Abstract
Henkelotherium guimarotae Krebs 1991 is an important Jurassic mammal for understanding therian evolution. We are presenting a new study of extensive, previously undescribed, mandibles and dentitions. The revised dental formula is: I4? or 5?/i4, C1/c1, P4/p4, M6/m7. The canine and premolars show an alternate replacement that ends with M4/m4 eruption, and is followed by a late sequential eruption of the last three lower (m5-7) and last two upper (M5-6) molars. The lower premolars erupted in the following order: p1 → p3 → p2 → p4, and the canine erupted most probably shortly before p4. The timing of the premolar replacement before the late molar eruption is similar to that of Dryolestes leiriensis, and is a characteristic of dryolestidans. Henkelotherium lower molars have subequal roots, a plesiomorphy of non-dryolestidan mammals, and the upper molars are supported by a strong, curved lingual root; a derived character. In the upper molars, the postvallum wear surface is contiguous to the parastyle wear surface of the succeeding molar, which differs from dryolestids. The parastylar lobe of the succeeding molar, and the postvallum of the preceding molar, are imbricated, and can develop strong, continuous wear surfaces, matching the prevallid crest of the lower molar. Henkelotherium differs from dryolestids in having an inflected, shelf-like mandibular angular process with a foramen. This large sample of Henkelotherium shows a significant variation gradient along the molar series, with the strongest wear occurring only in two to three consecutive molars. The extraordinarily long molar row is correlated with the late growth of jaws; and the jaw with late addition of molars sustained an effective mastication, much longer in older adults of dryolestidans than in other Mesozoic stem therians. The late eruption of several more molars after completion of antemolar replacement suggests that dryolestidans had either a longer-lived life, or slower life-history traits, or a combination of both, than crown therians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dryolestidans are the most stem-ward group of the cladotherian clade that includes living marsupials and placentals known as crown therians. They are fossil relatives of crown therians such as tribosphenid mammals (boreosphenidans) (Crompton 1971; McKenna 1975; Prothero 1981; Martin 1999; Luo et al. 2002; Rougier et al 2011; Wible and Rougier 2017). By current consensus, dryolestidans represent the proximal relatives to the common ancestor of “peramuran” and tribosphenid mammals known collectively as Zatheria (Kielan-Jaworowska et al. 2004; Martin 2018). In comparative morphology of Mesozoic mammals, dryolestidans are more derived than the spalacotherioid “symmetrodontans” in cranial and molar features, but more plesiomorphic than zatherians (including the paraphyletic “peramurans”) in dental, mandibular and petrosal characteristics (Krebs 1991; Sigogneau-Russell 1999; Luo and Wible 2005; Martin and Rauhut 2005; Rougier et al. 2007; Luo et al. 2007a, b, 2012; Ruf et al. 2009; Schultz and Martin 2014; Hughes et al. 2015; Grossnickle et al. 2022).
Dryolestidans have an extensive fossil record. The two diverse and abundant families are the Paurodontidae and Dryolestidae. The earliest fossil of the Paurodontidae, from the Balabansai Formation of Kyrgyzstan, is of Callovian age, Middle Jurassic (Martin and Averianov 2010), and the family extends to the Late Jurassic (Simpson 1929; Krebs 1991, 2000; Averianov and Martin 2015). It should be noted that in some recent analyses, the paurodontids have turned out to be paraphyletic (Rougier et al. 2012; Averianov et al. 2013; Wible and Rougier 2017; although see Martinelli et al. 2021). The earliest fossils of the family Dryolestidae are from the Middle Jurassic Berezovsk site of Siberia (Averianov et al. 2014) and the Late Bathonian, Middle Jurassic Forest Marble Formation of Great Britain (Freeman 1976, 1979).
The peak taxic diversity and specimen abundance, and the widest geographic distribution, of dryolestidans occurred in the Late Jurassic and Early Cretaceous, during which time the group is represented by relatively abundant fossils from the Morrison Formation of North America (Simpson 1929; Prothero 1981; Kielan-Jaworowska et al. 2004; Averianov and Martin 2015), the Late Jurassic Guimarota coal mine of Portugal (Martin and Krebs 2000) and the Late Jurassic Langenberg quarry of Germany (Martin et al. 2021), with a single putative record of Brancatherulum from the Late Jurassic in Africa (Heinrich 1991). Dryolestidans have a moderate diversity in the Lower Cretaceous of Great Britain, Spain, Germany, and Northern Africa (Simpson 1928; Henkel and Krebs 1969; Krebs 1971, 1985, 1993; Sigogneau-Russell 1991; Ensom and Sigogneau-Russell 1998; Martin 1998, 1999; Martin et al. 2022b; Lasseron et al. 2022), plus a putative dryolestidan fossil from Australia (Clemens et al. 2003). From the late Early Cretaceous and onward, dryolestidans are unknown among the faunas of the Laurasian continents, and have presumably disappeared. There are putative records from the Late Cretaceous of North America (Lillegraven and McKenna 1986), however, and from South America (Martin et al. 2022a).
Recent discoveries of meridiolestidan mammals from the Cretaceous of South America, and their inclusion in phylogenetic analyses, have altered the interpretation if paurodontids are still monophyletic, as previously assumed (e.g., Kielan-Jaworowska et al. 2004). Meridiolestidans were considered to be related to dryolestidans, when these fossils were first discovered and described (Bonaparte 1986, 1990, 1994, 2002; Kielan-Jaworowska et al. 2004; Chornogubsky 2011; Rougier et al. 2009, 2011, 2012, 2021b). However, a majority of the recent re-analyses of their relationships placed meridiolestidans and some paurodontids (with exception of Henkelotherium) as an independent clade from dryolestids (Rougier et al. 2012; Averianov et al. 2013; O’Meara and Thompson 2014; Wible and Rougier 2017), rendering paurodontids as a paraphyletic grouping. Other phylogenetic analyses with a smaller taxonomic sample still recognize the Paurodontidae as a clade (e.g., Martinelli et al. 2021), or recognize meridiolestidans as a group nested in the spalacotherioid clade, not related to dryolestidans (Averianov et al. 2013).
Eight endemic genera of meridiolestidans are known from the Cretaceous and Cenozoic of South America (Martinelli et al. 2021). The genus Peligrotherium is known from the Paleocene of Argentina, and the genus Necrolestes from the Miocene of Argentina is also considered to be a meridiolestidan (Rougier et al. 2012; Chimento et al. 2012; Wible and Rougier 2017). These fossils provide some interesting examples of a Mesozoic mammal group that survived the end of the Cretaceous mass extinction (Gelfo and Pascual 2001; Rougier et al. 2021a, b), well into the Neogene (Rougier et al. 2012). Pending a resolution of the competing hypotheses to place meridiolestidans with regards to paurodontids (Chimento et al. 2012; Rougier et al. 2012), or to spalacotherioids (Averianov et al. 2013), dryolestidans may be the longest-living Mesozoic mammal clade that survived well into the Neogene.
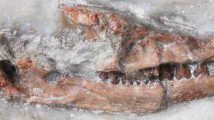
Henkelotherium guimarotae (Krebs 1991) is so far the best-preserved taxon (Fig. 1) of dryolestidans (Martin and Krebs 2000). It yielded significant information for the understanding of early mammal evolution, for the postcranial locomotor function, and for interpreting the ear region of therians (Krebs 1991; Vázquez-Molinero et al. 2001; Ruf et al. 2009; Luo et al. 2012; Hughes et al. 2015; Jäger et al. 2013a, b; 2019a). It is a key comparative taxon for the overall mammal phylogeny (Hu et al. 1997, 1998; Rougier et al. 1998; Ji et al. 1999; Luo et al. 2002), and for studying the ecological diversity of Mesozoic mammals (Krebs 2000; Luo 2007; Martin 2018; Jäger et al. 2019a).
Composite restoration of the mandible of Henkelotherium guimarotae Krebs (1991). a Lateral view. b Medial view. c Oblique dorsal (occlusal) view in paired stereo images. d Oblique ventral view in paired images. Composite from four mandibles: the posterior, and antemolar and symphyseal regions: Gui Mam 133/77; the mandible body and canine through m6 from Gui Mam 15/77; the mandibular angle from Gui Mam 15/77 and Gui Mam 44/80
On the basis of a larger, and previously unpublished sample of fossils of Henkelotherium guimarotae, we provide a detailed description of the mandibles and teeth, and document the character variation of the teeth, to augment the information on this important fossil mammal (Fig. 1). We also attempt to interpret the tooth replacement and eruption, and to characterize the late eruption of molars during the prolonged growth of the mandible, for the understanding of the paleobiology of Henkelotherium.
Materials and methods
Fossil specimens for this study (Tables 1 and 2; Appendix 1 in Supplementary Information) are from the Late Jurassic (Kimmeridgian) Guimarota coal mine, which has yielded a rich assemblage of fossil mammals, with over 32 species (Martin and Krebs 2000). Five species of dryolestidans have been recognized: the dryolestids Dryolestes leiriensis, Krebsotherium lusitanicum and Guimarotodus inflatus (Martin 1999), and the paurodontids Henkelotherium guimarotae (Krebs 1991) and Drescheratherium acutum (Krebs 1998). In abundance of specimens, Henkelotherium guimarotae is the fourth most common mammal species after the docodontan Haldanodon exspectatus and the dryolestids Dryolestes leiriensis and Krebsotherium lusitanicum from this site (Martin and Krebs 2000). For this study, we have available six specimens of the upper tooth series, some of which show replacement pattern in the antemolar (incisor, canine, premolar) positions (Table 1), and 21 mandibular specimens with lower teeth that cover the entire range of growth from juvenile to adult, and show the replacement of the teeth in the antemolar positions (Table 2; Appendix 1).
For a description of the molars (Figs. 2, 3, 4, 5), we followed the character definition of Prothero (1981) and Martin (1999), as modified by Kielan-Jaworowska et al. (2004: Fig. 10.2) and by Schultz and Martin (2011: Fig. 1). For stages of dental wear in molars, we follow the criteria of Schultz and Martin (2011). To avoid potential ambiguity of terms, terminology of molars is illustrated in Figs. 2, 3, 4, 5. For the morphology of the mandible, we follow the terminology of Krebs (1971) and Martin (1999), as augmented by this study (Figs. 1, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16).
Henkelotherium guimarotae (Dryolestida): morphological terminology of upper molars and their gradient of character variation. a and b Left M1 (unworn tooth) in tilted labial view and occlusal view (Gui Mam 114/77 with partial restoration). c and d Left M2 (with wear) in tilted labial view, and occlusal view (Gui Mam 94/75). e and f Left M2 crown and root structure, in anterior and posterior views (Gui Mam 94/75). g and h Labial (tilted) and occlusal views of an unworn left M4 (Gui Mam 114/77)
Henkelotherium guimarotae (Dryolestida): morphological terminology of the lower molars and their gradient of character variation. a and b Right m1 (with wear) in anterior and posterior views. c–g An unworn right m2 (Gui Mam 15/77): c occlusal view; d labial view; e anterior (mesial) view; f posterior (distal) view; g lingual view. h Right m5 (Gui Mam 78/76) linguo-posterior view. i Right m6 (Gui Mam 65/77) in occlusal view
Difference in root structure of Henkelotherium guimarotae (a, b and c) and Dryolestes leiriensis (d, e and f). a Henkelotherium, left M2 cross-sections of roots in ventral view (left) and roots viewed through transparent crown in dorsal view (right). b Henkelotherium left lower m2 roots in ventral view (left) and as seen through transparent crown with approximate root positions under the crown (right). c Henkelotherium lower m3 (Gui Mam 133/77) crown and roots by CT visualization, in anterior (mesial) view (left), in mesio-lingual view (middle), and posterior (distal) view (right). All views of Gui Mam 133/77 are left–right flipped for comparison to roots of right lower molar of Dryolestes. d Dryolestes leiriensis, left M2 cross-sections of roots in ventral view (left), and roots viewed through transparent crown in dorsal view (right). e Dryolestes left lower m4 cross-sections of roots in ventral (left) and roots viewed dorsally through transparent crown with approximate root positions under the crown (right). f Dryolestes leiriensis lower m4 (Gui Mam 82/79) by CT visualization, crown and roots in anterior (mesial) view (left), in mesio-lingual view (middle) and posterior view (right)
Variation of upper molar roots among dryolestidans. a Henkelotherium guimarotae (Gui Mam 91/75) right dP4-M3 in labial view. b Henkelotherium (Gui Mam 91/75) dP4-M3 roots in dorsal view (flipped from right for comparison). c Henkelotherium (Gui Mam 91/75) isolated M3 (right-left flipped) in anterior (mesial) view (left), and roots in dorsal view (right). d Schematic illustration of roots of left upper M1 (from Gui Mam 94/75). e Dryolestes, right upper P3 to M7 (Gui Mam 43/77) in labial view. f Dryolestes roots of upper P3 to M7 in dorsal view (images right-left flipped from Gui Mam 43/77). g M3 in mesial (anterior) view (Gui Mam 43/77, flipped). h Dryolestes M5 roots (with labial roots fully divided) (Gui Mam 43/77). i Dryolestes M6 roots in anterior view (left) and ventral root view (right)—M6 labial roots are fused and share a single root canal; and its lingual root is curved mesially (Gui Mam 43/77). j Hercynodon germanicus, an anterior upper molar (NLMH 105668, not on scale; Martin et al. 2021), comparable to a tooth locus between M3 and M5 of Dryolestes. Hercynodon and Dryolestes share similarities of the anterior upper molars in having a curved lingual root and prominent y-shaped connecting ridges between the three roots; k Minutolestes submersus, a posterior upper molar (WMNM P82304, not on scale; Martin et al. 2022a, b), comparable to either M6 or M7 of Dryolestes
Line illustration and CT rendering of a left mandible of Henkelotherium guimarotae (Gui Mam 133/77), the best-preserved specimen. a Illustration of the mandible in lateral view (before damage of the coronoid process). b CT visualization of the hollowed or trabeculated interior structure. c Illustration of mandible in occlusal view. d Illustration of mandible in lateral view. e CT visualization of the internal trabecular structures in lingual view. Abbreviations: IAN, canal of the inferior alveolar nerve (connected to the mandibular foramen; PDL, periodontal ligament. IAN-PDL space is a shared space of the inferior alveolar nerve passage and the periodontal ligamental tissues. The m6 crypt is a hollow space budding off from the mandibular canal
Mandibular characteristics of Henkelotherium guimarotae. a and b Mandibular surface and interior structures of the right mandible (a) and the exterior surface (b) in lingual view of CT rendering of Gui Mam 44/80. c Cross-section view of tooth roots, alveoli, mandibular “canal” space for inferior alveolar nerve (IAN) and the periodontal ligament (PDL) space around the molar roots in the mandibular body (Gui Mam 15/77). d Line drawing of right mandible (based on Gui Mam 44/80, lingual view). e Lingual view of right mandible (based on Gui Mam 133/77); the approximate position of m6 crypt detected by CT scanning. f Dorsal view (tilted slightly medially) of the posterior part of the mandible; composite reconstruction of Gui Mam 133/77 and Gui Mam 138/76, the holotype (stereo images). g Posterior view of the mandible, composite reconstruction from Gui Mam 133/77 and Gui Mam 48/78 (stereo images)
Growth series of mandibles and patterns of eruption and replacement of lower teeth of Henkelotherium guimarotae. a Gui Mam 48/78: one of the largest (presumably the oldest) specimens examined in this study, with six molars and all permanent antemolar teeth. The m7 crypt inside the mandible was detected by CT scanning. b Gui Mam 133/77: a specimen of advanced dental age, with five molars. The m6 crypt inside the mandible was detected by CT. c Gui Mam 48/75, a young adult or juvenile specimen with permanent p4 erupting, m1, alveoli for m2 through m4, and crypt for unerupted m5. d Gui Mam 44/80: a juvenile specimen with erupting permanent canine, dp4, permanent m1 and m2, alveoli for m3, m4 crown at bell stage inside the mandible (detected by CT), and a part of the trigonid of p4 already formed under dp4 (detected by CT); e Gui Mam 25/81: one of the smallest (presumably the youngest) specimens with all deciduous teeth from deciduous canine (dc) through dp1 and cusp of p1, dp2, dp4, and cap of p3; alveoli of m1 and m2, plus almost fully formed crown of m2 at bell stage, in its crypt inside the mandible
Henkelotherium guimarotae: CT visualization of tooth roots and unerupted teeth in the mandible. a and c Anterior teeth of Gui Mam 133/77 with partial restoration of incisors and canine to their anatomical position, in lateral view (a) and medial view (c) of transparent mandible. b and d CT visualization of the mandible, tooth roots and unerupted teeth of Gui Mam 44/80 in transparent (top) and solid (bottom) mandible models in lateral view (b) and medial view (d)
Growth series of mandibles, antemolar replacement and late molar eruption in Dryolestes leiriensis (Dryolestidae), for comparison to Henkelotherium guimarotae (Paurodontidae): Diphyodont replacement of incisors, canine and premolars, and late eruption of posterior molars. a Gui Mam 82/79: mandible of an old adult with permanent canine, premolars and nine molars (teeth and their roots based on CT visualization). b Gui Mam 129/75: mandible of a young individual exhibits early replacement of p1 and p3 (displaced p1 is here repositioned into its alveoli; dp2 is hypothetically reconstructed by comparison (modified from Martin 1997: Fig. 1, left–right flipped for comparison). c The youngest (juvenile) specimen available (Gui Mam 112/76) (modified from Martin 1997: Fig. 1, left–right flipped for comparison)
Henkelotherium guimarotae (Gui Mam 25/81) deciduous teeth of a juvenile mandible (the youngest available). a Occlusal view of deciduous canine, dp1, dp2, dp3, and dp4, with occlusal view of unerupted m3 to the right, all isolated by CT visualization. b Lingual view of deciduous canine, dp1 through dp4, with lingual view of unerupted m3 to the right, isolated by CT visualization. c Deciduous canine in labial view (Gui Mam 25/81 juvenile, flipped right from the left canine), and permanent canine in labial view of an adult (Gui Mam 133/77), both deciduous and permanent canines on same scale for comparison. d Lingual view of the split part of the right mandible, with dc, dp1 through dp4, and the exposed m1 alveoli, m2 alveoli, and the crypt of unerupted m3. e Composite restoration of lingual split counter-part of the right mandible, showing the position of unerupted m3 in the mandible. f Labial view the lateral split part of the right mandible
Scanning Electron Microscope (SEM) photographs of mandibles of Henkelotherium guimarotae. a and b Labial (lateral) and lingual (medial) views of a left mandible (Gui Mam 15/77). c Ventral view of the right mandible of the same individual (Gui Mam 15/77). d Medial view and e posterior view of a right mandible (Gui Mam 48/78)
Henkelotherium guimarotae mandible and lower dentition (Gui Mam 48/78) by SEM photographs and CT visualization. a and b Labial views of the complete right mandible with alveoli for i1, i2, i3, and teeth of i4-m6 in SEM photo (a) and CT visualization (b). c Preserved teeth with roots: i4 through m6 in labial view exposed by CT visualization. d and e, Lingual view of the mandible by SEM photo (d) and CT visualization (e). f Preserved teeth with roots: i4 through m6 in lingual view by CT visualization
Henkelotherium mandible and lower teeth with comparison of its persisting dp4. a through f Gui Mam 65/77. g through j Comparison of dp4, p4, and m1 among juvenile and adult H. guimarotae specimens. a Lingual view of the mandible and teeth with exposed roots (Gui Mam 65/77). b Labial view of the mandible and teeth with exposed roots. c Paired stereophotos of occlusal view. d Occlusal view of lower teeth (c-m6) of Gui Mam 65/77. e Lingual view of right lower canine and premolars (including dp4) through m1 (Gui Mam 65/77). f Persisting (permanent) dp4 and m1 in occlusal view (top) and lingual view (below). g Henkelotherium premolars with normal p4 (Gui Mam 48/78). h Henkelotherium with normal p4 (Gui Mam 133/77). i Deciduous premolar dp4 of a juvenile of Henkelotherium (Gui Mam 44/80). j Lower m1 of an adult of Henkelotherium (Gui Mam 48/78). d, e, g, h Comparison of persisting dp4 and m1 of an atypical adult (Gui Mam 65/77) with permanent p4 of a typical adult (Gui Mam 133/77). Box e and g Gui Mam 65/77 is an atypical individual with persisting dp4 and also larger p1 and p3, than the typical premolar sizes of Gui Mam 48/78. Box f, i, and j The persisting dp4 of Gui Mam 65/77 is identical to the (deciduous) dp4 of juvenile specimen Gui Mam 44/80 that is associated with p4 cusp (tooth cap): comparison in occlusal view (top) and lingual view (bottom)
SEM photographs of left lower premolars and molars (p2-m6) of Henkelotherium guimarotae (Gui Mam 78/76). a Paired stereophotos of occlusal view. b Paired stereophotos of lower molars in distolingual view. c Labial view. d Lingual view. Note that this is a relatively old individual with six fully erupted molars and the medial side of the mandible shows little or no sign of Meckel’s sulcus, and a well-developed mandibular symphysis
SEM photographs of left lower molars of Henkelotherium guimarotae (Gui Mam 15/77). a Paired stereophotos of lower m1–m2. b, c, d, and e Anterior, posterior, lingual and labial views of m1–m2. f Paired stereophotos of occlusal view of left lower m4–5. g Paired stereophotos of anterior view of m4. h Lingual view and i labial view of m4-5
Institutional Abbreviations: AMNH—American Museum of Natural History (New York, USA); Gui Mam—Guimarota Mammal Collection of the Geological Museum, National Laboratory of Energy and Geology (MG/LNEG), Lisbon, Portugal, currently under study at Universität Bonn (Germany). NLMH—Niedersächsisches Landesmuseum, Hannover, Germany. WMNM, LWL—Museum für Naturkunde/Westfälisches Landesmuseum mit Planetarium, Münster, Germany. For Geological Museum (MG/LNEG) repository specimen numbers see Supplementary Information.
Results
The body of the mandible (Corpus mandibulare) (Figs. 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16) is relatively deep, to accommodate the very long roots of the premolars and molars (Figs. 6, 7, 8, 9, 10, 11, 12, 13, 14, 15), as previously noted for Dryolestes and Krebsotherium (Martin 1999). The medial surface of the mandibular body is flatter, and has a greater vertical depth from the ventral margin to the tooth alveoli than the more convex lateral surface of the mandibular body, which has a shorter vertical depth. This was first observed in the H. guimarotae holotype by Krebs (1991) and can now be confirmed by additional mandibles examined in this study. We note that this difference between the medial depth and lateral depth of the mandible is complementary to the difference in the crown height difference on the lingual and labial sides of the tooth crown. Crowns of the lower molars are much taller on the labial side and shorter on the lingual side. The different depths of the medial and lateral surfaces of the mandibular body compensate for these differential heights of the lingual and labial sides of the molar crowns, such that the occlusal surface across the molar trigonids is transversely leveled, along the molar series. This feature is present in all dryolestidans (Krebs 1971; Martin 1999). It is also a common feature of therian mammals with zalambdodont molars (Ungar 2010; Kelt and Patton 2020)—the molars with steeply v-shaped trigonids that are also antero-posteriorly shortened.
The lingual alveolar margin of the mandible shows a slightly concave curvature in the premolar region (Figs. 1 and 6), such that the alveolar line along the anterior part of the tooth row shows a series of sigmoid curvatures (Figs. 1 and 12d). The lingual alveolar line is slightly raised (convex) dorsally under i4 and the canine, and then lower down to the lowest point under p1 and p2. The lingual alveolar line is raised from p3 and p4 through m1 and m2. The alveolar line is straight and leveled along most of the molar row (m2–m6) (Krebs 1971) (Fig. 12d).
The ventral margin of the mandible shows a rounded outline in cross-section (Figs. 1 and 12). Longitudinally, the posteroventral margin of the mandible is concave ventrally, from the level of the mandibular foramen to the apex of the angular process. This concave mandibular outline is related to the slight downward and medial orientation of the angular process. From the level of the mandibular foramen to the level of the posterior premolars, the mandibular ventral margin is gently convex. Further anteriorly from the premolar region, the ventral margin is straight. However, the anterior (mesial) end of the mandible is strongly convex under i1 and i2. Correspondingly, i1 and i2 have more vertically oriented crowns but long, posteriorly curved roots that are bent to conform to the symphyseal margin. The roots of i1 and i2 extend posteriorly to the medial side of the i3 and i4 roots. The canine has two roots that are also strongly curved posteriorly (Figs. 9, 10, 13, 14).
Anterior part of mandible
The mandible appears to be more constricted dorso-ventrally immediately posterior to the canine in both medial and lateral views, due to the concave curvature of the alveolar margin in the premolar region (Figs. 1 and 2). In the majority of the specimens, there are two mental foramina on the labial side of the incisor to premolar region: a circular mental foramen under i4, or between i4 and the canine, and a second, larger and oval mental foramen under the p2–p3 junction, or under p3. However, the number of foramina can be variable: two juvenile mandibles have three mental foramina (Gui Mam 44/80 and 25/81) (Figs. 9 and 11).
The variability of the mental foramina in Henkelotherium is similar in scope and in number to the variation pattern already known in Dryolestes (Martin 1999), most specimens of which have two mental foramina, one each under the canine, and under p2 or the p2–p3 junction. However, in one specimen (“Guimarota 6”), there are two foramina under p2 and p3 in a region where a single mental foramen is expected in other Dryolestes specimens (Martin 1999). In all dryolestids, the posterior-most position of the mental foramen is limited to the anterior premolar region, and usually anterior to the p3 locus and within the rostral 20% of the mandibular length. The relatively more anterior placement of the posterior-most mental foramen in both Henkelotherium and Dryolestes is similar to the anterior mandibular region in the mammaliaforms Morganucodon and Haldanodon, australosphenidan mammals, in spalacotherioids, and in meridiolestidans (Kermack et al. 1973; Krusat 1980; Crompton and Luo 1993; Rich et al. 1999, 2001, 2016; Asher et al. 2007; Ji et al. 2009; Rougier et al. 2011).
In Henkelotherium, the three foramina for the mental nerves are connected to the mandibular canal that carried the inferior alveolar nerve (Figs. 6, 7, 9). The more anterior placement of the mental foramina of dryolestidans is in contrast to the more derived zatherian mammals, as defined by the common ancestor of the Mesozoic “peramurans”, metatherians and eutherians. In zatherians, the posterior-most mental foramen is under the first lower molar (m1) for metatherians (Rougier et al. 1998, 2004; Luo et al. 2003), or under the ultimate premolar for the “peramuran” Peramus (Clemens and Mills 1971; Davis 2012), and in early eutherians such as Eomaia (Ji et al. 2002), Maelestes (Wible et al. 2009) and Juramaia (Luo et al. 2011), or in eutherians in general (Novacek 1986). Because “peramuran” mammals have a shorter molar row of only three-to-four molars (far fewer molars than most dryolestidans that have six-to-nine molars), a placement of the posterior-most mental foramen below the ultimate premolar or the first molar represents a more significant posterior shift of the mental foramina in “peramurans”, than the more anterior placement of this foramen in Dryolestes and Henkelotherium and other mammaliaforms more stem-wards in the phylogeny. The mental foramina are conduits for mental nerves (branches of the inferior alveolar nerve) that innervate the chin area and the anterior portion of the lower jaw in extant mammals (Evans 1993; Dyce et al. 1995). It is likely that the innervation area on the anterolabial surface of the lower jaw on the head surface is smaller in the more stem-ward dryolestidans, than in the phylogenetically more derived zatherians.
Symphysis
The symphysis is the contact between the two hemi-mandibles in mammals (Scapino 1965, 1981; Scott et al. 2012), and an unfused symphysis is a typical feature of mammaliaforms (Kermack et al. 1973; Crompton and Luo 1993; Luo and Martin 2007; Panciroli et al. 2019, 2021). The symphysis in Henkelotherium is an area of slightly rugose texture on the medial surface of the anterior part of the mandible from i1 to p2 (Figs. 1, 6, 7, 8, 9, 12, 13, 14, 15). The depth of the symphyseal contact between the two mandibles (hemi-mandibles) is about the half of the mandibular depth at the level of the canine. A symphyseal foramen, which is connected to the mandibular canal in the interior of the jaw, is present in the symphysis below i4 or just anterior to the canine root (Gui Mam 44/88, 133/77, 48/78).
The extent of the symphysis is variable with mandibular growth. It extends from the anterior (mesial-most) end, posteriorly to the level below the junction of p2 and p3 in juvenile and young adult specimens (Fig. 8). For example, in a juvenile mandible (Gui Mam 44/80) in which this structure is well preserved, the symphysis is relatively shorter, and ends below p1 (Fig. 9d). However, in the larger mandibles of presumably older individuals, the symphysis becomes longer proportionally and in absolute size, extending further posteriorly to below the junction of p2 and p3 in the next larger mandible (Gui Mam 133/77) (Fig. 6d). The symphysis was not fully exposed in the holotype specimen (Krebs 1991), but the visualization of CT scans shows that it appears to have a relatively longer symphysis, as in Gui Mam 48/78 (Fig. 17). In some of the largest available mandibles, such as Gui Mam 48/78, the symphysis extends further to the posterior edge of p3 (Figs. 8, 13). From the growth series of the mandible, it is clear that the extent of the symphysis has become longer with older growth stages of the mandible.
Henkelotherium guimarotae type specimen (Gui Mam 138/76) mandible and teeth. a Photograph of the local area of the right mandible and right upper teeth. b CT visualization to expose the anterior teeth in the right mandible invisible in photo (CT image on same scale as the photo in a). CT also shows m7 is single-rooted. Evenly spaced bumps on the ventral margin of the mandible are caused by tightly impressed upper molars (see photo of a). c CT visualization to expose the anterior right upper teeth invisible in the photograph; the dash-line indicates a fault line that shifted and distorted the P2 alveolus or alveoli
The symphysis is not fused in any of the mandibles in the large sample of this study. This suggests that the symphysis was mobile in life in Henkelotherium. Our interpretation is that the two mandibles were connected in an amphiarthrosis condition (sensu Scott et al. 2012), more likely by ligaments than by fibrocartilage. Both types of tissue structure are known in the symphysis of extant mammals (Scapino 1965, 1981). The two unfused mandibles were mobile relative to each other during jaw movement, as generally is the case in extant mammals with an unfused symphysis (Crompton and Hylander 1986; Scott et al. 2012). This is a prerequisite for the hemi-mandible to rotate on its long axis, for better matching of the occlusal surfaces of the tooth crowns during occlusion (Crompton 1974; Schultz and Martin 2011). The unfused symphysis of Henkelotherium (Figs. 8, 9, 12, 13, 14, 15) and Dryolestes (Martin 1999) is a general feature and plesiomorphy of other Mesozoic mammaliaforms (Kermack et al. 1973; Cifelli and Madsen 1999; Krebs 1991; Martin 1999).
Meckel’s sulcus
A distinctive Meckel’s sulcus is present on the medial surface of the mandibular body in the majority of the examined mandibles. The anterior part of the sulcus appears to extend into the rugose area of the mandibular symphysis in juveniles and some adults (Gui Mam 44/80; Gui Mam 133/77) (Figs. 6d and 9d). The sulcus extends posteriorly from the symphysis towards the margin of the pterygoid fossa, and either ends just below the posterior opening of the mandibular canal, or fades out below m5 without reaching the pterygoid fossa margin. However, the extent of this sulcus is dependent on growth stages, and is more prominent in juvenile and subadult specimens. It can be reduced or absent in older individuals with late erupting fifth to seventh molars, and with a longer mandibular body. When present, the sulcus is a shallow but very distinct groove, wider (relative to the mandibular depth) and fully continuous along the mandible in juvenile and young adult specimens (Gui Mam 44/80; Gui Mam 133/77). However, we note that the sulcus is variable in other individuals; it is fully visible in the left and right partial mandibles of Gui Mam 78/76 (a mandible of five molars). However, in Gui Mam 78/76 (with six molars), the anterior part of Meckel’s sulcus appears to be a weak and faint line from the symphysis, and the posterior part of the sulcus is absent (Fig. 15). In the longer jaws of the examined sample, with more late-erupted molars (Gui Mam 48/78, 65/77; 138/76 holotype) (Figs. 1, 12, 13, 14), the sulcus is absent, which represents the extreme of a wide range of variability of the Meckel’s sulcus.
In specimens of Henkelotherium in which Meckel’s sulcus is fully present, the sulcus is parallel to the ventral margin of the mandible, as in the dryolestids Dryolestes leiriensis and Krebsotherium (Martin 1999). Among mammaliaforms, the Meckel’s sulcus can either be parallel to the mandibular margin, such as in Sinoconodon and many eutriconodontans and spalacotherioids (Simpson 1928; Luo et al. 2007a, b; Gao et al. 2009; Ji et al. 2009; Meng et al. 2011; Mao et al. 2020), or intersecting with the ventral margin, as seen in Morganucodon and several docodontans (Kermack et al. 1973; Lillegraven and Krusat 1991; Luo 1994; Schultz et al. 2019; Panciroli et al. 2019). Henkelotherium shows the former condition (Fig. 8) (Krebs 1991).
The dryolestidan taxa show interspecific variation of Meckel’s sulcus. In Dryolestes leiriensis, the Meckel’s sulcus is present in all specimens, without exception (Krebs 1971; Martin 1999). The sulcus is also present in Krebsotherium (Martin 1999, 2000). Previously the sulcus was interpreted to be present only in the posterior-most part of the mandible in the Cretaceous dryolestid Crusafontia (Henkel and Krebs 1969; Krebs 1971), but a new CT visualization shows this sulcus to be very weak, if present at all (Martin et al. 2022b: figs. 11 and 12). The sulcus is absent in the dryolestid Beckumia (Martin et al. 2022b). Meckel’s sulcus is also well developed in the Late Cretaceous meridiolestid Cronopio (Rougier et al. 2011), but can be variable among other meridiolestidans, an endemic clade of South America (Rougier et al. 2021a).
Phylogenetic variation of Meckel’s sulcus is not uncommon for other Mesozoic stem therian groups (Luo 2011; Meng et al. 2011). At least two taxa of spalacotherioid “symmetrodontans” have a fully developed Meckel’s sulcus, linked with ossified Meckel’s element (Ji et al. 2009; Zhou et al. 2019; Mao et al. 2020), which is connected to the middle ear (Zhou et al. 2019; Luo and Manley 2020). By comparison, the more derived taxa of the same spalacotherioid clade lack this sulcus altogether (Cifelli and Madsen 1999; Tsubamoto et al. 2004). More recent discoveries show that the ossified Meckel’s element is variably retained in Mesozoic eutherian mammals (Wang et al. 2022), leaving a definitive sulcus in some specimens, but not in other taxa of the same group (Urban et al. 2017; Wang et al. 2022). The variability of Meckel’s sulcus here in Henkelotherium is consistent with the recent findings in eutherians (Wang et al. 2022).
The Meckel’s sulcus undergoes ontogenetic change in extant mammals. The sulcus is the interim contact site for the Meckel’s element, which is fully cartilaginous and associated with the mandible during embryonic and fetal stages in extant mammals (Anthwal et al. 2017; Urban et al. 2017). The sulcus on the surface of the mandible disappears after the bone remodeling in the aftermath of the incorporation of the middle part of Meckel’s cartilage into the mandible, and the resorption of the posterior part of the cartilage, in neonates of extant mammals (Zeller 1989; Anthwal et al. 2017; Urban et al. 2017).
Variation of the Meckel’s sulcus among individuals of Henkelotherium, and the systematic variation among taxa of dryolestidans may be interpreted in one of two scenarios. The first and more likely scenario is that the groove remained on the mandible after a cartilaginous Meckel’s element was re-absorbed in adults, and the sulcus has remained as a vestigial structure. The variability of this sulcus would be the consequence of uneven remodeling of the mandible bone post-resorption of the Meckel’s cartilage in neonate stages (Ramirez-Chavez et al. 2016; Anthwal et al. 2017).
An alternative scenario is also possible (although not probable) that Meckel’s element had been ossified, as in the rare preservation of this element in the eutherian Cokotherium (Wang et al. 2022), but the ossified Meckel’s element was so flimsy that it was not preserved in mandibular fossils. Several eutriconodontan mammals are known to have preserved an ossified Meckel’s cartilage with its original connection to the mandible (Wang et al. 2001; Luo et al. 2007a, b; Meng et al. 2011; Luo 2011; Mao et al. 2020). Among trechnotherian mammals (inclusive of dryolestidans), there are two spalacotherioid species, Maotherium asiaticum and Origolestes lii (Ji et al. 2009; Zhou et al. 2019; Plogschties and Martin 2020; Mao et al. 2020) in which an ossified Meckel’s element is connected to the relatively wide Meckel’s sulcus, and fully connected to the middle ear (Luo and Manley 2020). However, the relatively wide Meckel’s sulcus of spalacotherioids and eutriconodontans is quite different from the thin Meckel’s sulci in dryolestidans examined here. By this conspicuous difference, we argue that it is not probable that Meckel’s cartilage was ossified in life in Henkelotherium, because its Meckel’s sulcus is so thin and different from the broader sulcus of spalacotherioids and eutriconodontans known to have accommodated the stout Meckel’s element in full connection to the middle ear.
In earlier studies, which did not have the benefit of extensive new fossils of Meckel’s element in Mesozoic mammals, it was hypothesized that the sulcus would hold the mylohyoid nerve and artery, in addition to the association of Meckel’s cartilage (Krebs 1971), but given the strong fossil evidence in the last two decades, this earlier interpretation (Krebs 1971) is no longer tenable (see the discussion by Anthwal et al. 2017).
Comment on “splenial scar”
The splenial scar, as interpreted for Dryolestes leiriensis (Krebs 1971; Martin 1995, 1999), is a narrow triangular area anterior to the pterygoid fossa and between two faint grooves flanking the posterior-most part of the Meckel’s sulcus. This “scar” is interpreted to be the contact area for the vestigial splenial bone, although the splenial bone itself is not preserved in dryolestids (Krebs 1971, 1991; Martin 1995: Fig. 1, 1999, 2000). In specimens of Henkelotherium, this “scar” can be identified in one juvenile specimen (Gui Mam 44/80), where there is a part of the Meckel’s sulcus, which is wider in young individuals. However, this splenial “scar” is absent in the five well-preserved mandibles (including the holotype: Figs. 12, 13, 17) in which the periosteal surface of the mandible is intact and does not show any trace of a “scar” anterior to the pterygoid fossa. Other mandibles (more than seven specimens) are not well preserved enough, due to fractures and damages in this location, to be informative about the “splenial scar.” Given the wider sampling of more specimens, we interpret that the “splenial scar” is a variable feature of juveniles, and a part of the wider Meckel’s sulcus of early growth stages. However, it is absent in fully adult mandibles. Further, the splenial bone itself is not present in Henkelotherium, as the bone is also unknown in Dryolestes leiriensis (Krebs 1971; Martin 1999).
Coronoid scar
The scar of the coronoid fossa or facet is located at the corner between the crest-like anterior margin of the coronoid plate and the mandibular body, and the scar borders on the pterygoid fossa. The coronoid scar is a slightly rugose area on a platform slightly elevated from the surrounding bone. When well preserved, the outline of the rugose surface of the scar shows a roughly triangular shape in medial view (Gui Mam 133/77, 48/78) (Figs. 6, 13), but its outline can be variable, with a half-circle outline, or a narrow strap (Gui Mam 15/77), and even irregular among specimens in differing conditions of preservation. Despite the preservational variation, it is always present in all specimens in which the relevant region is preserved, regardless of the growth stage of a mandible. We have confirmed the coronoid fossa in eight mandibles in which this region is preserved (Appendix). The coronoid scar is a consistent feature of all dryolestids, as noted first by Krebs (1969, 1971) and corroborated by observation of Dryolestes leiriensis and Krebsotherium lusitanicum (Martin 1995: Fig. 1, 1999; Figs. 8a, 9, and 18a).
Upper teeth of two specimens of Henkelotherium guimarotae. a, b and c Young specimen (Gui Mam 91/75, either juvenile or subadult). a CT visualization of the preserved teeth in approximately occlusal view: dP2 was broken from its roots and restored virtually to its anatomical position; P3 was present in this individual, as indicated by its two alveoli that are distorted and displaced by a fault line through the P3 position in fossilization; dP4 still in place; M4 has fallen off but its broken alveoli are preserved. b, Reconstructive drawing of occlusal view of Gui Mam 91/75 (juvenile or subadult) with four premolar positions (dP4 still in place) and four molar positions (M1–3 present and M4 represented by broken alveoli). c, Reconstructive drawing of tilted occlusal view of upper teeth of Gui Mam 91/75 (juvenile or subadult). d Fully grown adult of Gui Mam 138/76 (holotype) with larger permanent canine, four premolars (P2 fallen off, but indicated by its alveoli), six molars (M1-5 preserved; M6 is represented by an outline of tooth socket, see Fig. 17), drawing adopted from Krebs (1991). Arrows indicate molar imbrication in which the distolabial corner of the preceding molar is overlapped by the parastylar lobe of the succeeding molar
Pterygoid fossa
The pterygoid fossa is a broad and shallow depression on the medial surface of the posterior region of the mandible (Figs. 1, 7, and 8). The dorsal part of the pterygoid fossa is bound anteriorly by a crest-like anterior margin on the coronoid process. The surface of the fossa is overall flat, but slightly convex medially in its dorsal part and more concave in the ventral part. Below the raised area of the coronoid fossa, it is bound by a curved margin that continues to the ventral border of the mandible; more posteriorly, the pterygoid fossa margin curves postero-dorsally and extends further to the articular condyle. The ventral and posterior margins of the pterygoid fossa are ridge-like and very distinctive in most specimens. The slightly convex dorsal part of the pterygoid fossa is likely for the insertion of the deep temporalis muscle which inserts on both the medial and lateral sides of the coronoid process. The distinctive ridge-like ventral and posterior margins of the pterygoid fossa are likely for insertion of the deep pterygoid muscle (M. pterygoideus internus) which may have further expanded to the inflected angular process (Turnbull 1970; Lautenschlager et al. 2017; Grossnickle 2017). The mandibular foramen (“Foramen dentale” of Martin 1999) is located in the deepest part in the ventral area of the fossa, just opposite to the posterior end of Meckel’s sulcus in juvenile specimens. The mandibular foramen is the posterior opening of the mandibular canal for entrance of the inferior alveolar nerve and its associated vessels into the mandible (Figs. 6, 7, 9, and 13).
Coronoid process and masseteric fossa
The coronoid process is plate-like, and dorsally curved to a posterior angle (preserved in Gui Mam 133/77) (Fig. 6). In side view, the lower part of the anterior border of the coronoid process is straight, but the top part of the coronoid margin curves posteriorly and forms a recurved apex of this process (Fig. 1; Appendix). The apophysis or the posterodorsal apex of the coronoid process is present, at least in one specimen (Gui Mam 133/77).
The straight part of the anterior margin forms an angle to the molar alveolar line from 50° to 65° (measured in lateral view on Gui Mam 48/78 and the holotype, reported by Krebs 1991). This angle is much smaller than the 80° to 90° angle in Dryolestes and Crusafontia, but overlaps the 60° angle in Krebsotherium (Martin 1999).
The masseteric fossa is well developed on the lateral aspect of the coronoid process. The fossa is bound anteriorly by the sharp anterior crest on the coronoid process, and the anterior margin of the fossa is flaring antero-laterally. The fossa is bound posteriorly by the gently convex lateral crest (= lateral ridge) (Figs. 1 and 6). The masseter fossa shows a gradational transition to the lateral side of the mandibular body, without a crest to demarcate its margin. Similar to other dryolestidans especially dryolestids (Krebs 1971; Martin 1999), the masseter fossa does not extend anteriorly onto the mandibular body, as in multituberculate mammals (Hahn and Hahn 2000; Kielan-Jaworowska and Hurum 2001), and it has no lateral mandibular foramen in the deep part of the masseteric fossa as in the Cretaceous stem zatherian Peramus, and the eutherians Prokennalestes and Juramaia (Kielan-Jaworowska and Dashzeveg 1989; Rougier et al. 1998; Luo et al. 2011).
The coronoid process shows an antecoronoid depression in its front base. Flanked by the lateral flaring of the anterior masseter margin and medially by the platform of the coronoid fossa, this depression is best seen in dorsal view (Figs. 1, 6, and 12). The antecoronoid depression is also present in Dryolestes leiriensis (Martin 1999) and Beckumia (Martin et al. 2022b), although not well developed in Crusafontia. This is a derived feature associated with the strong development of the crest for the anterior margin of the masseter fossa.
Below the recurved angle (= the apex) of the coronoid process, the posterior notch of the coronoid is strongly concave, but it has no crest in medial or lateral aspects. On the lateral aspect of the mandible, a low, broad crest arises from the ventral margin of the mandible to the articular condyle. The lateral crest is interpreted here to be the equivalent of the lateral ridge of the articular process of mammaliaforms (Crompton and Luo 1993; Schultz et al. 2019; Panciroli et al. 2019; Luo et al. 2022) (Figs. 1 and 6).
Articular process (“dentary condyle”)
The articular process is oriented postero-dorsally and it terminates in the dentary condyle. The condyle is raised well above the molar alveolar line. The dentary peduncle (sensu Luo et al. 2002), a neck-like constriction between the condyle and the rest of the coronoid part of mandible, is noticeable, although short and stout. The condyle and the peduncle are reinforced by a lateral crest forming the boundary of the masseter fossa on the lateral side, and are supported on the medial side by the crest-like posterior margin of the pterygoid fossa (Figs. 1 and 7). The condyle is cylindrical in dorsal view but shows a triangular outline in posterior view (Figs. 1 and 7) (Krebs 1991). The condyle appears to have a larger and more rounded lateral end, than medial end, due to being more expanded and rounded laterally than medially. Below the medial rim of the articular condyle, there is a small and slightly depressed area, likely to be for the insertion of the lateral pterygoid muscle or attachment of its associated tendon. A similar depression on the medial side of the articular process is present in Dryolestes. The articular process is similar in most characteristics to that of Dryolestes (Martin 1999).
Angular process
The angular process reaches posterior to the level of the dentary condyle but is separated from the dentary condyle by a wide, angular embayment, formed by the concave posterior margin of the dentary (sensu Martin 1999) (Figs. 1 and 6). The main part of the angular process is medially inflected and this inflection is best seen in ventral view, and also in posterior view (Figs. 1, 7, and 12). The ventral mandibular margin anterior to the angular process is noticeably curved (concave) in side view (Fig. 1).
The lateral face of the base of the angular process shows a flat area, which is bound by the posterior margin of the masseteric fossa, and by the lateral crest to the dentary condyle (Fig. 6a). The medial aspect of the inflected angular process is continuous with the prominent ventral margin of the pterygoid fossa. Near the base of the angular process is a triangular depression between the sharp crest of the pterygoid fossa posterior margin and the angular process. This depression houses the angular foramen, a vascular conduit to the trabecular space in the interior of the bone (Figs. 6, 7, 12, and 13). The angular foramen is present in all specimens of Henkelotherium where this region is preserved. This feature is generally absent in other dryolestidans. Among other Jurassic mammals, this foramen is present in the stem zatherian Nanolestes from the Guimarota mammal assemblage (Martin 2002), an exception and the only other example of this structure outside dryolestidans.
Henkelotherium differs from dryolestids in that the angular process of the latter group is not medially inflected, and has a straight and leveled ventral margin (Krebs 1971; Martin 1999). Henkelotherium is similar to meridiolestidans in the inflection of the angular process (Rougier et al. 2011, 2021a). Both the meridiolestidan Cronopio, and Necrolestes that is hypothesized to be related to meridiolestidans, have a prominent, medially inflected angular process (Asher et al. 2007; Rougier et al. 2012), although the degree of inflection and size of the angular process in Henkelotherium are much less than those of meridiolestidans. Averianov et al. (2013) re-interpreted that the inflected angular process was not present in meridiolestidans. Based on a review of meridiolestid specimens by Luo, we disagree with this interpretation. The degree of inflection of the angular process in Henkelotherium is similar to that of the mammaliaform Hadrocodium (Luo et al. 2001, 2022). One spalacotherioid mammal, Spalacolestes (Cifelli and Madsen 1999), has a medially flaring ventral margin of the pterygoid fossa, but the tip of the angular region of this fossil is not preserved to suggest a continuity of this margin to an inflected angle (Cifelli and Madsen 1999: Fig. 8), as in Henkelotherium. An inflected angular process continuous with the angular process is well known for metatherians (Sánchez-Villagra and Smith 1997), and the angular process and its related pterygoid shelf are attachment site for the medial pterygoid muscles in extant therians (Turnbull 1970; Lautenschlager et al. 2017; Grossnickle 2017). Here, Henkelotherium is interpreted similarly.
Interior structure of mandible
CT scanning and visualization have revealed the interior trabecular structure and spaces for periodontal attachment of the teeth, nerve conduit, and vascular channels. These hollow spaces are present inside the dentary condyle (Figs. 6 and 13: condylar plexus), and along the ventral part of the mandible through the entire length of the mandibular body to the symphysis, and in the angular region (angular plexus) (Figs. 6 and 7).
The roots of the lower teeth are held by their respective root alveoli that open ventrally into the shared space of the periodontal ligament (PDL) and inferior alveolar nerve (AIN) along the entire length of the mandibular body (Figs. 6 and 7: AIN-PDL spaces). The ventral one-third to one-half of the tooth roots are in the hollow space for the inferior alveolar nerve and its associated blood vessels, and the top one-half of the alveolar tubes are for soft tissues, holding the periodontal ligament (Figs. 6 and 7: PDL space) that is confluent with the mandibular canal.
In extant mammals, the mandibular canal houses the inferior alveolar nerve (IAN), a branch of the mandibular nerve of the trigeminus (cranial nerve V-3), and the major nerve that innervates the mandible and the lower teeth (Evans 1993; Dyce et al. 1995). We interpret that the IAN nerve entered the mandible through the mandibular foramen (“Foramen dentale”), and traversed the mandibular canal space along the root tips of the lower teeth (Fig. 7a: mandibular “canal”), through to the symphyseal region of the mandible, in Henkelotherium as in extant mammals. This nerve gave rise to the mental nerves that exited through mental foramina on the lateral surfaces of the mandible (as described earlier), and one branch of the nerve entered the symphyseal region through the symphyseal foramen. The hollowed space, beside the distal half and one-third of the roots (Fig. 7c), was filled with blood vessels, periodontal ligaments, and the associated soft tissues. The angular plexus inside the angular process is connected to the mandibular canal, and to the outside of the mandible via a vascular channel through the angular foramen.
The trabecular spaces associated with the mandibular canal and root alveoli are now documented in the dryolestids Crusafontia and Beckumia (Martin et al. 2022b), and are also newly visualized in Dryolestes here. Dryolestes is similar to Henkelotherium to the extent that the interior trabecular space, in the angular plexus inside the angular process, is anteriorly connected to the confluent space of the mandibular canal for the inferior alveolar nerve, its companion vessels with the periodontal ligament and related soft tissues. However, there are some minor differences: in Henkelotherium, this space is posteriorly connected to the angular foramen, a vascular conduit (Figs. 6 and 7: IAN-PDL space). In dryolestids, the angular plexus is present, but smaller relative to the size of the jaw, and not connected to the outside of the mandible, as the dryolestids examined so far lack an angular foramen.
Antemolar replacement/molar eruption
The several young and juvenile mandibles have made it feasible to establish a growth series and to interpret tooth replacement and eruption, in the context of mandibular growth (Figs. 7, 8, 9, and 11). The youngest specimen (Gui Mam 25/81; Figs. 8e and 11) is represented by the shortest of jaws (preserved length 11 mm) available for this study. This young individual has preserved the deciduous canine and deciduous premolars dp1 through dp4, with a cusp of permanent p1 and cap of permanent p3 (Figs. 8e and 11). This youngest specimen (Gui Mam 25/81) has preserved the two-rooted alveoli for m1 and m2—two root sockets for each tooth (although m1 and m2 are lost), plus an unerupted m3 crown at bell stage inside its crypt (Figs. 8e and 11).
The next juvenile specimen, represented by Gui Mam 44/80 (Fig. 8), suggests that canine, p1 and p3 have already replaced the deciduous canine, dp1 and dp3. However, this specimen has a developing (permanent) p4 in a crypt near the roots of the dp4 (Figs. 7a and 9), suggesting that dp4/p4 replacement had not been completed. We interpret the empty alveoli as for dp2. Under this interpretation, the replacements of dp2/p2 and dp4/p4 would occur in alternate canine/premolar loci of canine, p1 and p3. This suggests that Henkelotherium has a pattern of replacement at alternating premolar positions (p1 → p3, then p2 → p4), similar to that of Dryolestes, and of Zhangheotherium and Maotherium (Martin 1997; Luo and Ji 2005; Mao et al. 2020). Replacement of the canine and premolars at alternate loci appears to be a consistent feature of trechnotherian mammals through to Cretaceous eutherians (Kielan-Jaworowska 1981; Luckett 1993; Martin 1997; Luo et al. 2004). This alternating replacement is in contrast to the sequential replacement of premolars of mammaliaforms, which is interpreted to be plesiomorphic for mammaliaforms (Luo et al. 2004; Schultz et al. 2019).
As revealed by CT scans of the Gui Mam 44/80 specimen (Figs. 7, 8, 9), there is a developing m4 in the thickened bone in the posterior part of the mandibular body. The developing m4 crown has fully formed its trigonid at the bell stage of development (Figs. 7, 8, 9). CT scans have also revealed an empty space interpreted to be the m5 crypt, although an m5 crown is not detected, in this juvenile mandible. The m5 crypt is located inside the coronoid platform and posterior to the m4 bell crown (Fig. 9).
The two juvenile specimens here provide definitive evidence that the replacement sequence of premolars coincides with the successive eruption of m1 through m3; the replacement of dp4 by permanent p4 occurs approximately in synchrony with the eruption of m4. After replacement of all antemolars (incisors, canine and premolars) and eruption of m1–4, the later and successive eruption of m5 through m7 would further add to the molar row, concurrent with a prolonged growth of the mandible along the mandibular length (Fig. 8). The ramification of this mandibular growth pattern and similar pattern in the upper tooth row will be further explored in the “Discussion” section.
Upper dentition
Incisors
The ultimate right upper incisor is the only upper incisor preserved in the holotype specimen (Krebs 1991: 31) (Fig. 17). No other upper incisors are preserved in our study sample. The ultimate incisor is small, and shows a simple main cusp and a small mesial accessorial cuspule. The incisor has a long root (more than four times the crown height), which is curved distally and somewhat linguo-labially compressed (Krebs 1991: 36f). The tooth is associated with a bone fragment, here interpreted to be an incomplete premaxilla (Fig. 17, see also Krebs 1991). However, it cannot be ruled out that this incisor is in the maxilla, because the premaxilla–maxilla suture is not preserved in the type specimen. For the closely related Drescheratherium acutum, one, or possibly two, maxillary incisors have been reported (Krebs 1998: Figs. 1 and 2). Also, in the dryolestid Dryolestes leiriensis, the ultimate upper incisor (I5) is implanted in the maxilla (Martin 1999). The presence of maxillary incisors is a common feature for dryolestidans.
Canine
In the holotype specimen (Gui Mam 138/76), the upper canine crown is partially exposed from the lingual side (Fig. 17a); the labial side is visible through the transparent plastic matrix (Krebs 1991: 37). Our CT visualization of fully adult specimens of H. guimarotae shows that the upper canine has two long and fully divided and divergent roots, as exemplified by Gui Mam 114/77, or a two-rooted canine was indicated by two divided alveolar grooves as visualized for Gui Mam 17/76 (Figs. 19, 20, 21). The distal root is slightly stronger and straighter than the mesial one. In a juvenile (Gui Mam 91/75, with four molars), the crown of the canine has erupted, but the roots are not yet fully formed—this indicates that the full development of roots of the permanent canine occurs in late growth stages when M5 and M6 erupt (Fig. 18).
CT visualization of upper teeth and maxilla of Henkelotherium guimarotae (Gui Mam 17/76). a Lateral view of left maxilla and upper teeth: a two-rooted canine indicated by its alveoli, four premolars, and six molars. The position of missing M2 is indicated by its alveoli; M6 missing but indicated by its lingual root and broken labial root alveoli. b Occlusal view of left upper teeth. c Lateral (labial) view of exposed left upper teeth of Gui Mam 17/76. d Medial (lingual) view of exposed left upper teeth of Gui Mam 17/76
The infraorbital canal system and innervation of the upper dentition in dryolestidan maxillae. a Henkelotherium guimarotae (Gui Mam 177/76) maxilla and infraorbital canal system (the anterior opening of the infraorbital foramina is present, but the posterior opening is damaged). b Dryolestes leiriensis (Gui Mam 43/77) maxilla preserved with the anterior and middle parts of the canal for the superior alveolar nerve, and the canaliculi for the super dental nerve branches and the periodontal plexus
Eruption pattern of upper teeth in Henkelotherium guimarotae (lateral view of left upper teeth). a Gui Mam 17/76—the oldest individual examined in this study with all of its molars worn. b Gui Mam 114/77—a subadult individual with P2 just erupting (breaching the gum line), with fully developed roots of upper canine, and four upper molars. c Gui Mam 91/75—a juvenile, and the youngest individual examined by this study represented by upper dentition. Arrow of ontogeny indicates the successively older individuals of the growth series
The main cusp is acutely triangular, and the crown is tall and laterally compressed with a convex labial side and slightly convex lingual side, as described previously (Krebs 1991). The crown is recurved distally, and its mesial edge is convex and rounded, and the distal edge is slightly concave with a weak crest. The canine bears a small drop shaped apical wear facet that faces distally. On the mesial side and above the crown base, there is an accessorial cuspule which continues into a short cingular ledge onto the labial side. On the distal side sits a tiny accessory cuspule at the cervical end of the vertical distal crest. This cuspule is lingually placed, and slightly more elevated than the mesial cuspule.
Premolars
Krebs (1991: 37f) reported four upper premolars for the holotype specimen. This is now corroborated by new CT visualization (Fig. 17). Three premolars are preserved in situ (P1, P3, and P4), while P2 fell off during preparation and is conserved separately from the main specimen. The P2 position was interpreted to be represented by the gap between P1 and P3 (Krebs 1991: Fig. 3). According to Krebs (1991), P2, as the smallest upper premolar, is single rooted. Krebs’s (1991) interpretation can now be corroborated by our CT visualization revealing the P2 alveolus invisible on the surface, apparently fractured and shifted by a slight fault (Fig. 17c). P1, P3, and P4 have two roots in the holotype specimen, and can be seen from all aspects in the CT visualization.
In the holotype specimen, P1 is exposed only from the labial side. Here, we present supplementary information from Gui Mam 17/76 where all four premolars are preserved and are visible from all aspects. The main cusp of P1 is broad triangular and bilaterally compressed with the labial aspect somewhat more convex than the lingual aspect. The mesial margin is slightly convex with a small mesial accessorial cuspule near the crown base. A short cingular line is extending from the basal mesial cuspule posteriorly onto the lingual side until it fades in the middle. The distal margin of the crown is straight with an indistinct crest that ends in a distal accessorial cuspule at the crown base. The main cusp is somewhat pointing distally, but less so as illustrated by Krebs (1991: Fig. 3). The P1 bears a small apical wear facet which is oriented distally.
In Gui Mam 17/76, P2 is not yet fully erupted and corresponds in size and morphology to P1 except for a more pronounced distal heel that supports the distal cuspule. Differing from Krebs’s (1991) observation on the holotype specimen, P2 of Gui Mam 17/76 has two roots (Fig. 19).
The P3 main cusp is broadly triangular (Figs. 19 and 21). The crown is labio-lingually compressed with the labial side slightly more convex than the lingual side. The mesial margin of the crown is slightly convex and rounded, and the distal margin is concave with a crest which ends in a distal accessory cuspule sitting on a broad base. The main cusp is slightly pointing backwards and bears a small apical wear facet. On the mesial side, there is a small ledge near the crown base with an accessory cuspule. Although the crown of P3 is not yet fully erupted, its morphology corresponds to that of P1 except for a more pronounced distal cuspule. The P3 is twice the size of P1 and the estimated size of P2.
The P4 is the largest upper premolar and is fully erupted in Gui Mam 17/76 and 138/76 (Figs. 17, 19, and 21). The crown is only slightly compressed linguo-labially, and its main cusp is conical and is slightly pointing backwards and bears a small apical wear facet. The mesial margin of the crown is slightly convex in lateral view, and the distal margin is slightly concave with a distinct crest that ends in an accessory cuspule. The distal crest of P4 of Gui Mam 17/76 is not crenulated as suggested by (Krebs 1991). The labial aspect of the main cusp is convex, and the lingual aspect is mostly convex, except that the distolingual side is somewhat concave, but this concave curvature is less pronounced than was previously illustrated for Gui Mam 138/76 by Krebs (1991: Fig. 3). On the mesial margin of the crown base, there is a small ledge with a mesial accessory cuspule and an additional smaller cuspule on the lingual side. On the distal end of the crown base, there is a distinct heel bearing the distal cuspule and additional tiny knots.
Molars
Here, we provide new visual information from the new specimens of our study sample (Figs. 18, 19, 20, 21, 22, 23, 24, 25, 26, 27), beyond the previous illustrations and photographs on lingual or occlusolingual aspect of the teeth for the holotype specimen (Krebs 1991: Fig. 3). In several specimens (Gui Mam 91/75, 138/76, 17/76, 79/77, 9/82, and 114/77) (Figs. 17, 18, 19, 23, 24, 25, 26, 27), the size of the molars increases from M1 to M2; M3 and M4 are the largest, M5 corresponds in size to M2, and M6 would be the smallest as indicated by its small socket in the holotype and very small root in Gui Mam 17/76. In proportions, M1 is relatively the narrowest (labio-lingually) and longest (mesio-distally); M2 to M5 are increasingly wider and shorter. In five of the six examined specimens with upper molars, the parastylar corner of each succeeding molar overlaps the distolabial corner of the preceding molar, such that the upper molars form staggered contacts between the adjacent molars (Figs. 18, 23, 24, 25, 26, 27). The paracone of the more posterior molars (especially M4 and M5) appears to be slightly inclined anteriorly (Figs. 15, 20, 21, 23, and 25). We note that this staggered contact between upper molars and the changing orientation of the paracone constitute a morphological gradient along upper molars in many dryolestidans (Prothero 1981; Martin 1999), a distinctive feature of dryolestidans (Fig. 27).
Progression of molar wear pattern with age in Henkelotherium guimarotae. a Gui Mam 114/77 (left upper M1–M4, SEM photo). The youngest individual with upper molar series (only M1 and M2 show beginning stage of tooth wear along the preparacrista and postparacrista). b Gui Mam 79/77 (left upper M3–M5 SEM photo) shows beginning wear along preparacrista and postparacrista on M3–M5. c Gui Mam 9/82 (SEM photo of right upper M1–M4, and M5 position represented by alveolus, left–right flipped for comparison) illustrates significant wear on preparacrista and postparacrista on M1–3, and beginning wear on M4. d Gui Mam 17/76 (M1, M3–M5, and M6 position indicated by alveoli (CT visualization), the oldest available individual among specimens with upper teeth—all teeth through M5 are heavily worn
Gradient of variation of upper molars in Dryolestida. a Dryolestes leiriensis (Gui Mam 43/77) upper tooth row preserved in the maxilla: arrows indicating imbrication of a succeeding molar over the distolabial corner of its preceding molar; the curved arrow indicating medio-lingual rotation of the more posterior molars. b Curved roots of the posterior molars (M4-M6 and M7 roots of Gui Mam 43/77). c Henkelotherium guimarotae (Gui 17/76, left–right flipped for comparison) shows a similar pattern of imbrication, and mesio-lingual rotation of posterior molars relative to anterior molars. d Curvature of roots of the posterior molars (M4, M5, and M6) in Henkelotherium specimen Gui Mam17/76 (M3, M4, M5, and M6 roots). e Comotherium richi—as example of imbrication and rotation of the successively posterior molars in other dryolestidans (from Prothero 1981: Fig. 3, re-used with permission from D. R. Prothero)
Of the primary trigon, the paracone is the largest and highest cusp, followed by the stylocone and metacone (Figs. 2 and 22). The paracone is robust, and has a smooth, rounded lingual side that bears a slight bulge near the crown base on M2 and M3 (Figs. 18, 19, 22, 23, 24, 25), but this bulging can be indistinct or absent in other molars. The stylocone sits near the labial end of the preparacrista in the anterior molars (M1-2), and along the labial side, it marks the anterior end of the labial cingulum (Figs. 2a–d and 22a). In the more posterior molars, beginning with either M3 or M4, the stylocone is slightly shifted in lingual direction and no longer directly connected to the labial cingulum (Figs. 2h–i, 23, 24, 25, 26). In M4, the stylocone may develop a short cingular crest reaching towards the anterolingual aspect of the crown. In M5-6, the stylocone is shifted further in lingual direction, and becomes more separated by a curved groove around the labial side of the stylocone from the periphery of the tooth crown. In M4 and M5 of Gui Mam 79/77, the stylocone is strongly worn (Fig. 23). The preparacrista connects the tip of the paracone with the stylocone and borders the primary trigon mesially (Fig. 2a–i). It is strongly worn with dentine exposure in M1 (e.g., Gui Mam 9/82) and successively less worn in the more posterior molars. The postparacrista connects the paracone with the metacone which is more of a swelling on the postparacrista than a cusp and is mesio-distally compressed (Figs. 2a–h and 22a). The crest continues as metacrista to the metastyle which is shifted labially. Within the primary trigon basin, a bulge-like median ridge is running from the paracone in labial direction, and fades before reaching the labial edge of the crown (Fig. 2a–h). The median ridge varies somewhat in expression among different specimens, and is most strongly developed—almost crest-like—in the M4 of Gui Mam 79/77 (Fig. 22). The labial border of the crown is straight, except for the M5 of Gui Mam 79/77 where it exhibits a weakly developed ectoflexus (Fig. 22a). The labial cingulum bears a tiny mesostyle in the middle between stylocone and metastyle (Fig. 2a–h), and this small cusp can vary in size and expression between specimens. Close to the metastyle, a minute stylar cusp D can be variably present (e.g., in M3) (Fig. 2g). The parastylar lobe is well developed and separated from the labial cingulum in M1 (Fig. 2a, b). However, in the more posterior molars, it becomes more fully connected to the labial cingulum (Fig. 2c–h). The parastylar lobe and its cusp (if present) are larger and can bear strong apical wear in M1 through M3, but become much smaller in M4 and M5 (Figs. 23, 24, 25, 26).
The upper molars have three roots (Figs. 2, 4a, d, and 5), a lingual root supporting the paracone, and a mesiolabial root supporting the parastyle, as well as a distolabial root supporting the metastyle. The lingual root is the strongest, and the mesiolabial and distolabial roots are somewhat smaller (Figs. 2, 4a, d, and 5). The roots of M1 and M2 are separated from each other. However, on M3–M5 of Gui Mam 17/76, the three roots are connected by Y-shaped dentine ridges in the cervical region. The Y-shaped dentine ridges are variable in strength in different molars, and are best developed in the middle molars (M3, M4, and perhaps also M5), and also in older specimens with more fully erupted molars. By comparison, in Dryolestidae, these Y-shaped ridges between the upper molar roots are more strongly developed and more uniformly present along the molar series. In more derived taxa of the Dryolestidae, the labial roots can even be fused in posterior upper molars (Fig. 5), but in all Henkelotherium specimens, the mesiolabial and distolabial roots are always fully separated (Figs. 5a–d and 19). In Henkelotherium and several dryolestids (including Dryolestes), the lingual root is curved mesially, and this curvature is related to the successive rotation of the posterior upper molars (Fig. 27).
Molar wear pattern
In Gui Mam 9/82, the upper molars are strongly worn (Figs. 24 and 26). The paracone bears a well-developed apical wear facet with dentin exposure on M1, whereas the paracone apices of M2-4 are only slightly worn on M2 and hardly worn on M4. The preparacrista is strongly worn only in M1; in M2–M4, it exhibits only slight-to-moderate wear. Wear on the postparacrista/metacrista is much stronger with dentin exposure than on the preparacrista in the molars on Gui Mam 9/82 (Figs. 24 and 26). The wear of the postparacrista and the metacrista extends along their entire length (metacone and metastyle completely worn off) in M1 and M2. This wear facet on M1 is so much developed that it further extends onto the parastyle of M2, shearing off the M2 parastylar cusp (Figs. 24 and 26). The preparacrista and stylocone of M2 are only moderately worn. Although the postparacrista/metacrista of M2 are strongly worn, the parastylar cusp of M3 exhibits only a small apical wear facet. The same wear pattern occurs between the distal side of M3 and parastyle of M4.
Gui Mam 114/77 and 79/77 (Figs. 22, 23, 25, and 26) are two relatively younger maxillary specimens as indicated by their molar eruptions (Fig. 26), with the preparacrista slightly-to-moderately worn on M1, and to a lesser extent also on M2. On the more posterior molars, which presumably erupted later than M1–2, the preparacrista is unworn. The wear of the stylocone is stronger in M3 and M4 of Gui Mam 79/77, whereas in Gui Mam 114/77, it has only a tiny apical wear facet in the same molar positions. On the postvallum wear pattern in the molars of Gui Mam 114/77 (Fig. 26), the metacone is moderately worn in M1–2. This cusp is worn enough that it becomes connected to the postparacrista/metacrista in M1-2, and the wear is more developed in M1-2, than in M3-4. The paracone is worn in the M1 of both Gui Mam 79/77 and in Gui Mam 114/77 (Figs. 2, 22, 23, 25, and 26), but this cusp is less worn on M2-4 (or at all on M4) that would follow much later in tooth eruption (only tiny apical wear facet in M4). The parastyle exhibits wear in the M1 in both specimens, although less worn in Gui Mam 79/77 than in Gui Mam 114/77. In the more posterior molars, it is unworn. The metastyle exhibits slight wear on the M1 of Gui Mam 114/77, whereas in the other molars of both specimens, it is without noticeable wear. This suggests that the molar wear can have intraspecific variation.
In Henkelotherium (as in dryolestids), the upper molars form an imbricating pattern, such that the parastylar lobe of the succeeding molar overlaps the distolabial side of the preceding molar (Figs. 18 and 27). The wear continues to develop on the postvallum of the preceding molar, and the postvallum can form a continuous wear facet with the parastyle, and even the entire parastylar lobe (e.g., between M1-M2 of Gui Mam 9/82) (Fig. 24). This extensive wear surface contiguous across adjacent upper molars can match the prevallid crest of the lower molar. Therefore, we infer that the imbricating interlock of the upper molars has a masticatory function (Fig. 27).
Maxillary alveolar structure for upper teeth
The maxilla of Gui Mam 17/76 (Figs. 19 and 20a) contains the infraorbital canal system. The infraorbital canal system in the maxilla is here interpreted to have two external (facial) openings: the well-preserved anterior opening shows a circular outline and is located above P3 and P4 and between these two premolars. The posterior opening is broken but is here interpreted to be located above M2. Both these openings are identified by comparison to a well-preserved maxilla specimen of Dryolestes leiriensis (Gui Mam 43/77) (Fig. 20b). Although the maxilla (Gui Mam 17/76) is incomplete and distorted by fractures, we interpret that the infraorbital canal in the maxilla has two facial openings for Henkelotherium, similar to that of Dryolestes (Fig. 20).
Of the two external openings of the maxilla in Dryolestes, one opening is posteriorly positioned above M2, which we identify to be the posterior opening of the infraorbital canal (Fig. 20b). This is equivalent to the single infraorbital foramen in therians, as it corresponds to the common location of this foramen in extant therians (Evans 1993; Dyce et al. 1995; Benoit et al. 2020). This is equivalent to the “infraorbital foramen 2” as previously interpreted for Morganucodon (Kermack et al. 1981), and more recently re-interpreted by Benoit et al. (2020) to be the only opening that carried the infraorbital nerve, as seen in extant therians. The other facial opening in the maxilla of Dryolestes is more anteriorly positioned, above P4 at the level of the P3-P4 junction (Fig. 20b). This foramen is much larger than the aforementioned “posterior” infraorbital opening, and almost twice the size of the latter. This large foramen indicates that a major nerve branch through this foramen innervated the facial part of the skull, in addition to the infraorbital nerve branch through the posterior opening. The anterior opening corresponds to the “infraorbital foramen 1” of Morganucodon as identified by Kermack et al. (1981). But more recently, this foramen has been re-interpreted by Benoit et al. (2020) to be the foramen of the rostral alveolar nerve (= superior anterior alveolar nerve in Fig. 20b). The large openings for the infraorbital nerve have been interpreted to indicate tactile sensory acuity for placental mammals (Muchlinski and Kirk 2017).
We have visualized the connecting network of canaliculi of the rostral alveolar nerve canal (= superior anterior alveolar canal in Fig. 20b). These are clearly connected to tiny canalicular network around the roots of the anterior molars and posterior premolars, likely for the branches of the superior middle alveolar nerve (and its associated vessels) to these upper teeth. The channel of the superior middle alveolar nerve (and its associated vessels) for the posterior premolars is separated from the channel for the superior anterior alveolar nerve (and its vessels) for the incisors, canine, and anterior premolars (Fig. 20b). Each premolar has two alveoli in the maxilla, and each three-rooted upper molar has three alveoli in the maxilla. The alveolar sockets of the more posterior molars are successively deeper, corresponding to successively longer roots in these posterior molars—M4–6 have the longest roots. The roots of the last two molars are twisted in both Henkelotherium (Fig. 19) and Dryolestes (Fig. 20b), with the larger lingual root bent anteriorly. The curvature of bending increases from the penultimate to the ultimate molar (Figs. 20b and 27b).
In the well-preserved maxilla of Dryolestes (Gui Mam 43/77) (Figs. 20b, 27a, and b), the bony walls of the alveolar sockets in the maxilla are trabeculated and associated with a network of interconnected small channels that are dorsally connected to the larger canals of the infraorbital canal system in the maxilla (Fig. 20b). The nerves and vessels in these trabecular bones provide innervation and vascular supplies for the roots of the upper teeth. The two maxillary specimens of Henkelotherium (Gui Mam 91/75 and 17/76) are incomplete, but enough of their maxillary tooth alveoli are preserved to show some resemblance to the trabecular characteristics in a better-preserved maxilla of Dryolestes (Gui Mam 43/77). We interpret that Henkelotherium has a similar network of trabecular bone and channel network in the maxilla to innervate and supply the upper teeth.
Lower dentition
Incisors
In the holotype specimen of Henkelotherium (Gui Mam 138/76), no lower incisors are preserved (Krebs 1991: 40). Our description of the incisors is based on the left mandibles Gui Mam 133/77 (Figs. 1 and 6), and Gui Mam 44/80 (Figs. 7 and 8). In both specimens, four lower incisor loci are present (Figs. 1, 6, 7, 8). The root of i1 is preserved in Gui Mam 133/77, and in Gui Mam 44/80, the alveolus of i1 is preserved. The first incisor (i1) is procumbent and inserted about 45° to the long axis of the mandible. The i2 to i4 are implanted successively more steeply into the mandible, and i4 is almost vertically oriented in the mandible. The incisors are set apart from each other by small interdental gaps at the alveolar apertures of the single-rooted incisors. The incisor roots are long, about three-to-four times the crown length. The i2 and i3 have stronger curvature, but i1 and i4 are less curved. The cross-section of the roots is oval as the roots are mesio-distally compressed. The incisor crowns are spatulate with a single cusp. The labial side is convex and the lingual side is slightly concave, and the main cusp is slightly recurved distally. There is a faint lingual cingulid, which is curved and separates the concave lingual aspect from the convex aspect of the crown. On the distal side of the crown and near the crown base, there is a tiny bulge, which is cuspule-like. A faint crest connects the main cusp with that bulge.
Canine
In the holotype specimen of Henkelotherium, the labial aspect of the right lower canine is visible through the plastic matrix of the transferred specimen, whereas the lingual aspect is not exposed (Krebs 1991: 40). Here, we visualize this lower canine by CT cans (Fig. 17), and further describe the details of the canine, based on additional specimens (Gui Mam 133/77, 48/78, and 65/77) (Figs. 6, 9, 12, 13, 14). The crown of the canine is high triangular and labio-lingually compressed. Both the labial and the lingual sides are convex, and the labial side is more so. The lingual cingulid is distinctive and runs posterio-ventrally and then fades away at two-thirds of the crown length. The crown is slightly recurved distally with a gently convex mesial margin that ventrally joins the lingual cingulid. The distal margin is slightly concave and bears a faint crest. The apex of the main cusp shows a tiny round wear facet facing linguodistally. The distal crown base has a bulge-like swelling.
The canine has two large roots of which the mesial root is slightly larger. Both roots are recurved distally, but the mesial root is more curved than the distal root and the distal root extends below the mesial root of p1 (Fig. 9a). The much smaller deciduous canine has almost straight roots, while the adult canine roots are strongly curved. This indicates that the curvature of the roots may be dependent on the growth stage of the mandible (Fig. 11).
Premolars
The Henkelotherium holotype specimen possesses four lower premolars (p1–4) of which p4 is the largest, p3 is the second largest, followed (in decreasing size order) by p1 and the smallest p2 (Krebs 1991: 43). In the holotype specimen, right p3 and p4 are exposed from the lingual side, but the lingual sides of p1 and p2 are covered by bone fragments (Fig. 14). The labial sides of all premolars are embedded in plastic and are only visible from the backside of the transparent plastic. By CT visualization of the holotype, we have corroborated Krebs’s (1991: 43) observations on the number of premolars and the morphology of the lingual sides of p3 and p4. With new observation on additional specimens (Figs. 1, 6, 8, 9, 13, 14, 15), we are adding information on all aspects of the premolars, as well as their gradient of variation.
The premolars are linguo-labially compressed, and in side views, their crowns have a triangular profile. The mesial margin forms a crest that extends ventrally and ends in a tiny accessory cuspule at about half of the crown height. But dorsally, the crest is gently convex anteriorly, and fades out to become rounded surface in the apical part of the crown. The distal margin is gently concave, and the distal crest is more pronounced than the mesial crest. The tip of the main cusp is oriented distally and bears a tiny distally facing apical wear facet, in all premolars (Gui Mam 133/77, 48/78, 65/77, and 78/76). The mesial accessory cuspule is relatively larger in p1 and p2 than in p3 and p4. From the mesial cuspule, a lingual cingulid extends in cervico-distal direction, connecting to the distal accessory cuspule. The lingual cingulid is sharp and best developed in p4 and p3; in p1 and p2, it is less distinct and not sharp. The distal accessory cuspule is present in all four premolars, and on p4 and p3, it forms on a distal heel, which bears a short labial cingulid extending onto the labial side of the tooth. This distal accessory cuspule is less distinct on p1 and p2. Apart from a short labial cingulid at the distal heel in p3 and p4, the convex labial aspect of the crown is smooth without cingulid. The lingual side of the main cusp is mostly convex, except for a small and slightly concave distal area.
The p4 is the largest premolar and p3 is the second largest in all studied specimens. However, there is some size variation in p1 and p2. The p1 and p2 are of equal size in Gui Mam 133/77 (Figs. 1 and 6), and p1 is much smaller (about half size) than p2 in Gui Mam 48/78. However, in Gui Mam 65/77, the p1 is somewhat smaller than p2. The overall size of p1 and p2 of this specimen is atypical of Henkelotherium, as is its retention of a dp4 in adult stage (to be described below).
All premolars possess two roots of equal size, and the roots diverge slightly in cervical direction, as first noted by Krebs (1991: 43) and visualized by CT scanning of additional specimens (Figs. 6, 9, 13, and 14). The adjacent premolars are not in contact but separated from each other by small interdental gaps. In Gui Mam 78/76, 65/77, and 133/77, the premolars are evenly spaced, but there is a relatively large gap between the very small p1 and p2 in Gui Mam 48/78 (Figs. 1 and 6). There is also a space between p1 and c in all specimens for accommodation of the upper canine for full occlusion of the canines.
Molars
The holotype specimen has seven lower molars, but m7 is much smaller than the preceding molars and has only one root (Krebs 1991: 45; further corroborated by CT scanning) (Fig. 17). All other lower molars have two roots of equal size which are compressed mesio-distally and have an oval cross-section (Figs. 3, 4, 6, 9, and 13). Seven molars are the highest molar count that we observed in all of the studied specimens. In the holotype, the molars are only visible from their lingual side, the labial side is embedded in the matrix, and also, the occlusal aspect is obscured. We supplement the observation by Krebs (1991) with the newly studied mandibles, several of which are visualized by CT scans and SEM photos. In Gui Mam 138/76 (holotype) and Gui Mam 133/77, 78/76, and 48/78, m1 differs in proportions and size from the other molars in being relatively narrow linguo-labially and relatively long mesio-distally (Figs. 6, 13, and 15). However, this difference is less pronounced in a single exception of Gui Mam 65/77 (Fig. 14). The m3-4 are similar in morphology, proportions, and large size in all specimens. In Gui Mam 133/77, this applies to m2-4, and in Gui Mam 78/76 to m3-5. The m5 is slightly smaller than m2-4 in Gui Mam 48/78 and 65/77, and much smaller in Gui Mam 133/77. All m5s are double-rooted. The m6s are double-rooted, but the two roots are fused about half of their root length (Figs. 13 and 14). Because the roots are fused near the crown-root junction, the alveolus appears to be single (instead of two divided alveoli). Our CT visualization has detected the crypt of an m6 as it is budding from the mandibular canal (Fig. 6). In the other older mandibles (such as the holotype), posterior to m6, there is a small m7 with a single root, or the m7 is indicated by a single alveolus.
The lower molar crown consists of the three trigonid cusps, the protoconid, the paraconid, and the metaconid, plus a single-cusped talonid (the hypoconid) (Fig. 3). The trigonid cusps are relatively compact with blunt apices; the highest and most robust cusp is the protoconid, followed by the metaconid and paraconid. The hypoconid is the smallest cusp and is situated in a lingual position in m1–4 (Fig. 3b, c, f), but this cusp is shifted to a median position in m5 and m6 (Fig. 4).
The large protoconid is pyramidal in shape, and has a convex labial side with mesiolabially and distolabially facing flanks. The labial side is somewhat bulging just above the crown–root junction. The distal and lingual flanks are slightly concave. The protoconid is erect and two crests (paracristid and protocristid) run down from its apex. The paracristid runs in a mesio-lingual direction and connects to the paraconid, and the protocristid runs in a lingual direction and connects to the metaconid. The metaconid is the second-highest cusp and has a triangular cross-section. The lingual and mesial flanks are slightly convex, the distal flank is flat. Apically, the metaconid bears a wear facet with exposure of dentine that extends onto the protocristid. The metaconid protrudes slightly lingually in m3–5, but not so in the anterior and posterior molars. The paraconid is the third-largest cusp, shovel-like, and inclined mesially (~ 50°), called “procumbent” by other researchers of paurodontids (e.g., Prothero 1981). Its lingual flank is slightly convex, the mesial flank is flat, and the distal flank is slightly concave. The paracristid bears a large wear facet with exposure of dentine. The paraconid protrudes lingually beyond the metaconid and the talonid. The trigonid basin slopes mesially and is mesially opened by a v-shaped notch between paraconid and metaconid. The distal metacristid is indistinct.
The talonid is a mesio-distally short heel with a single cusp (hypoconid) that sits on the distal rim of the talonid. The heel is low and does not protrude lingually. The hypoconid is situated on the lingual side in more anterior molars (Fig. 3b, c, f). It is distally rounded. The talonid extends across two-thirds of the linguo-labial width of the crown and its distal border swings back in a gently s-shaped curve in a labial direction. Distally, the heel is bordered by a faint crested rim.
The lower molars are double rooted with a mesial and a distal root (Fig. 4b, c). Both roots are of equal size and are mesio-distally compressed, with an oval outline in horizontal section close to the root–crown junction, but the mesial root may show a longitudinal groove on its posterior (inter-radicular) surface. The mesial root supports the paraconid, and the distal root supports the protoconid, metaconid, and hypoconid. The roots are long relative to the crown height. The lengths of the lower molars correspond to depth (height) of the mandibular ramus. All molar roots are open at their distal ends, even in the anterior molars of older individuals (e.g., the holotype specimen). We further note that the roots of the posterior molars can fuse, and this fusion shows a gradient of variation along the molar row (more on this below). Henkelotherium is similar to other paurodontids in having two roots of equal size (Butler 1939; Averianov and Martin 2015), but very different from dryolestids that show a massively more expanded mesial root than the distal root of the lower molars (Figs. 4 and 10).
Variation of molar characteristics
As mentioned above, the molars exhibit considerable variation in several characters in a gradient along the tooth row. The first noteworthy gradient feature is that the long upper molar row becomes curved from the slight lingual rotation in the posterior molars and by the imbrication of all molars (Figs. 18 and 27). The imbrication of the molar series is formed by the parastylar lobe of each succeeding molar overlapping the distolabial corner of the preceding molar (thin arrows in Figs. 18 and 27). Along the row of staggered molars, the imbricating molars in posterior positions are also successively more rotated. The anterior molar(s) are transversely oriented and also have a more triangular outline than the more posterior molars, that have a mesio-distally compressed outline. The lingual cusp is rotated mesio-lingually in the last two or three molars (Fig. 27: curved arrow). As a consequence of these changes in tooth shape, orientation, and size, the postvallum surface of a molar is slightly more transverse in M1, but the postvallum surface is more oblique in the posterior molars (Fig. 27a). Furthermore, the rotation of the posterior molars is accompanied by mesio-lingually curved roots, and the curvature is more exaggerated in the lingual root, especially on the last two or three upper molars (Fig. 27). We note that this pattern of a curved molar row by molar imbrication and rotation is present in many dryolestidans with longer premolar–molar row (Figs. 5 and 27).
The second noteworthy variation is that the tooth size increases from m1 to the middle of the lower molar row and then decreases from the middle molars to the ultimate molars. Lower m1, m2, and m3 show a gradient of size increasing posteriorly. Lower molar 5, 6, or 7 (if the latter is present) show a gradient of size decreasing posteriorly in the three specimens with six molars erupted (Figs. 6, 13, 14, 15). The most mesial (m1) and the ultimate molars (m5, m6, or m7 if present) are linguo-labially narrower than the molars in the middle of the molar row (m2–m4). Usually, the teeth in the middle of the molar row (m3–m4) are the widest, and also have taller trigonids, than either the anterior (m1, m2) or the posterior (m6, m7) molars. For example, m1 and m6 are the narrowest molars, while m2–4 are wider in Gui Mam 133/77 (Fig. 6), in Gui Mam 48/78 (Fig. 13), and in Gui Mam 78/76 (Fig. 15). In the case of Gui Mam 65/77 (seven molars erupted, with m7 represented by alveolus only), m1 and m6 are also the narrowest teeth (Fig. 14). In overall size (length x width), either m3 or m4 (or both) are the largest molars, followed by m2, then m5 (in Gui Mam 78/76 m5 is the third-largest), m1, m6, and m7 representing the smallest (preserved only in the holotype specimen, see Krebs 1991) (Figs. 3, 6, 13, 14, 15).
The paracristid of the trigonid shows a prominent gradient variation in size and shape along the molar row. For example, in m1 and m2 of Gui Mam 78/76 (Fig. 15), the paraconid-crest and the protoconid-crest of the paracristid form an obtuse angle at the v-notch between the protoconid and the paraconid. The protoconid-crest is almost longitudinally oriented, while the lateral crest of the paraconid is oriented disto-labially (Fig. 15). Due to this angle in the paracristid, the trigonid appears to have a nearly rectangular outline in the occlusal view of m1 and m2. By contrast, the protoconid-crest and the paraconid-crest of the paracristid form a straighter alignment in the successively more posterior molars, from m5 towards m6 or m7.
Orientation of the protocristid between the protoconid and metaconid is transverse in m1 and m2 (Fig. 15). However, on m4 and the more posterior molars, the protocristid becomes successively more oblique to the long axis of the tooth (Fig. 15). Related to a more oblique orientation of the protocristid and the increasingly straighter paracristid, the triangle of the trigonid becomes wider in the successively more posterior molars, in contrast to the nearly rectangular trigonid outline of m1 and m2 (Figs. 3 and 15).
The shape and position of the hypoconid on the talonid also show significant gradient variation along the molar series. In the anterior molars, the hypoconid is a more pointed cusp and positioned closer to the lingual wall of the talonid (Figs. 3 and 16). However, in the successively more posterior molars, the hypoconid is wider, more obtuse and crescentic, and its position is shifted more labially to the middle point along the width of the talonid (Fig. 3 and 16). Compared to the lingually positioned hypoconid on m1 and 2, this cusp is slightly shifted labially in m3–5, and this cusp is in a median position in m6–7 (Fig. 3h, i). Thus labio-lingual placement of the hypoconid can change from the anterior to the more posterior molars.
The degree of division or the fusion of roots also show an obvious variation gradient along the molar row. The two roots can become partially fused in proximal parts of divided roots, or even completely fused. This partial fusion can occur in posterior molars, beginning with m5. The roots are fused by proximal one-third of the length in m5. However, the fusion of roots can develop along the full length of both roots in m6 and m7, as corroborated by CT scans of the holotype specimen. The increasingly greater development of fusion of the roots in more posterior lower molars is a common characteristic of Mesozoic mammaliaforms (Parrington 1973; Kermack et al. 1973; Gill 2004; Panciroli et al. 2019; Luo et al. 2022).
Molar wear pattern
The wear pattern on the lower molar row is variable, and in most mandibles, the wear development follows the eruption sequence of the molars, although this pattern is not uniform. In Gui Mam 48/78 and 65/77 (Figs. 12, 13, 14), m1 exhibits the strongest wear and the more posterior molars are successively less worn. This posteriorly decreasing gradient is particularly clearly developed in Gui Mam 65/77 and 78/76 (Figs. 14 and 15), where m1 exhibits moderate wear and the following molars are less and less worn, up to the unworn m6 (only the paraconid and the hypoconid are preserved on this tooth, both are unworn).
In Gui Mam 15/77 and 48/78 (Figs. 12, 13, and 16), m4 and m5 exhibit moderate-to-slight wear. The m1 exhibits the strongest wear, particularly on the paraconid, and the protoconid is almost equally (moderately) worn on m1–5; m5 exhibits only slight wear on the paraconid and metaconid. The ultimate m6 is unworn and shows only minute apical wear facets on the trigonid cusps. The talonid is slightly worn on the labial side of m3–5, and is unworn on m6. In Gui Mam 15/77, the labial side of the talonid is moderately worn on m1 and slightly worn on m2; in Gui Mam 48/78, this area is not visible in m1–2, obscured by sediment and glue.
The molar wear can show individual variation among the specimens. On Gui Mam 133/77, an otherwise very well-preserved mandible (Fig. 6), the wear pattern of the molars is more heterogeneous and does not show strictly an antero-posterior gradient. In m1, the paraconid and metaconid are worn down to the base, whereas the protoconid is only moderately worn apically. The talonid is strongly worn along the entire length of the hypoflexid groove. On m2, the paraconid is extremely worn and an oblique groove is developed which resembles the hypoflexid groove, but is on the mesial side of the tooth. The trigonid cusps are more heavily worn, and one-third of the metaconid is worn away apically. The protoconid has a moderately sized apical wear facet. The wear on m2 seems to be more extensive than on m1. The talonid is only moderately worn on the labial side. On m3, the trigonid cusps are moderately worn, but the hypoflexid groove is strongly worn away. On m4, the proto- and metaconid are slightly worn apically, but the paraconid and hypoflexid grooves are strongly worn. On m5, the protoconid is apically moderately worn, the paraconid and metaconid are slightly worn, and the talonid is unworn.
Gui Mam 78/76 also exhibits a heterogeneous wear pattern, of which the strong wear on m6 (much stronger than on m1 and m2) is most remarkable (Fig. 15). In m1 and m2, the main trigonid cusps are only moderately worn. The talonid shows a tiny wear facet on its labial side on m1 and a small wear facet in the same location on m2. On m3, the protoconid and metaconid are worn down to the deepest point of the v-shaped notch between them. The paraconid is strongly worn along its entire length. The talonid has only a moderately large wear facet on the labial side. On m4, protoconid and metaconid are strongly worn, but less than in m3. The paraconid is more strongly worn than on m3. The talonid has a large wear facet on the labial side which extends along the hypoflexid groove to its lingual side. On m5, protoconid and metaconid are moderately worn, and the paraconid is strongly worn, more so than on m4. Talonid and hypoflexid grooves are strongly worn. On m6, the protoconid and metaconid are worn down to the level of the v-shaped notch between them. The paraconid is strongly worn, and the talonid has a large labial wear facet that does not reach its lingual side.
Upper deciduous teeth
Deciduous canine
The only specimen with deciduous upper teeth is Gui Mam 91/75 (Fig. 18). The left maxilla shows the erupting permanent canine. Its crown is almost fully erupted, but its roots appear to be incomplete. The specimen has preserved P1, dP2, alveoli for P3, dP4, M1–3, and alveolus for M4.
Deciduous P2 and P4
The dP2 has shorter roots and a smaller distal root, than the permanent P2. The dP4 is highly distinctive in that its distolabial root and the lingual root have fused with each other, very different from most molars with three fully divided roots. The deciduous P4 crown is molariform but 15% shorter in length, and 10% narrower in width, than M1. Furthermore, the crown of dP4 is mesio-distally longer and labio-lingually shorter than the crowns of M1–3. Other differences are a prominent parastylar lobe (mesio-distally more elongate), lingually deflected from the labial side of the crown. The dP4 shows a shallower median ridge than M1. Its crown is more strongly worn than M1, and dP4 bears strong wear on the paracone, stylocone and the prevallum, and on the metacone which is worn down to its base.
Replacement sequence of upper antemolars
We infer that Henkelotherium has alternating mode of eruption of canine and premolars. This interpretation is based on the specimens Gui Mam 91/75 and 114/77 (Figs. 18 and 21). In the Gui Mam 91/75 specimen, the permanent canine is in a late stage of eruption, but its roots are not yet fully formed. We interpret that P1 is the permanent tooth as indicated by its straight and robust roots. This tooth is fully erupted. The deciduous status of dP2 is determined by its short and curved roots (more so than those of permanent P1), and dP2’s two roots are equal in diameter, unlike P1 and P3 that have a thicker mesial than distal root. Two other distinctive deciduous characters are a lower crown in dP2 than in P1, and presence of a low cingulum along the lingual side that is not present in permanent P2s of older specimens (Fig. 18). The P3 crown is not preserved, but our CT visualization of its two root alveoli is similar to P1 of Gui Mam 91/75. The dP4 is clearly a deciduous tooth by its unusually larger and longer parastylar lobe on a more heavily worn crown, plus a fusion of the lingual root with the distolabial root, different from all permanent molars.
From the evidence that Gui Mam 91/75 has four molars, we can conservatively interpret that C, P1, and P3 are permanent teeth of their respective loci, and dP2 and dP4 are still in place in Gui Mam 91/75 after M4 is already erupted as indicated by the M4 alveoli (Fig. 18). By comparison (Fig. 21), in older specimens of Gui Mam 114/77 (with five upper molars) and 17/76 (with six upper molars), replacement of all four premolars has progressed further than in Gui Mam 91/75. In Gui Mam 114/77, P1 and P3 are fully erupted, P2 is in the process of eruption, and P4 has almost fully erupted and replaced dP4 (Fig. 21b). The P2 is the last upper premolar to erupt, shortly after P4, coinciding with the presence of M1-5 in the same individual. The maxillary teeth of Gui Mam 114/77 indicate that the antemolar replacement is largely finished during the eruption of M5. This helps to establish the timing and sequence for upper teeth eruption. The oldest specimen Gui Mam 17/76 (preserved with full maxillary teeth with four premolars and six molars, with m6 indicated by its alveoli) corroborates that Henkelotherium has alternating replacements of canine and premolars, and that the premolar replacement is completed after the stage of four molars, and before full eruption of M5 in the maxilla.
Lower deciduous teeth
Deciduous lower canine
Gui Mam 25/81 (Figs. 8e and 11) is the ontogenetically youngest specimen. Its deciduous canine (dc) and dp1–dp4 are in place, together with m1 and m2 as evidenced by the m1 alveoli and the m2 alveoli, plus an almost complete crown of m3 at bell stage, inside the m3 crypt (Fig. 11). The dc has an obtuse-triangular shape in lateral profile; the anterior margin of the crown bears a crest, which is straight near the base and recurves distally near the apex. The distal margin is straight near the apex and convex towards the base, with a faint crest. The labial side of the distally recurved main cusp is convex, and the lingual side is separated by a slight vertical bulge in a mesial and distal shallow concave area. A faint lingual cingulid is present, and runs distally from the mid-height of the mesial margin, and then fades away near mid-length of the crown. On the distal end of the crown base, there is a well-developed accessory cuspule which is not present in the permanent canine.
The deciduous canine is half the size of the permanent canine (Fig. 11c), and its main cusp is much lower and mesio-distally narrower than the tall crown of the permanent lower canine with acute-triangular shape. The deciduous canine has two roots of equal size, only slightly curved, in contrast to the hypertrophied and strongly curved roots of the permanent canine of the Henkelotherium adult (Fig. 11c). However, it should be noted that the specimen Gui Mam 65/77 has a smaller permanent canine that other adult specimens (Fig. 14: Box e–g).
Deciduous lower premolars
The dp1 is the smallest deciduous premolar and has a premolariform crown. Its main cusp is low and shows an obtuse-triangular shape in lateral view (Fig. 11). The anterior margin of the main cusp is straight, with rounded crest, and the distal margin is slightly concave and bears a faint crest that ends in a very distinctive distal accessory cuspule at the crown base. The labial side of the main cusp is convex, the lingual side demarcated by a low, vertical bulge that separates two slightly concave areas anteriorly and posteriorly. The lingual side bears a faint cingulid that extends distally from the mesial end of crown, and fades out near one-third of the crown length.
The dp2 is similar in shape and proportion to dp1, but about 1.5 times larger. The well-developed distal accessory cuspule bears an apical wear facet. This deciduous tooth has two roots of which the distal root is more robust than the mesial one.
The dp3 and dp4 are much larger than dp2, such that the deciduous premolars form a size gradient (dp1 < dp2 < dp3 < dp4). The dp3 and dp4 are molariform and wider than the bilaterally compressed crowns of dp1 and dp2 (Fig. 11a). The dp3 and dp4 have full trigonids formed by the protoconid, paraconid, and metaconid, and a talonid with a single cusp (the hypoconid). The dp3 exhibits a mesio-distally elongate trigonid lingually wide open. The protoconid is the largest (although relatively low) cusp and is recurved distally; the paraconid is the second-largest cusp, it is also low (not erect) and about 45° procumbent mesially. The metaconid is the lowest trigonid cusp, is slightly inclined distally, and bulges lingually. The labial side of the trigonid is smooth, without a cingulid, but shows a slight bulge extending from the paraconid along the crown base in distal direction and fading out in the middle of the tooth. The talonid heel has a triangular shape, sits in a lingual position, and has a single, pointed hypoconid. The talonid is longer than those in dp4 and in the molars. The hypoflexid is slightly inclined labially and exhibits some wear. There are two roots of equal size.
The dp4 is 1.5 times larger than dp3, and fully molariform. The crown is linguo-labially wider relative to crown length, as compared to dp3 (Fig. 11). The protoconid is the highest cusp, the metaconid is the second highest, and the paraconid is the lowest. The paraconid is more erect than that of dp3, and the protoconid and metaconid are not inclined distally. The trigonid basin is lingually open and the metaconid bulges lingually. On the labial side, a bulge extends from the base of the paraconid in distal direction, before disappearing at the base of the protoconid. The talonid is shorter and wider than that of dp3 and its hypoconid is in a lingual position. The hypoflexid is inclined labially and has a worn groove with cervico-labially oriented striations. There are two roots of equal size.
The dp3 and dp4 are in contact via the talonid of dp3. The dp3, dp2, dp1, and the dc are separated by gaps from each other. The gap between dc and dp1 is the widest (length about half of dp1 length), the gap of dp1 and dp2 is intermediate, and the gap between dp2 and dp3 is the shortest. Overall, the interdental gaps between deciduous canine and premolars are decreasing in posterior direction.
Persisting dp4
Gui Mam 65/77 is a mandible of an adult as indicated by six erupted molars and the replaced canine. Its premolariforms p1–3 are already replaced (Fig. 14). All these features correspond in morphology to the teeth of other adult mandibles of Henkelotherium (e.g., Gui Mam 138/76, 48/78, and 133/77). However, Gui Mam 65/77 shows a variant pattern in its ultimate premolar position. The p4 position has a molariform tooth that closely resembles dp4 of Gui Mam 44/80, in which a tooth cap of the permanent p4 has been detected by CT (Figs. 9d and 14: Box f–i–j). No germ of a succeeding p4 has been found below dp4 of Gui Mam 65/77, but the space between its roots is somewhat wider than in p3 (Fig. 14) which is a deciduous premolar character. The crown of this tooth of Gui Mam 65/77 is identical to the dp4 of Gui Mam 44/80 in having an elongate but bilaterally compressed trigonid, a closer approximation of the protoconid and the metaconid (Fig. 14d: twinning), both of which are separated far apart from the paraconid, and a smaller and narrower talonid than in those in m1s of other Henkelotherium specimens. The molariform in p4 position in Gui Mam 65/77 also exhibits stronger wear than the permanent premolars and also as the following molars. We interpret this tooth as a deciduous p4 that persisted into adult age (also known as permanent dp4) (Fig. 14), as an intraspecific developmental variation. Persisting deciduous premolars (dp2) have previously been reported for Guimarotodus inflatus and Dryolestes, but in these taxa, the deciduous teeth persisted besides their permanent successors (Martin 1997, 1999). We interpret that in the mandible Gui Mam 65/77, no permanent p4 was formed. Further, this specimen (Gui Mam 65/77) is also different from other mandibles of Henkelotherium is that its p1 and p3 are larger than those in other adult specimens (Fig. 14: Box e–g), and the interdental gaps are smaller.
Comparison with Dryolestes leiriensis
The lower deciduous canine of Dryolestes leiriensis differs from the permanent canine, by its smaller size and a larger distal accessorial cuspule (Martin 1997, 1999) (Fig. 10). The difference between the deciduous and the permanent canine in Dryolestes (Fig. 10) is comparable to that seen in Henkelotherium (Figs. 8 and 11). Like in Henkelotherium, the dp1 and dp2 of Dryolestes leiriensis are premolariform with a low triangular outline of the main cusp in side view, in contrast to an acute-triangular, and tall main cusp in the permanent p1–2; dp1 is smaller than dp2. Differing from Henkelotherium, the dp1–2 of Dryolestes leiriensis have larger mesial and distal accessory cuspules (Martin 1997, 1999). In Henkelotherium, there are no mesial accessory cuspules in dp1–2, and the distal accessory cuspule is small in dp1 and somewhat larger in dp2.
As in Henkelotherium, dp3 and dp4 of Dryolestes leiriensis are molariform and much larger than dp1 and dp2. The protoconid of dp3 is erect in Dryolestes leiriensis, not recurved distally as in Henkelotherium, and the paraconid is more erect than in the molars, whereas it is procumbent in Henkelotherium. The roots of dp3 of Dryolestes leiriensis are, as in Henkelotherium, of equal size. The anterior root of the Dryolestes leiriensis dp4 is somewhat larger than the posterior root (but the size disparity is less than in the molars). By contrast, the dp4 roots are of equal size in Henkelotherium. The canine and dp1–dp3 of Dryolestes leiriensis are separated by gaps from each other, of which the largest gap is between dc and p. The dp3 and dp4 are in contact via the talonid of dp3 and the paraconid of dp4 (Martin 1997, 1999). In this feature, Dryolestes and Henkelotherium are about the same.
Henkelotherium corroborates the early observation on Dryolestes (Martin 1997) that dp3 and dp4 are molariform in stem therians and are replaced by premolariform successors. The stem zatherian Nanolestes has five premolariform lower permanent premolars, but the posterior deciduous premolars (dP?3–5) are molariform (Martin 2002). Palaeoxonodon oolithicus has five lower premolars, but the shape of p5 is not known (Sigogneau-Russell 2003; Close et al. 2015). In the more derived zatherian Peramus p5 is semimolariform (McKenna 1975), and has originally been interpreted as m1 (Simpson 1928; Mills 1964; Clemens and Mills 1971), but is now generally accepted as p5 instead of m1 (Prothero 1981; Novacek 1986; Butler and Clemens 2001; Martin 2002), which has now been corroborated by CT visualization (Davis 2012). Therefore, a molariform crown in the ultimate lower premolar is a wide-spread pattern among cladotherians.
Discussion
Talonid characteristics
In general, paurodontids (including Henkelotherium) can be distinguished by a number of lower molar characters from dryolestid species, such as Dryolestes leiriensis, Krebsotherium lusitanicum, and Guimarotodus inflatus (Martin 1999, 2000). The more rectangular trigonid with an angled paracristid in the anterior lower molars, as best seen in Henkelotherium, is distinct from the triangular trigonid with the relatively straight paracristid of dryolestid species. The protocristid is transversely oriented in all lower molars of dryolestids and the triangulation of the trigonid is more acute. By contrast, in the most posterior molars of paurodontids, the protocristid is oblique and the triangulation of the trigonid cusps appears to be more obtuse (Figs. 3, 14, 15, 16).
The labial position of the hypoconid on the talonid was adopted to be a distinguishing feature of paurodontids from dryolestids (Prothero 1981; Krebs 1991; Kielan-Jaworowska et al. 2004). However, this feature in Henkelotherium shows a variation: the labial placement of the hypoconulid only occurs, or appears to be more prominent, on the more posterior lower molars (Fig. 3). This feature on the posterior molars is still a valid diagnostic character for paurodontids, but the distinction of this character is less so for the anterior lower molars (Figs. 15a and 16).
Termination of dental development
We interpret that in mandibles with high number of molars, the conspicuous size decrease in posterior molars, accompanied by a fusion of lower molar roots and a bending of upper molar roots, are indication that such specimens are approaching the terminal phase of jaw development. In several mandibles of Henkelotherium, the posterior m5 and m6 show a decrease in size, and a partial fusion of the roots of m6, and completely fused roots into a single structure (or reduced to a single root) in m7. The ultimate molar m7 is the smallest of the molars and has a single root, as noted earlier by Krebs (1991) and confirmed by CT scanning. Now, we have also found, by further CT scans of adult Dryolestes leiriensis specimens (with nine molar loci to the fullest extent), similar patterns that the last molars have decreased crown size, and that the roots are partially fused in m7 and m8, and fully fused in m9, in mandibles with eight-to-nine lower molars (Gui Mam 82/79 and 171/74) (Fig. 10). Similarly, in the maxillary specimen of Dryolestes leiriensis with eight upper molars, the two mesiolabial and distolabial roots are fused into one labial root on M8 (Gui Mam 51/75). The latter pattern also occurs in other dryolestids, such as Minutolestes (Martin et al. 2022a, b) (Fig. 5).
Several major Mesozoic mammaliaform groups show a pattern that the penultimate and ultimate molars tend to decrease in crown size and their roots tend to be increasingly fused, or completely fused, as the sequential eruption of molars approaches the end, concurrently with termination of growth of the mandible (Luo et al. 2004, 2022; O’Meara and Asher 2016). This pattern has now been much better documented, with relatively good specimen samples, for a diversity of Mesozoic mammaliaform taxa: Morganucodon watsoni (Parrington 1973; Kermack et al. 1973; Jäger et al. 2019b), Morganucodon oehleri (Luo et al. 2022), Kuehneotherium praecursoris (Kermack et al. 1968; Gill 2004), the docodontans Docodon victor, Borealestes serendipitus, and Haldanodon exspectatus (Schultz et al. 2019; Panciroli et al. 2019), possibly also some spalacotherioid species among crown Mesozoic mammals (pers. observation).
From this comparative information, we postulate that Henkelotherium guimarotae reached the end of its jaw growth and molar eruption at the stage of lower m7 and/or at upper M6, by the fact that the lower m7 in Henkelotherium guimarotae holotype (Gui Mam 138/76) is very small and single-rooted, characteristic of the ultimate molar in other Mesozoic mammaliaforms (Parrington 1973; Panciroli et al. 2019; Luo et al. 2022). We also suggest that Dryolestes leiriensis reached its termination of mandibular growth and molar eruption at the nine lower molar stage or eight upper molar stage, on the same reasoning. This provides a basis for further discussion on the prolonged mandibular growth and late eruption of posterior molars in dryolestidans, as follows.
Sequence and timing of tooth replacement
For the upper dentition of Henkelotherium, information on the sequence of replacement is limited, and there is no information for the incisors. Thus, we focus on the premolars (Fig. 21). The P1 and P3 positions are the first antemolars to be replaced and the permanent P1 and P3 have replaced the dP1 and dP3 in Gui Mam 91/75, followed by eruption of the permanent canine – its crown is almost fully erupted at this stage. In the same specimen, M4 was evidenced by its alveoli and it is fully erupted in next older specimen (Fig. 21: Gui Mam 114/77). The dP4 is replaced by its permanent successor at this stage (Gui Mam 17/76, 114/77). The dP2 is the last antemolar to be replaced, as the permanent P2 is in the process of eruption, concurrent with the eruption of M5 (M5 evidenced by its broken alveoli) (Fig. 21).
For the lower dentition (Fig. 8), more data on the sequence of tooth replacement are available for the canine and premolars, although there is no information for incisor replacement. In the ontogenetically youngest mandible (Gui Mam 25/81), the deciduous canine and premolars are still in place and m1 is in the process of eruption. In the next older specimen (Gui Mam 44/80), dp4 is still in place when m1–2 are erupted and m3 is in the process of eruption, and m4 bell is developed enough for its protoconid emerged to the surface, followed by m5 crypt (Figs. 8 and 9). The canine is in process of eruption. The p1 and p3 are replaced, and in the dp2/p2 position, only the alveoli are preserved. In Gui Mam 48/75 with the same molar configuration, p1 and p3 are replaced, and permanent p4 is in the process of eruption. At the dp2/p2 position, only two alveoli are preserved. All premolars are replaced when m1–4 are erupted and the crypt of m5 is evident on the ascending ramus slope (Gui Mam 48/75). No information on the canine is available for this stage, but we speculate that the permanent canine was probably in place no later than m5 eruption. An alternating replacement pattern with early replacement of dp1 and dp3, and late replacement of dc, dp2, and dp4 can be reconstructed, with the antemolar replacement completed when the crypt of m5 is evident on the slope of the ascending ramus. The premolar eruption sequence of Henkelotherium is p1 → p3 → p2 → p4, with the canine erupting most likely shortly before p4. This replacement pattern of Henkelotherium is consistent with the pattern observed for Dryolestes leiriensis where dp4 is the last premolar position to be replaced. Replacement of dp4 occurs in Dryolestes leiriensis at the same time as that of the deciduous canine (Martin 1997, 1999).
Late molar eruption with prolonged jaw growth
Dryolestidans show a late addition of more molars as older mandibles have grown longer. In Henkelotherium (Fig. 8), after full replacement of the antemolars, two more molars—m6 and m7—were added to the tooth row, accompanied by a late growth in mandibular length. A comparable pattern occurs in Dryolestes leiriensis (Fig. 10) in which the posterior molars (m6-m9) were sequentially added to the molar row after antemolar replacement (Martin 1997, 1999).
The occurrence of supernumerary molars is an exceptional feature for Mesozoic mammaliaforms in general, and by comparison to extant therians. Fossils of Dryolestidae from the Jurassic and Cretaceous of Laurasian landmasses have more molars, up to seven or eight upper molars and eight or nine lower molars (Simpson 1929; Krebs 1971; Prothero 1981; Martin 1999; Butler and Clemens 2001). In addition to Dryolestes leiriensis (Fig. 10: new CT scans), Krebsotherium lusitanicum has nine lower molars and eight upper molars. Laolestes eminens and Amblotherium have up to nine lower molars (Prothero 1981; Butler and Clemens 2001).
Paurodontids show more molars than other Mesozoic mammals, although less extreme than dryolestids. Henkelotherium guimarotae, as a representative of the Paurodontidae, is confirmed here to have seven lower molars and six upper molars from a large sample. And Paurodon valens has at least five lower molars (Averianov and Martin 2015). The supernumerary molars of these dryolestidans also correspond to the substantially longer mandibular body (horizontal ramus), which appears to have experienced a prolonged growth, to accommodate the posterior molars (m5, m6, and m7) that were added to the longer molar row well after the termination of antemolar replacement. We propose that this is a common feature in dryolestids and Henkelotherium (Prothero 1981; Krebs 1991; Martin 1999) (Figs. 8 and 18).
This dryolestidan pattern of supernumerary molars on longer mandibles is in contrast to crown therians and a majority of the zatherian taxa closer to Theria than to dryolestidans (Kielan-Jaworowska et al. 2004; Martin 2018): Nanolestes and Palaeoxonodon—two stem therians of zatherian clade—have five molars, respectively (Martin 2002; Close et al. 2015). Peramus has three molars (McKenna 1975; Butler and Clemens 2001; Davis 2012). Vincelestes—a mammal possibly closer to boreosphenidans (including crown Theria)—has three molars (Rougier et al. 2021a). Among zatherians closer to crown therians than dryolestidans, Amphitherium is an outlier with seven molars (Prothero 1981; Butler and Clemens 2001), presumably representing a convergent case in evolution of supernumerary molars as Amphitherium is phylogenetically distinct from dryolestidans in the latest phylogenetic studies (Close et al. 2015; Panciroli et al. 2018).
The boreosphenidan mammals have either three or four molars, eutherians (including placentals) have three molars, and metatherians (including marsupials) have four molars (Kielan-Jaworowska et al. 2004). Thus, the supernumerary molars (up to seven or nine) of derived dryolestidans are autapomorphic, and distinct from the shorter molar row of 3–4 in crown therians, and their zatherian relatives, with the exception of Amphitherium. We propose two hypotheses for these dryolestidans with uncommon supernumerary molars and longer mandibles. The first of these is based on our observation of the development of the occlusal pattern of the molar row; and the second is based on timing differences of molar eruptions, relative to the timing of antemolar replacement, as seen in Dryolestidae (Martin 1997, 1999) and Henkelotherium (Figs. 8 and 18).
Regarding the occlusal dental function, the older individuals of Henkelotherium had continued their molar development with eruption of M4, M5, and M6, after full replacement of the upper canine and premolars. The more posterior molars M4–M6 have sustained some modest wear in the holotype specimen, a relatively old individual (Figs. 17 and 18), but these teeth show extensive wear in the oldest available specimen Gui Mam 17/76 (Fig. 19). In posterior lower molars of mandibles old enough to have six molars, which include the holotype (Krebs 1991: Fig. 3; photos of Fig. 17) and three other specimens (Figs. 13, 14, 15), the posterior m4–m5 have extensive wear on the paracristid and the protocristid of the trigonids, as well as visible wear on the slope of the hypoflexid. Even the last functioning molar m6 shows the beginning stage of apical wear on the trigonid and wear on the hypoflexid (Figs. 14, 15). We postulate that the higher number of molars in longer molar rows is functionally advantageous, to maintain a more consistent contact for occlusion by the late eruption of fresh molars in the more posterior part of the molar row. Thus, a longer tooth row, with late eruption of two or more molars well after the completion of premolar replacement, would prolong the masticatory function more consistently, and convey an adaptive advantage by sustaining effective occlusion in later stages, and over the longer lifespan of a mammal with a long molar row.
Second, we note that in late growth stages of longer jaws of dryolestidans, the more posterior molars continue to erupt long after the termination of the replacement of the antemolars (incisors, canine, and premolars), as exemplified by canine and premolars (we note that the deciduous incisors are not preserved in our examined specimens). This sequence shows a major timing difference in continued eruption of posterior molars, relative to the timing of replacement for antemolars in these dryolestidans (Martin 1997) (Figs. 8, 10, and 21), from the sequence of antemolar replacement and molar eruption in crown Theria.
Eutherians show a range of differences in timing and sequence of eruption of the molars, relative to replacement of the antemolars (Schultz 1935, 1960; Smith 1994, 2000; Asher and Lehmann 2008; Geiger and Asher 2019). In general, in slow-growing and long-lived placentals with life-history events occurring over greater timespan, such as slower skull growth, the eruption and replacement of the antemolars (e.g., premolars) tend to occur early, relative to the eruption sequence of molars. Usually, with this pattern, the last premolar can be replaced well before, or in synchrony with the eruption of the last molar. By comparison, in fast-growing placentals with life-history events occurring over shorter time span, such as faster skull growth, the eruption and replacement of antemolars tend to occur later, relative to the eruption sequence of molars, and eruption of some (or all) molars may occur before replacements of some premolars, or most premolars.
These comparative patterns, as first noticed by Schultz (1935, 1960), are now called Schultz’s rule by other researchers who have characterized these patterns in great details and over a wider diversity of therian mammals (e.g., Smith 2000; Forasiepi and Sanchez-Villagra 2014; Asher et al. 2017), and also in different contexts (Geiger and Asher 2019). Patterns of Schultz’s rule are well known from ungulates, carnivores, primates, and insectivorous placentals (Smith 2000; Henderson 2007), and in afrotherians especially from hyracoids (Asher and Lehmann 2008; Asher et al. 2017). However, there are noted exceptions to these patterns among taxa in some primate clades (Godfrey et al. 2005), or absence of such patterns among artiodactyl clades (Monson and Hlusko 2018a). They have been demonstrated to be weak or absent in domesticated mammals in comparison to their ancestral (or closely related) wild species (Geiger and Asher 2019). It has been also debated whether these patterns of timing and sequence in dental replacement are influenced by differences in “fast” or “slow” life histories (Smith 2000), or if they are constrained and influenced mainly by phylogenies (Monson and Hlusko 2018b), or both (Smith 2000).
Marsupials have the most specialized antemolar replacement, and the only antemolars replaced are the last premolars (dP3/dp3) (Luckett 1993; van Nievelt and Smith 2005), which is hypothesized to be correlated with the specialized lactation of the marsupial neonates (Luckett 1993; Maier 1999). In marsupials, the eruption of P3/p3 to replace dP3/dp3 tends to be in synchrony with the ultimate molars M3/m4, or M4/m4 depending on species (van Nievelt and Smith 2005; Astua and Leiner 2008; Forasiepi and Sanchez-Villagra 2014). This pattern appears in the Cretaceous metatherians (Cifelli et al. 1996) that are related to extant marsupials. Even with the premolar replacement of only one position (P3/p3), in relative timing of the last premolar replacement to last molar eruption, marsupials appear to be more similar to the slow-growing placentals, than to the fast-growing placentals.
In dryolestidans, eruption of m1 occurs before the replacement of the anterior premolars in Dryolestes (Martin 1997) and also in Henkelotherium (Figs. 8–10). The eruption of m4 is in approximate synchrony with the eruption of p4 to replace dp4 in Henkelotherium (Figs. 8 and 9), and eruption of m6 is in synchrony with p4 replacing dp4 in Dryolestes (Fig. 10) (Martin 1997: Table 1). For this part of the premolar replacement and molar eruption, the timing and sequence are comparable to those placentals with slow life history and possibly a longer life (Smith 2000; Henderson 2007; Asher et al 2017) and also consistent with the timing and sequence of marsupials (van Nievelt and Smith 2005; Astua and Leiner 2008).
By comparison to crown Theria, dryolestidans surpass extant placentals and marsupials by a more prolonged molar eruption sequence, with late eruption of several molars long after the ending of replacement of premolars. The successive eruptions of m5 through m7 in Henkelotherium (Fig. 8), and m6 through m8, or m9 in dryolestid taxa (Martin 1997, 1999), all occurred after the premolar replacement. The late sequences of m5 to m7 in Henkelotherium and m6 to m9 concurrent with prolonged mandibular growth in dryolestid taxa are not represented in the relatively truncated, and shorter sequence of eruption of m1–m3 in eutherians, and m1–m4 in metatherians.
By these characteristics, dryolestidans have either a longer-lived life, or slower life-history traits, or a combination of both, by the proxy of the timing and sequence of the premolar replacements, relative to their late eruption of several more molars and by their much longer molar series, than crown therians (Smith 2000; van Nievelt and Smith 2005) that have short molar series and relatively shorter jaw, with a manifest of a determinate growth that starts fast and plateaus earlier (Luo et al. 2004; O’Meara and Asher 2016). It cannot be ruled out that such a transition in life-history pattern in therian mammal evolution could occur with zatherian sister-clades (such as Peramus) that are also more crown therian-like in such features as shorter molar series on relatively shorter mandibles.
Data availability
Specimens are deposited in the cited collections and institutions. Images of segmentation of all specimens scanned by uCT for this study, and SEM images of all specimens examined by Scanning Electron Microscopy are presented in text figures. The supplementary information provides further data in photographs of additional 15 specimens, and three movies on the composite reconstruction of adult mandibles, of the juvenile specimen Gui Mam 44/80, and of a restoration of incisors and canine of Gui Mam 133/77. Additional data are available from authors upon reasonable request.
References
Anthwal, N., D.J. Urban, Z.-X. Luo, K.E. Sears, and A.S. Tucker. 2017. Meckel’s cartilage breakdown offers clues to mammalian middle ear evolution. Nature Ecology & Evolution 1: 0093. https://doi.org/10.1038/s41559-017-0093(www.nature.com/natecolevol).
Asher, R.J., G.F. Gunnell, E.R. Seiffert, D. Pattinson, R. Tabuce, L. Hautier, and H.M. Sallam. 2017. Dental eruption and growth in Hyracoidea (Mammalia, Afrotheria). Journal of Vertebrate Paleontology 37: e1317638.
Asher, R.J., I. Horovitz, T. Martin, and M. Sánchez-Villagra. 2007. Neither a rodent nor a platypus: A reexamination of Necroleste patagonensis Ameghino. American Museum Novitates 3546: 1–40.
Asher, R.J., and T. Lehmann. 2008. Dental eruption in afrotherian mammals. BMC Biology 6: 14.
Astua, D., and N.O. Leiner. 2008. Tooth eruption sequence and replacement pattern in woolly opossums, genus Caluromys (Didelphimorphia: Didelphidae). Journal of Mammalogy 89: 244–251.
Averianov, A.O., and T. Martin. 2015. Ontogeny and taxonomy of Paurodon valens (Mammalia, Cladotheria) from the Upper Jurassic Morrison Formation of USA. Proceedings of the Zoological Institute Russian Academy of Sciences 319: 326–340.
Averianov, A.O., T. Martin, and A.V. Lopatin. 2013. A new phylogeny for basal Trechnotheria and Cladotheria and affinities of South American endemic Late Cretaceous mammals. Naturwissenschaften 100: 311–326.
Averianov, A.O., T. Martin, and A.V. Lopatin. 2014. The oldest dryolestid mammal from the Middle Jurassic of Siberia. Journal of Vertebrate Paleontology. 34: 924–931. https://doi.org/10.1080/02724634.2014.837471.
Benoit, J., I. Ruf, J.A. Miyamae, V. Fernandez, P.G. Rodrigues, and B.S. Rubidge. 2020. The evolution of the maxillary canal in Probainognathia (Cynodontia, Synapsida): Reassessment of the homology of the infraorbital foramen in mammalian ancestors. Journal of Mammalian Evolution 27: 329–348. https://doi.org/10.1007/s10914-019-09467-8.
Bonaparte, J.F. 1986. Sobre Mesungulatum houssayi y nuevos mamíferos cretácicos de Patagonia. Actas IV Congreso Argentino de Paleontología y Bioestratigrafía 2: 48–61.
Bonaparte, J.F. 1990. New Late Cretaceous mammals from the Los Alamitos Formation, northern Patagonia. National Geographic Research 6: 63–93.
Bonaparte, J.F. 1994. Approach to the significance of the Late Cretaceous mammals of South America. Berliner Geowissenschaftliche Abhandlungen E 13: 31–44.
Bonaparte, J.F. 2002. New Dryolestida (Theria) from the Late Cretaceous of Los Alamitos, Argentina, and paleogeographical comments. Neues Jahrbuch Für Geologie Und Paläontologie, Abhandlungen 224: 339–371.
Butler, P.M. 1939. The teeth of the Jurassic Mammals. Proceedings of the Zoological Society B 109: 329–356.
Butler, P.M., and W.A. Clemens. 2001. Dental morphology of the Jurassic holotherian mammal Amphitherium, with a discussion of the evolution of mammalian post-canine dental formulae. Palaeontology 44: 1–20.
Chimento, N.R., F.L. Agnolin, and F.E. Novas. 2012. The Patagonian fossil mammal Necrolestes: a Neogene survivor of Dryolestoidea. Revista del Museo Argentino de Ciencias Naturales 14: 261–306.
Chornogubsky, L. 2011. New remains of the dryolestoid mammal Leonardus cuspidatus from the Los Alamitos Formation (Late Cretaceous, Argentina). Paläontogische Zeitschrift 85: 1–8.
Cifelli, R.L., and S.K. Madsen. 1999. Spalacotheriid symmetrodonts (Mammalia) from the medial Cretaceous (Upper Albian or lower Cenomnian) Mussentuchit local fauna, Cedar Mountain Formation, Utah, USA. Geodiversitas 21: 167–214.
Cifelli, R.L., T.B. Rowe, W.P. Luckett, J. Banta, R. Reyes, and R.I. Howes. 1996. Origin of marsupial pattern of tooth replacement: Fossil evidence revealed by high resolution X-ray CT. Nature 379: 715–718.
Clemens, W.A., and J.R.E. Mills. 1971. Review of Peramus tenuirostris. Bulletin of the British Museum (Natural History). Geology 20: 89–113.
Clemens, W.A., G.P. Wilson, and R. Molnar. 2003. An emigmatic (synapsid?) tooth from the Early Cretaceous of New South Wales, Australia. Journal of Vertebrate Paleontology 23: 232–237.
Close, R.A., B.M. Davis, S. Walsh, A.S. Wolniewicz, M. Friedman, and R.B.J. Benson. 2015. A lower jaw of Palaeoxonodon from the Middle Jurassic of the Isle of Skye, Scotland, sheds new light on the diversity of British stem therians. Palaeontology. https://doi.org/10.1111/pala.12218.
Crompton, A.W. 1971. The origin of the tribosphenic molar. In Early mammals, ed. D.M. Kermack and K.A. Kermack, 65–87. Zoological Journal of the Linnean Society 50, supplement 1.
Crompton, A.W. 1974. The dentition and relationships of the southern African Triassic mammals, Erythrotherium parringtoni and Megazostrodon rudnerae. Bulletin of the British Museum (Natural History), Geology 24: 397–437.
Crompton, A.W., and W.L. Hylander. 1986. Changes in mandibular function following the acquisition of a dentary-squamosal joint. In The Ecology and Biology of Mammal-like Reptiles, ed. N. Hotton, P.D. Macean, and J.J. Roth, 263–282. Washington: Smithsonian Institution Press.
Crompton, A.W., and Z.-X. Luo. 1993. Relationships of the Liassic mammals Sinoconodon, Morganucodon, and Dinnetherium. In Mammal phylogeny: Mesozoic differentiation, multituberculates, monotremes, early therians, and marsupials, ed. F.S. Szalay, M.J. Novacek, and M.C. McKenna, 30–44. New York: Springer-Verlag.
Davis, B.M. 2012. Micro-computed tomography reveals a diversity of peramuran mammals from the Purbeck Group (Berriasian) of England. Palaeontology 55: 789–817.
Dyce, K.M., W.O. Sack, and C.J.G. Wensing. 1995. Textbook of veterinary anatomy. Saunders Elsevier.
Ensom, P.C., and D. Sigogneau-Russell. 1998. New dryolestoid mammals from the basal Cretaceous Purbeck Limestone Group of southern England. Palaeontology 41: 35–55.
Evans, H.E. 1993. Miller’s anatomy of the dog. Philadelphia: Saunders Company.
Forasiepi, A.M., and M.R. Sanchez-Villagra. 2014. Heterochrony, dental ontogenetic diversity, and the circumvention of constrains in marsupial mammals and extinct relatives. Paleobiology 40: 2220237.
Freeman, E.F. 1976. Mammal teeth from the Forest Marble (Middle Jurassic) of Oxfordshire, England. Science 194: 1053–1055.
Freeman, E.F. 1979. A Middle Jurassic mammal bed from Oxfordshire. Palaeontology 22: 135–166.
Gao, C.-L., G.P. Wilson, Z.-X. Luo, A.M. Maga, Q.-J. Meng, and X.-R. Wang. 2009. A new mammal skull from the Lower Cretaceous of China with implications for the evolution of obtuse angled molars and amphilestid eutriconodonts. Proceedings of the Royal Society B (london) 277: 237–246. https://doi.org/10.1098/rspb.2009.1014.
Geiger, M., and R.J. Asher. 2019. Schultz’s rule in domesticated mammals. Mammalian Biology 96: 36–42. https://doi.org/10.1016/j.mambio.2019.07.002.
Gelfo, J.N., and R. Pascual. 2001. Peligrotherium tropicalis (Mammalia, Dryolestida) from the early Paleocene of Patagonia, a survival from a Mesozoic Gondwanan radiation. Geodiversitas 23: 369–379.
Gill, P.G. 2004.Kuehneotherium from the Mesozoic Fissure Fillings of South Wales. Ph.D. Dissertation, University of Bristol, Bristol, United Kingdom, 1–286. http://research-information.bristol.ac.uk
Godfrey, L.R., K.E. Samonds, P.C. Wright, and S.J. King. 2005. Schultz’s unruly rule: Dental developmental sequences and schedules in small-bodied, folivorous lemurs. Folia Primatologica 76: 77–99.
Grossnickle, D.M. 2017. The evolutionary origin of jaw yaw in mammals. Scientific Reports 7: 1–13. https://doi.org/10.1038/srep45094.
Grossnickle, D.M., L.N. Weaver, K.R.K. Jäger, and J.A. Schultz. 2022. The evolution of anteriorly directed molar occlusion in mammals. Zoological Journal of the Linnean Society 194: 349–365.
Hahn, G., and R. Hahn. 2000. Multituberculates from the Guimarota mine. In Guimarota: A Jurassic ecosystem, ed. T. Martin and B. Krebs, 97–108. München: Verlag Dr. Friedrich Pfeil.
Heinrich, W.-D. 1991. Über Brancatherulum tendagurense Dietrich, 1927 (Mammalia: Eupantotheria) aus dem Oberjura von Tendaguru, Tansania. Mitteilungen aus dem Zoologischen Museum Berlin 67: 97–104. https://doi.org/10.1002/mmnz.19910670114.
Henderson, E. 2007. Platyrrhine dental eruption sequence. American Journal of Physical Anthropology 134: 226–239.
Henkel, S., and B. Krebs. 1969. Zwei Säugetier-Unterkiefer aus der Unteren Kreide von Uña (Prov. Cuenca, Spanien). Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 1969: 449–463.
Hu, Y.-M., Y.-Q. Wang, C.-K. Li, and Z.-X. Luo. 1998. Morphology of dentition and forelimb of Zhangheotherium. Vertebrata PalAsiatica 36: 102–125.
Hu, Y.-M., Y.-Q. Wang, Z.-X. Luo, and C.-K. Li. 1997. A new symmetrodont mammal from China and its implications for mammalian evolution. Nature 390: 137–142.
Hughes, E.M., J.R. Wible, M. Spaulding, and Z.-X. Luo. 2015. Mammalian petrosal from the Upper Jurassic Morrison Formation of Fruita, Colorado. Annals of Carnegie Museum 83: 1–17.
Jäger, K.R.K., P.G. Gill, I.J. Corfe, and T. Martin. 2019b. 3D Occlusion and dental function of Morganucodon and Megazostrodon. Journal of Vertebrate Paleontology 39: e1635135. https://doi.org/10.1080/02724634.2019.1635135.
Jäger, K., Z.-X. Luo, and T. Martin. 2013a. CT scanning and 3D image analysis of the postcranial skeleton of Henkelotherium guimarotae (Cladotheria, Mammalia) from the Late Jurassic of Portugal and its locomotor adaptations. Society of Vertebrate Paleontology 73rd Annual Meeting, Program and Abstracts: 147.
Jäger K.R.K, Z.-X. Luo, and T. Martin. 2013b. Reinvestigation of the postcranial skeleton of Henkelotherium guimarotae (Cladotheria, Mammalia) from the Late Jurassic of Portugal using MicroCT data. Joint Meeting of Paläontologische Gesellschaft and of Palaeontological Society of China in Göttingen.
Jäger, K.R.K., Z.-X. Luo, and T. Martin. 2019a. Postcranial skeleton of Henkelotherium guimarotae (Cladotheria, Mammalia) and locomotor adaptation. Journal of Mammalian Evolution 27: 349–372. https://doi.org/10.1007/s10914-018-09457-2.
Ji, Q., Z.-X. Luo, and S.-A. Li. 1999. A Chinese triconodont mammal and mosaic evolution of the mammalian skeleton. Nature 398: 326–330.
Ji, Q., Z.-X. Luo, C.-X. Yuan, R.J. Wible, J.P. Zhang, and J.A. Georgi. 2002. The earliest known eutherian mammal. Nature 416: 816–822.
Ji, Q., Z.-X. Luo, X. Zhang, C.-X. Yuan, and L. Xu. 2009. Evolutionary development of the middle ear in Mesozoic therian mammals. Science 326: 278–281.
Kelt, D.A., and J.L. Patton. 2020. A manual of the mammalia: An Homage to Lawlor’s “Handbook to the orders and families of living mammals.” The University of Chicago Press.
Kermack, D.M., K.A. Kermack, and F. Mussett. 1968. The Welsh pantothere Kuehneotherium praecursoris. Journal of the Linnean Society (zoology) 47: 407–423.
Kermack, K.A., F. Mussett, and H.W. Rigney. 1973. The lower jaw of Morganucodon. Zoological Journal of Linnean Society 53: 87–175.
Kermack, K.A., F. Mussett, and H.W. Rigney. 1981. The skull of Morganucodon. Zoological Journal of the Linnean Society 71: 1–158.
Kielan-Jaworowska, Z. 1981. Evolution of the therian mammals in the Late Cretaceous of Asia. Part IV. Skull structure in Kennalestes and Asioryctes. Palaeontologia Polonica 42: 25–78.
Kielan-Jaworowska, Z., R.L. Cifelli, and Z.-X. Luo. 2004. Mammals from the age of dinosaurs: Origins, evolution and structure. New York: Columbia University Press.
Kielan-Jaworowska, Z., and D. Dashzeveg. 1989. Eutherian mammals from the Early Cretaceous of Mongolia. Zoologica Scripta 18: 347–355.
Kielan-Jaworowska, Z., and J.H. Hurum. 2001. Phylogeny and systematics of multituberculate mammals. Palaeontology 44: 389–429.
Krebs, B. 1969. Nachweis eines rudimentären Coronoids im Unterkiefer der Pantotheria (Mammalia). Paläontologische Zeitschrift 43: 57–63.
Krebs, B. 1971. Evolution of the mandible and lower dentition in dryolestids (Pantotheria, Mammalia). In Early mammals, ed. D.M. Kermack and K.A. Kermack, 89–103. Zoological Journal of the Linnean Society 50, supplement 1.
Krebs, B. 1985. Theria (Mammalia) aus der Unterkreide von Galve (Provinz Teruel, Spanien). Berliner geowissenschaftliche Abhandlungen A 60: 29–48.
Krebs, B. 1991. Das Skelett von Henkelotherium guimarotae gen. et sp. nov. (Eupantotheria, Mammalia) aus dem Oberen Jura von Portugal. Berliner geowissenschaftliche Abhandlungen A 133: 1–110.
Krebs, B. 1993. Das Gebiß von Crusafontia (Eupantotheria, Mammalia) - Funde aus der Unter-Kreide von Galve und Uña (Spanien). Berliner geowissenschaftliche Abhandlungen E 9: 233–252.
Krebs, B. 1998. Drescheratherium acutum gen. et sp. nov., ein neuer Eupantotherier (Mammalia) aus dem Oberen Jura von Portugal. Berliner geowissenschaftliche Abhandlungen E 28: 91–111.
Krebs, B. 2000. The henkelotheriids from the Guimarota mine. In Guimarota: A Jurassic ecosystem, ed. T. Martin and B. Krebs, 121–128. München: Verlag Dr. Friedrich Pfeil.
Krusat, G. 1980. Contribuçăo para o conhecimento da fauna do Kimeridgiano da mina de lignito Guimarota (Leiria, Portugal). IV Parte. Haldanodon exspectatus Kühne & Krusat 1972 (Mammalia, Docodonta). Memórias dos Serviços Geológicos de Portugal 27: 1–79.
Lasseron, M., T. Martin, R. Allain, H. Haddoumi, N.-E. Jalil, S. Zouhri, and E. Gheerbrant. 2022. An African radiation of “Dryolestoidea” (Donodontidae, Cladotheria) and ist significance forr mammalian evolution. Journal of Mammalian Evolution. https://doi.org/10.1007/s10914-022-09613-9.
Lautenschlager, S., P. Gill, Z.-X. Luo, M.J. Fagan, and E.J. Rayfield. 2017. Morphological evolution of the mammalian jaw adductor complex. Biological Reviews 92: 1910–1940. https://doi.org/10.1111/brv.12314.
Lillegraven, J.A., and G. Krusat. 1991. Cranio-mandibular anatomy of Haldanodon exspectatus (Docodonta; Mammalia) from the Late Jurassic of Portugal and its implications to the evolution of mammalian characters. Contributions to Geology, University of Wyoming 28: 39–138.
Lillegraven, J.A., and M.C. McKenna. 1986. Fossil Mammals from the “Mesaverde” Formation (Late Cretaceous, Judithian) of the Bighorn and Wind River basins, Wyoming, with definitions of Late Cretaceous North American landmammal “ages.” American Museum Novitates 2840: 1–68.
Luckett, W.P. 1993. An ontogenetic assessment of dental homologies in therian mammals. In Mammal phylogeny: Mesozoic differentiation, multituberculates, monotremes, early therians, and marsupials, ed. F.S. Szalay, M.J. Novacek, and M.C. McKenna, 182–204. New York: Springer-Verlag.
Luo, Z.-X. 1994. Sister taxon relationships of mammals and the transformations of the diagnostic mammalian characters. In In the shadow of dinosaurs—early Mesozoic tetrapods, ed. N.C. Fraser and H.-D. Sues, 98–128. Cambridge: Cambridge University Press.
Luo, Z.-X. 2007. Transformation and diversification in the early mammalian evolution. Nature 450: 1011–1019.
Luo, Z.-X. 2011. Developmental patterns in Mesozoic evolution of mammal ears. Annual Review of Ecology, Evolution and Systematics 42: 355–380. https://doi.org/10.1146/annurev-ecolsys-032511-142302.
Luo, Z.-X., B.-A.S. Bhullar, A.W. Crompton, A.I. Neander, and T.B. Rowe. 2022. Reexamination of the mandibular and dental morphology of the Early Jurassic mammaliaform Hadrocodium wui. Acta Palaeontologica Polonica 67: 95–113. https://doi.org/10.4202/app.00949.2021.
Luo, Z.-X., P.-J. Chen, G. Li, and M. Chen. 2007a. A new eutriconodont mammal and evolutionary development in early mammals. Nature 446: 288–293.
Luo, Z.-X., A.W. Crompton, and A.-L. Sun. 2001. A new mammal from the Early Jurassic and evolution of mammalian characteristics. Science 292: 1535–1540.
Luo, Z.-X., and Q. Ji. 2005. New study on dental and skeletal features of the Cretaceous mammal Zhangheotherium. Journal of Mammalian Evolution 12: 337–357.
Luo, Z.-X., Q. Ji, J.R. Wible, and C.-X. Yuan. 2003. An Early Cretaceous tribosphenic mammal and metatherian evolution. Science 302: 1934–1940.
Luo, Z.-X., Q. Ji, and C.-X. Yuan. 2007b. Convergent dental evolution in pseudotribosphenic and tribosphenic mammals. Nature 450: 93–97.
Luo, Z.-X., Z. Kielan-Jaworowska, and R.L. Cifelli. 2002. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontologica Polonica 47: 1–78.
Luo, Z.-X., Z. Kielan-Jaworowska, and R.L. Cifelli. 2004. Evolution of dental replacement in mammals. In Fanfare for an uncommon paleontologist—Festschrift in honor of Dr. Malcolm C. McKenna, 36th ed., ed. M.R. Dawson and J.A. Lillegraven, 159–175. The Carnegie Museum of Natural History Bulletin 36.
Luo, Z.-X., and G.A. Manley. 2020. Origins and evolution of mammalian ears and hearing function. In The senses: A comprehensive reference (volume 2), 2nd ed., ed. B. Fritzsch and B. Grothe, 207–252. Amsterdam: Elsevier Academic Press. https://doi.org/10.1016/B978-0-12-805408-6.00033-6.
Luo, Z.-X., and T. Martin. 2007. Analysis of molar structure and phylogeny of docodont genera. Carnegie Museum of Natural History Bulletin 39: 27–47.
Luo, Z.-X., I. Ruf, and T. Martin. 2012. The petrosal and inner ear of the Late Jurassic cladotherian mammal Dryolestes leiriensis and implications for evolution of ear in therian mammals. Zoological Journal of Linnean Society (London) 166: 433–463.
Luo, Z.-X., and J.R. Wible. 2005. A new Late Jurassic digging mammal and early mammalian diversification. Science 308: 103–107.
Luo, Z.-X., C.-X. Yuan, Q.-J. Meng, and Q. Ji. 2011. A Jurassic eutherian mammal and the divergence of marsupials and placentals. Nature 476: 442–445. https://doi.org/10.1038/nature10291.
Maier, W. 1999. On the evolutionary biology of early mammals— with methodological remarks on the interaction between ontogenetic adaptation and phylogenetic transformation. Zoologischer Anzeiger 238: 55–74.
Mao, F.-Y., Y.-M. Hu, Y.-Q. Wang, M.H. Chase, A.L. Smith, and J. Meng. 2020. Integrated hearing and chewing modules decoupled in a Cretaceous stem therian mammal. Science 367: 305–308. https://doi.org/10.1126/science.aay9220.
Martin, T. 1995. Dryolestidae from the Kimmeridge of the Guimarota coal mine (Portugal) and their implications for dryolestida systematics and phylogeny. In Sixth Symposium on Mesozoic terrestrial ecosystems and biota, ed. A.-L. Sun and Y. Wang, 229–231. Beijing: Ocean Press.
Martin, T. 1997. Tooth replacement in Late Jurassic Dryolestidae (Eupantotheria, Mammalia). Journal of Mammalian Evolution 4: 1–18.
Martin, T. 1998. The premolars of Crusafontia cuencana (Dryolestidae, Mammalia) from the Early Cretaceous (Barremian) of Spain. Berliner geowissenschaftliche Abhandlungen E 28: 119–126.
Martin, T. 1999. Dryolestidae (Dryolestoidea, Mammalia) aus dem Oberen Jura von Portugal. Abhandlungen der senckenbergischen naturforschenden Gesellschaft 550: 1–119.
Martin, T. 2000. The dryolestids and the small “peramurid” from the Guimarota coal mine. In Guimarota: A Jurassic ecosystem, ed. T. Martin and B. Krebs, 109–120. München: Verlag Dr. Friedrich Pfeil.
Martin, T. 2002. New stem-line representatives of Zatheria (Mammalia) from the Late Jurassic of Portugal. Journal of Vertebrate Paleontology 22: 332–348.
Martin, T. 2018. Mesozoic mammals early mammalian diversity and ecomorphological adaptations. In Handbook of zoology, mammalia. Mammalian evolution, diversity and systematics, ed. F.E. Zachos and R.J. Asher, 199–299. Berlin: De Gruyter.
Martin, T., and A.O. Averianov. 2010. Mammals from the Middle Jurassic Balabansai formation of the fergana depression, Kyrgyzstan. Journal of Vertebrate Paleontology 30: 855–871.
Martin, T., A.O. Averianov, J.A. Schultz, R. Schellhorn, and A.H. Schwermann. 2022a. First spalacotheriid and dryolestid mammals from the Cretaceous of Germany. Acta Palaeontologica Polonica 67: 155–175.
Martin, T., A.O. Averianov, J.A. Schultz, A.H. Schwermann, and O. Wings. 2021. A derived dryolestid mammal indicates possible insular endemism in the Late Jurassic of Germany. The Science of Nature 108: 23. https://doi.org/10.1007/s00114-021-01719-z).
Martin, T., F.J. Goin, J.A. Schultz, and J.N. Gelfo. 2022b. Early Late Cretaceous mammals from southern Patagonia (Santa Cruz Province, Argentina). Cretaceous Research 133: 105127. https://doi.org/10.1016/j.cretres.2021.105127.
Martin, T., and B. Krebs, eds. 2000. Guimarota: A Jurassic Ecosystem. München: Verlag Dr. Friedrich Pfeil.
Martin, T., and O.W.M. Rauhut. 2005. Mandible and dentition of Asfaltomylos patagonicus (Australosphenida, Mammalia) and the evolution of tribosphenic teeth. Journal of Vertebrate Paleontology 25: 414–425.
Martinelli, A.G., S. Soto-Acuña, F.J. Goin, J. Kaluza, J.E. Bostelmann, P.H.M. Fonseca, M.A. Reguero, M. Leppe, and A.O. Vargas. 2021. New cladotherian mammal from southern Chile and the evolution of mesungulatid meridiolestidans at the dusk of the Mesozoic era. Scientific Reports 11: 7594. https://doi.org/10.1038/s41598-021-87245-4.
McKenna, M.C. 1975. Toward a phylogenetic classification of the mammalia. In Phylogeny of the primates, ed. W.P. Luckett and F.S. Szalay, 21–46. New York: Plenum Publishing Corporation.
Meng, J., Y.-Q. Wang, and C.-K. Li. 2011. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature 472: 181–185.
Mills, J.R.E. 1964. The dentitions of Peramus and Amphitherium. Proceedings of the Linnean Society of London 175: 117–133.
Monson, T.A., and L.J. Hlusko. 2018a. The evolution of dental eruption sequence in artiodactyls. Journal of Mammalian Evolution 25: 15–26.
Monson, T.A., and L.J. Hlusko. 2018b. Breaking the rules: Phylogeny, not life history explains dental eruption sequence in primates. American Journal of Physical Anthropology 167: 217–233.
Muchlinski, M.N., and E.C. Kirk. 2017. A comparative analysis of infraorbital foramen size in Paleogene euarchontans. Journal of Human Evolution 105: 57–68. https://doi.org/10.1016/j.jhevol.2017.01.017.
Novacek, M.J. 1986. The primitive eutherian dental formula. Journal of Vertebrate Paleontology 6: 191–196.
O’Meara, R.N., and R.J. Asher. 2016. The evolution of growth patterns in mammalian versus non-mammalian cynodonts. Paleobiology 42: 439–464.
O’Meara, R.N., and R.S. Thompson. 2014. Were there Miocene meridiolestidans? Assessing the phylogenetic placement of Necrolestes patagonensis and the presence of a 40 million year meridiolestidan ghost lineage. Journal of Mammalian Evolution 21: 271–284. https://doi.org/10.1007/s10914-013-9252-3.
Panciroli, E.L., R.B.J. Benson, and R.J. Butler. 2018. New partial dentaries of Palaeoxonodon ooliticus (Mammalia, Amphitheriidae) from Scotland, and posterior dentary morphology in stem cladotherians. Acta Palaeontologica Polonica 63: 197–206.
Panciroli, E.L., R.B.J. Benson, V. Fernandez, R.J. Butler, N.C. Fraser, Z.-X. Luo, and S. Walch. 2021. New species of mammaliaform and the cranium of Borealestes (Mammaliformes: Docodonta) from the Middle Jurassic of the British Isles. Zoological Journal of the Linnean Society 192: 1323–1362.
Panciroli, E.L., R.B.J. Benson, and Z.-X. Luo. 2019. The mandible and dentition of Borealestes serendipitus (Docodonta) from the Middle Jurassic of Skye, Scotland. Journal of Vertebrate Paleontology. https://doi.org/10.1080/02724634.2019.1621884.
Parrington, F.R. 1973. The dentitions of the earliest mammals. Zoological Journal of the Linnean Society 52: 85–95.
Plogschties, T., and T. Martin. 2020. New information on the maxilla, dentary, and dentition of Maotherium sinense, with comments on the zhangheotheriid dental formulae. PalZ 94: 155–164. https://doi.org/10.1007/s12542-019-00460-3).
Prothero, D.R. 1981. New Jurassic Mammals from Como Bluff, Wyoming, and the Interrelationships of Non-tribosphenic Theria. Bulletin of the American Museum of Natural History 167: 277–326.
Ramírez-Chaves, H.E., V. Weisbecker, S. Wroe, and M.J. Phillips. 2016. Resolving the evolution of the mammalian middle ear using Bayesian inference. Frontiers in Zoology 13: 39. https://doi.org/10.1186/s12983-016-0171-z.
Rich, T.H., T.F. Flannery, P. Trusler, A. Constantine, L. Kool, N. van Klaveren, and P. Vickers-Rich. 2001. An advanced ausktribosphenid from the Early Cretaceous of Australia. The Records of the Queen Victoria Museum 110: 1–9.
Rich, T.H., J.A. Hopson, P.G. Gill, P. Trusler, S. Rogers-Davidson, S. Morton, R.L. Cifelli, D. Pickering, L. Kool, K. Siu, F.A. Burgmann, T. Senden, A.R. Evans, B.E. Wagstaff, D. Seegets-Villiers, I.J. Corfe, T.F. Flannery, K. Walker, A.M. Musser, M. Archer, R. Pian, and P. Vickers-Rich. 2016. The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri. Alcheringa 40: 475–501. https://doi.org/10.1080/03115518.2016.1180034.
Rich, T.H., P. Vickers-Rich, A. Constantine, T.F. Flannery, L. Kool, and N. van Klaveren. 1999. Early Cretaceous mammals from Flat Rocks, Victoria, Australia. Records of the Queen Victoria Museum 106: 1–35.
Rougier, G.W., S. Apesteguía, and L.C. Gaetano. 2011. Highly specialized mammalian skulls from the Late Cretaceous of South America. Nature 479: 98–102.
Rougier, G.W., A.M. Forasiepi, R.V. Hill, and M.J. Novacek. 2009. New mammalian remains from the Late Cretaceous La Colonia Formation, Patagonia, Argentina. Acta Palaeontologica Polonica 54: 195–212.
Rougier, G.W., A.G. Martinelli, and A.M. Forasiepi. 2021a. Mesozoic mammals from South America and their forerunners. Springer Earth System Sciences. https://doi.org/10.1007/978-3-030-63862-7.
Rougier, G.W., A.G. Martinelli, A.M. Forasiepi, and M.J. Novacek. 2007. New Jurassic mammals from Patagonia, Argentina: A reappraisal of australosphenidan morphology and interrelationships. American Museum Novitates 3566: 1–54.
Rougier, G.W., G.F. Turazzinni, M.S. Cardozo, T. Harper, A.I. Lires, and L.A. Canessa. 2021b. New specimens of Reigitherium bunodontum from the Late Cretaceous La Colonia Formation, Patagonia, Argentina and meridiolestidan diversity in South America. Journal of Mammalian Evolution 2021 (28): 1051–1081. https://doi.org/10.1007/s10914-021-09585-2.
Rougier, G.W., J.R. Wible, R.M.B. Beck, and S. Apesteguía. 2012. The Miocene mammal Necrolestes demonstrates the survival of a Mesozoic notherian lineage inthe the late Cenozoic of South America. Proceedings of National Academy of Sciences USA 109: 20053–20058.
Rougier, G.W., J.R. Wible, and M.J. Novacek. 1998. Implications of Deltatheridium specimens for early marsupial history. Nature 396: 459–463.
Rougier, G.W., J.R. Wible, and M.J. Novacek. 2004. New specimens of Deltatheroides cretacicus (Metatheria, Deltatheroida) from the Late Cretaceous of Mongolia. Bulletin of Carnegie Museum of Natural History 36: 245–266.
Ruf, I., Z.-X. Luo, J.R. Wible, and T. Martin. 2009. Petrosal Anatomy and Inner Ear Structure of the Late Jurassic mammal Henkelotherium and the ear region characters of basal therian mammals. Journal of Anatomy 214: 679–693. https://doi.org/10.1111/j.1469-7580.2009.01059.x.
Sánchez-Villagra, M.R., and K.K. Smith. 1997. Diversity and evolution of the marsupial mandibular angular process. Journal of Mammalian Evolution 4: 119–144.
Scapino, R.P. 1965. The third joint of the canine jaw. Journal of Morphology 116: 23–50.
Scapino, R.P. 1981. Morphological investigation into functions of the jaw symphysis in carnivorans. Journal of Morphology 167: 339–375.
Schultz, A.H. 1935. Eruption and decay of the permanent teeth in primates. American Journal of Physical Anthropology 19: 489–581.
Schultz, A.H. 1960. Age changes in primates and their modification in man. In Human growth, ed. J.M. Tanner, 1–20. Oxford: Pergamon Press.
Schultz, J.A., B.-A.S. Bhullar, and Z.-X. Luo. 2019. Re-examination of the Jurassic mammaliaform Docodon victor by computed tomography and occlusal functional analysis. Journal of Mammalian Evolution. 26 (9–38): 2017. https://doi.org/10.1007/s10914-017-9418)(publishedonlinein.
Schultz, J.A., and T. Martin. 2011. Wear pattern and functional morphology of dryolestoid molars (Mammalia, Cladotheria). Paläontologische Zeitschrift 85: 269–285.
Schultz, J.A., and T. Martin. 2014. Function of pretribosphenic and tribosphenic mammalian molars inferred from 3D animation. Naturwissenschaften 101: 771–781.
Scott, J.E., A.S. Hogue, and M.J. Ravosa. 2012. The adaptive significance of mandibular symphyseal fusion in mammals. Journal of Evolutionary Biology 25: 661–673.
Sigogneau-Russell, D. 1991. Nouveaux mammifères theriens du Crétacé inférieur du Maroc. Comptes Rendus de l’académie des Sciences 313: 279–285.
Sigogneau-Russell, D. 1999. Réévaluation des Peramura (Mammalia, Theria) sur la base de nouveaux spécimens du Crétacé inférieur d’Angleterre et du Maroc. Geodiversitas 21: 93–127.
Sigogneau-Russell, D. 2003. Holotherian mammals from the forest marble (Middle Jurassic of England). Geodiversitas 25: 501–537.
Simpson, G.G. 1928. A catalogue of the mesozoic mammalia in the Geological Department of the British Museum (Natural History). London: Trustees of the British Museum.
Simpson, G.G. 1929. American mesozoic mammalia. Memoires of the Peabody Museum 3: 1–235.
Smith, B.H. 1994. Sequence of emergence of the permanent teeth in Macaca, Pan, Homo, and Australopithecus: Its evolutionary significance. American Journal of Human Biology 6: 61–76.
Smith, B.H. 2000. ‘Schultz’s rule’ and the evolution of tooth emergence and replacement patterns in primates and ungulates. In Development, function and evolution of teeth, ed. M.F. Teaford, M.M. Smith, and M.W.J. Ferguson, 212–227. Cambridge: Cambridge University Press.
Tsubamoto, T., G.W. Rougier, S. Isaji, M. Manabe, and A.M. Forasiepi. 2004. New Early Cretaceous spalacotheriid “symmetrodont” mammal from Japan. Acta Palaeontologica Polonica 49: 329–346.
Turnbull, W.D. 1970. Mammalian masticatory apparatus. Fieldiana Geology 18: 147–356.
Ungar, P.S. 2010. Mammal teeth: Origin, evolution, and diversity. Baltimore: Johns Hopkins University Press.
Urban, D.J., N. Anthwal, Z.-X. Luo, J.A. Maier, A. Sadier, A.S. Tucker, and K.E. Sears. 2017. A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw. Proceedings of Royal Society B 284: 1–8. https://doi.org/10.1098/rspb.2016.2416.
van Nievelt, A.F.H., and K.K. Smith. 2005. The significance of reduced functional tooth replacement in marsupial and placental mammals. Paleobiology 31: 324–346.
Vázquez-Molinero, R., T. Martin, M.S. Fischer, and R. Frey. 2001. Comparative anatomical investigations of the postcranial skeleton of Henkelotherium guimarotae Krebs, 1991 (Eupantotheria, Mammalia) and their implications on its locomotion. Mitteilungen aus dem Museum für Naturkunde in Berlin, Zoologische Reihe 77: 207–216.
Wang, H.-B., S. Hoffmann, D.-C. Wang, and Y.-Q. Wang. 2022. A new mammal from the Lower Cretaceous Jehol Biota and implications for eutherian evolution. Philosophical Transactions of the Royal Society B 377: 20210042. https://doi.org/10.1098/rstb.2021.0042.
Wang, Y.-Q., Y.-M. Hu, J. Meng, and C.-K. Li. 2001. An ossified Meckel’s cartilage in two Cretaceous mammals and origin of the mammalian middle ear. Science 294: 357–361.
Wible, J.R., and G.W. Rougier. 2017. Craniomandibular anatomy of the subterranean meridiolestidan Necrolestes patagonensis Ameghino, 1891 (Mammalia, Cladotheria) from the Early Miocene of Patagonia. Annals of Carnegie Museum 84: 183–252.
Wible, J.R., G.W. Rougier, M.J. Novacek, and R.J. Asher. 2009. The eutherian mammal Maelestes gobiensis from the late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bulletin of the American Museum of Natural History 327: 1–123.
Zeller, U. 1989. Die Entwicklung und Morphologie des Schädels von Ornithorhyncus anatinus (Mammalia: Prototheria: Monotremata). Abhandlungen der senckenbergischen naturforschenden Gesellschaft 545: 1–188.
Zhou, C.-F., B.-A.S. Bhullar, A.I. Neander, T. Martin, and Z.-X. Luo. 2019. New Jurassic mammaliaform sheds light on early evolution of mammal-like hyoid bones. Science 365: 276–279. https://doi.org/10.1126/science.aau9345.
Acknowledgements
Field work in Portugal was generously supported by the Serviços Geológicos de Portugal (Lisbon). Special thanks are due to the former director of this institution F. Moitinho de Almeida and his successor M. Ramalho for their long-term goodwill. E. Eggert (Drescher) masterfully prepared the fossils. M. Bulang-Lörcher (both Free University Berlin) drew the mandible of Fig. 6. D. Kranz provided the drawings of Fig. 8, and G. Oleschinski (both University of Bonn) assisted at the SEM; he and Sven Tränkner (Senckenberg Research Institute, Frankfurt) provided the photographs. April I. Neander (The University of Chicago) scanned the fossil specimens and meticulously executed a large volume of 3D renderings for this study. During this study, we benefited from extensive discussion from A. O. Averianov, K. R. K. Jäger, and G. W. Rougier. Luo likes to thank G. W. Rougier for the opportunity to see the meridiolestidan specimens. D. R. Prothero graciously granted the permission to re-use graphics. Our manuscript benefitted from constructive comments by reviewers Pam Gill (University of Bristol) and Elsa Panciroli (University of Oxford).The excavations in the Guimarota mine (1973-1982) were financed by the Freie Universität Berlin and the German Research Foundation (DFG). Z.-X. Luo was funded by a Research Award by the Alexander von Humboldt-Foundation (Bonn) and Division of Biological Sciences of the University of Chicago. T. Martin was granted a sabbatical leave by the University of Bonn.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling Editor: Thomas Mörs.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, ZX., Martin, T. Mandibular and dental characteristics of the Late Jurassic mammal Henkelotherium guimarotae (Paurodontidae, Dryolestida). PalZ 97, 569–619 (2023). https://doi.org/10.1007/s12542-023-00651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-023-00651-z