Abstract
A new parasitic copepod, Abyssotaurus vermiambatus gen. et sp. nov. of the family Serpulidicolidae Stock (Copepoda, Cyclopoida), was discovered during the RV Meteor cruise M48/1 (DIVA 1) in the deep sea of the southeast Atlantic Ocean. Despite the doubtless affiliation of A. vermiambatus gen. et sp. nov. to Serpulidicolidae, the new species cannot be allocated into one of the currently known genera. It presents three autapomorphies: (i) fusion of the cephalosome with the remaining body somites, (ii) development of a derived labrum, and (iii) modification of the mandibular gnathobase into a strong masticatory apparatus. In addition to a detailed species description, a comprehensive phylogenetic evaluation of Serpulidicolidae using 52 morphological characters was realized. It confirms the monophyly of Serpulidicolidae, whose family diagnosis had to be amended, and reveals its closest relation to Nereicolidae Claus. Furthermore, some brief remarks on the biology of Serpulidicolidae, including an updated species checklist, are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Serpulidicolidae Stock (Copepoda: Cyclopoida) is a small and poorly known group of Copepoda, which are found to be associated with polychaetes (Gravier 1912, 1913; Southward 1964; Stock 1979, 1989; Humes and Grassle 1979) and corals (Humes 1985). The body segmentation is partially or completely lost, and the extremities can be strongly modified. Until now, the Serpulidicolidae consisted of five genera, i.e., Serpulidicola Southward, 1964 (type genus; four species), Serpulidicoloides Boxshall & Halsey, 2004, Rhabdopus Southward, 1964, Rhynchopus Stock, 1979, and Parangium Humes, 1985 (all four monotypic). So far, Serpulidicolidae are known from coastal shallow waters from the northern Atlantic, the Gulf of Mexico, the Antarctic Sea, and the Banda Sea in the Indo-Pacific (Gravier 1912; Southward 1964; Humes and Grassle 1979; Stock 1979, 1989; Humes 1985). Depth ranges from the littoral down to a depth of 2506 m in the northern Atlantic (Southward 1964; Humes and Grassle 1979; Stock 1979, 1989; Humes 1985).
The DIVA 1 expedition (“Latitudinal gradients of bioDIVersity in the deep Atlantic”) was realized from July 6th to August 2nd, 2000 (research cruise M48/1 of German RV Meteor), aiming to study the benthic biodiversity in the Angola deep-sea Basin (Martínez Arbizu and Schminke 2005; Balzer et al. 2006).
In the material collected during the cruise, a single female specimen of a new copepod was discovered. While detailed comparison with the corresponding literature confirms its assignation to Serpulidicolidae, extending the distribution range of that family towards the southern Atlantic Ocean and to >5000 m depth, the specimen shows several deviating morphological features that inhibit its allocation in any of the currently known genera. Thus, a new genus, Abyssotaurus gen. nov., is established. The specimen is described as A. vermiambatus sp. nov. in the contribution on hand. Its systematic position as well as the phylogenetic relations of and within Serpulidicolidae is discussed. Furthermore, some brief remarks on the biology of Serpulidicolidae are added.
Materials and methods
One female was found in an Agassiz trawl at station #399, start position 18° 19.430′ S, 004° 42.059′ E (defined as locus typicus), end position 18° 24.553′ S, 004° 43.854′ E (Fig. 1), during research cruise M48/1 DIVA 1 of RV Meteor (Balzer et al. 2006).
On board, the specimen was preserved in 70% ethanol and subsequently transferred onto a slide, fixed with phenol containing “Kaisers glycerine-gelatine” stained with “light green” in a paraffin frame, and covered (Platt and Warwick 1988). Drawings were made of the whole specimen, using an Olympus interference contrast microscope equipped with a digital camera and a camera lucida. Since there was just a single specimen available, no further preparations or dissections were made. The holotype is deposited in the Zoological Museum Hamburg, Germany, ZMH no. K-40185.
Abbreviations used in the text: A1: antennula; A2: antenna; enp: endopod; exp.: exopod; FR: furcal rami; md: mandible; mxl: maxillula; mx: maxilla; mxp: maxilliped; P1–P6: first to sixth swimming legs.
Results
Taxonomy
Subclass: Copepoda Milne Edwards, 1840
Order: Cyclopoida Burmeister, 1834
Family: Serpulidicolidae Stock, 1979
Genus: Abyssotaurus gen. nov.
Type species: Abyssotaurus vermiambatus gen. et sp. nov.; monotypic
Cyclopoida versus “Poecilostomatoida”
For many years, it has been discussed if the “Poecilostomatoida” constitute a distinct copepod order or if they form a quite derived component of Cyclopoida. The authors follow Martínez Arbizu (2000) and Boxshall and Halsey (2004), who integrated “Poecilostomatoida” into Cyclopoida.
Abyssotaurus vermiambatus gen. et sp. nov.
Locus typicus: Angola Basin (southeast Atlantic Ocean), geographical position 18° 19.430′ S, 04° 42.059′ E, 5419–5443 m depth, sampled on 22nd July 2000 during RV Meteor cruise M48/1 (DIVA 1) (Balzer et al. 2006).
Material examined: Female holotype, put on one slide, collection number ZMH K-40185. Male unknown.
Etymology: The generic name is composed of “abysso” from Greek abyssos = the depth, “taurus” from Latin taurus = bull, as the antennules resemble the horns of a bull. The epitheton vermiambatus derives from the Latin vermis = worm and from the Greek ambates = rider, and is given because of the hypothetical way of living on hosts (cf. Southward 1964: 214, fig. A), reminding of a horseman.
Description of the female holotype
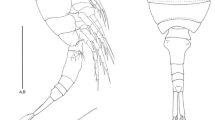
Habitus (Fig. 2a, b) vermiform, swollen, length 1560 μm, being composed by the prosome (cephalosome plus thoracic somites bearing P1–P5), followed by small genito-anal somite (last thoracic somite plus telson). Cephalosome and all body somites fused, former separations still indicated by transverse creases. Body surface wrinkled, only weakly sclerotized. Rostrum short, robust, situated mediofrontally as a blunt spine-like projection. Mouth opening (Fig. 3b) rounded, frontally bordered by a semi-circular small labrum, caudal edge mainly formed by a deepened, semi-circular area. Telson (Fig. 2a, b) with massive furcal rami being widely divergent. Anus located ventro-terminally.
A1 (Fig. 3a, b) five-segmented, first segment reaching 2/3rds of the total antennular length; at anterior subapical margin with 2 strong spines, apically with 1 spine and 2 slender bare setae. Second to fifth segments roughly equal in length, with the second and fifth segments being slightly longer than the third and fourth ones. Second and third segments without setae, fourth segment of left A1 subapically with 1 seta that is missing at counterpart. The fifth segment of left A1 terminally equipped with 3 fused setae, while its counterpart bears 4 setae (Fig. 3b).
A2 (Fig. 3a, b) uniramous, composed of 3 partly fused segments. Distal segment with 1 bare spine at its tip.
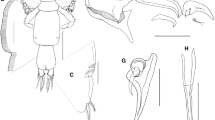
Labrum (Fig. 3b, b*) underneath the proximal mouth opening limitation, semi-circular, with four slightly bulbous elevations, each showing about 20 small circular structures.
md (Fig. 3b, b*) elongated and slender, gnathobase broadened, forming a strong masticatory apparatus; mandibular palp completely lost.
mxl (Fig. 3b, b*) arising beside and above the md basis, small, round, without setae. Apically with a stiletto-like thorn protruding into mouth opening.
mx (Fig. 3b, b*) consisting of two rounded humps. Anterior hump with four massive crescent-shaped teeth, posterior hump with fine setae.
mxp (Fig. 3b, b*) 4-segmented, subchelate, consisting of syncoxa, basis, and 2-segmented enp. All segments unarmed, except enp-2 that is equipped with a massive claw.
P1–P4 (Fig. 4a–h) directed outwardly, biramous, lacking intercoxal sclerites; coxa and basis not clearly separated/distinguishable. Exopods well developed, 3-segmented, apparently fused to basis. P1–P4 with two fields of (sensillar?) setules (fs in Fig. 4d) distally on first exp. segment; on exp.-1 and exp.-2 with 1 multipinnate, ladle-shaped spine at distal outer margin (Fig. 4e*) that is basally fused to the segment. P1 and P2 (Fig. 4a–d) exp.-2 additionally with 1 setular (sensillar?) field. P3 and P4 exp.-3 (Fig. 4e–h) as well as left P1 and P2 exp.-3 (Fig. 4a, c) subapically and apically with 1 multipinnate, ladle-shaped spine, but with only 1 respective spine on the right counterpart (Fig. 4b, d). Terminally, left P1 and P2 (Fig. 4a, c) as well as P3 (both limbs; Fig. 4e, f) with 1 small bare spine, which is missing at P4 exp.-3 (Fig. 4h). Endopods (Fig. 2b) tiny, 1-segmented and unarmed. For the setal formula, see Table 1.
P5 (Fig. 5a, b) stronger than P1–P4, without articulations, bending latero-caudally after a quarter of its length. Outer basal element produced into 1 bare spine; baseoendopod not discernible, exp. long and cylindrical, apically with 2 claw-like spines (Fig. 5b*).
P6 (Fig. 5c, d) small, flattened, and fin-like. Inserting dorsolaterally, reaching over dorsal surface of furcal rami. On posterior margin left limb medially with 2 broad spines, one of which serrated and bearing slender element (Fig. 5c). Right limb carrying only 1 serrated spine, lacking additional slender appendage (Fig. 5d).
FR (Fig. 2a, b) directing laterally, each ramus with 2 bare setae apically that reach almost half of the length of the ramus.
Amended diagnosis of Serpulidicolidae Stock, 1979
Cyclopoida. Adult female: cyclopiform or vermiform, body length between 0.77 and 2.5 mm; pedigerous somites separated or fused; in the latter case, cephalosome separated from or fused with pedigerous trunk; genital somite and following abdominal somites showing variable grades of fusion; paired genital apertures situated dorsally. Furcal rami diverging, in some species up to 180°. A1 3- to 6-segmented, first antennular segment often enlarged; A2 uniramous, 3-segmented, reduced in size or enlarged and transformed into clasping appendage. Mouthparts highly variable, often miniaturized; maxilliped 4-segmented, sometimes transformed in strong clasping organ with apical claw, sometimes with distal segment transformed in rounded, spinulose pad. Swimming legs P1–P5 located ventrolaterally, directed laterally. Intercoxal sclerites of P2–P4 always and of P1 sometimes missing. P1–P4 exopods 1- to 3-segmented, endopods absent or 1- to 2-segmented, tiny. P5 absent or 1-segmented, then often of increased size, being sometimes longer than P1–P4. P6 vestigial, with up to 4 elements. Male cyclopiform, dwarfed in comparison with female, body length 0.42–0.9 mm, attached to female (not confirmed for all species); body consisting of 5-segmented prosome and 6-segmented urosomes; all somites and cphs separate. FR not diverging. A1 7-segmented; A2 4-segmented and uniramous. Maxilliped large, produced into mighty clasping appendage, apically equipped with strong claw. Swimming legs located ventrally, biramous, well-developed, intercoxal sclerites present; exopods 3, endopods 2- to 3-segmented. P5 with coxobasis linked by small intercoxal sclerite, and with 1-segmented exopod.
Nine species in six genera: Serpulidicola Southward, 1964 (S. josephellae Humes & Grassle, 1979, S. omphalopomae Southward, 1964, S. placostegi Southward, 1964, S. segmentatus Stock, 1990); Parangium Humes, 1985 (P. abstrusum Humes, 1985); Rhabdopus Southward, 1964 (R. salmacinae Southward, 1964); Rhynchopus Stock, 1979 (R. catinatus Stock, 1979); Serpulidicoloides Boxshall & Halsey, 2004 (S. cystopomati (Gravier, 1912)); Abyssotaurus gen. nov. (A. vermiambatus gen. et sp. nov.).
Diagnostic key to species of Serpulidicolidae (females):
-
1.
Maxilliped proximally with strong, backwardly-pointed tooth…………………………............…Serpulidicola...2
Maxilliped without proximal strong, backwardly-pointed tooth; vermiform body shape, thoracic somites 1–4 fused …………………………….……..….........…5
-
2.
Body cyclopiform, pedigerous somites 1–4 separated….........…..……….S. segmentatus Stock, 1990
Body vermiform, pedigerous somites 1–4 fused………...................................................…………3
-
3.
Legs 1–4 with 2-segmented endopods……..........……...4
Legs 1–4 with 1-segmented endopods…S. josephellae Humes & Grassle, 1979
-
4.
Mandible with long, stiletto-like gnathobase; leg 5 2-segmented…….…..............…S. placostegi Southward, 1964
Mandible with short, curved gnathobase; leg 5 1-segmented…….…...S. omphalopomae Southward, 1964
-
5.
Exopods of legs 3 and 4 3-segmented; leg 5 1–2-segmented..……...……............…………………………..…….6
Exopods of legs 3 and 4 1-segmented, leg 5 absent…….....……..Parangium abstrusum Humes, 1985
-
6.
Legs 1–4 with 1–2-segmented endopods.......………..…7
Legs 1–4 lacking endopods ………............................8
-
7.
Legs 1–4 with 2-segmented endopods………Serpulidicoloides cystopomati (Gravier, 1912)
Legs 1–4 with 1-segmented endopods………Abyssotaurus vermiambatus gen. et sp. nov.
-
8.
Fifth leg 1-segmented, shorter than swimming legs 1–4…........................…..Rhynchopus catinatus Stock, 1979
Fifth leg 2-segmented, longer than swimming legs 1–4…..…….........Rhabdopus salmacinae Southward, 1964.
Discussion
The description of Abyssotaurus vermiambatus gen. et sp. nov. came about with a re-examination of serpulidicolid characters provided in the literature, uncovering, indeed, several discrepancies and perhaps even sporadic mistakes in some species descriptions. An exhaustive phylogenetic discussion would exceed the scope of the contribution on hand; it demanded detailed re-examination of (the type) material of all corresponding species, which cannot be achieved here. Moreover, the single serpulidicolid species presents special morphological adaptations like, e.g., the (at least partly) fusion of certain body sections and the strongly modified mouthparts, due to the respective specialized parasitic mode of life. That leads to uncertainties regarding the recognition of potential synapomorphic states of corresponding characters. Here, molecular taxonomy might be a fruitful approach in the future. Nonetheless, some morphology-based remarks on the phylogenetic relations within Serpulidicolidae shall be made. First, however, some confusion regarding particular morphological features shall be summed. Due to their ambiguous character, they had to be neglected in the following phylogenetic discussion.
Female body shape
In his family diagnosis of Serpulidicolidae, Stock (1979, p. 2 ff.) characterizes the female body shape as follows: “Female vermiform. Four pedigerous metasomites fused”. If compared with the four taxa that, according to Stock (1979), are closely related to Serpulidicolidae—Catiniidae Bocquet & Stock (Bocquet and Stock 1957), Clausidiidae Embleton (Embleton 1901), Clausiidae Giesbrecht (Giesbrecht 1895), and Nereicolidae Claus (Claus 1875)—the female vermiform body shape constitutes the derived state, whereas in the first three mentioned relatives, the females retain the ancestral cyclopiform body shape (e.g., Boxshall and Halsey 2004), while Nereicolidae Claus (Claus 1875) presents another deviation, with the female body becoming strongly swollen and rounded (e.g., Krøyer 1837; Keferstein 1863; Bresciani 1964; Boxshall and Halsey 2004). However, the vermiform body cannot be interpreted as autapomorphy for Serpulidicolidae, since the females of Serpulidicola segmentatus Stock, 1990 present the ancestral cyclopiform body shape, with the four pedigerous somites being separated. Therefore, the development of a vermiform body with fused pedigerous somites does not belong to the familiar ground pattern but must have evolved subsequently (and convergent) within Serpulidicolidae.
Fusion of urosomal somites
Another familiar character is, according to Stock (1979, p. 2), the presence of “only 1 (genito-anal) or 2 (genital and anal) somites behind fifth pedigerous somite” in serpulidicolid females. However, that becomes questionable with respect to Humes’ (1985) description of Parangium abstrusum Humes, 1985. On page 284, that author clearly notes “three postgenital segments”. Although that statement is contradicted by the author’s own illustrations (Humes 1985, p. 283, fig. 2a, b, e), which clearly show a fusion of the genital and the first postgenital somite, leading that, to only 2 postgenital somites, the number of segments following the fifth pedigerous somite is 3, which does not concur with Stock’s (1979) family diagnosis. That inconsistency has not yet been attended; Stock (1989) ignored it, whereas Boxshall and Halsey (2004, p. 656) simply stated that serpulidicolid females bear “an unsegmented posterior genitoabdomen representing fused genital and abdominal somites”.
Enlargement of female P5
According to Stock (1979), Serpulidicolidae share another unique morphological feature, namely a remarkable enlargement of the female fifth swimming leg. Stock (1979, p.4) assumed that such enlarged P5 is possibly “used for attachment inside the host’s tube”. However, Serpulidicola josephellae Humes & Grassle, 1979, S. placostegi Southward, 1964, and S. segmentatus lack such enlarged female P5, and it disappeared completely in Parangium abstrusum. Due to the difficulty in interpreting a decreased size of that limb as either the ancestral state or a secondary development from a former enlarged one, the enlarged P5 cannot be assumed as apomorphic for the family.
Males cyclopiform, dwarfed, attached to female
In parasitic Copepoda, the males are often dwarfed, retain the original cyclopiform body shape, and are attached to the females (e.g., Kabata 1979). Stock (1979) assigns that character as unique for Serpulidicolidae, if compared with the closely related families named above. Despite the potential phylogenetic relevance of that character, it has to be remarked that males are known only from three out of nine serpulidicolid species, i.e., Rhynchopus catinatus Stock, 1979, Serpulidicola placostegi, and S. omphalopomae Southward, 1964, while remaining unknown from Abyssotaurus vermiambatus gen. et sp. nov., Parangium abstrusum, Rhabdopus salmacinae Southward, 1964, Serpulidicola josephellae, S. segmentatus, and Serpulidicoloides cystopomati (Gravier, 1912). Thus, it may be somewhat precipitate concluding that the development of dwarfed males that are attached to the females may be an autapomorphy for Serpulidicolidae.
Exopods “versus” endopods
Gravier (1912, 1913) provided the first description of a serpulidicolid species, Serpulidicoloides cystopomati. According to the author, the swimming legs P1–P4 consist of rudimentary 2-segmented exopods and large 3-segmented endopods. This condition, already noted by Stock (1979), is absolutely the reverse compared to all remaining Serpulidicolidae, where the exopods are large and 3-segmented, whereas the endopods are minute, 1–2-segmented, or even absent. A plausible conclusion might be that Gravier (1912, 1913) erred, confounding the exopodal and endopodal limbs. Otherwise, it would mean that, in S. cystopomati, the swimming legs developed absolutely contrary to all other Serpulidicolidae, leading, however, to exactly the same shape of legs as in the other species; in our opinion, it is a quite implausible and dissatisfying explanation. Nevertheless, that discrepancy is completely ignored by Stock (1979, 1989) and Boxshall and Halsey (2004). These authors tacitly pass over Gravier’s (1912, 1913) probable mistake (if it is a mistake at all), noting down in their family diagnoses“(endopodite lacking or strongly reduced)” (Stock 1979, p.4), respectively “Female legs 1 to four typically biramous with 3-segmented exopod and extremely reduced, unarmed, 1 or 2-segmented endopods” (Boxshall and Halsey 2004, p. 656). Future careful examination of additional material of S. cystopomati has to elucidate if Gravier (1912, 1913) erred, or if that species actually presents quite derived swimming legs.
Autapomorphies of Serpulidicolidae
For the phylogenetic analysis, 52 relevant characters were recognized. They are listed in Table 2 and serve for the following character discussion. The results of the phylogenetic analysis are graphically summarized in Fig. 6.
According to Stock (1979), Serpulidicolidae belongs to the “clausidiid—clausiid—catiniid—nereicolid group”, with Clausidiidae as the most ancestral and Nereicolidae as the most derived taxon. As Serpulidicolidae presents a large number of derived features and shares four derived characters only with Nereicolidae (see below), it is concluded that, within the “clausidiid—clausiid—catiniid—nereicolid—serpulidicolid group”, the latter two families may be sister groups. Thus, Nereicolidae was selected for outgroup comparison. Synapomorphies of Serpulidicolidae and Nereicolidae are the fusion of at least some abdominal somites with the genital somite (Table 2, character 1) and the reduction of P2–P4 endopodal segments to at most 2 (characters 2–4), while Cataniidae, Clausidiidae, and Clausiidae retain all postgenital somites separated and present the ancestral state of 3-segmented P2–P4 endopods.
Leaving aside the above named unclear deviations regarding the body shape, composition of the female abdomen, enlarged female P5, and dwarfed males, and compared with the selected outgroup Nereicolidae, Serpulidicolidae can be characterized by nine autapomorphies (characters 5–13). Their females present divergent FR (character 5) least expressed in Serpulidicola segmentatus Stock, 1990 (57°) and Rhynchopus catinatus Stock, 1979 (62°), while reaching almost 180° divergence in Abyssotaurus vermiambatus gen. et sp. nov., Parangium abstrusum Humes, 1985, Rhabdopus salmacinae Southward, 1964, and Serpulidicola placostegi Southward, 1964. The ancestral cyclopiform shape of FR is observable in the described males of Rhynchopus catinatus, Serpulidicola omphalopomae, and S. placostegi (Southward 1964; Stock 1979). Moreover, it is retained in the families Clausidiidae, Clausiidae, and Cataniidae. Instead, Nereicolidae presents (among other apomorphies) a derived female body becoming strongly swollen and rounded (character 14), which encloses an extreme diminution of most appendages, including the FR. Thus, the monophyletic status of Serpulidicolidae is well supported by the named nine autapomorphies.
Assignment of Abyssotaurus vermiambatus gen. et sp. nov. to Serpulidicolidae
The female specimen of Abyssotaurus vermiambatus gen. et sp. nov. described here can doubtlessly be assigned to Serpulidicolidae, as it shares all supposed familiar autapomorphies 5–13 (Table 1, Fig. 6). Nevertheless, an allocation into any of the currently known genera is not possible, as each genus presents a series of supposed autapomorphies (Table 2) that are not shared by the new species described here. Furthermore, A. vermiambatus gen. et sp. nov. also shows three derived characters (Table 2, characters 44*–46), regarded as autapomorphies and, thus, justifying the establishment of the new species. For detailed discussion on the systematic position of the new species, see below.
Phylogenetic relationships within Serpulidicolidae
All serpulidicolid genera except Serpulidicola Southward, 1964 are monotypic and, thus, characterized by autapomorphies of the respective species. For phylogenetic comparison, only females are considered, due to the lack of knowledge of most males (see above).
Parangium abstrusum appears to be the most basal serpulidicolid species (Fig. 6), despite its high number of derived features. As shown in Table 2, that species is well characterized by eight autapomorphies missing in all remaining Serpulidicolidae (characters 24–31). Beyond its vermiform body shape including the fusion of the body somites, that species seems to evolve towards a “true vermiform” organism by reducing also its appendages: it presents a tiny 3-segmented A1 (characters 24 and 25), while all other Serpulidicolidae retain 4 (Rhynchopus), 5 (Abyssotaurus gen. nov., Rhabdopus, Serpulidicola), or 6 (Serpulidicoloides) antennular segments (outgroup Nereicolidae: 7 antennular segments). The A2 of that species is prehensile (character 26), enabling the animal to clasp on the host, a unique condition in Serpulidicolidae. That condition, which is clearly visible from Humes’ (1985, p. 283, Fig. 3f) detailed illustration of the buccal area, contradicts Stock’s (1979) family diagnosis, but is acknowledged by Boxshall and Halsey (2004). Furthermore, P. abstrusum shows 1-segmented P3 and P4 exopods (characters 27 and 28), these being 3-segmented in the remaining Serpulidicolidae. Moreover, P3 and P4 lack endopods (characters 29 and 30), which are at least 1-segmented in the remaining Serpulidicolidae. Finally, P. abstrusum lacks a P5 (character 31) that is present in all other serpulidicolid taxa. In contrast to the P5-related difficulties named above, the complete loss of the P5 can doubtlessly be interpreted as deviation, so it was included into the character matrix. Further indication for an ongoing reduction in P. abstrusum is the small size of the P1, the loss of endopods in P3 and P4, and the representation of the P6 by 2 min setae (Humes 1985). But because these features are also sporadically present in other serpulidicolid taxa, their phylogenetic evaluation must be postponed. The isolated position of P. abstrusum is substantiated by the fact that it is, so far, the only known serpulidicolid species not parasitizing on serpulid Polychaeta but on a scleractinian coral.
The remaining serpulidicolid taxa share apomorphies 15–23 (Table 2, Fig. 6). They present an enlarged first antennular segment (character 15), probably resulting from the fusion of former segments 1–3, as can still be noted in Serpulidicola omphalopomae. Moreover, they bear modified elements on the exopodal segments of P1–P4 (characters 16–23). These were named “lateroterminal, spinuliferous, pointed processes” by Stock (1979) and are considered here as homologue, derived spines that became ladle-shaped, at least in Abyssotaurus vermiambatus gen. et sp. nov. (cf. Figs. 2b* and 4).
Serpulidicoloides cystopomati forms the next branch (Fig. 6). It can be separated from the remaining species because of the particular shape of its P5 (character 35). It bears two apical opposite spines, reminding on a pair of nippers (Gravier 1913). Such an armature is unique in Serpulidicolidae and is, therefore, regarded as autapomorphy of that taxon.
The remaining Serpulidicolidae can be characterized by three apomorphies: all of them bear a “lateroterminal, spinuliferous, pointed process” also on P2–P4 exp.-2 (characters 32–34), which is missing in S. cystopomati.
The next branch is formed by Serpulidicola (Fig. 6), enclosing four species. The monophyly of that genus is well justified by 3 autapomorphies (Table 2). The md is slender and stiletto-like (character 36), the mxp presents a basal segment that is produced into a large, backwardly directed spine-like process (character 37), and its distal segment is rounded and densely covered with hairy setules (character 38). None of these derived features has evolved in the remaining Serpulidicolidae, so they are regarded as apomorphies of Serpulidicola. Within that genus, however, the systematic relationships remain unclear, due to lacking morphological characters. The descriptions of S. josephellae, S. omphalopomae, S. placostegi, and S. segmentatus are not detailed enough for in-depth morphological comparison. Thus, a corresponding phylogenetic analysis at the species level in that genus is pending.
The sister group of Serpulidicola is formed by Abyssotaurus gen. nov., Rhabdopus Southward, 1964, and Rhynchopus Stock, 1979 (Fig. 6), which share the derived loss of an intercoxal sclerite in the P1 (character 39). Whereas the derived loss of intercoxal sclerites in swimming legs P2–P4 took place in all Serpulidicolidae (see characters 11–13), these retained that sclerite in P1, with the exception of the three above-named genera. Thus, that character is regarded as synapomorphic for the group Abyssotaurus gen. nov.—Rhabdopus/Rhynchopus. Within that monophylum, Abyssotaurus gen. nov. can be characterized by three autapomorphies. Two of them are exclusively found in A. vermiambatus gen. et sp. nov., i.e., the particular shape of the labrum that, at its front, presents 4 bulbous elevations, each of which is furnished with a series of small circular structures (character 45) and the special shape of the mandibular gnathobase (character 46). Both character states are not only unique within Serpulidicolidae but also in the outgroup Nereicolidae used here. Therefore, they are considered as autapomorphic for Abyssotaurus gen. nov. It has to be kept in mind, however, that, especially in parasites, the buccal area may often be extremely transformed due to the adaptation to the respective hosts and the specialized mode of life. The third supposed autapomorphy (character 44*) seems to be a convergence, as Parangium also presents a fusion of the cephalosome with the body somites. Nevertheless, as stated above, Parangium is a highly derived serpulidicolid taxon presenting an advanced vermiform transformation. Thus, the fusion of cphs and body somites is a consequent step. On the other hand, that taxon lacks all apomorphies shared by the remaining Serpulidicolidae, including Abyssotaurus gen. nov. (Table 2, Fig. 6). Therefore, a convergent fusion of cphs and body somites in both Parangium and Abyssotaurus gen. nov. is most plausible and parsimonious.
Similar applies to the characters that confirm the monophyly of the Rhabdopus/Rhynchopus group. From the four synapomorphies of these two genera found (characters 40*–43, i.e., the complete loss of the P1–P4 endopods), two (characters 40* and 41*) are also present in Parangium. That taxon shares the endopodal loss of P3 and P4 with both Rhabdopus and Rhynchopus, which, in addition, lost the endopods of P1 and P2. Like for character 44, it appears most plausible and parsimonious assuming a convergent loss of P3 and P4 endopods in Parangium and the Rhabdopus/Rhynchopus group.
Finally, Rhabdopus and Rhynchopus can each be characterized by autapomorphies. Rhabdopus is the only taxon in Serpulidicolidae presenting a dense coverage with fine setules on the whole outer margins of P1–P4 (characters 47–50), considered here as autapomorphic. Instead, Rhynchopus shows an exclusively derived labrum (character 51) and paragnaths (character 52) that are entirely different from those of all remaining Serpulidicolidae, as well as from Nereicolidae.
Remarks on the biology of Abyssotaurus vermiambatus gen. et sp. nov.
Since there are neither observations of the living animal nor any further data about its habitat—beside the fact that it was found in an Agassiz trawl sample—only speculations about the biology of Abyssotaurus vermiambatus gen. et sp. nov. can be presented.
The sediment in the Angola Basin is dominated by foraminiferan shells and other biogenic detritus mixed with fine clay. Kröncke and Türkay (2003) measured a salinity of 34.8, a temperature of 2.48 °C near the sea bottom, a poor availability of nutrients, and a huge, up to 30 cm thick and oxidized sediment surface layer.
According to current knowledge, the members of the Serpulidicolidae are living parasitically or commensally on polychaetes or corals (Southward 1964; Stock 1979; Humes 1985; Boxshall and Halsey 2004). The species described here is equipped with four pairs of well-developed limbs, which would allow an active movement on the ground or on a larger host organism. Probably, the well-developed fifth thoracic limbs are used for clinging to the substrate or a host organism. At DIVA 1 station #399, the following polychaete taxa have been found: Orbiniidae: Scoloplos (Leodamas) sp. 1 (N = 1); Terebellidae: Pista sp. 1 (N = 20); Ampharetidae: Melinnopsis sp. 1 (N = 22); Oweniidae: Owenia sp. 1 (N = 1); Serpulidae: Protula sp. 1 (N = 9); Siboglinidae: Lamellisabella denticulata Southward, 1978 (N = 1); Lumbrineridae sp. (N = 1); and Maldanidae sp. (N = 1).
As most Serpulidicolidae have been found on Serpulidae (Table 3), which was also reported at DIVA 1 station #399, it might be speculated that Abyssotaurus vermiambatus gen. et sp. nov. possibly lives as a commensal or parasite on that polychaete species. Future investigations providing more material of A. vermiambatus gen. et sp. nov. might elucidate the answer to that question.
References
Balzer W, Alheit J, Emeis K-C, Lass HU, Türkay M (eds) (2006) South-East Atlantic 2000, Cruise No. 41, 6 July - 3 November 2000. METEOR-Berichte, Universität Hamburg 06–05:1–219
Bocquet C, Stock JH (1957) Copépodes parasites d’invertébrés des côtes de France. IVc. Le double parasitisme de Sipunculus nudus nov. gen., nov. sp. et Catadinia plana nov. gen., nov. sp., copepodes cyclopoïdes très remarkables. Proc K Ned Akad Wet (C) 60(3):425–431
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity, vol 166, part 1. The Ray Society, London
Bresciani J (1964) Sigecheres brittae gen. et sp. nov., a parasitic copepod from the polychaete Sige fusigera Malmgren. Ophelia 1(2):295–301
Claus C (1875) Beiträge zur Kenntnis parasitischer Copepoden nebst Bemerkungen über das System derselben. Z Wiss Zool 25:327–360
Embleton AL (1901) Goidelia japonica—a new entozoic copepod from Japan, associated with an infusorian (Trichodina). J Linn Soc Lond (Zool) 28:211–229
Giesbrecht W (1895) Mittheilungen über Copepoden. 10 u. 11. Mitt Zool Stn Neapel 12(1):217–226
Gravier MC (1912) Sur un type nouveau de Crustacé parasite d’un Serpulien de l’Antarctique sud-Americaine (Bactropus nov. g. cystopomati nov. sp.). Bull Mus nat Hist nat Zoology 18:67–71
Gravier MC (1913) Crustacés parasites. Deuxiéme Exped. franc. Sci Nat Doc Sci Paris: 27–77
Humes AG (1985) Poecilostomatoid copepods parasitic in the Scleractinian coral genus Goniastrea in the Moluccas. Publ Seto Mar Biol Lab 30(4/6):277–286
Humes AG, Grassle JF (1979) Serpulidicola josephellae sp. nov. (Copepoda, Cyclopoida) from a deep-water Polychaete west of Ireland. Crustaceana 36(3):309–315
Kabata Z (1979) Parasitic Copepoda of British fishes. The Ray Society, London
Keferstein W (1863) Ueber einen neuen Schmarotzerkrebs (Nereicola ovata Kef.) von einer Annelide. Z Wiss Zool 12:461–464
Kröncke I, Türkay M (2003) Structural and functional aspects of the benthic communities in the deep Angola Basin. Mar Ecol Prog Ser 260:43–53
Krøyer H (1837) Om Snyltekrebsene, isaer med Hensyn til den danske Fauna. Naturh Tidsskr 1(5):476–506
Martínez Arbizu P (2000) The paraphyly of Cyclopinidae Sars, 1913, and the phylogenetic position of poecilostome families within Cyclopoida Burmeister, 1835 (Copepoda: Crustacea). PhD thesis, University of Oldenburg, 345 pp
Martínez Arbizu P, Schminke HK (2005) DIVA-1 expedition to the deep sea of the Angola Basin in 2000 and DIVA-1 workshop in 2003. Org Divers Evol 5(S1):1–2
Platt HM, Warwick RM (1988) Free-living marine nematodes. Part II. British Chromadorids. In: Kermack DM, Barnes RSK (eds) Synopses of the British Fauna (new series), vol 38. E.J. Brill/Backhuys, Leiden, pp 14–21
Southward EC (1964) On three new cyclopoid copepods associated with deep-water polychaetes in the north-east Atlantic. Crustaceana 6(3):207–219
Stock JH (1979) Serpulidicolidae, a new family of Copepoda associated with tubicolous polychaetes, with descriptions of a new genus and species from the Gulf of Mexico. Mem Hourglass Cruises 5(2):1–11
Stock JH (1989) Présence de la famille des Serpulicolidae, Copépodes Poecilostomatoida associés aux Polychètes Serpulidés, dans l’Indo-Pacifique: Description d’une nouvelle espèce d’Indonésie. Indo-Malayan Zool 6:165–171
Acknowledgments
The authors would like to thank Prof F. J. Cristobo Rodriguez and Prof V. Urgorri (Universidade de Santiago de Compostela, Spain), who discovered and provided this unique specimen. Thanks to Prof. Dr J.-W. Wägele (Zoologische Forschungsmuseum Alexander Koenig, Bonn, Germany) and also thanks to Prof. G.A. Boxshall (Natural History Museum, London), PhD. J. Ho (California State University, Long Beach), Prof Dr. T. Schram (University of Oslo), Dr. T. Hansknecht, Dr. D. Defaye (Muséum national d’Histoire naturelle, Paris), PhD. G. W. Benz (Tennessee Aquarium and Research Institute, Chattanooga), and Dr. B. Jones (Department of Fisheries South Perth, Australia), who kindly supported this work with helpful comments and discussions. We are grateful to Dr. B. Ebbe (Alfred Wegener Institut für Polar- und Meeresforschung, Bremerhaven, Germany) and A. Allspach and Dr. D. Fiege (Senckenberg Forschungsinstitut und Naturmuseum, Frankfurt/Main, Germany) for their help with the identification of the Polychaeta. The authors would like to thank A. Kogelheide and I. Manstedt (University of Bochum) for their help with the illustrating work. This study was supported by grants of the Deutsche Forschungsgemeinschaft (DFG) under contract nos. WA 530/27-1 and WA 530/23-2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Gollner
This species is registered in ZooBank under: urn:lsid:zoobank.org:act:C848361A-2CA9-4C75-B21C-8B4539940811
Rights and permissions
About this article
Cite this article
Brenke, N., Fanenbruck, M. & George, K.H. A new parasitic deep-sea copepod from the Angola Basin (southeast Atlantic Ocean): Abyssotaurus vermiambatus gen. et sp. nov. (Copepoda: Cyclopoida: Serpulidicolidae Stock, 1979), with remarks on serpulidicolid systematics and a key to the species. Mar Biodiv 48, 517–530 (2018). https://doi.org/10.1007/s12526-017-0724-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-017-0724-1