Abstract
The sequence of eruption of the second generation of teeth varies across taxa, is highly functional, and is strongly influenced by genetic effects. We assessed postcanine dental eruption sequence across artiodactyls in order to test two hypotheses: 1) dental eruption sequence is a good phylogenetic character for artiodactyls; and, 2) eruption sequence is adaptive and associated with life history variables like postnatal growth and longevity in artiodactyls (Schultz’s Rule). We examined postcanine eruption sequence in 81 genera (100 species) spanning ten families of Artiodactyla. Our ancestral state reconstruction supports the interpretation that the third molar erupted last in the ancestor of Artiodactyla, and that the fourth premolar erupted after the third molar in the ancestor of Ruminantia. Our results indicate that eruption of the third molar last evolved secondarily in the caprines, likely sometime in the Miocene. Overall, our results support the hypothesis that dental eruption sequence is phylogenetically conserved in artiodactyls. Caprines occupy high elevation habitats, and we hypothesize that evolution of their unique dental eruption sequence may be associated with limited resource availability in high elevation mountain systems and the necessity to process a wide range of vegetation types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most mammals are diphyodont meaning they have two generations of teeth: the deciduous (milk) teeth and the adult teeth. These two generations of teeth allow for overall size and complexity to increase while growing from juvenile to adult, matching changes in overall body size and shape (Hillson 2005). The timing of tooth eruption is highly correlated between teeth, particularly in the postcanine dentition (Smith 1994), and is generally considered to be adaptive. This tight correlation is not surprising when considering that the timing of tooth eruption is constrained by many other factors, including the size of the face and dental arch, timing of permanent tooth development, juvenile and adult diets, life history, and even group dynamics, as represented by intrasex competition (Schultz 1935, 1956; Bryant 1990; Smith 1994, 2000; Leigh et al. 2008).
Many dental eruption studies have focused on primates and found that the sequence of eruption of the second generation of teeth varies across taxa, is highly functional, and is strongly influenced by genetic effects (Schultz 1935, 1956; Bryant 1990; Smith 1994, 2000; Wise et al. 2002; Suri et al. 2004; Leigh et al. 2008). There are generally two lines of thinking about dental eruption sequence: 1) dental eruption sequence is a good phylogenetic character in primates, and likely other mammals (Schwartz 1974; Tattersall and Schwartz 1974; Byrd 1981; Veitschegger and Sánchez-Villagra 2016), or 2) dental eruption sequence is adaptive and associated with life history variables like postnatal growth and longevity (Schultz 1956, 1960; Smith 1994, 2000).
Schultz (1956, 1960) originally hypothesized that dental eruption sequence is associated with the rate of postnatal growth in primates. This led to the proposal of Schultz’s Rule, which states that replacement teeth erupt earlier in long-lived species with a slower rate of growth, also known as a slower life history (Smith 2000). As the molars have no deciduous complement in heterodont mammals (e.g., Osborn and Crompton 1973; Järvinen et al. 2009), the premolars are the replacement teeth of the postcanine dentition. Smith (2000) tested Schultz’s Rule in ungulates and found that several faster-growing species erupt all the molars before other permanent teeth, while slower-growing species erupt the molars later in the sequence. Smith (2000) also found that maximum lifespan across 20 species of ungulates is significantly correlated with dental eruption sequence (calculated by the eruption of either I1 or P3 relative to the mandibular molars), suggesting that ungulates tend to follow Schultz’s Rule, although primates appear to adhere to it even more so. In order to test the hypothesis that dental eruption sequence is a good phylogenetic character across mammals, we conducted a much more comprehensive examination of dental eruption sequence across artiodactyls and incorporated the most appropriate and up-to-date phylogenetic analyses.
Artiodactyls are a large group of hooved mammals (with more than 200 species; Geisler et al. 2007; Hassanin et al. 2012) that evolved approximately 70 Ma in Eurasia (Hassanin et al. 2012) and are nested paraphyletically within Artiodactyla (Geisler et al. 2007). The geographic origin of artiodactyls is somewhat obscured in the fossil record, as fossil artiodactyls have been recovered in North America, Europe, and Asia, all dated to the early Eocene (~55 Ma; Theodor et al. 2007). These small-bodied early artiodactyls have been classified taxonomically as Dichobunidae (McKenna and Bell 1997), or alternatively, broken down in two families, Dichobunidae and Diacodexeidae (Theodor et al. 2007). While artiodactyls are rare in the early Eocene, a presumed morphological radiation led to a diversity of forms by the middle Eocene (Theodor et al. 2007). Since then, Artiodactyla has diversified to include more than 200 extant terrestrial taxa, as well as the cetaceans (Geisler et al. 2007; Hassanin et al. 2012).
Artiodactyls are globally distributed, almost exclusively herbivorous, and play significant roles in their local ecosystems (Vrba and Schaller 2000a). Additionally, many species are habitat specialists that respond strongly to environmental change (e.g., Vrba and Schaller 2000a; Brooks et al. 2001; Buzan et al. 2013). With the exception of Suina, artiodactyls are obligate herbivores, and many of the groups have evolved specialized feeding techniques including a dependency on bacterial fermentation (Vrba and Schaller 2000a). As herbivorous grazers consuming large quantities of grasses and plant matter, artiodactyls have a significant impact on ecosystems and ecology (Owen-Smith et al. 2010), and grazing and foraging by artiodactyls has been linked to vegetation changes in density, succession sequence, and composition (e.g., Molvar et al. 1993; Bowyer 1997).
Artiodactyls also represent the group most heavily domesticated by humans: pigs, goats, sheep, cows, and llamas are some of the domestic artiodactyls utilized by humans around the world (Vrba and Schaller 2000a). The impact that artiodactyls have had in human history is unrivaled by any other mammalian group, in terms of agriculture and economics, as well as in environmental impact (Gentry 2000). The majority of human-use domestic animals are artiodactyls, and they serve as the world’s largest source of farmed meat, milk, skins, and wools (Gentry 2000). Overall, artiodactyls as a group share many features including a generalized body plan, life history, and diet (Vrba and Schaller 2000a). Artiodactyls also have a well-sampled fossil record, and many share a relatively ancestral dental formula with teeth in all four dental classes (i.e., molars, premolars, incisors, and canines in some groups) (Hillson 2005).
Our work investigates generic-level variation in dental eruption sequence in artiodactyls. Previous work has demonstrated the modularity of the dentition in primates and mice, where the anterior teeth are genetically and phenotypically independent of the postcanine teeth (Hlusko et al. 2011). With this in mind, we restrict our study to the functional grinding teeth in herbivores, the postcanine dentition (premolars and molars). Specifically, we hypothesize that: H1) dental eruption sequence is phylogenetically conserved in artiodactyls, or more similar in more closely related genera, and H2) eruption of the third molar last is the ancestral condition in this group.
Materials and Methods
Materials
For this study, we examined postcanine dental eruption sequence in 81 genera (100 species) spanning ten families of Artiodactyla (Table 1). All examined specimens are held in the collections of the Museum of Vertebrate Zoology, Berkeley, CA (n = 726) or the National Museum of Natural History, Washington, D.C. (n = 3445). We visually examined specimens across ontogenetic stages. Eruption sequence was defined as either having the third molar erupt before the fourth premolar, vice versa, or simultaneous (Fig. 1). When eruption of the fourth premolar and third molar seemed to be at the same stage in multiple specimens, the dental eruption sequence for the genus was scored as simultaneous. At least two occurrences of eruption sequence for each genus were required to confidently assess the relationship between eruption of the fourth premolar and the third molar (exceptions are Bos, Bubalus, Catagonus, Hippocamelus, Hylochoerus, Nilgiritragus, Ozotoceros, Pelea, Phacochoerus, Pseudois, and Rucervus; Table 2). We examined eruption sequence in both the maxilla and mandible of each specimen where available, and from here forward, we use the mandibular notation (tooth type abbreviation followed by tooth number in subscript) when discussing particular teeth. While we found no difference in eruption sequence between maxilla and mandible, we did note that eruption tends to occur earlier in the mandible than in the maxilla of an individual.
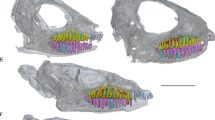
Examples of different eruption sequences in the mandibles of terrestrial Artiodactyla. a Eruption of the M3 last in Capra caucasica. b Eruption of the P4 after the M3 in Odocoileus hemionus. c Simultaneous eruption of M3 and P4 in Oreamnos americanus. M is molar, P is premolar, d is deciduous, and the number denotes tooth number. E.g., dP4 is deciduous premolar 4
Intraspecific Variation in Dental Eruption Sequence
We systematically surveyed all Ovis craniodental specimens available at the Museum of Vertebrate Zoology (Ovis canadensis n = 176, Ovis dalli n = 9) to assess intraspecific variation in dental eruption sequence. We classified the state of eruption of each postcanine tooth from 1 to 3, where a score of 1 is unerupted, 2 is erupting, and 3 is fully erupted. Our definition of erupting (Score 2) is when the tooth has erupted above the level of the maxilla/mandible when viewed lingually, but is not yet fully erupted. All four postcanine quadrants were scored when available (left and right, maxillary, and mandibular). In cases where a tooth was missing by wear, but the rest of the jaw was clearly fully erupted and fully worn, a score of fully erupted was given for that tooth.
Of the 185 Ovis specimens systematically surveyed at the MVZ, n = 48 are in the process of erupting one or more of the postcanine teeth. The sample is further reduced when comparing third molar and fourth premolar eruption. Only n = 15 specimens, all Ovis canadensis, are in the process of erupting either the fourth premolar, the third molar, or both. Within that small sample, n = 4 specimens show the fourth premolar fully erupted while the third molar is still in the process of erupting. There is no variability among these four specimens: all postcanine teeth are fully erupted except the third molar. In contrast, not a single specimen has a third molar that is fully erupted while the fourth premolar is still erupting or unerupted. Within this large sample size, we find no variability in the phenotype of the third molar erupting last. Based on this analysis, we are confident in assuming that our determination of eruption sequence based on sample sizes of at least two observations is fairly sound. Given the large sample sizes needed to test this more fully, absolute certainty will be difficult to accomplish. The full data from the test for intraspecific variation are available in Online Resource 1.
Ancestral State Reconstruction
In order to quantify the evolutionary context of the phenotype, we ran an ancestral state reconstruction using the distribution of extant dental eruption sequences. Also known as character mapping, ancestral state reconstruction statistically predicts the state of each node in the phylogeny based on the distribution of eruption sequences across extant taxa (Felsenstein 1985; Coddington 1988; Donoghue 1989). For this reconstruction, we mapped the dental eruption sequences onto the artiodactyl phylogeny by creating a character state matrix. We ran the ancestral state reconstruction in Mesquite with the Mk1 likelihood method (Maddison and Maddison 2015) using molecular data (six autosomal and 14 mitochondrial genes) from the 10ktrees database (Arnold et al. 2010). In cases where we have phenotypic data for a species but molecular data are not available, we used congeners in the phylogeny (marked by an asterisk). We also ran the ancestral state reconstruction using parsimony, but it gave the same results and we decided to defer to the likelihood method for further analysis. It is important to note that debate continues on the phylogenetic relationships between crown artiodactyls. In particular, the exact timing and sequence of divergence of Suina, Camelidae, Hippopotamidae, and Ruminantia are still under considerable debate (Matthee et al. 2001; Murphy et al. 2001; Spaulding et al. 2009; Meredith et al. 2011; Zhou et al. 2011; Hassanin et al. 2012). We chose to use the phylogeny from 10ktrees, and the divergence of Suina is placed basal to Camelidae and Hippopotamidae in this study. Specimens classified as “likely” erupting P4 after M3 were excluded from the ancestral state reconstruction.
Test for Phylogenetic Signal
In order to test for phylogenetic signal in dental eruption sequence, we ran the D-statistic in the R statistical program v3.1.2 (R Core Team 2016) using caper (Orme et al. 2015). The D-statistic tests the hypothesis that a phenotype is more phylogenetically conserved across the tree than expected under Brownian motion (Orme et al. 2015). The D-statistic is used expressly for binary traits and was therefore only applied to dental eruption sequence in this study. A D-value less than 1 indicates phylogenetic conservatism relative to random Brownian motion (Orme et al. 2015).
To test the non-binary life history and body size traits, we ran a Blomberg’s K analysis using phylosignal in the picante package (Kembel et al. 2010). Blomberg’s K tests whether a particular character is present in related taxa more frequently than expected by Brownian motion (Blomberg et al. 2003). The K-value for a trait can be either greater than 1, equal to 1, or less than 1. A K-value of K = 1 generally indicates neutral evolution of the trait, while K > 1 generally suggests that the trait is phylogenetically conserved relative to Brownian motion. In contrast, K < 1 is generally interpreted as less associated with phylogeny than expected from the null, although rapid divergence or heterogeneous rates of genetic drift can also result in a low K-value (Blomberg et al. 2003; Revell et al. 2008).
For these phylogenetic analyses, we trimmed the tree down to only those species for which dental eruption sequence was confidently assessed (i.e., not “likely” or simultaneous), and for which molecular data were available. In order to account for phylogenetic uncertainty, we ran both the D-statistic and Blomberg’s K through 25 iterations, using 25 of the likely phylogenies generated by the 10ktrees analyses. The averages of the 25 iterations were taken as our values of D and K for analysis of phylogenetic signal.
Life history and body size variables. We collected life history and body size data for the majority of artiodactyls included in this study. Ranges for each trait were taken from the literature, with the exception of average maximum lifespan, the ranges for which were collected from AnAge, part of the Human Ageing Genomic Resources database (Tacutu et al. 2013). For this study, we investigated average maximum lifespan, litter size, length (cm), height (cm), and body weight (cm). Body weight values were taken from the average for the male of the species, but length and height values were not specified by sex. All values, with the exception of litter size, were log-transformed for the analyses. We calculated phylogenetic independent contrasts across our phylogenetic tree using the ape package in R (Paradis et al. 2004). This statistic allows for the comparison of means between traits while taking into account the phylogeny and the number of independent trait occurrences (Ricklefs and Starck 1996). All independent contrasts were run through 100 iterations to account for phylogenetic uncertainty. Raw data and references for life history and body size variables are available in Online Resource 2.
Results
Variation in Eruption Sequence across Artiodactyls
We definitively assessed postcanine eruption sequence in 62 genera (81 species). Of those, 45 genera have a sequence where the fourth premolar (P4) erupts later than the third molar (M3), 14 have a sequence where the M3 erupts last, and three have a sequence with approximately simultaneous eruption of P4 and M3 (Table 2). We found four more genera that likely have the P4 erupting after the M3, and one genus that likely has the M3 erupting last, but we need a larger sample size to definitively confirm these sequences. We were unable to definitely assess dental eruption sequence in 14 genera based on a lack of available specimens at appropriate ontogenetic stages. We found no evidence of intrageneric variation in dental eruption sequence in this study.
Overall, we successfully sampled the breadth of the artiodactyl phylogeny, and our results clearly demonstrate a phylogenetic pattern (Fig. 2). Genera sampled in Artiodactyla families Camelidae, Hippopotamidae, Suidae, and Tayassuidae all erupt the third molar last. In contrast, nested within the artiodactyl clade, Ruminantia families Bovidae, Moschidae, Cervidae, Antilocapridae, and Tragulidae almost exclusively erupt the P4 last, although there are some exceptions. Of the 52 ruminant genera we examined, 45 erupt the P4 last. The exceptional genera are Capra, Hemitragus, Nilgiritragus, Oreamnos, Oryx, Ourebia, and Ovis.
Ancestral state reconstruction of dental eruption sequence across Artiodactyla. Yellow indicates that the third molar (M3) erupts last. Black indicates that the fourth premolar (P4) erupts after the M3. Blue indicates that M3 and P4 erupt simultaneously. See methods for information about construction of the phylogeny. See Table 3 for dates and likelihoods at numbered nodes. Asterisks denote congeners used in place of related species for which we have phenotypic but no molecular data. Double asterisks denote species with an alternate genus level classification
Capra, Hemitragus, Nilgiritragus, and Ovis all erupt the M3 last, uniquely among Ruminantia. These four genera are all part of subfamily Caprinae. Due to small sample sizes, we were unable to definitely assess dental eruption sequence in some of the other members of Caprinae (Arabitragus, Ammotragus, and Pantholops). But several of the caprine genera sampled here erupt the M3 last. Based on the distribution of dental eruption sequences across the artiodactyl phylogeny, and supported by our ancestral state reconstruction, the eruption of the M3 last is either a reversal to the ancestral state, or secondarily derived, in subfamily Caprinae.
Oryx, Ourebia, and Oreamnos appear to erupt the P4 and M3 approximately simultaneously, but this approximation could be the result of limited sample size. With a larger sample spanning a greater ontogenetic timeframe, it may be possible to refine this approximation and definitively assess whether the P4 or M3 erupts later. Oryx (tribe Hippotragini) and Ourebia (tribe Antilopini) are both African bovids with a primary diet of grasses supplemented by other vegetation. Recent analyses suggest that Hippotragini and Antilopini diverged 15 to 13 Ma (Bibi 2013). There is some suggestion in the literature that Oreamnos may erupt the M3 after the P4 (Brandborg 1950), but other reports are contradictory (Kerr 1966). The North American mountain goat (Oreamnos americanus) is closely related to Capra, Hemitragus, Ovis, and other genera in subfamily Caprinae. Overall, our data show that subfamily Caprinae is unique among Ruminantia in having a cluster of genera that erupt the M3 last (Capra, Hemitragus, Nilgiritragus and Ovis), as well as one with simultaneous eruption (Oreamnos).
Ancestral State Reconstruction
Our ancestral state reconstruction supports that the third molar erupted last in the ancestor of Artiodactyla with 89.0% likelihood (Fig. 2; Table 3). An equally high likelihood of 86.1% supports that the ancestor of artiodactyls (excepting Suina) erupted the third molar last. The likelihood of the third molar erupting last is reduced to 78.8% for the ancestor of Ruminantia + Hippotamidae. After the divergence of Hippotamidae, our ancestral state reconstruction finds 87.4% likelihood that the P4 erupted after the M3 in the ancestor of Ruminantia. It is important to note that, as our test of intraspecific variation was completed in a ruminant taxon, it is possible that non-ruminant artiodactyls are more variable in their eruption sequence. Greater sampling, particularly of Camelidae and Hippopotamidae, and their fossil relatives, will further resolve the support for changes in dental eruption sequence at more basal nodes of the phylogeny.
The most likely character state is more ambiguous at the base of subfamily Caprinae. Greater sampling, as well as a more resolved phylogeny, could likely clarify the state at these nodes. Our ancestral state reconstruction supports that the ancestor of Caprinae is 0.0% likely to have erupted the third molar last. The likelihood that the third molar erupted last at the node between Pseudois and Capra is slightly higher at 1.3%. It is not until the ancestor of Hemitragus and Capra that we find 98% likelihood that the third molar erupted last. A similar pattern is found along the branch of the phylogeny leading to Ovis and Nilgiritragus.
Test for Phylogenetic Signal
All of the life history and body size variables analyzed here have a significant phylogenetic signal (p < 0.05), meaning that the distribution of each trait across the phylogeny is significantly different from a neutral model of Brownian motion (Table 4). Dental eruption sequence is very phylogenetically conserved (D-value <0). Average litter size is also phylogenetically conserved (K-value 1.20). A K-value <1 is indicative of a trait being less phylogenetically conserved than expected when compared to the neutral model of Brownian motion, although low K-values can also result from rapid divergence or heterogeneous rates of genetic drift (Revell et al. 2008). Average body weight, height, length, and maximum lifespan all have K-values significantly less than 1 in this study. Overall, average height and length have the lowest K-values, and, with the exception of dental eruption sequence, average litter size is the only life history or body size trait with a K > 1, suggesting strong phylogenetic signal for this trait.
Life History and Body Size Variables
Although the life history and body size variables have significant phylogenetic signals, none of them are significantly correlated with dental eruption sequence. None of the phylogenetic independent contrasts between life history or body size variables and dental eruption sequence in artiodactyls are significant (Table 5). These data suggest that dental eruption sequence and the life history and body size variables sampled here, including maximum lifespan, are not correlated.
Discussion
Our results demonstrate that eruption of the fourth premolars after the third molars is an apomorphy for Ruminantia within Artiodactyla. Based on the fossil record and molecular data for this group, this eruption sequence probably evolved ~40–55 Ma (Gentry 2000; Métais and Vislobokova 2007; Hassanin et al. 2012). The fossil record of Ruminantia extends to approximately 55 Ma, spanning North America and Asia (Métais and Vislobokova 2007; Theodor et al. 2007). Within the extant artiodactyls, the ruminant clade is comprised of all artiodactyls excepting the suids, tayassuids, hippopotamids, and camelids, and is defined by three primary hard tissue features: the fusion of the cuboid and navicular tarsals, the loss of upper incisors, and the incisiform lower canines (Métais and Vislobokova 2007). Ruminantia are also characterized by a suite of morphological and behavioral phenotypes that are associated with their unique diet and obligate herbivory (Vrba and Schaller 2000a). We would add eruption of the fourth premolar after the third molar to this list, excepting subfamily Caprinae and the two bovid genera with simultaneous eruption.
We find no evidence that major life history or body size variables correlate with this difference in eruption sequence. Rather, the synapomorphic chewing complex shared by Ruminantia may have played a role in the evolution of this derived dental eruption sequence. Evolution of delayed premolar eruption in the ancestor of Ruminantia may be associated with diet, jaw morphology, and/or the biomechanics of chewing. Ruminantia are characterized by an unfused mandible and forward placement of the masseter muscle, one of the most important chewing muscles in mammals (e.g., Janis 1995; Hogue and Ravosa 2001). Additionally, interproximal tooth wear at the junction between the P4 and M1 is common in Ruminantia (pers. obs.), frequently resulting in a marked concavity. This suggests that factors such as intense chewing strain and force during feeding, enamel thickness, and mesial drift may contribute to extensive wear at the P4/M1 junction (Fortelius 1985; Pfretzschner 1992; Macho and Berner 1993). Later eruption of the fourth premolars may have evolved as a buffer to this strain, ensuring that a strong chewing surface is available for a longer period of time before wear causes serious problems and even death.
The early Miocene was characterized by a period of relatively warm and stable temperatures, referred to as the Miocene Climatic Optimum (Böhme 2003). During this Optimum (17–15 Ma), many groups of animals, including the artiodactyls, radiated rapidly, evolving new traits and exploiting the new habitats created by changing climate and vegetation shifts (Janis 1989; Zachos et al. 2001; Zhou et al. 2012). Changes in pressure systems also changed aridity in other areas of the world expanding open grasslands, savannah, and steppe plains at both high and low elevations (Janis 1989). After the warmer Miocene, temperatures cooled in the early Pliocene resulting in the further spread of grasslands (Strömberg 2011). Also during this time, the uplifting of mountain ranges like the Himalayas, the East African Rift System, and the Rockies created new mountain habitats yet to be occupied by the radiating artiodactyls (Wolfe 1985; Janis 1989). As competition increased, the ancestor of the caprines moved into a high elevation niche, likely exploiting this novel habitat in response to changing ecosystems.
Our results indicate that either an evolutionary reversal or a secondary evolution of the ancestral dental eruption sequence occurred in subfamily Caprinae at least once and possibly twice. Based on our ancestral state reconstruction, the ancestor of Hemitragus and Capra was erupting the third molar last with 98.0% likelihood. The molecular split between these two genera likely occurred ~4.5 Ma (Ropiquet and Hassanin 2005), although fossils date the first appearance of Capra around 1.9 Ma (Bibi et al. 2012). Molecular data and the fossil record place the evolution of subfamily Caprinae sometime in the Miocene, approximately 6–15 Ma: the wide range reflects the range of opinions on the precise date (Fernández and Vrba 2005; Lalueza-Fox et al. 2005; Ropiquet and Hassanin 2006). The earliest fossil occurrence of subfamily Caprinae is hypothesized to be Protoryx enanus from the middle Miocene of Europe (Köhler 1987; Gentry and Heizmann 1996). Like many ungulate groups, caprines underwent a major radiation in the Miocene (Gentry 2000; Vrba and Schaller 2000b). The exact phylogenetic relationships of extant taxa in subfamily Caprinae are still under much debate (e.g., Hassanin et al. 1998; Hassanin and Douzery 1999; Shafer and Hall 2010; Bibi 2013).
To this day, caprines are characterized by their utilization of high elevation habitats, rare among artiodactyls, and they can be found at the tops of mountains around the world (Vrba and Schaller 2000b). Vegetation quality, density, and composition changes along an altitudinal gradient (Vázquez and Givnish 1998; Shaheen and Shinwari 2012). The vegetation at high elevations is less diverse (e.g., Tranquillini 1964), and many plants die back completely under the snow (e.g., Khan et al. 2013). It is possible that evolution of the unique dental eruption sequence in caprines may be associated with resource availability in high elevation habitats. A shift in diet due to ancestral changes in habitat occupation may have altered the chewing stroke and bite force in goats and their relatives. Later eruption of the third molars guarantees that grinding teeth will be available longer in the lifespan of the animal, potentially improving overall fitness. There are a few other unique ruminants that occupy high elevation niches, particularly in the Tibetan plateau (e.g., cervid Cervus albirostris [formerly Przewalskium], bovid Bos mutus, and caprine Pantholops hodgsonii; Leslie 2010; Ge et al. 2013), but we were not able to sample these taxa in the current study. Their rarity is reflected in their poor representation in many museum collections. Further work examining these high elevation species, as well as directly comparing the quality and diversity of diets in Caprinae compared to other high elevation and non-elevation ruminants, will allow for more thorough testing of this hypothesis.
Fossil Evidence
An overview of artiodactyl fossil evidence supports the timeline produced by our ancestral state reconstruction. Fossil suid specimen KNM-SH 38051 (Nyanzachoerus), a right mandible with P4-M3, clearly shows the fourth premolar erupted and in occlusion while the third molar is still in the process of erupting (Tsujikawa 2005). This Miocene fossil provides evidence that suids have been erupting the third molar last for at least ten million years, and likely since their purported divergence with the ancestral artiodactyl group in the Eocene (Kumar and Hedges 1998). In contrast, Diplobune, a European artiodactyl that lived approximately 35 Ma, erupted the fourth premolars after the third molars (Sudre 1974; Blondel 2001; Erfurt and Métais 2007). The date of this fossil and dental eruption sequence accord directly with our ancestral state reconstruction detailing the evolution of this phenotype approximately 40–55 Ma, and suggest that either this trait is homoplastic or that Diplobune may have been a ruminant.
While we were unable to definitively assess dental eruption sequence for extant Giraffidae in this study, personal examination of a fossil giraffe from the Pleistocene of Ethiopia (BOU-VP-1/30) gives at least one example of dental eruption sequence in this group. BOU-VP-1/30 is an excellently preserved right maxilla that clearly shows the M3 fully erupted while the P4 is in the process of erupting. This fossil provides some limited evidence that Giraffidae, like other ruminants, likely erupts the P4 after the M3.
Fossil evidence from subfamily Caprinae also supports the results of our ancestral state reconstruction. Myotragus, a fossil goat from the Balearic Islands of Spain, erupts the third molars last like other extant caprines (Jordana et al. 2013). This extinct animal lived exclusively on the Balearic Islands from approximately 5 Ma until about 10,000 years ago (Jordana and Köhler 2011). Interestingly, eruption of the third molars in this island goat is even later than is seen in the rest of the subfamily (Bover and Alcover 1999; Jordana and Köhler 2011). Jordana and Köhler (2011) argued that the limited resources of the island habitat further influenced the timing of eruption in this group.
Conclusion
These data show a clear phylogenetic signal for variation in dental eruption sequence across Artiodactyla. All four extant artiodactyl families basal to Ruminantia erupt the third molar last (Suidae, Tayassuidae, Camelidae, and Hippopotamidae). In contrast, almost all Ruminantia erupt the fourth premolar after the third molar. Based on fossil and molecular evidence, the character state change, from erupting M3 last to erupting P4 after the M3, occurred in the ancestor of Ruminantia between 40 and 55 Ma, during the Eocene.
Uniquely among Ruminantia, four genera in subfamily Caprinae erupt the third molar last. Another genus in the subfamily has almost simultaneous eruption of the fourth premolar and third molar, a character state that is relatively rare among artiodactyls. Parsimony suggests that the evolution of this dental eruption sequence, either as a reversal or a secondary derivation, occurred at least once and possibly twice in the Miocene ancestors of Capra and Ovis.
Overall, these data support the hypothesis that dental eruption sequence is phylogenetically conserved in artiodactyls, and fail to support the hypothesis that either lifespan or other life history and body size traits are significantly associated with dental eruption sequence. In the case of the artiodactyls, Schultz’s Rule does not apply. By highlighting a dental trait with a strong phylogenetic signal that extends into the Eocene, we offer an additional phenotype that can be used to weigh in on ongoing phylogenetic debates about relationships among extant and extinct taxa. As we explore the phylogenetic signal of dental eruption sequence in other taxa, we will be able to better understand the potential drivers of variation in this trait.
References
Arnold C, Matthews LJ, Nunn CL (2010) The 10kTrees website: a new online resource for primate phylogeny. Evol Anthropol 19:114–118
Bibi F (2013) A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol Biol 13(1):166–171
Bibi F, Vrba E, Fack F (2012) A new African fossil caprin and a combined molecular and morphological bayesian phylogenetic analysis of caprini (Mammalia: Bovidae). J Evol Biol 25(9):1843–1854
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57(4):717–745
Blondel C (2001) The Eocene–Oligocene ungulates from Western Europe and their environment. Palaeogeogr Palaeoclimatol Palaeoecol 168(1):125–139
Böhme M (2003) The Miocene Climatic Optimum: evidence from ectothermic vertebrates of Central Europe. Palaeogeogr Palaeoclimatol Palaeoecol 195(3):389–401
Bover P, Alcover JA (1999) The evolution and ontogeny of the dentition of Myotragus balearicus Bate, 1909 (Artiodactyla, Caprinae): evidence from new fossil data. Biol J Linn Soc 68(3):401–428
Bowyer R (1997) The role of moose in landscape processes: effects of biogeography, population dynamics and predation. In: Bissonette J (ed) Wildlife and Landscape Ecology: Effects of Sequence and Scale. Springer-Verlag, New York, pp 265–287
Brandborg SM (1950) The life history and ecology of the mountain goat in Idaho and Montana. Dissertation, University of Idaho
Brooks DM, Tarifa T, Rojas JM, Vargas RJ, Aranibar H (2001) A preliminary assessment of the mammalian fauna of the eastern Bolivian panhandle. Mammalia 65(4):509–520
Bryant HN (1990) Implications of the dental eruption sequence in Barbourofelis (Carnivora, Nimravidae) for the function of upper canines and the duration of parental care in sabretoothed carnivores. J Zool 222(4):585–590
Buzan EV, Bryja J, Zemanová B, Kryštufek B (2013) Population genetics of chamois in the contact zone between the Alps and the Dinaric Mountains: uncovering the role of habitat fragmentation and past management. Conserv Genet 14(2):401–412
Byrd KE (1981) Sequences of dental ontogeny and callitrichid taxonomy. Primates 22(1):103–118
Coddington JA (1988) Cladistic tests of adaptational hypotheses. Cladistics 4(1):3–22
Donoghue MJ (1989) Phylogenies and the analysis of evolutionary sequences, with examples from seed plants. Evolution 43(6):1137–1156
Erfurt J, Métais G (2007) Endemic European Paleogene artiodactyls. In: Prothero DR, Foss SE (eds) The Evolution of Artiodactyls. Johns Hopkins University Press, Baltimore, pp 59–84
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Fernández MH, Vrba ES (2005) A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of the extant ruminants. Biol Rev 80(2):269–302
Fortelius M (1985) Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zoologica Fennica 180:1–76
Ge RL, Cai Q, Shen YY, San A, Lan M, Zhang Y, Yi X, Chen Y, Yang L, Huang Y, He R, Hui Y, Hao M, Li Y, Wang B, Ou X, Xu J, Zhang Y, Wu K, Geng C, Zhou W, Zhou T, Irwin D, Yang Y, Ying L, Bao H, Kim J, Larkin D, Ma J, Lewin H, Xing J, Platt II R, Ray D, Auvil L, Capitanu B, Zhang X, Zhang G, Murphy R, Wang J, Zhang Y, Wang J (2013) Draft genome sequence of the Tibetan antelope. Nat Commun 4:1–7
Geisler JH, Theodor JM, Uhen MD, Foss SE (2007) Phylogenetic relationships of cetaceans to terrestrial artiodactyls. In: Prothero DR, Foss S (eds) The Evolution of Artiodactyls. John Hopkins University Press, Baltimore, pp 19–31
Gentry AW (2000) The ruminant radiation. In: Vrba ES, Schaller GB (eds) Antelopes, Deer, and Relatives. Yale University Press, New Haven, pp 11–25
Gentry AW, Heizmann EPJ (1996) Miocene ruminants of the central and eastern Tethys and Paratethys. In: Bernor R, Fahlbusch V, Mittmann H (eds) The Evolution of Western Eurasian Neogene Mammal Faunas. Columbia University Press, New York, pp 378–391
Green MJB (1987) Scent-marking in the Himalayan musk deer (Moschus chrysogaster). J Zool 1:721–737
Grzimek B (1990) Grzimek’s animal Life Encyclopedia: Mammals I-IV. McGraw-Hill Publishing Company, New York
Hassanin A, Douzery EJ (1999) The tribal radiation of the family Bovidae (Artiodactyla) and the evolution of the mitochondrial cytochrome b gene. Mol Phylogenet Evol 13(2): 227–243
Hassanin A, Pasquet E, Vigne JD (1998) Molecular systematics of the subfamily Caprinae (Artiodactyla, Bovidae) as determined from cytochrome b sequences. J Mammal Evol 5(3):217–236
Hassanin, A, Delsuc F, Ropiquet A, Hammer C, van Vuuren BJ, Matthee C, Ruiz-Garcia M, Catzeflis F, Areskoug V, Nguyen TT, Couloux A (2012) Sequence and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. C R Biol 335(1):32–50
Hillson S (2005) Teeth. Cambridge University Press, Cambridge
Hlusko LJ, Sage RD, Mahaney MC (2011) Modularity in the mammalian dentition: mice and monkeys share a common dental genetic architecture. J Exp Zool B Mol Dev Evol 316(1):21–49
Hogue AS, Ravosa MJ (2001) Transverse masticatory movements, occlusal orientation, and symphyseal fusion in selenodont artiodactyls. J Morphol 249(3):221–241
Janis CM (1989) A climatic explanation for patterns of evolutionary diversity in ungulate mammals. Palaeontology 32(3):463–481
Janis CM (1995) Correlations between craniodental morphology and feeding behavior in ungulates: reciprocal illumination between living and fossil taxa. In: Thomason J (ed) Functional Morphology in Vertebrate Paleontology. Cambridge University Press, New York, pp 76–98
Järvinen E, Tummers M, Thesleff I (2009) The role of the dental lamina in mammalian tooth replacement. J Exp Zool B Mol Dev Evol 312(4):281–291
Jordana X, Köhler M (2011) Enamel microstructure in the fossil bovid Myotragus balearicus (Majorca, Spain): implications for life-history evolution of dwarf mammals in insular ecosystems. Palaeogeogr Palaeoclimatol Palaeoecol 300(1):59–66
Jordana X, Marín-Moratalla N, Moncunill-Solé B, Bover P, Alcover JA, Köhler M (2013) First fossil evidence for the advance of replacement teeth coupled with life history evolution along an anagenetic mammalian lineage. PLoS One 8(7):e70743
Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO (2010) Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26:1463–1464
Kerr GR (1966) The ecology of mountain goats in west central Alberta. Dissertation, University of Alberta (Calgary)
Khan SM, Page S, Ahmad H, Ullah Z, Shaheen H, Ahmad M, Harper DM (2013) Phyto-climatic gradient of vegetation and habitat specificity in the high elevation western Himalayas. Pak J Bot 45:223–230
Köhler M (1987) Boviden des türkischen Miozäns (Känozoikum und Braunkohlen der Türkei). Paleontologia i Evolució 21:133–246
Kumar S, Hedges SB (1998) A molecular timescale for vertebrate evolution. Nature 392(6679):917–920
Lalueza-Fox C, Castresana J, Sampietro L, Marquès-Bonet T, Alcover JA, Bertranpetit J (2005) Molecular dating of caprines using ancient DNA sequences of Myotragus balearicus, an extinct endemic Balearic mammal. BMC Evol Biol 5:70–81
Leigh SR, Setchell JM, Charpentier M, Knapp LA, Wickings EJ (2008) Canine tooth size and fitness in male mandrills (Mandrillus sphinx). J Hum Evol 55(1):75–85
Leslie DM Jr (2010) Przewalskium albirostre (Artiodactyla: Cervidae). Mammal Species 42(1):7–18
Macho GA, Berner ME (1993) Enamel thickness of human maxillary molars reconsidered. Am J Phys Anthropol 92(2):189–200
Maddison WP, Maddison DR (2015) Mesquite: a modular system for evolutionary analysis (Version 3.04). http://mesquiteproject.org
Matthee CA, Burzlaff JD, Taylor JF, Davis SK (2001) Mining the mammalian genome for artiodactyl systematics. Syst Biol 50(3):367–390
McKenna MC, Bell SK (1997) Classification of Mammals above the Species Level. Columbia University Press, New York
Meredith R, Janečka J, Gatesy J, Ryder O, Fisher C, Teeling E, Goodbla A, Eizirik E, Simão T, Stadler T, Rabosky D, Honeycutt R, Flynn J, Ingram C, Steiner C, Williams T, Robinson T, Burk-Herrick A, Westerman M, Ayoub N, Springer M, Murphy W (2011) Impacts of the Cretaceous terrestrial revolution and KPg extinction on mammal diversification. Science 334(6055):521–524
Métais G, Vislobokova N (2007) Basal ruminants. In: Prothero DR, Foss S (eds) The Evolution of Artiodactyls. John Hopkins University Press, Baltimore, pp 189–212
Molvar E, Bowyer R, Van Ballenberghe V (1993) Moose herbivory, browse quality and nutrient cycling in an Alaskan tree line community. Oecologia 94:472–479
Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA, O’Brien SJ (2001) Molecular phylogenetics and the origins of placental mammals. Nature 409(6820):614–618.
Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W (2015) The caper package: comparative analysis of phylogenetics and evolution in R. R package version 5(2)
Osborn JW, Crompton AW (1973) The evolution of mammalian from reptilian dentitions. Breviora 399:1–18
Owen-Smith N, Fryxell JM, Merrill EH (2010) Foraging theory upscaled: the behavioural ecology of herbivore movement. Phil Trans R Soc Lond B Biol Sci 365(1550):2267–2278
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20(2):289–290
Pfretzschner HU (1992) Enamel microstructure and hypsodonty in large mammals. In: Smith P, Tchernov E (eds) Structure, function and evolution of teeth. Freund Publishing House, London, pp 147–162
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org/
Revell LJ, Harmon LJ, Collar DC (2008) Phylogenetic signal, evolutionary process, and rate. Syst Biol 57(4):591–601
Ricklefs RE, Starck JM (1996) Applications of phylogenetically independent contrasts: a mixed progress report. Oikos 77(1):167–172
Ropiquet A, Hassanin A (2005) Molecular evidence for the polyphyly of the genus Hemitragus (Mammalia, Bovidae). Mol Phylogenet Evol 36(1):154–168.
Ropiquet A, Hassanin A (2006) Hybrid origin of the Pliocene ancestor of wild goats. Mol Phylogenet Evol 41(2):395–404
Schultz AH (1935) Eruption and decay of the permanent teeth in primates. Am J Phys Anthropol 19(1):489–581
Schultz AH (1956) Postembryonic age changes. In: Hofer H, Schultz AH, Starck D (eds) Primatologia. Karger, Basel, pp 887–964
Schultz AH (1960) Age changes in primates and their modification in man. In: Tanner JM (ed) Human Growth vol III. Pergamon Press, Oxford, pp 1–20
Schwartz JH (1974) Dental development and eruption in the prosimians and its bearing on their evolution. Dissertation, Columbia University
Shafer A, Hall J (2010) Placing the mountain goat: a total evidence approach to testing alternative hypotheses. Mol Phylogenet Evol 55(1):18–25
Shaheen H, Shinwari ZK (2012) Phyto diversity and endemic richness of Karambar Lake vegetation from Chitral, Hindukush-Himalayas. Pak J Bot 44(1):17–21
Smith BH (1994) Sequence of emergence of the permanent teeth in Macaca, Pan, Homo, and Australopithecus: its evolutionary significance. Am J Hum Biol 6:61–76
Smith BH (2000) ‘Schultz’s rule’ and the evolution of tooth emergence and replacement patterns inprimates and ungulates. In: Teaford MF, Smith MM, Ferguson MW (eds) Development, function and evolution of teeth. Cambridge University Press, Cambridge, pp 212–227
Spaulding M, O’Leary MA, Gatesy J (2009) Relationships of Cetacea (Artiodactyla) among mammals: increased taxon sampling alters interpretations of key fossils and character evolution. PLoS One 4(9):e7062
Strömberg CA (2011) Evolution of grasses and grassland ecosystems. Annu Rev Earth Planet Sci 39:517–544
Sudre J (1974) D’importants restes de Diplobune minor Filhol à Itardies (Quercy). Palaeovertebrata 6:47–54
Suri L, Gagari E, Vastardis H (2004) Delayed tooth eruption: pathogenesis, diagnosis, and treatment. Am J Orthod Dentofacial Orthop 126(4):432–445
Tacutu R, Craig T, Budovsky A, Wuttke D, Lehmann G, Taranukha D, Costa J, Fraifeld VE, de Magalhaes JP (2013) Human ageing genomic resources: integrated databases and tools for the biology and genetics of ageing. Nucleic Acids Res 41(D1):D1027-D1033
Tattersall I, Schwartz JH (1974) Craniodental morphology and the systematics of the Malagasy lemurs (Primates, Prosimii). Anthropol Pap Am Mus Nat Hist 52:139–192
Theodor JM, Erfurt J, Métais G (2007) The earliest artiodactyls. In: Prothero DR, Foss S (eds) The Evolution of Artiodactyls. John Hopkins University Press, Baltimore, pp 32–58
Tranquillini W (1964) The physiology of plants at high altitudes. Annu Rev Plant Biol 15(1):345–362
Tsujikawa H (2005) The updated late Miocene large mammal fauna from Samburu Hills, northern Kenya. Afr Study Monogr Suppl 32:1–50
Vázquez GJA, Givnish TJ (1998) Altitudinal gradients in tropical forest composition, structure, and diversity in the Sierra de Manantlan. J Ecol 86(6):999–1020
Veitschegger K, Sánchez-Villagra MR (2016) Tooth eruption sequences in cervids and the effect of morphology, life history, and phylogeny. J Mammal Evol 23:251–263
Vrba ES, Schaller GB (2000a) Introduction. In: Vrba ES, Schaller GB (eds) Antelopes, Deer, and Relatives. Yale University Press, New Haven, pp 1–10
Vrba ES, Schaller GB (2000b) Phylogeny of bovidae based on behavior, glands, skulls and postcrania. In: Vrba ES, Schaller GB (eds) Antelopes, Deer, and Relatives. Yale University Press, New Haven, pp 203–222
Wise GE, Frazier-Bowers S, D’souza RN (2002) Cellular, molecular, and genetic determinants of tooth eruption. Crit Rev Oral Biol Med 13(4):323–335
Wolfe JA (1985) Distribution of major vegetational types during the tertiary. In: Sundquist ET, Broecker WS (eds) The Carbon Cycle and Atmospheric CO: Natural Variations Archean to Present. American Geophysical Union, Washington, D.C., pp 357–375
Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292(5517):686–693
Zhou L, Su YC, Thomas DC, Saunders RM (2012) ‘Out-of-Africa’ dispersal of tropical floras during the Miocene climatic optimum: evidence from Uvaria (Annonaceae). J Biogeogr 39(2):322–335
Zhou X, Xu S, Yang Y, Zhou K, Yang G (2011) Phylogenomic analyses and improved resolution of Cetartiodactyla. Mol Phylogenet Evol 61(2):255–264
Acknowledgments
The authors thank Chris Conroy (MVZ), Esther Langan (NMNH), Darrin Lunde (NMNH), and John Ososky (NMNH) for access to specimens. We would like to thank Marianne Brasil, Andrew Weitz, and the MVZ community for providing helpful feedback and discussion, and Madeleine Zuercher for assistance with the literature review. The authors would also like to thank Eva Bärmann, one anonymous reviewer, and Editor-in-Chief John R. Wible for their thoughtful comments and suggestions that greatly improved this manuscript. Funding for this study and presentation of results was generously provided by the Museum of Vertebrate Zoology, the J. Desmond Clark Human Evolution Research Center, and the Department of Integrative Biology, UC Berkeley.
Author Contributions
TAM collected the data, conducted the analyses, and wrote the manuscript. LJH supervised the project and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monson, T.A., Hlusko, L.J. The Evolution of Dental Eruption Sequence in Artiodactyls. J Mammal Evol 25, 15–26 (2018). https://doi.org/10.1007/s10914-016-9362-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-016-9362-9