect, there is in fact so far no obvious reason known why a monomer by itself should not be catalytically active. In the case of the activation cycle, however, the significance of the trimeric state seems to be clear because of the fact that the C-terminal domain of each subunit interacts with the cytoplasmic surface of the adjacent subunit. However, this still could be a relatively trivial mechanism, if each C-terminal domain would exert its regulatory impact only back to the function of the subunit to which it is covalently connected. An attractive mode of regulation, however, would involve inhibition of the adjacent monomer by each C-terminal domain, possibly via blocking the respective substrate access site at the cytoplasmic surface of this subunit or by direct conformational interaction. This would result in a complicated mechanism in which domains from different monomers would collaborate for the correct function of the activation cycle. This, however, is still a matter of speculation.
Conclusions and perspectives
Among the three acknowledged model systems of osmoregulated transporters, ProP from E. coli, OpuA from L. lactis and BetP from C. glutamicum, BetP currently seems to be the system with the deepest knowledge concerning a detailed structure/function analysis. What did we learn so far from this system with respect to bacterial sensing and signaling?
At least in the case of BetP, the cell has selected a relatively specific and, at the same time, interestingly indirect aspect for a proper stimulus which is connected with the event of hyperosmotic challenge from the external medium, namely the intracellular K+ concentration. This is clearly different in the other two model systems, which seems to respond to much broader stimuli, like ionic strength or protein hydration, respectively. Both in the case of the transporter BetP and the membrane sensor MtrB, stimuli from the cytoplasmic side are used in spite of the fact the primary event, i.e., a shift to hyperosmotic conditions, happens at the outside of the cell, and in spite of the fact that both proteins would in principle provide prominent hydrophilic elements available also at the outside as putative sensor elements.
The interesting question whether the membrane is somehow involved in osmosensing and signaling is not yet completely resolved, at least not for osmoregulated transport proteins. There is no serious doubt that the kinds of stimuli identified to far should be correct, but there is some doubt, whether the list is complete. The major conclusions in this respect have all been made in reconstituted systems and these artificial test systems are, although being ideally suited to reduce the complexity, not fully competent for answering this question in all aspects. For example, proteoliposomes, per definition, lack any turgor related phenomena and are thus not possible to detect stimuli related to this kind of events.
BetP, however, seems to be a well-suited object to study sensing and regulation phenomena in transporters which are related to protein-protein interaction. There are numerous transporters available which are found to occur in oligomeric state, and in general, it is not known whether this state is of functional significance for the catalytic cycle of a carrier, or for its regulation. In the case of BetP, it seems probable that the trimeric organization is a prerequisite for the mechanism of osmotic activation (activation cycle), and it may well be possible that this holds also true for the catalytic cycle of BetP.
References
Biemans-Oldehinkel E, Mahmood NA, Poolman B (2006) A sensor for intracellular ionic strength. Proc Natl Acad Sci USA 103:10624–10629
Bolen DW, Rose GD (2008) Structure and energetics of the hydrogen-bonded backbone in protein folding. Annu Rev Biochem 77:339–362
Booth IR, Edwards MD, Black S, Schumann U, Miller S (2007) Mechanosensitive channels in bacteria: signs of closure? Nat Rev Microbiol 5:431–440
Botzenhardt J, Morbach S, Krämer R (2004) Activity regulation of the betaine transporter BetP of Corynebacterium glutamicum in response to osmotic compensation. Biochim Biophys Acta 1667:229–240
Bremer E, Krämer R (2000) Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes. In: Storz G, Hengge-Aronis R (eds) Bacterial stress responses. ASM Press, Washington, pp 79–97
Dinnbier U, Limpinsel E, Schmid R, Bakker E (1988) Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K12 to elevated sodium chloride concentrations. Arch Microbiol 150:348–357
Farwick M, Siewe RM, Krämer R (1995) Glycine betaine uptake after hyperosmotic shift in Corynebacterium glutamicum. J Bacteriol 177:4690–4695
Grammann K, Volke A, Kunte HJ (2002) New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581. J Bacteriol 184:3078–3085
Hamann K, Zimmann P, Altendorf KH (2008) Reduction of turgor is not the stimulus for the sensor kinase KdpD of Escherichia coli. J Bacteriol 190:2360–2367
Janausch IG, Garcia-Moreno I, Unden G (2002) Function of DcuS from Escherichia coli as a fumarate-stimulated histidine protein kinase in vitro. J Biol Chem 277:39809–39814
Jung K, Hamann K, Revermann A (2001) K+ stimulates specifically the autokinase activity of purified and reconstituted EnvZ of Escherichia coli. J Biol Chem 272:10847–10852
Kempf B, Bremer E (1998) Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol 170:319–330
Kneuper H, Janausch IG, Vijayan V, Zweckstetter M, Bock V, Griesinger C, Unden F (2005) The nature of the stimulus and of the fumarate binding site of the fumarate sensor DcuS of Escherichia coli. J Biol Chem 280:20596–20603
Kočan M, Schaffer S, Ishige T, Sorger-Herrmann U, Wendisch VW, Bott M (2006) Two-component systems of Corynebacterium glutamicum: deletion analysis and involvement of the PhoS–PhoR system in the phosphate starvation response. J Bacteriol 188:724–732
Krämer R, Morbach S (2004) BetP of Corynebacterium glutamicum, a transporter with three different functions: betaine transport, osmosensing, and osmoregulation. Biochim Biophys Acta 1658:31–36
Krämer R, Ziegler C (2009) Regulative interactions of the osmosensing C-terminal domain in the trimeric glycine-betaine transporter BetP from Corynebacterium glutamicum. Biol Chem (in press)
Möker N, Brocker M, Schaffer S, Krämer R, Morbach S, Bott M (2004) Deletion of the genes encoding the MtrA–MtrB two-component system of Corynebacterium glutamicum has a strong influence on cell morphology, antibiotics susceptibility and expression of genes involved in osmoregulation. Mol Microbiol 54:420–438
Möker N, Krämer J, Unden G, Krämer R, Morbach S (2007a) In vitro analysis of the two-component system MtrB–MtrA from Corynebacterium glutamicum. J Bacteriol 189:3645–3649
Möker N, Reihlen P, Krämer R, Morbach S (2007b) Osmosensing properties of the histidine kinase MtrB from Corynebacterium glutamicum. J Biol Chem 282:27666–27677
Morbach S, Krämer R (2004) Osmoregulation and osmosensing by uptake carriers for compatible solutes in bacteria. In: Boles E, Krämer R (eds) Molecular mechanisms controlling transmembrane transport, topics in current genetics, vol 9. Springer, Berlin, pp 155–178
Morbach S, Krämer R (2005a) Osmoregulation in Corynebacterium glutamicum. In: Eggeling L, Bott M (eds) Corynebacterium glutamicum. CRC Press LLC, Boca Raton, pp 417–438
Morbach S, Krämer R (2005b) Structure and Function of the betaine uptake system BetP of Corynebacterium glutamicum: strategies to sense osmotic and chill stress. J Mol Microbiol Biotechnol 10:143–153
Morbach S, Krämer R (2008) Environmental stress response of Corynebacterium glutamicum. In: Burkovski A (ed) Corynebacterium glutamicum genomics and molecular biology. Caister Academic Press, Norfolk, pp 313–334
Nottebrock D, Meyer U, Krämer R, Morbach S (2003) Molecular and biochemical characterization of mechanosensitive channels in Corynebacterium glutamicum. FEMS Microb Lett 218:305–309
Ott V, Koch J, Späte K, Morbach S, Krämer R (2008) Regulatory properties and interaction of the C- and N-terminal domains of BetP, an osmoregulated betaine transporter from Corynebacterium glutamicum. Biochemistry 47:12208–12218
Özcan N, Krämer R, Morbach S (2005) Chill activation of compatible solute transporters in Corynebacterium glutamicum at the level of transport activity. J Bacteriol 187:4752–4759
Özcan N, Ejsing CS, Shevchenko A, Lipski A, Morbach S, Krämer R (2007) Osmolality, temperature, and membrane lipid composition modulate the activity of betaine transporter BetP in Corynebacterium glutamicum. J Bacteriol 189:7485–7496
Peter H, Burkovski A, Krämer R (1996) Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J Bacteriol 178:5229–5234
Peter H, Burkovski A, Krämer R (1998a) Osmosensing by N- and C-terminal extensions of the glycine betaine uptake system BetP of Corynebacterium glutamicum. J Biol Chem 273:2567–2574
Peter H, Weil B, Burkovski A, Krämer R, Morbach S (1998b) Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing and characterization of the proline/ectoine uptake system ProP, and the ectoine/proline/glycine betaine carrier EctP. J Bacteriol 180:6005–6012
Poolman B, Spitzer JJ, Wood JM (2004) Bacterial osmosensing: roles of membrane structure and electrostatics in lipid–protein and protein–protein interactions. Biochim Biophys Acta 1666:88–104
Ressl S, Terwissha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C (2009) Molecular basis of transport and regulation of the Na+/betaine symporter BetP. Nature 458:47–52
Rübenhagen R, Rönsch H, Jung H, Krämer R, Morbach S (2000) Osmosensor and osmoregulator properties of the betaine carrier BetP from Corynebacterium glutamicum in proteoliposomes. J Biol Chem 275:735–741
Rübenhagen R, Morbach S, Krämer R (2001) The osmoreactive betaine carrier BetP from Corynebacterium glutamicum is a sensor for cytoplasmic K+. EMBO J 20:5412–5420
Ruffert S, Berrier C, Krämer R, Ghazi A (1999) Identification of mechanosensitive ion channels in the cytoplasmic membrane of Corynebacterium glutamicum. J Bacteriol 181:1673–1676
Schiller D, Rübenhagen R, Krämer R, Morbach S (2004a) The C-terminal domain of the betaine carrier BetP of Corynebacterium glutamicum is directly involved in sensing K+ as an osmotic stimulus. Biochemistry 43:5583–5591
Schiller D, Krämer R, Morbach S (2004b) Cation specificity of osmosensing by the betaine carrier BetP of Corynebacterium glutamicum. FEBS Lett 563:108–112
Schiller D, Ott V, Krämer R, Morbach S (2006) Influence of membrane composition on osmosensing by the betaine carrier BetP from Corynebacterium glutamicum. J Biol Chem 281:7737–7746
Tsatskis Y, Khambati J, Dobson M, Bogdanov M, Dowhan W, Wood JM (2005) The osmotic activation of transporter ProP is tuned by both ist C-terminal coiled-coil and osmotically induced changes in phospholipid composition. J Biol Chem 280:1387–1394
Van der Heide T, Stuart MCA, Poolman B (2001) On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J 20:7022–7032
Voelkner P, Puppe W, Altendorf KH (1993) Characterization of the KdpD protein, the sensor kinase of the K+ translocating Kdp system of Escherichia coli. Eur J Biochem 217:1019–1026
Wood JM (1999) Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev 63:230–262
Wood JM (2006) Osmosensing by bacteria. Science STKE pe43
Wood JM (2007) Bacterial osmosensing transporters. Methods Enzymol 478:77–107
Ziegler C, Morbach S, Schiller D, Krämer R, Tziatzios C, Schubert D, Kühlbrandt W (2004) Projection structure and oligomeric state of the osmoregulated sodium/glycine betaine symporter BetP of Corynebacterium glutamicum. J Mol Biol 337:1137–1147
Acknowledgment
I am indebted to Christine Ziegler, Frankfurt, for Fig. 5c, and the Deutsche Forschungsgemeinschaft for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krämer, R. Osmosensing and osmosignaling in Corynebacterium glutamicum . Amino Acids 37, 487–497 (2009). https://doi.org/10.1007/s00726-009-0271-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-009-0271-6