Abstract
Neoargestes laevis sp. nov. (Copepoda, Harpacticoida, Argestidae), the third species of the genus, is described from the Clarion Clipperton Fracture Zone (Pacific Ocean), and a re-description of the holotype of Neoargestes incertus Becker, 1979 is provided. The generic diagnosis of Neoargestes Drzycimski, 1967 is amended and its allocation to Argestidae is confirmed. The monophyly of Neoargestes is recognized by six autapomorphies: transformation of the mandibular gnathobase into a strong masticating apparatus with a broad front, reduction in size of the first endopodal segment in P2–P4, and reduction in size of the P5 baseoendopod and exopod. The presence of 3-segmented endopods in P2–P4 in Neoargestes points to a rather basal position of the genus within Argestidae. Its affinities to Argestinae, Bodinia George, 2004 and Odiliacletodes Soyer, 1964 as well as its intrageneric systematics are briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Representatives of the family Argestidae Por, 1986 show a world-wide distribution (George 2004) and form one of the dominant groups of meiobenthic deep-sea Harpacticoida (George et al. 2014; Rose et al. 2005). To date, neither a clear family diagnosis nor an unambiguous phylogenetic characterization of Argestidae has been available (Boxshall and Halsey 2004). This has resulted in the exclusion and subsequent re-allocation of genera (e.g., Argestigens Willey, 1935 was excluded from Argestidae by Huys et al. (1996), re-allocated to Argestidae by Wells (2007), and excluded again by Huys et al. (2009)), and in rather tentative allocations of genera to Argestidae (e.g. Austrocletodes Pallares, 1979 by Fiers (1987); Argestoides Huys and Conroy-Dalton, 1997 by Huys and Conroy-Dalton (1997); Bodinia George, 2004 by George (2004)). Huys and Conroy-Dalton (1997) and Huys et al. (2009) noted that family boundaries of Ameiridae and Argestidae are not well defined, and molecular analyses indicate that Argestidae may be paraphyletic, encompassing a monophyletic Ameiridae as the terminal clade (Huys et al. 2009). Nonetheless, George (2004, 2008, 2011) as well as Corgosinho and Martínez Arbizu (2010) listed a series of putative morphological apomorphies indicating the monophyly of Argestidae, and George (2011) characterized the monophyletic subfamily Argestinae Por, 1986.
To further elucidate the phylogeny of Argestidae, the description of new species may provide valuable phylogenetic information. During a study of the harpacticoid copepods from an area within the Clarion Clipperton Fracture Zone (Pacific Ocean) licensed for the exploration of polymetallic nodules, a single female was found of a new representative of the genus Neoargestes Drzycimski, 1967. This genus was established by Drzycimski (1967) to include Neoargestes variabilis Drzycimski, 1967 found in muddy sediments in Husnesfjorden, western Norway at a depth of 520 m. Becker (1979) added a second species, Neoargestes incertus Becker, 1979, described from a single female collected in the Iberian deep sea at a depth of 3820 m. Since then, Neoargestes has been reported in a number of deep-sea ecological studies: the Slope of Sergipe, northeast of Brazil, Atlantic Ocean (Vasconcelos 2008); the Pacific Nodule Province, northeast Pacific Ocean (Mahatma 2009); the Kuril and Ryukyu regions, western Pacific Ocean (Kitahashi et al. 2014); and the Porcupine Abyssal Plain, northeast Atlantic Ocean (V. Kalogeropoulou, personal communication). While these findings confirm a wide distribution of the genus, they provide no additional species level information.
Drzycimski (1967) included the genus Neoargestes into Cletodidae T. Scott, 1905. Por (1986) subsequently revised the Cletodidae and established the family Argestidae to encompass Neoargestes and 13 other genera. The primitive setation and segmentation of the swimming legs of Neoargestes suggests that this genus occupies a rather basal position within Argestidae (cf. Drzycimski 1967; Becker 1979). A revision of Neoargestes will help to clarify the phylogeny of the genera within the Argestidae and to define the monophyletic status of Neoargestes. A description of the new species, as well as the re-description of N. incertus and remarks on the systematic position of Neoargestes are presented herein.
Material and methods
The holotype of the new species of Neoargestes was collected with a multicorer during expedition GSRNOD15A to the Global Sea mineral Resources (GSR) exploration area in the Clarion-Clipperton Fracture Zone (CCFZ, Pacific Ocean) in September–October 2015. It has been deposited in the Invertebrate Collections of the Royal Belgian Institute of Natural Sciences (Brussels, Belgium; labeled COP). More details on sample processing and environmental data can be found in Pape et al. (2017).
Additional material of the new species and of Neoargestes incertus was collected from the Porcupine Abyssal Plain (NE Atlantic Ocean) during cruises RRS Challenger 135, and RRS Discovery 226 and 229. For detailed sampling and sample treatment information, see Kalogeropoulou et al. (2010). This material has been deposited in the collection of the Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt, Germany.
Specimens were dissected under a dissecting microscope, mounted in glycerine, and preparations were sealed with transparent nail varnish. All drawings were made using a drawing tube on a Leica DMLB microscope equipped with differential interference contrast (max. magnification × 1000). The descriptive terminology used in the text is adopted from Huys et al. (1996). Abbreviations are A1, antennule; A2, antenna; aes, aesthetasc; benp, baseoendopod; cphth, cephalothorax; exp, exopod; enp, endopod; exp(enp)-1(2,3) for the proximal (middle, distal) segment of the respective ramus; FR, furcal rami; GF, genital field; P1–P6, first to sixth legs; md, mandible; mxl, maxillule; mx, maxilla; and mxp, maxilliped.
Results
Systematics
Family Argestidae Por, 1986
Genus Neoargestes Drzycimski, 1967
Type species: Neoargestes variabilis Drzycimski, 1967
Other species: Neoargestes incertus Becker, 1979; Neoargestes laevis sp. nov.
Generic diagnosis (females only; males unknown)
Body cylindrical, ~ 400–1000 μm long, distinction between prosome and urosome inconspicuous. Cphth and body somites smooth. Rostrum small, fused to cphth; the latter approximately one-fourth of total body length. Genital double-somite, subdivided by (dorso-)lateral sutures. Telson squarish, as long as or slightly longer than preceding somite; anal operculum smooth. FR squarish to almost three times longer than wide, set wide apart, with seven setae. A1 6–7-segmented, with bare and pinnate setae/spines, aesthetascs on 4th and last segments. A2 with allobasis, or basis and enp-1 not completely fused; exp small, 1-segmented, with 1–2 setae. Mandibular gnathobase short, with broad masticating front; palpus 2-segmented, basis elongated, with 1 strong pinnate seta and at most 1 accompanying small seta; enp 1-segmented, with 5 setae; exp absent. Mxl with small basis; enp and exp absent or each represented by up to two setae. Mx with two endites (both Drzycimski (1967) and Becker (1979) erroneously interpreted the basis as third endite), distal endite with one very strong spine, basis with claw and one additional spine or seta; enp small, carrying or being represented by 1–2 setae. Mxp prehensile; syncoxa with several spinules, and 1–2 pinnate setae; basis with spinules; enp small, with long claw and 1–2 bare setae. Coxae of swimming legs larger than bases; endopodal and exopodal rami displaced towards outer margin of basis. P1 not prehensile, with 3-segmented exp and 2–3-segmented enp; exp-1 without inner seta, exp-2 with inner seta, exp-3 with 3 outer spines and 2 apical setae. P2–P4 with 3-segmented rami; endopods shorter than exopods; enp-1 smallest; exp-3 with 3 outer spines. P5 small, with reduced benp bearing 1–3 setae; exp small, distinct or fused to benp, with 3–4 setae. GF represented by single gonopore; P6 represented by one seta and two tube pores, or two setae.
Neoargestes incertus Becker, 1979
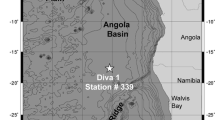
Material examined. Holotype female, Cop. No. 1077; deposited in the Zoologisches Museum der Christian-Albrechts-Universität in Kiel, Germany. The material was collected on 19 March 1970 by K.-H. Becker during cruise M19 of German RV METEOR to the Iberian deep sea (Becker 1979). Additional material from the Porcupine Abyssal Plain (NE Atlantic Ocean): one female (body length including FR: 1094 μm) from station 13077#24, coordinates 48° 49.97′ N/16° 30.39′ W, 4844 m depth, collected in March 1997 during cruise RRS Discovery 226, slide reference 13077#24#4-5(6); one female (body length including FR: 1009 μm) dissected on three slides, from station 13200#1, coordinates 48° 49.98′ N/16° 30.00′ W, 4843 m depth, collected in July 1997 during cruise RRS Discovery 229, slide reference 13200#1#2-3(1) (Kalogeropoulou 2014). This material is deposited in Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt, Germany.
Type locality. Iberian deep sea, station #263, 37° 44′ N/10° 31′ W; depth 3820 m.
Re-description of female. Habitus (Fig. 1a, b) cylindrical. Cphth about one-fourth of total body length. Whole body with smooth, weakly sclerotized integument, with pattern of sensilla and pores as figured. No clear distinction between prosome and urosome, the latter slightly tapering posteriorly. Second and third urosomites fused to form genital double somite, original segmentation indicated by a weak dorso-lateral chitinous bar. Rostrum small, triangular, fused to cphth. Posterior margin of cphth and all somites with broad, smooth hyaline frill, margin not denticulate. Genital double-somite, and fourth and fifth urosomites with row of spinules ventrally near posterior margin.
Telson (Figs. 1a, b and 2d) slightly broader than long, with row of ventral spinules and few spinules near posterior margin. Anal operculum with smooth margin, weakly developed, flanked by pair of sensilla.
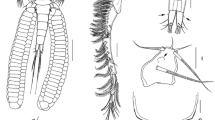
Neoargestes incertus. Female from Porcupine Abyssal Plain. a Right furcal ramus, dorsal (furcal setae numbered I–VII); b Right furcal ramus, ventral; c Right furcal ramus, lateral. Female holotype; d Telson and furcal rami; e Detail of middle part of furcal seta V; f Rostrum and left antennule, dorsal (setation of segments 3, 4, and 7 omitted); g Left antennule, segments 3 and 4; h Left antennule, segment 7
FR (Fig. 2a–c) about 1.5 times as long as wide, smooth, with seven setae. Seta I very small, inserted next to seta II; seta III with two spinules at base; seta I, II, III, and VII inserted subapically; seta III and VI subequal in length; seta IV and V longest (seta V, 746 μm long), inserted apically, not fused; seta VI inserted apically; dorsal seta VII close to inner margin, tri-articulated at base. Hyaline tube pore near middle of ventral surface and pore at one-third of outer margin.
A1 (Fig. 2f–h) seven-segmented. Third segment longest, with one bi-articulated seta. Second segment slightly shorter than third. Fourth segment with aes (length not discernible). Fifth segment smallest. Seventh segment with slight suture; six setae bi-articulated at their bases, and aes, fused to one seta. Setal formula: 1/1; 2/8; 3/8; 4/4 + aes; 5/2; 6/3; 7/9 + (1 + aes).
A2 (Fig. 3a). Coxa short, with some fine spinules. Basis and enp-1 partially fused, both with some fine spinules along abexopodal margin, abexopodal seta absent. Enp-2 with strong inner spinules and slender subapical outer spinules, two bipinnate lateral spines, and six apical elements: two bipinnate inner spines, two geniculate setae, and one geniculate outer seta bearing long pinnules near geniculation and fused basally to small outermost slender seta. Exp one-segmented, bearing two setae.
Md (Fig. 3b). Short gnathobase, with broad masticating front and tooth-like projection. Basis of mandibular palp with one strong pinnate seta and one slender, small and bare seta. Enp 1-segmented, with five bare setae. Exp absent.
Mxl (Fig. 3c–g). Praecoxal arthrite (Fig. 3c, d) with seven apical spines, three of which strongly unipinnate, and two bare surface setae. Coxa (Fig. 3e, f) drawn out into strong and blunt claw, with six setae and a row of short spinules at its base. Basis (Fig. 3g) with three apical setae. Enp and exp each represented by two setae.
Mx (Figs. 4a and 5a). Syncoxa with two endites. Proximal endite small and bulbous proximally, with two fused setae. Distal endite with one strong unipinnate spine fused to endite, and two slender setae. Basis with unipinnate claw (fused to basis) and one additional seta. Enp 1-segmented, small, bearing two bare setae.
Mxp (Fig. 4b) prehensile. Syncoxa with sparse short inner spinules and two apical setae, one of which plumose, thick and very long. Basis with long, slender outer spinules, and shorter medial inner spinules. Enp produced into long claw with two strong pinnules, and two bare setae at base of claw, one of which approximately same length as claw.
P1 (Fig. 4c). Coxa rectangular in shape, slightly broader and larger than more triangularly-shaped basis. Basis with inner and outer bipinnate spines subequal in length. Coxa and basis with several rows of spinules. Exp and enp 3-segmented, subequal in length. Exp-1 without inner seta. Exp-2 with short, inner seta. Exp-3 with three bipinnate outer spines, one outer terminal spine and one inner terminal seta. Enp-1 with inner seta, bearing short row of pinnae near tip. Enp-2 with one bipinnate inner seta. Enp-3 with two bipinnate terminal setae, and one bipinnate outer spine.
P2–P4 (Figs. 5b, c and 6a). Exp and enp 3-segmented. Intercoxal sclerites with three strong spinules on each side. Coxa rectangular, distinctly larger than basis. Basis approximately triangular, about twice as broad as long, with outer basal seta. Praecoxa, coxa and basis with spinule rows. Exp-1 and exp-2 subequal in length, each with short inner seta and bipinnate outer spine. Exp-3 almost as long as exp-1 and exp-2 combined, with three bipinnate outer spines, one outer spine and one inner seta apically, and two short inner setae (P2; Fig. 5b), three inner setae (P3; Fig. 5c) of which proximal and distal short and subequal in length, middle one long, or with three inner setae (P4; Fig. 6a) of which distal one short, slender and bare, proximal and middle ones long, bipinnate and almost equal in length. Endopod of P2 reaching barely beyond tip of exp-2 (Fig. 5b); enp of P3 not reaching tip of exp-2 (Fig. 5c), enp of P4 reaching the middle of exp-2 (Fig. 6a). Enp-3 slightly longer than enp-1 and enp-2 combined. Enp-1 short, with one inner, short, and slender seta (bipinnate in P2, bare in P3 and P4). Enp-2 with one inner, pinnate seta. Enp-3 with two inner and two apical bipinnate setae, and one pinnate outer spine. Setal formulae of P1–P4 as in Table 1.
P5 (Fig. 6b, c). Endopodal lobe poorly developed, with one bipinnate seta. Exp distinct, bearing two long, bipinnate apical setae and two short outer elements, and with one long tube pore.
P6 (Fig. 6d) represented by two short setae. Genital field near middle of second urosomite, with single gonopore.
Male unknown.
Neoargestes laevis sp. nov.
Material examined. Holotype female, dissected onto 12 slides (COP 10500/1-12; I.G. 33763), deposited in the Invertebrate Collections of the Royal Belgian Institute of Natural Sciences (Brussels, Belgium). The material was collected on 7 October 2015 with a multiple corer from station B4N01 (MUC deployment MUC009) (see Pape et al. 2017). Additional material from the Porcupine Abyssal Plain (NE Atlantic Ocean): one female (body length including FR: 376 μm) from station 54301#9, coordinates 48° 50.50′ N/16° 31.3′ W, depth 4843 m, collected on 22 October 1997 during RRS Challenger 135, slide reference 54301#9#0-1(5) (Kalogeropoulou 2014). This specimen is deposited in Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt, Germany.
Type locality. GSR exploration area in the Clarion-Clipperton Fracture Zone (Pacific Ocean), coordinates 14° 42′ 23.36′′ N/125° 26′ 31.34′′ W, depth 4501 m.
Etymology. The Latin adjective laevis (meaning smooth) refers to the smooth body surface of this species.
Description of female. Habitus (Fig. 7a, c) moderately slender, body length including FR approximately 443 μm. Cphth about one quarter of total body length. Whole body with smooth, weakly sclerotized integument, with pattern of sensilla and pores as figured. No clear distinction between prosome and urosome, urosome slightly tapering posteriorly. Second and third urosomites fused to form genital double-somite, original segmentation indicated by a weak dorso-lateral chitinous bar. Rostrum small, approximately triangular, fused to cphth. Posterior margin of cphth and all somites with broad, smooth hyaline frill, margin not denticulate. Fourth and fifth urosomites with row of spinules ventrally near posterior margin.
Telson (Figs. 8a and 9a) approximately as long as penultimate somite, with row of short spinules near insertion of furcal rami and row of ventral spinules covered by hyaline frill of penultimate somite. Anal operculum weakly developed, covered entirely by hyaline frill of penultimate somite (margin not entirely visible). Pair of sensilla associated with anal operculum displaced posteriorly.
FR (Fig. 8b, c) almost three times as long as wide, smooth, with few spinules on inner and outer apical margins, with six setae. Seta I absent; seta II, III, and VII inserted subapically: seta II on dorsal surface, with pore near insertion; seta III inserted ventrally, longer than seta II; setae IV and V longest, fused, inserted apically, seta IV pinnate, seta V broken; seta VI shortest, inserted apically; seta VII dorsally, tri-articulated at base. Pore at two thirds of ventral surface.
A1 (Fig. 9b–f) six-segmented. Segment one with few, short spinules along inner margin and one bipinnate seta apically. Second segment with three pinnate and five bare setae, and one strong, long and bipinnate spine, the latter inserted on ventral surface. Third segment about as long as second segment, with three unipinnate and three bare setae. Fourth segment small, with two bare setae and aes. Fifth segment smallest, with one unipinnate seta. Sixth segment with ten setae, six of which bi-articulate at base, and acrothek (consisting of aes, one unipinnate seta and one bipinnate seta fused at base). Setal formula: 1/1; 2/9; 3/6; 4/2 + aes; 5/1; 6/10 + acrothek.
A2 (Fig. 10a) with allobasis and one-segmented exp. Exp bearing two bipinnate setae. Allobasis without abexopodal seta, with long spinules along abexopodal margin. Endopod with long spinules along margin, short subapical spinules, two bipinnate lateral spines, five apical setae (three of which bipinnate), and one small, slender seta fused at base to neighboring seta.
Labrum (Fig. 10b) with one medial and two lateral rows of spinules.
Paragnaths (Fig. 10e) on each side of labrum with row of strong spinules.
Md (Fig. 10c, d, h) Gnathobase (Fig. 10c, d) with broad masticating front and dorsal seta. Basis of mandibular palp (Fig. 10h) with two setae, one of which strong and bipinnate. Enp with five bare setae. Exp absent.
Mxl (Fig. 10f, g) in bad condition, description tentative. Praecoxal arthrite with six strong terminal elements, two of which pinnate; with two long, slender surface setae (broken in Fig. 10g). Coxa (Fig. 10f) with strong claw and 1 seta. Basis (Fig. 10f) with at least four setae.
Mx (Figs. 10i and 11a, b) probably damaged. Syncoxa with one endite armed with one strong unipinnate spine fused to endite and two setae. Proximal endite possibly lost. Basis with unipinnate claw (fused to basis) and one additional strong spine. Enp represented by two bare setae.
Mxp (Fig. 11c) prehensile, basis 1.5 times as long as syncoxa. The latter with spinules and with two long apical setae (one broken, one bipinnate). Basis with patch of spinules. Enp 1-segmented, with long distinct claw carrying few, strong pinnules, enp additionally with two bare, short setae subapically.
P1 (Fig. 11d, e) with three-segmented exp and two2-segmented enp, enp slightly longer than exp. Intercoxal sclerite short. Coxa slightly broader than basis, both with several short spinule rows. Basis with strong, bipinnate inner spine reaching middle of enp-2, and minute outer seta inserted on posterior surface, hardly discernible (see arrow in Fig. 11d). Exp-1 without inner seta, exp-2 with short inner seta. Exp-3 with five elements (three bipinnate outer spines and two bipinnate apical setae). Enp-1 with one bipinnate inner seta. Enp-2 with one inner and two apical bipinnate setae, and one bipinnate outer spine.
P2–P4 (Figs. 12b and 13a, b) with three-segmented exp and three-segmented enp. Intercoxal sclerites short. Coxa slightly larger than basis. Basis about twice as broad as long. Praecoxa, coxa and basis with short spinule rows as figured. Outer basal setae short, slender and bare. Exp-3 almost as long as exp-1 and exp-2 combined, exp-1 slight shorter than exp-2. Exp-1 without inner seta, with outer bipinnate spine. Exp-2 with one short, slender, and pinnate inner seta and one bipinnate outer spine. Exp-3 with three bipinnate outer spines, two slender, short inner setae and apically with one bipinnate outer spine and one bipinnate inner seta. Enp of P2 reaching insertion site of proximal inner seta of exp-3 (Fig. 12b); enp of P3 (Fig. 13a) and P4 (Fig. 13b) reaching slightly beyond tip of exp-2. Enp-3 slightly longer than enp-1 and enp-2 combined. Enp-1 short. Enp-1 and enp-2 with one bipinnate inner seta. Enp-3 with two inner and two apical bipinnate setae, and one bipinnate outer spine. Setal formulae of P1–P4 as in Table 1.
P5 (Fig. 12c, d) strongly reduced, endopodal lobe and exopod fused, each forming a weakly protruded lobe. Endopodal lobe with three setae (innermost pinnate), exopodal lobe with three setae and one tube pore. Basal seta on short setophore.
P6 (Fig. 12e) represented by one short seta and two tube pores. Genital field and P6 located near anterior margin of genital double-somite, with single gonopore.
Male unknown.
Variability. The single female of Neoargestes laevis sp. nov. (Figs. 10h, i and 12a) from the Porcupine Abyssal Plain differs only slightly from the holotype in the following characters:
Mx (Fig. 10i) carries a proximal endite with two setae (presumably lost in the type material); the inner basal spine on P1 carries fewer and longer spinules (Fig. 12a).
Discussion
Neoargestes is a small genus apparently restricted to the deep sea. So far, only two species have been described, N. variabilis and N. incertus. Neoargestes variabilis has only been found in Husnesfjord (Bergen, Norway; Drzycimski 1967), while N. incertus has been collected from two distant localities in the eastern Atlantic Ocean (Iberian Abyssal Plain and Porcupine Abyssal Plain) (Becker 1979; present contribution). Here, we describe a third species, Neoargestes laevis sp. nov., from the Clarion Clipperton Fracture Zone (Pacific Ocean) and from the Porcupine Abyssal Plain (Atlantic Ocean). All three Neoargestes species fit the generic diagnosis by Drzycimski (1967). However, Drzycimski’s (1967) diagnosis does not discriminate between apomorphic and plesiomorphic characters. The placement of Neoargestes into Argestidae and its monophyletic status are discussed.
Assignment of Neoargestes to Argestidae
George (2011) listed several presumptive apomorphies of the monophylum Argestidae. However, these apomorphies [plesiomorphic conditions in square brackets] have not been proved for all argestid taxa (e.g., Corallicletodes Soyer, 1966, Hypalocletodes Por, 1967, Leptocletodes Sars, 1921), and, following George (2004, 2011), are considered here as provisional:
-
1.
Integument weakly sclerotized [cuticle of regular strength]
-
2.
Telson nearly square, large [telson tapering distally, shorter than preceding abdominal somite]
-
3.
Anal operculum displaced anteriorly [anal operculum at posterior margin of telson]
-
4.
FR set wide apart at outer corners of telson [FR apically on telson, not widely apart]
-
5.
A2 exp at most one-segmented [A2 exp at least two-segmented]
-
6.
P2 rami displaced toward outer margin of basis [no displacement]
-
7.
P3 rami displaced toward outer margin of basis [no displacement]
-
8.
P4 rami displaced toward outer margin of basis [no displacement]
-
9.
Mx distal endite armed with strong spine [armed with moderate seta/spine]
-
10.
Mx basis with claw and strong spine [claw accompanied by moderate seta]
Characters 6–8 were pooled by George (2011) into one single apomorphy. However, for detailed phylogenetic comparison, we considered characters 6–8 separately, raising to 10 the number of probable autapomorphies for Argestidae. George (2004) proposed two further derived characters, (i) the presence of, at the most, one seta on A2 exp and (ii) the loss of the accompanying seta on the maxillipedal claw. However, the ancestral states of both characters (i.e., the presence of a second seta on A2 exp and/or at least one accompanying seta on the maxillipedal claw) are present in single argestid taxa (e.g., Argestinae, Argestigens, Mesocletodes Sars, 1909, Megistocletodes Por, 1986, Neoargestes) and therefore, characters (i) and (ii) cannot be regarded as apomorphies of Argestidae.
Neoargestes shares all ten presumptive apomorphies listed above, although some are weakly developed. For example, N. variabilis (Drzycimski 1967) and N. incertus possess a large, squarish telson (character 2), while the telson of N. laevis sp. nov. is broader than long and, at most, as long as the preceding somite. Also, the rami of P2–P4 are displaced toward outer basal margin in N. variabilis and N. laevis sp. nov. (characters 6–8), but N. incertus shows only a slight displacement. Further, in N. incertus, the claw on Mx basis is accompanied by a moderate seta rather than a claw (character 10). Nevertheless, as Neoargestes meets characters 1, 3–5, and 9 (and with certain reservations characters 2, 6–8, and 10), its allocation to Argestidae is confirmed.
Systematic position of Neoargestes within Argestidae
These presumptive apomorphies 2, 6, 7, and 8 confirm that Neoargestes holds a relatively basal position within Argestidae, as suggested by Drzycimski (1967) and Becker (1979). Neoargestes shares the three-segmented endopods of P2–P4 with Argestinae (Argestes Sars, 1910 and Fultonia T. Scott, 1902; note that George (2011) commented on the possible allocation of Dizahavia Por, 1979 to this subfamily), Bodinia peterrummi George, 2004, and Odiliacletodes Soyer, 1964. However, the three species of Neoargestes differ remarkably from the other genera. For example, Neoargestes cannot be assigned to Argestinae since it lacks the dense dorsal and lateral spinular ornamentation of cphth and body somites, the development of a strong, long apical seta on the sixth antennular segment, and the strong elongation of the dorsal thoracic sensilla (see George 2011). Furthermore, it lacks the derived apron that is characteristic for Bodinia, and it also differs clearly from Odiliacletodes in several morphological features, like, e.g., the size and shape of the P5 and P2–P4 endopods. Instead, Neoargestes is characterized here by six autapomorphies that are considered indicative of its monophyletic status [plesiomorphic states in square brackets]:
-
11.
Mandibular gnathobase transformed into broad masticating front [gnathobase equipped with cuspidate teeth]
-
12.
P2 enp-1 strongly reduced in size, at most half as long as enp-3 [enp-1 at least half as long as enp-3]
-
13.
P3 enp-1 strongly reduced in size, at most half as long as enp-3 [enp-1 at least half as long as enp-3]
-
14.
P4 enp-1 strongly reduced in size, at most half as long as enp-3 [enp-1 at least half as long as enp-3]
-
15.
P5 benp strongly reduced in size [benp lobate]
-
16.
P5 exp strongly reduced in size, at most 1.5 times as long as broad [exp at least twice as long as broad]
A gnathobase with a broad masticating front (character 11) is also present in Mesocletodes and Fultonia (Argestinae). However, as discussed above, Neoargestes cannot be assigned to Fultonia or even Argestinae. Similarly, it differs from Mesocletodes, a derived representative of Argestidae, characterized by several apomorphies, including the presence of a robust protrusion bearing a strong, backwardly pointing bipinnate seta on the second antennular segment, a reduced proximal outer spine on P1 exp-3, and further deviations regarding the size and the shape of swimming legs, furcal rami and others (cf. Menzel and George 2009). Thus, it is concluded here that the formation of a mandibular gnathobase with a strong and broad masticating front has occurred several times within Argestidae and must be regarded as convergent in Fultonia, Mesocletodes, and Neoargestes.
Neoargestes, Argestinae (Argestes-Fultonia), Bodinia peterrummi, Dizahavia, and Odiliacletodes also share the primitive three-segmented endopod on P2–P4, while the remaining Argestidae possess a reduced number of segments on the P2–P4 endopod. Neoargestes, however, is unique in the drastic reduction of the first endopodal segment of P2–P4 (characters 12–14), and in the strongly reduced female P5 benp (character 15), which are regarded here as derived conditions, and therefore interpreted as autapomorphic for this taxon. Contrary to the apomorphic condition of the strongly reduced P5 endopodal lobe of Neoargestes, Bodinia, and Odiliacletodes possess a well-developed endopodal lobe of P5 and is regarded here as plesiomorphic. As demonstrated above, both Argestinae and Mesocletodes present several apomorphies that are not shared by Neoargestes, so a closer relation between the latter and Argestinae/Mesocletodes can be ruled out. Thus, both Argestinae and Mesocletodes are excluded from further comparison. The strongly reduced P5 endopodal lobe observed in Dizahavia and Neoargestes might suggest a closer relationship between these genera. However, Dizahavia lacks apomorphies 11–16, and following Por’s (1979) description, Dizahavia might be closely related to Argestes and Fultonia, thus possibly forming part of Argestinae as suggested by George (2011).
Finally, the small P5 exp (character 16), which is distinct or fused to the baseoendopod, is exclusively present in Neoargestes. This strong reduction of the P5 exopod is unique within Argestidae, and is interpreted here as apomorphic for the genus.
Systematic relationships within Neoargestes
The three species so far attributed to Neoargestes share characters 11–16, which are regarded here as synapomorphies supporting the monophyletic status of the genus. Relationships between these species remain vague and the re-description of the type species, N. variabilis, is still pending. Type material of N. variabilis deposited at Bergen University Zoological Museum and other material deposited at the Marine Biology Station in Blomsterdalen could not be found and is presumed lost. Comparison of all three Neoargestes species reveals that N. variabilis shares two derived characters with N. laevis sp. nov. [plesiomorphic state present in N. incertus]:
-
17.
Maxillary endopod lost, represented by 1–2 setae [endopod 1-segmented, with 2 setae]
-
18.
P5 exopod and baseoendopod fused [P5 exopod distinct]
A third tentative apomorphy refers to the segmentation of the P1 enp. In N. laevis, it is two-segmented, and also Drzycimski (1967, Table 1, p. 204) lists nine individuals of N. variabilis presenting a two-segmented P1 enp. However, that author noticed a remarkable variability in the segmentation of the P1, reaching from a three-segmented enp to a partial and even complete fusion of P1 enp-2 and enp-3. That variability applies even to both P1 legs of single specimens, for which reason it is not considered here.
These mutually derived features might point towards a close relationship between N. variabilis and N. laevis sp. nov. In addition, both species share the presence of only two inner setae in P3 and P4 exp-3, whereas N. incertus bears three inner setae. However, it cannot be confirmed if N. variabilis and N. laevis sp. nov. lost homologous setae and their exact relationship cannot be determined.
Neoargestes variabilis and N. incertus also share one derived character [plesiomorphic state present in N. laevis sp. nov.]:
-
19.
Maxillipedal enp fused with apical strong claw [enp and claw still distinct]
A maxillipedal enp carrying a distinct apical claw forms part of the podogennontan groundpattern (Willen 2000; Seifried 2003) and, therefore, is regarded as the ancestral state in those families composing that taxon. Consequently, the fusion of the enp with the apical claw constitutes the derived state. Within Argestidae, the ancestral state is distributed quite randomly over the different genera and appears sporadically, for example, in some Eurycletodes Sars, 1909 species (e.g., Eurycletodes (E.) laticauda Sars, 1909, E. (E.) serratus Sars, 1921, E. (Oligocletodes) peruanus Becker, 1979), in Leptocletodes sp. (Soyer, 1964), Mesocletodes duosetosus Schriever, 1985, M. kunzi Schriever, 1985, and Neoargestes laevis sp. nov. Regarding Neoargestes, the presence of the derived state (maxillipedal endopod and endopodal claw fused) in both N. incertus and N. variabilis may support their close relationship, while N. laevis sp. nov. (maxillipedal endopod and endopodal claw distinct) may represent the ancestral state within Neoargestes.
The phylogenetic relationships within Neoargestes remain unclear. The re-description of the type species N. variabilis would certainly provide valuable data, potentially allowing all species to be characterized by distinct autapomorphies. According to Drzycimski’s (1967) description, N. variabilis is unique in possessing four autapomorphies [plesiomorphic conditions in square brackets]:
-
20.
A2 exp carrying only 1 seta [with two setae]
-
21.
Maxillary endopod represented by only 1 seta [mx enp with two setae]
-
22.
Maxillipedal syncoxa with only 1 spine [with two spines]
-
23.
P2 third endopodal segment with one inner seta [with two inner setae].
N. laevis sp. nov. also presents at least four autapomorphies [plesiomorphic conditions in square brackets] in comparison to N. variabilis and N. incertus:
-
24.
A2 with allobasis [A2 with basis]
-
25.
P2 exp-1 without inner seta [with one inner seta]
-
26.
P3 exp-1 without inner seta [with one inner seta]
-
27.
P4 exp-1 without inner seta [with one inner seta]
Autapomorphies 20–27 show fusion of segments or the loss of setae or spines. We have applied the general oligomerization principle (Huys 1996), whereby the retention of segments, setae, or spines is considered to be the plesiomorphic state, and their fusion or loss as the derived state.
Finally, N. incertus shows at least three unique autapomorphies [plesiomorphic conditions in square brackets]:
-
28.
Subapical seta of maxillipedal syncoxa multipinnate [corresponding seta uni- to bipinnate]
-
29.
Subapical seta of maxillipedal syncoxa extremely elongated and strengthened, as long as syncoxa and basis combined [seta slender, at most reaching length of syncoxa]
-
30.
Female P5 baseoendopod with 1 endopodal seta [with at least two setae]
In contrast to characters 20–27, apomorphies 28–29 present neither fusions nor reductions but qualitative modifications of the respective elements. The development of multipinnate setae (character 28) is relatively unusual, being bare, uni- or bipinnate elements more common: the maxillipedal seta of the remaining Neoargestes species and even other Argestidae is at most bipinnate. Therefore, the development of a multipinnate syncoxal seta on the mxp is interpreted as autapomorphic for N. incertus. Similarly, the extreme elongation and strengthening of that seta (character 29) is unique not only within Neoargestes but the whole Argestidae. Finally, according to the general oligomerization principle, character 30, the reduction to 1 baseoendopodal seta in the female P5 of N. incertus, is regarded as apomorphic.
Briefly, Neoargestes constitutes a monophylum composed of three known species, N. incertus, N. laevis sp. nov., and N. variabilis, sharing six apomorphic characters. The allocation of Neoargestes to Argestidae is confirmed. Within the genus, each species is characterized by a series of autapomorphies, but the relationships between them remain unclear due to missing morphological data.
References
Becker K-H (1979) Eidonomie und Taxonomie abyssaler Harpacticoida (Crustacea, Copepoda). Teil II. Paramesochridae, Cylindropsyllidae und Cletodidae. Meteor Forsch.-Ergebnisse (D). 31:1–37
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity. The Ray Society, London
Corgosinho PHC, Martínez Arbizu P (2010) Ameiridae Boeck and Argestidae Por revisited, with establishment of Parameiropsidae, a new family of Harpacticoida (Crustacea, Copepoda) from deep-sea sediments. Helgol Mar Res 64:223–254
Drzycimski I (1967) Zwei neue Cletodidae (Copepoda Harpacticoida) aus dem westnorwegischen Küstengebiet. Sarsia 29:199–206
Fiers F (1987) Intercletodes interita n. gen., n. sp. and Orthopsyllus coralliophilus n. sp., two new copepods from the northern coast of Papua New Guinea. (Copepoda, Harpacticoida). Bull Inst Roy Sci Nat Belgique Biol 57:123–132
George KH (2004) Description of two new species of Bodinia, a new genus incertae sedis in Argestidae Por, 1986 (Copepoda, Harpacticoida), with reflections on argestid colonization of the Great Meteor Seamount plateau. Org Divers Evol 4:241–264
George KH (2008) Argestes angolaensis sp. nov. (Copepoda: Harpacticoida: Argestidae) from the Angola Basin (Southeast Atlantic), and the phylogenetic characterization of the taxon Argestes Sars, including the redescription of A. mollis Sars, 1910, and A. reductus (Itô, 1983). Zootaxa 1866:223–262
George KH (2011) Revision of the taxon Fultonia T. Scott (Copepoda: Harpacticoida: Argestidae), including the (re)description of some species, discontinuation of the genus Parargestes Lang, and erection of Argestinae Por, subfam. nov. Meiofauna Marina 19:127–160
George KH, Veit-Köhler G, Martínez Arbizu P, Seifried S, Rose A, Willen E, Bröhldick K, Corgosinho PH, Drewes J, Menzel L, Moura G, Schminke HK (2014) Community structure and species diversity of Harpacticoida (Crustacea: Copepoda) at two sites in the deep sea of the Angola Basin (Southeast Atlantic). Org Divers Evol 14:57–73
Huys R (1996) Superornatiremidae fam. nov. (Copepoda: Harpacticoida): an enigmatic family from North Atlantic anchihaline caves. Sci Mar 60:497–542
Huys R, Conroy-Dalton S (1997) Discovery of hydrothermal vent Tantulocarida on a new genus of Argestidae (Copepoda: Harpacticoida). Cah Biol Mar 38:235–249
Huys R, Gee JM, Moore CG, Hamond R (1996) Marine and brackish water harpacticoid copepods. Part 1. Field studies council, Shrewsbury
Huys R, Mackenzie-Dodds J, Llewellyn-Hughes J (2009) Cancrincolidae (Copepoda, Harpacticoida) associated with land crabs: a semiterrestrial leaf of the ameirid tree. Mol Phylogenet Evol 51:143–156
Kalogeropoulou V (2014) Structural and functional biodiversity of the deep-sea metazoan meiobenthic communities at the Porcupine Abyssal Plain, NE Atlantic. Dissertation, Carl von Ossietzky-Universität
Kalogeropoulou V, Bett BJ, Gooday AJ, Lampadariou N, Martinez Arbizu P, Vanreusel A (2010) Temporal changes (1989–1999) in deep-sea metazoan meiofaunal assemblages on the Porcupine Abyssal Plain, NE Atlantic. Deep-Sea Res II 57:1383–1395
Kitahashi T, Kawamura K, Kojima S, Shimanaga M (2014) Bathymetric patterns of α and β diversity of harpacticoid copepods at the genus level around the Ryukyu trench, and turnover diversity between trenches around Japan. Prog Oceanogr 123:54–63
Mahatma R (2009) Meiofauna communities of the Pacific Nodule Province: abundance, diversity and community structure. Dissertation, Carl von Ossietzky-Universität
Menzel L, George KH (2009) Description of four new species of Mesocletodes Sars, 1909 (Copepoda, Harpacticoida, Argestidae) and redescription of Mesocletodes robustus Por, 1965 from the South Atlantic, including remarks on the Mesocletodes abyssicola-group. Zootaxa 2096:214–256
Pallares RE (1979) Copépodos harpacticoides marinos de Tierra del Fuego (Argentina). Isla de Los Estados III. Contr Cient CIBIMA 142:1–22
Pape E, Bezerra TN, Hauquier F, Vanreusel A (2017) Limited spatial and temporal variability in Meiofauna and nematode communities at distant but environmentally similar sites in an area of interest for deep-sea mining. Front Mar Sci 4:205
Por FD (1967) Level bottom Harpacticoida (Crustacea, Copepoda) from Elat (Red Sea), Part I. Israel J Zool 16:101–165
Por FD (1979) The Copepoda of Di Zahav Pool (Gulf of Elat, Red Sea). Crustaceana 37:13–30
Por FD (1986) A re-evaluation of the family Cletodidae Sars, Lang (Copepoda, Harpacticoida). Syllogeus 58:420–425
Rose A, Seifried S, Willen E, George KH, Veit-Köhler G, Bröhldick K, Drewes J, Moura G, Martínez Arbizu P, Schminke HK (2005) A method for comparing within-core alpha-diversity values from repeated multicorer samplings, shown for abyssal Harpacticoida (Crustacea: Copepoda) from the Angola Basin. Org Divers Evol 5(suppl. 1):3–17
Sars GO (1909) Copepoda Harpacticoida. Parts XXVII & XXVIII. Cletodidae (concluded), Anchorabolidae, Cylindropsyllidae, Tachidiidae (part). An Account of the Crustacea of Norway, with short descriptions and figures of all the species 5:305–336
Sars GO (1910) Copepoda Harpacticoida. Parts XXIX & XXX. Tachidiidae (concluded), Metidae, Balaenophilidae, supplement (part). An Account of the Crustacea of Norway, with short descriptions and figures of all the species. Bergen Museum Bergen 5:337–368
Sars GO (1921) Copepoda Supplement. Parts IX & X. Harpacticoida (concluded), Cyclopoida. An Account of the Crustacea of Norway, with short descriptions and figures of all the species. Bergen Museum Bergen 7:93–121
Schriever G (1985) New Harpacticoida (Crustacea, Copepoda) from the North Atlantic Ocean. VII. The description of five new species of the genus Mesocletodes Sars (Cletodidae). Mitteilungen aus dem Zoologischen Museum und Universität Kiel 2:1–12
Scott T (1902) Notes on gatherings of Crustacea collected by the Fishery Steamer “Garland” and the steam trawlers “Star of Peace” and “Star of Hope”, of Aberdeen, during the year 1901. Fisheries Scotland Sci Invest 20(3):447–485
Scott T (1905) On some new and rare Crustacea from the Scottish seas. Fisheries, Scotland, Sci Invest 23(3):141–153
Seifried S (2003) Phylogeny of Harpacticoida (Copepoda): revision of “Maxillipedasphalea” and Exanechentera. Cuvillier Verlag, Göttingen
Soyer J (1964) Copépodes harpacticoïdes de l’étage bathyal de la région de Banyuls-sur-Mer. V. Cletodidae T. Scott. Vie Milieu 15:573–643
Soyer J (1966) Copépodes harpacticoïdes de Banyuls-sur-Mer. 3. Quelques formes du coralligène. Vie Milieu 17B:303–344
Vasconcelos DM (2008) Distribuição dos copepoda harpacticoida da meiofauna em área de talude no litoral de Sergipe, Brasil. Dissertation, Universidade Federal de Pernambuco
Wells JBJ (2007) An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa 1568:1–872
Willen E (2000) Phylogeny of the Thalestridimorpha Lang, 1944 (Crustacea, Copepoda). Cuvillier Verlag, Göttingen
Willey A (1935) Harpacticoid Copepoda from Bermuda. – Part II. Ann Mag Nat Hist 15(10):50–100
Acknowledgments
The authors are grateful to Dr. Dirk Brandis and Lina Rosotta (Kiel Museum, Germany) for lending the type material of Neoargestes incertus. Jon Anders Kongsrud (University of Bergen, Museum of Zoology, Norway), Dr. Thorolf Magnesen and Dr. Torleiv Brattegard (Espegrend Marine Research Field Station), and Dr. Teresa Radziejewska (University of Szczecin, Poland) are kindly thanked for providing information about the Neoargestes material of Dr. I. Drzycimski. Dr. Vicky Kalogeropoulou (Hellenic Centre of Marine Research, Greece) is kindly thanked for allowing to study her specimens of Neoargestes.
Thanks are also due to the captain and the crew of the RV ‘Mt. Mitchell,’ François Charlet (GSR), Tom De Wachter (GSR), Niels Viaene (Marine Biology Research Group, UGent), Dr. Freija Hauquier (Marine Biology Research Group, UGent), Liesbet Colson (Marine Biology Research Group, UGent), Dr. Ellen Pape (Marine Biology Research Group, UGent), Alison Proctor (OFG), Phil and Tony Wass (OFG), and Nick Eloot (G-TEC) for their help during the GSRNOD15A expedition. The authors are grateful to Annick Van Kenhove, Guy De Smet and Dr. Ellen Pape (Marine Biology Research Group, UGent) for their help during sample processing. Special thanks go to Dr. Natalie Barnes (United Kingdom) for reviewing the English text of the manuscript. We are indebted to three anonymous reviewers for their helpful and constructive comments and recommendations.
Funding
The environmental baseline survey in the GSR license area is supported by a service arrangement between the private company Global Sea Mineral Resources N.V. and Ghent University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Sampling and field studies
Permits for sampling were not required.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under urn:lsid:zoobank.org:pub:45F0141D-4D98-40DC-B9EC-331EF6BF7901
Neoargestes laevis is registered in ZooBank under urn:lsid:zoobank.org
zoobank.org:act:D1DCE3B3-31A1-4E9A-A7D1-B90A348F8E77
Communicated by L. Menzel
Rights and permissions
About this article
Cite this article
Gheerardyn, H., George, K.H. Description of a new species of Neoargestes Drzycimski, 1967 (Copepoda, Harpacticoida, Argestidae) from the Clarion Clipperton Fracture Zone (Pacific Ocean), with remarks on the systematics of the genus. Mar Biodiv 49, 1891–1912 (2019). https://doi.org/10.1007/s12526-019-00951-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-019-00951-1