Abstract
The aim of this study was to evaluate compositional differences between harpacticoid (Crustacea, Copepoda) assemblages at two widely separated abyssal locations. During the DIVA 1 cruise of RV METEOR (July/August 2000) to the Angola Basin (Southeast Atlantic), two deep-sea stations, approximately 300 nautical miles apart (Stations 325 and 346), were sampled repeatedly by Multicorer (MUC). For quantitative analyses, 5 MUC samples were selected at random from each of 15 deployments at both stations, totalling 75 cores. Across the study, 7,081 Harpacticoida specimens were encountered and of these 31.4 % were adults and could be analysed to species level: 682 species were identified, with 99.3 % new to science. At northern Station 346, a total of 600 species were recorded—the highest harpacticoid species number ever recorded for a single deep-sea locality. Most species (56 %) were represented by singletons. Multivariate tests identified significant differences between community compositions at the two stations. Diversity, species richness and species density were higher at Station 346, whilst taxonomic distinctness, evenness, and rarefaction were similar between stations. Regression and correlation analyses showed that the difference in species diversity was best explained by species densities rather than species richness. Under-sampling due to low densities was an issue at the southern Station 325. Nevertheless, our study demonstrated significant differences in regional-scale harpacticoid community structures within a single deep-sea basin that would usually be considered a uniform and stable habitat. These observed differences are thought to reflect differences in food availability at the two stations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meiofauna organisms are ubiquitous, and are the numerically dominant metazoans at all water depths (Rex et al. 2006; Wei et al. 2010). Concerning the deep sea, our knowledge of the large-scale distributional patterns of metazoan meiofauna species has grown over the last years. Communities of deep-sea meiobenthic taxa (primarily Nematoda and Harpacticoida) have been investigated across complete geographical areas (e.g. Baguley et al. 2006; Lambshead et al. 2000, 2001, 2002; Lampadariou et al. 2009) or over certain time periods (Shimanaga et al. 2004). Species turnover of abyssal Harpacticoida has been studied over large scales, showing that distribution ranges of single harpacticoid species can span thousands of kilometres (e.g. Gheerardyn and Veit-Köhler 2009; Kottmann et al. 2013; Menzel 2011a; Menzel et al. 2011; Pointner et al. 2013; Seifried and Martínez Arbizu 2008). Nevertheless, diversity of mega- and macro-fauna and their role in the ecosystem are far better documented, and species-level studies on all Harpacticoida in deep-sea communities are scarce (Coull 1972; Dinet 1973; Soltwedel 1997; Thistle 2001; Baguley et al. 2006).
Besides sediment characteristics, oxygen supply, hydrologic conditions (currents), and catastrophic physical disturbance, food supply plays an important role in structuring meiobenthic communities and species diversity in the deep sea (cf. Coull 1988; Lambshead et al. 2000, 2001, 2002; Levin et al. 2001; Soltwedel 2000; Tietjen 1984, 1992; Vanhove et al. 1995; Vanreusel et al. 1995a, b). Increased food resources should result in an increased number of corresponding feeders, and this theory has been supported by a number of recent studies (e.g. Baguley et al. 2006; Danovaro et al. 2000; Gutzmann et al. 2004; Lampadariou et al. 2009; Raes et al. 2010; Rose et al. 2005; Shimanaga et al. 2000). Moreover, it is known that primary productivity is higher near the coasts than offshore, even in deep-sea areas (e.g. Brown et al. 1991; Levin et al. 2001; Rex et al. 1993; Smith and Hinga 1983; Soltwedel 2000; Thistle 2003; Wasmund et al. 2005). However, environmental conditions in the deep sea are thought to change only slightly even over great distances (cf. Ramírez-Llodra et al. 2010; Thistle 2003; Türkay 2006; Tyler 2003).
The DIVA project (Latitudinal Gradients of Deep-Sea Biodiversity in the Atlantic Ocean), initiated in 2000, aims to develop a comprehensive data set to enable a reliable estimate of deep-sea biodiversity. So far, the bottom fauna, from the nano- to the megabenthos, have been studied across five Atlantic deep-sea basins during three DIVA expeditions (Balzer et al. 2006; Martínez Arbizu and Schminke 2005). In addition to an extensive taxonomic inventory of the benthos and the description of new species, a primary objective has been the comparison of different deep-sea basins regarding their taxonomic composition and species diversity (Türkay 2006). The three DIVA expeditions (DIVA 1, 2000; DIVA 2, 2005 and DIVA 3, 2009) have sampled extensively the deep-sea basins of the southern Atlantic Ocean, i.e. the Cape, Angola, Guinea, Argentine, and Brazil Basins. The study presented here relates to the DIVA 1 cruise to the Angola Basin.
DIVA 1 concentrated on a transect approximately 700 km long in the Angola Basin (Türkay 2006). One aim of this cruise was to evaluate whether the presumed environmental uniformity and stability within a single deep-sea basin holds true (e.g. Levin et al. 2001). Kröncke and Türkay (2003) recorded decreased productivity, biomass, and abundance of the mega- and macrobenthos from north to the south along the investigated DIVA 1 transect, casting doubt on the assumption of environmental uniformity for the Angola Basin.
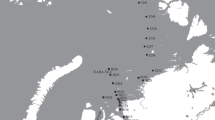
Two Stations of this transect (Fig. 1), one southern (325) and one northern (346), were sampled repeatedly by Multicorer (MUC). This was the first study where a comparable, intense sampling effort has been carried out at two stations. The replicated, quantitative data set was used to compare these stations regarding their taxonomic composition and species diversity. Rose et al. (2005) demonstrated a significantly higher within-core alpha diversity of harpacticoid copepods at the species-level, and higher individual densities at the northernmost Station 346 compared to the southern Station 325. This earlier study calculated only within-core diversities since species designations had not been integrated across the stations but the data compiled allows a hitherto unmatched investigation of community diversity in the deep sea.
Based on the results of Rose et al. (2005), and taking into account the different productivity regimes of the two investigated stations, the following null hypothesis was tested:
The Copepoda Harpacticoida assemblages of the two stations are statistically indistinguishable in their structure (taxa composition) and diversity.
Materials and methods
Sampling protocol
Sampling took place from 6 July to 2 August 2000 during the DIVA 1 campaign of RV METEOR (cruise M48/1) to the Angola Basin (Southeast Atlantic). Two stations, Stations 325 and 346 were sampled repeatedly by Multicorer (MUC)—the most quantitative sampling device for soft sediments (Barnett et al. 1984; Bett et al. 1994; Shirayama and Fukushima 1995), each deployment providing ten cores (inner diameter, 9.6 cm, so one core covers 72.4 cm2). Station 325 (19°58.2΄S, 02°59.8΄E; depth, 5,448 m, 550 nautical miles west from the African coast) was located 300 nautical miles south-west of Station 346 (16°17.0΄S, 05°27.0΄E; depth, 5,389 m, 350 nautical miles west from the African coast) (Fig. 1). The stations correspond to “working areas” 3 and 6 in Kröncke and Türkay (2003) and Fiege et al. (2010), and were characterised by differing productivity resulting in divergent biomass and abundance of the mega- and macrobenthos. They were sampled eight times each, every deployment being considered as a replicate, with five of the ten cores from each replicate selected at random to remove operator bias in an attempt to achieve representative subsampling of the population (corresponding to 362.0 cm2 per replicate). At Station 325, only seven replicates were successful, providing a total of 35 cores (subsamples) (corresponding to 2,534.0 cm2), whilst at Station 346 all eight replicates were successful yielding 40 subsamples for further analysis (corresponding to 2,896.0 cm2). Hence, 15 replicates comprising 75 subsamples (i.e., 5,430.0 cm2 = 0.54 m2) were analysed quantitatively for the present study.
To get the meiofauna, the upper 5 cm layer of sediment was taken from each subsample. The sediment was preserved in 5 % formaldehyde solution on ship. In the laboratory, the fixed subsamples were washed with tap water through a 40 μm-mesh sieve. Meiofauna and organic material were then extracted from the remaining sediment by centrifugation with a colloidal silica aquasol (Levasil®) as the flotation medium, using kaolin to cover the heavier particles (Somerfield et al. 2005). Each subsample was centrifuged three times at 4,000 rpm for 6 min, after each spin the floating matter was decanted on to a 40 μm-mesh sieve and rinsed with tap water. Copepods were subsequently transferred to glycerine, and the undamaged adult harpacticoid copepods were analysed quantitatively at species level (Appendix A: species-stations matrix).
Species identification
Assignment to species of the harpacticoid taxa was made in accordance with the recent nomenclature, using a variety of identification keys (Boxshall and Halsey 2004; Huys et al. 1996; Lang 1948; Wells 2007) and original descriptions. For the systematic status of Dactylopusiidae Lang, 1936, and Pseudotachidiidae Lang, 1936, we follow Willen (2000) and Willen (2002) for Miraciidae Dana, 1846. Idyanthidae Lang, 1944, Neobradyidae Olofsson, 1917, Tisbidae Stebbing, 1910, and Zosimeidae Seifried, 2003, are assigned according to Seifried (2003), and Aegisthidae Giesbrecht, 1892, and Rometidae Seifried and Schminke, 2003 according to Seifried and Schminke (2003).
Species identification resulted in an extensive species-station matrix (Appendix A) that formed the basis of community analysis. Adult individuals were identified to species level, whether or not assignment to a known genus or family of Harpacticoida was possible. In such cases, species were assigned to the lowest possible supraspecific taxon level (e.g. Podogennonta sp. 1, Ameiroidea sp. 10, Diosaccinae sp.). No species of Ameiridae was assigned to generic level.
Statistical data analysis
Similarity analysis
Similarity analysis was performed using Cosine Similarity. According to Pfeifer et al. (1998), the Cosine Similarity, as a more “qualitative” similarity measure, may reveal the structural properties of communities more clearly than more “quantitative” ones. In particular the Bray-Curtis coefficient, which is widely accepted in ecology, “has the major drawback that it separates ‘similar’ monitoring units if the difference is mainly due to multiples in abundance” (Pfeifer et al. 1998: p 65). Therefore it often requires data transformation (e.g. fourth root), especially when comparing different sampling sites. However, data transformation reduces the acquainted data (here: abundance values) into direction of the level of ranks, which results in a loss of information. Thus, the Cosine Similarity is increasingly preferred as an adequate similarity measure in faunistic analyses (e.g. Pfeifer et al. 1998; Rose 2001; Shimanaga et al. 2004; George 2005; Veit-Köhler et al. 2008) and also adopted in the present contribution. To visualise potential differences between the two assemblages, a two-dimensional non-metrical multidimensional scaling (nMDS) was applied. In order to test for significant differences, a minimum spanning tree-test (MST; cf. Schleier and van Bernem 1996 for detailed description of the test procedure) was made, analogous to Rose et al. (2005). Like the commonly applied “analysis of similarities” (ANOSIM; Clarke 1993), the MST is based on the estimated similarity values of all pairs of samples (Schleier and van Bernem 1996), i.e. upon the similarity matrix. In a first step, the samples (here: seven replicates of Station 325 and eight replicates of Station 346) were ordered (Schleier and van Bernem 1996). The minimum spanning tree consists of a series of lines connecting directly those samples which are closest together. After tree building, all lines that connect samples of different stations are removed, leaving “subtrees” that link only samples from the same station. The resulting number of subtrees is the test statistic (Schleier and van Bernem 1996). If significantly fewer subtrees than expected under randomised conditions were left, the two stations would be significantly different. The nMDS ordination served as a graphic basis for a drawing of the tree (Rose et al. 2005). As the original software SPANTREE 1.0 was not available to the authors, the MST-test was performed using the software PAST 2.00 (Hammer 2010).
Diversity analyses
As a first qualitative comparison of the absolute species number, S pointed towards much higher species diversity at Station 346 than at Station 325, estimation of species diversity was undertaken using several different coefficients, aiming to elucidate if possible differences might be related to species richness or species density. Very first insights into species diversity are provided by the N/S ratio (cf. Rose et al. 2005), which is expressed by the relationship between number of individuals (N) and species number (S). Also Shannon’s H’, Pielou’s Evenness J’, and Simpson’s Evenness 1-λ consider the distribution of species over individuals. As each coefficient focusses on particular aspects of diversity (H’: measures the “average uncertainty” when predicting to which species a specimen selected randomly from given S and N belongs; J’: expresses H’ in relation to H’ max ; 1-λ: gives the probability that two specimens picked randomly from a given assemblage belong to the same species; Ludwig and Reynolds 1988), they may complement each other, delivering comprehensive insights into species diversity. In order to account for the different number of replicates collected at the stations, rarefaction (Hurlbert 1971) was additionally applied to directly compare estimates of expected species numbers [E(S n )] based on the same number of individuals. In contrast to the above mentioned diversity and Evenness indices, rarefaction does not refer to all species S and individuals N of the different stations, but rather interpolates the species numbers expected in a sample of n individuals (Hurlbert 1971; Ludwig and Reynolds 1988). This flattens the differences in size and amount of samples and replicates, enabling their direct comparison.
To detect potential influence of phylogenetic structures within the harpacticoid assemblages of both stations 325 and 346, average taxonomic diversity Δ, and average taxonomic distinctness Δ* (Clarke and Warwick 1998; Warwick and Clarke 1995) were calculated based on the 352 species for which genera and family associations could be determined. Following Warwick and Clarke (1995), taxonomic distances (ω ij ) ranged between 0 and 100; the higher the Δ and Δ* values, the larger the taxonomic distance within the community.
To estimate the number of species potentially overlooked during the study, three extrapolative non-parametric methods were applied to calculate expected number of species: Chao1, Chao2, and Jackknife1 (cf. Baltanás 1992; Chao 1984, 1987; Colwell and Coddington 1994; Heltshe and Forrester 1983; Palmer 1990, 1991). Although such extrapolative estimates of species numbers should be interpreted with caution, due to their tendency to produce biased values (mainly underestimating species numbers, cf. Rose 2001), they may give an approximation of expected species number provided no under-sampling took place.
All listed analyses were performed with the software PAST 2.00 (Hammer 2010) and PRIMER 6.1.6 (Clarke and Warwick 2001).
Results
Overall data
In total, the seven replicates (35 subsamples) from Station 325 and the eight replicates (40 subsamples) from Station 346 combined yielded 7,081 Harpacticoida specimens, of which 4,860 (68.6 %) were copepodids and 2,221 (31.4 %) adults. The adults were 76.2 % female and 23.8 % male. Of the adult specimens, 69 (3.1 %) were badly damaged and therefore unidentifiable; quantitative analyses at species-level were based on the remaining 2,152 adult Harpacticoida (Table 1).
Supraspecific taxa
Altogether, 20 known families were identified (Table 1), with an additional 53 individuals that could not be assigned to any a known family pooled as “Harpacticoida incertae sedis (i.s.)”. Five families reached dominance values >8 % (N >150), with Pseudotachidiidae showing highest abundance values (Table 1). A further five families each represented < 0.5 % of relative abundance (N <10), with Rometidae, Dactylopusiidae, and Rhizotrichidae being represented by 2, 1, and 1 specimen, respectively (Table 1).
Generally, Station 346 presented considerably higher abundance (N) values than Station 325 (Table 1, Fig. 2). Also, both stations exhibited different taxonomic compositions: Canuellidae, Tisbidae, Rometidae, and Dactylopusiidae were found exclusively at Station 346, with a total of 19 families recorded here, whilst 16 families were found at Station 325, with only Rhizotrichidae being restricted to that station (Table 1). The remaining 15 families were reported from both stations.
Harpacticoid families collected from Stations 325 and 346 (Angola Basin). Families are sorted according to the total number of collected individuals N (columns) and dominance (pie charts). “Others” refer to the following families (in decreasing order from left to right): Station 325 = Paramesochridae, Huntemanniidae, Ancorabolidae, Cletodidae, Aegisthidae, Miraciidae, Rhizotrichidae, Canuellidae; Station 346 = Neobradyidae, Paramesochridae, Zosimeidae, Canthocamptidae, Idyanthidae, Miraciidae, Huntemanniidae, Cletodidae, Aegisthidae, Ancorabolidae, Tisbidae, Canuellidae, Rometidae, Dactylopusiidae
At both stations, Pseudotachidiidae was the most abundant taxon (Table 1, Fig. 2). At Station 325 it was followed by Neobradyidae and Ectinosomatidae, whereas at Station 346 Argestidae, Ameiridae, and Ectinosomatidae showed next highest abundance values (Fig. 2).
Approximately half (51.3 %) of all determined species could be assigned to known genera. In addition to the 53 individuals named “Harpacticoida i.s.” (see above), none of the Ameiridae (i.e., Ameirinae + Stenocopiinae) species could be assigned to a known genus. It was remarkable that in some of the more species-rich families (Aegisthidae, Huntemanniidae, Zosimeidae) all collected species belonged to known genera, whereas in others (Canthocamptidae, Neobradyidae) more than 40 % did not (cf. Appendix A). The total number of determined genera was 58 (Appendix B). Of these 28 genera were collected at both stations (grey fields in Appendix B), while 3 genera were restricted to Station 325, and 27 genera were found exclusively at Station 346.
Species of Harpacticoida
From the 2,152 individuals of adult Harpacticoida collected, 682 species were identified. A condensed species list is shown in Appendix A. Only five of these species (0.7 %) were known to science at the time of sampling, 677 species (99.3 %) were new to science. The known species were: Marsteinia parasimilis (Dinet, 1974) (Neobradyidae), Mesocletodes robustus Por, 1965 (Argestidae), Microsetella norvegica (Boeck, 1865) (Ectinosomatidae), Selenopsyllus dahmsi Moura and Pottek, 1998 (Canthocamptidae), and Styracothorax gladiator Huys, 1993 (Idyanthidae). As part of the taxonomic goal of the CeDAMar Programme (http://www.cedamar.org), a further 27 harpacticoid species have been described recently (Bröhldick 2005; Bruch et al. 2011; Corgosinho and Martínez Arbizu 2010; George 2006a, b, 2008; George and Müller 2013; Gheerardyn and George 2010; Menzel 2011a, b; Menzel and George 2009; Pointner et al. 2013; Seifried and Martínez Arbizu 2008 Seifried and Schminke 2003; Seifried et al. 2007; Veit-Köhler 2004, 2005; Veit-Köhler and Drewes 2009; Willen 2005, 2008, 2009).
Harpacticoid densities were comparatively low, with average values of 1.37 individuals (ind.)/10 cm2 (standard deviation 0.27) and 6.02 ind./10 cm2 (standard deviation 1.18) at Stations 325 and 346, respectively.
Nearly 75 % of all recorded species belonged to just five families (25 %), namely Ameiridae, Pseudotachidiidae, Ectinosomatidae, Argestidae, and Neobradyidae (Table 1).
Cletodidae showed the highest N/S ratio (38.0), as this family comprised only one species with 38 individuals across the stations. Next highest N/S ratios were calculated for Paramesochridae and Ancorabolidae. In contrast, within the species-rich families of Ameiridae, Ectinosomatidae, and Pseudotachidiidae on average only few individuals were recorded per species, resulting in low N/S ratios (Table 1).
In total, 384 species (56.3 % of species, 17.9 % of individuals) were represented by singletons (Appendix A) and a further 111 species (16.3 %) were represented by two individuals. The most frequently recorded species were: Argestes angolaensis George, 2008 (N = 92), Paradanielssenia sp. 1 (N = 67), Argestes sp. 1 (N = 50), Ameiridae sp. 10 (N = 47), Cletodidae sp. (N = 38), Neobradyidae sp. 1 (N = 36), and Bradya kurtschminkei Seifried and Martínez Arbizu, 2008 (N = 26) (Appendix A). Argestes angolaensis, Ameiridae sp. 10, and Bradya kurtschminkei were found only at Station 346 (Appendix A, cf. George 2008; Seifried and Martínez Arbizu 2008). The remaining four species were present at both stations.
Numbers of individuals and species in single cores were almost always higher at Station 346 than at Station 325 (Fig. 3). At Station 325, a total of 172 species were found (N = 348), whilst 600 species were recorded at Station 346 (N = 1804) (Table 1, Appendix A). The stations shared 90 species (Appendix A), whilst 82 and 510 species were exclusive to Station 325 and Station 346, respectively. Thus, 592 species (86.8 %) were restricted to one station only.
Community analysis
The taxonomic distribution of the species over the families also differed between the stations. At Station 325 the community was dominated by species of Pseudotachidiidae, Ectinosomatidae, Ameiridae, and Neobradyidae, which together accounted for 100 (58.1 %) of the 172 species sampled at that station (Table 1, Fig. 2). In contrast, Ameiridae was the most species-rich taxon at Station 346, followed by Pseudotachidiidae, Ectinosomatidae, and Argestidae, the four families representing 421 (70.2 %) of the 600 species recorded (Table 1, Fig. 2).
Similarity analyses
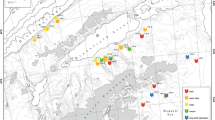
The result of Cosine Similarity analysis is shown in Fig. 4. The non-metric MDS plot (Fig. 4) visualises the separation of Stations 325 and 346 due to species composition and abundance values. The stress value (0.15) indicates a relatively unproblematic down-scaling of the measured similarities (Appendix C: similarity matrix) to the two dimensions of the nMDS plot. The northern Station 346 (red diamonds in Fig. 4 representing replicates 1–8) forms a highly distinct group, with all eight replicates located closely to each other and distant from Station 325 replicates (green circles in Fig. 4).
Two-dimensional non-metrical multidimensional scaling (nMDS) ordination plot of replicates based on all 682 collected harpacticoid species (green: Station 325, replicates 2–8; red: Station 346, replicates 1–8). Similarity index: Cosine Similarity (no data transformation, stress: 0.15). Blue line Minimum spanning tree, dotted line sole connection between the two stations
The MST-test (Fig. 4: blue lines between replicates), reveals that only two subtrees were left (continuous lines in Fig. 4) after removing all connections between replicates of different stations (just one such connection: dotted line in Fig. 4). This was significantly different (P = 0.0003) from the expected number of trees under random conditions (N = 8.47).
Hence, with respect to the taxa composition the null hypothesis must be rejected, since the similarity analyses showed that the assemblages were significantly statistically different.
Diversity analyses
Comparison of different diversity indices (Table 2) revealed a certain complexity of species diversity. When looking at absolute species number, S, Station 346 had a clearly higher species richness compared to Station 325, a result closely related to the much higher abundance at Station 346 (Table 2, Figs. 3, 5A). Also, Shannon’s H’ (Table 2, Fig. 5B) confirmed a higher species diversity at Station 346, values about three times higher than at Station 325. In contrast to the calculated much higher species diversity at Station 346, only a slightly higher rarefaction curve was calculated for Station 346 (Fig. 6). A detailed analysis of replicate level [sums of five subsamples] did not identify differences for E(S 30 ) but did find differences for E(S 100 ) estimates (Table 2).
a–d Diversity measures calculated for the replicates from Stations 325 and 346. a S Total species numbers, N abundance, Z Median, Min. minimum, Max. maximum, Q 1 quartile 1 (25 % of all measured data below that value), Q 3 quartile 3 (75 % of all measured data below that value). b H’ values. c Equitability measures, J’ and 1-λ. d Average taxonomic diversity Δ and average taxonomic distinctness Δ*
Evenness was high at both stations (both J’ and 1-λ > 0.9); however, J’ values were significantly higher at Station 325 than at Station 346 (P < 0.01) (Fig. 5C, Table 2). No significant difference (P > 0.05) was found in Simpson’s Evenness 1-λ between the stations.
Plotting number of individuals against number of species for the single cores, combined for both stations (Fig. 7) combined with a regression analysis gave an almost perfect linear regression (y = 0.8333x), S and N being strongly correlated (R 2 = 0.9821).
Average taxonomic diversity and average taxonomic distinctness
Table 2 provides Δ and Δ* values for all replicates from Station 325 and Station 346 (each replicate: five pooled subsamples). These were significantly (P < 0.05) higher for Station 325 (Δ = 72.58–75.94) than Station 346 (Δ = 72.07–74.13). Significant differences (P < 0.001) were also found for the Δ* values (Fig. 5D, Table 2).
Estimation of diversity via extrapolation
Table 3 lists the estimated species numbers for Stations 325 and 346 as calculated with Chao1, Chao2, and Jackknife1. The percentage of species recovered by our study at Station 325 lies between 39.7 % (Chao1) and 61.2 % (Jackknife1), with an average percentage of 47.7 %. This ratio is higher for Station 346 (average 54.4 %), ranging between 48.95 % (Chao2) and 63.6 % (Jackknife1). Roughly, the number of collected species accounts for approximately one-half of what could have been expected at the sampled stations. However, all estimates strongly depend on scale and intensity of sampling. Thus, the outcome (1) might be affected by under-sampling (especially for Station 325), and (2) cannot be extrapolated to other spatial scales (e.g. sub-regions of the Angola Basin).
Therefore, also with respect to the species diversity, the above formulated hypothesis must be rejected, since the diversity analyses showed that the assemblages were significantly statistically different.
Discussion
In the past 40 years, several quantitative studies on deep-sea Harpacticoida have been published (e.g. Coull 1972; Dinet 1973; Thiel 1983; Vincx et al. 1994; Soltwedel 1997; Thistle 2001; Baguley et al. 2006; Ramírez-Llodra et al. 2010; Kitahashi et al. 2012). Two of these studies, those by Dinet (1973) and Soltwedel (1997), included stations close to our sampling area.
Dinet (1973) compared meiobenthos sampled at both sides of the Walvis Ridge. From the 11 sampled stations, station no. 02 is located most closely to the DIVA 1 stations (Fig. 1, black square A). Soltwedel (1997) studied the distribution pattern of meiobenthos with respect to sedimentation of organic matter at 13 transects along the whole western African coast. The deepest station on transect 13 was near the DIVA 1 sampling area (Fig. 1, black square B). Both authors used different sampling methods to the current study (only three replicate cores sub-sampled from box corers, each study using different types of box core) and therefore direct data comparison was not possible. However, both Dinet (1973) and Soltwedel (1997) provided harpacticoid abundance data, as ind./10 cm2, which were also estimated for the DIVA 1 Stations 325 and 346. Abundances were considerably lower at the DIVA 1 stations. Dinet (1973) observed a clear difference in population density on each side of the Walvis Ridge, with highest densities in the Cape Basin (south of the ridge) and it might be assumed that abundance increased from north to south. However, this is not consistent with our own observations within the Angola Basin, which found higher abundances at the northern Station 346. Instead, it may be that in this earlier study the recorded abundance pattern reflected distance from the continental slope.
Among other factors, food supply is important for the structure and species diversity of meiobenthic communities (cf. Coull 1988; Lambshead et al. 2000, 2001, 2002; Levin et al. 2001; Soltwedel 2000; Tietjen 1984, 1992; Vanhove et al. 1995; Vanreusel et al. 1995a, b). A number of recent studies from the Antarctic (Gutzmann et al. 2004; Raes et al. 2010), the Mediterranean (Danovaro et al. 2000; Lampadariou et al. 2009), the Gulf of Mexico (Baguley et al. 2006), the Sagami Bay (Shimanaga et al. 2000) or the two stations from the Angola Basin presented herein (Rose et al. 2005) showed that increased food supply resulted in a higher number of organisms at the sea floor. However, Shimanaga et al. (2007) suggested for the Sulu Sea that food quantity had limited importance to organism density. Additionally, a trend of higher primary productivity near the coasts compared to offshore stations has been observed even for deep-sea areas (e.g. Brown et al. 1991; Levin et al. 2001; Rex et al. 1993; Smith and Hinga 1983; Soltwedel 2000; Thistle 2003; Wasmund et al. 2005).
Thus, since Dinet’s (1973) station no. 02 (although at almost the same depth) is located much closer to the African coast than DIVA 1 Stations 325 and 346, increased food supply may explain the higher abundances recorded there, and may also explain the lower abundances found at Station 325 compared to Station 346, given that it is furthest from the continental shelf. However, this discussion is qualified by the data provided by Soltwedel (1997), his station located nearest to the DIVA 1 study area was even closer to the coast than Dinet’s (1973) station 02, yet supported lower abundances (Table 4). Soltwedel (1997) observed discrepancies between potential organic matter input and meiobenthic abundance, and postulated that this resulted from fractionated sedimentation to the sea floor. This may also explain the different abundance values recorded at DIVA 1 Stations 325 and 346.
Comparing the faunistic results for Harpacticoida to those for macrobenthic groups studied during the DIVA 1 expedition, it is notable that no macrobenthic taxon has diversity values comparable to the harpacticoids. Of the 58 macrofaunal major taxa listed by Kröncke and Türkay (2003), only 6 have been investigated to species-level to date. Highest species and abundance values were reported for Isopoda (S = 100; N = 1,326: Brandt et al. 2005). They are followed by Polychaeta (S = 83; N = 1,047: Fiege et al. 2010), Tanaidacea (S = 50; N = 194), Cumacea (S = 45; N = 479), Amphipoda (S = 39; N = 150), and Mysidacea (S = 7; N = 34) (Brandt et al. 2005). The results confirm that, in the deep sea, the meiobenthos generally have a much higher diversity and abundance than the macrobenthos (e.g. Lambshead 1993; Rex 1983; Thiel 1983; Thistle 2001). Moreover, it has to be emphasised that the extraordinarily high number of harpacticoid species recorded here (682 species) was collected from only two stations, with 600 species at only one station (Station 346), while the macrobenthic studies in the same area considered five to seven stations along a complete transect (Brandt et al. 2005; Fiege et al. 2010; Kröncke and Türkay 2003). An even higher number of Harpacticoida species might be expected if all stations were considered.
Supraspecific taxa
Almost all of the 20 families found in the Angola Basin have been reported previously from the deep sea; two families were previously unreported (Parameiropsidae: Corgosinho and Martínez Arbizu 2010; Rometidae: Seifried and Schminke 2003). However, the systematics of many families has changed in the past decades, and therefore comparison at suprageneric level with data from other publications may be difficult (cf. Seifried 2004). Also, 35 species (5.1 %) did not belong to any known family of Harpacticoida, showing that in the deep sea new taxa can be discovered even at family level.
Comparing our data with those in the literature, two findings were evident:
-
1.
All studies revealed a similar taxonomic composition, confirming the cosmopolitan nature of Harpacticoida at family level, as concluded by Wells (1986), and extending this hypothesis into the deep sea (Gheerardyn & Veit-Köhler 2009; Menzel 2011a; Menzel et al. 2011);
-
2.
There are certain differences regarding the composition of dominant families between marine realms. The northeast Atlantic deep sea seems to be dominated by Cletodidae, Diosaccinae, Ectinosomatidae, Tisbidae, and particularly Cerviniinae (cf. Vincx et al. 1994). In the northern Gulf of Mexico, Tisbidae, Ectinosomatidae, Diosaccinae, Ameiridae, and Argestidae appear to dominate (Hicks & Coull 1983; Baguley et al. 2006). In the Pacific Ocean Ectinosomatidae, Ameiridae, Tisbidae (here including Zosimeidae for reasons of comparability), Argestidae and Miraciidae (Ahnert and Schriever 2001; Kitahashi et al. 2012) are dominant. The new data presented here show that the Angola Basin was dominated by Pseudotachidiidae, Argestidae, Ameiridae, Ectinosomatidae, and Neobradyidae, tallying with the North-Atlantic and Pacific data only partly. Only Ectinosomatidae may be regarded as dominant in the whole Atlantic deep sea.
Some families were dominant in the South-eastern Atlantic (Pseudotachidiidae, Neobradyidae) but only subdominant to subrecedent in the northern part, and conversely others (Diosaccinae, Cletodidae, Tisbidae, and Cerviniinae) may be dominant in the northern Atlantic but do not play a major role in the South-eastern Atlantic harpacticoid assemblage. Future deep-sea expeditions have to evaluate the validity of such trends. However, the partial absence of certain families from the Angola Basin (346, no Rhizotrichidae; 325, no Tisbidae, Canuellidae, Rometidae or Dactylopusiidae) must be considered an artefact of under-sampling. They were collected in very low frequencies, which indicate a rarity in this area. Thus, not to find a certain family at one or even both of the stations does not necessarily indicate their absence.
Stations 325 and 346 appeared to show different assemblage structures, the former station presented a relatively homogenous distribution of specimens over several families, whilst at the latter station most individuals belonged to few families that dominated the assemblage. Communities dominated by few taxa are more commonly found under conditions of higher productivity and often indicate the decrease phase of the unimodal productivity-diversity relationship (e.g. Rosenzweig 1995).
At genus level the number of unknown taxa was considerably higher: of the 647 species that could be assigned to a known family, 136 (21.0 %) did not belong to a known genus. These results clearly demonstrate the vast number of unknown deep-sea Harpacticoida, particularly at species level.
Species of Harpacticoida
Recently, Baguley et al. (2006) reported 696 harpacticoid species (distributed over 22Footnote 1 families) from the northern Gulf of Mexico. This impressively high number arises, however, from 43 stations covering an area of 1,130 x 273 km (see Fig. 1 in Baguley et al. 2006). Moreover, station depths range from 212 to 3,150 m, from the continental shelf down to the lower abyssal. Compared to those data, the 682 species collected from only two deep-sea stations in the Angola Basin during the DIVA 1 expedition is remarkable and unexpected. Although being separated by approximately 300 nautical miles, both stations were of approximately the same depth (5,400 m) and were in a generally uniform environment (Fiege et al. 2010; Kröncke and Türkay 2003). Also, 87.8 % of the species were sampled at Station 346 alone: 600 Harpacticoida species at one deep-sea station is the highest ever record from any deep-sea locality so far.
The high percentage (99.3 %) of undescribed species is greater than any other known record (e.g. George 2005, 84.4 %; George and Schminke 2002, 96.4 %; Shimanaga et al. 2004, p. 1099, did not identify to species level). However, in shallower studies, from the sublittoral down to the abyssal (cf. Humes 1994; George 2005, Schminke 2007; Seifried 2004; Veit-Köhler et al. 2010), a high number of previously unknown Harpacticoida species are generally recorded. Thus, in a global context, most Harpacticoida species are doubtlessly unknown to science. Consequently, a significant increase in taxonomic research is needed to address this situation (e.g. Boero 2010; Brökeland and George 2009; Mallet and Willmott 2003; Wheeler 2004; Zhang 2008).
Despite our attempt to optimise the experimental sampling design by conducting eight MUC deployments per station and treating each deployment as a single replicate consisting of five (randomly chosen) cores, some restrictions to quantitative analysis still remain. For instance, no statement can be made regarding the biased occurrence of a species, particularly if it was recorded in low density. The absence of a taxon from one station is not necessarily evidence for its absence from that locality but may rather reflect an insufficient sampling effort (see above). Even if a species was quite frequent at one station but not reported from the other one (e.g. Argestes angolaensis), a possible presence at the latter station could not be excluded. Bradya kurtschminkei and Emertonia andeep (Veit-Köhler, 2004), for example, were not recorded at Station 325, but B. kurtschminkei is known from the Guinea Basin, the Cape Basin, and the Porcupine Abyssal Plain (Seifried and Martínez Arbizu 2008), and E. andeep has been reported from the Guinea Basin and the Weddell Sea (Gheerardyn and Veit-Köhler 2009).
A wide distribution in the deep sea has been shown previously for species of the family Paramesochridae (Gheerardyn and Veit-Köhler 2009) and the argestid genus Mesocletodes (Menzel 2011a; Menzel et al. 2011). Also most of the five species already known show a wide distribution range: Mesocletodes robustus shows an Atlantic-wide distribution and was found also in Antarctic waters, the Mediterranean, and in the Eastern Pacific in depths ranging from 219 down to 5,000 m (see Menzel et al. 2011 for review); Microsetella norvegica is distributed world-wide, due to its planktonic living; however, its report from the deep sea is the first (cf. World Register of Marine Species, http://www.marinespecies.org); Selenopsyllus dahmsi was found in the Antarcic Weddell Sea at 2,000 m depth (Moura and Pottek 1998), and Styracothorax gladiator was collected northwest of Manila (Philippines, Pacific Ocean) in 2,050 m depth (Huys 1993). In contrast, Marsteinia parasimilis is the only species that had been reported previously from the Walvis Ridge in the Angola Basin (Dinet 1974).
Such wide distribution of the mentioned species is thought possible because, among other factors, environmental conditions change only slightly even over great distances (cf. Ramírez-Llodra et al. 2010; Thistle 2003; Türkay 2006; Tyler 2003). Against this background, it seems somewhat astonishing that only 13.2 % of the species recorded here in the Angola Basin (90 out of 682 species) were present at both stations. The stations were not separated by geological barriers, had the same depth and a similar sediment composition (but lower productivity) (Kröncke and Türkay 2003). At Station 346, a total of 600 species were recorded, of which 510 were exclusive to this site. Only 14.8 % of these 600 species (90 species) were also recorded at Station 325, where an additional 82 species were recorded. Thus from a total of 172 species, Station 325 shared 47.7 % species with Station 346. At Station 325, the low densities of species and individuals led to under-sampling compared to Station 346; this probably concealed much higher species numbers—possibly akin to Station 346. However, to sample these additional species, a much higher number of replicates than at Station 346 would have been necessary.
Community analysis
Qualitative comparisons between Stations 325 and 346 already indicated remarkable differences between their harpacticoid population structures. Species and specimen numbers per core per replicate (five cores per replicate) were much higher at Station 346 than Station 325. Multivariate similarity analysis confirmed this: the stations differed significantly regarding their community structure, as shown both by the MST-test. This is presumably caused by the lower absolute numbers of individuals in the replicates from Station 325. Similarly, differences in the number of individuals and species within Station 325 between cores also resulted in the higher relative differences between replicates in this low-abundance station, compared to the high-abundance Station 346. Hence, the greater dissimilarity of replicates from Station 325, as shown in the nMDS ordination, may be at least partly a mathematical artefact of the lower absolute abundances/under-sampling at that station.
As demonstrated by J’ and 1-λ values (Fig. 5C), both stations show a high evenness; however, the proportion of singletons was higher at Station 325 (65 %) than at Station 346 (57 %; Appendix A). This meant that at Station 325 replicates were more distinct from each other, than at Station 346, where densities were higher and under-sampling less severe. Data from Station 346 support the conclusions of Rose et al. (2005) regarding harpacticoid within-core diversity: the station showed low local-scale variability with respect to species composition and frequency, with all replicates being quite similar. In contrast, considerable local-scale differences were found at Station 325, and these occasionally exceeded differences at the regional scale (see Fig. 4, replicates 325/3, 325/4). However, under-sampling is more severe when densities are low, causing strong “pseudo-turnover” between replicates (i.e. measured turnover due to overlooked species; Whittaker 1998). Pseudo-turnover appears to be a strong factor at Station 325, artificially rendering the replicates from this station more dissimilar than those from Station 346. Nonetheless, the exact proportion of pseudo-turnover affecting our results remains unknown. Thus, for the Harpacticoida we reject the null hypothesis: the harpacticoid assemblages of Stations 325 and 346 are statistically distinct in their structure (taxa composition) and diversity.
Average taxonomic diversity Δ and average taxonomic distinctness Δ*, were slightly higher at Station 325 compared to Station 346. However, due to the currently limited knowledge of deep-sea Harpacticoida this is difficult to interprete. The relatively high Δ and Δ* values at both stations (>70) underline the existence of phylogenetically heterogeneous assemblages that are not formed by different but closely related species deriving from few common ancestors. That would have been a hint for a rather isolated fauna that possibly conquered the environment by radiation. In contrast, it seems more likely that large-scale taxonomic exchange occurs, which has in the meantime been confirmed for Mesocletodes species by Menzel et al. (2011), resulting in quite heterogeneous, species-rich assemblages at Stations 325 and 346, whose diversity becomes manifest even in higher taxonomic levels.
Regarding classical diversity analyses, harpacticoid density (ind./10 cm2) is about 4.5x higher at Station 346 than at Station 325 and, if comparing absolute S, Station 346 is much more diverse than Station 325. If including abundance, N, in an estimation of species diversity, H’, this still holds true: H’ ranges from 3.21 to 3.84 at Station 325, and from 4.30 to 4.94 at Station 346. However, when comparing species numbers accounting for abundance by rarefaction, diversity differences are not significant [e.g. for E(S 30 )]. Even though in this study the Multicorer sampling effort per abyssal deep-sea station was greater than ever before, our results indicate potential under-sampling at Station 325; we might have recorded higher species richness, similar to Station 346, if a greater number of individuals had been collected.
Furthermore, a qualitative look at the data reveals that, on average, at Station 325 every second specimen resulted in an additional species (N/S = 2.03), whereas at Station 346 three specimens were needed to add another species (N/S = 3.01). This again qualifies the characteristics of higher diversity, as S, at the northern Station 346, and is supported also by evenness J’, which is higher at Station 325 than at Station 346. It was estimated by Simpson’s 1-λ whether the number of frequent species at stations 346 and 325 was high or low, the index calculating the probability that two specimens chosen randomly belong to the same species. As the index was almost identical at the two stations, the probability of finding two specimens of the same species is not significantly different between Stations 325 and 346. The values are high for both stations, emphasising the low number of common species.
As also shown by Rose et al. (2005) for within-core alpha-diversity of harpacticoid copepods, a higher measured species richness was found at Station 346, since almost all cores from that station provided more species and specimens than those of Station 325. The almost perfect linearity seen in Fig. 7 indicates that the more specimens collected, the more species found, and that, even with the high sampling effort employed here, we are probably far from the asymptotic part of the collectors curve. Assuming severe under-sampling at least for Station 325, an increase in N might have led to a linear increase in S at that station for a considerable number of further individuals, possibly reaching similar species numbers as Station 346 with similar individual numbers. This leads to the conclusion that the difference in collected species numbers between the stations may be explained by species density rather than by species richness. However, under-sampling was probably also an issue for Station 346 as indicated by the linearity of the regression curve for this station also. This is confirmed by both the Rarefaction analysis as well as by extrapolative species estimation, as both methods suggest possible hyper-diversity but at the same time under-sampling.
Different productivity levels between the stations might explain the different densities recorded. This issue was discussed extensively by Rose et al. (2005) for both stations and can be summarised as follows: in contrast to Station 325, Station 346 was positioned in an area of upwelling with higher productivity. Under the assumption that deep-sea habitats generally show lower productivity compared to other marine or terrestrial habitats (Grassle 1989; Thiel 1983; Tietjen 1992; Valiela 1995), productivity can be regarded as a major limiting factor in deep-sea environments under normal circumstances. A peak or subsequent descending, productivity–diversity relationship will probably not be reached within the abyssal productivity range (following Rosenzweig 1995, p. 351: “As productivity rises from very low to moderate levels, diversity also rises”). However, we have shown that, at family-level, the descending part of the unimodal productivity–diversity curve might already have been reached. Thus, large-scale heterogeneity in food availability could be an important factor in structuring harpacticoid communities in the abyss of the Angola Basin, and possibly also in other deep-sea regions (Rose et al. 2005). Thus, it can be stated that Stations 325 and 346 differ noticeably in some aspects of species diversity, but less in others. By showing this, our investigation reflects the complexity of the term ‘diversity’. The study gives evidence for pronounced structural difference between the harpacticoid communities at Stations 325 and 346, which is expressed mainly by different abundance and species densities, probably caused by varying food availability in the Angola Basin.
References
Ahnert, A., & Schriever, G. (2001). Response of abyssal Copepoda Harpacticoida (Crustacea) and other meiobenthos to an artificial disturbance and its bearing on future mining for polymetallic nodules. Deep-Sea Research II, 48, 3779–3794.
Baguley, J. G., Montagna, P. A., Lee, W., Hyde, L. J., & Rowe, G. T. (2006). Spatial and bathymetric trends in Harpacticoida (Copepoda) community structure in the Northern Gulf of Mexico deep sea. Journal of Experimental Marine Biology and Ecology, 330, 327–341.
Baltanás, A. (1992). On the use of some methods for the estimation of species richness. OIKOS, 65, 484–492.
Balzer, W., Alheit, J., Emeis, K.-C., Lass, H.U., & Türkay, M. (2006). South-East Atlantic 2000. Cruise No. 48, 6 July 2000─3 November 2000. Meteor-Berichte, Universität Hamburg, 06–05
Barnett, P. R. O., Watson, J., & Connelly, D. (1984). A multiple corer for taking visually undisturbed samples from shelf, bathyal and abyssal sediments. Oceanologica Acta, 7, 399–408.
Bett, B. J., Vanreusel, A., Vincx, M., Soltwedel, T., Pfannkuche, O., Lambshead, P. J. D., Gooday, A. J., Ferrero, T., & Dinet, A. (1994). Sampler bias in the quantitative study of deep-sea meiobenthos. Marine Ecology Progress Series, 104, 197–203.
Boero, F. (2010). The study of species in the era of biodiversity: a tale of stupidity. Diversity, 2, 115–126.
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to Copepod Diversity, parts I and II. London: The Ray Society.
Brandt, A., Brenke, N., Andres, H. G., Brix, S., Guerrero-Kommritz, J., Mühlenhardt-Siegel, U., & Wägele, J. W. (2005). Diversity of peracarid crustaceans (Malacostraca) from the abyssal plain of the Angola Basin. Organisms, Diversity & Evolution, 5(1), 105–112.
Bröhldick, K. (2005). A new taxon of Idyanthidae (Copepoda, Harpacticoida) from the deep sea of the Angola Basin. Organisms, Diversity & Evolution, 5(1), 43–57.
Brökeland, W., & George, K. H. (2009). Editorial: Deep-sea taxonomy—a contribution to our knowledge of biodiversity. Zootaxa, 2096, 6–8.
Brown, P. C., Painting, S. J., & Cochrane, K. L. (1991). Estimates of phytoplankton and bacterial biomass and production in the northern and southern Benguela ecosystems. South African Journal of marine Science, 11, 537–564.
Bruch, K., Glatzel, T., & Veit-Köhler, G. (2011). Isthmiocaris laurae sp. nov. (Crustacea, Copepoda, Harpacticoida) from the Angola Basin—first deep-sea species of the genus with first remarks on its copepodid development. Meiofauna Marina, 19, 173–193.
Chao, A. (1984). Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics, 11, 265–270.
Chao, A. (1987). Estimating the population size for capture-recapture data with unequal catchability. Biometrics, 43(4), 783–791.
Clarke, K. R. (1993). Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18, 117–143.
Clarke, K. R., & Warwick, R. M. (1998). A taxonomic distinctness index and its statistical properties. Journal of Applied Ecology, 35, 523–531.
Clarke, K.R., & Warwick, R.M. (2001). Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: 2nd Edn, PRIMER-E.
Colwell, R. K., & Coddington, J. A. (1994). Estimating terrestrial biodiversity through extrapolation. Philosphical Transactions of the Royal Society of London Series B, Biological Sciences, 345, 101–118.
Corgosinho, P. H. C., & Martínez Arbizu, P. (2010). Ameiridae Boeck and Argestidae Por revisited, with establishment of Parameiropsidae, a new family of Harpacticoida (Crustacea, Copepoda) from deep-sea sediments. Helgoland Marine Research, 64, 223–255.
Coull, B. C. (1972). Species diversity and faunal affinities of meiobenthic Copepoda in the deep sea. Marine Biology, 1, 48–51.
Coull, B. C. (1988). Ecology of the marine meiofauna. In R. P. Higgins & H. Thiel (Eds.), Introduction to the Study of Meiofauna (pp. 18–38). Washington: Smithsonian Institution Press.
Danovaro, R., Tselepides, A., Otegui, A., & Della Croce, N. (2000). Dynamics of meiofaunal assemblages on the continental shelf and deep-sea sediments of the Cretan Sea (NE Mediterranean): relationships with seasonal changes in food supply. Progress in Oceanography, 46, 367–400.
Dinet, A. (1973). Distribution quantitative du méiobenthos profond dans la région de la dorsale de Walvis (Sud-Ouest-Africain). Marine Biology, 20, 20–26.
Dinet, A. (1974). Espèces nouvelles des copepodes harpacticoïdes (Crustacea) des sediments profonds de la dorsale de Walvis. Archives de Zoologie experimentale et génerale, 115, 549–577.
Fiege, D., Ramsey, P. A., & Ebbe, B. (2010). Diversity and distributional patterns of Polychaeta in the deep South Atlantic. Deep-Sea Research I. doi:10.1016/j.dsr.2010.06.012.
George, K. H. (2005). Sublittoral and bathyal Harpacticoida (Crustacea: Copepoda) of the Magellan region. Composition, distribution and species diversity of selected major taxa. Scientia Marina, 69, 147–158.
George, K. H. (2006a). New Ancorabolinae Sars, 1909 (Copepoda: Harpacticoida: Ancorabolidae) of the Atlantic and the Pacific Ocean. The taxa Ceratonotus Sars, and Dendropsyllus Conroy-Dalton. Meiofauna Marina, 15, 87–122.
George, K. H. (2006b). Ancorabolinae Sars (Copepoda: Harpacticoida: Ancorabolidae) of the deep Atlantic Ocean. Ancorabolina chimaera gen. et sp. nov. and remarks to the evolution of the first natatorial leg in comparison with Laophontoidea T. Scott. Meiofauna Marina, 15, 157–176.
George, K. H. (2008). Argestes angolaensis sp. nov. (Copepoda: Harpacticoida: Argestidae) from the Angola Basin (Southeast Atlantic), and the phylogenetic characterization of the taxon Argestes Sars, including the rediscription of A. mollis Sars, 1910, and A. reductus (Itô, 1983). Zootaxa, 1866, 223–262.
George, K. H., & Müller, F. (2013). Characterization of a monophylum Echinocletodes, its exclusion from Ancorabolinae (Copepoda, Harpacticoida), and displacement of E. bodini and E. walvisi to Cletodes, including the description of two new species. Zootaxa, 3666, 101–136.
George, K. H., & Schminke, H. K. (2002). Harpacticoida (Crustacea, Copepoda) of the Great Meteor Seamount, with first conclusions as to the origin of the plateau fauna. Marine Biology, 141, 887–895.
Gheerardyn, H., & George, K. H. (2010). New representatives of the genus Ancorabolina George, 2006 (Copepoda, Harpacticoida, Ancorabolidae) including remarks on ancorabolid phylogeny. Zoological Journal of the Linnean Society, 158, 16–55.
Gheerardyn, H., & Veit-Köhler, G. (2009). Diversity and large-scale biogeography of Paramesochridae (Copepoda, Harpacticoida) in South Atlantic abyssal plains and the deep Southern Ocean. Deep-Sea Research I, 56(10), 1804–1815. doi:10.1016/j.dsr.2009.05.002.
Grassle, J. F. (1989). Species diversity in deep-sea communities. Trends in Ecology & Evolution, 4, 12–15.
Gutzmann, E., Martínez Arbizu, P., Rose, A., & Veit-Köhler, G. (2004). Meiofauna communities along an abyssal depth gradient in the Drake Passage. Deep-Sea Research II, 51(14–16), 1617–1628. doi:10.1016/j.dsr2.2004.06.
Hammer, Ø. (2010). PAST—PAleontological STatistics, Version 2.00. Oslo: Reference Manual. Natural History Museum, University of Oslo 1999–2010.
Heltshe, J. F., & Forrester, N. E. (1983). Estimating species richness using the Jackknife Procedure. Biometrics, 39, 1–11.
Hicks, G. R. F., & Coull, B. C. (1983). The ecology of marine meiobenthic harpacticoid copepods. Oceanography and Marine Biology Annual Review, 21, 67–175.
Humes, A. G. (1994). How many copepods? Hydrobiologia, 292(293), 1–7.
Hurlbert, S. H. (1971). The non-concept of species diversity: a critique and alternative parameters. Ecology, 52(4), 577–586.
Huys, R. (1993). Styracothoracidae (Copepoda: Harpacticoida), a new family from the Philippine deep sea. Journal of Crustacean Biology, 13, 769–783.
Huys, R., Gee, J. M., Moore, C. G., & Hamond, R. (1996). Marine and brackish water harpacticoid copepods. Part 1. Synopsis of British Fauna, 51, 1–352.
Kitahashi, T., Kawamura, K., Veit-Köhler, G., Danovaro, R., Tietjen, J., Kojima, S., & Shimanaga, M. (2012). Assemblages of Harpacticoida (Crustacea: Copepoda) from the Ryukyu and Kuril Trenches, north-west Pacific Ocean. Journal of the Marine Biological Association of the United Kingdom, 92(2), 275–286. doi:10.1017/S0025315411001536.
Kottmann, J., Kihara, T. C., Glatzel, T., & Veit-Köhler, G. (2013). A new species of Wellsopsyllus (Copepoda, Harpacticoida, Paramesochridae) from the deep Southern Ocean and remarks on its biogeography. Helgoland Marine Research, 67, 33–48. doi:10.1007/s10152-012-0302-7.
Kröncke, I., & Türkay, M. (2003). Structural and functional aspects of the benthic communities in the deep Angola Basin. Marine Ecology Progress Series, 260, 43–53.
Lambshead, P. J. D. (1993). Recent developments in marine benthic biodiversity research. Océanis, 19(6), 5–24.
Lambshead, P. J. D., Tietjen, J., Ferrero, T., & Jensen, P. (2000). Latitudinal diversity gradients in the deep sea with special reference to North Atlantic nematodes. Marine Ecology Progress Series, 194, 159–167.
Lambshead, P. J. D., Tietjen, J., Moncrieff, C. B., & Ferrero, T. J. (2001). North Atlantic latitudinal diversity patterns in deep-sea marine nematode data: a reply to Rex et al. Marine Ecology Progress Series, 210, 299–301.
Lambshead, P. J. D., Brown, C. J., Ferrero, T. J., Mitchell, N. J., Smith, C. R., Hawkins, L. E., & Tietjen, J. (2002). Latitudinal diversity patterns of deep-sea marine nematodes and organic fluxes: a test from the central equatorial Pacific. Marine Ecology Progress Series, 236, 129–135.
Lampadariou, N., Tselepides, A., & Hatziyanni, E. (2009). Deep-sea meiofaunal and foraminiferal communities along a gradient of primary productivity in the eastern Mediterranean Sea. Scientia Marina, 73(2), 337–345.
Lang, K. (1948). Monographie der Harpacticiden. Königstein: Otto Koeltz Science Publishers.
Levin, L. A., Etter, R. J., Rex, M. A., Gooday, A. J., Smith, C. R., Pineda, J., Stuart, C. T., Hessler, R. R., & Pawson, D. (2001). Environmental influences on regional deep-sea species diversity. Annual Review of Ecology and Systematics, 32, 51–93.
Ludwig, J. A., & Reynolds, J. F. (1988). Statistical Ecology. New York: Wiley.
Mallet, J., & Willmott, K. (2003). Taxonomy: renaissance or Tower of Babel? Trends in Ecology and Evolution, 18(2), 57–59.
Martínez Arb, P., & Schminke, H. K. (2005). Editorial: DIVA-1 expedition to the deep sea of the Angola Basin in 2000 and DIVA-1 workshop in 2003. Organisms Diversity & Evolution, 5(1), 1–2.
Menzel, L. (2011a). First descriptions of copepodid stages, sexual dimorphism and intraspecific variability of Mesocletodes Sars, 1909 (Copepoda, Harpacticoida, Argestidae), including the description of a new species with broad abyssal distribution. ZooKeys, 96, 39–80.
Menzel, L. (2011b). A new species of Eurycletodes Sars, 1909 (Copepoda: Harpacticoida: Argestidae) from the southern hemisphere including remarks on the phylogeny of and within this genus. Helgoland Marine Research, 65, 479–493.
Menzel, L., & George, K. H. (2009). Description of four new species of Mesocletodes Sars, 1909 (Copepoda, Harpacticoida, Argestidae) and redescription of Mesocletodes robustus Por, 1965 from the South Atlantic, including remarks on the Mesocletodes abyssicola-group. Zootaxa, 2096, 214–256.
Menzel, L., George, K. H., & Martínez Arbizu, P. (2011). Submarine ridges do not prevent large-scale dispersal of abyssal fauna: a case study of Mesocletodes (Crustacea, Copepoda, Harpacticoida). Deep-Sea Research I, 58, 839–864.
Moura, G., & Pottek, M. (1998). Selenopsyllus, a new genus of Cylindropsyllinae (Copepoda, Harpacticoida) from Atlantic and Antarctic deep waters. Senckenbergiana maritima, 28, 185–209.
Palmer, M. W. (1990). The estimation of species richness by extrapolation. Ecology, 71(3), 1195–1198.
Palmer, M. W. (1991). Estimating species richness: the second-order Jackknife reconsidered. Ecology, 72(4), 1512–1513.
Pfeifer, D., Bäumer, H. P., Dekker, R., & Schleier, U. (1998). Statistical Tools for Monitoring Benthic Communities. Senckenbergiana maritima, 29(1/6), 63–76.
Pointner, K., Kihara, T. C., Glatzel, T., & Veit-Köhler, G. (2013). Two new closely related deep-sea species of Paramesochridae (Copepoda, Harpacticoida) with extremely differing geographical range sizes. Marine Biodiversity. doi:10.1007/s12526-013-0158-3.
Raes, M., Rose, A., & Vanreusel, A. (2010). Response of nematode communities after large-scale iceshelf collapse events in the Antarctic Larsen area. Global Change Biology, 16, 1618–1631.
Ramírez-Llodra, E., Brandt, A., Danovaro, R., De Mol, B., Escobar, E., German, C. R., Levin, L. A., Martínez Arbizu, P., Menot, L., Buhl-Mortensen, P., Narayanaswamy, B. E., Smith, C. R., Tittensor, D. P., Tyler, P. A., Vanreusel, A., & Vecchione, M. (2010). Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences, 7, 2851–2899.
Rex, M. A. (1983). Georgaphic patterns of species diversity in the deep-sea benthos. In G. T. Rowe (Ed.), Deep-Sea Biology (pp. 453–472). New York: Wiley.
Rex, M. A., Etter, R. J., Morris, J. S., Crouse, J., McClain, C. R., Johnson, N. A., Stuart, C. T., Deming, J. W., Thies, R., & Avery, R. (2006). Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Marine Ecology Progress Series, 317, 1–8.
Rex, M. A., Stuart, C. T., Hessler, R. R., Allen, J. R., Sanders, H. L., & Wilson, G. D. F. (1993). Global-scale latitudinal patterns of species diversity in the deep-sea benthos. Nature, 365, 636–639.
Rose, A. (2001). Räumliche und zeitliche Verteilungsmuster der Kurzflügelkäfer (Coleoptera, Staphylinidae) auf Nordsee-Düneninseln unterschiedlicher Sukzessionsstadien. Archiv zoologischer Publikationen, 5, 1–220.
Rose, A., Seifried, S., Willen, E., George, K. H., Veit-Köhler, G., Bröhldick, K., Drewes, J., Moura, G., Martínez Arbizu, P., & Schminke, H. K. (2005). A method for comparing within-core alpha diversity values from repeated Multicorer samplings, shown for abyssal Harpacticoida (Crustacea: Copepoda) from the Angola Basin. Organisms, Diversity & Evolution, 5(1), 3–17. doi:10.1016/j.ode.2004.10.001.
Rosenzweig, M. L. (1995). Species diversity in space and time. Cambridge (UK): Cambridge Universiy Press.
Schleier, U., & van Bernem, K.-H. (1996). A method to compare samples of soft bottom communities. Senckenbergiana maritima, 26(3/6), 135–144.
Schminke, H. K. (2007). Entomology for the copepodologist. Journal of Plankton Research, 29(1), i149–i162.
Seifried, S. (2003). Phylogeny of Harpacticoida (Copepoda): revision of “Maxillipedasphalea” and Exanechentera. Göttingen: Cuvillier.
Seifried, S. (2004). The importance of a phylogenetic system for the study of deep-sea harpacticoid diversity. Zoological Studies, 43(2), 8–19.
Seifried, S., & Martínez Arbizu, P. (2008). A new and exceptional species of Bradya Boeck, 1873 (Copepoda: Harpacticoida: Ectinosomatidae) from the abyssal plain of the Angola Basin and the variability of deep-sea Harpacticoida. Zootaxa, 1866, 303–322.
Seifried, S., & Schminke, H. K. (2003). Phylogenetic relationships at the base of Oligoarthra (Copepoda, Harpacticoida) with a new species as the cornerstone. Organisms, Diversity & Evolution, 3, 13–37.
Seifried, S., Plum, C., & Schulz, M. (2007). A new species of Parabradya Lang, 1944 (Copepoda: Harpacticoida: Ectinosomatidae) from the abyssal plain of the Angola Basin. Zootaxa, 1432, 1–21.
Shimanaga, M., Kitazato, H., & Shirayama, Y. (2000). Seasonal patterns of vertical distribution between meiofaunal groups in relation to phytodetritus deposition in the bathyal Sagami Bay, Central Japan. Journal of Oceanography, 56, 379–387.
Shimanaga, M., Kitazato, H., & Shirayama, Y. (2004). Temporal patterns in diversity and species composition of deep-sea benthic copepods in bathyal Sagami Bay, central Japan. Marine Biology, 144, 1097–1110.
Shimanaga, M., Nomaki, H., Suetsugu, K., Murayama, M., & Kitazato, H. (2007). Standing stock of deep-sea metazoan meiofauna in the Sulu Sea and adjacent areas. Deep-Sea Research II, 54, 131–144.
Shirayama, Y., & Fukushima, T. (1995). Comparisons of deep-sea sediments and overlying water collected using Multiple Corer and Box Corer. Journal of Oceanography, 51, 75–82.
Smith, K. L., & Hinga, K. R. (1983). Sediment community respiration in the deep sea. In G. T. Rowe (Ed.), Deep-Sea Biology (pp. 331–370). New York: Wiley.
Soltwedel, T. (1997). Meiobenthos distribution pattern in the tropical East Atlantic: indication for fractionated sedimentation of organic matter to the sea floor? Marine Biology, 129, 747–756.
Soltwedel, T. (2000). Metazoan meiobenthos along continental margins: a review. Progress in Oceanography, 46, 59–84.
Somerfield, P. J., Warwick, R. M., & Moensm, T. (2005). Meiofauna techniques. In A. Eleftheriou & A. McIntyre (Eds.), Methods for the study of marine benthos (pp. 229–272). Oxford: Blackwell Science.
Thiel, H. (1983). Meiobenthos and nanobenthos of the deep sea. In G. T. Rowe (Ed.), Deep-Sea Biology (pp. 167–230). New York: Wiley.
Thistle, D. (2001). Harpacticoid copepods are successful in the soft-bottom deep sea. Hydrobiologia, 453(454), 255–259.
Thistle, D. (2003). The deep-sea floor: an overview. In P. A. Tyler (Ed.), Ecosystems of the deep oceans (pp. 5–37). Amsterdam: Elsevier.
Tietjen, J. H. (1984). Distribution and species diversity of deep-sea nematodes in the Venezuela Basin. Deep-Sea Research, 31(2), 119–132.
Tietjen, J. H. (1992). Abundance and biomass of metazoan meiobenthos in the deep sea. In G. T. Rowe & V. Pariente (Eds.), Deep-sea food chains and the global carbon cycle (pp. 45–62). Dordrecht: Kluwer.
Türkay, M. (2006). Research program. In: W. Balzer, J. Alheit, K.-C. Emeis, H.U. Lass, & M. Türkay (Eds.), South-East Atlantic 2000. Cruise No. 48, 6 July 2000─3 November 2000. Meteor-Berichte, Universität Hamburg, 06–05, 1–3
Tyler, P. A. (2003). Introduction. In P. A. Tyler (Ed.), Ecosystems of the deep oceans (pp. 1–3). Amsterdam: Elsevier.
Valiela, I. (1995). Marine ecological processes. Berlin: Springer.
Vanhove, S., Wittoeck, J., Desmet, G., Van den Berghe, B., Herman, R. L., Bak, R. P. M., Nieuwland, G., Vosjan, J. H., Boldrin, A., Rabitti, S., & Vincx, M. (1995). Deep-sea meiofauna communities in Antarctica: structural analysis and relation with the environment. Marine Ecology Progress Series, 127, 65–76.
Vanreusel, A., Vincx, M., Bett, B. J., & Rice, A. L. (1995a). Nematode biomass spectra at two abyssal sites in the NE Atlantic with a contrasting food supply. Internationale Revue der gesamten Hydrobiologie, 80(2), 287–296.
Vanreusel, A., Vincx, M., Schram, D., & Van Gansbeke, D. (1995b). On the vertical distribution of the metazoan meiofauna in shelf break and upper slope habitats of the NE Atlantic. Internationale Revue der gesamten Hydrobiologie, 80(2), 313–326.
Veit-Köhler, G. (2004). Kliopsyllus andeep sp. n. (Copepoda: Harpacticoida) from the Antarctic deep sea – a copepod closely related to certain shallow-water species. Deep-Sea Research II, 51(14–16), 1629–1641. doi:10.1016/j.dsr2.2004.06.027.
Veit-Köhler, G. (2005). First deep-sea record of the genus Kliopsyllus Kunz, 1962 (Copepoda: Harpacticoida) with the description of Kliopsyllus diva sp. n.—the most abundant member of Paramesochridae at two different sites of the Angola Basin. Organisms, Diversity & Evolution, 5, 29–42. doi:10.1016/j.ode.2004.10.001.
Veit-Köhler, G., Laudien, J., Knott, J., Velez, J., & Sahade, R. (2008). Meiobenthic colonisation of soft sediments in arctic glacial Kongsfjorden (Svalbard). Journal of Experimental Marine Biology and Ecology, 363, 58–65. doi:10.1016/j.jembe.2008.06.018.
Veit-Köhler, G., & Drewes, J. (2009). Kliopsyllus schminkei sp. n. (Copepoda, Harpacticoida, Paramesochridae)—a new copepod from the southeast Atlantic deep sea (Angola Basin). Zootaxa, 2096, 313–326, http://www.mapress.com/zootaxa/2009/f/zt02096p326.pdf.
Veit-Köhler, G., De Troch, M., Grego, M., Bezerra, T. N., Bonne, W., De Smet, G., Folkers, C., George, K. H., Guotong, C., Herman, R., Huys, R., Lampadariou, N., Laudien, J., Martínez Arbizu, P., Rose, A., Schratzberger, M., Seifried, S., Somerfield, P., Vanaverbeke, J., Vanden Berghe, E., Vincx, M., Vriser, B., & Vandepitte, L. (2010). Large-scale diversity and biogeography of benthic copepods in European waters. Marine Biology, 157(8), 1819–1835. doi:10.1007/s00227-010-1454-0.
Vincx, M., Bett, B. J., Dinet, A., Ferrero, T., Gooday, A. J., Lambshead, P. J. D., Pfannkuche, O., Soltwedel, T., & Vanreusel, A. (1994). Meiobenthos of the deep northeast Atlantic. Advances in Marine Biology, 30, 1–87.
Warwick, K. R., & Clarke, R. M. (1995). New “biodiversity” measures reveal a decrease in taxonomic distinctness with increasing stress. Marine Ecology Progress Series, 129, 301–305.
Wasmund, N., Lass, H.-U., & Nausch, G. (2005). Distribution of nutrients, chlorophyll and phytoplankton primary production in relation to hydrographic structures bordering the Benguela-Angolan frontal region. African Journal of Marine Science, 27(1), 177–190.
Wei, C.-L., Rowe, G. T., Escobar-Briones, E., Boetius, A., Soltwedel, T., Caley, M. J., Soliman, Y., Huettmann, F., Qu, F., Yu, Z., Pitcher, C. R., Haedrich, R. L., Wicksten, M. K., Rex, M. A., Baguley, J. G., Sharma, J., Danovaro, R., MacDonald, I. R., Nunnally, C. C., Deming, J. W., Montagna, P., Lévesque, M., Weslawski, J. M., Wlodarska-Kowalczuk, M., Ingole, B. S., Bett, B. J., Billett, D. S. M., Yool, A., Bluhm, B. A., Iken, K., & Narayanaswamy, B. E. (2010). Global patterns and predictions of seafloor biomass using random forests. PloS ONE, 5(12), e15323.
Wells, J. B. J. (1986). Biogeography of benthic harpacticoid copepods of the marine littoral and continental shelf. Syllogeus, 58, 126–135.
Wells, J. B. J. (2007). An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa, 1568, 1–872.
Wheeler, Q. D. (2004). Taxonomic triage and the poverty of phylogeny. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 359, 571–583.
Whittaker, R. J. (1998). Island Biogeography: Ecology, Evolution and Conservation. Oxford: Oxford University Press.
Willen, E. (2000). Phylogeny of the Thalestridimorpha Lang, 1944 (Crustacea, Copepoda). Göttingen: Cuvillier.
Willen, E. (2002). Notes on the systematic position of Stenheliinae (Copepoda, Harpacticoida) within the Thalestridimorpha and description of two new species from Motupore Island, Papua New Guinea. Cahiers de Biologie Marine, 43, 27–42.
Willen, E. (2005). A new species of Paranannopus Lang, 1936 (Copepoda, Harpacticoida, Pseudotachidiidae) with atrophic mouthparts from the abyssal of the Angola Basin. Organisms, Diversity & Evolution, 5(1), 19–27.
Willen, E. (2008). Pseudotachidiidae (Copepoda: Harpacticoida) from the Angola Basin and the Antarctic deep sea, with the description of a new species of Paradanielssenia Soyer,1970. Organisms Diversity & Evolution, 8(4), 249–250.
Willen, E. (2009). Nyxis rostrocularis, a new genus and species of Paranannopinae Por, 1986 (Copepoda, Harpacticoida) from the Southern Atlantic deep sea. Zootaxa, 2096, 299–312.
Zhang, Z.-Q. (2008). Contributing to the progress of descriptive taxonomy. Zootaxa, 1968, 65–68.
Acknowledgements
Sample treatment in the laboratories was carried out by Mrs Iris Zaehle (Carl von Ossietzky Universität Oldenburg, Germany). Mrs Viola Siegler (DZMB, Wilhelmshaven, Germany) helped with the preparation of illustrations. Special thanks are due to Dr. Natalie Barnes (London, UK) for correcting the English text and thus improving the quality of the manuscript. The participation of K.H.G. and E.W. on M48/1 DIVA 1 was supported financially by the Deutsche Forschungsgemeinschaft (DFG). The support provided to S. Seifried and L. Menzel by the DFG (SCHM 352/30, GE 1086/6, GE 1086–11) as well as that to P.H. Corgosinho by CeDAMar is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A
Species-stations matrix of the Copepoda Harpacticoida collected from the single subsamples of each replicate deployment at Stations 325 and 346 during the DIVA 1 expedition. Species in grey are present at both stations (N = 90) (XLSX 246 kb)
Appendix B
List of genera (presence/absence) collected at Stations 325 and 346 during the DIVA 1 expedition. In addition, the number of species recorded per genus is indicated. Genera in grey are present at both stations (XLSX 40 kb)
Appendix C
Similarity matrix based on Appendix A. Cosine Similarity, non-transformed data (XLSX 33 kb)
Rights and permissions
About this article
Cite this article
George, K.H., Veit-Köhler, G., Arbizu, P.M. et al. Community structure and species diversity of Harpacticoida (Crustacea: Copepoda) at two sites in the deep sea of the Angola Basin (Southeast Atlantic). Org Divers Evol 14, 57–73 (2014). https://doi.org/10.1007/s13127-013-0154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-013-0154-2