Abstract
Zearalenone (ZEN) is a non-steroidal estrogenic mycotoxin produced by Fusarium fungi. ZEN has endocrine disruptor effects and could impair the hormonal balance. Here, we aimed at investigating possible effects of ZEN on metabolism-related pathways and its relation to epigenetic mechanisms in breast adenocarcinoma (MCF7) and breast epithelial (MCF10F) cells. Using the MTT and neutral red uptake (NRU) cell viability tests, IC50 values of ZEN after 24 h were found to be 191 μmol/L and 92.6 μmol/L in MCF7 cells and 67.4 μmol/L and 79.5 μmol/L in MCF10F cells. A significant increase on global levels of 5-methylcytosine (5-mC%) was observed for MCF7 cells, correlating with the increased expression of DNA methyltransferases. No alterations were observed on levels of 5-mC% and expression of DNA methyltransferases for MCF10F cells. Further, at least threefold upregulation compared to control was observed for several genes related to nuclear receptors and metabolism in MCF7 cells, while some of these genes were downregulated in MCF10F cells. The most notably altered genes were IGF1, HK2, PXR, and PPARγ. We suggested that ZEN could alter levels of global DNA methylation and impair metabolism-related pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zearalenone (ZEN) is a non-steroidal estrogenic mycotoxin produced by Fusarium species that contaminates cereals and other crops (Battorico 1998). ZEN binds to estrogen receptors (ER) and causes alteration of hormone production and reproductive toxic effects (Shier et al. 2001; Frizzell et al. 2011). Exposure to ZEN results in inhibition of protein and DNA synthesis, and triggers endoplasmic reticulum and mitochondrial stress, lipid peroxidation, oxidative damage, and apoptosis (Abid-Essefi et al. 2004; Ayed-Boussema et al. 2007; Bouaziz et al. 2008; Banjerdpongchai et al. 2010; Cai et al. 2019; Cheng et al. 2019; Kowalska et al. 2019; Wang et al. 2019). Moreover, ZEN has immunotoxic, hepatotoxic, and nephrotoxic properties (Maaroufi et al. 1996; Bouaziz et al. 2008; Gao et al. 2013; Lee et al. 2013; Hueza et al. 2014; Jia et al. 2014; Pistol et al. 2015; Islam et al. 2017; Gao et al. 2018; Zhang et al. 2018). There is limited evidence for the carcinogenicity of ZEN in experimental animals and it is classified as Group 3 (IARC 1993).
DNA methylation, one of the most studied epigenetic modifications, plays crucial roles in aging, cell proliferation, and various diseases such as cancer and diabetes (Baylin 1997; Richardson and Yung 1999; Robertson and Wolffe 2000; Moggs et al. 2004; Dean et al. 2005; Ulrey et al. 2005; Jones and Baylin 2007; Kulis and Esteller 2010; Anderson et al. 2012; Bansal and Pinney 2017). DNA methylation contributes to alterations in gene expression of key molecular pathways in several ways, including global genomic DNA hypomethylation, hypomethylation of individual genes, and tumor suppressor gene silencing through hypermethylation of CpG islands of genes (Baylin et al. 1986; Watson and Goodman 2002). Especially, DNA methyltransferase 1 (DNMT1) manages maintenance of methyltransferase activity that conserves the methylation state across DNA replication (Pradhan et al. 1999; Das and Singal 2004). In this case, O6-methylguanine-DNA methyltransferase (MGMT) repairs one of the most mutagenic alkylations at the O6-position of guanine nucleotide to cancer prevention by transfer of the methyl group from guanine to a cysteine residue (Pegg et al. 1995). It appears that DNMT1 and MGMT have crucial roles in cell cycle process, cell proliferation, and DNA repair (Kleihues et al. 1983; Pfohl-Leszkowicz and Dirheimer 1986; Jaenisch and Bird 2003; Guo et al. 2004; Sabharwal and Middleton 2006; Pathania et al. 2015; Wang and Li 2017). Although ZEN has genotoxic properties in some test systems (JECFA 2000), reveals DNA adduct formation in treated mice (Pfohl-Leszkowicz et al. 1995a; Grosse et al. 1997), and causes DNA damage, it has been suggested that mutagenic and carcinogenic properties of ZEN were still controversial (Ouanes-Ben Othmen et al. 2008; Abassi et al. 2016; Mandal et al. 2018). However, there are few studies that have limited relevance to epigenetic modifications including global or gene-specific DNA methylation and histone modifications caused by ZEN (Kouadio et al. 2007; Zhu et al. 2014a; Zhu et al. 2014b; Han et al. 2015; Ren et al. 2015; Zhang et al. 2017).
Breast cancer is one of the most common diseases in women, and obesity is one of the currently known risk factors of breast cancer (Lorincz and Sukumar 2006; Székely et al. 2010). Liu and Lin (2004) have also shown that commercial form of ZEN was able to transform human normal breast epithelial cell and increase cell proliferation in a dose-dependent manner. In the view of these data, it has been thought that ZEN exposure could be related to mechanisms of breast cancer progression. We aimed to investigate the global levels of DNA methylation and the related enzymes (DNMT1 and MGMT) to observe the role of epigenetic alterations in the ZEN toxicity. Moreover, we showed effects of ZEN on expression levels of metabolism-related genes and nuclear receptor genes in human breast adenocarcinoma (MCF7) and human breast epithelial (MCF10F) cell lines.
Materials and methods
Chemicals
ZEN (99% purity) was obtained from Sigma-Aldrich (St Louis, Missouri, USA). A stock solution of ZEN (1000 μmol/L) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) in a sterile glass volumetric flask and kept at − 20 °C. Cell culture media and all other supplements were purchased from Wisent Bioproducts (Saint-JeanBaptiste, QC, Canada) and sterile plastic materials were purchased from Nest Biotechnology (Jiangsu, China). DNA, RNA isolation kits, and cDNA synthesis kit and syber green master mix were obtained from Roche Life Sciences (Penzberg, Upper Bavaria, Germany). 5-Methylcytosine (5-mC) DNA ELISA kits were purchased from Epigentek (Farmingdale, NY). Primers for gene expressions were obtained from Sentromer DNA Technologies (Istanbul, Turkey).
Cell culture and treatments
The human breast adenocarcinoma MCF7 (ATCC® HTB-22™) and human breast epithelial MCF10F cell lines (ATCC® CRL-10318™) were obtained from American Type Culture Collection. MCF7 cells were maintained in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM F-12) medium containing 0.01 mg/mL human recombinant insulin, 10% heat-inactivated fetal bovine serum (FBS), and penicillin–streptomycin (100 U–100 μg/mL) at 37 °C in a humidified atmosphere with 5% CO2. MCF10F cells were cultured in DMEM F-12 Ham (Sigma, D8900) medium containing 0.006 g/L CaCl2·2H2O (Sigma-Aldrich, C8106) and 1.2 g/L NaHCO3 (Sigma, S5761) supplemented with 5% horse serum, 20 ng/mL epidermal growth factor, 100 ng/mL cholera toxin, 0.01 ng/mL insulin, 500 ng/mL hydrocortisone (Sigma, H4001), and penicillin–streptomycin (100 U–100 μg/mL) at 37 °C in a humidified atmosphere with 5% CO2. Subculturing was performed when the cells reached 70–80% confluence (every 2–3 days) using trypsinization. Exposure to ZEN was observed at the 8th to 16th round of subculture for both cell lines.
For gene expression and DNA methylation analysis, 1 × 106 were cultured in a 25-cm2 culture flask for 24 h in CO2 incubator prior to treatment. ZEN was treated in the concentrations of 1, 10, and 50 μmol/L in MCF7 cells and 0.1, 1, and 10 μmol/L in MCF10F cells, and 1% DMSO (exposure concentration in culture media) was used as solvent control in both cells. Cells were trypsinized, collected, and counted by Luna cell counter (Virginia, USA) with trypan blue staining. For all concentrations, it was tested in triplicates and each test was repeated twice.
Based on our cytotoxicity results and also previous studies in different cell types (Venkataramana et al. 2014; Sang et al. 2016; Tatay et al. 2014; Xie et al. 2017; Zhou et al. 2017), in the present study we selected 1, 10, and 50 μmol/L and 0.1, 1, and 10 μmol/L exposure concentrations of ZEN for 24 h for MCF7 and MCF10F cells, respectively. We could not apply the highest dose for the MCF10F because of the high cell death; therefore, we chose the 0.1 μmol/L concentration for the lowest and third concentration for the exposure of ZEN in MCF10F cells.
Cell viability
Effects of ZEN on cell viability were assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide, a tetrazole] and neutral red uptake (NRU) cytotoxicity tests. In the principle of the MTT test, yellow MTT is reduced to purple formazan in the mitochondria of living cells by the enzyme succinate dehydrogenase and it measures cell viability and proliferation (Mosmann 1983; Alley et al. 1988). The NRU test is a cell viability test method based on reduction in the uptake of neutral red dye into the lysosomes of cells (Borenfreund and Puerner 1985; Repetto et al. 2008).
For cytotoxicity tests, cells (1 × 104 in 100 μL medium) were seeded in 96-well plates and exposed to ZEN in the range of 7.81–250 μmol/L and 3.12–100 μmol/L of concentrations for MCF7 and MCF10F, respectively, and 1% DMSO (exposure concentration in culture media) was used as solvent control for 24 h. The absorbance of formed colored solution was measured at 590 nm for MTT test and 540 nm for NRU test using a microplate spectrophotometer system (Biotek-Epoch, Winooski, USA). The cytotoxicity results were calculated as a relative percentage to the control cells and expressed as 50% of inhibitory concentration (IC50) of the compound that caused 50% inhibition of the enzyme activity in the cells.
Global DNA methylation analysis
Genomic DNA was isolated from MCF7 and MCF10F cells using the High Pure PCR Template Preparation kit (Roche Applied Science, Mannheim, Germany) according to the instructions provided by the manufacturer. 5-mC analysis was performed using the MethylFlash™ Methylated DNA Quantification kit (Epigentek, Farmingdale, NY) according to the instructions provided by the manufacturer using 100 ng of input genomic DNA. DNA samples were treated with binding solution and incubated at 37 °C. Plate was washed with buffer. DNA samples were incubated with anti-5-mC monoclonal antibody and detection antibody. After addition of enhancer and developer solution, the absorbance was read at 450 nm using a microplate spectrophotometer system (Biotek-Epoch, Winooski, USA).
Gene expression analysis
Gene expressions of DNA methyltransferase genes including DNA methyltransferase 1 (DNMT1), O-6-methylguanine-DNA methyltransferase (MGMT), and metabolism-related genes including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), glucose transporter 2 (GLUT2), insulin-like growth factor 1 (IGF1), liver fatty acid-binding protein (L-FABP), sterol regulatory element-binding protein 1 (SREBP1c), hexokinase 2 (HK2), and nuclear receptor genes including pregnane X receptor (PXR), aryl hydrocarbon receptor (AhR), peroxisome proliferator activated receptor gamma (PPARɣ), estrogen receptor alpha (ERα), and estrogen receptor beta (ERβ) were performed by real-time PCR analysis. Total RNA was extracted from control and ZEN-treated groups in MCF7 and MCF10F cell lines using a High Pure RNA Isolation kit (Roche Life Science) according to the instructions provided by the manufacturer. First-strand cDNA was prepared from 500 ng of total RNA with the mixture of anchored-oligo(dT) and random hexamer primers by Transcriptor First Strand cDNA Synthesis kit (Roche Life Science). Real-time PCR reactions were performed using LightCycler® 480 Sybr Green master mix (Roche, Mannheim, Germany) under the following cycling conditions: 95 °C for 10 min, followed by 45 cycles of 95 °C for 30 s, annealing temperature for 30 s, 72 °C for 40 s, melting curve, and cooling. The primer sequences and the annealing temperatures of the genes are provided in Table 1. Ct of real-time PCR specific for nuclear receptor and metabolism-related genes and the reference gene (β-actin) were determined. The relative expression was evaluated by the comparative Ct method.
Statistical analysis
Global methylation levels (5-mC%) and cytotoxicity results were represented as mean ± standard deviation (SD). Statistical analysis was performed by ANOVA followed by Dunnett’s multiple comparison test using SPSS version 21.0 for Windows (IBM Analytics, New York, USA). P values less than 0.05 and 0.001 were selected as the levels of significance.
Results
Effects of ZEN on cell viability in MCF7 and MCF10F cells
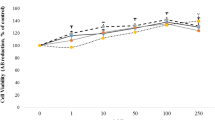
To assess the effects of ZEN on cell viability, cells were treated with 7.81–250 μmol/L and 3.12–100 μmol/L of concentrations for MCF7 and MCF10F, respectively, for 24 h then analyzed by MTT and NRU tests. IC50 value of ZEN was determined as 191 and 92.6 μmol/L in MCF7 cells and 67.4 and 79.5 μmol/L in MCF10F cells for 24 h by MTT and NRU tests, respectively. Figure 1a and b shows that treatments of ZEN for 24 h decreased the cell viability of MCF7 cells and MCF10F cells in comparison to the vehicle control group for MTT and NRU tests, respectively.
Effects of ZEN on global DNA methylation in MCF7 and MCF10F cells
Fifty micromoles per liter of ZEN exposure resulted in a significant increase in 5-mC% status (8.14-fold, p ˂ 0.001) compared to control group in MCF7 cells for 24 h (Fig. 2a). However, we found no changes in levels of 5-mC% in MCF10F cells after exposure to ZEN for 24 h (Fig. 2b). Figure 3a and b shows an increase on the expression levels of DNMT1 (> 4.28-fold) and MGMT (> 4.72-fold) genes significantly after 24-h exposure to ZEN in MCF7 cells. However, expression levels of DNMT1 and MGMT showed no changes in MCF10F cells (Fig. 3c, d).
Effects of ZEN (1, 10, and 50 μmol/L) on the levels of 5-mC% in MCF7 cells and MCF10F cells after 24-h exposure. Genomic DNA was extracted, then 5-mC% levels were detected using the ELISA kit. Data are presented as mean ± SD. Statistical analysis was performed by ANOVA–Dunnett post hoc test. Statistically significant changes are indicated by an asterisk (p < 0.001)
Effects of ZEN (1, 10, and 50 μmol/L) on gene expressions of DNMT1 and MGMT by real-time PCR in MCF7 (a) cells after 24-h exposure. Effects of ZEN (0.1, 1, and 10 μmol/L) on gene expressions of DNMT1 and MGMT by real-time PCR in MCF10F cells (b) after 24-h exposure. Data are presented as mean ± SD. Statistical analysis was performed by ANOVA–Dunnett post hoc test. Statistically significant changes are indicated by asterisks (p < 0.001)
Effects of ZEN on gene expression in MCF7 and MCF10F cells
In Fig. 4a, our data showed that exposure to ZEN significantly increased expression levels of GAPDH (> 5.19-fold), IGF1 (> 11.2-fold), L-FABP (> 6.19-fold), HK2 (> 19.46-fold), PXR (> 6.4-fold), PPARɣ (> 5.05-fold), ERα (> 3.25-fold), and ERβ (> 5.72-fold), while expression levels of GLUT2, SREBP1c, and AhR did not show any changes in MCF7 cells. However, expression levels of GAPDH (< 5.88-fold), AhR (< 3.03-fold), and PPARɣ (< 5.88-fold) significantly decreased while IGF1 (> 8.72-fold) and HK2 (> 2.26-fold) increased after ZEN exposure for 24 h in MCF10F cells (Fig. 4b). ZEN did not change expression levels of L-FABP, SREBP1c, PXR, and ERβ genes in MCF10F cells for 24 h. Additionally, ERα and GLUT2 were not expressed in MCF10F cells, even in control samples.
Quantification of metabolism-related genes and nuclear receptor genes expressions by real-time PCR. Statistical analysis was performed by ANOVA + Dunnett post hoc test. (*p < 0.001, **p < 0.05). (a) MCF7 cells were treated with 1, 10, and 50 μmol/L of ZEN for 24 h prior to extraction of total RNAs. (b) MCF10F cells were treated with 0.1, 1, and 10 μmol/L of ZEN for 24 h prior to extraction of total RNAs
Discussion
ZEN is a non-steroidal estrogenic mycotoxin produced by Fusarium species. It has been reported that ZEN was well known as an estrogenic exposure source in the environment and it could be accepted as one of the important endocrine disruptors. Main source of exposure to ZEN consists of food such as grain, breakfast cereals, bread, wine, beer, and dried fruits; therefore, ZEN affects human and animal health through the food chain (Kriszt et al. 2012; EFSA 2017). It has been shown that ZEN could induce various health problems such as alteration of hormone levels, reproductive and developmental disorders, modulation of cell cycle control, inflammation, and cancer (Fink-Gremmels and Malekinejad 2007; Zinedine et al. 2007; Escrivá et al. 2015; Kowalska et al. 2016; Gao et al. 2018; Zhang et al. 2018; Wang et al. 2019).
In the present study, we aimed to investigate the effects of ZEN on the expressions of the genes which are related to metabolism pathways in breast cell lines; in addition, we observed the effects of ZEN on global DNA methylation. The MTT test converts yellow MTT tetrazolium salt to purple formazan crystals by mitochondrial succinate dehydrogenase in viable cells (Mosmann 1983) while the NRU test determines the incorporation of neutral red dye into lysosomes of uninjured cells (Borenfreund and Puerner 1985). ZEN decreased cell viability in the concentrations more than 31.25 μmol/L and 25 μmol/L in MCF7 and MCF10F, respectively. IC50 value of ZEN was determined as 191 and 92.6 μmol/L in MCF7 cells and 67.4 and 79.5 μmol/L in MCF10F cells for 24 h by MTT and NRU tests, respectively. Venkataramana et al. (2014) have observed effects of 1–200 μmol/L concentrations of ZEN on cell viability in SH-SY5Y human neuroblastoma cell line for 24 h. After 24, 48, and 72 h of ZEN exposure (12.5–100 μmol/L) in ovarian CHO-K1 cells, it has been shown that the IC50 value was > 100 μmol/L for 24 h (Tatay et al. 2014). In the other study, ZEN (3–300 μmol/L) treated in HEK-293 human embryo kidney cells for 24 h and the IC50 value has been determined as 80 μmol/L by WST-8 assay (Sang et al. 2016).
Investigating epigenetic alterations such as DNA methylation and histone modifications has been useful biomarkers for the toxicity assessment of endocrine-disrupting chemicals (Zhang and Ho 2011; Greally and Jacobs 2013; Casati et al. 2015; Maqbool et al. 2016). However, there have been limited studies on epigenetic modifications of ZEN. Our results showed that 50 μmol/L of ZEN exposure for 24 h increased the levels of 5-mC% (8.14-fold) in MCF7 cells. Similar to our results, Zhu et al. (2014a) showed that ZEN increased global DNA methylation level in the high-dose group (50 μmol/L) of mouse oocytes for 12 h. Kouadio et al. (2007) studied the effects of combinations of Fusarium mycotoxins (ZEN, deoxynivalenol, fumonisin B1) on the global DNA methylation and found that ZEN increased the 5-mC% levels in Caco-2 human intestinal cell line DNA at 40 μmol/L for 24 h. In ZEN-treated porcine oocytes, levels of 5-mC% increased at 30 μmol/L of ZEN for 30 h (Han et al. 2015). Although we observed increases in the levels of 5-mC status in MCF7 cells, interestingly, we did not find any significant changes after 24-h exposure to ZEN in MCF10F cells. As correlated with global DNA methylation results, ZEN induced expression levels of DNMT1 and MGMT at 50 μmol/L in MCF7 cells; however, expressions of these genes did not change in ZEN-treated MCF10F cells. Similarly to our results in MCF7 cells, Han et al. (2015) have found that ZEN increased global DNA methylation level and mRNA levels of DNA methyltransferases (DNMT3a and DNMT3b) significantly increased compared to control at 30 μmol/L of ZEN in the oocytes. However, Zhu et al. (2014a) examined gene expressions of DNA methyltransferases (DNMT1, DNMT3a, DNMT3b, and DNMT3L) in the ZEN-treated oocytes for 8.5 and 12 h and the DNMTs expression levels did not differ from the control group. Therefore, exposure time and different cell culture could affect alterations on global DNA methylation and gene expression levels by ZEN. Additionally, several studies have demonstrated that DNMT1 and MGMT levels were higher in tumors than in their normal tissues (Pfohl-Leszkowicz et al. 1995b; Pieper 1997; Gerson 2004; Sabharwal and Middleton 2006; Sharma et al. 2009; Shi et al. 2012; Roll et al. 2013; Yu et al. 2014). Wang and Li (2017) have observed that DNMT1 regulates the cell cycle, proliferation, and apoptosis process. Increased methyltransferase activity and higher expressions of methyltransferases have also been shown in breast cancer cells (Ottaviano et al. 1994; Roll et al. 2013).
It has been suggested that commercial form of ZEN, zeranol (Ralgro), accelerated breast cancer cell growth at low concentrations and induced human normal breast epithelial cell transformation to neoplastic cell (Liu and Lin 2004; Yuri et al. 2006). Xu et al. (2009, 2010, 2011) have found that zeranol had mitogenic activity on breast cancer cells and there was an interaction between leptin and zeranol. It has been suggested that zeranol promoted proliferation in human breast cancer cells and obese individuals could have a higher risk of developing zeranol-induced breast cancer (Xu et al. 2009). In summary, it is well known that interactions between breast cancer and obesity have been studied for many years and zeranol is used as growth promoter for cattle. Therefore, in the present study, for further analysis we aimed to examine the effects of ZEN on metabolism-related genes in human breast epithelial cells.
Altered energy metabolism of cancer cells provides rapid growing for tumor cells than normal cells using higher rate of glucose metabolism (Hanahan and Weinberg 2011). Here, we have observed the effects of ZEN on transformed energy metabolism as a hallmark of cancer in breast cancer cells by analysis of gene expression levels. Our results showed that ZEN exposure altered mRNA levels of GAPDH, IGF1, L-FABP, HK2, AhR, PXR, and PPARγ genes significantly for 24 h in both cell lines. Various studies have shown that dysregulations in these genes could disrupt carbohydrate and lipid metabolism and could be associated with both metabolic dysfunctions and cancer progress (Gordon and Lowe 1985; Lee et al. 2010; Ahn et al. 2014; Cave et al. 2016; Lee et al. 2017). We observed that IGF1 was significantly upregulated in both MCF7 and MCF10F cells after 10 μmol/L of ZEN for 24 h. In the present study, our results have shown that all exposure groups of ZEN significantly increased mRNA levels of L-FABP and HK2 in MCF7 cells for 24 h, whereas only 10 μmol/L of ZEN increased HK2 expression in MCF10F cells. Song et al. (2012) observed that differentially expressed proteins in tumor tissues compared to normal tissue were related to glycolysis/gluconeogenesis among other pathways, including GAPDH. Harami-Papp et al. (2016) found that GAPDH expression was higher in the p53 mutant group between two breast cancer lines. Ter Braak et al. (2017) have reported that IGF1 signaling axis plays a major role in tumorigenesis and IGF1 overexpression has a strong and significant proliferative effect and mitogenic potential in mammary gland tumors. Besides, fatty acid synthase has a crucial role in the epithelial–mesenchymal transition of breast cancer cells, related to cell migration, metastasis, and L-FABP in its downstream proteins (Li et al. 2014). Furthermore, it has been shown that L-FABP was upregulated and could play a key role in the progress of invasiveness and metastasis in human breast cancer (Li et al. 2007). HK2 performs the first step in most glucose metabolism pathways that is overexpressed in many cancer cell types, and HK2 upregulation is related to the chemoresistance phenotypes in breast cancer cells (Lyon et al. 1988; Kaplan et al. 1990; Shinohara et al. 1994; Mathupala et al. 2009). Gao et al. (2017) have also demonstrated that HK2 plays a role in the process of inflammation-driven migration in breast cancer cells.
Interplay between nuclear receptor function and breast cancer has been studied for several years, especially estrogen receptors. After nuclear receptor activation, growth factor signaling pathways could be coordinately activated. Interaction between nuclear receptors and epithelial cell growth is clinically associated with breast cancer (reviewed in Conzen 2008). Overexpressions of PPARɣ and ERα have proliferative and antiapoptotic effects in breast cancer (Harvey et al. 1999; Suzuki et al. 2006). Our results have also shown that ZEN increased mRNA levels of PPARɣ and ERα in MCF7 cells. Besides, it has been reported that PPARɣ and ERs play a role on the regulation of HK2 expression directly (Onishi et al. 2010; Tennessen et al. 2011; Panasyuk et al. 2012). Moreover, it was observed that after ZEN exposure, PPARɣ and AhR was downregulated in MCF10F cells. Similar to our MCF10F results, downregulation of PPARɣ and AhR in mice has exhibited an increase in mammary adenocarcinomas, ductal hyperplasia, and mammary growth in mammary gland and primary culture of mammary epithelial cells in carcinogen-mediated carcinogenesis (Nicol et al. 2004; Miret et al. 2017).
Consequentially, this is the first study to investigate the effects of ZEN on metabolism-related genes and global DNA methylation levels in MCF7 and MCF10F cells. Our findings could contribute that ZEN might affect epigenetic regulation and could induce progress of breast cancer. Especially, we found significant dose-related changes of IGF1, L-FABP, HK2, PXR, and PPARɣ genes in MCF7 cells. Altered DNA methylation may have resulted in the abnormal gene expression of the key regulator genes which involve ZEN toxicity. Furthermore, we also highlight the role of IGF1, HK2, PXR, and PPARγ genes in the mechanism of ZEN toxicity.
Abbreviations
- 5-mC:
-
5-Methylcytosine
- AhR:
-
Aryl hydrocarbon receptor
- Caco-2:
-
Human colorectal adenocarcinoma cell line
- CHO-K1:
-
Chinese hamster ovary cell line
- DNMT1:
-
DNA methyltransferase 1
- ERα:
-
Estrogen receptor alpha
- ERβ:
-
Estrogen receptor beta
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GLUT2:
-
Glucose transporter 2
- HK2:
-
Hexokinase 2
- IC50 :
-
50% of inhibitory concentration
- IGF1:
-
Insulin-like growth factor 1
- L-FABP:
-
Liver fatty acid-binding protein
- MCF7:
-
Human breast adenocarcinoma cell line
- MCF10F:
-
Human breast epithelial cell line
- MGMT:
-
O6-Methylguanine-DNA methyltransferase
- MTT:
-
[3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide, a tetrazole]
- NRU:
-
Neutral red uptake
- PPARɣ:
-
Peroxisome proliferator-activated receptor gamma
- PXR:
-
Pregnane X receptor
- SH-SY5Y:
-
Human neuroblastoma cell line
- SREBP1c:
-
Sterol regulatory element-binding protein 1
References
Abassi H, Ayed-Boussema I, Shirley S, Abid S, Bacha H, Micheau O (2016) The mycotoxin zearalenone enhances cell proliferation, colony formation and promotes cell migration in the human colon carcinoma cell line HCT116. Toxicol Lett 254:1–7
Abid-Essefi S, Ouanes Z, Hassen W, Baudrimont I, Creppy EE, Bacha H (2004) Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol in Vitro 18(4):467–474
Ahmadnejad M, Amirizadeh N, Mehrasa R, Karkhah A, Nikougoftar M, Oodi A (2017) Elevated expression of DNMT1 is associated with increased expansion and proliferation of hematopoietic stem cells co-cultured with human MSCs. Blood Res 52(1):25–30
Ahn SB, Jang K, Jun DW, Lee BH, Shin KJ (2014) Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci 59(12):2975–2982
Alley MC, Scudiere DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48:589–601
Anderson OS, Sant KE, Dolinoy DC (2012) Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem 23(8):853–859
Ayed-Boussema I, Ouanes Z, Bacha H, Abid S (2007) Toxicities induced in cultured cells exposed to zearalenone: apoptosis or mutagenesis? J Biochem Mol Toxicol 21(3):136–144
Ayed-Boussema I, Pascussi JM, Rjiba K, Maurel P, Bacha H, Hassen W (2011) The mycotoxin, patulin, increases the expression of PXR and AhR and their target cytochrome P450s in primary cultured human hepatocytes. Drug Chem Toxicol 35(3):241–250
Banjerdpongchai R, Kongtawelert P, Khantamat O, Srisomsap C, Chokchaichamnankit D, Subhasitanont P, Svasti J (2010) Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J Hematol Oncol 3(1):50
Bansal A, Pinney SE (2017) DNA methylation and its role in the pathogenesis of diabetes. Pediatr Diabetes 18(3):167–177
Battorico A (1998) Fusarium diseases of cereals: species complex and related mycotoxin profiles. Eur J Plant Pathol 80:85–103
Baylin SB (1997) Tying it all together: epigenetics, genetics, cell cycle, and cancer. Science 277(5334):1948–1949
Baylin SB, Hoppener JW, de Bustros A, Steenbergh PH, Lips CJ, Nelkin BD (1986) DNA methylation patterns of the calcitonin gene in human lung cancers and lymphomas. Cancer Res 46:2917–2922
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24(2):119–124
Bouaziz C, El Golli E, Abid-Essefi S, Brenner C, Lemaire C, Bacha H (2008) Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology 254(1):19–28
Cai G, Si M, Li X, Zou H, Gu J, Yuan Y, Liu X, Liu Z, Bian J (2019) Zearalenone induces apoptosis of rat Sertoli cells through Fas–Fas ligand and mitochondrial pathway. Environ Toxicol 34:424–433. https://doi.org/10.1002/tox.22696
Casati L, Sendra R, Sibilia V, Celotti F (2015) Endocrine disrupters: the new players able to affect the epigenome. Front Cell Dev Biol 3:37
Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, Sidey J, Shi H, Aqel BA, McClain CJ, Prough RA (2016) Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta Gene Regul Mech 1859(9):1083–1099
Cheng Q, Jiang SZ, Huang LB, Ge JS, Wang YX, Yang WR (2019) Zearalenone induced oxidative stress in the jejunum in postweaning gilts through modulation of the Keap1–Nrf2 signaling pathway and relevant genes. J Anim Sci. https://doi.org/10.1093/jas/skz051
Das PM, Singal R (2004) DNA methylation and cancer. J Clin Oncol 22(22):4632–4642
Dean W, Lucifero D, Santos F (2005) DNA methylation in mammalian development and disease. Birth Defects Res C Embryo Today 75(2):98–111
Escrivá L, Font G, Manyes L (2015) In vivo toxicity studies of Fusarium mycotoxins in the last decade: a review. Food Chem Toxicol 78:185–206
European Food Safety Authority (EFSA) (2017) Risks for animal health related to the presence of zearalenone and its modified forms in feed. The EFSA Journal 15(7):4851
Fink-Gremmels J, Malekinejad H (2007) Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim Feed Sci Technol 137(3):326–341
Frizzell C, Ndossi D, Verhaegen S, Dahl E, Eriksen G, Sørlie M, Ropstad E, Muller M, Elliott CT, Connolly L (2011) Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol Lett 206(2):210–217
Gao F, Jiang LP, Chen M, Geng CY, Yang G, Ji F, Zhong LF, Liu XF (2013) Genotoxic effects induced by zearalenone in a human embryonic kidney cell line. Mutat Res Genet Toxicol Environ Mutagen 755(1):6–10
Gao Y, Yang Y, Yuan F, Huang J, Xu W, Mao B, Yuan Z, Bi W (2017) TNFα-YAP/p65-HK2 axis mediates breast cancer cell migration. Oncogenesis 6(9):e383
Gao X, Xiao ZH, Liu M, Zhang NY, Khalil MM, Gu CQ, Qi DS, Sun LH (2018) Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J Nutr 148(8):1209–1216
Gerson SL (2004) MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer 4:296–307
Gordon JI, Lowe JB (1985) Analyzing the structures, functions and evolution of two abundant gastrointestinal fatty acid binding proteins with recombinant DNA and computational techniques. Chem Phys Lipids 38(1–2):137–158
Greally JM, Jacobs MN (2013) In vitro and in vivo testing methods of epigenomic endpoints for evaluating endocrine disruptors. Altex 30(4):445–471
Grosse Y, Chekir-Ghedira L, Huc A, Obrecht-Pflumio S, Dirheimer G, Bacha H, Pfohl-Leszkowicz A (1997) Retinol, ascorbic acid and α-tocopherol prevent DNA adduct formation in mice treated with the mycotoxins ochratoxin A and zearalenone. Cancer Lett 114(1–2):225–229
Guo G, Wang W, Bradley A (2004) Mismatch repair genes identified using genetic screens in Blm-deficient embryonic stem cells. Nature 429:891–895
Han J, Wang T, Fu L, Shi LY, Zhu CC, Liu J, Zhang Y, Cui XS, Kim NH, Sun SC (2015) Altered oxidative stress, apoptosis/autophagy, and epigenetic modifications in zearalenone-treated porcine oocytes. Toxicol Res 4(5):1184–1194
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Hao Q, Li T, Zhang X, Gao P, Qiao P, Li S, Geng Z (2014) Expression and roles of fatty acid synthase in hepatocellular carcinoma. Oncol Rep 32(6):2471–2476
Harada K, Isse K, Kamihira T, Shimoda S, Nakanuma Y (2005) Th1 cytokine–induced downregulation of PPARγ in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology 41(6):1329–1338
Harami-Papp H, Pongor LS, Munkácsy G, Horváth G, Nagy ÁM, Ambrus A, Hauser P, Szabó A, Tretter L, Győrffy B (2016) TP53 mutation hits energy metabolism and increases glycolysis in breast cancer. Oncotarget 7(41):67183
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Hueza IM, Raspantini PCF, Raspantini LER, Latorre AO, Górniak SL (2014) Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 6(3):1080–1095
International Agency for Research on Cancer (IARC) (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. In: IARC (Ed.), IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans, vol. 56. Lyon, France, pp 397–444
Islam MR, Kim JW, Roh YS, Kim JH, Han KM, Kwon HJ, Lim CW, Kim B (2017) Evaluation of immunomodulatory effects of zearalenone in mice. J Immunotoxicol 14(1):125–136
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(3s):245–254
Jang EH, Jang SY, Cho IH, Hong D, Jung B, Park MJ, Kim JH (2015) Hispolon inhibits the growth of estrogen receptor positive human breast cancer cells through modulation of estrogen receptor alpha. Biochem Biophys Res Commun 463(4):917–922
Jia Z, Liu M, Qu Z, Zhang Y, Yin S, Shan A (2014) Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ Toxicol Pharmacol 37(2):580–591
Joint FAO/WHO Expert Committee on Food Additives (JECFA) (2000). Meeting, & World Health Organization. Safety Evalution of certain food additives and contaminants Vol. 44
Jones PA, Baylin SB (2007) The epigenomics of cancer. Cell 128(4):683–692
Kaplan O, Navon G, Lyon RC, Faustino PJ, Straka EJ, Cohen JS (1990) Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res 50(3):544–551
Kleihues P, Hodgson RM, Veit C, Schweinsberg F, Wiessler M (1983) DNA modification and repair in vivo: towards a biochemical basis of organ-specific carcinogenesis by methylating agents. In: Organ and species specificity in chemical carcinogenesis. Springer, Boston, MA, pp 509–529
Kouadio JH, Dano SD, Moukha S, Mobio TA, Creppy EE (2007) Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, DNA methylation and fragmentation, and viability in Caco-2 cells. Toxicon 49(3):306–317
Kowalska K, Habrowska-Górczyńska DE, Piastowska-Ciesielska AW (2016) Zearalenone as an endocrine disruptor in humans. Environ Toxicol Pharmacol 48:141–149
Kowalska K, Habrowska-Górczyńska DE, Urbanek KA, Domińska K, Sakowicz A, Piastowska-Ciesielska AW (2019) Estrogen receptor β plays a protective role in zearalenone-induced oxidative stress in normal prostate epithelial cells. Ecotoxicol Environ Saf 172:504–513
Kriszt R, Krifaton C, Szoboszlay S, Cserháti M, Kriszt B, Kukolya J, Czéh A, Fehér-Tóth S, Török L, Szőke Z, Kovács KJ, Barna T, Ferenczi S (2012) A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PLoS One 7(9):e43608
Kulis M, Esteller M (2010) DNA methylation and cancer. Adv Genet 70(10):27–56
Lai JC, Cheng YW, Goan YG, Chang JT, Wu TC, Chen CY, Lee H (2008) Promoter methylation of O 6-methylguanine-DNA-methyltransferase in lung cancer is regulated by p53. DNA Repair 7(8):1352–1363
Lee JH, Wada T, Febbraio M, He J, Matsubara T, Lee MJ, Gonzalez FJ, Xie W (2010) A novel role for the dioxin receptor in fatty acid metabolism and hepatic steatosis. Gastroenterology 139(2):653–663
Lee H, Kang C, Yoo YS, Hah DY, Kim CH, Kim E, Kim JS (2013) Cytotoxicity and the induction of the stress protein Hsp 70 in Chang liver cells in response to zearalenone-induced oxidative stress. Environ Toxicol Pharmacol 36(2):732–740
Lee KS, Chun SY, Kwon YS, Kim S, Nam KS (2017) Deep sea water improves hypercholesterolemia and hepatic lipid accumulation through the regulation of hepatic lipid metabolic gene expression. Mol Med Rep 15(5):2814–2822
Li H, Lu Q, Dong LH, Xue H, Zhou HY, Yang HJ (2007) Expression of fatty acid binding protein in human breast cancer tissues. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 23(4):312–316
Li J, Dong L, Wei D, Wang X, Zhang S, Li H (2014) Fatty acid synthase mediates the epithelial–mesenchymal transition of breast cancer cells. Int J Biol Sci 10(2):171–180
Liu S, Lin YC (2004) Transformation of MCF-10A human breast epithelial cells by zeranol and estradiol-17beta. Breast J 10(6):514–521
Lorincz AM, Sukumar S (2006) Molecular links between obesity and breast cancer. Endocr Relat Cancer 13(2):279–292
Lyon RC, Cohen JS, Faustino PJ, Megnin F, Myers CE (1988) Glucose metabolism in drug-sensitive and drug-resistant human breast cancer cells monitored by magnetic resonance spectroscopy. Cancer Res 48(4):870–877
Maaroufi K, Chekir L, Creppy EE, Ellouz F, Bacha H (1996) Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon 34(5):535–540
Mandal P, Rai A, Mishra S, Tripathi A, Das M (2018). Mutagens in food. In Mutagenicity: assays and applications (pp. 133–160)
Maqbool F, Mostafalou S, Bahadar H, Abdollahi M (2016) Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci 145:265–273
Mathupala SP, Ko YH, Pedersen PL (2009) Hexokinase-2 bound to mitochondria: cancer's stygian link to the “Warburg effect” and a pivotal target for effective therapy. Semin Cancer Biol 19:17–24
Miret N, Rico-Leo E, Pontillo C, Zotta E, Fernández-Salguero P, Randi A (2017) A dioxin-like compound induces hyperplasia and branching morphogenesis in mouse mammary gland, through alterations in TGF-β1 and aryl hydrocarbon receptor signaling. Toxicol Appl Pharmacol 334:192–206
Moggs JG, Goodman JI, Trosko JE, Roberts RA (2004) Epigenetics and cancer: implications for drug discovery and safety assessment. Toxicol Appl Pharmacol 196(3):422–430
Mollerup S, Jørgensen K, Berge G, Haugen A (2002) Expression of estrogen receptors α and β in human lung tissue and cell lines. Lung Cancer 37(2):153–159
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63
Nicol CJ, Yoon M, Ward JM, Yamashita M, Fukamachi K, Peters JM, Gonzalez FJ (2004) PPARɣ influences susceptibility to DMBA-induced mammary, ovarian and skin carcinogenesis. Carcinogenesis 25:1747–1755
Onishi A, Peng GH, Poth EM, Lee DA, Chen J, Alexis U, de Melo J, Chen S, Blackshaw S (2010) The orphan nuclear hormone receptor ERRβ controls rod photoreceptor survival. Proc Natl Acad Sci U S A 107(25):11579–11584
Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE (1994) Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res 54(10):2552–2555
Ouanes-Ben Othmen Z, Essefi S, Bacha H (2008) Mutagenic and epigenetic mechanisms of zearalenone: prevention by vitamin E. World Mycotoxin J 1(3):369–374
Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, Pontoglio M, Ferré P, Scoazec JY, Birnbaum MJ, Ricci JE, Pende M (2012) PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun 3:672
Pathania R, Ramachandran S, Elangovan S, Padia R, Yang P, Cinghu S, Veeranan-Karmegam R, Arjunan P, Gnana-Prakasam JP, Sadanand F, Pei L, Chang CS, Choi JH, Shi H, Manicassamy S, Prasad PD, Sharma S, Ganapathy V, Jothi R, Thangaraju M (2015) DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat Commun 6:6910
Pegg AE, Dolan ME, Moschel RC (1995). Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. In Progress in nucleic acid research and molecular biology (Vol. 51, pp. 167–223). Academic Press
Pfohl-Leszkowicz A, Dirheimer G (1986) Changes in de novo DNA (cytosine-5-)-methyltransferase activity in oncogenically susceptible rat target tissues induced by N-methyl-N-nitrosourea. Cancer Res 46(3):1110–1113
Pfohl-Leszkowicz A, Chekir-Ghedira L, Bacha H (1995a) Genotoxicity of zearalenone, an estrogenic mycotoxin: DNA adduct formation in female mouse tissues. Carcinogenesis 16(10):2315–2320
Pfohl-Leszkowicz A, Grosse Y, Carriere V, Cugnenc PH, Berger A, Carnot F, Huc A, Beaune P, Waziers ID (1995b) Impaired DNA methylation and DNA adduct concentrations in human peritumoral and tumoral colorectal samples and normal colon. Clin Chem 41(12):1924–1925
Pieper RO (1997) Understanding and manipulating O6-methylguanine-DNA methyltransferase expression. Pharmacol Ther 74:285–297
Pistol GC, Braicu C, Motiu M, Gras MA, Marin DE, Stancu M, Calin L, Israel-Roming F, Berindan-Neagoe I, Taranu I (2015) Zearalenone mycotoxin affects immune mediators, MAPK signalling molecules, nuclear receptors and genome-wide gene expression in pig spleen. PLoS One 10(5):e0127503
Pradhan S, Bacolla A, Wells RD, Roberts RJ (1999) Recombinant human DNA (cytosine-5) methyltransferase I. Expression, purification, and comparison of de novo and maintenance methylation. J Biol Chem 274(46):33002–33010
Reimer RA, Leone-Vautravers P, Zbinden I, Harris CC, Pfeifer AM, Macé K (2004) Dysregulation of fatty acid synthase mRNA in immortalized human hepatocyte cell lines in response to high glucose and the absence of GLUT2. Nutr Res 24(5):321–336
Ren Y, Suzuki H, Jagarlamudi K, Golnoski K, McGuire M, Lopes R, Pachnis V, Rajkovic A (2015) Lhx8 regulates primordial follicle activation and postnatal folliculogenesis. BMC Biol 13
Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3(7):1125–1131
Richardson B, Yung R (1999) Role of DNA methylation in the regulation of cell function. J Lab Clin Med 134(4):333–340
Robertson KD, Wolffe AP (2000) DNA methylation in health and disease. Nat Rev Genet 1(1):11–19
Roll JD, Rivenbark AG, Sandhu R, Parker JS, Jones WD, Carey LA, Livasy CA, Coleman WB (2013) Dysregulation of the epigenome in triple-negative breast cancers: basal-like and claudin-low breast cancers express aberrant DNA hypermethylation. Exp Mol Pathol 95(3):276–287
Rosa SC, Gonçalves J, Judas F, Mobasheri A, Lopes C, Mendes AF (2009) Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther 11(3):R80
Sabharwal A, Middleton MR (2006) Exploiting the role of O6-methylguanine-DNA-methyltransferase (MGMT) in cancer therapy. Curr Opin Pharmacol 6:355–363
Sang Y, Li W, Zhang G (2016) The protective effect of resveratrol against cytotoxicity induced by mycotoxin, zearalenone. Food Funct 7(9):3703–3715
Sharma S, Salehi F, Scheithauer BW, Rotondo F, Syro LV, Kovacs K (2009) Role of MGMT in tumor development, progression, diagnosis, treatment and prognosis. Anticancer Res 29:3759–3768
Shi JF, Li XJ, Si XX, Li AD, Ding HJ, Han X, Sun YJ (2012) ERα positively regulated DNMT1 expression by binding to the gene promoter region in human breast cancer MCF-7 cells. Biochem Biophys Res Commun 427(1):47–53
Shier WT, Shier AC, Xie W, Mirocha CJ (2001) Structure–activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 39(9):1435–1438
Shinohara Y, Yamamoto K, Kogure K, Ichihara J, Terada H (1994) Steady state transcript levels of the type II hexokinase and type 1 glucose transporter in human tumor cell lines. Cancer Lett 82(1):27–32
Song MN, Moon PG, Lee JE, Na M, Kang W, Chae YS, Park JY, Park H, Baek MC (2012) Proteomic analysis of breast cancer tissues to identify biomarker candidates by gel-assisted digestion and label-free quantification methods using LC-MS/MS. Arch Pharm Res 35(10):1839–1847
Srinivasa S, Suresh C, Mottla J, Hamarneh SR, Irazoqui JE, Frontera W, Torriani M, Stanley T, Makimura H (2016) FNDC5 relates to skeletal muscle IGF-I and mitochondrial function and gene expression in obese men with reduced growth hormone. Growth Hormon IGF Res 26:36–41
Suzuki T, Hayashi S, Miki Y, Nakamura Y, Moriya T, Sugawara A, Ishida T, Ohuchi N, Sasano H (2006) Peroxisome proliferator-activated receptor ɣ in human breast carcinoma: a modulator of estrogenic actions. Endocr Relat Cancer 13:233–250
Székely B, Szentmártoni G, Kulka J, Szász AM, Langmár Z, Dank M (2010) Primary systemic therapy in breast cancer—an update for gynecologic oncologists. Eur J Gynaecol Oncol 32(6):636–641
Tatay E, Meca G, Font G, Ruiz MJ (2014) Interactive effects of zearalenone and its metabolites on cytotoxicity and metabolization in ovarian CHO-K1 cells. Toxicol in Vitro 28(1):95–103
Tennessen JM, Baker KD, Lam G, Evans J, Thummel CS (2011) The Drosophila estrogen-related receptor directs a metabolic switch that supports developmental growth. Cell Metab 13(2):139–148
Ter Braak B, Siezen CL, Lee JS, Rao P, Voorhoeve C, Ruppin E, Van der Laan JW, Van de Water B (2017) Insulin-like growth factor 1 receptor activation promotes mammary gland tumor development by increasing glycolysis and promoting biomass production. Breast Cancer Res 19(1):14
Ulrey CL, Liu L, Andrews LG, Tollefsbol TO (2005) The impact of metabolism on DNA methylation. Hum Mol Genet 14(suppl_1):R139–R147
Venkataramana M, Nayaka SC, Anand T, Rajesh R, Aiyaz M, Divakara ST, Murali HS, Prakash HS, Lakshmana Rao PV (2014) Zearalenone induced toxicity in SHSY-5Y cells: the role of oxidative stress evidenced by N-acetyl cysteine. Food Chem Toxicol 65:335–342
Wang X, Li B (2017) DNMT1 regulates human endometrial carcinoma cell proliferation. Onco Targets Ther 10:1865–1873
Wang G, Gong Y, Anderson J, Sun D, Minuk G, Roberts MS, Burczynski FJ (2005) Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology 42(4):871–879
Wang M, Wu W, Li L, He J, Huang S, Chen S, Chen J, Long M, Yang S, Li P (2019) Analysis of the miRNA expression profiles in the zearalenone-exposed TM3 Leydig cell line. Int J Mol Sci 20(3):E635
Watson RE, Goodman JI (2002) Epigenetics and DNA methylation come of age in toxicology. Toxicol Sci 67(1):11–16
Xie H, Hu J, Xiao C, Dai Y, Ding X, Xu Y (2017) Exploration of ZEA cytotoxicity to mouse endometrial stromal cells and RNA-seq analysis. J Biochem Mol Toxicol 31(4)
Xu P, Ye W, Jen R, Lin SH, Kuo CT, Lin YC (2009) Mitogenic activity of zeranol in human breast cancer cells is enhanced by leptin and suppressed by gossypol. Anticancer Res 29(11):4621–4628
Xu P, Ye W, Li H, Lin SH, Kuo CT, Feng E, Lin YC (2010) Zeranol enhances leptin-induced proliferation in primary cultured human breast cancer epithelial cells. Mol Med Rep 3(5):795–800
Xu P, Ye W, Zhong S, Jen R, Li H, Feng E, Lin S, Liu J, Lin YC (2011) Zeranol may increase the risk of leptin-induced neoplasia in human breast. Oncol Lett 2(1):101–108
Yu Z, Xiao Q, Zhao L, Ren J, Bai X, Sun M, Wu H, Liu X, Song Z, Yan Y, Mi X, Wang E, Jin F, Wei M (2014) DNA methyltransferase 1/3a overexpression in sporadic breast cancer is associated with reduced expression of estrogen receptor-alpha/breast cancer susceptibility gene 1 and poor prognosis. Mol Carcinog 54(9):707–719
Yuri T, Tsukamoto R, Miki K, Uehara N, Matsuoka Y, Tsubura A (2006) Biphasic effects of zeranol on the growth of estrogen receptor-positive human breast carcinoma cells. Oncol Rep 16(6):1307–1312
Zhang X, Ho SM (2011) Epigenetics meets endocrinology. J Mol Endocrinol 46(1):R11–R32
Zhang GL, Sun XF, Feng YZ, Li B, Li YP, Yang F, Nyachoti CM, Shen W, Sun S, Li L (2017) Zearalenone exposure impairs ovarian primordial follicle formation via down-regulation of Lhx8 expression in vitro. Toxicol Appl Pharmacol 317:33–40
Zhang W, Zhang S, Zhang M, Yang L, Cheng B, Li J, Shan A (2018) Individual and combined effects of Fusarium toxins on apoptosis in PK15 cells and the protective role of N-acetylcysteine. Food Chem Toxicol 111:27–43
Zhao S, Liu H, Liu Y, Wu J, Wang C, Hou X, Chen X, Yang G, Zhao L, Che H, Bi Y, Wang H, Peng F, Ai J (2013) miR-143 inhibits glycolysis and depletes stemness of glioblastoma stem-like cells. Cancer Lett 333(2):253–260
Zhou H, George S, Hay C, Lee J, Qian H, Sun X (2017) Individual and combined effects of aflatoxin B 1, deoxynivalenol and zearalenone on HepG2 and RAW 264.7 cell lines. Food Chem Toxicol 103:18–27
Zhu CC, Hou YJ, Han J, Liu HL, Cui XS, Kim NH, Sun SC (2014a) Effect of mycotoxin-containing diets on epigenetic modifications of mouse oocytes by fluorescence microscopy analysis. Microsc Microanal 20(4):1158–1166
Zhu CC, Hou YJ, Han J, Cui XS, Kim NH, Sun SC (2014b) Zearalenone exposure affects epigenetic modifications of mouse eggs. Mutagenesis 29(6):489–495
Zinedine A, Soriano JM, Molto JC, Manes J (2007) Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol 45(1):1–18
Acknowledgements
This work was supported in part by the TUBITAK (2211-C/2015-2 grant for E.F.K.) and Scientific Research Projects Coordination Unit of Istanbul University (project no. TOA-2016-20307).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karaman, E.F., Ozden, S. Alterations in global DNA methylation and metabolism-related genes caused by zearalenone in MCF7 and MCF10F cells. Mycotoxin Res 35, 309–320 (2019). https://doi.org/10.1007/s12550-019-00358-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-019-00358-8